Multifaceted Effects of Thymoquinone on Platelet Calcium Homeostasis
Highlights
- Thymoquinone (TQ) strongly and acutely inhibited agonist-induced platelet activation and aggregation. TQ differentially regulates GPCR and GPVI receptor activation by enhancing [Ca2+]i mobilization by GPCR-induced activation and inhibition of GPVI-induced [Ca2+]i mobi-lization.
- Presented data are the first example in which complete inhibition of ADP- and Trap-6-, but not CRP-induced, aggregation is accompanied by high [Ca2+]i levels.
- TQ could be a valuable molecule for the analysis of calcium homeostasis in platelets and other cells.
- TQ is now considered a promising therapeutic agent against cancer, and our results, that TQ is a potent inhibitor of platelets, should be taken into account, especially in pathological situations with possible bleeding complications.
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Materials
2.3. Preparation of Platelet-Rich Plasma (PRP) and Washed Human Platelets (WPs)
2.4. Analysis of TQ-Induced Aggregation and Shape Change Reaction by the Laser Diffraction Method
2.5. Analysis of Platelet Ca2+ Mobilization
2.6. Flow Cytometry Analysis
2.7. Western Blot Analysis
2.8. Data Analysis
3. Results
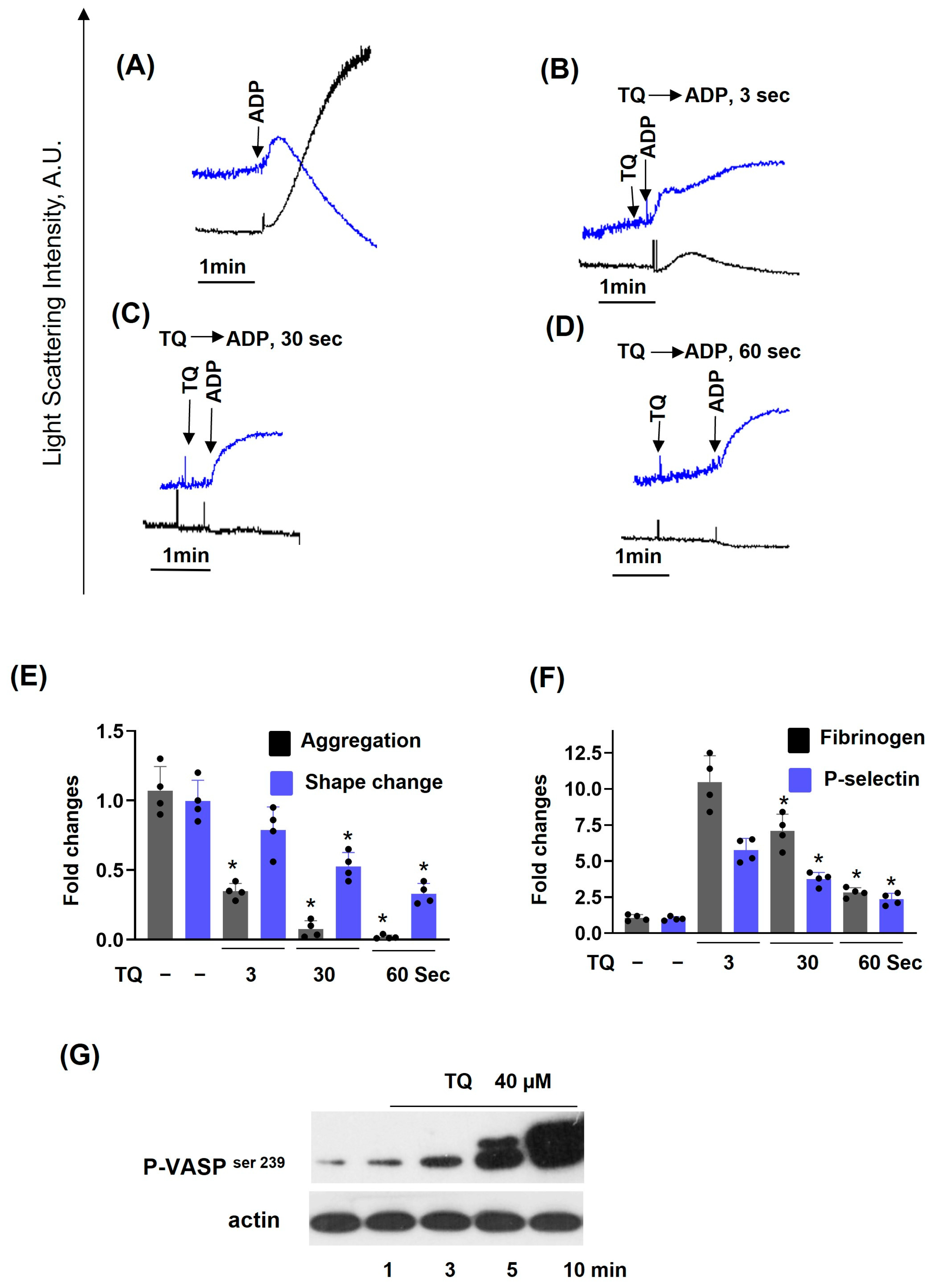
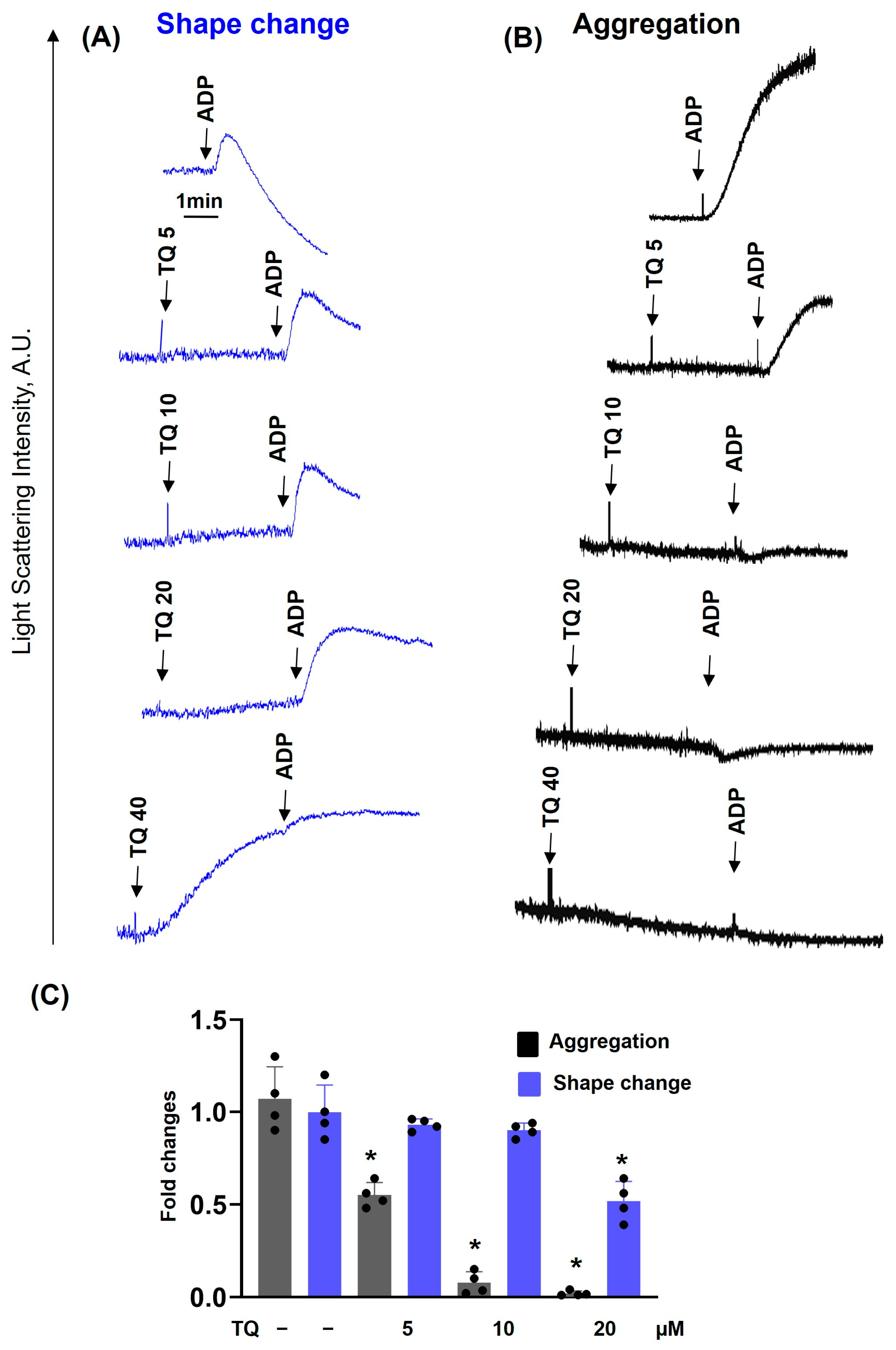
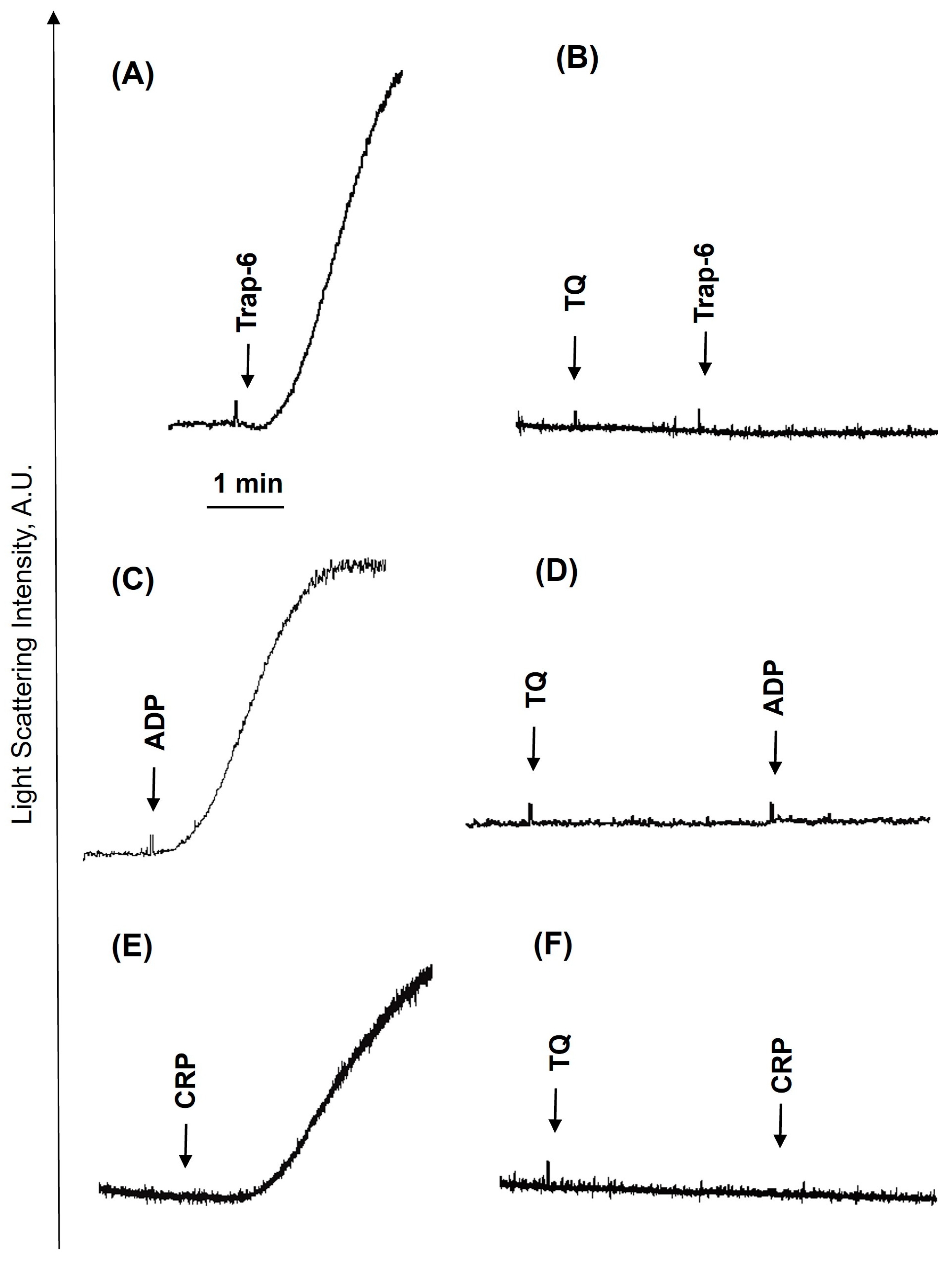
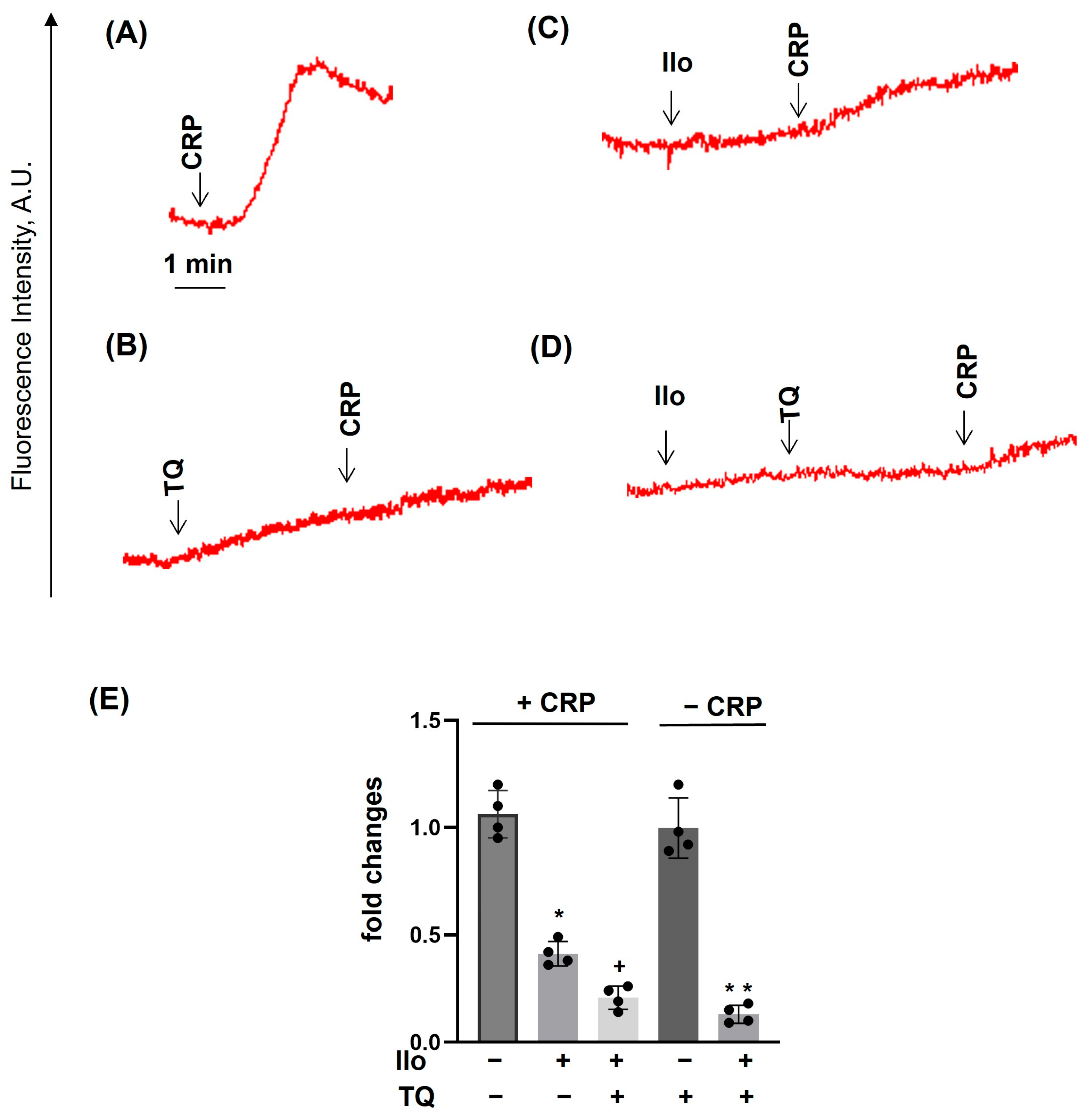
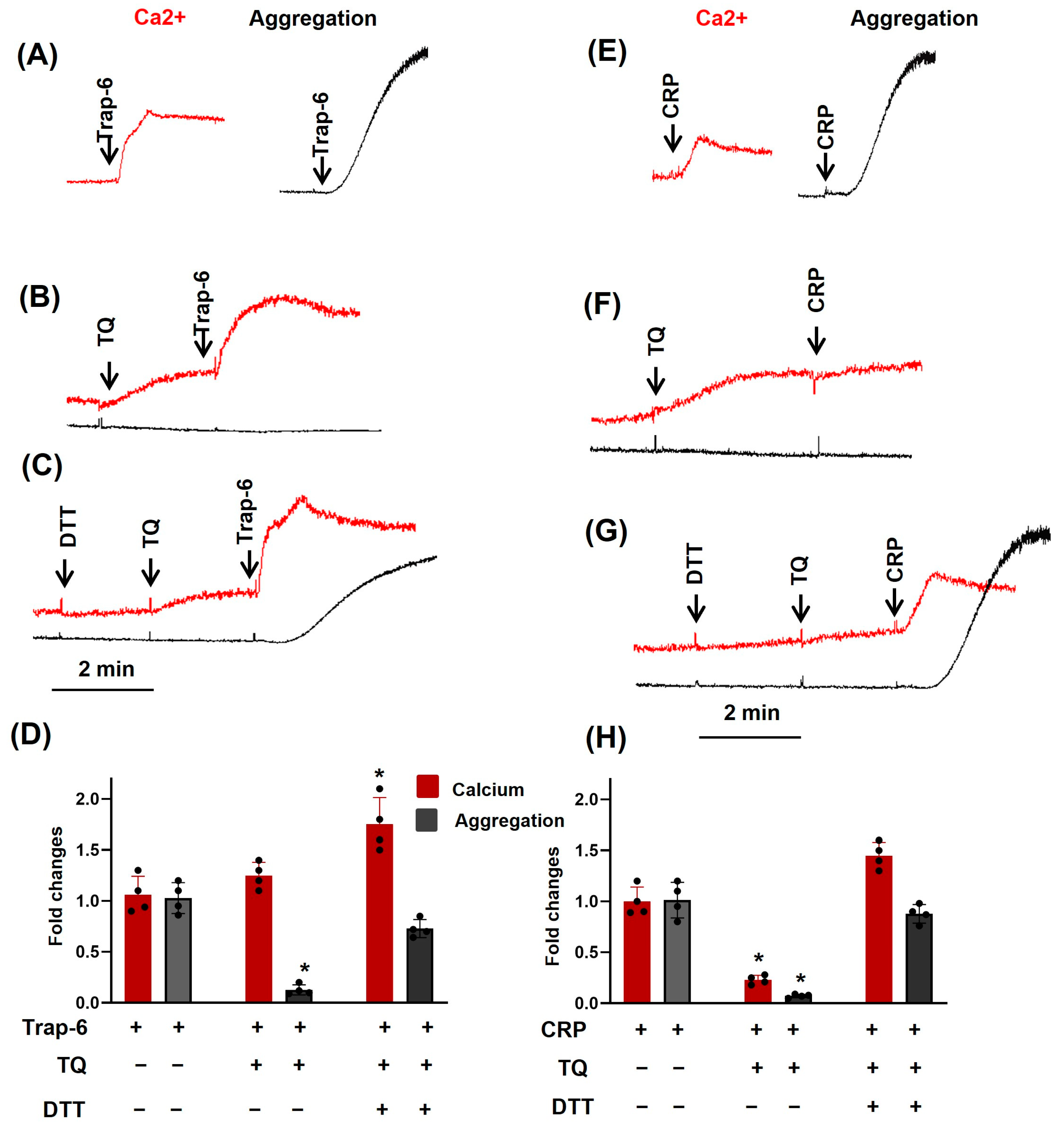
3.1. TQ Inhibited Agonist-Induced Platelet Activation
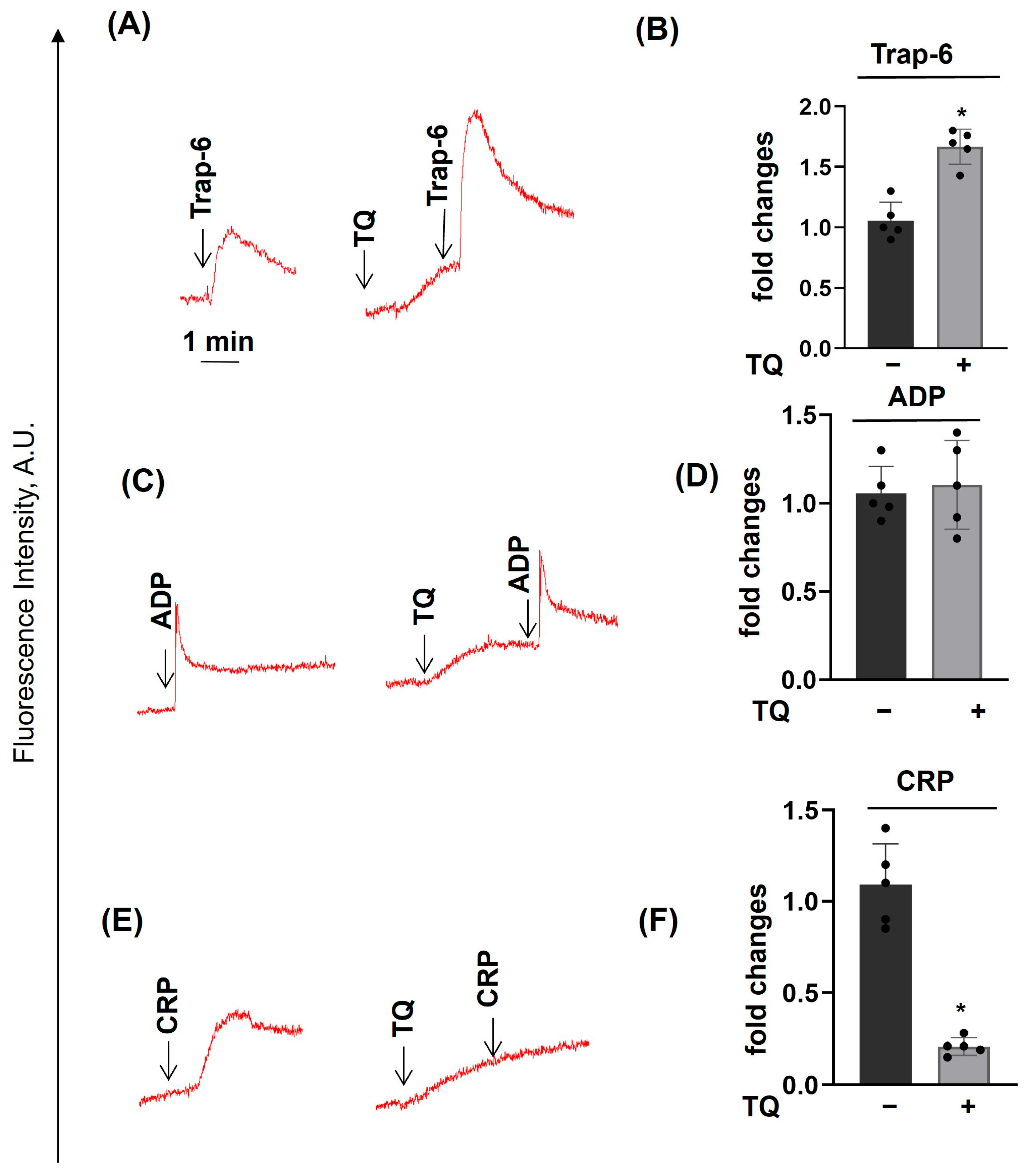
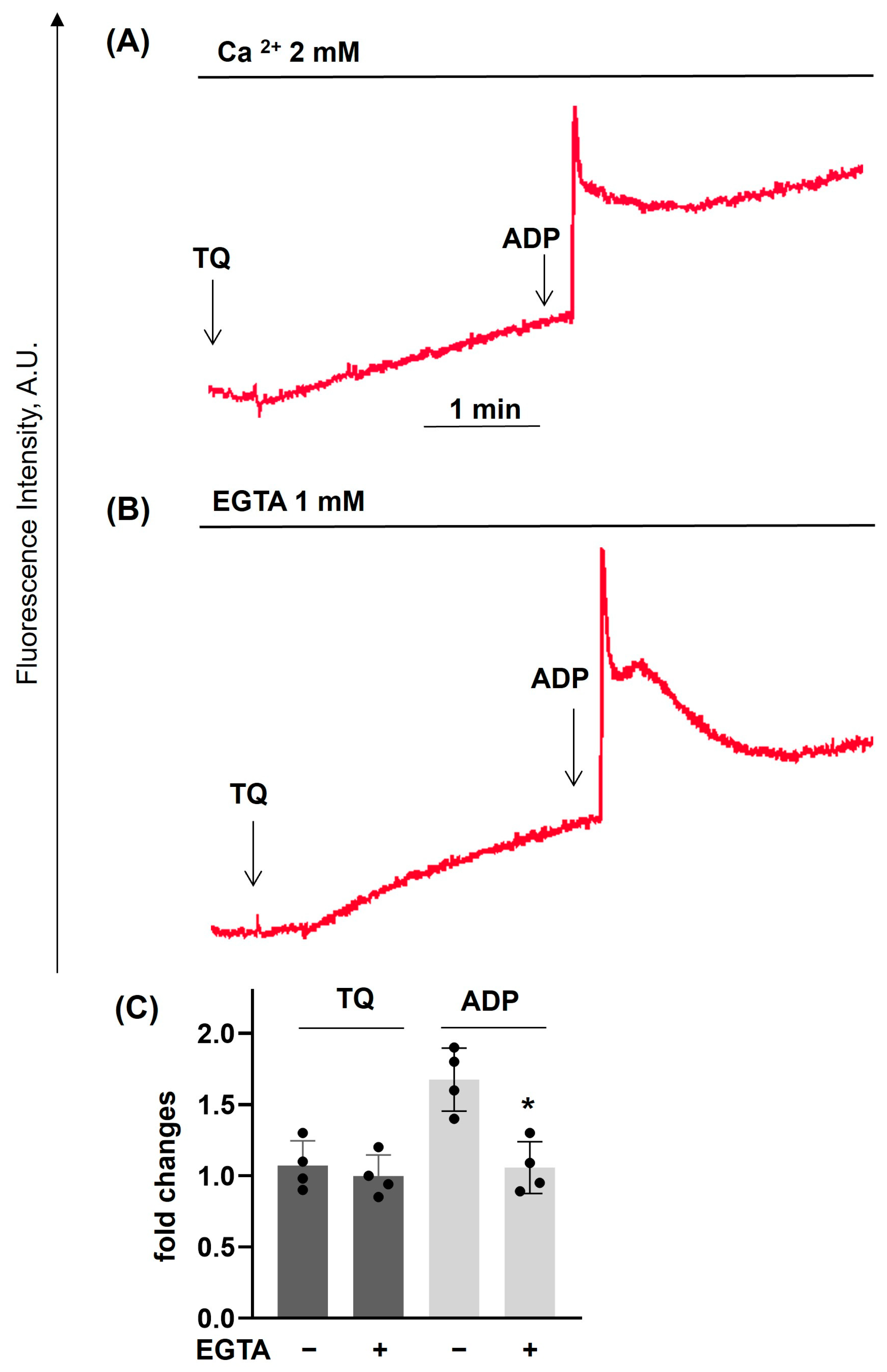
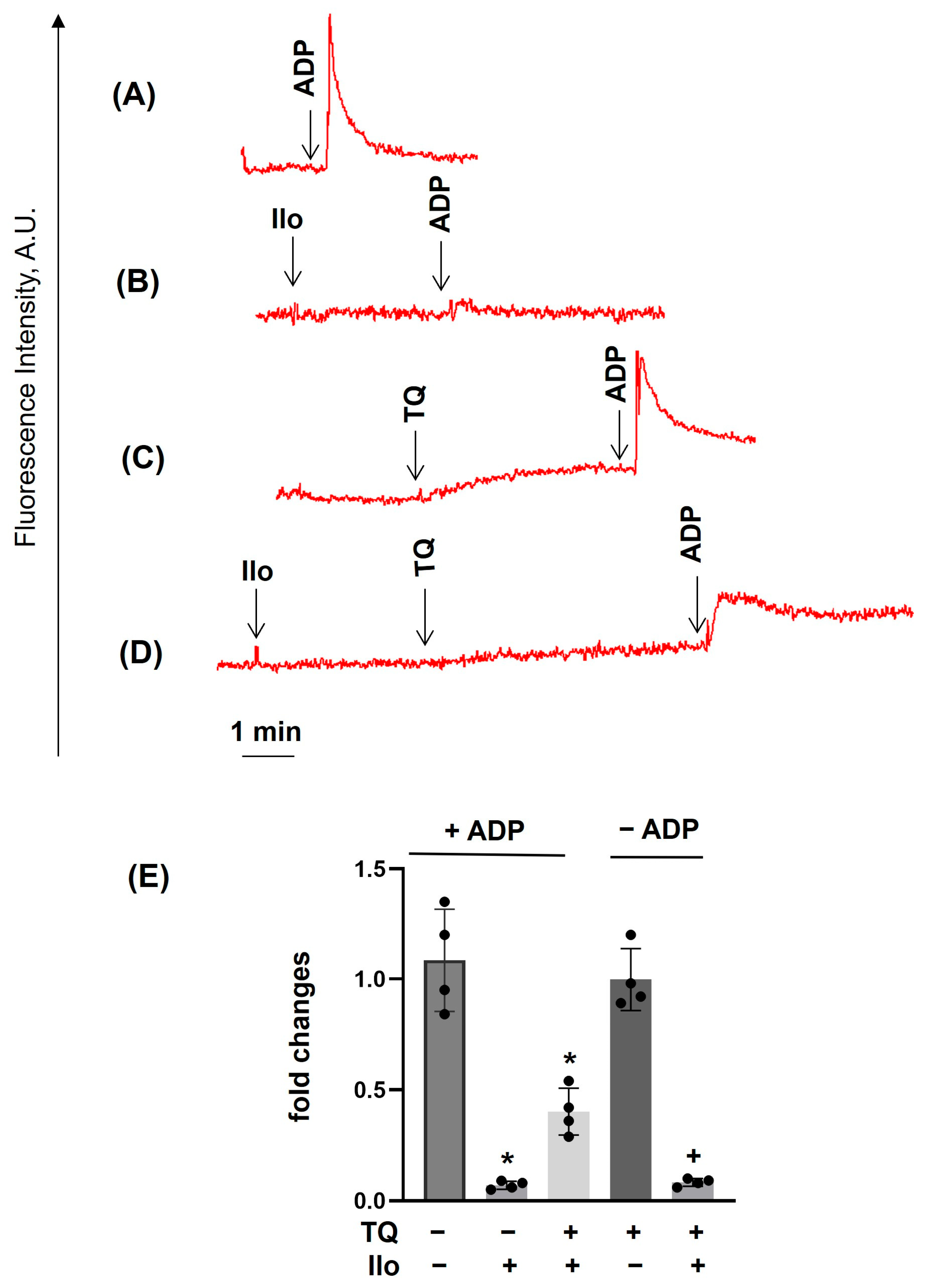
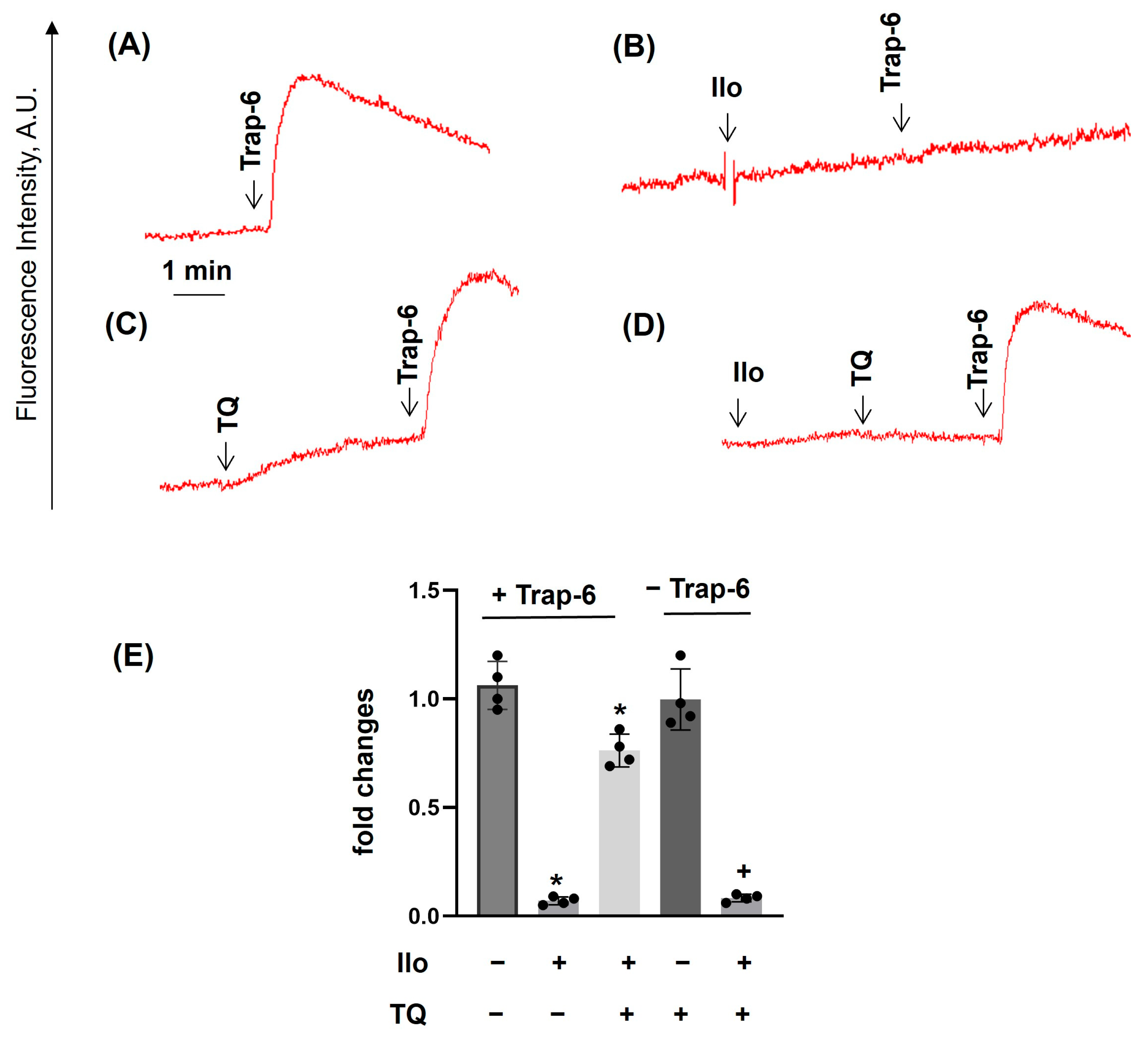
3.2. TQ Differentially Regulates Agonist-Induced Intracellular Calcium Mobilization
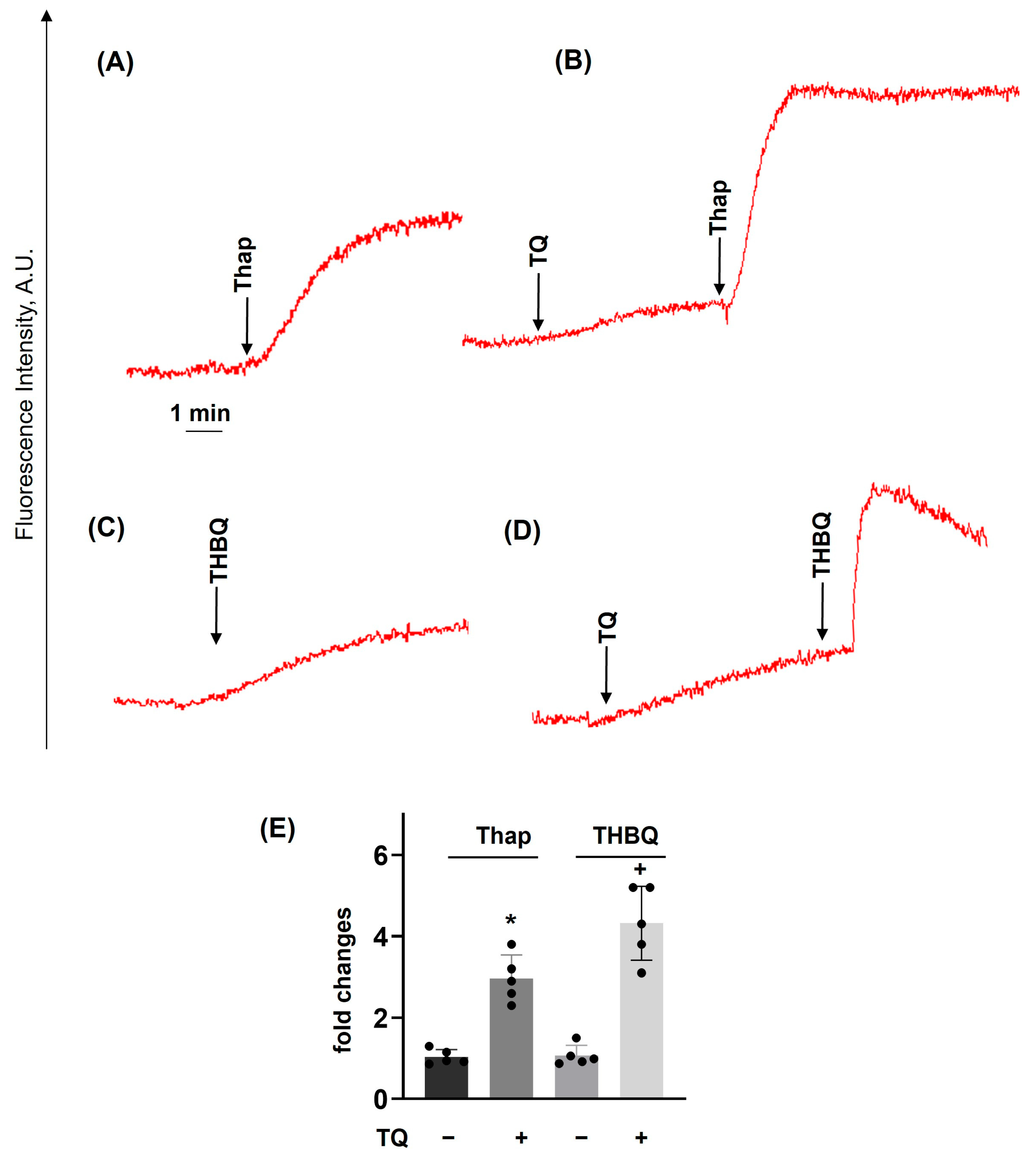
3.3. TQ Potentiated the Effects of Thapsigargin and THBQ
3.4. PKA Activation Completely Inhibited Agonist-Induced [Ca2+]i, Whereas TQ Partly Reversed This Effect
3.5. Dithiothreitol Prevented TQ-Induced Effects in Platelets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadeghi, E.; Imenshahidi, M.; Hosseinzadeh, H. Molecular mechanisms and signaling pathways of black cumin (Nigella sativa) and its active constituent, thymoquinone: A review. Mol. Biol. Rep. 2023, 50, 5439–5454. [Google Scholar] [CrossRef] [PubMed]
