Converging Molecular Mechanisms of Nucleated Cell Death Pathways and Procoagulant Platelet Formation
Abstract
1. Introduction
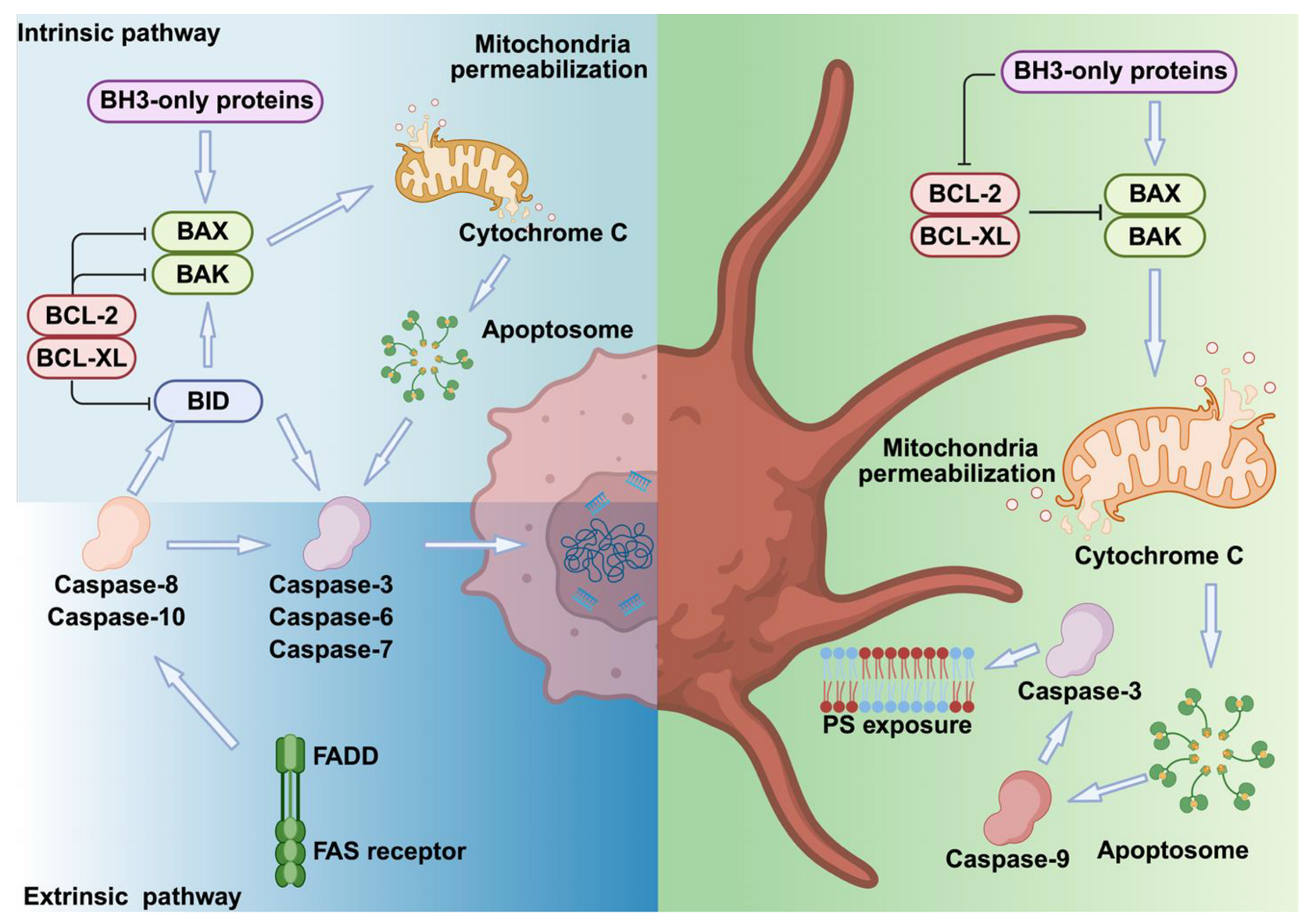
2. Apoptosis Pathways in Nucleated Cells Versus Platelets
2.1. Extrinsic and Intrinsic Apoptosis Pathway Activation Mechanisms
2.2. Intrinsic Apoptosis Pathway Activation Mechanisms in MKs and Platelets
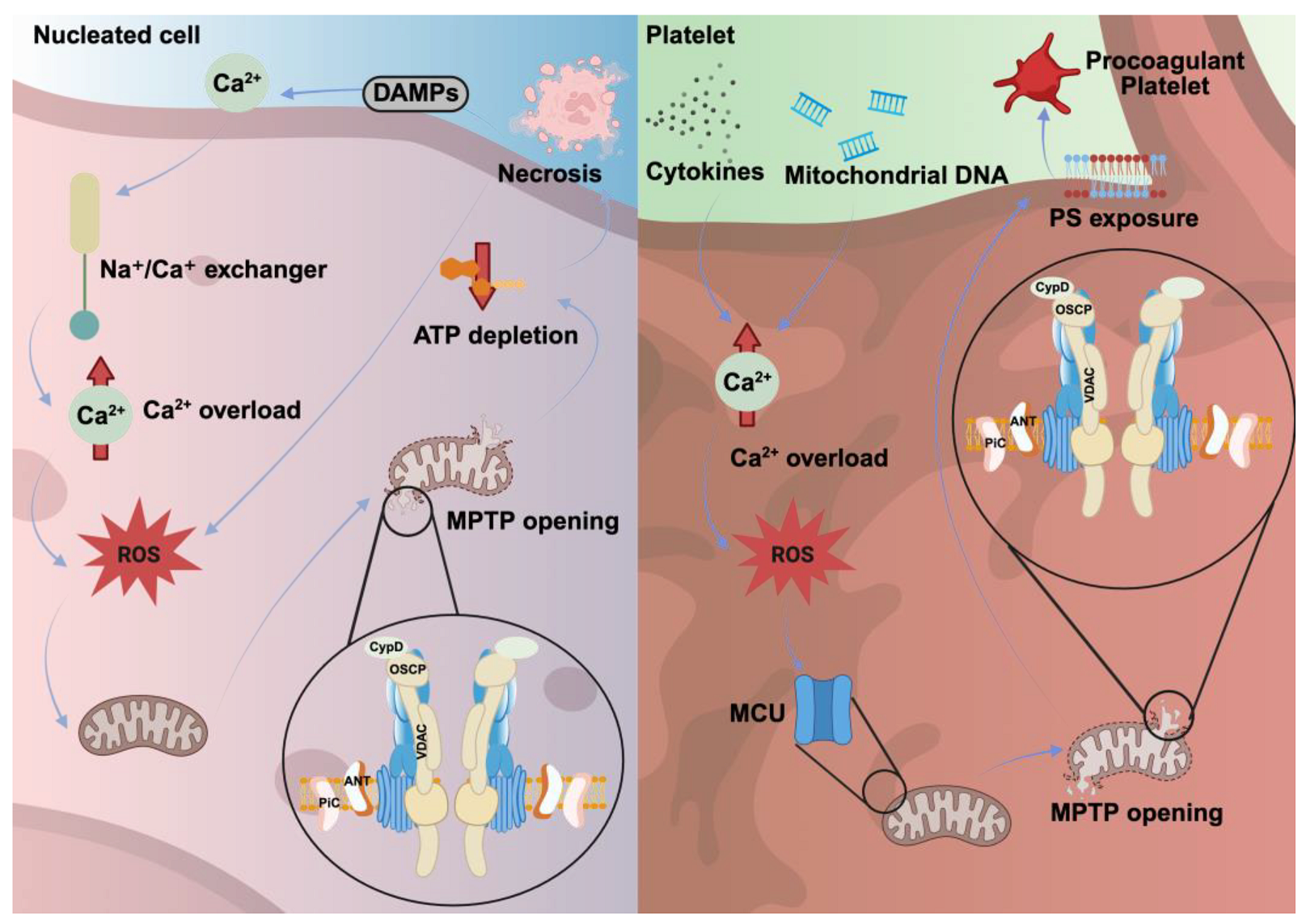
3. Necrosis in Nucleated Cells Versus Platelets
3.1. Mitochondrial Dysfunction as a Trigger for Necrosis in Nucleated Cells
3.2. Procoagulant Activity as a Hallmark of Platelet Necrosis
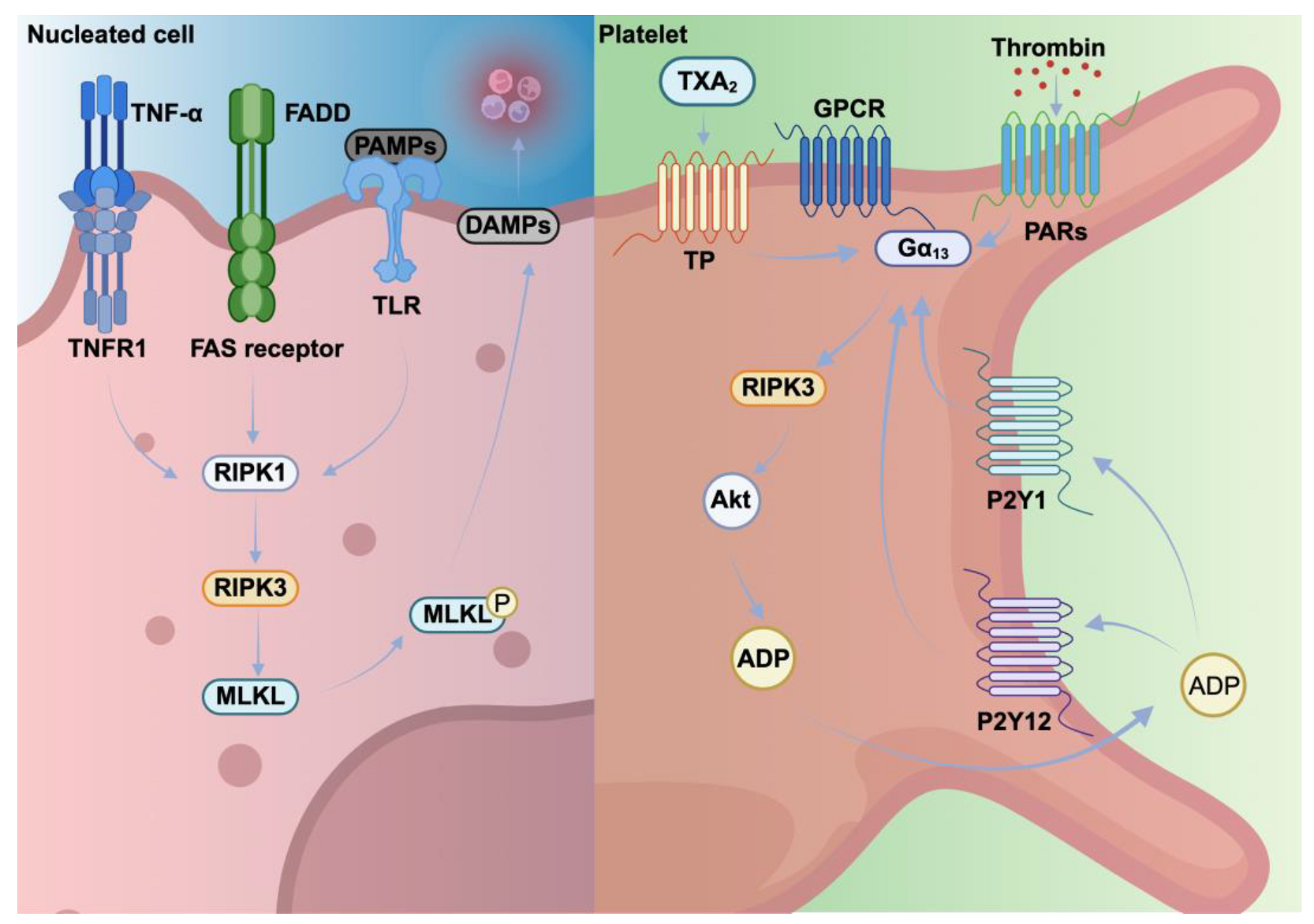
4. Necroptosis Pathways in Nucleated Cells Versus Platelets
4.1. Necroptosis as a Controlled Alternative Death Pathway in Nucleated Cells
4.2. Necroptotic Platelets: Regulators of Hemostasis and Thrombosis
5. Pyroptosis Pathways in Nucleated Cells Versus Platelets
5.1. Pyroptosis Is an Inflammatory Form of Cell Death
5.2. Pyroptotic Platelets: Drivers of Inflammatory Death
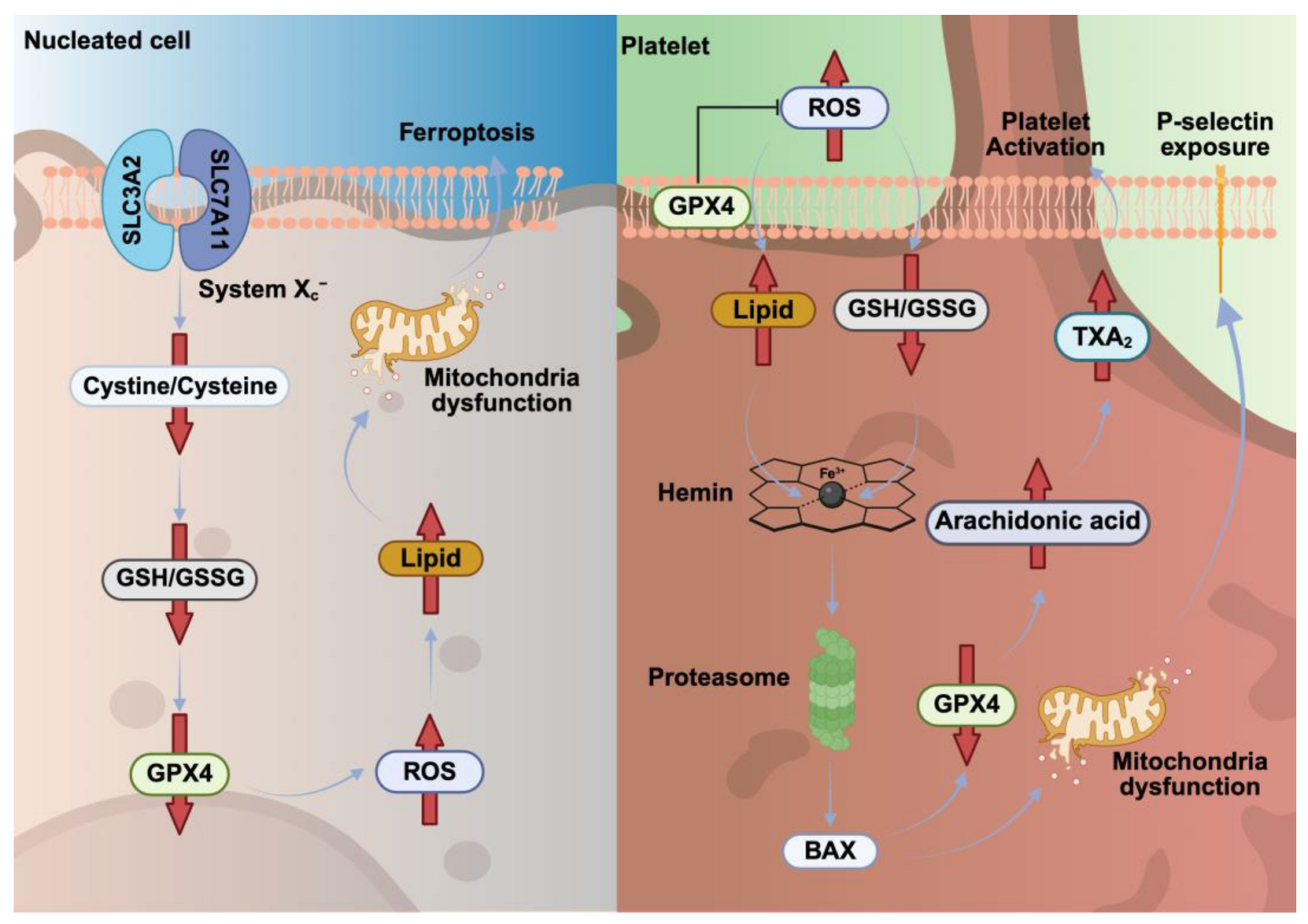
6. Ferroptosis Pathways in Nucleated Cells Versus Platelets
6.1. Ferroptosis Depends on the Balance Between ROS Generation and Antioxidant Defenses
6.2. Heme-Induced Ferroptosis Promotes Platelet Activation and Thrombosis
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| ANT | Adenine nucleotide translocator |
| ATP | Adenosine triphosphate |
| BAK | BCL-2 homologous antagonist/killer |
| BAX | BCL-2-associated X protein |
| BCL-2 | B-cell lymphoma 2 |
| BCL-XL | B-cell lymphoma-extra large |
| BID | BH3 interacting-domain death agonist |
| Ca2+ | Calcium |
| cGMP-cGKI | Guanosine monophosphate-cyclic guanosine monophosphate kinase I |
| CLEC-2 | C-type lectin-like receptor-2 |
| CXCR7 | C-X-C chemokine receptor type 7 |
| CypD | Cyclophilin D |
| DAMPs | Damage-associated molecular patterns |
| DIC | Disseminated intravascular coagulation |
| DISC | Death-inducing signaling complex |
| DNA | Deoxyribonucleic acid |
| DR4 | Death receptor 4 |
| DR5 | Death receptor 5 |
| FADD | Fas-associated death domain |
| Fas CD45 | Cluster of differentiation CD45 |
| FasL | Fas ligand |
| FSP1 | Ferroptosis suppressor protein 1 |
| FTH1 | Ferritin heavy chain 1 |
| GPCR | G-protein-coupled receptor |
| GPVI | Glycoprotein VI |
| GPX4 | Glutathione peroxidase 4 |
| GSDMD | Gasdermin D |
| GSDME | Gasdermin E |
| GSH | Glutathione |
| GSH/GSSG | Glutathione/oxidized glutathione |
| HMGB1 | High-mobility group box 1 |
| IL-18 | Interleukine 18 |
| IL-1β | Interleukine 1 beta |
| ITP | Immune thrombocytopenic purpura |
| LPS | Lipopolysaccharides |
| LTCC | L-type calcium channel |
| MCU | Mitochondrial calcium uniporter |
| MKs | Megakaryocytes |
| MLKL | Mixed lineage kinase domain-like protein |
| MLT | Melatonin |
| MPTP | Mitochondrial permeability transition pore |
| NETs | Neutrophil extracellular traps |
| N-GSDMD | N-terminal domain of GSDMD |
| NLRP1 | Nod-like receptor pyrin domain containing 1 |
| NLRP3 | Nod-like receptor pyrin domain containing 3 |
| NLRs | Nod-like receptors |
| OSCP | Oligomycin-sensitive binding protein |
| P2Y1 | Purinergic receptor P2Y1 |
| P2Y12 | Purinergic receptor P2Y12 |
| PAMPs | Pathogen-associated molecular patterns |
| PARs | Protease-activated receptors |
| PiC | Mitochondrial inorganic phosphate carrier |
| PS | Phosphatidylserine |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 |
| RIPK1 | Receptor-interacting protein kinase 1 |
| RIPK3 | Receptor-interacting protein kinase 3 |
| ROS | Reactive oxygen species |
| SCD | Sickle cell disease |
| SMAC | Second mitochondria-derived activator of caspases |
| SOCE | Store-operated calcium entry |
| TF | Tissue factor |
| TLRs | Toll-like receptors |
| TNFR1 | Tumor necrosis factor receptor |
| TNF-α | Tumor necrosis alpha |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TRPM2 | Transient receptor potential cation channel, subfamily M, member 2 |
| TRPs | Transient receptor potential channels |
| TXA2 | Thromboxane A2 |
| UCB | Unconjugated bilirubin |
| VDAC | Voltage-dependent anion channel |
References
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ofengeim, D. A guide to cell death pathways. Nat. Rev. Mol. Cell Biol. 2024, 25, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Riera Romo, M. Cell death as part of innate immunity: Cause or consequence? Immunology 2021, 163, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, E.C.; White, M.J.; Dowling, M.R.; Kile, B.T. Platelet life span and apoptosis. Methods Mol. Biol. 2012, 788, 59–71. [Google Scholar] [CrossRef]
