FGF12 Enhances Prostate Cancer Cell Survival via the YB1-lncRNA Axis
Highlights
- FGF12 is upregulated in t-NEPC across different models.
- FGF12 enhances PCa cell survival under chemotherapeutic stress by interacting with YB1 and promoting YB1-mediated binding and stabilization of oncogenic lncRNAs, NEAT1 and MALAT1.
- These findings reveal a novel FGF12-YB1-lncRNA axis in advanced PCa.
- Targeting this signaling axis could provide new therapeutic opportunities.
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Public RNA-Seq Data Analysis
2.3. Quantitative Real-Time PCR (qRT-PCR)
2.4. Western Blotting
2.5. Immunohistochemistry (IHC)
2.6. Plasmid Construction and Cell Transfection
2.7. Drug Treatment and Cell Viability Assays
2.8. RNA-Seq and Gene Set Enrichment Analysis (GSEA)
2.9. Co-Immunoprecipitation (Co-IP)
2.10. RNA Co-Immunoprecipitation (RIP)
2.11. siRNA Knockdown
2.12. Statistics
3. Results
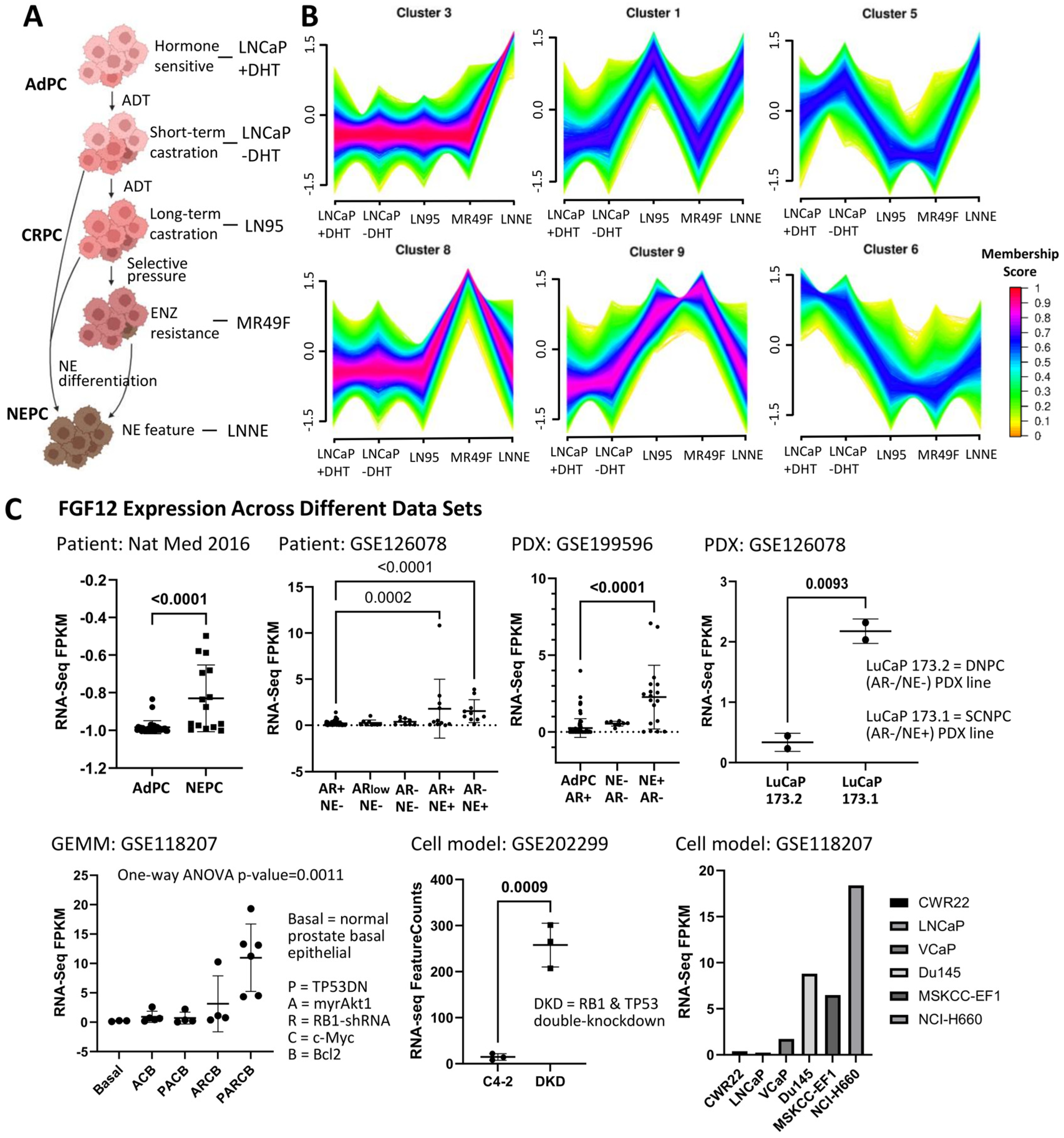
3.1. FGF12 Is Upregulated in Different NEPC Models
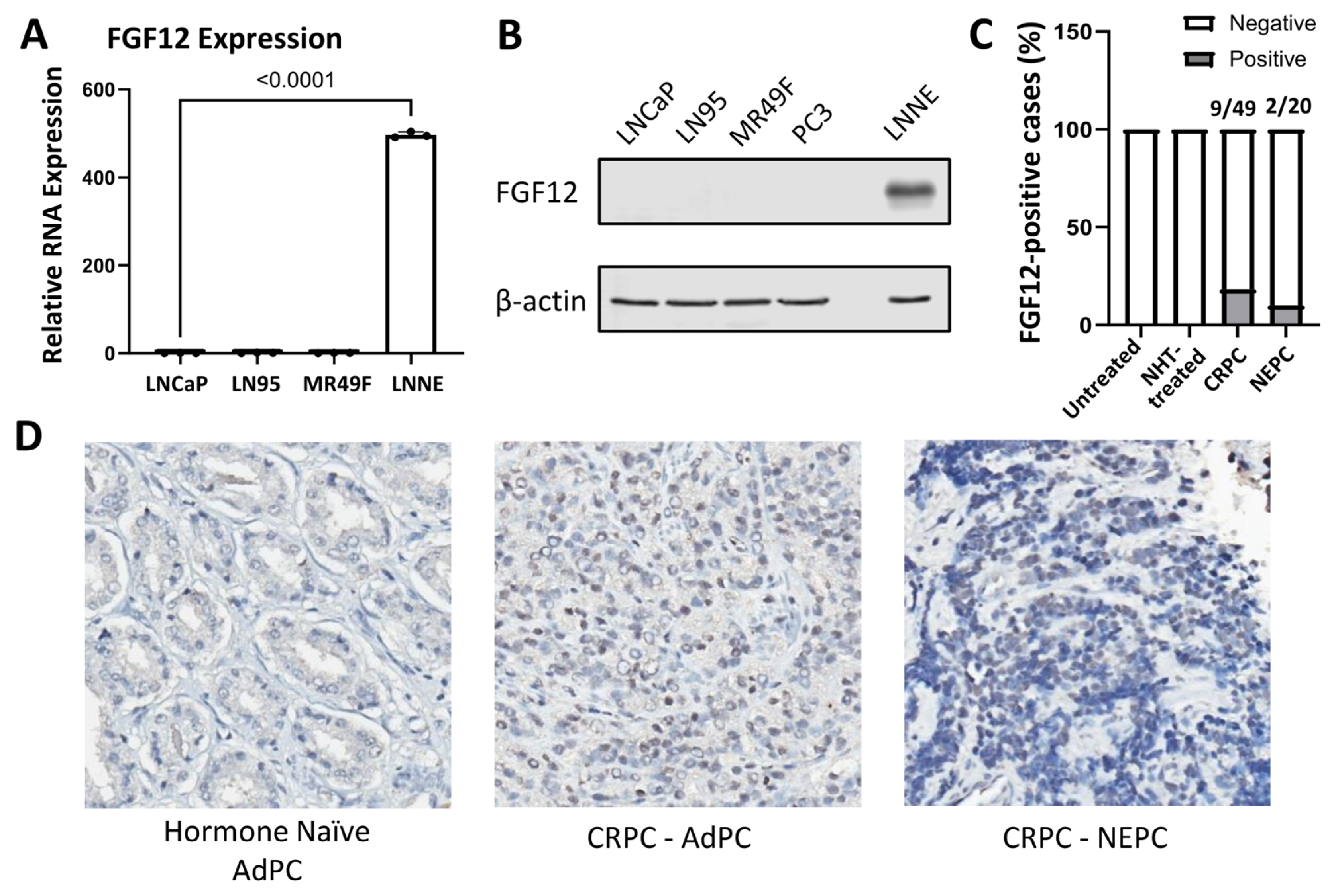
3.2. FGF12 Expression Is Elevated in NEPC Cell Line and Clinical Samples
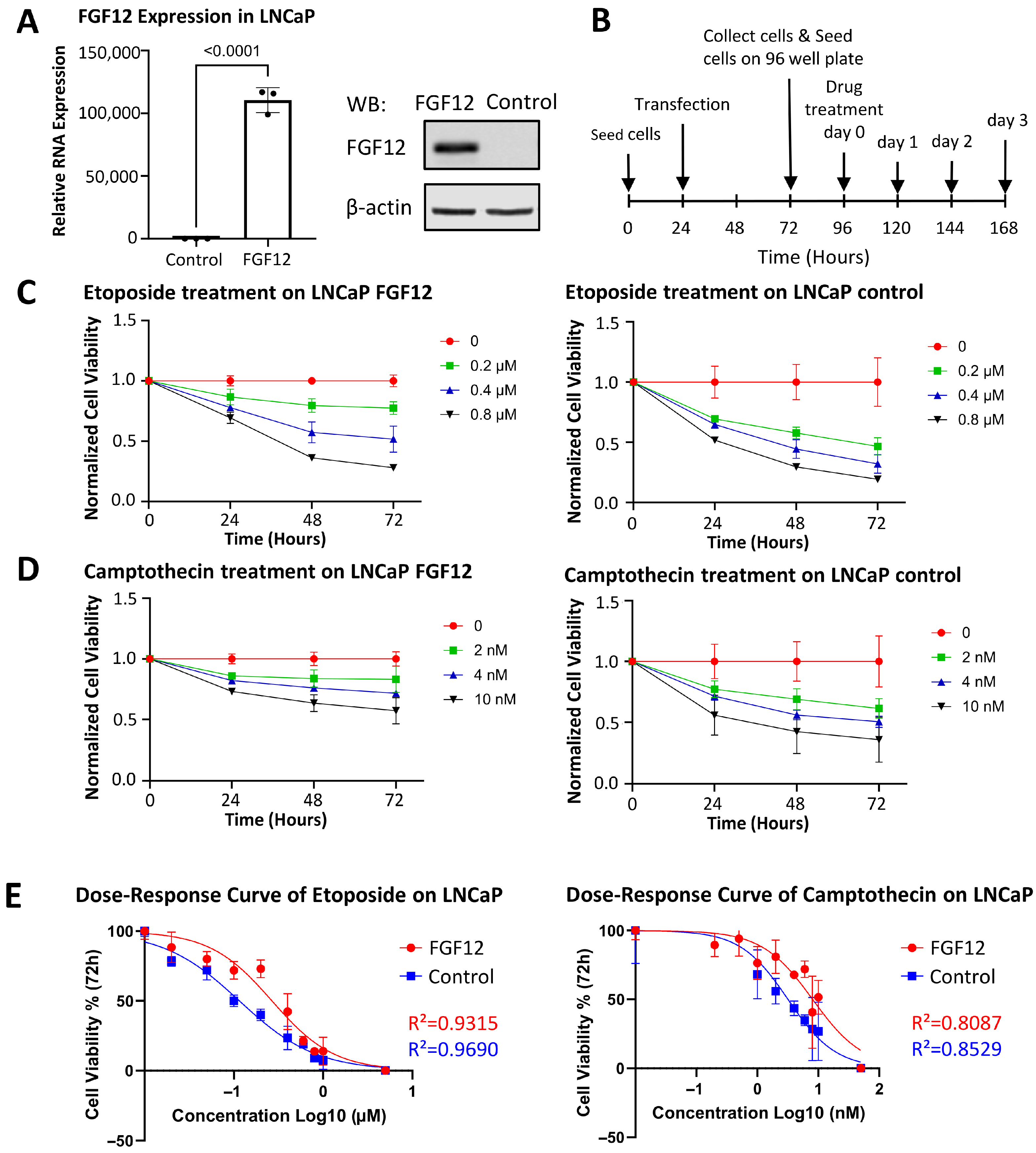
3.3. FGF12 Expression Enhances LNCaP Cell Survival Under Stress
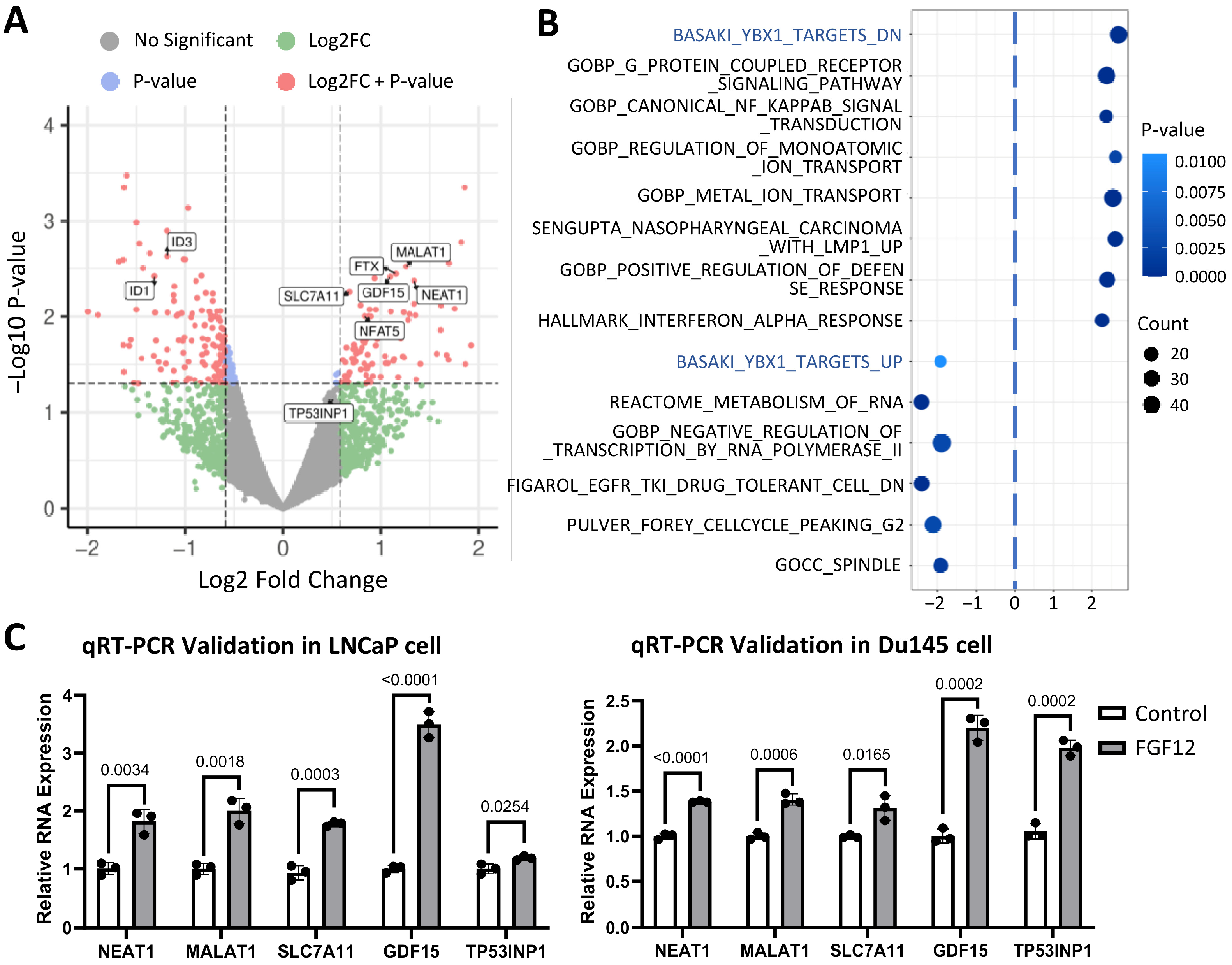
3.4. Transcriptomic Profiling Reveals FGF12 Regulates lncRNAs and YB1-Related Gene Set
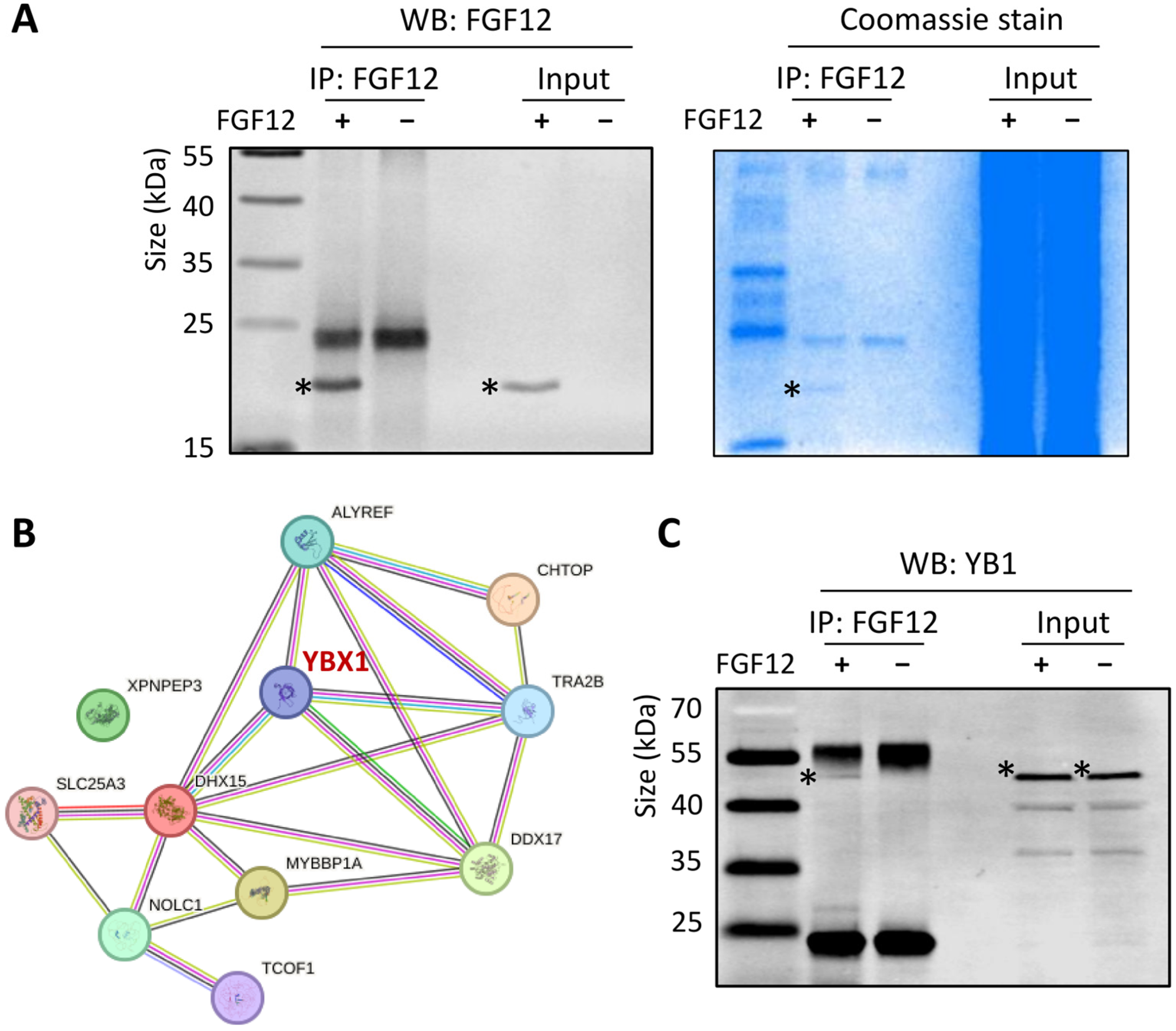
3.5. FGF12 Interacts with YB1 in LNCaP Cells
