1. Introduction
Patients with World Health Organization (WHO) grade III–IV central nervous system (CNS) gliomas exhibit a median overall survival of approximately 12–18 months following conventional surgical intervention [
1]. Owing to the limited sensitivity of these tumors to radiotherapy and chemotherapy [
2,
3], novel therapeutic modalities such as immunotherapy [
4], cellular therapy, and oncolytic virotherapy have been extensively explored in preclinical research [
5,
6]. Dendritic cell (DC) vaccination represents a promising immunotherapeutic strategy [
7]. This approach involves the differentiation of autologous peripheral blood-derived monocytes into DCs, which are subsequently pulsed with tumor lysate antigens ex vivo and reinfused into the patient. As professional antigen-presenting cells (APCs), DCs process and present the loaded antigens to CD8
+ and CD4
+ T cells within lymphoid organs, thereby indirectly eliciting antitumor immune responses [
8,
9]. However, similar to other emerging therapies, DC-based vaccines face significant challenges, including immune evasion mechanisms and the highly immunosuppressive tumor microenvironment (TME) characteristic of CNS gliomas [
10]. Although clinical trials of DC vaccines for glioma patients are underway, the autologous nature of these vaccines presents inherent limitations: DCs are often terminally differentiated and exhibit poor antigen-presenting capacity, insufficient T cell activation, and impaired migratory ability [
11,
12,
13,
14,
15]. Furthermore, the complex and costly procedures for DC extraction and induction, coupled with their suboptimal biological functionality, impose substantial physiological and economic burdens on patients. Toll-like receptors (TLRs) play pivotal roles in innate immunity. Previous studies have reported that activation of TLR3 and TLR9 can enhance the expression of antitumor surface markers on DCs [
16,
17,
18,
19]. Members of our research group have previously reported that combined activation of TLR3 and TLR9 may enhance the antitumor function of microglia through a synergistic effect, thereby offering a new strategy for glioma immunotherapy [
20]. Since both dendritic cells and macrophages are professional antigen-presenting cells, we hypothesize that co-activation of TLR3 and TLR9 can potentiate the antitumor efficacy of dendritic cell vaccines. This study aims to develop a cost-effective strategy to augment the antitumor efficacy of DC vaccines and to elucidate the underlying mechanisms.
Using a murine glioma model, we investigated a modified DC vaccine strategy involving the synergistic ex vivo activation of TLR9 and TLR3 with CpG ODN and poly(I:C), respectively. This combined stimulation enhanced DC migration, antigen presentation, and CD8+ T cell activation capacity. In vivo experiments demonstrated that this approach significantly delayed tumor progression and extended the survival of glioma-bearing mice. Integrated transcriptomic sequencing and Western blot analyses revealed that the synergistic mechanism may involve TLR9-mediated upregulation of TLR3 expression. Further experiments indicated that sequential administration of the agonists (TLR9 activation followed by TLR3 activation) yielded more pronounced enhancements in DC function and antitumor efficacy, likely due to the time-dependent receptor upregulation requirement.
This study provides a novel strategy for improving the efficacy of human-derived DC vaccines and offers preliminary insights into the associated mechanistic pathways.
2. Materials and Methods
2.1. Ethical Approval
The study protocol was reviewed and approved by the Animal Ethics Committee (TJH-24-07-046, Approval Date: 19 December 2024) and the Clinical Ethics Committee (TJ-IRB20220325, Approval Date: 7 September 2022) of Tongji Hospital, Huazhong University of Science and Technology. Written informed consent was obtained from both volunteers who provided blood samples.
2.2. Cell Culture and Processing
The mouse glioma cell lines (GL261 and CT2A) and human glioma cell lines (LN229 and U251) used in this study were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and confirmed to be free of mycoplasma contamination. All glioma cell lines were maintained in high-glucose DMEM medium (G4612, ServiceBio, Wuhan, China) supplemented with 10% fetal bovine serum (FBS, A5661701, Gibco, Plainville, MA, USA) and 1% penicillin-streptomycin solution (G4003, ServiceBio, Wuhan, China) at 37 °C in a humidified incubator. The culture medium was replaced every 24–48 h. When cells reached 70–80% confluence as observed under an optical microscope (Olympus, Tokyo, Japan), they were either subjected to experimental procedures or passaged/frozen using trypsin digestion solution (G4001, ServiceBio, Wuhan, China). Mouse-derived dendritic cells (DCs) were isolated from the bone marrow of femurs of 6–10-week-old male C57BL/6 mice. These cells were induced to differentiate in RPMI-1640 medium (G4531, ServiceBio, Wuhan, China) containing recombinant GM-CSF (HY-P7361, MCE, Shanghai, China) and IL-4 (HY-P70644, MCE, Shanghai, China) for 7 days before experimental use. Mouse-derived lymphocytes were extracted from the spleens of 6–10-week-old male C57BL/6 mice using lymphocyte separation medium (DKW33, DAKEWE, Shenzhen, China) and cultured in RPMI-1640 medium. Human CD8+ T cells were obtained from the same two volunteers who provided peripheral blood samples for human dendritic cell isolation. CD8+ T cells were isolated from peripheral blood and used for co-culture experiments.