- Almajali, B.; Johan, M.F.; Al-Wajeeh, A.S.; Wan Taib, W.R.; Ismail, I.; Alhawamdeh, M.; Al-Tawarah, N.M.; Ibrahim, W.N.; Al-Rawashde, F.A.; Al-Jamal, H.A.N. Gene Expression Profiling and Protein Analysis Reveal Suppression of the C-Myc Oncogene and Inhibition JAK/STAT and PI3K/AKT/mTOR Signaling by Thymoquinone in Acute Myeloid Leukemia Cells. Pharmaceuticals 2022, 15, 307. [Google Scholar] [CrossRef]
- Karim, S.; Burzangi, A.S.; Ahmad, A.; Siddiqui, N.A.; Ibrahim, I.M.; Sharma, P.; Abualsunun, W.A.; Gabr, G.A. PI3K-AKT Pathway Modulation by Thymoquinone Limits Tumor Growth and Glycolytic Metabolism in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 2305. [Google Scholar] [CrossRef]
- Idris, S.; Refaat, B.; Almaimani, R.A.; Ahmed, H.G.; Ahmad, J.; Alhadrami, M.; El-Readi, M.Z.; Elzubier, M.E.; Alaufi, H.A.A.; Al-Amin, B.; et al. Enhanced in vitro tumoricidal effects of 5-Fluorouracil, thymoquinone, and active vitamin D(3) triple therapy against colon cancer cells by attenuating the PI3K/AKT/mTOR pathway. Life Sci. 2022, 296, 120442. [Google Scholar] [CrossRef]
- Kou, B.; Kou, Q.; Ma, B.; Zhang, J.; Sun, B.; Yang, Y.; Li, J.; Zhou, J.; Liu, W. Thymoquinone inhibits metastatic phenotype and epithelial—Mesenchymal transition in renal cell carcinoma by regulating the LKB1/AMPK signaling pathway. Oncol. Rep. 2018, 40, 1443–1450. [Google Scholar] [CrossRef]
- Wei, C.; Zou, H.; Xiao, T.; Liu, X.; Wang, Q.; Cheng, J.; Fu, S.; Peng, J.; Xie, X.; Fu, J. TQFL12, a novel synthetic derivative of TQ, inhibits triple-negative breast cancer metastasis and invasion through activating AMPK/ACC pathway. J. Cell Mol. Med. 2021, 25, 10101–10110. [Google Scholar] [CrossRef]
- Pei, X.; Li, X.; Chen, H.; Han, Y.; Fan, Y. Thymoquinone Inhibits Angiotensin II-Induced Proliferation and Migration of Vascular Smooth Muscle Cells Through the AMPK/PPARγ/PGC-1α Pathway. DNA Cell Biol. 2016, 35, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhuo, C.; Zu, A.; Yuan, S.; Zhang, H.; Zhao, J.; Zheng, L. Thymoquinone ameliorates pressure overload-induced cardiac hypertrophy by activating the AMPK signalling pathway. J. Cell Mol. Med. 2022, 26, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, R.; El-Bakoush, A.; Lepiarz, I.; Ogunrinade, F.; Olajide, O.A. AMPK and SIRT1 activation contribute to inhibition of neuroinflammation by thymoquinone in BV2 microglia. Mol. Cell Biochem. 2017, 435, 149–162. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Ma, J.; Wang, Y.; Pei, Z.; Ding, H. Effects of thymoquinone against angiotensin II—Induced cardiac damage in apolipoprotein E—Deficient mice. Int. J. Mol. Med. 2022, 49, 1–12. [Google Scholar] [CrossRef]