3.6. FGF12 Enhances YB1-Associated lncRNA Binding and Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saad, F.; Hotte, S.J. Guidelines for the management of castrate-resistant prostate cancer. Can. Urol. Assoc. J. 2010, 4, 380–384. [Google Scholar] [CrossRef]
- Labrecque, M.P.; Alumkal, J.J.; Coleman, I.M.; Nelson, P.S.; Morrissey, C. The heterogeneity of prostate cancers lacking AR activity will require diverse treatment approaches. Endocr. Relat. Cancer 2021, 28, T51–T66. [Google Scholar] [CrossRef]
- Parimi, V.; Goyal, R.; Poropatich, K.; Yang, X.J. Neuroendocrine differentiation of prostate cancer: A review. Am. J. Clin. Exp. Urol. 2014, 2, 273–285. [Google Scholar]
- Beltran, H.; Rickman, D.S.; Park, K.; Chae, S.S.; Sboner, A.; MacDonald, T.Y.; Wang, Y.; Sheikh, K.L.; Terry, S.; Tagawa, S.T.; et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011, 1, 487–495. [Google Scholar] [CrossRef]
- Dardenne, E.; Beltran, H.; Benelli, M.; Gayvert, K.; Berger, A.; Puca, L.; Cyrta, J.; Sboner, A.; Noorzad, Z.; MacDonald, T.; et al. N-Myc Induces an EZH2-Mediated Transcriptional Program Driving Neuroendocrine Prostate Cancer. Cancer Cell 2016, 30, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.Y.; Rosario, S.; Wang, Y.; Mu, P.; Seshadri, M.; Goodrich, Z.W.; Goodrich, M.M.; Labbe, D.P.; Gomez, E.C.; Wang, J.; et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017, 355, 78–83. [Google Scholar] [CrossRef]
- Mu, P.; Zhang, Z.; Benelli, M.; Karthaus, W.R.; Hoover, E.; Chen, C.C.; Wongvipat, J.; Ku, S.Y.; Gao, D.; Cao, Z.; et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 2017, 355, 84–88. [Google Scholar] [CrossRef]
