Highlights
What are the main findings?
- Flavonoids, terpenoids, glycosides, alkaloids, and polyphenolics show promise in the treatment of intervertebral disc degeneration and low back pain.
- Pro-resolving anti-inflammatory lipids (lipoxin A4, resolvin D1, protectins, and maresins) and statins show promise in the inhibition of intervertebral disc degeneration and promote repair processes.
What is the implication of the main finding?
- Many plant compounds show potential in the repair of the intervertebral disc.
- Biological therapies for the treatment of disc disease warrant further investigation.
Abstract
This comprehensive narrative review of bioactive plant compounds, pro-resolving anti-inflammatory lipids, and statins shows their potential in the inhibition of intervertebral disc degeneration (IVDD), pain resolution, tissue repair, and disc regeneration. IVDD is a multifactorial disease involving a multitude of signaling pathways, leading to the loss of normal disc function. An influx of nociceptive mechanoreceptors generate low back pain (LBP). IL6 and IL8 levels are elevated in patients undergoing spinal fusion to alleviate LBP, indicating these pro-inflammatory mediators may be major contributors to the generation of LBP. Apoptosis of disc cells leads to the depletion of key extracellular matrix components that equip the disc with its weight-bearing properties. A biomechanically incompetent degenerated IVD stimulates nociceptor mechanoreceptor activity, generating pain. Myo-tendinous, vertebral body, muscle, and facet joint tissues also contain pain receptors. Disturbance of the normal architecture of the IVD also generates pain in these tissues. Plant compounds have been used in folkloric medicine for centuries. This review attempts to provide a scientific basis for their purported health benefits; however, further studies are still required to substantiate this. Until this evidence is available, it would be prudent to be cautious in the use of such compounds. A diverse range of plant compounds (flavonoids, terpenoids, glycosides, alkaloids, and polyphenolics) inhibit inflammation and apoptosis, reduce spinal pain, and stimulate tissue repair by targeting cell signaling pathways in IVDD. Pro-resolving lipid mediators (lipoxin A4, resolvin D1, protectins, and maresins) also reduce inflammation, maintaining disc health and function. Cholesterol lowering statins disrupt phosphorylation in cell signaling pathways inhibiting IVDD, promoting tissue repair and regeneration.
1. Introduction
The intervertebral disc (IVD) is a major contributor to the weight-bearing properties and flexibility of the spine [], and when it degenerates, it is a major contributor to the generation of low back pain (LBP) due to the mechanical sensitization of pain-generating nerves and mechanoreceptors that grow into the degenerated IVD []. LBP is the most impactful of any musculoskeletal condition. A 10-year global study of 291 major human diseases acknowledged that LBP was the most consequential musculoskeletal condition [,], and ~80% of the general population will be affected by LBP some time in their life, with sufficient severity to warrant intervention by a physician []. It is estimated that about 40% of the world’s human population suffers from LBP [] (632 million). A total of 5.5% of these have symptomatic intervertebral disc degeneration (IVDD), which contributes to this condition []. LBP has been the leading cause of years lived with disability since 1990, and its treatment remains a significant global public healthcare challenge and a major strain on healthcare resources.
Identification of specific bioactive molecules in plant materials in traditional folkloric medicine is confounded by the complex mixture of bioactive compounds present in such preparations. Advances in functional analytical procedures for plant compounds using computational molecular docking [,,,], computer-based AI design for biomolecule assembly, and pharmaceutical network analysis for complex medicinal products [,,,,,,,], plant genomics, and informatics [], has improved the identification of roles of specific compounds in these healing procedures. A number of bioactive plant compounds have potential uses in the treatment of IVDD [,,,]. Plants have been used for healing purposes for centuries [,] in traditional Chinese and Indian medicine over the last 3000 years []. Botanical products with antioxidant, anticarcinogenic, antiallergenic, anti-inflammatory, antimutagenic, and antimicrobial activities have been harnessed in a diverse array of biomedical applications stemming from original observations gleaned in folkloric medicine [,,,].
Specific aims of this study.
The aim of this narrative review is to illustrate the potential of bioactive plant compounds, pro-resolving anti-inflammatory lipids, and statins as potential therapeutic agents for the treatment of IVDD and LBP. This is the number one musculoskeletal condition and has global socioeconomic significance.
1.1. The Complexity of IVDD and Natural Plant-Based Therapeutic Interventions
With IVDD, a number of proinflammatory mediators and cytokines, such as interleukin-6 (IL-6), interleukin-8 (IL-8), prostaglandin E2 (PGE2), nitric oxide (NO), and matrix metalloproteases (MMPs), are produced, leading to tissue inflammation, tissue destruction, and stimulation of nociceptors, resulting in the generation of pain [,,]. Tissue destruction due to elevated MMP levels leads to the depletion of key ECM components, which provides the IVD with its weight-bearing properties. Degenerate disc tissue thus has decreased biomechanical competence, resulting in increased load being transmitted to nociceptors and mechanoreceptors, exacerbating pain generation. IL6 and IL8 levels are elevated in patients undergoing spinal fusion to alleviate LBP, indicating that these pro-inflammatory mediators produced in the NP may be major contributors to the generation of LBP []. The effects of inflammatory mediators may be more dominant in the aging spine [], which is consistent with elevated levels of LBP with ageing. Adults aged 50 years and older are especially vulnerable to LBP [], with its prevalence increasing up to 80 years of age. Studies show that up to 85 percent of people will experience some form of LBP some time in their lifetime [].
Degeneration of the IVD is a multifactorial disease of considerable complexity. At least ten cell signaling pathways have roles in IVD degenerative processes [,,,,,,] (Table 1). A diverse range of plant compounds can target specific aspects of these cell signaling pathways. Many of these plant compounds are ingested as dietary components or as nutritional supplements and are processed by gut bacteria into smaller, more bioavailable metabolites []. A gut–IVD axis has been demonstrated [,,], and presumably, blood vessels serving the gut epithelium and lumbar arteries arising from the aorta transport these to the IVD, where they gain access to the IVD by diffusive processes []. Degenerated IVDs are depleted of aggrecan, and their collagen networks are damaged [], resulting in the diminishment of IVD properties, which normally exclude diffusive entry of metabolites, making degenerated IVDs more accessible to small metabolites.

Table 1.
IVDD signaling pathways that interventional plant compounds affect.
1.2. General Comments on Plant Therapeutic Healthcare Products
Five classes of plant compounds have found applications in biomedicine: flavonoids, terpenoids, polyphenolics, glycosides, and alkaloids (Table 2).

Table 2.
Some examples of the therapeutic properties of plant compounds.
1.2.1. Flavonoids
Over 10,000 flavone/flavonoid compounds have been characterized so far []. Plant phenolic compounds are also a biodiverse family of plant compounds (Figure 1). The majority of these have therapeutic properties as anticancer, antimicrobial, antiviral, antiangiogenic, antimalarial, antioxidant, neuroprotective, antitumor, and anti-proliferative agents [,,,]. Many flavonoid and phenolic compounds have found application in the treatment of IVDD by inhibiting degenerative processes and promoting tissue repair and regeneration [,].
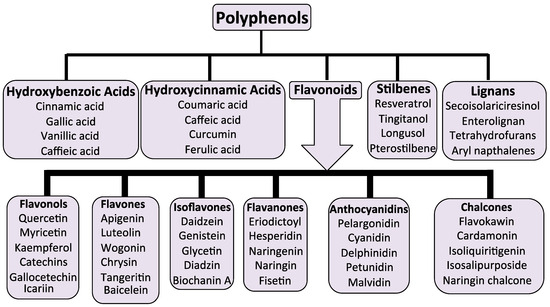
Figure 1.
The biodiversity of phenolic plant compounds and their sub-classes.
1.2.2. Terpenoids
Terpenoids, phenolics, alkaloids, and glycosides display a diverse range of properties in cell signaling pathways that affect inflammation, apoptosis, ECM stabilization, cell viability, and autophagy [,,,,,,,]. Terpenoids display an immense level of structural diversity, with at least 40,000 structural presentations identified. Some terpenoids have potent anti-inflammatory properties [,] and are volatile components of essential oils, imparting distinctive aromatic signatures to plants such as eucalyptus, peppermint, lemon, pine, lavender, and various herbs. The volatile nature of these compounds provides a novel drug delivery system in aromatherapy to treat inflammatory diseases [,]. The few terpenoids that have been used to treat IVDD are discussed in detail later in this review [,].
1.2.3. Glycosides
Phenolic acid and flavonoid glycosides form a varied class of naturally occurring compounds with potent anti-oxidant and anti-inflammatory properties and are more soluble than their aglycone forms and more bioavailable, making them more effective interactive molecules []. Glycoside flavonoids are transported in the gut by active transport, whereas their aglycone forms are transported through the gut by passive diffusion. Glycosides are also more soluble []. Glycosylation can also reduce any toxic effects that may be evident in some aglycone forms of these molecules, and in some cases, can improve their bioactivity [,,]. Glycosides exert anti-inflammatory, antioxidative, and anti-apoptotic effects through PI3K/Akt or JAK/STAT signaling. Some glycosides (ginsenosides, notoginsenoside, astragaloside IV, dioscin, kinsenoside, and crocin) are of interest for the treatment of IVDD [,,,,,].
1.2.4. Alkaloids
Naturally occurring alkaloid compounds have been used in global traditional healing practices since ancient times, and many are still in use today [,]. Many alkaloids were originally isolated from weedy plants, which have an enhanced ability to colonize disturbed habitats through highly bioactive compounds, giving them greater adaptability to altered growth conditions. Alkaloids can also have toxic properties and require careful initial assessment, but this inherent toxicity has also proved useful in the eradication of infective organisms [,]. Present-day use of alkaloid medicines has benefited from the experience gleaned by traditional medical practitioners who first documented the usefulness of alkaloids as medicines []. Nigella sativa is a good example of a medicinal plant that has been extensively studied and shown to produce a wide range of compounds with pharmacological properties useful in antidiabetic, anticancer, immunomodulator, analgesic, antimicrobial, anti-inflammatory, spasmolytic, bronchodilator, and hepato-, reno-, and gastro-protective applications []. N. sativa is a herb native to the Mediterranean, North Africa, Middle East, and Western Asia and has been used as a spice and herbal medicine for many centuries. Moringa oleifera is another medicinal plant that contains alkaloid compounds of medicinal importance, which display antimicrobial, antitumor, and anti-hypertensive properties. Thiocarbamate alkaloids isolated from M. oleifera have been evaluated in clinical trials for type II diabetes, osteoporosis, dyslipidemias, and HIV infection and are cardio-protective [,,]. Some present-day alkaloid medicinal compounds had their origins in medicinal plants. These include well-known compounds such as vincristine, vinblastine, taxol, morphine, ephedrine, colchicine, codeine, cocaine, berberine, and atropine []. Newer plant compounds have also been identified with useful pain relief properties. Chuanxiong rhizome from Conioselinum anthriscoides has been used to treat low back pain [] and stroke [] in traditional Chinese medicine for centuries. Ligustrazine, an alkyl pyrazine, alkaloid isolated from Chuanxiong, relieves pain, suppresses inflammation [,,,], is chondroprotective [,], and protects the IVD from degenerative effects [].
2. Plant Compounds as Therapeutic Agents for the Treatment of IVDD
A large number of plant compounds are antioxidant and anti-inflammatory molecules that provide tissue protection. These have been specifically investigated to assess if they have protective properties over IVD NP cells and the chondrocytes of the CEP in the chemical and weight-bearing environments they are exposed to during IVDD. These are summarized in Table 3, Table 4, Table 5, Table 6 and Table 7.