- Nicolai, L.; Pekayvaz, K.; Massberg, S. Platelets: Orchestrators of immunity in host defense and beyond. Immunity 2024, 57, 957–972. [Google Scholar] [CrossRef]
- An, O.; Deppermann, C. Platelet lifespan and mechanisms for clearance. Curr. Opin. Hematol. 2024, 31, 6–15. [Google Scholar] [CrossRef]
- Harper, M.T.; Poole, A.W. Chloride channels are necessary for full platelet phosphatidylserine exposure and procoagulant activity. Cell Death Dis. 2013, 4, e969. [Google Scholar] [CrossRef]
- Shin, H.W.; Takatsu, H. Phosphatidylserine exposure in living cells. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 166–178. [Google Scholar] [CrossRef]
- Jackson, S.P.; Schoenwaelder, S.M. Procoagulant platelets: Are they necrotic? Blood 2010, 116, 2011–2018. [Google Scholar] [CrossRef]
- Hua, V.M.; Chen, V.M. Procoagulant platelets and the pathways leading to cell death. Semin. Thromb. Hemost. 2015, 41, 405–412. [Google Scholar] [CrossRef]
- Denorme, F.; Campbell, R.A. Procoagulant platelets: Novel players in thromboinflammation. Am. J. Physiol. Cell Physiol. 2022, 323, C951–C958. [Google Scholar] [CrossRef] [PubMed]
- Agbani, E.O.; Poole, A.W. Procoagulant platelets: Generation, function, and therapeutic targeting in thrombosis. Blood 2017, 130, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Nair, P.; Lu, M.; Petersen, S.; Ashkenazi, A. Apoptosis initiation through the cell-extrinsic pathway. Methods Enzym. 2014, 544, 99–128. [Google Scholar] [CrossRef]
- Westaby, D.; Jimenez-Vacas, J.M.; Padilha, A.; Varkaris, A.; Balk, S.P.; de Bono, J.S.; Sharp, A. Targeting the Intrinsic Apoptosis Pathway: A Window of Opportunity for Prostate Cancer. Cancers 2021, 14, 51. [Google Scholar] [CrossRef]
- Li, J.; Xia, Y.; Bertino, A.M.; Coburn, J.P.; Kuter, D.J. The mechanism of apoptosis in human platelets during storage. Transfusion 2000, 40, 1320–1329. [Google Scholar] [CrossRef]
- Josefsson, E.C.; Burnett, D.L.; Lebois, M.; Debrincat, M.A.; White, M.J.; Henley, K.J.; Lane, R.M.; Moujalled, D.; Preston, S.P.; O’Reilly, L.A.; et al. Platelet production proceeds independently of the intrinsic and extrinsic apoptosis pathways. Nat Commun 2014, 5, 3455. [Google Scholar] [CrossRef]
- Plenchette, S.; Moutet, M.; Benguella, M.; N’Gondara, J.P.; Guigner, F.; Coffe, C.; Corcos, L.; Bettaieb, A.; Solary, E. Early increase in DcR2 expression and late activation of caspases in the platelet storage lesion. Leukemia 2001, 15, 1572–1581. [Google Scholar] [CrossRef]
- Josefsson, E.C. Platelet intrinsic apoptosis. Thromb. Res. 2023, 231, 206–213. [Google Scholar] [CrossRef]
- Schleicher, R.I.; Reichenbach, F.; Kraft, P.; Kumar, A.; Lescan, M.; Todt, F.; Gobel, K.; Hilgendorf, I.; Geisler, T.; Bauer, A.; et al. Platelets induce apoptosis via membrane-bound FasL. Blood 2015, 126, 1483–1493. [Google Scholar] [CrossRef]
- Wagner, K.U.; Claudio, E.; Rucker, E.B., 3rd; Riedlinger, G.; Broussard, C.; Schwartzberg, P.L.; Siebenlist, U.; Hennighausen, L. Conditional deletion of the Bcl-x gene from erythroid cells results in hemolytic anemia and profound splenomegaly. Development 2000, 127, 4949–4958. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, E.C.; James, C.; Henley, K.J.; Debrincat, M.A.; Rogers, K.L.; Dowling, M.R.; White, M.J.; Kruse, E.A.; Lane, R.M.; Ellis, S.; et al. Megakaryocytes possess a functional intrinsic apoptosis pathway that must be restrained to survive and produce platelets. J. Exp. Med. 2011, 208, 2017–2031. [Google Scholar] [CrossRef] [PubMed]
- Schoenwaelder, S.M.; Yuan, Y.; Josefsson, E.C.; White, M.J.; Yao, Y.; Mason, K.D.; O’Reilly, L.A.; Henley, K.J.; Ono, A.; Hsiao, S.; et al. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood 2009, 114, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nimmer, P.M.; Tahir, S.K.; Chen, J.; Fryer, R.M.; Hahn, K.R.; Iciek, L.A.; Morgan, S.J.; Nasarre, M.C.; Nelson, R.; et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007, 14, 943–951. [Google Scholar] [CrossRef]
- Mason, K.D.; Carpinelli, M.R.; Fletcher, J.I.; Collinge, J.E.; Hilton, A.A.; Ellis, S.; Kelly, P.N.; Ekert, P.G.; Metcalf, D.; Roberts, A.W.; et al. Programmed anuclear cell death delimits platelet life span. Cell 2007, 128, 1173–1186. [Google Scholar] [CrossRef]