- Dalli, T.; Beker, M.; Terzioglu-Usak, S.; Akbas, F.; Elibol, B. Thymoquinone activates MAPK pathway in hippocampus of streptozotocin-treated rat model. Biomed. Pharmacother. 2018, 99, 391–401. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Mehri, S.; Abnous, K.; Hosseinzadeh, H. Neuroprotective Effects of Thymoquinone in Acrylamide-Induced Peripheral Nervous System Toxicity Through MAPKinase and Apoptosis Pathways in Rat. Neurochem Res. 2019, 44, 1101–1112. [Google Scholar] [CrossRef]
- Yang, J.; Kuang, X.R.; Lv, P.T.; Yan, X.X. Thymoquinone inhibits proliferation and invasion of human nonsmall-cell lung cancer cells via ERK pathway. Tumour. Biol. 2015, 36, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ting, W.J.; Gao, J.; Kang, Z.F.; Huang, C.Y.; Weng, Y.J. Erk phosphorylation reduces the thymoquinone toxicity in human hepatocarcinoma. Environ. Toxicol. 2021, 36, 1990–1998. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, T.; Sun, P.; Lian, L.-H.; Yao, Y.-L.; Zheng, H.-X.; Li, X.; Li, J.-B.; Wu, Y.-L.; Nan, J.-X. Thymoquinone, a bioactive component of Nigella sativa Linn seeds or traditional spice, attenuates acute hepatic failure and blocks apoptosis via the MAPK signaling pathway in mice. RSC Adv. 2015, 5, 7285–7290. [Google Scholar] [CrossRef]
- Kordestani, Z.; Shahrokhi-Farjah, M.; Yazdi Rouholamini, S.E.; Saberi, A. Reduced IKK/NF- kB Expression by Nigella Sativa Extract in Breast Cancer. Middle East J. Cancer 2020, 11, 150–158. [Google Scholar] [CrossRef]
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Soliman, K.F.A. Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFκB pathway signaling targets in LPS/IFNγ -activated BV-2 microglia cells. J. Neuroimmunol. 2018, 320, 87–97. [Google Scholar] [CrossRef]
- Chae, I.G.; Song, N.Y.; Kim, D.H.; Lee, M.Y.; Park, J.M.; Chun, K.S. Thymoquinone induces apoptosis of human renal carcinoma Caki-1 cells by inhibiting JAK2/STAT3 through pro-oxidant effect. Food Chem. Toxicol. 2020, 139, 111253. [Google Scholar] [CrossRef]