2.1. Flavonoids That Inhibit IVDD and Promote Tissue Repair and Regeneration
The knowledge base acquired over generations of use of plants in traditional medicine has been invaluable for the identification of plant compounds appropriate for further investigation as prospective therapeutic agents for the treatment of IVDD. Flavonoids are a particularly diverse family of plant compounds with valuable tissue protective and tissue reparative properties, making them appropriate candidates. Flavonoids have been categorized into six sub-classes, as shown in Figure 2e–j, while they are also categorized as polyphenolic compounds and can occur as glycosylated and aglycone forms.
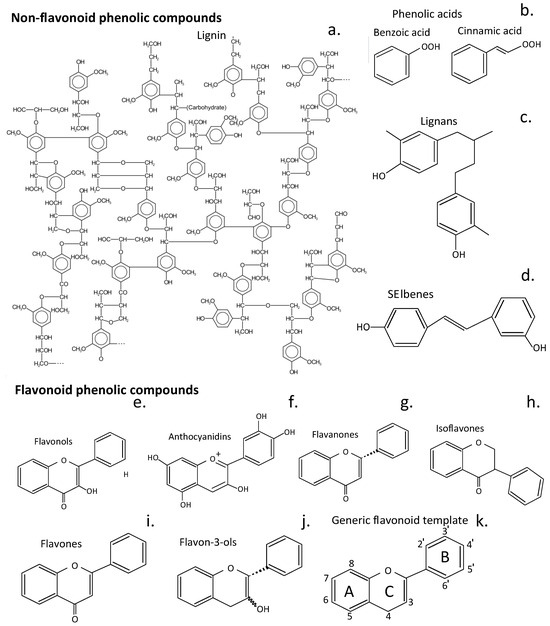
Figure 2.
Structures of phenolic plant compound classes that have been used in therapeutic procedures to treat human diseases. An idealized presentation of a soft wood lignin (a), phenolic acids (b), lignans (c), and stilbenes (d). Structures of flavonoid phenolic compounds of the sub-classes of flavonoids, flavonols (e), anthocyanidins (f), flavonones (g), isoflavones (h), flavones (i), and flavan-3-ols (j). The generic structure of flavonoids and the numbering system 3-8 and 2′-6′ for their 3 rings (A, C, B) is shown (k). The structures shown are the aglycone structures for each class of compound; however, these compounds also occur as glycosylated isoforms of diverse functions. Glycosylation of flavonoids strongly enhances their water solubility and thus increases their bioavailability [].

Table 3.
Flavonoids that have useful properties in the inhibition of IVDD and stimulation of repair and regeneration.
Table 3.
Flavonoids that have useful properties in the inhibition of IVDD and stimulation of repair and regeneration.
| Compound | Mode of Action |
|---|---|
| Hyperoside | Hyperoside significantly mitigates TNF-α-induced apoptosis in human NP cells by upregulating SIRT1 and Nrf2, and reduces ECM degradation and apoptosis mediated by ER stress []. Cell culture data. |
| Quercetin | Protects NP cells from apoptosis by inhibiting p38 MAPK-mediated autophagy, prevents ECM degeneration and IVDD in a rat tail puncture model []. Suppresses apoptosis and ECM degradation through activation of the SIRT1-autophagy signaling pathway []. Quercetin is a senolytic agent that binds Keap1-Nrf2 complex and inhibits the NF-κB pathway, reducing the expression of senescence-associated secretory phenotypic factors in IL-1β-stimulated NP cells []. Inhibits oxidative stress-induced senescence through the regulation of the miR-34a/SIRT1 axis []. Cell culture and animal model data. |
| Apigenin | Enhances autophagy through the AMP-activated protein kinase (AMPK)/mTOR/transcription factor signaling cascade, alleviates oxidative stress-induced senescence in NP cells, suppresses the expression of TNF-α-mediated pro-inflammatory cytokines, mitigating disc degeneration in rat IVDD models []. Animal model data. |
| Butein | Butein is a chalcone-type flavonoid [], with antioxidant, anti-inflammatory, antiangiogenic, anticancer, and antidiabetic activities []. In vitro and in vivo studies show butein activates SIRT1 and suppresses p53 acetylation, protecting NP cells from apoptosis and senescence triggered by hyperglycemia. Significantly alleviates degenerative effects in diabetic IVDD rat models, where increased NP expression of SIRT1 and decreased p53 acetylation is evident []. Cell culture and animal model data. |
| Baicalein | Baicalein inhibits activation of NF-κB and MAPK signaling, reducing inflammatory cytokine expression, prostaglandin E2 (PGE2), TNF-α, and IL-6 in IL-1β-stimulated NP cells []. Prevents ECM degradation and loss of aggrecan and type II collagen []. Baicalin alleviates IVDD by inhibiting the p38 MAPK signaling pathway []. Cell culture data. |
| Kaempferol | Network pharmacology data suggest kaempferol may be a key component of traditional Chinese medicines used to treat IVDD []. An injectable kaempferol-loaded fibrin gel used in a rat IVDD model reduced inflammation, promoted aggrecan and type II collagen synthesis, and reduced IVDD []. Kaempferol inhibits phosphorylation of ERK1/2, downregulates MMP3 and ADAMTS4 expression, significantly restores cell viability, and reduces ROS accumulation and apoptosis in NP cells []. Slows IVDD by modifying LPS-induced osteogenesis/adipogenesis imbalance and inflammatory [] response [], induces chondrogenesis in ATDC5 cells through activation of ERK/BMP-2 signaling []. Injectable kaempferol-loaded fibrin glue inhibits inflammation in IVDD []. Cell culture and animal model data. |
| Luteolin | Luteolin suppresses MMP13, p53, and p21 expression but promotes CDK2, CDK4, and Col2α1 expression in endplate chondrocytes and alleviates cellular senescence []. Luteolin also reduces apoptosis of NP cells and reverses TNF-α-induced senescence and inflammation through activation of SIRT6 and inhibition of NF-κB cell signaling []. This prevents progressive degenerative changes in IVD tissues. Cell culture data. |
| Luteoloside | Luteoloside is the 7 O-glycoside of luteolin. Luteoloside inhibits IL-1β-induced apoptosis and catabolism in NP cells and ameliorates IVDD []. Cell culture data. |
| Naringin and Naringenin | Naringin and its aglycone naringenin are effective anti-inflammatory agents in the treatment of low back pain arising from IVDD []. Naringin upregulates expressions of Sox-6, BMP2, and aggrecan in IVD cells isolated from degenerated IVDs but downregulates TNF-α and MMP3 expression and promotes NP cell proliferation recovery from IVDD []. Naringin suppresses the NF-κB pathway and p53 expression [], protects endplate chondrocytes from apoptosis by promoting SIRT3-mediated mitophagy, and suppresses NLRP3 inflammasome activation [] and its contributions to IVDD []. Naringin protects human NP cells against TNF-α-induced inflammation, oxidative stress by enhancing autophagic flux via AMPK/SIRT1 activation []. Naringin inhibits apoptosis induced by cyclic stretch in AF cells diminishing IVDD by inhibition of ROS/NF-κB signaling []. Age-related degeneration of NP cells is lowered by inhibition of IGFBP3 activity []. Naringin diminishes autophagy-driven oxidative stress-induced apoptosis in NP cells []. Cell culture data. |
| Icariin | Icariin is a traditional Chinese medicine flavonoid glycoside with diverse pharmacological properties [] affecting bone, inflammation, cancer, immunity, the cardiovascular system, and CNS [,,,]. Icariin has NP and CEP cell-protective effects in IVDD through its anti-inflammatory and antioxidant properties and promotes ECM synthesis. Icariin prevents IL-1β-induced apoptosis of NP cells via the PI3K/AKT pathway [] and H2O2-induced apoptosis of NP cells via PI3K/Akt signaling []. It inhibits IL-1β-induced MAPK and NF-κB cell signaling pathways, reduces secretion of proinflammatory factors and degradative enzymes, and alleviates oxidative stress []. Icariin activates the Nrf2/HO-1 pathway to promote mitophagy, inhibit ferroptosis, maintain mitochondrial function, and enhance cell survival [,]. Chemokines, such as IGF-1, TGF-β, and SDF-1 are upregulated by icariin-promoting tissue repair []. Cell culture data. |
| Fisetin | Fisetin has antioxidant, anti-inflammatory, anticancer, anti-aging, and nephroprotective properties [,,,,]. Fisetin protects NP cells by inhibiting oxidative stress and apoptosis, and maintains ECM structural organization [] acting through the Nrf2/HO-1 pathway to inhibit oxidative stress-induced ferroptosis, reducing disc cell death []. Cell culture data. |
| Acetacetin | Acacetin is a monomethoxy flavonoid with broad therapeutic potential stemming from its anti-inflammatory, antimicrobial, antioxidant, anticancer, anti-obesity, and cardiovascular protective properties [,,,,,]. Acacetin also mitigates the degeneration of NP cells in vitro and IVD tissues in rat IVDD models. In vitro, acacetin activates the Nrf2 pathway and upregulates antioxidant proteins such as HO-1, NADQO-1, and SOD, inhibiting ROS production, reducing COX-2 and iNOS-mediated inflammation. Acetacetin also inhibits the degradation of aggrecan and type II collagen in IVDD models [,]. Inhibition of the phosphorylation of p38, JNK, and ERK1/2 by acacetin moderates degenerative effects on NP cells and significantly ameliorates IVDD in rat puncture IVD models []. Animal model and cell culture data. |
| Orientin | Orientin is an 8-C flavone glucoside of luteolin with antioxidant and anti-inflammatory properties. Orientin downregulates the NF-κB pathway, prevents NF-κB translocation to the nucleus, limiting synthesis of TNF-α, IL-6, and IL-1β by inhibiting IκB kinase []. Orientin reduces the expression of iNOS and COX-2, reducing the production of pro-inflammatory mediators, such as NO and prostaglandins. MAPK is targeted by orientin, attenuating the activation of p38 MAPK and JNK, which are crucial for inflammation. Orientin downregulates oxidative ER stress and mitochondrial dysfunction through the AMPK/SIRT1 pathway in rat NP cells in vitro and attenuates IVDD in vivo []. Animal model and cell culture data. |
| Cardamonin | Cardamonin protects NP cells from IL-1β-induced inflammation and catabolism via the Nrf2/NF-κB axis []. Cell culture data. |
| Morin | Morin attenuates pyroptosis of NP cells and ameliorates IVDD via inhibition of the TXNIP/NLRP3/caspase-1/IL-1β signaling pathway []. Cell culture data. |
| Glycitin | Protects against IVDD through antagonizing inflammation and oxidative stress in NP cells []. Cell culture data. |
| Genkwanin | Genkwanin is an O-methylated flavone that regulates IVDD through the ITGA2/PI3K/AKT pathway and by inhibiting apoptosis and senescence []. Animal model and cell culture data. |
| Wogonin | Mitigates IVDD through the Nrf2/ARE and MAPK cell signaling pathways []. Animal model and cell culture data. |
| Isoliquiritigenin | Inhibits IVDD induced by oxidative stress and mitochondrial dysfunction through a PPARγ-dependent pathway []. Animal model and cell culture data. |
| Myrcetin | Myrcetin protects against IVDD through regulation of Nrf2/HO-1/NF-κB signaling. Dihydromyricetin inhibits IVDD through inhibition of NLRP3 inflammasome activation via the Keap1/Nrf2/HO-1 pathway [] and restores autophagy attenuating IVDD by negative regulation of the JAK2/STAT3 pathway []. Animal model and cell culture data. |
| Hesperidin | Mitigation of oxidative stress-induced ferroptosis in NP cells via the Nrf2/NF-κB axis reduces IVDD []. Animal model and cell culture data. |
| Cyanidin | Procyanidin B3 alleviates IVDD via interaction with the TLR4/MD-2 complex []. Proanthocyanidins inhibit the apoptosis and aging of NP cells via the PI3K/Akt pathway, delaying IVDD []. Procyanidin C1 ameliorates acidic stress-induced NP degeneration through SIRT3/FOXO3-mediated mitochondrial dynamics [], attenuates apoptosis of NP cells and IVDD via the JAK2/STAT3 signal pathway [], and attenuates the high hydrostatic pressure-induced degradation of NP ECM by blocking the Wnt/β-catenin signaling []. Cell culture data. |
