Role of Lipocalin-2 in Brain Injury After Subarachnoid Hemorrhage in Female Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Subarachnoid Hemorrhage Induction
2.2. Immunohistochemistry and Immunofluorescence Staining
2.3. Western Blotting
2.4. Fluoro-Jade C Staining
2.5. Magnetic Resonance Imaging (MRI) Scanning and T2 Lesion Measurement
2.6. Neurological Score
2.7. Statistical Analysis
3. Results
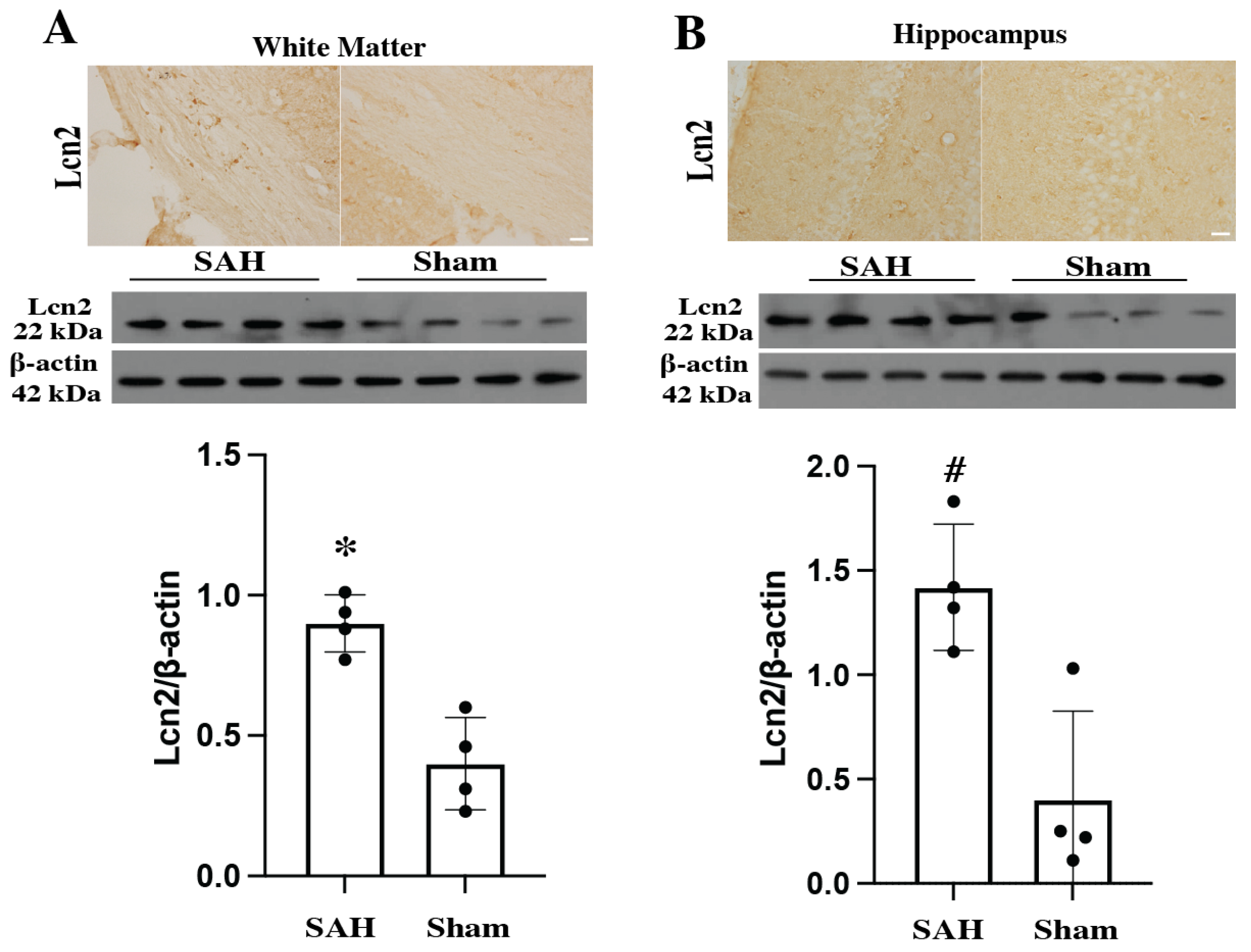
3.1. Lcn2 Expression Was Elevated in Different Parts of Brain at Day 1 After SAH
3.2. Lcn2 Expression Located with Microglia, Astrocytes but Not Neurons
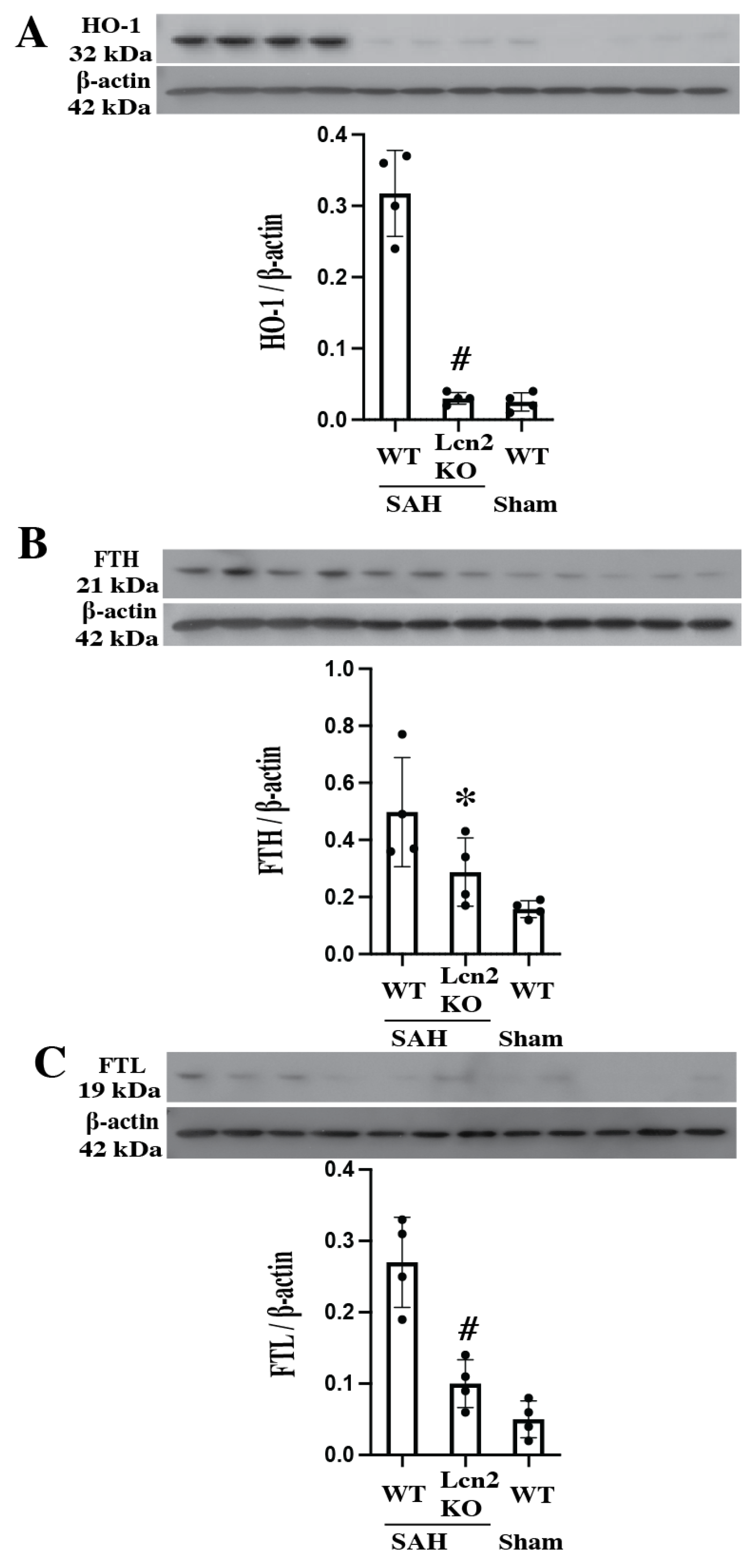
3.3. Lcn2 KO Reduced Heme Oxygenase-1 and Ferritin Upregulation at Day 1 After SAH
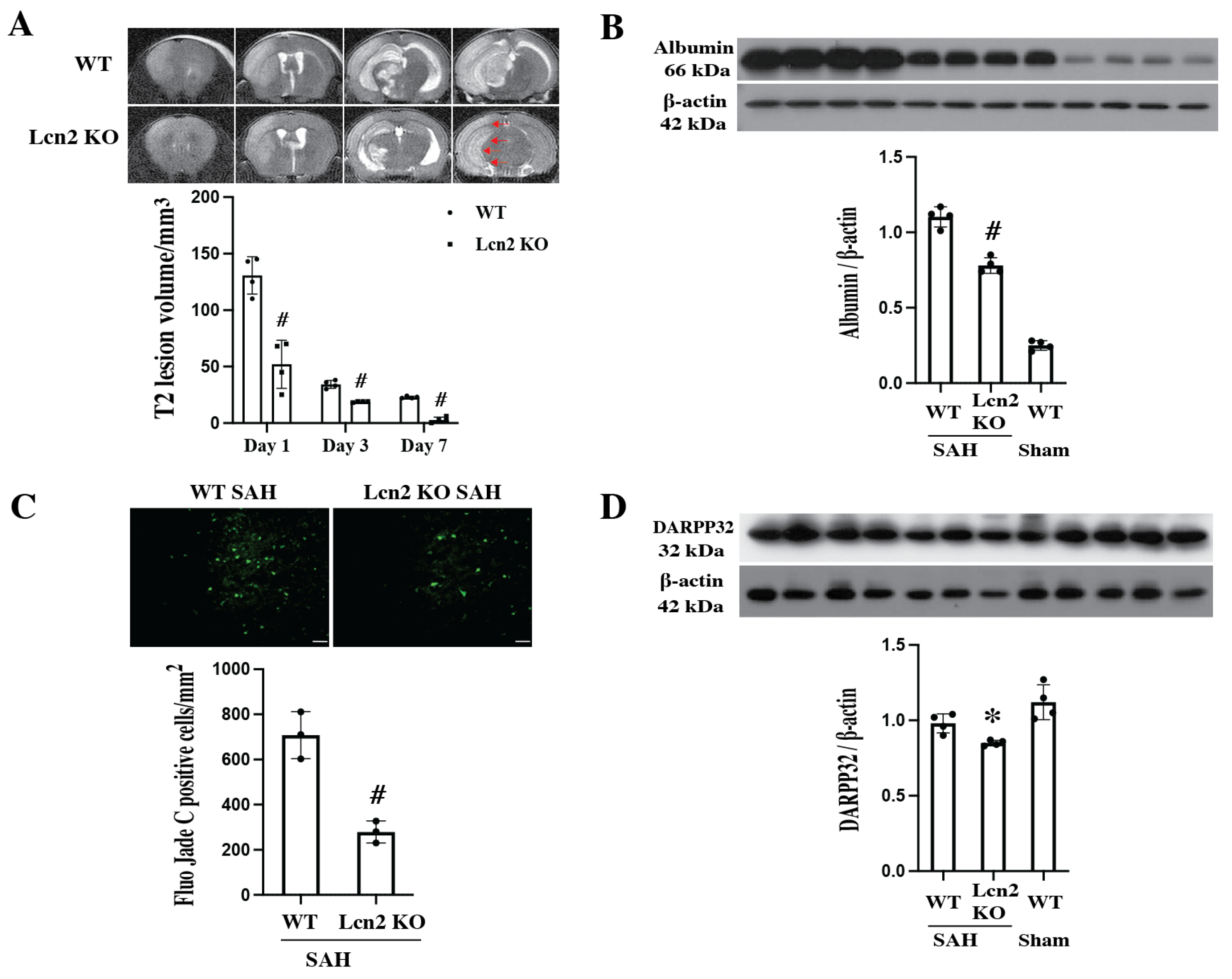
3.4. SAH-Induced Smaller MRI T2 Lesion, Less BBB Disruption and Decreased Neuronal Death in Lcn2 KO Mice
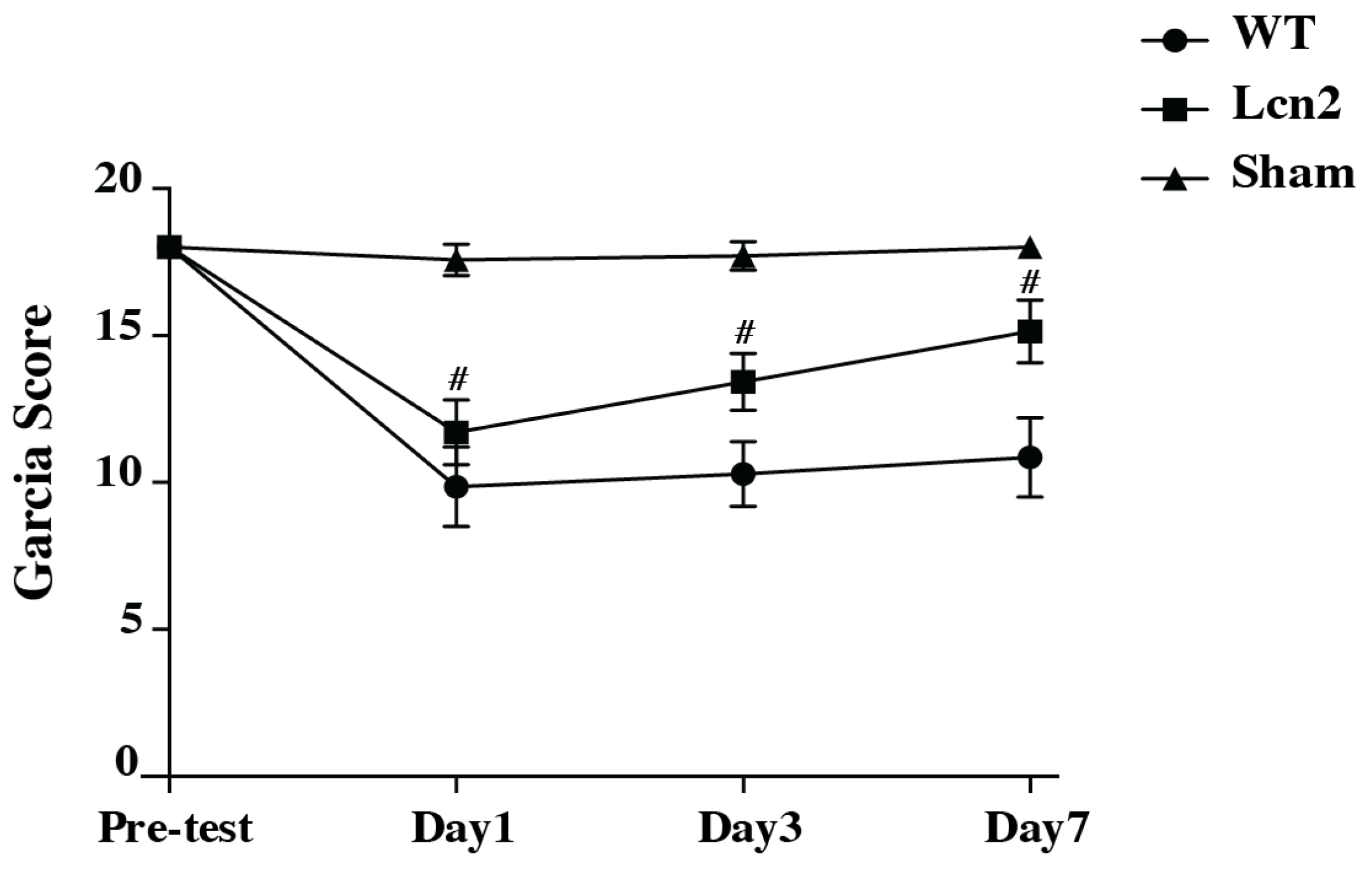
3.5. SAH Resulted in Reduced Neurological Deficits in Lcn2 KO Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SAH | Subarachnoid hemorrhage |
| BBB | Blood–brain barrier |
| Lcn2 | Lipocalin 2 |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| FTL | Ferritin light chain |
| FTH | Ferritin heavy chain |
| KO | Knockout |
| WT | Wild type |
| ICH | Intracerebral hemorrhage |
| CSF | Cerebrospinal fluid |
| MRI | Magnetic resonance imaging |
| SD | Standard deviation |
| ECA | External carotid artery |
| CCA | Common carotid artery |
| ICA | Internal carotid artery |
| HO-1 | Heme oxygenase-1 |
References
- Claassen, J.; Park, S. Spontaneous subarachnoid haemorrhage. Lancet 2022, 400, 846–862. [Google Scholar] [CrossRef] [PubMed]