- Biadun, M.; Karelus, R.; Krowarsch, D.; Opalinski, L.; Zakrzewska, M. FGF12: Biology and function. Differentiation 2024, 139, 100740. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, P.M.; Munoz-Sanjuan, I.; Tong, P.; Macke, J.P.; Hendry, S.H.; Gilbert, D.J.; Copeland, N.G.; Jenkins, N.A.; Nathans, J. Fibroblast growth factor (FGF) homologous factors: New members of the FGF family implicated in nervous system development. Proc. Natl. Acad. Sci. USA 1996, 93, 9850–9857. [Google Scholar] [CrossRef]
- Schoorlemmer, J.; Goldfarb, M. Fibroblast growth factor homologous factors are intracellular signaling proteins. Curr. Biol. 2001, 11, 793–797. [Google Scholar] [CrossRef]
- Liu, C.; Dib-Hajj, S.D.; Waxman, S.G. Fibroblast growth factor homologous factor 1B binds to the C terminus of the tetrodotoxin-resistant sodium channel rNav1.9a (NaN). J. Biol. Chem. 2001, 276, 18925–18933. [Google Scholar] [CrossRef]
- König, H.G.; Fenner, B.J.; Byrne, J.C.; Schwamborn, R.F.; Bernas, T.; Jefferies, C.A.; Prehn, J.H. Fibroblast growth factor homologous factor 1 interacts with NEMO to regulate NF-κB signaling in neurons. J. Cell Sci. 2012, 125, 6058–6070. [Google Scholar] [CrossRef]
- Sochacka, M.; Karelus, R.; Opalinski, L.; Krowarsch, D.; Biadun, M.; Otlewski, J.; Zakrzewska, M. FGF12 is a novel component of the nucleolar NOLC1/TCOF1 ribosome biogenesis complex. Cell Commun. Signal 2022, 20, 182. [Google Scholar] [CrossRef]