2.3. Isolation of Human CD8+ T Cells
Human CD8+ T cells were isolated from heparinized peripheral blood of healthy volunteers. Briefly, the blood sample was diluted with a 10-fold volume of cold isolation buffer. Cell isolation was then carried out using the Dynabeads™ Untouched™ Human CD8+ T Cells Kit (11348D, Thermo Fisher, Waltham, MA, USA) according to the manufacturer’s protocol, which is based on a negative selection principle to obtain untouched, high-purity CD8+ T cells.
2.4. Animals
C57BL/6 mice (6–10 weeks old) used in this study were obtained from Hubei BIONt Biological Technology Co., Ltd. (Wuhan, China). The mice were housed under specific pathogen-free (SPF) conditions in the animal facility of the Research Building at Tongji Hospital.
2.5. Preparation of Dendritic Cell Vaccines
Tumor lysate-derived whole antigen was prepared from glioma cell lines. Briefly, the harvested glioma cells were subjected to five cycles of rapid freezing in liquid nitrogen for 3 min followed by immediate thawing in a water bath at 37 °C for 2 min. The resulting lysate was then filtered through a 40 um cell strainer to obtain the whole antigen preparation. For mouse bone marrow-derived dendritic cells (BMDCs), the whole antigen derived from GL261 or CT2A glioma cell lines was added to the dendritic cell culture medium at a ratio of 2:1 (glioma cells to dendritic cells) and incubated for 24 h to allow antigen uptake. For human engineered dendritic cells, the whole antigen prepared from LN229 or U251 glioma cell lines was loaded at a ratio of 4:1 (glioma cells to dendritic cells) under the same incubation conditions. The antigen-loaded dendritic cells were then used as dendritic cell vaccines.