- Etminan, N.; Chang, H.S.; Hackenberg, K.; de Rooij, N.K.; Vergouwen, M.D.I.; Rinkel, G.J.E.; Algra, A. Worldwide Incidence of Aneurysmal Subarachnoid Hemorrhage According to Region, Time Period, Blood Pressure, and Smoking Prevalence in the Population: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Schupper, A.J.; Hardigan, T.A.; Mehta, A.; Yim, B.; Yaeger, K.A.; De Leacy, R.; Fifi, J.T.; Mocco, J.; Majidi, S. Sex and Racial Disparity in Outcome of Aneurysmal Subarachnoid Hemorrhage in the United States: A 20-Year Analysis. Stroke 2023, 54, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.M.; Duckles, S.P. Vascular actions of estrogens: Functional implications. Pharmacol. Rev. 2008, 60, 210–241. [Google Scholar] [CrossRef]
- Crago, E.A.; Sherwood, P.R.; Bender, C.; Balzer, J.; Ren, D.; Poloyac, S.M. Plasma Estrogen Levels Are Associated With Severity of Injury and Outcomes After Aneurysmal Subarachnoid Hemorrhage. Biol. Res. Nurs. 2015, 17, 558–566. [Google Scholar] [CrossRef]
- Lauzier, D.C.; Jayaraman, K.; Yuan, J.Y.; Diwan, D.; Vellimana, A.K.; Osbun, J.W.; Chatterjee, A.R.; Athiraman, U.; Dhar, R.; Zipfel, G.J. Early Brain Injury After Subarachnoid Hemorrhage: Incidence and Mechanisms. Stroke 2023, 54, 1426–1440. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, W.; Yin, P.; Zhao, X.; Fang, X.; Zhou, Y. Dynamic correlation between brain edema scores and blood–brain barrier permeability predicts the outcome of aneurysmal subarachnoid hemorrhage. Clin. Neurol. Neurosurg. 2025, 257, 109086. [Google Scholar] [CrossRef]