- Biadun, M.; Sochacka, M.; Karelus, R.; Baran, K.; Czyrek, A.; Otlewski, J.; Krowarsch, D.; Opalinski, L.; Zakrzewska, M. FGF homologous factors are secreted from cells to induce FGFR-mediated anti-apoptotic response. FASEB J. 2023, 37, e23043. [Google Scholar] [CrossRef] [PubMed]
- Volkomorov, V.; Grigoryeva, E.; Krasnov, G.; Litviakov, N.; Tsyganov, M.; Karbyshev, M.; Zavyalova, M.; Afanasyev, S.; Cherdyntseva, N.; Lisitsyn, N.; et al. Search for potential gastric cancer markers using miRNA databases and gene expression analysis. Exp. Oncol. 2013, 35, 2–7. [Google Scholar] [PubMed]
- Bhushan, A.; Singh, A.; Kapur, S.; Borthakar, B.B.; Sharma, J.; Rai, A.K.; Kataki, A.C.; Saxena, S. Identification and Validation of Fibroblast Growth Factor 12 Gene as a Novel Potential Biomarker in Esophageal Cancer Using Cancer Genomic Datasets. Omics 2017, 21, 616–631. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Ren, F.; Li, X.; Ren, D.; Dong, M.; Chen, G.; Song, Z.; Chen, J. Impact of genetic alterations on outcomes of patients with stage I nonsmall cell lung cancer: An analysis of the cancer genome atlas data. Cancer Med. 2020, 9, 7686–7694. [Google Scholar] [CrossRef]
- Miao, Y.; Dong, M.; Zhou, Q.; Thiel, J.; Li, N.; Cai, Y.; Yuan, D.; Wang, H.; Jin, S.H.; Yang, H.; et al. Single-cell RNA-seq reveals FGF12 as a prognostic biomarker in low-grade endometrial stromal sarcoma. Front. Immunol. 2024, 15, 1513076. [Google Scholar] [CrossRef]