| Epigallocatechin 3-gallate and Urolithin A | Suppresses IL-1-induced inflammatory responses in IVD and reduces radiculopathic pain [], protects H2O2-induced NP cell apoptosis and inflammation by inhibiting cGAS/Sting/NLRP3 activation [] and oxidative stress []. Urolithin A is a flavonoid metabolite generated from dietary epigallocatechin 3-gallate by gut bacteria. Urolithin A has potent anti-inflammatory and antioxidant properties [], inhibits TNF alpha induced inflammation [] and TNF alpha catabolic effects on NP cells and IVDD []. Animal model and cell culture data. |
| Sesamin | Sesamin inhibits LPS-induced inflammation and ECM catabolism in the rat IVD []. Intradiscal injection of sesamin protects IVDs from lesion-induced IVDD []. Animal model and cell culture data. |
| Casticin | Casticin is a methoxylated flavonol with some hydroxyl groups in the flavonoid structure replaced by methyl groups. Castacin inhibits LPS-stimulated oxidative stress, inflammation, and ECM degradation by activating the Nrf2/HO-1 signaling axis and indirectly blocks the NF-κB pathway, preventing the progression of IVDD rat models. Casticin promotes the nuclear translocation of Nrf2 and blocks the NF-κB pathway, resulting in decreased levels of iNOS, TNF-α, IL-1β, PGE2, MMP-13, ADAMTS-5, and ROS []. Animal model and cell culture data. |
Abbreviations: SIRT-1; Keap-1, Nrf2, nuclear factor erythroid 2-related factor 2; ECM, extracellular matrix; ER, endoplasmic reticulum; Kelch-like ECH-associated protein 1 (Keap1); AMPK, AMP-activated protein kinase; p38 MAPK, p38 mitogen-activated protein kinase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; mTOR, mammalian target of rapamycin; TNF-α, tumor necrosis factor alpha; IVDD, intervertebral disc degeneration; IL-1β, interleukin-1 beta; MAPK, mitogen-activated protein kinase; PGE2, prostaglandin E2; IL-6, interleukin-6; Sox-6, SRY-box 6 transcription factor; BMP2, bone morphogenetic protein; SIRT-1, NAD-dependent deacetylase sirtuin-1; MMP3, matrix metalloprotease 3; ADAMTS4, a disintegrin and metalloproteinase with thrombospondin motifs 4; ROS, reactive oxygen species; CDK, cyclin-dependent kinase; SDF-1, stromal-derived factor; HO-1, heme oxygenase-1; NADQO, NAD(P)H:quinone oxidoreductase 1; p38, mitogen-activated protein kinase; JNK, c-Jun N-terminal kinase; ERK1/2, extracellular signal regulated kinase 1/2; COX-2, cyclooxygenase-2, prostaglandin–endoperoxide synthase 2; iNOS, inducible nitric oxide synthase; PPAR, protease-activated receptor; Wnt, wingless-type MMTV integration site family; TLR4/MD-2 complex, Toll-like receptor-4/MD-2 complex; SIRT3/FOXO3, NAD-dependent deacetylase sirtuin-3/Forkhead box O3 protein; LPS, lipopolysaccharide; Nrf2/ARE, Nuclear factor erythroid 2-related factor 2/antioxidant response elements; ITGA2/PI3K/AKT, integrin alpha 2/phosphatidylinositol 3-kinase/protein kinase B; AMPK, 5’AMP-activated protein kinase; iNOS, inducible nitric oxide synthase.
Four widely published flavonoids (quercetin, baicalein, icariin, and naringin, Figure 3) were selected for more detailed discussion.
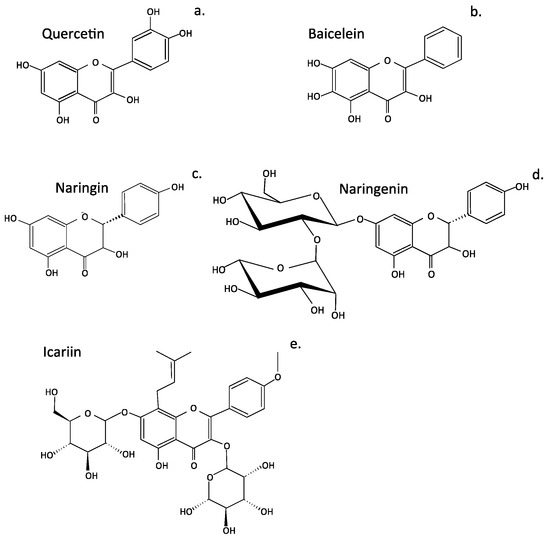
Figure 3.
Structures of four flavonoids that show promise for the treatment of intervertebral disc disease. Quercetin (a), baicalein (b), naringin (c), naringenin (d), and icariin (e).
2.1.1. Quercetin as an IVD Therapeutic Agent
Quercetin has been extensively investigated for therapeutic medical purposes []. Quercetin is a plant flavonol from the flavonoid group of polyphenols and, like all flavonoids, has potent antioxidant and anti-inflammatory properties, which are the basis of many of its medicinal properties (Figure 3a). Quercetin is a widely distributed pigment in fruits, vegetables, teas, wine, and cereals and one of the most abundant antioxidants in the human diet. Quercetin has important roles in preventing ROS damage to tissues. Quercetin inhibits p38 MAPK signaling, an enzyme of the serine and threonine (Ser/Thr) protein kinase family, associated with the progression of IVDD []. Promotion of cell apoptosis and senescence suppresses cell proliferation and autophagy. p38 MAPK thus represents a logical therapeutic target in the prospective treatment of IVDD. However, p38 MAPK is but one cell signaling pathway operative during IVDD. Quercetin protects NP cells from apoptosis by inhibiting p38 MAPK-mediated autophagy, and prevented IVDD in a rat tail IVD puncture model []. Quercetin also suppresses apoptosis and ECM degradation through activation of the SIRT1-autophagy cell signaling pathway []. With aging, the IVD loses hydration and structural integrity, resulting in elevation in cell tress levels that can lead to a dysfunctional state known as cellular senescence [,,,]. Senescent cells are metabolically active, but their cycle cell has ceased, and the cells are unable to divide. However, they release a range of damaging molecules in a senescence-associated secretory phenotype. Quercetin is a senolytic agent that binds Keap1-Nrf2 complex and inhibits the NF-κB pathway, reducing the expression of senescence-associated secretory phenotypic factors in IL-1β-stimulated NP cells []. Inhibition of oxidative stress-induced senescence by quercetin is also achieved through regulation of the miR-34a/SIRT1 axis []. Thus, quercetin is a multi-functional IVD protective compound.
2.1.2. Protective Roles for Baicalein in IVD Tissues
Baicalein has a similar structure to quercetin but is not hydroxylated on the 3′ and 4′ positions of the flavonoid B ring. It is hydroxylated on position 6 on ring A, which is absent in quercetin (Figure 2k). Baicalein is the aglycone form of baicalin and a natural health supplement originally extracted from the roots of the Chinese herbs Scutellaria baicalensis and Scutellaria lateriflora. Baicalein’s tissue protective properties stems from its ability to scavenge ROS and to interact with signaling molecules operative in apoptosis, inflammation, autophagy, and mitochondrial dynamics. Baicalein reduces inflammatory cytokine expression by inhibiting the activation of NF-κB and p38 MAPK cell signaling []. This also reduces prostaglandin E2 (PGE2), TNF-α, and IL-6 production in IL-1β-stimulated NP cells [] and prevents ECM degradation and loss of aggrecan and type II collagen from the IVD [].
2.1.3. Naringin and Naringenin Therapeutic Properties in the Treatment of IVDD
Citrus fruits are rich sources of naringin and naringenin. These are flavonoids with strong anti-inflammatory and antioxidant activities. Naringin and naringenin prevent the oxidation of LDL and reduce total cholesterol and HDL levels, but LDL, VLDL, and tri-glycerol levels are unaffected, showing their potential in the treatment of hyperlipidemia. Naringin and its aglycone naringenin are also effective anti-inflammatory agents in the treatment of LBP arising from IVDD []. Naringin upregulates the expression of Sox-6, BMP2, and aggrecan in IVD cells isolated from degenerated IVDs but downregulates TNF-α and MMP3 expression and promotes NP cell proliferation and recovery from IVDD []. Naringin suppresses the NF-κB pathway and p53 expression [], demonstrating its anti-inflammatory properties. This protects endplate chondrocytes from apoptosis by promoting SIRT3-mediated mitophagy and suppresses NLRP3 inflammasome activation [] and IVDD []. Naringin protects human NP cells against TNF-α-induced inflammation and oxidative stress by enhancing autophagic flux via AMPK/SIRT1 activation [] and inhibits apoptosis induced by cyclic stretch in AF cells, diminishing IVDD and ROS/NF-κB signaling []. Naringin diminishes autophagy-driven oxidative stress-induced apoptosis in NP cells [].
2.1.4. Icariin and Its Potential Roles in the Treatment of IVDD
Horny goat weed and some herbs are sources of icariin []. Icariin is a prenylated flavonol traditional Chinese medicine with diverse pharmacological properties [] affecting bone, inflammation, cancer, immunity, the cardiovascular system, and CNS [,,,]. Icariin prevents IL-1β-induced apoptosis of NP cells via the PI3K/AKT pathway [] and H2O2-induced apoptosis of NP cells via PI3K/Akt signaling []. Inhibition of IL-1β-induced MAPK and NF-κB cell signaling pathways by icariin reduces the secretion of proinflammatory mediators, degradative enzymes, and alleviates oxidative stress []. Icariin activates the Nrf2/HO-1 cell signaling pathway to promote mitophagy, inhibit ferroptosis, maintain mitochondrial function, and enhance cell survival [,]. Chemokines such as IGF-1, TGF-β, and SDF-1 are upregulated by icariin, promoting tissue repair []. Ferroptosis is a type of programmed cell death that is dependent on iron and characterized by the accumulation of lipid peroxides []. It is distinct from other forms of regulated cell death, such as apoptosis and necroptosis.
3. Terpenoids Displaying Potential in Tissue Protection and Treatment of IVDD
Terpenoids are diverse natural secondary plant metabolites formed from isoprene units (C-5) (Figure 4). These have a wide range of biological properties, including antioxidant, antimicrobial, anti-inflammatory, antiallergic, anticancer, antimetastatic, antiangiogenic, and apoptotic properties []. Some terpenoids (aucubin, morronside, and celastrol) have biological properties applicable to the treatment of IVDD. Aucubin represses the activation of the NF-κB-NLRP3 inflammasome in chondrocytes in the CEP []. Aucubin is an iridoid glycoside that has extensive biological properties as an antioxidant, anti-aging, anti-inflammatory, anti-fibrotic, anticancer, hepatoprotective, neuroprotective, and osteo protective agent []. Morronside is a traditional Chinese herbal preparation that has been used for centuries in traditional medical practice to protect the nervous system, treat OA, inhibit platelet aggregation, prevent diabetic angiopathies and renal damage, and reduce bone resorption []. NP cell senescence is also attenuated, alleviating IVDD via inhibition of the ROS-Hippo-p53 cell signaling pathway []. Celastrol is a pentacyclic nortriterpene quinone member of the terpenoid family, which has anti-inflammatory, antioxidant, and anticancer activities. Celastrol has been applied to the treatment of chronic inflammatory and autoimmune diseases (e.g., RA, MS, SLE, inflammatory bowel disease, and psoriasis) []. Kongensin A is a diterpene plant product isolated from Croton kongensis, a tropical shrub, which is a potent inhibitor of necroptosis and an inducer of apoptosis []. Kongensin A covalently binds to HSP90, dissociating it from its co-chaperone CDC37, leading to the inhibition of receptor-interacting serine/threonine-protein kinase 3 (RIP3)-mediated necroptosis and the promotion of apoptosis in multiple cancer cell lines []. Kongensin inhibits mitogen-activated protein kinase kinase kinase 7 (TAK1, Map3k7), a key regulator of innate immunity, cell death, inflammation, and cellular homeostasis. Kongensin upregulates TAK1 expression in NP cells during IVDD, inhibiting PANoptosis by suppressing oxidative stress. This delays IVDD progression and is a promising novel therapeutic approach to the treatment of IVDD [].