- Kodama, T.; Takehara, T.; Hikita, H.; Shimizu, S.; Shigekawa, M.; Li, W.; Miyagi, T.; Hosui, A.; Tatsumi, T.; Ishida, H.; et al. BH3-only activator proteins Bid and Bim are dispensable for Bak/Bax-dependent thrombocyte apoptosis induced by Bcl-xL deficiency: Molecular requisites for the mitochondrial pathway to apoptosis in platelets. J. Biol. Chem. 2011, 286, 13905–13913. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Kelly, P.N.; White, M.J.; Goschnick, M.W.; Fairfax, K.A.; Tarlinton, D.M.; Kinkel, S.A.; Bouillet, P.; Adams, J.M.; Kile, B.T.; Strasser, A. Individual and overlapping roles of BH3-only proteins Bim and Bad in apoptosis of lymphocytes and platelets and in suppression of thymic lymphoma development. Cell Death Differ. 2010, 17, 1655–1664. [Google Scholar] [CrossRef]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef]
- Millington-Burgess, S.L.; Harper, M.T. Cytosolic and mitochondrial Ca(2+) signaling in procoagulant platelets. Platelets 2021, 32, 855–862. [Google Scholar] [CrossRef]
- Choo, H.J.; Saafir, T.B.; Mkumba, L.; Wagner, M.B.; Jobe, S.M. Mitochondrial calcium and reactive oxygen species regulate agonist-initiated platelet phosphatidylserine exposure. Arter. Thromb. Vasc. Biol. 2012, 32, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- Pasalic, L.; Wing-Lun, E.; Lau, J.K.; Campbell, H.; Pennings, G.J.; Lau, E.; Connor, D.; Liang, H.P.; Muller, D.; Kritharides, L.; et al. Novel assay demonstrates that coronary artery disease patients have heightened procoagulant platelet response. J. Thromb. Haemost. 2018, 16, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Robichaux, D.J.; Harata, M.; Murphy, E.; Karch, J. Mitochondrial permeability transition pore-dependent necrosis. J Mol Cell Cardiol 2023, 174, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Li, G.; Feng, Q.; Hou, M.; Peng, J. Reduced intracellular antioxidant capacity in platelets contributes to primary immune thrombocytopenia via ROS-NLRP3-caspase-1 pathway. Thromb. Res. 2021, 199, 1–9. [Google Scholar] [CrossRef]
- Nakagawa, T.; Shimizu, S.; Watanabe, T.; Yamaguchi, O.; Otsu, K.; Yamagata, H.; Inohara, H.; Kubo, T.; Tsujimoto, Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005, 434, 652–658. [Google Scholar] [CrossRef]
- Jobe, S.M.; Wilson, K.M.; Leo, L.; Raimondi, A.; Molkentin, J.D.; Lentz, S.R.; Di Paola, J. Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis. Blood 2008, 111, 1257–1265. [Google Scholar] [CrossRef]
- Su, M.; Chen, C.; Li, S.; Li, M.; Zeng, Z.; Zhang, Y.; Xia, L.; Li, X.; Zheng, D.; Lin, Q.; et al. Gasdermin D-dependent platelet pyroptosis exacerbates NET formation and inflammation in severe sepsis. Nat. Cardiovasc. Res. 2022, 1, 732–747. [Google Scholar] [CrossRef]
- Liu, F.; Gamez, G.; Myers, D.R.; Clemmons, W.; Lam, W.A.; Jobe, S.M. Mitochondrially mediated integrin alphaIIbbeta3 protein inactivation limits thrombus growth. J. Biol. Chem. 2013, 288, 30672–30681. [Google Scholar] [CrossRef]
- Hua, V.M.; Abeynaike, L.; Glaros, E.; Campbell, H.; Pasalic, L.; Hogg, P.J.; Chen, V.M. Necrotic platelets provide a procoagulant surface during thrombosis. Blood 2015, 126, 2852–2862. [Google Scholar] [CrossRef]
- Tomasiak, M.; Rusak, T.; Gacko, M.; Stelmach, H. Cyclosporine enhances platelet procoagulant activity. Nephrol. Dial. Transplant. 2007, 22, 1750–1756. [Google Scholar] [CrossRef]
- Arachiche, A.; Kerbiriou-Nabias, D.; Garcin, I.; Letellier, T.; Dachary-Prigent, J. Rapid procoagulant phosphatidylserine exposure relies on high cytosolic calcium rather than on mitochondrial depolarization. Arter. Thromb. Vasc. Biol. 2009, 29, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Guo, H.; Zhang, Y.; Qiao, R. Procoagulant platelets: Generation, characteristics, and therapeutic target. J. Clin. Lab. Anal. 2021, 35, e23750. [Google Scholar] [CrossRef] [PubMed]
- Wernig, F.; Xu, Q. Mechanical stress-induced apoptosis in the cardiovascular system. Prog. Biophys. Mol. Biol. 2002, 78, 105–137. [Google Scholar] [CrossRef] [PubMed]
- Masselli, E.; Pozzi, G.; Vaccarezza, M.; Mirandola, P.; Galli, D.; Vitale, M.; Carubbi, C.; Gobbi, G. ROS in Platelet Biology: Functional Aspects and Methodological Insights. Int. J. Mol. Sci. 2020, 21, 4866. [Google Scholar] [CrossRef]
- Kaiser, R.; Escaig, R.; Kranich, J.; Hoffknecht, M.L.; Anjum, A.; Polewka, V.; Mader, M.; Hu, W.; Belz, L.; Gold, C.; et al. Procoagulant platelet sentinels prevent inflammatory bleeding through GPIIBIIIA and GPVI. Blood 2022, 140, 121–139. [Google Scholar] [CrossRef]