- Yang, B.S.K.; Gusdon, A.M.; Ren, X.S.; Jeong, H.G.; Lee, C.H.; Blackburn, S.; Choi, H.A. Update on Strategies to Reduce Early Brain Injury after Subarachnoid Hemorrhage. Curr. Neurol. Neurosci. Rep. 2024, 25, 14. [Google Scholar] [CrossRef]
- Galaris, A.; Fanidis, D.; Tsitoura, E.; Kanellopoulou, P.; Barbayianni, I.; Ntatsoulis, K.; Touloumi, K.; Gramenoudi, S.; Karampitsakos, T.; Tzouvelekis, A.; et al. Increased lipocalin-2 expression in pulmonary inflammation and fibrosis. Front. Med. 2023, 10, 1195501. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.; Kim, J.; Kwon, H.J.; Lee, Y. SARS-CoV-2 infection induces expression and secretion of lipocalin-2 and regulates iron in a human lung cancer xenograft model. BMB Rep. 2023, 56, 669–674. [Google Scholar] [CrossRef]
- Zhao, R.Y.; Wei, P.J.; Sun, X.; Zhang, D.H.; He, Q.Y.; Liu, J.; Chang, J.L.; Yang, Y.; Guo, Z.N. Role of lipocalin 2 in stroke. Neurobiol. Dis. 2023, 179, 106044. [Google Scholar] [CrossRef]
- Xiao, X.; Yeoh, B.S.; Vijay-Kumar, M. Lipocalin 2: An Emerging Player in Iron Homeostasis and Inflammation. Annu. Rev. Nutr. 2017, 37, 103–130. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Zheng, M.; Xi, G.; Keep, R.F.; Hua, Y. Role of lipocalin-2 in brain injury after intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2015, 35, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Xi, G.; Keep, R.F.; Hua, Y. Role of Lipocalin-2 in Thrombin-Induced Brain Injury. Stroke 2016, 47, 1078–1084. [Google Scholar] [CrossRef]
- Yu, F.; Saand, A.; Xing, C.; Lee, J.W.; Hsu, L.; Palmer, O.P.; Jackson, V.; Tang, L.; Ning, M.; Du, R.; et al. CSF lipocalin-2 increases early in subarachnoid hemorrhage are associated with neuroinflammation and unfavorable outcome. J. Cereb. Blood Flow Metab. 2021, 41, 2524–2533. [Google Scholar] [CrossRef]