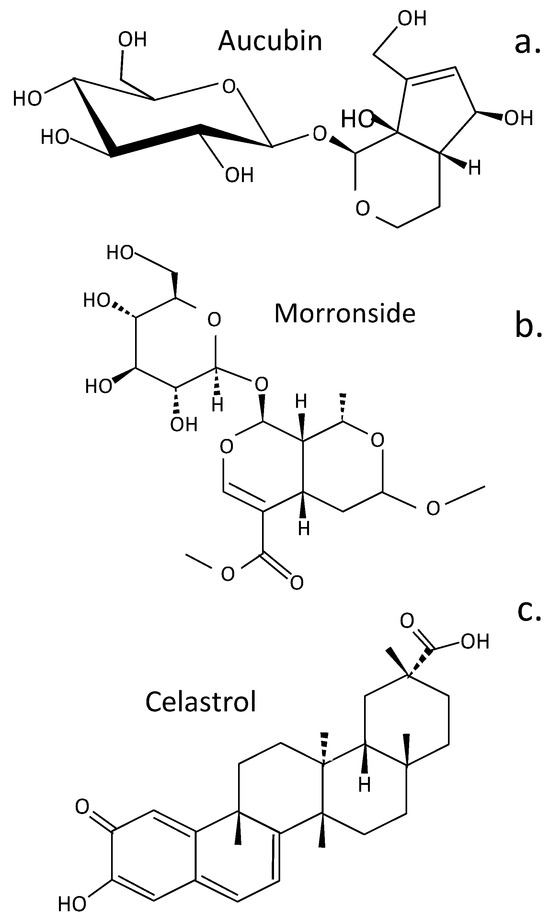
Figure 4.
Structure of terpenoids that have been evaluated for the treatment of IVDD. Aucubin (a), Morronside (b), Celastol (c).

Table 4.
Terpenoids with useful therapeutic properties for the treatment of IVDD.
Table 4.
Terpenoids with useful therapeutic properties for the treatment of IVDD.
| Compound | Therapeutic Properties in IVDD |
|---|---|
| Aucubin | Represses NF-κB-NLRP3 inflammasome activation in CEP chondrocytes []. Cell culture data. |
| Morroniside | Attenuates NP cell senescence to alleviate IVDD via inhibition of the ROS-Hippo-p53 pathway []. Cell culture data. |
| Celastrol | Reduces IL-1β-induced ECM catabolism, oxidative stress, and inflammation in NP cells attenuating rat IVDD in vivo []. Animal model and cell culture data. |
| Kongensin | Kongensin upregulates TAK1 expression in NP cells during IVDD and inhibits PANoptosis, suppressing oxidative stress delaying IVDD progression []. Animal model and cell culture data. |
4. Phenolic Compounds Displaying Potential as IVD Protective Agents
Polyphenolic (derived from the Greek word polus, meaning many) compounds are widely distributed in the plant kingdom and are a large family of structurally diverse molecules spanning phenolic acids, flavonoids, tannic acid, stilbenes, lignans, lignins, and ellagitannins []. Polyphenolics have broad antioxidant and anti-inflammatory properties (Figure 5, Table 5).
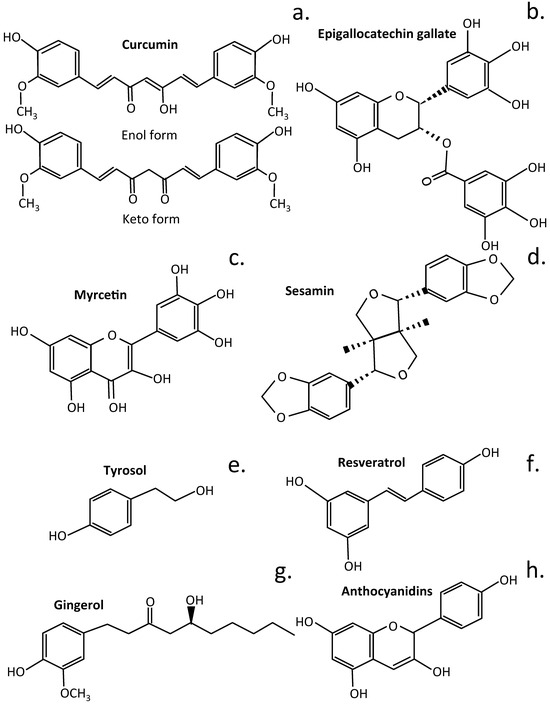
Figure 5.
Structures of some phenolic plant compounds that have been used to treat IVDD. Curcumin in its enol and keto forms (a), Epigallocatechin gallate (b), Myrcetin (c), Sesamin (d), Tyrosol (e), Resveratrol (f), Gingerol (g), Anthocyanidins (h).
Curcumin attenuates NF-κB expression [] and regulates iNOS, COX-2, TGF-β1/2, MMP-9, and BDNF in IVDD [], thus producing anti-inflammatory and anti-catabolic effects on human IVD cells []. Curcumin also has a protective effect on endplate chondrocytes, inhibiting IL-1β-induced apoptosis mediated through Bcl-2/Bax []. Myricetin is a member of the flavonol class of flavonoids but is also classified as a polyphenolic compound. Myrcetin attenuates IVDD through its regulatory effects over the Nrf2/HO-1/NF-κB cell signaling pathway []. Sesamin inhibits inflammation induced by LPS, preventing degradative events that lead to catabolism of key function IVD components []. Intradiscal injection of sesamin prevents ECM catabolism in animal models of lesion-induced IVDD by upregulation of the macroautophagy and autophagy family member Beclin 2 (BECN2). This is a regulator of G-protein coupled receptor turnover, which facilitates phosphatidylinositol 3-kinase binding and activity in the phosphatidylinositol 3-kinase-GPRASP1 (G Protein-Coupled Receptor Associated Sorting Protein 1) complex as a regulator of autophagy and as a regulator of G-protein-coupled receptor turnover []. Sesamin also ameliorates CEP degeneration in models of IVDD [].
Dried green tea leaves have a high content of Epigallocatechin Gallate (EGCG), white tea has appreciable levels, and black tea has lower EGCG levels. EGCG has potent regulatory properties over molecular pathways that control inflammation, oxidative stress, and apoptosis [,,]. EGCG has protective effects over IVD cells and provides protection from oxidative stress []. It also suppresses IL-1β-induced inflammatory responses, reduces radiculopathic pain [], and protects the IVD from H2O2-induced NP cell apoptosis and inflammation by inhibiting cGAS/Sting/NLRP3 activation [] and the activation of the NLRP3 inflammasome and secretion of IL-1β []. The EGCG component of green tea is a potent inhibitor of fatty acid synthase []. Low bioavailability of tea catechins, however, can limit their effectiveness as therapeutic agents. Methods have therefore been investigated to depolymerize EGCG into metabolites with improved bioavailability using enzymatic treatment with tannase and pectinases []. The gut microbiota also transform EGCG into more bioavailable phenolic metabolites that retain or actually have improved biological activity [,,,]. Fermentation of green tea to form black tea also results in the bioconversion of EGCG into smaller bioactive metabolites []. Urolithins are generated from EGCG by the gut microbiota [,], and these have greater bioavailability and anti-inflammatory, antioxidant, antitumor, and anti-aging properties than EGCG []. γ-Valerolactones are also generated by gut bacteria by the bioconversion of EGCG [].
Resveratrol has potent antioxidant and anti-inflammatory properties in the laboratory and similar protective properties in tissues, provided that therapeutic doses are achieved. Resveratrol stimulates anabolic processes in the IVD, stimulating IVDD repair [,,,]. Anthocyanin is the glycoside form of anthocyanidin and a specific type of berry fruit flavonoid of elderberry, chokeberry, black raspberry, bilberry, blackcurrant, and blueberry [,]. Anthocyanidins are responsible for the blue, red, and purple coloration of flowerheads and berry fruits []. Anthocyanins undergo phase II reactions in the gut, where the aglycone anthocyanidin is subjected to methylation, sulphation, and glucosidation. The gut microbiota then transform the anthocyanins into protocatechuic acid [] and phlorglucinaldehyde (1,4,6-trihydroxy benzaldehyde) metabolites, which have greater bioavailability []. Cyanidins attenuate NP cell apoptosis in IVDD through the JAK2/STAT3 cell signaling pathway [], protect against high glucose-induced injury in human NP cells by regulating Nrf2/HO-1 signaling [], and attenuate high hydrostatic pressure-induced ECM degradation by blocking Wnt/β-catenin signaling []. Tyrosol is a biophenol secondary metabolite found in olive oil and wine with antioxidant, stress-protective, and anti-inflammatory properties []. It forms part of the health-promoting Mediterranean diet []. Tyrosol upregulates Sirt1 expression, suppressing apoptosis and inflammation in IL-1β-stimulated human NP cells through activation of the PI3K/Akt pathway []. Gingerol is a phenolic compound with antioxidant, antitumor, and anti-inflammatory properties of general application in the treatment of chronic diseases [], and it also attenuates IVDD by inhibiting IL-1β-mediated NLRP3 cell signaling [].

Table 5.
Phenolic compounds with therapeutic properties for the treatment of IVDD.
Table 5.
Phenolic compounds with therapeutic properties for the treatment of IVDD.
| Compound | Therapeutic Properties in IVDD |
|---|---|
| Curcumin | Attenuates NF-κB expression in rat lumbar IVDD [], regulates the expression of iNOS, COX-2, TGF-β1/2, MMP-9, and BDNF in a rat model of IVDD [], exhibits anti-inflammatory and anti-catabolic effects on human IVD cells by reducing TLR2 expression and JNK activity [], and protects rat CEP chondrocytes from IL-1β-induced apoptosis via Bcl-2/Bax regulation []. Animal model and cell culture data. |
| Myrcetin | Myricetin is structurally similar to fisetin, luteolin, and quercetin and is reported to have many of the same functions as these other members of the flavonol class of flavonoids but is also a polyphenolic compound. Myrcetin attenuates IVDD through regulation of the Nrf2/HO-1/NF-κB signaling pathway []. Animal model and cell culture data. |
| Sesamin | Inhibits LPS-induced inflammation and ECM catabolism in rat IVD []. Animal model and cell culture data. |
| Epigallocatechin Gallate | Epigallocatechin gallate protects IVD cells from oxidative stress []. Epigallocatechin 3-gallate suppresses interleukin-1β-induced inflammatory responses in IVD cells, reduces radiculopathic pain [], and protects the IVD from H2O2-induced NP cell apoptosis and inflammation by inhibiting cGAS/Sting/NLRP3 activation []. Animal model and cell culture data. |
| Resveratrol | Has antioxidant and anti-inflammatory properties inhibiting IVDD and stimulates anabolic properties in IVD repair [,,,]. Animal model and cell culture data. |
| Anthocyanidins | Cyanidin attenuates the apoptosis of rat NP cells and IVDD via the JAK2/STAT3 signal pathway []. Cyanidin-3-glucoside protects against high glucose-induced injury in human NP cells by regulating Nrf2/HO-1 signaling []. Cyanidin attenuates high hydrostatic pressure-induced ECM degradation by blocking Wnt/β-catenin signaling []. Animal model and cell culture data. |
| Tyrosol | Upregulates Sirt1 expression, suppresses apoptosis and inflammation, and modulates ECM remodeling in IL-1β-stimulated human NP cells through activation of the PI3K/Akt pathway []. Cell culture data. |
| Gingerol | Ameliorates IVDD by inhibiting IL-1β-mediated NLRP3 cell signaling []. Cell culture data. |
5. Alkaloids Displaying Therapeutic Potential in the Treatment of IVDD
Ligustrazine (tetramethylpyrazine) is a small alkyl pyrazine plant compound of the Chinese herb ligusticum chuanxiong hort (chuanxiong). It has been used for centuries in traditional Chinese medicine to treat LBP. In more recent times, ligustrazine has been identified as a useful molecular template amenable to chemical modifications to produce customized pharmaceutical compounds for specific human diseases [,] (Figure 6). Ligustrazine has been used to provide pain relief in knee OA []. Ligustrazine also suppresses aberrant TGFβ activation in NP cells, preventing the development of IVDD []. It also has IVD protective properties [] and inhibits CEP hypertrophy via suppression of TGF-β1 activity in models of IVDD [].