2.6. Co-Culture System
2.6.1. CFSE Labeling of CD8+ T Cells
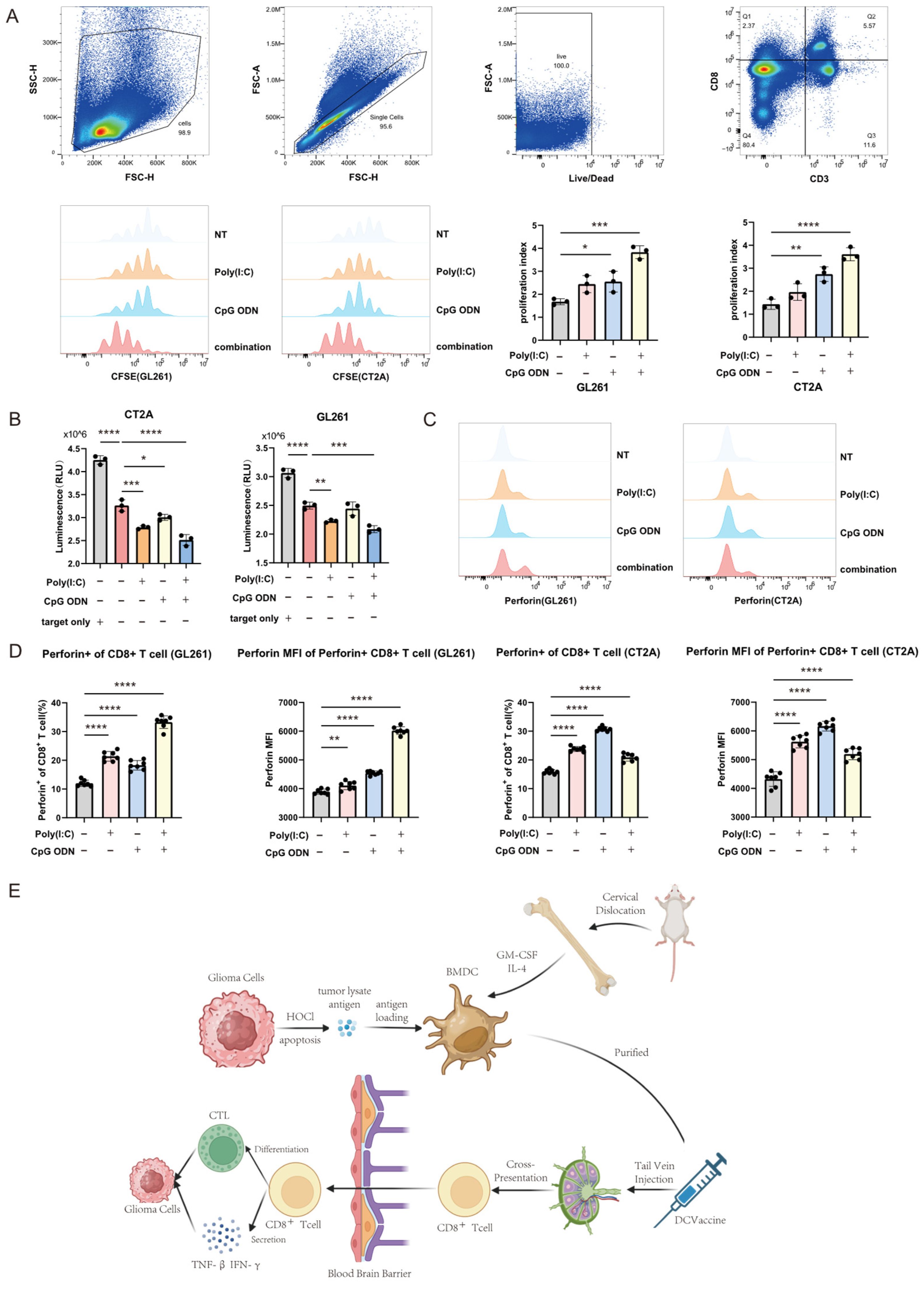
Mouse splenic lymphocytes or human peripheral blood-derived CD8+ T cells were pre-labeled with CFSE dye (E-CK-A345, Elabscience, Wuhan, China) at a concentration of 5 uM for 20 min at 37 °C protected from light. After incubation, the staining was quenched by adding a five-fold volume of complete culture medium (RPMI 1640 supplemented with 10% FBS). The cells were then washed thoroughly three times with phosphate-buffered saline (PBS) to remove any residual unbound dye and resuspended in fresh complete medium. Prior to formal experiments, the proportion and purity of CD8+ T cells were confirmed by flow cytometric analysis using anti-CD3 (300311, 155606, DAKEWE, Shenzhen, China) and anti-CD8 (100707, 344706, DAKEWE, Shenzhen, China) antibodies.
2.6.2. Preparation of Dendritic Cell (DC) Vaccines
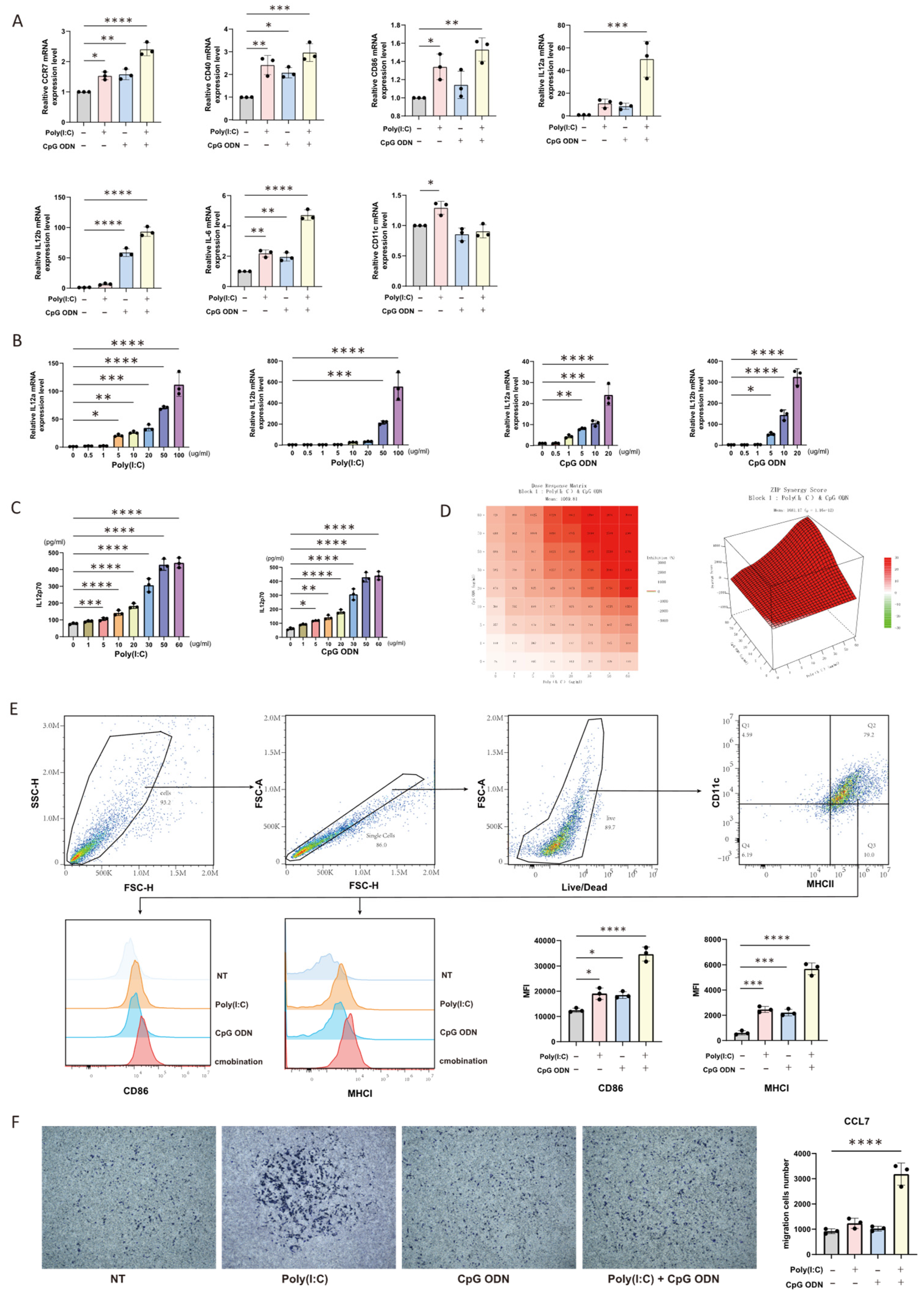
Mouse Bone Marrow-Derived DC Vaccines: Mouse bone marrow-derived dendritic cells (BMDCs) were stimulated for 24 h under one of the following four conditions: (1) PBS (untreated control); (2) 10 ug/mL poly(I:C) alone; (3) 10 ug/mL CpG ODN alone; (4) a combination of 10 ug/mL poly(I:C) and 10 ug/mL CpG ODN. Following stimulation, the DCs were pulsed with whole tumor lysate antigen (at a DC to tumor cell ratio of 1:3) for an additional 24 h to generate antigen-loaded DC vaccines.