- Shishido, H.; Egashira, Y.; Okubo, S.; Zhang, H.; Hua, Y.; Keep, R.F.; Xi, G. A magnetic resonance imaging grading system for subarachnoid hemorrhage severity in a rat model. J. Neurosci. Methods 2015, 243, 115–119. [Google Scholar] [CrossRef]
- Egashira, Y.; Shishido, H.; Hua, Y.; Keep, R.F.; Xi, G. New grading system based on magnetic resonance imaging in a mouse model of subarachnoid hemorrhage. Stroke 2015, 46, 582–584. [Google Scholar] [CrossRef]
- Garcia, J.H.; Wagner, S.; Liu, K.F.; Hu, X.J. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 1995, 26, 627–634; Discussion 635. [Google Scholar] [CrossRef]
- Egashira, Y.; Hua, Y.; Keep, R.F.; Xi, G. Acute white matter injury after experimental subarachnoid hemorrhage: Potential role of lipocalin 2. Stroke 2014, 45, 2141–2143. [Google Scholar] [CrossRef]
- Toyota, Y.; Wei, J.; Xi, G.; Keep, R.F.; Hua, Y. White matter T2 hyperintensities and blood-brain barrier disruption in the hyperacute stage of subarachnoid hemorrhage in male mice: The role of lipocalin-2. CNS Neurosci. Ther. 2019, 25, 1207–1214. [Google Scholar] [CrossRef]
- Anzabi, M.; Ardalan, M.; Iversen, N.K.; Rafati, A.H.; Hansen, B.; Ostergaard, L. Hippocampal Atrophy Following Subarachnoid Hemorrhage Correlates with Disruption of Astrocyte Morphology and Capillary Coverage by AQP4. Front. Cell. Neurosci. 2018, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Regnier-Golanov, A.S.; Dundar, F.; Zumbo, P.; Betel, D.; Hernandez, M.S.; Peterson, L.E.; Lo, E.H.; Golanov, E.V.; Britz, G.W. Hippocampal Transcriptome Changes After Subarachnoid Hemorrhage in Mice. Front. Neurol. 