- Atteia, H.H.; Arafa, M.H.; Mohammad, N.S.; Amin, D.M.; Sakr, A.T. Thymoquinone upregulates miR-125a-5p, attenuates STAT3 activation, and potentiates doxorubicin antitumor activity in murine solid Ehrlich carcinoma. J. Biochem. Mol. Toxicol. 2021, 35, e22924. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; D’Angelo, A.; Maffioli, P.; Cucinella, L.; Nappi, R.E. The Use of Nigella sativa in Cardiometabolic Diseases. Biomedicines 2024, 12, 405. [Google Scholar] [CrossRef]
- Alberts, A.; Moldoveanu, E.T.; Niculescu, A.G.; Grumezescu, A.M. Nigella sativa: A Comprehensive Review of Its Therapeutic Potential, Pharmacological Properties, and Clinical Applications. Int. J. Mol. Sci. 2024, 25, 13410. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Al-Samaraie, S.; Terro, T.M. Molecular Mechanisms and Signaling Pathways Underlying the Therapeutic Potential of Thymoquinone Against Colorectal Cancer. Molecules 2024, 29, 5907. [Google Scholar] [CrossRef]
- Rahjoo, T.; Motamedzadeh, A.; Ferdosi, F.; Dadgostar, E.; Aschner, M.; Mirzaei, H.; Ghesmatpour, S.; Nabavizadeh, F.; Rahmati-Dehkordi, F.; Tamtaji, O.R. Potential role of thymoquinone to treat gastrointestinal cancers: Insights into its molecular mechanisms. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 9471–9497. [Google Scholar] [CrossRef]
- Nicolai, L.; Pekayvaz, K.; Massberg, S. Platelets: Orchestrators of immunity in host defense and beyond. Immunity 2024, 57, 957–972. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, L.; Yuan, Y.; Zhang, S.; Xue, R. Metabolic crosstalk between platelets and cancer: Mechanisms, functions, and therapeutic potential. Semin. Cancer Biol. 2025, 110, 65–82. [Google Scholar] [CrossRef]
- Yang, J.; Xu, P.; Zhang, G.; Wang, D.; Ye, B.; Wu, L. Advances and potentials in platelet-circulating tumor cell crosstalk. Am. J. Cancer Res. 2025, 15, 407–425. [Google Scholar] [CrossRef]
- Rukoyatkina, N.; Butt, E.; Subramanian, H.; Nikolaev, V.O.; Mindukshev, I.; Walter, U.; Gambaryan, S.; Benz, P.M. Protein kinase A activation by the anti-cancer drugs ABT-737 and thymoquinone is caspase-3-dependent and correlates with platelet inhibition and apoptosis. Cell Death Dis. 2017, 8, e2898. [Google Scholar] [CrossRef]
- Towhid, S.T.; Schmidt, E.M.; Schmid, E.; Münzer, P.; Qadri, S.M.; Borst, O.; Lang, F. Thymoquinone-induced platelet apoptosis. J. Cell Biochem. 2011, 112, 3112–3121. [Google Scholar] [CrossRef] [PubMed]