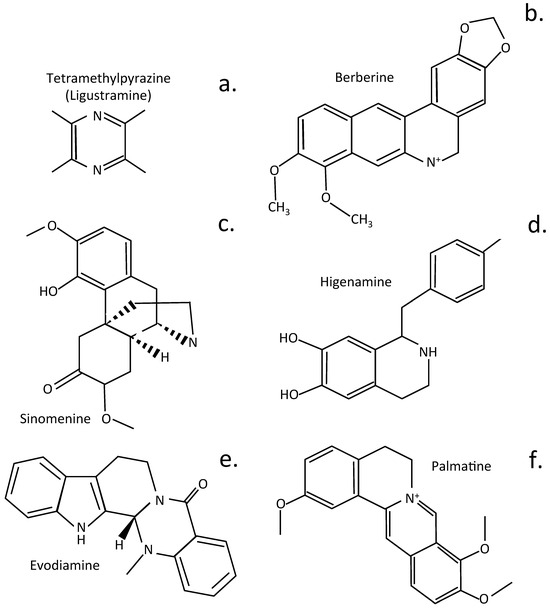
Figure 6.
Structure of alkaloid plant compounds that have been used to treat IVDD. Ligustramine (a), Berberine (b), Sinomenine (c), Higenamine (d), Evodiamine (e), Palmatine (f).
Berberine is a bioactive herbal quaternary ammonium salt of the protoberberine group of isoquinoline alkaloids and has anti-inflammatory properties useful for application in a number of chronic disease [,,,,]. Berberine has protective properties over disc cell populations in disease, ameliorates oxidative stress and its effects, resulting in induction of apoptosis by modulating ER stress and autophagy in human NP cells []. Suppression of apoptosis and ECM degradation in NP cells ameliorates IVDD in the intact IVD []. In human NP cells, berberine inhibits the NFκB pathway, preventing the development of inflammatory conditions in the disc. It also has protective properties, shielding intervertebral disc cells from the effects of IL-1β-induced ECM degradation, altered IVD mechanics, and the induction of apoptosis [].
Sinomenine is an alkaloid isolated from Caulis sinomenii, and Sinomenium acutum and has been used for 30 years in traditional Chinese medicine as an anti-inflammatory drug []. Sinomenine displays bioactivities relevant to the treatment of RA and alleviation of inflammation []. Sinomenine also ameliorates IL-1β-induced IVDD in rat models through suppression of inflammation and oxidative stress, mediated by the Keap1/Nrf2/NF-κB cell signaling pathway []. It also inhibits apoptosis and autophagy in vitro and in vivo [].
The root of the Chinese herbal plant called Fuzi, (Radix Aconiti lateralis praeparata) is a source of the alkaloid drug higenamine. This is a widely used drug in traditional Chinese medicine to treat various cardiovascular and skeletal medical disorders []. Higenamine inhibits IL-1β-induced human NP cell apoptosis by mediating ROS free radical effects through PI3K/Akt signaling []. It also inhibits IL-1β-induced inflammation in human NP cells []. Higenamine also inhibits acute and chronic inflammatory pain through the modulation of TRPV4 channels; thus, its therapeutic value in the treatment of IVDD may be enhanced by its ability to prevent the generation of LBP [].
Evodiamine is a quinolone alkaloid isolated from the fruit of Evodia rutaecarpa. This is a drug with broad application in folkloric Chinese medicine and is a popular traditional Chinese herb []. Evodiamine has also been shown to ameliorate IVDD through inhibitory effects on Nrf2 and MAPK cell signaling pathways [] and by the activation of the PI3K/AKT cell signaling pathway to block IVDD [].
Palmatine is a natural isoquinoline alkaloid and has a wide range of pharmacological properties []. Palmatine activates TFEB [] (transcription factor EB), enhances autophagy, and alleviates ER stress in IVDD []. TFEB is a pivotal transcription factor, with roles in the regulation of lysosomal biogenesis and autophagy [,].

Table 6.
Alkaloids displaying therapeutic properties for the treatment of IVDD.
Table 6.
Alkaloids displaying therapeutic properties for the treatment of IVDD.
| Compound | Therapeutic Properties in IVDD |
|---|---|
| Ligustrazine | Suppresses aberrant TGFβ activation of NP cells to prevent IVDD []. It has IVD protective properties [] and inhibits CEP hypertrophy via suppression of TGF-β1 activity []. Cell culture data. |
| Berberine | Ameliorates oxidative stress-induced apoptosis by modulating ER stress and autophagy in human NP cells [], suppresses apoptosis and ECM degradation in NP cells, ameliorates IVDD [], and prevents human NP cells from IL-1β-induced ECM degradation and apoptosis by inhibiting the NFκB pathway []. Cell culture data. |
| Sinomenine | Ameliorates IL-1β-induced IVDD in rats through suppression of inflammation and oxidative stress via Keap1/Nrf2/NF-κB cell signaling [] and ameliorates IVDD via inhibition of apoptosis and autophagy in vitro and in vivo []. Animal model and cell culture data. |
| Higenamine | Mitigates IL-1β-induced human NP cell apoptosis by ROS-mediated PI3K/Akt signaling [], inhibits IL-1β-induced inflammation in human NP cells []. Cell culture data. |
| Evodiamine | Ameliorates IVDD through the Nrf2 and MAPK cell signaling pathways [], activates PI3K/AKT cell signaling pathway to block IVDD []. Animal model and cell culture data. |
| Palmatine | Activates TFEB, enhances autophagy, and alleviates ER stress in IVDD []. Cell culture data. |
6. Glycoside IVDD Treatment Compounds
6.1. Ginsenosides
Ginseng is a widely used herbal nutraceutical traditional Chinese medicine [] and has antioxidant and anti-inflammatory activities []. Ginsenoside bioactive components of ginseng are triterpenoid saponins, and more than 180 types have been identified [] (Figure 7). Ginsenoside Rg1 is of major interest for the treatment of IVDD since it regulates disc homeostasis and its hydration and inhibits apoptosis, inflammation, and ECM degradation, delaying IVDD progression. Rg1 improves the proliferation of NP cells and reduces apoptosis. Prevention of IVDD by Rg1 is affected through inhibition of the Wnt/β-catenin signaling pathway [] and by suppressing the activation of the Yes-associated protein (YAP)/transcriptional coactivator with the PDZ-binding motif (TAZ-1) transcriptional coactivator Hippo cell signaling pathway. This significantly increases the mechanical strength of IVDs in rat IVDD models []. Rg1 prevents the activation of the NF-κB signaling pathway, inhibits apoptosis, suppresses IL-6 and TNF-α expression in IL-1-treated NP cells, and stimulates aggrecan and collagen II biosynthesis, an inhibitor of kappa B kinase (IκK) []. Rg3 ginsenoside also reverses IL-1β-induced apoptosis through inactivation of the p38 MAPK pathway. This significantly reduces MMP2, MMP3, ADAMTS-4, and ADAMTS-5 activity in IVDD. However, Rg3 not only addresses degenerative changes in the NP but also restores AF lamellar organization and the functional properties of the IVD as a weight-bearing structure [].
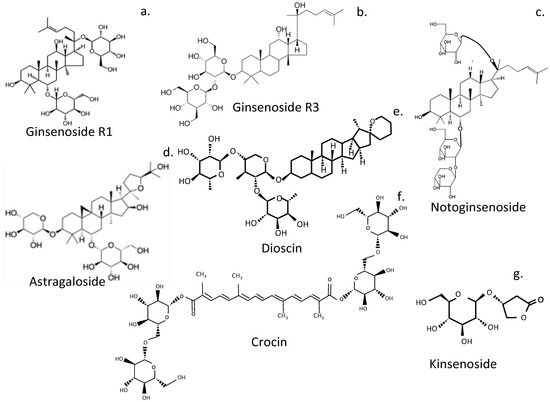
Figure 7.
Glycoside plant compounds that show promise in the treatment of IVDD. Ginsenoside R1 (a), Ginsenoside R3 (b), Notoginsenoside (c), Astragaloside (d), Dioscin (e), Crocin (f), Kinsenoside (g).
6.2. Notoginsenosides
Notoginsenoside R1 (NR1) suppressed IVDD in a rat annular puncture model, restored IVD functional properties, and suppressed mechanical and thermal hyperalgesia []. Moreover, NR1 promoted ECM synthesis in vivo and in vitro and decreased proinflammatory cytokine mRNA expression, inactivated NF-κB/NLRP3 cell signaling pathways, and obviated inflammation in the IVD []. A cellular environment in the IVD was thus established by NR1, conducive to NP cellular activity. NR1 protects the IVD from degeneration through suppression of the NF-κB/NLRP3 cell signaling pathway.
6.3. Astragaloside IV
Network pharmacology and molecular docking procedures have been used to investigate the mechanism of action of Astragaloside IV in the treatment of IVDD in a lumbar spine IVD instability mouse model []. Disc height and volume and matrix metabolism were improved, along with Col2α1 and aggrecan expression. Network pharmacology analysis revealed 11 key core genes, including ALB (albumin gene), MAPK1, MAPK14 (p38 MAPK), EGFR, TGFBR1, MAPK8, MMP3, ANXA5 [], ESR1, CASP3, and IGF1. ANXA5 has anti-inflammatory properties, is neuroprotective, promotes osteogenic differentiation, and takes part in chondrocyte apoptosis and mineralization []. ESR1 encodes an estrogen receptor. IVD protective effects were mediated through inhibition of the EGFR/MAPK signaling pathway.
6.4. Dioscin
Dioscin is a multi-targeting bioactive steroid saponin phytocompound from traditional Chinese medicine []. Dioscin has shown potential as a prospective therapeutic agent for the treatment of IVDD through attenuation of IL-1β-induced catabolism and apoptosis, mediated by TLR4-NF-κB signaling in human NP cells [].
6.5. Kinsenoside
Kinsenoside, ameliorates IVDD through the activation of the AKT-ERK1/2-Nrf2 cell signaling pathway [].
6.6. Crocin
Crocin, a glycosylated carotenoid of Crocus sativus L. (saffron) [] has antioxidant, anti-inflammatory, neuroprotective, anti-retinopathy, anticancer, and antidepressant properties [,,,]. Crocin inhibits inflammation and IVDD catabolic processes [], significantly suppresses LPS-induced overexpression of MMP-1, MMP-3, MMP-13, ADAMTS-4, ADAMTS-5, and proinflammatory IL-1β, TNF-α, IL-6, iNOS, and TLR-2 in-vitro.

Table 7.
Glycosides displaying therapeutic properties in the treatment of IVDD.
Table 7.