Human Engineered DC Vaccines: Human engineered dendritic cells were subjected to one of four distinct activation protocols: (1) PBS treatment for 24 h (control); (2) simultaneous treatment with 10 ug/mL poly(I:C) and 15 ug/mL CpG ODN for 24 h; (3) simultaneous treatment with 15 ug/mL poly(I:C) and 10 ug/mL CpG ODN for 24 h; (4) sequential treatment involving pretreatment with 10 ug/mL CpG ODN for 24 h followed by 10 ug/mL poly(I:C) for another 24 h. Subsequently, all groups were loaded with whole tumor lysate antigen (at a DC to tumor cell ratio of 1:6) for 24 h to form the final DC vaccines.
2.6.3. Co-Culture and Sample Collection
Both mouse and human antigen-loaded DC vaccines were harvested, washed extensively with PBS to remove residual culture components, and counted. The washed DC vaccines were then co-cultured with the pre-prepared CFSE-labeled CD8+ T cells at an effector-to-target ratio of 4:1 (CD8+ T cells to DCs) in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (dual antibiotics). The co-culture system was maintained in a humidified incubator at 37 °C with 5% CO2 for 48 h. After the incubation period, the co-culture supernatant was carefully collected for subsequent cytokine analysis by ELISA, while the cell pellets were harvested for flow cytometric analysis of T cell proliferation (based on CFSE dilution) and activation markers. The measurements for all parameters within the co-culture system were conducted in three biologically independent replicates.
2.7. Cytotoxicity Assay of CD8+ T Cells
The in vitro CD8+ T cell cytotoxicity was evaluated using a co-culture system comprising CD8+ T cells, dendritic cells (DCs), and luciferase-transfected tumor cells. Following 48 h of co-culture, the cells were lysed, and potassium luciferin substrate was added to the system. The bioluminescence intensity, which inversely correlates with the number of viable tumor cells, was measured using a multifunctional microplate reader within 5–25 min after substrate addition.
2.8. Engineered Dendritic Cells
The human-derived engineered dendritic cell (DC) vaccine was developed and provided by Beijing Celarts Biosciences Group Co., Ltd. (Beijing, China). Peripheral blood samples, used for the induction of dendritic cells and the isolation of CD8+ T cells, were obtained from two healthy adult volunteers recruited at Tongji Hospital, Huazhong University of Science and Technology.