- Kaefer, A.; Yang, J.; Noertersheuser, P.; Mensing, S.; Humerickhouse, R.; Awni, W.; Xiong, H. Mechanism-based pharmacokinetic/pharmacodynamic meta-analysis of navitoclax (ABT-263) induced thrombocytopenia. Cancer Chemother. Pharmacol. 2014, 74, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M.; Braun, Y.; Smith, V.M.; Westhoff, M.A.; Pereira, R.S.; Pieper, N.M.; Anders, M.; Callens, M.; Vervliet, T.; Abbas, M.; et al. The BCL2 family: From apoptosis mechanisms to new advances in targeted therapy. Signal Transduct. Target Ther. 2025, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, A.; Batra, V. Severe Thrombocytopenia Associated With Black Seed Oil and Evening Primrose Oil. Cureus 2020, 12, e8390. [Google Scholar] [CrossRef]
- Berehab, M.; Rouas, R.; Akl, H.; Duvillier, H.; Journe, F.; Fayyad-Kazan, H.; Ghanem, G.; Bron, D.; Lewalle, P.; Merimi, M. Apoptotic and Non-Apoptotic Modalities of Thymoquinone-Induced Lymphoma Cell Death: Highlight of the Role of Cytosolic Calcium and Necroptosis. Cancers 2021, 13, 3579. [Google Scholar] [CrossRef]
- Phua, C.Y.H.; Teoh, Z.L.; Goh, B.H.; Yap, W.H.; Tang, Y.Q. Triangulating the pharmacological properties of thymoquinone in regulating reactive oxygen species, inflammation, and cancer: Therapeutic applications and mechanistic pathways. Life Sci. 2021, 287, 120120. [Google Scholar] [CrossRef] [PubMed]
- Kale, E.; Kale, A.; Bozali, K.; Gulgec, A.S.; Ozdemir, M.; Yalcin, B.; Guler, E.M. TQ-Ox, a novel synthetic derivative of thymoquinone on ovarian cancer cells in vitro. Nat. Prod. Res. 2023, 37, 3015–3024. [Google Scholar] [CrossRef]
- Guler, E.M.; Bozali, K. Synthesised thymoquinone-oxime induces cytotoxicity, genotoxicity and apoptosis in hepatocellular cancer cells: In vitro study. Nat. Prod. Res. 2024, 38, 1695–1703. [Google Scholar] [CrossRef]
- Shehwar, D.; Barki, S.; Aliotta, A.; Calderara, D.B.; Veuthey, L.; Portela, C.P.; Alberio, L.; Alam, M.R. Platelets and mitochondria: The calcium connection. Mol. Biol. Rep. 2025, 52, 276. [Google Scholar] [CrossRef]
- Varga-Szabo, D.; Braun, A.; Nieswandt, B. Calcium signaling in platelets. J. Thromb. Haemost. 2009, 7, 1057–1066. [Google Scholar] [CrossRef]
- Fernández, D.I.; Kuijpers, M.J.E.; Heemskerk, J.W.M. Platelet calcium signaling by G-protein coupled and ITAM-linked receptors regulating anoctamin-6 and procoagulant activity. Platelets 2021, 32, 863–871. [Google Scholar] [CrossRef]