Glycosides displaying therapeutic properties in the treatment of IVDD.
| Compound | Therapeutic Properties in IVDD |
|---|---|
| Ginsenosides | Ginsenoside Rg1 inhibits NP cell apoptosis, inflammation, and ECM degradation via YAP1/TAZ/Hippo cell signaling []. Rg1 relieves rat IVDD and inhibits IL-1β-induced NP cell apoptosis and inflammation via NF-κB signaling [,]. Ginsenoside Rg3 exhibited anti-catabolic and anti-apoptotic effects in IL-1β-treated human disc NP cells and in a rat model of IVDD by inactivating the MAPK cell signaling pathway [], ginsenoside Rg3 inhibited NF-κB signaling in TNF-α-stimulated human NP cells inhibiting IVDD []. Animal model and cell culture data. |
| Notoginsenoside | Notoginsenoside R1 suppresses the inflammatory response/pyrop tosis in NP cells via inactivation of NF-κB/NLRP3 cell signaling []. Animal model and cell culture data. |
| Astragaloside IV | Attenuates IL-1β-induced IVDD through inhibition of the NF-κB pathway [], relieves IL-1β-induced human NP cell degeneration through modulating PI3K/Akt signaling pathway [], inhibits miR-223/JAK2/STAT1 signaling to alleviate LPS-induced damage in NP [], activates telomerase activity protecting NP cells from high glucose-induced senescence and apoptosis []. Animal model and cell culture data. |
| Dioscin | Attenuates IL-1β-induced catabolism and apoptosis through modulation of TLR4-NF-κB signaling in human NP cells []. Cell culture data. |
| Kinsenoside | Ameliorates IVDD through the activation of AKT-ERK1/2-Nrf2 signaling pathway []. Animal model and Cell culture data. |
| Crocin | Has anti-inflammatory and anti-catabolic effects on rat IVDs through suppression of JNK signaling activation []. Animal model and cell culture data. |
7. The Role of Pigmented Compounds and Lipids in IVD Bioregulation
7.1. Emodin
Emodin is a bioactive anthraquinone that upregulates anabolic markers (COL2A1, aggrecan) and negatively regulates catabolic markers (MMP3, MMP13) in cultured NP cells, inhibiting cell apoptosis in the inflammatory environment of degenerated IVDs [] and effectively alleviating IVDD in a rat model. Emodin inhibits inflammation-induced NF-ĸB activation through suppression of the degradation of LRP1 via the proteasome pathway []. Emodin treatment prevents reduced NP cell viability induced by IL-1β by reducing elevated ROS levels, secretion of IL-6 and TNF-α, and caspase-3 activity to abolish IL-1β-induced inflammation in NP cells []. Emodin is a potent and selective inhibitor of NLRP3 inflammasome activation, suppressing casein kinase II (CK2)-mediated phosphorylation of FUNDC1, a pivotal mitophagy receptor. This prevents mitochondrial ROS-induced NLRP3 inflammasome assembly [].
7.2. Rhein
Rhein (RH, 4,5-dihydroxyanthraquinone 2-carboxylic acid) is a lipophilic anthraquinone that enhances the synthesis of ECM components and inhibits production of inflammatory mediators in the IVD. The metabolic precursor of rhein, diacerein has significant pain-relieving properties in OA and provides functional improvement in joint function. RH may be a useful compound to evaluate for the treatment of IVDD since it has the ability to diminish IL-1-induced apoptosis and secretion of MMPs and aggrecanases [,,,].
7.3. Physcion (Parietin)
Physcion is an anthraquinone from rhubarb that displays antimicrobial, antitumor, antioxidant, and anti-inflammatory properties that counter inflammation and tissue damage [,,].
8. Statins, Cholesterol, and Animal Models for Experimental IVDD
Statins are cholesterol-lowering drugs originally isolated from fungi [,]. Monascus spp., Penicillium spp., Aspergillus terreus, and Pleurotus ostreatus were used to characterize the first naturally occurring statin, mevastatin (compactin) [,]. Statin drugs are now produced by pharmaceutical companies and are probably the most frequently prescribed global drug. Besides their cholesterol-lowering properties, statins have beneficial effects in the treatment of IVDD, inhibiting degenerative changes in the IVD and stimulating repair [,,]. Intradiscal administration of lovastatin upregulates BMP-2 and SOX9 expression and promotes chondrogenesis of rat caudal discs after needle puncture injury []. Simvastin promotes IVD repair processes, as evident in radiologic, histologic, and genetic assessments in a rat IVDD model []. Simvastin upregulates BMP2 expression and stimulates chondrogenic processes in experimental IVDD []. Rosuvastatin inhibits mechanical pressure-induced IVDD []. Rats fed a high-cholesterol diet display degenerative features in lumbar IVDs compared to rats fed a standard diet. This effect could be abolished by the cholesterol-lowering drug atorvastatin. Cholesterol levels are higher in NP cells treated with TNF-α and IL-1β, implicating cholesterol in the progression of IVDD, accelerated pyroptosis in NP cells, and ECM degradation. ER stress was responsible for this cholesterol-induced pyroptosis and ECM degradation [].
9. Miscellaneous Plant Compounds as Prospective IVDD Treatment Agents
9.1. Aloin
Aloin (barbaloin), an ancient traditional medicine, is a yellow-, orange-, and red-pigmented glycosylated anthraquinone from aloe []. It has been used to treat skeletal degenerative diseases [], displays curative effects on ECM metabolism and apoptosis in TNF-α-treated NP cells, and inhibits oxidative stress and proinflammatory mediators suppressing the TGF-β-activated kinase 1 (TAK1)/NF-κB pathway, downregulating the NLPR3 inflammasome in TNF-α-treated NP cells [] (Figure 8).
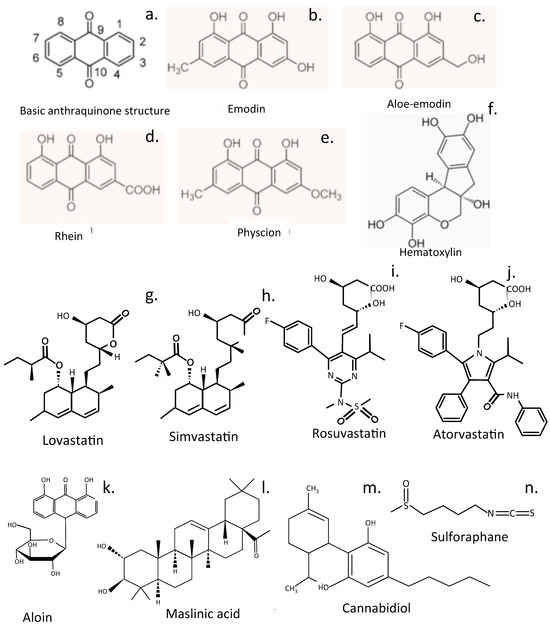
Figure 8.
Miscellaneous compounds that have shown promise in the treatment of IVDD. The ring numbering system for these compounds is presented in (a). Anthraquinones (b–e), haematoxylin (f), statins (g–j), aloin (k), maslinic acid (l), cannabidiol (m), and sulforaphane (n).
9.2. Maslinic Acid
Maslinic acid (MA), an anti-inflammatory compound found in olive plants (Olea europaea) and many herbs mitigates cellular senescence, upregulates aggrecan and collagen II biosynthesis, and downregulates MMP and ADAMTS levels in NP cells. MA impedes the progression of IVDD in rat models through inhibition of the PI3K/AKT and NF-κB pathways. Molecular docking studies show that MA binds to PI3K, resulting in dysfunction of the PI3K/AKT pathway []. The health-promoting properties of olive oil that prevent a toxic build-up of saturated fatty acids in tissues reduces the risk of diseases linked to oxidative stress and chronic inflammation, where fatty acids are oxidized by ROS []. MA contributes to the tissue-protective properties of olive oil.
9.3. Cannabidiol
Cannabidiol reduces LBP by reducing inflammation, combats the anxiety associated with long-term LBP, and helps with sleep. In animal studies cannabidiol inhibited hydrogen peroxide-induced apoptosis [], inflammation, and oxidative stress in NP cells, and showed tissue-protective properties in lesion-induced IVDD []. Cannabidiol reduced vertebral bone loss in rats with severe spinal cord injury.
9.4. Sulforaphane
Sulforaphane is an isothiocyanate polyphenolic compound that is generated in mustard and other cruciferous vegetables when tissue damage occurs. Glucoraphanin, a precursor of sulforaphane, is degraded by myrosinase, a β-thioglucosidase, to generate sulforaphane in plants []. Sulforaphane is also generated from glucoraphanin by intestinal bacteria and alleviates intestinal inflammation and oxidative stress, maintaining gut barrier integrity []. Sulforaphane delays IVDD by alleviating ER stress in NP cells [] and also inhibits cellular senescence in the IVD [].
10. The Role of Lipids in the Metabolism of Resident Disc Cell Populations
Excessive cholesterol levels have been shown to promote IVDD. Metabolic lipid disorders are associated with CEP senescence and calcification (EPC) in IVDD [,]. Oxidized low-density lipoprotein (ox-LDL) and lectin-like oxidized low-density lipoprotein receptor 1 (LOX-1) in human degenerative EPC is associated with hyperlipidemia (HLP) []. Hyperlipidemic conditions result in ox-LDL/LOX-1-induced EPC, mediated through LOX-1 receptor by the ROS/P38-MAPK/NF-κB cell signaling pathway. ox-LDL, formed by the lipid peroxidation of LDL, is a primary pathogenic factor in metabolic lipid disorders [,]. The LOX-1 receptor is the principal cell membrane receptor of ox-LDL. LOX-1 is expressed by IVD cells and is prominent in the CEP during EPC and IVDD []. Induction of EPC in rat IVDs by high-fat diets is mediated through the p38 MAPK/NF-κB cell signaling pathway []. HLP aggravates IVDD through the induction of inflammatory mediators and catabolic effects on NP and AF cells [], promoting apoptosis in NP cells [].
11. Pro-Resolving Anti-Inflammatory Lipids Rescue Degenerated IVDs
While peroxidation of lipids can generate ROS-promoting inflammatory conditions in the IVD and degenerative tissue changes, some polyunsaturated IVD fatty acid lipid metabolites have been identified with potent anti-inflammatory properties (Figure 9). These include lipoxin A4 (LXA4), formed from AA (arachidonic acid); E series resolvins, formed from EPA (eicosapentaenoic acid); and D series resolvins, protectins, and maresins, formed from DHA (docosahexaenoic acid) []. These rescue the degenerated IVD by re-balancing lipid profiles. LXA4 inhibits ROS generation, NFkB activation, and the generation of pro-inflammatory IVD cytokines (e.g., IL8, IL13, IL12, and IL5) [,]. Intrathecal injection of LXA4 alleviated the development of neuropathic pain, inhibited the upregulation of pro-inflammatory cytokines (TNF-α and IL-1β), upregulated the expression of anti-inflammatory cytokines (TGF-β1 and IL-10), and attenuated the activation of NF-κB/p65, p-ERK, and p-JNK, but not that of p-p38, in a dose-dependent manner [].
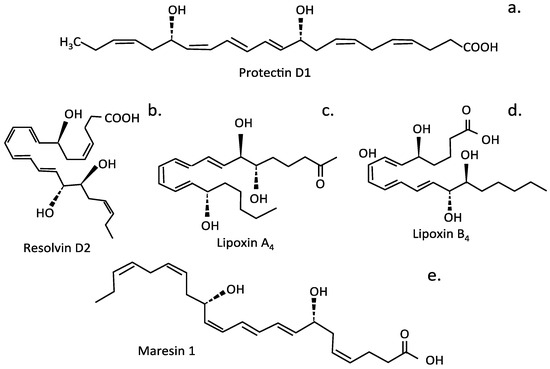
Figure 9.
Bioactive lipid metabolites with properties beneficial for the treatment of IVDD. Protectoin D1 (a), Resolvin D2 (b), Lipoxin A4 (c), Lipoxin B4 (d), Maresin 1 (e).