- Kaiser, R.; Dewender, R.; Mulkers, M.; Stermann, J.; Rossaro, D.; Di Fina, L.; Li, L.; Gold, C.; Schmid, M.; Kaab, L.; et al. Procoagulant platelet activation promotes venous thrombosis. Blood 2024, 144, 2546–2553. [Google Scholar] [CrossRef]
- Denorme, F.; Manne, B.K.; Portier, I.; Eustes, A.S.; Kosaka, Y.; Kile, B.T.; Rondina, M.T.; Campbell, R.A. Platelet necrosis mediates ischemic stroke outcome in mice. Blood 2020, 135, 429–440. [Google Scholar] [CrossRef]
- Schaubaecher, J.B.; Smiljanov, B.; Haring, F.; Steiger, K.; Wu, Z.; Ugurluoglu, A.; Luft, J.; Ballke, S.; Mahameed, S.; Schneewind, V.; et al. Procoagulant platelets promote immune evasion in triple-negative breast cancer. Blood 2024, 144, 216–226. [Google Scholar] [CrossRef]
- Grootjans, S.; Vanden Berghe, T.; Vandenabeele, P. Initiation and execution mechanisms of necroptosis: An overview. Cell Death Differ. 2017, 24, 1184–1195. [Google Scholar] [CrossRef]
- Shi, C.; Mammadova-Bach, E.; Li, C.; Liu, D.; Anders, H.J. Pathophysiology and targeted treatment of cholesterol crystal embolism and the related thrombotic angiopathy. FASEB J. 2023, 37, e23179. [Google Scholar] [CrossRef]
- DeRoo, E.; Khoury, M.; Zhou, T.; Yang, H.; Stranz, A.; Luke, C.; Henke, P.; Liu, B. Investigating the role of receptor interacting protein kinase 3 in venous thrombosis. JVS Vasc. Sci. 2022, 3, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Moujalled, D.; Gangatirkar, P.; Kauppi, M.; Corbin, J.; Lebois, M.; Murphy, J.M.; Lalaoui, N.; Hildebrand, J.M.; Silke, J.; Alexander, W.S.; et al. The necroptotic cell death pathway operates in megakaryocytes, but not in platelet synthesis. Cell Death Dis. 2021, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, D.; Desai, J.; Steiger, S.; Muller, S.; Devarapu, S.K.; Mulay, S.R.; Iwakura, T.; Anders, H.J. Activated platelets induce MLKL-driven neutrophil necroptosis and release of neutrophil extracellular traps in venous thrombosis. Cell Death Discov. 2018, 4, 6. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Yan, R.; Tian, J.; Zhang, Y.; Zhang, J.; Chen, M.; Cui, Q.; Zhao, L.; Hu, R.; et al. Receptor-interacting protein kinase 3 promotes platelet activation and thrombosis. Proc. Natl. Acad. Sci. USA 2017, 114, 2964–2969. [Google Scholar] [CrossRef]
- Kamal, A.M.; Nabih, N.A.; Rakha, N.M.; Sanad, E.F. Upregulation of necroptosis markers RIPK3/MLKL and their crosstalk with autophagy-related protein Beclin-1 in primary immune thrombocytopenia. Clin. Exp. Med. 2023, 23, 447–456. [Google Scholar] [CrossRef]
- Andonegui, G.; Kerfoot, S.M.; McNagny, K.; Ebbert, K.V.; Patel, K.D.; Kubes, P. Platelets express functional Toll-like receptor-4. Blood 2005, 106, 2417–2423. [Google Scholar] [CrossRef]
- Broz, P. Pyroptosis: Molecular mechanisms and roles in disease. Cell Res. 2025, 35, 334–344. [Google Scholar] [CrossRef]
- Vande Walle, L.; Lamkanfi, M. Pyroptosis. Curr. Biol. 2016, 26, R568–R572. [Google Scholar] [CrossRef]
- Imre, G. Pyroptosis in health and disease. Am. J. Physiol. Cell Physiol. 2024, 326, C784–C794. [Google Scholar] [CrossRef]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Wu, J.; Cai, J.; Tang, Y.; Lu, B. The noncanonical inflammasome-induced pyroptosis and septic shock. Semin. Immunol. 2023, 70, 101844. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, T.; Qiao, R. Pyroptosis in platelets: Thrombocytopenia and inflammation. J. Clin. Lab. Anal. 2023, 37, e24852. [Google Scholar] [CrossRef] [PubMed]
- Yarovinsky, T.O.; Su, M.; Chen, C.; Xiang, Y.; Tang, W.H.; Hwa, J. Pyroptosis in cardiovascular diseases: Pumping gasdermin on the fire. Semin. Immunol. 2023, 69, 101809. [Google Scholar] [CrossRef] [PubMed]
- Lien, T.S.; Chan, H.; Sun, D.S.; Wu, J.C.; Lin, Y.Y.; Lin, G.L.; Chang, H.H. Exposure of Platelets to Dengue Virus and Envelope Protein Domain III Induces Nlrp3 Inflammasome-Dependent Platelet Cell Death and Thrombocytopenia in Mice. Front. Immunol. 2021, 12, 616394. [Google Scholar] [CrossRef]
- Hottz, E.D.; Lopes, J.F.; Freitas, C.; Valls-de-Souza, R.; Oliveira, M.F.; Bozza, M.T.; Da Poian, A.T.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, F.A.; et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood 2013, 122, 3405–3414. [Google Scholar] [CrossRef]
- Lien, T.S.; Sun, D.S.; Wu, C.Y.; Chang, H.H. Exposure to Dengue Envelope Protein Domain III Induces Nlrp3 Inflammasome-Dependent Endothelial Dysfunction and Hemorrhage in Mice. Front. Immunol. 2021, 12, 617251. [Google Scholar] [CrossRef]