2021, 12, 691631. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.H.; Kim, J.H.; Seo, J.W.; Han, H.S.; Lee, W.H.; Mori, K.; Nakao, K.; Barasch, J.; Suk, K. Lipocalin-2 Is a chemokine inducer in the central nervous system: Role of chemokine ligand 10 (CXCL10) in lipocalin-2-induced cell migration. J. Biol. Chem. 2011, 286, 43855–43870. [Google Scholar] [CrossRef] [PubMed]
- Dekens, D.W.; Naude, P.J.W.; Keijser, J.N.; Boerema, A.S.; De Deyn, P.P.; Eisel, U.L.M. Lipocalin 2 contributes to brain iron dysregulation but does not affect cognition, plaque load, and glial activation in the J20 Alzheimer mouse model. J. Neuroinflamm. 2018, 15, 330. [Google Scholar] [CrossRef]
- Zhao, N.; Xu, X.; Jiang, Y.; Gao, J.; Wang, F.; Xu, X.; Wen, Z.; Xie, Y.; Li, J.; Li, R.; et al. Lipocalin-2 may produce damaging effect after cerebral ischemia by inducing astrocytes classical activation. J. Neuroinflamm. 2019, 16, 168. [Google Scholar] [CrossRef]
- Dong, M.; Xi, G.; Keep, R.F.; Hua, Y. Role of iron in brain lipocalin 2 upregulation after intracerebral hemorrhage in rats. Brain Res. 2013, 1505, 86–92. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Okauchi, M.; Hua, Y.; Keep, R.F.; Xi, G. Deferoxamine reduces neuronal death and hematoma lysis after intracerebral hemorrhage in aged rats. Transl. Stroke Res. 2013, 4, 546–553. [Google Scholar] [CrossRef]
- Jin, H.; Xi, G.; Keep, R.F.; Wu, J.; Hua, Y. DARPP-32 to quantify intracerebral hemorrhage-induced neuronal death in basal ganglia. Transl. Stroke Res. 2013, 4, 130–134. [Google Scholar] [CrossRef]