2.9. Brain Tissue Sectioning and Staining
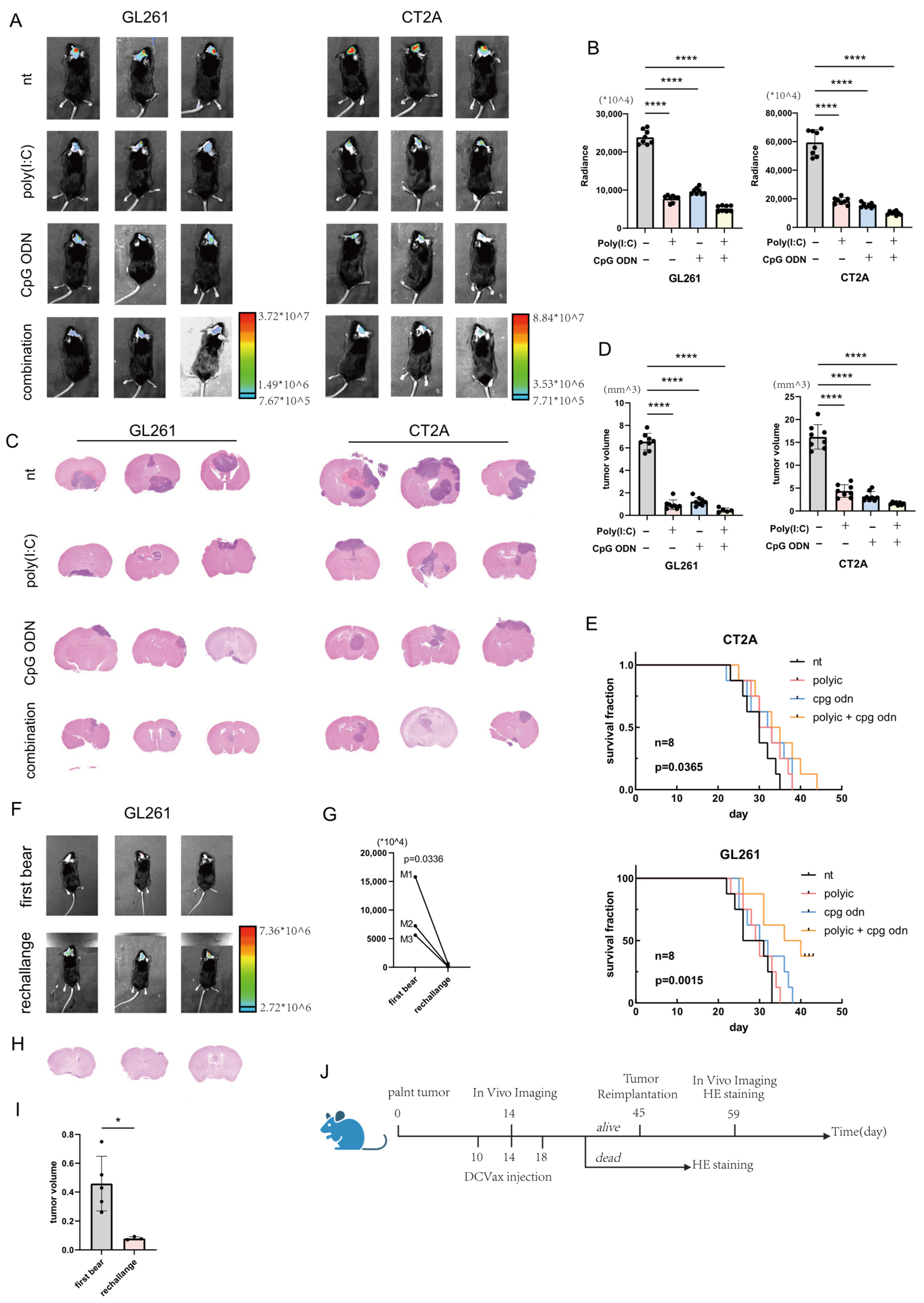
When glioma-bearing mice exhibited clear tumor-related symptoms such as hemiplegia, convulsions, and significant weight loss, they were administered muscle relaxants and an overdose of anesthetic. The limbs were secured, and transcardial perfusion was performed using phosphate-buffered saline (PBS). Subsequently, the brain tissue was carefully extracted for sectioning and hematoxylin-eosin (H&E) staining. The processes of brain tissue sectioning, H&E staining, and slide scanning were conducted by Biossci Company (Wuhan, China).
2.10. Establishment and Monitoring of Orthotopic Glioma Models
On day 0, glioma cells stably transfected with the luciferase gene were digested, washed and then resuspended at a density of 50,000 cells per mouse. The cell suspension was intracranially injected into the right hemisphere of anesthetized mice using a stereotactic instrument (68801, RWD, Shenzhen, China). On days 10, 14, and 18, each mouse received 200,000 dendritic cell vaccines via tail vein injection. On day 14, anesthetized mice were intraperitoneally injected with 150 mg/kg D-luciferin sodium (HY-12591, MCE, Shanghai, China). Bioluminescence intensity in the brain was monitored using a small animal in vivo imaging system (LAGO X, Spectral Instruments Imaging, Tucson, AZ, USA) within 10–40 min post-injection. Mice surviving beyond day 45 without significant symptoms underwent tumor reimplantation. Bioluminescence signals were reassessed on day 59. The longest diameter (L) and the perpendicular shortest diameter (W) of the tumor region were measured in the scanned sections using CaseViewer software (version 2.4.0.119028), and the tumor volume was estimated using the formula V = 1/2 × L × W2.
2.11. Protein Sample Preparation
Dendritic cells were pelleted by centrifugation, and the supernatant was carefully aspirated and discarded. The cell pellet was then resuspended in phosphate-buffered saline (PBS) and washed twice to thoroughly remove any residual culture medium. A working solution of RIPA lysis buffer was prepared by supplementing it with phenylmethylsulfonyl fluoride at a recommended ratio (typically 100:1, v/v) to inhibit protease activity. This mixture was added to the cell pellet, followed by incubation on ice for 30 min with intermittent vortexing to ensure complete lysis. The lysate was subsequently centrifuged at 12,000× g for 15 min at 4 °C to precipitate insoluble debris. The resulting supernatant, containing the soluble protein fraction, was carefully collected. The total protein concentration was quantified using a bicinchoninic acid (BCA) protein assay kit (G2026, ServiceBio, Wuhan, China), according to the manufacturer’s instructions. Finally, the protein samples were mixed with protein loading buffer (WB2001, NCM Biotech, Suzhou, China) at an appropriate volume ratio (typically 1:4, sample/buffer) and denatured by boiling at 95–100 °C for 5–10 min to ensure complete dissociation and linearization of the proteins for subsequent electrophoretic analysis.