- Xue, R.; Li, M.; Zhang, G.; Zhang, W.; Han, L.; Bo, T.; Zhong, H.; Yao, D.; Deng, Y.; Chen, S.; et al. GSDME-mediated pyroptosis contributes to chemotherapy-induced platelet hyperactivity and thrombotic potential. Blood 2024, 144, 2652–2665. [Google Scholar] [CrossRef]
- Butenas, S.; Krudysz-Amblo, J. Decryption of tissue factor. Thromb. Res. 2012, 129 (Suppl. S2), S18–S20. [Google Scholar] [CrossRef][Green Version]
- Ryan, T.A.J.; Preston, R.J.S.; O’Neill, L.A.J. Immunothrombosis and the molecular control of tissue factor by pyroptosis: Prospects for new anticoagulants. Biochem. J. 2022, 479, 731–750. [Google Scholar] [CrossRef]
- Cornelius, D.C.; Travis, O.K.; Tramel, R.W.; Borges-Rodriguez, M.; Baik, C.H.; Greer, M.; Giachelli, C.A.; Tardo, G.A.; Williams, J.M. NLRP3 inflammasome inhibition attenuates sepsis-induced platelet activation and prevents multi-organ injury in cecal-ligation puncture. PLoS ONE 2020, 15, e0234039. [Google Scholar] [CrossRef]
- Vogel, S.; Kamimura, S.; Arora, T.; Smith, M.L.; Almeida, L.E.F.; Combs, C.A.; Thein, S.L.; Quezado, Z.M.N. NLRP3 inflammasome and bruton tyrosine kinase inhibition interferes with upregulated platelet aggregation and in vitro thrombus formation in sickle cell mice. Biochem. Biophys. Res. Commun. 2021, 555, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, S.E.; Halai, R.; Cooper, M.A. Pharmacological Inhibition of the Nod-Like Receptor Family Pyrin Domain Containing 3 Inflammasome with MCC950. Pharmacol. Rev. 2021, 73, 968–1000. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Conrad, M. Ferroptosis: When metabolism meets cell death. Physiol. Rev. 2025, 105, 651–706. [Google Scholar] [CrossRef] [PubMed]
- Januel, C.; El Hentati, F.Z.; Carreras, M.; Arthur, J.R.; Calzada, C.; Lagarde, M.; Vericel, E. Phospholipid-hydroperoxide glutathione peroxidase (GPx-4) localization in resting platelets, and compartmental change during platelet activation. Biochim. Biophys. Acta 2006, 1761, 1228–1234. [Google Scholar] [CrossRef]
- Lagarde, M.; Calzada, C.; Vericel, E. Pathophysiologic role of redox status in blood platelet activation. Influence of docosahexaenoic acid. Lipids 2003, 38, 465–468. [Google Scholar] [CrossRef]
- Chu, B.; Kon, N.; Chen, D.; Li, T.; Liu, T.; Jiang, L.; Song, S.; Tavana, O.; Gu, W. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat. Cell Biol. 2019, 21, 579–591. [Google Scholar] [CrossRef]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 2022, 608, 778–783. [Google Scholar] [CrossRef]
- Song, B.; Miao, W.; Cui, Q.; Shi, B.; Zhang, J.; Qiu, H.; Zhang, L.; Han, Y. Inhibition of ferroptosis promotes megakaryocyte differentiation and platelet production. J. Cell Mol. Med. 2022, 26, 3582–3585. [Google Scholar] [CrossRef]
- Fortuna, V.; Lima, J.; Oliveira, G.F.; Oliveira, Y.S.; Getachew, B.; Nekhai, S.; Aschner, M.; Tizabi, Y. Ferroptosis as an emerging target in sickle cell disease. Curr. Res. Toxicol. 2024, 7, 100181. [Google Scholar] [CrossRef]
- Mikaelsdottir, M.; Vidarsson, B.; Runarsson, G.; Bjarnadottir, U.; Onundarson, P.T.; Sigurjonsson, O.E.; Halldorsdottir, A.M. A comparison of platelet quality between platelets from healthy donors and hereditary hemochromatosis donors over seven-day storage. Transfusion 2021, 61, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Brissot, E.; Troadec, M.B.; Loreal, O.; Brissot, P. Iron and platelets: A subtle, under-recognized relationship. Am. J. Hematol. 2021, 96, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Fibach, E.; Rachmilewitz, E.A. Iron overload in hematological disorders. Presse Med. 2017, 46 Pt 2, e296–e305. [Google Scholar] [CrossRef] [PubMed]
- Barradas, M.A.; Jeremy, J.Y.; Kontoghiorghes, G.J.; Mikhailidis, D.P.; Hoffbrand, A.V.; Dandona, P. Iron chelators inhibit human platelet aggregation, thromboxane A2 synthesis and lipoxygenase activity. FEBS Lett. 1989, 245, 105–109. [Google Scholar] [CrossRef]
- Dimitrov, J.D.; Roumenina, L.T.; Perrella, G.; Rayes, J. Basic Mechanisms of Hemolysis-Associated Thrombo-Inflammation and Immune Dysregulation. Arter. Thromb. Vasc. Biol. 2023, 43, 1349–1361. [Google Scholar] [CrossRef]
- Oishi, S.; Tsukiji, N.; Otake, S.; Oishi, N.; Sasaki, T.; Shirai, T.; Yoshikawa, Y.; Takano, K.; Shinmori, H.; Inukai, T.; et al. Heme activates platelets and exacerbates rhabdomyolysis-induced acute kidney injury via CLEC-2 and GPVI/FcRgamma. Blood Adv. 2021, 5, 2017–2026. [Google Scholar] [CrossRef]