Resolvin D1 (RvD1) has potent anti-inflammatory and antinociceptive properties, alleviating neuropathic pain by regulating the inflammatory mediator NF-κB/p65 and p-ERK pathways []. Protectin PD1, an endogenous stereoselective lipid mediator, also has potent analgesic properties and regulated SIRT1-mediated CGRP signaling in a model of non-compressive lumbar disc herniation []. Maresin1 (MaR1), a macrophage-derived mediator of inflammation resolution, also displayed potent anti-inflammatory activity through inhibition of the NLRP3 inflammasome and NF-κB signaling []. LXA4, resolvins, protectins, and maresins inhibit the production and action of IL-6, TNF-α, and other pro-inflammatory cytokines. Therefore, they are of interest for the treatment of IVDD [,,,,,], as they can prevent excessive inflammation, restoring tissue homeostasis. Resolvin D2 (RvD2) suppresses the expression of pro-IL-1β, reducing the secretion of mature IL-1β by macrophages and deactivating the NLRP3 inflammasome. Injections of LXA4, resolvins, protectins, and maresins at sites of IVDD could be of significant therapeutic benefit.
A large number of compounds have been covered in this review; however, a few guiding comments are required to outline the most promising plant phytochemicals for the treatment of IVDD and potential IVD regeneration.
12. The Most Effective Plant Phytochemicals for Therapeutic Medical Applications
Flavonoids are a biodiverse family of bioactive plant phytochemicals that have, as a group, found widespread application in biomedicine, with over 10,000 flavonoids listed. It was beyond the scope of this study to cover all of these compounds. Selected flavonoid members that have shown promise in the treatment of IVDD have been covered in this review.
Quercetin, resveratrol, myrcetin, hyperoside, baicalein, kaempferol, luteolin, naringin, icariin, hesperidin, epigallocatechin 3-gallate (EGCG)/Urolithin A, and sesamin are all useful therapeutic plant phytochemicals that have been evaluated in the treatment of IVDD (Table 3). The properties of these compounds are briefly outlined in Table 3 and more extensively in Section 2.1.1, Section 2.1.2, Section 2.1.3 and Section 2.1.4 demonstrating why they have been evaluated in the treatment of IVDD and their potential to promote the regeneration of degenerated IVDs. These flavonoids have potent antioxidant and anti-inflammatory properties that counteract the changes induced in IVDs by NFkB cell signaling pathways, which play central roles in the generation of inflammatory and degradative pain-generating conditions in the IVD. Several flavonoid members also inhibit the expression of NFkB in disc tissues, and overlap between compounds classified as phenolics and flavonoids is evident. Curcumin and resveratrol are two phenolic compounds that display potent properties that counteract degradative events occurring in IVDD. Curcumin inhibits NFkB expression in IVD cells, produces anti-inflammatory and anti-catabolic conditions in IVDs, and counteracts chondrocyte apoptosis in the CEP. Sesamin also has CEP-protective properties, promoted by induction of PI3K/AKT/mTOR cell signaling. Myrcetin is a flavonoid and a phenolic compound that counteracts NFkB activity by promoting Nrf2/HO-1 cell signaling (Table 5). EGCG has potent regulatory properties over signaling pathways that control inflammation, oxidative stress, and apoptosis, providing protective effects over IVD cells. Resveratrol has potent antioxidant and anti-inflammatory properties in vitro and stimulates anabolic processes in IVDs, stimulating IVDD repair in vivo. Berberine is a promising alkaloid that ameliorates oxidation-induced apoptosis by modulating ER stress and autophagy in human NP cells. It also suppresses ECM degradation and IVDD by inhibiting the NFκB cell signaling pathway (Table 6).
Ginseng is a widely used herbal glycoside nutraceutical traditional Chinese medicine with antioxidant and anti-inflammatory properties. Ginsenoside Rg1 is of major interest for the treatment of IVDD since it regulates disc homeostasis, inhibits apoptosis, inflammation, and ECM degradation, delaying IVDD progression. Rg1 improves the proliferation of NP cells. Its IVDD inhibitory properties are affected through inhibition of the Wnt/β-catenin cell signaling pathway. Ginsenoside Rg1 also prevents activation of the NF-κB signaling pathway through the induction of IκK, inhibiting secretion of inflammatory mediators and stimulating aggrecan and collagen II biosynthesis. Ginsenoside Rg3 inactivates the p38 MAPK pathway, significantly reducing MMP2, MMP3, ADAMTS-4, and ADAMTS-5 degradative activity in the NP and restoring AF lamellar structure and function in the IVD composite structure (Table 7).
13. Key Cell Signaling Pathways Operative in IVDD
Mitochondrial dysfunction is critical in the pathogenesis of IVDD, influencing numerous cellular processes crucial for disc health. This dysfunction primarily results in excessive production of reactive oxygen species (ROS), leading to oxidative stress, mitochondrial DNA damage, and disrupted cellular bioenergetics []. These conditions activate apoptotic pathways, especially in NP cells, causing cell death and ECM degradation [].
Enhanced mitophagy with agents such as urolithin A promotes mitochondrial homeostasis. The use of SIRT3 activators also protects cells from mitochondrial-induced damage [].
Oxidative stress plays a central role in the development of IVDD by disrupting the balance between mitochondrial ROS production and the antioxidant defense system. In healthy IVDs, ROS levels are tightly regulated by antioxidants. However, in IVDD, aging, mechanical stress, and inflammation lead to an imbalance in ROS activity. This can cause cellular damage, lipid peroxidation, protein oxidation, and DNA damage, leading to apoptosis or dysfunction of NP and AF cells, critical for the maintenance of IVD structure and function.
Molecular pathways identified as key modulators of ROS in IVDD offer potential therapeutic targets. Manipulation of the activity of SIRT3 mitigates oxidative stress-induced senescence in NP cells []. The Keap1/Nrf2 axis plays a crucial role in the improvement of antioxidant defenses, providing protection against ROS-induced damage [,]. Activation of this pathway counteracts oxidative stress and slows IVDD processes. Hesperidin and glycitin modulate specific cellular signaling pathways, mitigating oxidative damage within the IVD [,]. Therapeutic strategies that reduce oxidative stress and enhance cellular antioxidant activity are promising strategies to inhibit IVDD and promote normal IVD functions.
13.1. Nrf2 Cell Signaling and IVDD
As shown in Figure 10, nuclear factor erythroid 2-related factor 2 (Nrf2), is an important antioxidant transcription factor that plays a crucial role in the modulation of the pathogenesis and progression of IVDD. It maintains redox homeostasis by protecting NP cells from oxidative stress, damage through inflammatory processes, ECM degradation, cell senescence, cell death, and the generation of LBP []. Nrf2 is a master antioxidant transcription factor with protective properties that counteract oxidative stress by mediating cellular damage in IVDD. Nrf2 is negatively regulated by Kelch-like ECH-associated protein 1 (Keap1). Nrf2 regulates the transcription of downstream antioxidant genes expressed by disc cells by binding to antioxidant response elements (AREs) in promoter regions in genes, such as heme oxygenase-1 (HO-1), glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and NADPH quinone dehydrogenase 1 (NQO1) []. This antioxidant defense system regulates cell apoptosis, senescence, ECM turnover, inflammatory responses displayed by NP cells, autophagy, and calcification of the CEP in IVDD []. Heme oxygenase-1 (HO-1) has antioxidant, anti-inflammatory, and anti-apoptotic protective properties regulated by Nrf2 and plays a particularly important role in the protection of IVD cells during IVDD []. Sulforaphane (SFN) is a natural compound found in the Brassica plant family that displays potent Nrf-2 agonist activity. It also has antioxidant properties in vitro and in vivo that counteract the degradative events that occur in IVDD []. SFN can promote the entry of Nrf-2 into the nucleus and increase the expression level of heme oxygenase 1 (HO-1) in vitro. This aids in the clearance of ROS accumulation in IVD cells, which can induce ER stress, thereby delaying the progression of IVDD.
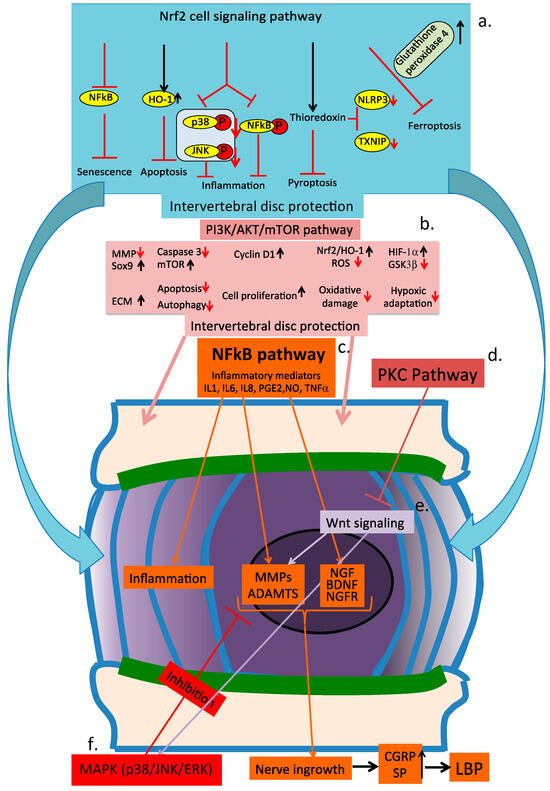
Figure 10.
Schematic of the major cell signaling pathways of the intervertebral disc operating during disc degeneration. (a) Nrf2 cell signaling pathway, (b) PI3/AKT/mTOR pathway, (c) NFκB pathway, (d) PKS pathway, (e) Wnt cell signaling pathway, and (f) MAPK (p38/JNK/ERK) cell signaling pathway.
A widespread range of phenolic phytochemicals display potent antioxidant and anti-inflammatory properties that protect IVD tissues from damage during IVDD []. Many of these plant phenolic phytochemicals also promote expression of the Nrf2/Kelch system, providing cell and tissue protection in IVDD [,,]. The Nrf2 cell signaling pathway inhibits cellular senescence, apoptosis, and inflammation.
Some examples of these phenolic compounds have been discussed earlier in this review, including quercetin, curcumin, icariin, myrcetin, epigallocatechin 3-gallate (EGCG), baicalein, resveratrol, and SFN; however, this is an extremely diverse group of compounds, with 10,000 flavonoids listed alone [,].
13.2. PI3/AKt/mTOR Cell Signaling in IVDD
Activation of the phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR pathway also has cell-protective properties through inhibition of IVDD, producing an increase in ECM synthesis, inhibition of cell apoptosis, promotion of cell proliferation, induction or prevention of cell autophagy, reduced oxidative damage, and facilitative adaptation to a hypoxic microenvironment []. A large number of plant phytochemicals can activate the PI3K/Akt cell signaling pathway, including quercetin, resveratrol, curcumin, proanthocyanidins, kaempferol, tyrosol, icariin, naringin, myrcetin, curcumin, hesperidin, and baicalein, indicating that these have potential in the treatment of IVDD [,].