2.12. Western Blot Analysis
The prepared protein samples were loaded onto an SDS-PAGE gel for electrophoretic separation. Following electrophoresis, the proteins were transferred onto a methanol-activated PVDF membrane (IPFL00005, Millipore, Burlington, MA, USA) using a wet transfer system. After successful transfer, the membrane was blocked with 5% bovine serum albumin to prevent non-specific binding. The blocked membrane was then washed and incubated overnight at 4 °C with the following primary antibodies: TLR3 (A11778, ABclonal, Wuhan, China), p38 MAPK (14064-1-AP, Proteintech, Wuhan, China), phospho-p38 MAPK (28796-1-AP, Proteintech, Wuhan, China), ATF3 (A13469, ABclonal, Wuhan, China), GAPDH (10494-1-AP, Proteintech, Wuhan, China), and β-actin. After primary antibody incubation, the membrane was thoroughly washed and subsequently incubated with species-appropriate HRP-conjugated secondary antibodies. Following a final series of washes, the membrane was treated with a chemiluminescent substrate (P10060, NCM Biotech, Suzhou, China) according to the manufacturer’s instructions. The immunoreactive signals were detected and visualized using the GeneGnome GRQ imaging system (Syngene, Cambridge, UK).
2.13. Enzyme-Linked Immunosorbent Assay (ELISA)
Supernatants were collected from dendritic cell cultures following centrifugation. According to the manufacturer’s instructions provided with the Mouse IL-12 (p70) ELISA Kit (EK0422, Boster, Wuhan, China) or Human IL-12 (p70) ELISA Kit (EK0421, Boster, Wuhan, China), the supernatants, a series of diluted standard solutions, and sample dilution buffer were aliquoted into the pre-coated wells of a microplate. The plate was sealed with an adhesive membrane and incubated for 90 min. After incubation, the liquid was aspirated, and a biotin-conjugated detection antibody working solution was added to each well, followed by a 60-min incubation. The wells were then thoroughly washed, and an Avidin-Biotin-Peroxidase Complex (ABC) working solution was added, with subsequent incubation for 30 min. Following another washing step, Tetramethylbenzidine (TMB) substrate solution was added, and the plate was incubated for 20 min in the dark to allow color development. The enzymatic reaction was terminated by adding stop solution. The optical density (O.D.) of each well was immediately measured at a wavelength of 450 nm using a microplate reader. A standard curve was generated by plotting the mean O.D. values of the standard concentrations versus their corresponding concentrations. The concentration of IL-12 in each test sample was determined by interpolating its O.D. value from the standard curve.
2.14. Flow Cytometry
Cells were harvested and washed thoroughly with phosphate-buffered saline (PBS). According to the manufacturer’s instructions (423106, BioLegend, San Diego, CA, USA), an appropriate amount of flow cytometry antibody was added to the cell suspension. The mixture was thoroughly mixed and incubated protected from light. After incubation, cells were washed again with PBS to remove unbound antibodies. For samples requiring intracellular staining, after the aforementioned steps, cells were fixed using FluoroFix™ Buffer (422101, BioLegend, San Diego, CA, USA). Fixed cells were washed with diluted 10× Intracellular Staining Permeabilization Wash Buffer (421002, BioLegend, San Diego, CA, USA). Subsequently, the corresponding intracellular flow antibody was added, and the mixture was thoroughly mixed and incubated for 60 min protected from light. After incubation, cells were washed again with Intracellular Staining Permeabilization Wash Buffer and resuspended. The prepared samples were analyzed on a CytoFLEX flow cytometer (Beckman Coulter, Indianapolis, IN, USA). The acquired data were statistically processed and analyzed using FlowJo software (version 10.9.0).