- Jobe, S.M.; Wilson, K.M.; Leo, L.; Raimondi, A.; Molkentin, J.D.; Lentz, S.R.; Di Paola, J. Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis. Blood 2008, 111, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Walkon, L.L.; Strubbe-Rivera, J.O.; Bazil, J.N. Calcium Overload and Mitochondrial Metabolism. Biomolecules 2022, 12, 1891. [Google Scholar] [CrossRef] [PubMed]
- Mindukshev, I.; Gambaryan, S.; Kehrer, L.; Schuetz, C.; Kobsar, A.; Rukoyatkina, N.; Nikolaev, V.O.; Krivchenko, A.; Watson, S.P.; Walter, U.; et al. Low angle light scattering analysis: A novel quantitative method for functional characterization of human and murine platelet receptors. Clin. Chem. Lab. Med. 2012, 50, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Mindukshev, I.; Fock, E.; Dobrylko, I.; Sudnitsyna, J.; Gambaryan, S.; Panteleev, M.A. Platelet Hemostasis Reactions at Different Temperatures Correlate with Intracellular Calcium Concentration. Int. J. Mol. Sci. 2022, 23, 10667. [Google Scholar] [CrossRef]
- Mikhailova, D.M.; Sudnitsyna, J.; Kovgan, P.; Naida, L.; Kharazova, A.; Mindukshev, I.; Gambaryan, S. Analysis of Ferric Protoporphyrin IX Effects on Human Platelets: Hematin Is a More Potent Agonist than Hemin. Cells 2025, 14, 255. [Google Scholar] [CrossRef]
- Rukoyatkina, N.; Mindukshev, I.; Walter, U.; Gambaryan, S. Dual role of the p38 MAPK/cPLA2 pathway in the regulation of platelet apoptosis induced by ABT-737 and strong platelet agonists. Cell Death Dis. 2013, 4, e931. [Google Scholar] [CrossRef]
- Rosado, J.A.; Pariente, J.A.; Salido, G.M.; Redondo, P.C. SERCA2b activity is regulated by cyclophilins in human platelets. Arter. Thromb. Vasc. Biol. 2010, 30, 419–425. [Google Scholar] [CrossRef]
- Smolenski, A. Novel roles of cAMP/cGMP-dependent signaling in platelets. J. Thromb. Haemost. 2012, 10, 167–176. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, C.; Flaumenhaft, R.; Huang, M. Recent advances in vascular thiol isomerases: Insights into structures, functions in thrombosis and antithrombotic inhibitor development. Thromb. J. 2025, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Essex, D.W.; Wang, L. Recent advances in vascular thiol isomerases and redox systems in platelet function and thrombosis. J. Thromb. Haemost. 2024, 22, 1806–1818. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, J.Y.; Lee, M.Y.; Chung, S.M.; Bae, O.N.; Chung, J.H. Association of quinone-induced platelet anti-aggregation with cytotoxicity. Toxicol. Sci. 2001, 62, 176–182. [Google Scholar] [CrossRef]