- Bourne, J.H.; Colicchia, M.; Di, Y.; Martin, E.; Slater, A.; Roumenina, L.T.; Dimitrov, J.D.; Watson, S.P.; Rayes, J. Heme induces human and mouse platelet activation through C-type-lectin-like receptor-2. Haematologica 2021, 106, 626–629. [Google Scholar] [CrossRef]
- Tsukiji, N.; Yokomori, R.; Takusagawa, K.; Shirai, T.; Oishi, S.; Sasaki, T.; Takano, K.; Suzuki-Inoue, K. C-type lectin-like receptor-2 in platelets mediates ferric chloride-induced platelet activation and attenuates ferroptosis of endothelial cells. J. Thromb. Haemost. 2024, 22, 1749–1757. [Google Scholar] [CrossRef]
- Rohlfing, A.K.; Kremser, M.; Schaale, D.; Dicenta-Baunach, V.; Laspa, Z.; Fu, X.; Zizmare, L.; Sigle, M.; Harm, T.; Munzer, P.; et al. cGMP modulates hemin-mediated platelet death. Thromb. Res. 2024, 234, 63–74. [Google Scholar] [CrossRef]
- Manikanta; NaveenKumar, S.K.; Thushara, R.M.; Hemshekhar, M.; Sumedini, M.L.; Sunitha, K.; Kemparaju, K.; Girish, K.S. Counteraction of unconjugated bilirubin against heme-induced toxicity in platelets. Thromb. Res. 2024, 244, 109199. [Google Scholar] [CrossRef]
- Cuperus, F.J.; Hafkamp, A.M.; Hulzebos, C.V.; Verkade, H.J. Pharmacological therapies for unconjugated hyperbilirubinemia. Curr. Pharm. Des. 2009, 15, 2927–2938. [Google Scholar] [CrossRef] [PubMed]
- Brites, D.; Silva, R.F.M. Bilirubin neurotoxicity: A narrative review on long lasting, insidious, and dangerous effects. Pediatr. Med. 2021, 4, 34. [Google Scholar] [CrossRef]
- Bohm, A.; Lauko, V.; Dostalova, K.; Balanova, I.; Varga, I.; Bezak, B.; Jajcay, N.; Moravcik, R.; Lazurova, L.; Slezak, P.; et al. In-vitro antiplatelet effect of melatonin in healthy individuals and patients with type 2 diabetes mellitus. J. Endocrinol. Investig. 2023, 46, 2493–2500. [Google Scholar] [CrossRef]
- Vicente, J.M.; Lescano, C.H.; Bordin, S.; Monica, F.Z.; Gobbi, G.; Anhe, G.F. Agomelatine inhibits platelet aggregation through melatonin receptor-dependent and independent mechanisms. Life Sci. 2023, 328, 121906. [Google Scholar] [CrossRef]
- NaveenKumar, S.K.; Hemshekhar, M.; Kemparaju, K.; Girish, K.S. Hemin-induced platelet activation and ferroptosis is mediated through ROS-driven proteasomal activity and inflammasome activation: Protection by Melatonin. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2303–2316. [Google Scholar] [CrossRef]
- Tripathi, J.K.; Sharma, A.; Sukumaran, P.; Sun, Y.; Mishra, B.B.; Singh, B.B.; Sharma, J. Oxidant sensor cation channel TRPM2 regulates neutrophil extracellular trap formation and protects against pneumoseptic bacterial infection. FASEB J. 2018, 32, 6848–6859. [Google Scholar] [CrossRef]
- Hobai, I.A.; Edgecomb, J.; LaBarge, K.; Colucci, W.S. Dysregulation of intracellular calcium transporters in animal models of sepsis-induced cardiomyopathy. Shock 2015, 43, 3–15. [Google Scholar] [CrossRef]
- D’Elia, J.A.; Weinrauch, L.A. Calcium Ion Channels: Roles in Infection and Sepsis Mechanisms of Calcium Channel Blocker Benefits in Immunocompromised Patients at Risk for Infection. Int. J. Mol. Sci. 2018, 19, 2465. [Google Scholar] [CrossRef]
- Benson, J.C.; Trebak, M. Too much of a good thing: The case of SOCE in cellular apoptosis. Cell Calcium 2023, 111, 102716. [Google Scholar] [CrossRef]
- Saavedra-Torres, J.S.; Pinzon-Fernandez, M.V.; Ocampo-Posada, M.; Nati-Castillo, H.A.; Jimenez Hincapie, L.A.; Cadrazo-Gil, E.J.; Arias-Intriago, M.; Rojas-Cadena, M.; Tello-De-la-Torre, A.; Osejos, W.; et al. Inflammasomes and Signaling Pathways: Key Mechanisms in the Pathophysiology of Sepsis. Cells 2025, 14, 930. [Google Scholar] [CrossRef]
- Kaestner, L.; Bogdanova, A.; Egee, S. Calcium Channels and Calcium-Regulated Channels in Human Red Blood Cells. Adv. Exp. Med. Biol. 2020, 1131, 625–648. [Google Scholar] [CrossRef] [PubMed]
- Lew, V.L. The Calcium Homeostasis of Human Red Blood Cells in Health and Disease: Interactions of PIEZO1, the Plasma Membrane Calcium Pump, and Gardos Channels. Annu. Rev. Physiol. 2025, 87, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Patane, G.T.; Calderaro, A.; Barreca, D.; Tellone, E.; Putaggio, S. Crosstalk Between Sickle Cell Disease and Ferroptosis. Int. J. Mol. Sci. 2025, 26, 3675. [Google Scholar] [CrossRef] [PubMed]