- Matsumoto, H.; Yamamoto, Y.; Shiota, M.; Kuruma, H.; Beraldi, E.; Matsuyama, H.; Zoubeidi, A.; Gleave, M. Cotargeting Androgen Receptor and Clusterin Delays Castrate-Resistant Prostate Cancer Progression by Inhibiting Adaptive Stress Response and AR Stability. Cancer Res. 2013, 73, 5206–5217. [Google Scholar] [CrossRef]
- Hu, R.; Lu, C.; Mostaghel, E.A.; Yegnasubramanian, S.; Gurel, M.; Tannahill, C.; Edwards, J.; Isaacs, W.B.; Nelson, P.S.; Bluemn, E.; et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012, 72, 3457–3462. [Google Scholar] [CrossRef]
- Li, Y.; Chen, R.; Bowden, M.; Mo, F.; Lin, Y.Y.; Gleave, M.; Collins, C.; Dong, X. Establishment of a neuroendocrine prostate cancer model driven by the RNA splicing factor SRRM4. Oncotarget 2017, 8, 66878–66888. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.; Varambally, S.; et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef]
- Labrecque, M.P.; Coleman, I.M.; Brown, L.G.; True, L.D.; Kollath, L.; Lakely, B.; Nguyen, H.M.; Yang, Y.C.; da Costa, R.M.G.; Kaipainen, A.; et al. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J. Clin. Investig. 2019, 129, 4492–4505. [Google Scholar] [CrossRef]
- Coleman, I.M.; DeSarkar, N.; Morrissey, C.; Xin, L.; Roudier, M.P.; Sayar, E.; Li, D.; Corey, E.; Haffner, M.C.; Nelson, P.S. Therapeutic Implications for Intrinsic Phenotype Classification of Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2022, 28, 3127–3140. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lee, J.K.; Sheu, K.M.; Wang, L.; Balanis, N.G.; Nguyen, K.; Smith, B.A.; Cheng, C.; Tsai, B.L.; Cheng, D.; et al. Reprogramming normal human epithelial tissues to a common, lethal neuroendocrine cancer lineage. Science 2018, 362, 91–95. [Google Scholar] [CrossRef]