- Liebeke, M.; Pöther, D.C.; van Duy, N.; Albrecht, D.; Becher, D.; Hochgräfe, F.; Lalk, M.; Hecker, M.; Antelmann, H. Depletion of thiol-containing proteins in response to quinones in Bacillus subtilis. Mol. Microbiol. 2008, 69, 1513–1529. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Hu, J.; Cai, Z.; Ju, H. Dual quinone tagging for MALDI-TOF mass spectrometric quantitation of cysteine-containing peptide. Anal. Chem. 2014, 86, 8275–8280. [Google Scholar] [CrossRef]
- Cleland, W.W. Dithiothreitol, a New Protective Reagent for SH Groups. Biochemistry 1964, 3, 480–482. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Fernandes, D.A.; Gomes, A.N.P.; da Silva, C.M.; de Medeiros Henriques, I.S.; de Menezes, R.P.B.; Scotti, M.T.; Teles, Y.C.F.; Edrada-Ebel, R.; de Fatima Vanderlei de Souza, M. Natural Phenolic Compounds with Antithrombotic and Antiplatelet Effects: A Drug-likeness Approach. Curr. Med. Chem. 2024, 31, 4138–4159. [Google Scholar] [CrossRef]
- Sharma, B.; Shekhar, H.; Sahu, A.; Haque, S.; Kaur, D.; Tuli, H.S.; Sharma, U. Deciphering the anticancer potential of thymoquinone: In-depth exploration of the potent flavonoid from Nigella sativa. Mol. Biol. Rep. 2025, 52, 268. [Google Scholar] [CrossRef]
- Cheah, L.T.; Hindle, M.S.; Khalil, J.S.; Duval, C.; Unsworth, A.J.; Naseem, K.M. Platelet Reactive Oxygen Species, Oxidised Lipid Stress, Current Perspectives, and an Update on Future Directions. Cells 2025, 14, 500. [Google Scholar] [CrossRef] [PubMed]
- Fernández, D.I.; Provenzale, I.; Cheung, H.Y.F.; van Groningen, J.; Tullemans, B.M.E.; Veninga, A.; Dunster, J.L.; Honarnejad, S.; van den Hurk, H.; Kuijpers, M.J.E.; et al. Ultra-high-throughput Ca(2+) assay in platelets to distinguish ITAM-linked and G-protein-coupled receptor activation. iScience 2022, 25, 103718. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Nagy, M.; Heemskerk, J.W.M.; Nieswandt, B.; Braun, A. Store-operated calcium entry in thrombosis and thrombo-inflammation. Cell Calcium 2019, 77, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Riondino, S.; Lotti, L.V.; Cutini, L.; Pulcinelli, F.M. Collagen-induced platelet shape change is not affected by positive feedback pathway inhibitors and cAMP-elevating agents. J. Biol. Chem. 2005, 280, 6504–6510. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.C.; Kavanagh, D.M.; Watson, S.; Pike, J.A.; Andrews, R.K.; Gardiner, E.E.; Poulter, N.S.; Hill, S.J.; Watson, S.P. Adenosine and Forskolin Inhibit Platelet Aggregation by Collagen but not the Proximal Signalling Events. Thromb. Haemost. 2019, 119, 1124–1137. [Google Scholar] [CrossRef]
- Margaritis, A.; Priora, R.; Frosali, S.; Di Giuseppe, D.; Summa, D.; Coppo, L.; Di Stefano, A.; Di Simplicio, P. The role of protein sulfhydryl groups and protein disulfides of the platelet surface in aggregation processes involving thiol exchange reactions. Pharmacol. Res. 2011, 63, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zucker, M.B.; Masiello, N.C. Platelet aggregation caused by dithiothreitol. Thromb. Haemost. 1984, 51, 119–124. [Google Scholar] [CrossRef] [PubMed]
| Compound | Stock Solutions Solvents | Stock Solutions Concentrations | Final Concentrations |
|---|---|---|---|
| TBHQ | DMSO ** | 20 mM | 20 μM |
| TQ * | DMSO | 40 mM | 5–40 μM |
| Thapsigargin | DMSO | 1 mM | 1 μM |
| TRAP-6 | DMSO | 10 mM | 10μM |
| ADP | Water | 10 mM | 1 μM |
| CRP | PBS | 5 mg/mL | 10 µg/mL |
| Iloprost | Ethanol | 55 μM | 5 nM |
| DTT | Water | 10 mM | 100 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rukoyatkina, N.; Mindukshev, I.; Mikhailova, D.M.; Panteleev, M.A.; Gambaryan, S. Multifaceted Effects of Thymoquinone on Platelet Calcium Homeostasis. Cells 2025, 14, 1827. https://doi.org/10.3390/cells14221827
Rukoyatkina N, Mindukshev I, Mikhailova DM, Panteleev MA, Gambaryan S. Multifaceted Effects of Thymoquinone on Platelet Calcium Homeostasis. Cells. 2025; 14(22):1827. https://doi.org/10.3390/cells14221827
Chicago/Turabian StyleRukoyatkina, Natalia, Igor Mindukshev, Diana M. Mikhailova, Mikhail A. Panteleev, and Stepan Gambaryan. 2025. "Multifaceted Effects of Thymoquinone on Platelet Calcium Homeostasis" Cells 14, no. 22: 1827. https://doi.org/10.3390/cells14221827
APA StyleRukoyatkina, N., Mindukshev, I., Mikhailova, D. M., Panteleev, M. A., & Gambaryan, S. (2025). Multifaceted Effects of Thymoquinone on Platelet Calcium Homeostasis. Cells, 14(22), 1827. https://doi.org/10.3390/cells14221827