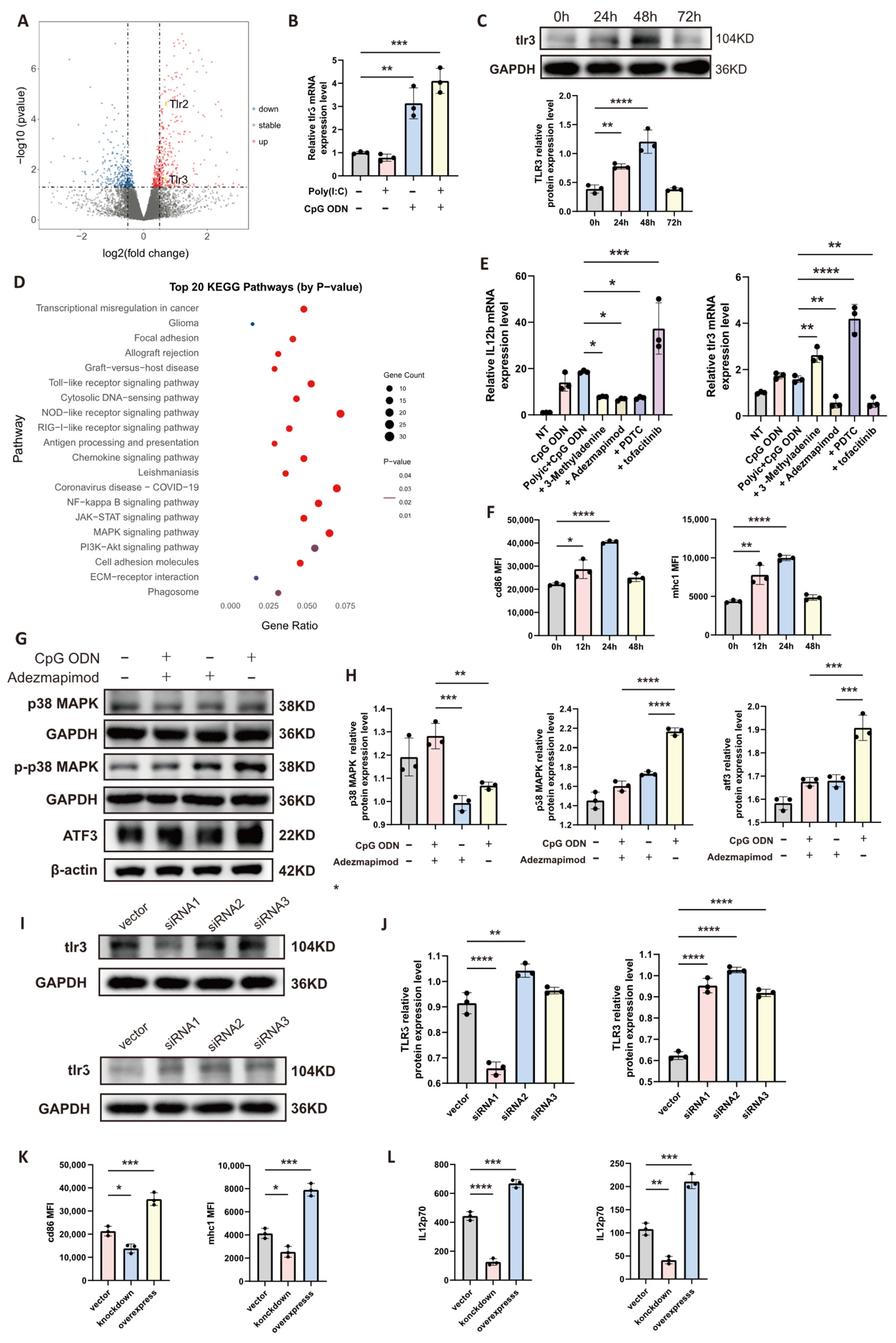
2.15. Transcriptome Sequencing and Bioinformatic Analysis
Mature dendritic cells (DCs) cultured in vitro were distributed into six independent culture dishes. Three dishes were designated as the untreated control group, while the other three were stimulated with a combination of 10 ug/mL CpG ODN and 10 ug/mL poly(I:C) for 24 h. Total RNA was subsequently extracted from all cell samples for transcriptome sequencing. The sequencing was performed on an Illumina platform, with an average sequencing data volume of approximately 6 Gb per sample to ensure sufficient transcriptome coverage. The Q30 base calling accuracy rate for all samples exceeded 85%, guaranteeing the reliability of the raw data. Bioinformatic analysis followed a standardized pipeline. The raw sequencing data were first subjected to quality control filtering using fastp software (version 0.23.4) to remove adapter sequences and low-quality reads. The clean data were then aligned to the appropriate reference genome using the HISAT2 software (version 2.2.1). Gene expression levels were quantified using HTSeq software (version 0.11.2), presented as raw read counts, and further converted into FPKM values for cross-sample comparisons. Differential expression analysis was conducted using the DESeq2 R package (version 1.36.0). Its internal pipeline includes a normalization step that corrects for sequencing depth variation in the raw read counts. Significantly differentially expressed genes were identified using a threshold of an adjusted p-value less than 0.05 and an absolute log2 fold change greater than 1. Based on this analysis, a volcano plot was constructed to visualize the overall distribution of differential genes. For genes significantly upregulated in the combination treatment group, KEGG pathway enrichment analysis was performed using the cluster Profiler tool. The enriched pathways were ranked according to the adjusted p-value of the enrichment results. The top 20 most significantly enriched signaling pathways were selected, and their enrichment significance along with the expression patterns of associated genes were visualized using a heatmap.