- Lee, S.; Jha, M.K.; Suk, K. Lipocalin-2 in the Inflammatory Activation of Brain Astrocytes. Crit. Rev. Immunol. 2015, 35, 77–84. [Google Scholar] [CrossRef]
- Chia, W.J.; Dawe, G.S.; Ong, W.Y. Expression and localization of the iron-siderophore binding protein lipocalin 2 in the normal rat brain and after kainate-induced excitotoxicity. Neurochem. Int. 2011, 59, 591–599. [Google Scholar] [CrossRef]
- Rathore, K.I.; Berard, J.L.; Redensek, A.; Chierzi, S.; Lopez-Vales, R.; Santos, M.; Akira, S.; David, S. Lipocalin 2 plays an immunomodulatory role and has detrimental effects after spinal cord injury. J. Neurosci. 2011, 31, 13412–13419. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, L.R.; Gazin, C.; Zhu, X.; Green, M.R. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell 2005, 123, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, J.Y.; Lee, W.H.; Kim, H.; Park, H.C.; Mori, K.; Suk, K. Lipocalin-2 is an autocrine mediator of reactive astrocytosis. J. Neurosci. 2009, 29, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Jeong, E.A.; Lee, J.Y.; An, H.S.; Jang, H.M.; Ahn, Y.J.; Lee, J.; Kim, K.E.; Roh, G.S. Lipocalin-2 Deficiency Reduces Oxidative Stress and Neuroinflammation and Results in Attenuation of Kainic Acid-Induced Hippocampal Cell Death. Antioxidants 2021, 10, 100. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Y.; Brockman, D.A.; Hahn, W.; Bernlohr, D.A.; Chen, X. Lipocalin 2 deficiency alters estradiol production and estrogen receptor signaling in female mice. Endocrinology 2012, 153, 1183–1193. [Google Scholar] [CrossRef][Green Version]