- Islam, R.; Mishra, J.; Polavaram, N.S.; Bhattacharya, S.; Hong, Z.; Bodas, S.; Sharma, S.; Bouska, A.; Gilbreath, T.; Said, A.M.; et al. Neuropilin-2 axis in regulating secretory phenotype of neuroendocrine-like prostate cancer cells and its implication in therapy resistance. Cell Rep. 2022, 40, 111097. [Google Scholar] [CrossRef]
- Bates, M.; Boland, A.; McDermott, N.; Marignol, L. YB-1: The key to personalised prostate cancer management? Cancer Lett. 2020, 490, 66–75. [Google Scholar] [CrossRef]
- Dong, X.; Chen, R. Understanding aberrant RNA splicing to facilitate cancer diagnosis and therapy. Oncogene 2020, 39, 2231–2242. [Google Scholar] [CrossRef]
- Lovnicki, J.; Gan, Y.; Feng, T.; Li, Y.; Xie, N.; Ho, C.H.; Lee, A.R.; Chen, X.; Nappi, L.; Han, B.; et al. LIN28B promotes the development of neuroendocrine prostate cancer. J. Clin. Investig. 2020, 130, 5338–5348. [Google Scholar] [CrossRef]
- Samsonova, A.; El Hage, K.; Desforges, B.; Joshi, V.; Clément, M.J.; Lambert, G.; Henrie, H.; Babault, N.; Craveur, P.; Maroun, R.C.; et al. Lin28, a major translation reprogramming factor, gains access to YB-1-packaged mRNA through its cold-shock domain. Commun. Biol. 2021, 4, 359. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Wang, X.; Li, S.; Tang, L. Substrate Stiffness Drives Epithelial to Mesenchymal Transition and Proliferation through the NEAT1-Wnt/β-Catenin Pathway in Liver Cancer. Int. J. Mol. Sci. 2021, 22, 12066. [Google Scholar] [CrossRef]