2.16. Statistical Analysis
All data analyses and visualizations were performed using GraphPad Prism software (version 9.0.0). Data are presented as the mean ± standard deviation, and individual data points are displayed on column bar graphs. Spearman’s correlation analysis was employed to assess the relationship between two variables. For comparisons between multiple groups, one-way analysis of variance (ANOVA) was conducted, while an unpaired Student’s t-test was used for comparisons between two groups. Statistical significance was defined as a p-value less than 0.05. Specific significance levels were denoted as follows: * = p < 0.05, ** = p < 0.01, *** = p < 0.001, and **** = p < 0.0001.
4. Discussion
In the investigation of immunotherapeutic strategies for glioma, our in vitro experiments demonstrated that individual application of the Toll-like receptor 9 (TLR9) agonist CpG ODN or the TLR3 agonist poly(I:C) moderately upregulates the expression of certain antitumor-related genes (e.g., CD86, MHC-I, IL-12) in dendritic cells (DCs). However, the magnitude of this upregulation was relatively limited. Notably, a synergistic enhancement in the expression of these immunologically active markers was observed when a combined agonist strategy was employed, with the effect being substantially superior to that of any single agonist treatment. This synergistic effect is likely attributable to cross-talk between TLR signaling pathways. Of particular significance, our mechanistic exploration suggests that TLR9 activation may indirectly upregulate TLR3 expression via the p38 MAPK–ATF3 signaling axis, thereby amplifying the responsiveness of DCs to subsequent TLR3 stimulation. This likely constitutes a crucial molecular basis for the synergy observed with combined treatment. ATF3 (activating transcription factor 3) has conventionally been characterized in oncological research as a stress-inducible transcription factor that typically exerts a negative regulatory influence on the p38 MAPK pathway within cells, potentially suppressing tumor growth and invasion [
21]. Similarly, our study reveals a divergent role for ATF3 in DCs: it functions as a key intermediary downstream of TLR9, facilitating the upregulation of TLR3. Leveraging this property, we implemented a sequential activation strategy (CpG ODN followed by poly(I:C)), which significantly augmented the adjuvant effect of DC vaccines, thereby potentiating the antitumor immune response. In in vivo experiments, mice administered DC vaccines pre-activated synergistically exhibited significant tumor growth suppression and prolonged survival. The mechanism underlying this efficacy involves the potent activation of peripheral lymphocytes by these activated DC vaccines, promoting their proliferation and differentiation, which consequently improves the prognosis of mice bearing central nervous system (CNS) gliomas. This finding aligns with a research confirming the presence of resident T cells within the CNS and their role in immune surveillance [
22]. Nevertheless, the observed infiltration of T cells into the CNS in an orthotopic glioma model could also be partially ascribed to the compromise of the blood–brain barrier (BBB) resulting from tumor growth [
23,
24]. Additionally, our histological analysis indicated that tumors with significant hemorrhagic foci demonstrated relatively constrained growth and a delayed onset of clinical symptoms. This suggests that alterations in the vascular microenvironment (e.g., inflammatory reactions or antigen release potentially triggered by hemorrhage) may influence the potency or nature of the antitumor immune response. In the final phase of our research, we employed an engineered dendritic cell (DC) line. Its paramount advantage lies in its capacity for unlimited proliferation in vitro (immortalization), and even in the absence of TLR3 or TLR9 agonists, its baseline expression of immunologically active markers (e.g., CD80, CD86, MHC-II) was significantly higher than that of DCs conventionally differentiated from peripheral blood mononuclear cells (PBMCs). Both the sequential TLR9 and TLR3 activation protocol and such engineered DC vaccines present promising novel avenues for the application of DCs in glioma therapy. Although sequential administration of poly(I:C) and CpG ODN demonstrated promising efficacy in improving the prognosis of tumor-bearing mice in preclinical studies, significant attention must be paid to the clinical safety profile of these Toll-like receptor (TLR) agonists. The systemic toxicity risk associated with poly(I:C) and CpG ODN primarily stems from their potential to induce excessive immune activation. In severe cases, this may lead to systemic inflammatory response or cytokine release syndrome, particularly when administered intravenously or at high doses. While adoptive T cell therapy may partially mitigate these risks, researchers should remain cautious of these limitations during clinical translation. In terms of mechanistic insights, classical oncology literature posits that tumor cells can upregulate the expression of PD-L1 through transcription factors such as ATF3, thereby exhausting effector T-cell function and achieving immune evasion [
25,
26]. This apparent contradiction may be attributed to the divergent roles of ATF3 in tumor cells versus dendritic cells. Furthermore, in the present study, dendritic cells were not chronically exposed to the complex tumor microenvironment, which could also explain why ATF3 exhibited antitumor effects in our experimental setting. Based on these findings, future research directions emerging from this study include utilizing the immortal nature of engineered DCs to construct cellular therapies capable of the sustained autonomous expression of immune-stimulatory molecules (e.g., cytokines, co-stimulatory ligands) and employing nanoparticle-based delivery systems for the controlled release or targeted delivery of TLR agonists or other small-molecule immunomodulators within DCs. Such strategies hold the potential to overcome the temporal and operational complexities associated with sequential administration, paving the way for the development of a new generation of highly efficient and intelligent DC vaccines.