- Drew, B.G.; Hamidi, H.; Zhou, Z.; Villanueva, C.J.; Krum, S.A.; Calkin, A.C.; Parks, B.W.; Ribas, V.; Kalajian, N.Y.; Phun, J.; et al. Estrogen receptor (ER)alpha-regulated lipocalin 2 expression in adipose tissue links obesity with breast cancer progression. J. Biol. Chem. 2015, 290, 5566–5581. [Google Scholar] [CrossRef]
- Krizanac, M.; Mass Sanchez, P.B.; Weiskirchen, R.; Schroder, S.K. Overview of the expression patterns and roles of Lipocalin 2 in the reproductive system. Front. Endocrinol. 2024, 15, 1365602. [Google Scholar] [CrossRef]
- Fiocchetti, M.; Ascenzi, P.; Marino, M. Neuroprotective effects of 17beta-estradiol rely on estrogen receptor membrane initiated signals. Front. Physiol. 2012, 3, 73. [Google Scholar] [CrossRef]
- Stragier, H.; Vandersmissen, H.; Ordies, S.; Thiessen, S.; Mesotten, D.; Peuskens, D.; Ten Cate, H. Pathophysiological mechanisms underlying early brain injury and delayed cerebral ischemia in the aftermath of aneurysmal subarachnoid hemorrhage: A comprehensive analysis. Front. Neurol. 2025, 16, 1587091. [Google Scholar] [CrossRef]
- de Oliveira, J.; Denadai, M.B.; Costa, D.L. Crosstalk between Heme Oxygenase-1 and Iron Metabolism in Macrophages: Implications for the Modulation of Inflammation and Immunity. Antioxidants 2022, 11, 861. [Google Scholar] [CrossRef]
- Ahmad, S.; Sultan, S.; Naz, N.; Ahmad, G.; Alwahsh, S.M.; Cameron, S.; Moriconi, F.; Ramadori, G.; Malik, I.A. Regulation of iron uptake in primary culture rat hepatocytes: The role of acute-phase cytokines. Shock 2014, 41, 337–345. [Google Scholar] [CrossRef]
- Xiao, R.; Pan, J.; Yang, M.; Liu, H.; Zhang, A.; Guo, X.; Zhou, S. Regulating astrocyte phenotype by Lcn2 inhibition toward ischemic stroke therapy. Biomaterials 2025, 317, 123102. [Google Scholar] [CrossRef]