- Khamseekaew, J.; Kumfu, S.; Chattipakorn, S.C.; Chattipakorn, N. Effects of Iron Overload on Cardiac Calcium Regulation: Translational Insights Into Mechanisms and Management of a Global Epidemic. Can. J. Cardiol. 2016, 32, 1009–1016. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, B.; Lv, X.; Chen, C.; Li, K.; Wang, Y.; Liu, J. Ferroptosis: Roles and molecular mechanisms in diabetic cardiomyopathy. Front. Endocrinol. 2023, 14, 1140644. [Google Scholar] [CrossRef]
- Kazandzhieva, K.; Mammadova-Bach, E.; Dietrich, A.; Gudermann, T.; Braun, A. TRP channel function in platelets and megakaryocytes: Basic mechanisms and pathophysiological impact. Pharmacol. Ther. 2022, 237, 108164. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, Z.; Liu, X.; Yin, H.Y.; Tang, Y.; Cao, X. P2X7 Receptor-Mediated Inflammation in Cardiovascular Disease. Front. Pharmacol. 2021, 12, 654425. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Nagy, M.; Heemskerk, J.W.M.; Nieswandt, B.; Braun, A. Store-operated calcium entry in thrombosis and thrombo-inflammation. Cell Calcium 2019, 77, 39–48. [Google Scholar] [CrossRef]
- Ni, D.; Lei, C.; Liu, M.; Peng, J.; Yi, G.; Mo, Z. Cell death in atherosclerosis. Cell Cycle 2024, 23, 495–518. [Google Scholar] [CrossRef]
- Stanzione, R.; Forte, M.; Cotugno, M.; Bianchi, F.; Marchitti, S.; Rubattu, S. Relevance of stromal interaction molecule 1 (STIM1) in experimental and human stroke. Pflug. Arch. 2022, 474, 141–153. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, N.; Ni, Y.S.; Yang, J.M.; Ma, L.; Lan, X.B.; Wu, J.; Niu, J.G.; Yu, J.Q. TRPM2 in ischemic stroke: Structure, molecular mechanisms, and drug intervention. Channels 2021, 15, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zou, Y.; Miao, Z.; Jiang, L.; Zhao, X. Transient receptor potential ion channels and cerebral stroke. Brain Behav. 2023, 13, e2843. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, Y.; Feng, H. P2X7 Receptor-Associated Programmed Cell Death in the Pathophysiology of Hemorrhagic Stroke. Curr. Neuropharmacol. 2018, 16, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Rinald, J.H.; Troy, C.M. A review of cell death pathways in hemorrhagic stroke. Front. Cell Dev. Biol. 2025, 13, 1570569. [Google Scholar] [CrossRef]

| Nucleated Cells | Platelets | ||
|---|---|---|---|
| Triggering factors | Death receptors | Cellular stress factors | Do not express FAS or TRAIL receptors |
| Pathways | Extrinsic | Intrinsic | Intrinsic |
| Triggering mechanism | Ligand binding → adaptor proteins → caspases | Caspases 8 → BID → BAX/BAK → Mitochondrial damage | BAX/BAK → Mitochondrial damage |
| Key regulatory proteins | Caspases 3/6/7, BID | BCL-XL, BAX, BAK | |
| Morphological signs | Cell shrinkage, DNA fragmentation, chromatin condensation, membrane blebbing and PS exposure | PS exposure and membrane blebbing | |
| Outcomes | Cell death | Procoagulant activity (BH3-only proteins–mimetism) Severe thrombocytopenia (BCL-XL deficiency) Prolonged platelet lifespan (BAD, BAK deficiency) | |
| Nucleated Cells | Platelets | |
|---|---|---|
| Triggering factors | Ischemia, trauma and infection | Mechanical stress and high ROS |
| Pathways | Ca2+ ↑ → MPTP formation → Δψm collapse → ROS increase → | Ca2+ ↑ → MPTP formation via MCU and CypD → PS exposure |
| Morphological changes | Membrane rupture, cytoplasmic disintegration and nuclear fragmentation | Platelet swelling and fragmentation |
| Key regulatory proteins | CypD, components of MPTP | Cyclophilin D, MCU |
| Outcomes | Extensive tissue inflammation and damage | Coagulation and clot stabilization |
| Nucleated Cells | Platelets | |
|---|---|---|
| Triggering factors | TNF-α, PAMPs, TLR activation | TNF-α, oxidative stress, TLR4 activation, thrombosis |
| Pathways | TNFR1 or TLR activation → RIPK1→ RIPK3 → MLKL→ Membrane pore formation | TLR4 activation → RIPK1 → RIPK3 → MLKL |
| Morphological changes | Plasma membrane swelling, organelle swelling, rupture of plasma membrane, nuclear fragmentation and cellular lysis | Platelet activation and granule release |
| Key regulatory proteins | RIPK1, RIPK3, MLKL | RIPK3, MLKL |
| Outcomes | Cell lysis, DAMPs, and cytokines release and tissue damage | Clot formation, thrombosis and thromboinflammation |
| Nucleated Cells | Platelets | |
|---|---|---|
| Triggering factors | PAMPs or DAMPs | PAMPs, DAMPs, oxidative stress and microbial infections |
| Pathways | Canonical: NLRP3 → Caspase-1 → GSDMD → Membrane Pores | NLRP3 → Caspase-1 → GSDMD → membrane pores |
| Non-canonical: Caspase-4/5 (or Caspase-11 in mice) →LPS activation | ||