- Zhang, M.; Xing, J.; Zhao, S.; Lu, M.; Liu, Y.; Lin, L.; Gao, W.; Chen, L.; Li, W.; Shang, J.; et al. Exosomal YB-1 facilitates ovarian restoration by MALAT1/miR-211-5p/FOXO(3) axis. Cell Biol. Toxicol. 2024, 40, 29. [Google Scholar] [CrossRef]
- Nanni, S.; Bacci, L.; Aiello, A.; Re, A.; Salis, C.; Grassi, C.; Pontecorvi, A.; Gaetano, C.; Farsetti, A. Signaling through estrogen receptors modulates long non-coding RNAs in prostate cancer. Mol. Cell Endocrinol. 2020, 511, 110864. [Google Scholar] [CrossRef]
- Chakravarty, D.; Sboner, A.; Nair, S.S.; Giannopoulou, E.; Li, R.; Hennig, S.; Mosquera, J.M.; Pauwels, J.; Park, K.; Kossai, M.; et al. The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat. Commun. 2014, 5, 5383. [Google Scholar] [CrossRef]
- Yamazaki, T.; Souquere, S.; Chujo, T.; Kobelke, S.; Chong, Y.S.; Fox, A.H.; Bond, C.S.; Nakagawa, S.; Pierron, G.; Hirose, T. Functional Domains of NEAT1 Architectural lncRNA Induce Paraspeckle Assembly through Phase Separation. Mol. Cell 2018, 70, 1038–1053.e1037. [Google Scholar] [CrossRef]
- Pisani, G.; Baron, B. NEAT1 and Paraspeckles in Cancer Development and Chemoresistance. Noncoding RNA 2020, 6, 43. [Google Scholar] [CrossRef]
- Ren, S.; Liu, Y.; Xu, W.; Sun, Y.; Lu, J.; Wang, F.; Wei, M.; Shen, J.; Hou, J.; Gao, X.; et al. Long noncoding RNA MALAT-1 is a new potential therapeutic target for castration resistant prostate cancer. J. Urol. 2013, 190, 2278–2287. [Google Scholar] [CrossRef]