- Zheng, N.; Li, X.; Zhou, N.; Luo, L. Identification of Novel LCN2 Inhibitors Based on Construction of Pharmacophore Models and Screening of Marine Compound Libraries by Fragment Design. Mar. Drugs 2025, 23, 24. [Google Scholar] [CrossRef]
- Suk, K. Lipocalin-2 as a therapeutic target for brain injury: An astrocentric perspective. Prog. Neurobiol. 2016, 144, 158–172. [Google Scholar] [CrossRef]
- Wang, G.; Weng, Y.C.; Chiang, I.C.; Huang, Y.T.; Liao, Y.C.; Chen, Y.C.; Kao, C.Y.; Liu, Y.L.; Lee, T.H.; Chou, W.H. Neutralization of Lipocalin-2 Diminishes Stroke-Reperfusion Injury. Int. J. Mol. Sci. 2020, 21, 6253. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Wan, Y.; Koduri, S.; Hua, Y.; Xi, G.; Keep, R.F. Role of Lipocalin-2 in Brain Injury After Subarachnoid Hemorrhage in Female Mice. Cells 2025, 14, 1770. https://doi.org/10.3390/cells14221770
Zhao H, Wan Y, Koduri S, Hua Y, Xi G, Keep RF. Role of Lipocalin-2 in Brain Injury After Subarachnoid Hemorrhage in Female Mice. Cells. 2025; 14(22):1770. https://doi.org/10.3390/cells14221770
Chicago/Turabian StyleZhao, Hao, Yingfeng Wan, Sravanthi Koduri, Ya Hua, Guohua Xi, and Richard F. Keep. 2025. "Role of Lipocalin-2 in Brain Injury After Subarachnoid Hemorrhage in Female Mice" Cells 14, no. 22: 1770. https://doi.org/10.3390/cells14221770
APA StyleZhao, H., Wan, Y., Koduri, S., Hua, Y., Xi, G., & Keep, R. F. (2025). Role of Lipocalin-2 in Brain Injury After Subarachnoid Hemorrhage in Female Mice. Cells, 14(22), 1770. https://doi.org/10.3390/cells14221770