| Morphological changes | Cell swelling, chromatin condensation and membrane rupture | Platelet swelling, membrane pore formation and IL-1β release |
| Key regulatory proteins | NLRP3, caspase-1, GSDMD, IL-1β, IL-18 | NLRP3, caspase-1, GSDMD, TLR4, S100A8/A9 |
| Outcomes | Cytokine release (IL-1β, IL-18) and immune cell recruitment | Thrombosis and inflammation |
| Nucleated Cells | Platelets | |
|---|---|---|
| Triggering factors | Iron-dependent ROS production, neurodegeneration and cancer | Iron overload (e.g., heme/hemin), hemolysis and rhabdomyolysis |
| Pathways | ROS → Fe2+ ↑ → Lipid peroxidation ↑→ GSH depletion → GPX4 inactivation → Ferroptosis | Eryptosis → Hemin → Fe2+ → ROS ↑ → Lipid peroxidation ↑ → GSH depletion ↓ → GPX4 translocation → TXA2 ↑ → Platelet activation |
| Molecular components | Transferrin, ferritin, ferroportin, GPX4, FSP1 | Transferrin, ferritin, GPX4, FSP1, GSDMD, hemin, S100A8/A9, TLR4 |
| Morphological changes | Lipid peroxidation → Membrane rupture → Cell death | Lipid peroxidation → Membrane pore→ Platelet swelling and activation |
| Outcomes | Cell death accompanied with DAMPs and cytokine release | Platelet activation and aggregation, cytokine release and thrombosis |
| Disease Context | Cell Death Pathways | Calcium Channels | ROS Production | Crosstalk |
|---|---|---|---|---|
| Sepsis/ Systemic Inflammation | Apoptosis, Necroptosis Pyroptosis Ferroptosis | TRPM2 [96] CICR [97] SOCE [98,99] | Activation of NLRP3 complex; Mitochondrial damage and cytokine /ROS release; Accumulation of free iron enhances ROS production, lipid peroxidation. | Pyroptosis drives inflammation. Necroptosis, ferroptosis and apoptosis occur in parallel, triggering multiorgan failure (coagulopathy, microthrombus formation, endothelial dysfunction, immunoparalysis) [100]. |
| Sickle Cell Disease (SCD) | Ferroptosis Pyroptosis Necrosis | Piezo TRPs [101,102] | Hemolysis-associated ROS production; ROS-mediated thrombosis, abnormal red blood cell and platelet adhesion and lipid peroxidation. | Heme-mediated NLRP3 activation and pyroptosis. Ferroptosis is driven in parallel by lipid ROS and iron overload [103]. |
| Iron Overload/ Cardiomyopathy | Ferroptosis Apoptosis | LTCC [104] | Iron overload-induced ROS production triggering both apoptosis and ferroptosis. | Iron overload-mediated lipid peroxidation and ferroptosis. Mitochondrial ROS-induced endothelial dysfunction and apoptosis. Both pathways converge on ROS [105]. |
| Atherosclerosis/ Thrombosis | Apoptosis Pyroptosis Necroptosis | TRPs [106] P2X7 [107] SOCE [108] | Endothelial dysfunction and inflammasome activation and foam cell death | Apoptosis-mediated endothelial damage; endothelial and macrophage apoptosis destabilizing necrotic plaques; pyroptotic macrophages triggers vascular inflammation; necroptosis-induced plaque rupture and thrombosis, monocyte and platelet adhesion [109]. |
| Stroke/Ischemia–Reperfusion Injury | Necrosis Apoptosis Ferroptosis Necroptosis | SOCE [110] TRPs [111,112] P2X7 [113] | Lipid peroxidation | Pyroptosis-mediated inflammatory cell death; blood–brain barrier rupture; Intracerebral hemorrhage-mediated hemolysis triggering ferroptosis and necroptosis; Necroptosis-mediated neurodegeneration; Intracranial mechanical force-induced necrosis; Mitochondrial Ca2+ overload-induced apoptosis and necrosis. ROS and iron accumulation triggering ferroptosis [114]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Braun, A.; Zu, J.; Gudermann, T.; Mammadova-Bach, E.; Anders, H.-J. Converging Molecular Mechanisms of Nucleated Cell Death Pathways and Procoagulant Platelet Formation. Cells 2025, 14, 1075. https://doi.org/10.3390/cells14141075
Li C, Braun A, Zu J, Gudermann T, Mammadova-Bach E, Anders H-J. Converging Molecular Mechanisms of Nucleated Cell Death Pathways and Procoagulant Platelet Formation. Cells. 2025; 14(14):1075. https://doi.org/10.3390/cells14141075
Chicago/Turabian StyleLi, Cong, Attila Braun, Juan Zu, Thomas Gudermann, Elmina Mammadova-Bach, and Hans-Joachim Anders. 2025. "Converging Molecular Mechanisms of Nucleated Cell Death Pathways and Procoagulant Platelet Formation" Cells 14, no. 14: 1075. https://doi.org/10.3390/cells14141075
APA StyleLi, C., Braun, A., Zu, J., Gudermann, T., Mammadova-Bach, E., & Anders, H.-J. (2025). Converging Molecular Mechanisms of Nucleated Cell Death Pathways and Procoagulant Platelet Formation. Cells, 14(14), 1075. https://doi.org/10.3390/cells14141075