- Wang, D.; Ding, L.; Wang, L.; Zhao, Y.; Sun, Z.; Karnes, R.J.; Zhang, J.; Huang, H. LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget 2015, 6, 41045–41055. [Google Scholar] [CrossRef]
- Wu, H.; Liu, A. Long non-coding RNA NEAT1 regulates ferroptosis sensitivity in non-small-cell lung cancer. J. Int. Med. Res. 2021, 49, 300060521996183. [Google Scholar] [CrossRef]
- Gupta, S.; Silveira, D.A.; Mombach, J.C.M.; Hashimoto, R.F. DNA Damage-Induced Ferroptosis: A Boolean Model Regulating p53 and Non-Coding RNAs in Drug Resistance. Proteomes 2025, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Kung, S.H.Y.; Adomat, H.; Oo, H.Z.; Forbes, C.; Hach, F.; Dong, X. FGF12 Enhances Prostate Cancer Cell Survival via the YB1-lncRNA Axis. Cells 2025, 14, 1828. https://doi.org/10.3390/cells14221828
Huang Z, Kung SHY, Adomat H, Oo HZ, Forbes C, Hach F, Dong X. FGF12 Enhances Prostate Cancer Cell Survival via the YB1-lncRNA Axis. Cells. 2025; 14(22):1828. https://doi.org/10.3390/cells14221828
Chicago/Turabian StyleHuang, Zechao, Sonia H. Y. Kung, Hans Adomat, Htoo Zarni Oo, Connor Forbes, Faraz Hach, and Xuesen Dong. 2025. "FGF12 Enhances Prostate Cancer Cell Survival via the YB1-lncRNA Axis" Cells 14, no. 22: 1828. https://doi.org/10.3390/cells14221828
APA StyleHuang, Z., Kung, S. H. Y., Adomat, H., Oo, H. Z., Forbes, C., Hach, F., & Dong, X. (2025). FGF12 Enhances Prostate Cancer Cell Survival via the YB1-lncRNA Axis. Cells, 14(22), 1828. https://doi.org/10.3390/cells14221828