13.3. NFkB Cell Signaling Plays a Central Role in Inflammatory Processes in IVDD
The nuclear factor NF-kappa B cell signaling pathway is central to inflammation in the IVD. NF-kappa B regulates the expression of proinflammatory genes controlling the synthesis and secretion of cytokines, chemokines, and adhesion molecules []. Persistent inflammation is associated with IVDD due to prolonged nuclear factor κB (NFκB) activation and leads to recruitment of active macrophages []. Macrophages have important roles in inflammation-driven IVDD, stemming from conversion to a catabolic phenotype []. M2 macrophages promote inflammatory processes in the IVD and regulate IVD cellular activities, affecting IVD ECM synthesis and degradation, vascularization, and innervation in the IVD, which contributes to the progression of IVDD and generation of LBP. The NFκB family of transcription factors regulate immune development, immune responses, inflammation, and cancer through interactions between NFκB dimers, IκB regulators, and IKK complexes []. The IKK complex is a central regulator of NFkB activation []. The IKK complex consists of two kinases (IKKalpha and IKKbeta) and a regulatory subunit, NEMO/IKKgamma []. IKKalphaand IKKbeta mediate phosphorylation of IkB proteins in signal transduction pathways that lead to NFkB activation. IKKbeta(and IKKgamma) have essential roles in the rapid activation of NFkB in response to proinflammatory signaling cascades, triggered by TNF alpha or LPS. Related IKKs bearing structural similarity to IKKalpha and IKKbeta are important for the activation of interferon response factor 3 (IRF3) and IRF7, transcription factors that play key roles in the induction of type I interferon (IFN-I). NF-kB is a cytoplasmic transcription factor, which, upon activation, translocates to the nucleus, where it controls the expression of 400 different genes, making it a master regulator of inflammatory processes and innate and adaptive immune responses. A wide range of plant phenolic phytochemicals can inhibit the activation of NFkB, controlling inflammatory processes and the degradative changes they induce in IVDD [,].
13.4. MAPK (p38, ERK, JNK) Cell Signaling in IVDD
This review has shown that in IVDD, elevated expression and synthesis of MMPs and inflammatory mediators is mediated by NFkB- and MAPK-regulated pathways [,]. NFkB and MAPK are considered major regulators of inflammation and catabolism in processes that lead to IVDD []. c-Jun N-terminal kinase (JNK) is a key branch of the MAPK signaling pathway [] in IVDD [] and interacts with the PI3K/Akt and NF-ĸB cell signaling pathways, influencing cell growth, survival, and metabolism [,,].
Baicalin [], berberine [], glycyrrhizin [], sesamin [], and crocin [] all inhibit aspects of JNK cell signaling. Flavonoids (e.g., quercetin, hyperoside), glycosides (e.g., ginsenosides, notoginsenosides), terpenoids (e.g., aucubin, celastrol), phenolic compounds (e.g., curcumin, resveratrol), and alkaloids (e.g., berberine) all exert therapeutic effects on IVDD by modulating key signaling pathways, including Sirtuin-1 (SIRT1), MAPK, PI3K/Akt, and Nrf2 [], thus providing potent anti-inflammatory, antioxidant, anti-apoptotic, anti-senescence, and IVD regenerative properties [,]. NFκB signaling is associated with pain-related neuropeptide expression and pain generation in IVDD [,]. Inflammatory mediators induced by NFkB, such as IL-1beta and TNF alpha stimulate production of NGF by IVD cells [,]. NGF, stimulate IVD cells, resulting in ECM degradation and the promotion of IVDD. NGF levels are elevated in degenerated IVDs compared to normal IVDs []. BDNF levels are also elevated in degenerated IVDs [].
13.5. PKC Signaling Inhibits Wnt-Mediated Processes in IVDD
Activation of PKC signaling leads to an increase in ECM synthesis and cell proliferation, inhibiting IVD degeneration through inhibition of Wnt signaling []. These processes are summarized in Figure 10.
14. Limitations on the Therapeutic Application of Plant Phytochemicals
Bioavailability of Plant Compounds
Phytochemicals hold immense potential for improving health; however, they face challenges that limit their application in mainstream therapeutics and use as dietary supplements due, in some cases, to their low bioavailability, poor solubility, and stability. The full potential of polyphenolic and carotenoid phytochemicals is therefore challenged, preventing their full therapeutic potential from being realized []. Future research should further investigate the role of gut microbiota and how they influence the bioavailability of dietary plant phytochemicals. The gut microbiome can process phytochemicals into fragments such as urolithin A, retaining the bioactivity of the native molecule that is more easily transported and making them more bioavailable []. Polyphenols, isothiocyanates, and curcumin can modulate the gut microbiota, impacting health outcomes. Polyphenols promote beneficial bacteria, such as Bifidobacterium and Lactobacillus, improving gut and metabolic health-reducing inflammation in obesity and diabetes []. Furthermore, isothiocyanates found in cruciferous vegetables support gut microbe diversity and reduce cancer risk by modulating gut inflammation []. Moreover, curcumin impacts the gut–brain axis, reducing neuroinflammation in neurodegenerative diseases like Alzheimer’s [] and Parkinson’s [,,]. Understanding the interaction between phytochemicals and gut microbiota could unveil new therapeutic avenues, leading to personalized nutritional strategies based on individual metabolic profiling. The inclusion of phytochemical supplements, functional foods, and dietary interventions may make it possible to realize the health benefits of phytochemicals in a secure and efficient manner.
15. Effective Delivery of Phytochemicals into the IVD
This review has established the chemical properties of plant compounds with antioxidant and anti-inflammatory properties with potential application in IVDD. However, a critical step that needs to be resolved is how these compounds are delivered to the IVD. Degenerated IVDs have depleted levels of aggrecan, their main space-filling proteoglycan, making them more amenable to the diffusive entry of metabolites. Blood vessels penetrate deeper into degenerated IVDs since the internal pressure is lower than in normal IVDs, where blood vessels do not penetrate past the outermost lamellar layer in the annulus. The elevated ingrowth of blood vessels into degenerated IVDs represents a potential entry route for plant compounds carried in the blood stream to central regions of the IVD, where tissue damage may be advanced. Further studies need to be undertaken to determine the diffusive properties of IVDs to plant compounds to develop an effective means of administering these into the IVD.
A gut–IVD axis has been established, with blood networks supplying the lumbar spinal region serving as conduits for phytochemicals to reach degenerated IVDs []. Innovative delivery systems, such as nanoparticles, liposomes, and micro-encapsulation, also show promise in enhancing the absorption and stability of administered phytochemicals, aiding their full therapeutic potential. Multifunctional stimuli-responsive nanoparticles show potential for their capacity to provide precise targeting and controlled therapeutic release of cargo chemicals, offering improved localization and sustained delivery of phytochemicals into the IVD []. This may overcome the limitations of conventional therapeutic treatment modalities, enabling more effective, targeted management strategies for IVDD []. Immune-defense microspheres have emerged with critical properties that inhibit IVDD through modulation of the inflammatory microenvironment, while also promoting disc regeneration []. Nanocomposite EGCG-coated hydroxyapatite composites with O-carboxymethyl chitosan cross-linked to HA have proved useful for this application.
16. Future Research on Plant Phytochemicals
Nature has provided a highly diverse array of molecules in plants, which are an immense resource. Plant compounds have diverse properties and have been harnessed in medical applications to combat disease for centuries. It should be noted that the origins of many successful present-day pharmaceuticals had their origins in plant compounds that were noted to have bioactive properties of potential application in biomedicine [,]. A total of 80% of 122 plant-derived drugs are reported to have had their origins in ethnopharmacological folkloric medicine []. The biodiversity of these compounds and their many applications in biomedicine are clearly evident. Flavonoids in particular have many potential applications in biomedicine, but this is only one class of compounds covered in this review (Figure 11). This is an area that warrants further investigation in the future. History shows the likelihood of this being a fruitful area of investigation, particularly in problematic present-day medicinal areas, such as antiviral development. This may potentially uncover compounds that may be used in a preventative capacity rather than more costly approaches requiring the development of vaccines and therapeutic antibodies to treat viral symptoms. Pentosan polysulfate, a sulfated semi-synthetic plant polysaccharide, is an example of a cost-effective pan-specific antiviral compound that prevents SARS CoV-2 infections []. It also has pleiotropic cell and tissue-protective properties of therapeutic application in long COVID disease [].
Figure 11.
Some examples of the diverse areas in biomedicine where plant phytochemicals or drugs developed from these have found therapeutic application. Abbreviations: ROS, reactive oxygen species; BChE, butyrylcholinesterase, serine hydrolase; AChE, acetylcholinesterase; BACE-1, beta-secretase 1, also known as beta-site amyloid precursor protein cleaving enzyme 1; NDM-1, New Delhi metallo beta lactamase.
Other areas that warrant further research are optimized phytochemical delivery systems to the IVD, the impact of the gut microbiome on the processing of dietary plant phytochemicals, and how this effects the delivery of bioactive phytochemical species into the IVD via the gut–IVD axis. For example, a study using a smart responsive injectable hydrogel loaded with icariin has just been published, and similar delivery systems are expected to follow in the near future []. This delivery system with icariin inhibited ferroptosis and promoted regeneration of the NP.
Funding
This study was funded by the Melrose Personal Research Fund, Sydney, Australia.
Data Availability Statement
No new data was generated in this study, all data is directly available from the cited references.
Acknowledgments
This review was written in entirety by JM. The author declares that this research was conducted in the absence of any commercial or financial relationships that could be construed to be potential conflicts of interest. JM has received consultancy fees from Arthopharm-Fidia Pharmaceutical, which had no input into the writing or interpretation of the findings presented herein.
Conflicts of Interest
The author has no conflicts to report.
Abbreviations
| IVDD | Intervertebral disc degeneration |
| ADAMTS4 | A disintegrin and metalloproteinase with thrombospondin motifs 4 |
| Akt | Protein kinase B |
| AMPK | 5′ AMP-activated protein kinase; iNOS, inducible nitric oxide synthase. |
| AMP | Adenosine monophosphate |
| ARE | Antioxidant response elements |
| BDNF | Brain-derived neurotrophic factor (abrineurin) |
| BECN2 | Macroautophagy and autophagy family member beclin 2 (Bcl2/Bax) |
| BMP-2 | Bone morphogenetic protein-2 |
| CDK | Cyclin-dependent kinase |
| cGAS/Sting | Cyclic GMP-AMP synthase–stimulator of interferon genes |
| COX-2 | Cyclooxygenase-2, prostaglandin–endoperoxide synthase 2 |
| E2 | Prostaglandin E2 |
| EGCG | Epigallocatechin gallate |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| FOXO3 | Forkhead box O3 protein |
| GPRASP1 | G protein-coupled receptor associated sorting protein 1 |
| PI3K | Phosphatidylinositol 3-kinase |
| HDL | High-density lipoprotein |
| HO-1 | Heme oxygenase-1 |
| IL-6 | Interleukin 6 |
| IL-1β | Interleukin-1 beta |
| iNOS | Inducible nitric oxide synthase |
| ITGA2 | Integrin alpha 2 |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| Keap-1 | Kelch-like ECH-associated protein 1 |
| LDL | Low-density lipoprotein |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MMP3 | Matrix metalloprotease 3 |
| mTOR | Mammalian target of rapamycin. |
| MS | Muscular sclerosis |
| NADQO | NAD(P)H:quinone oxidoreductase 1 |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| P38 | Mitogen-activated protein kinase |
| P53 | Tumor protein p53, a regulatory transcription factor protein |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PPAR | Protease activated receptor |
| PANoptosis | A prominent innate immune, inflammatory, and lytic cell death pathway initiated by innate immune sensors driven by caspases and receptor-interactive protein kinases |
| RA | Rheumatoid arthritis |
| RIP3 | Receptor-interacting serine/threonine-protein kinase 3 |
| ROS | Reactive oxygen species |
| SIRT-1 | Sirtuin 1, NAD-dependent deacetylase |
| SLE | Systemic lupus erthythematosus |
| SOX6 | SRY box 6 transcription factor |
| SDF-1 | Stromal-derived factor |
| STAT | Signal transducer and activator of transcription |
| TAK1 | Mitogen-activated protein kinase kinase kinase 7(Map3k7) |
| TLR | Toll-like receptor |
| TLR4 | Toll-like receptor 4 |
| TLR4/MD-2 | Toll like receptor-4/Muscarinic acetylcholine receptor-2 complex |
| TNF-α | Tumor necrosis factor alpha |
| VLDL | Very-low-density lipoprotein |
| Wnt | Wingless-type MMTV integration site family |
References
- Schäfer, R.; Trompeter, K.; Fett, D.; Heinrich, K.; Funken, J.; Willwacher, S.; Brüggemann, G.-P.; Platen, P. The mechanical loading of the spine in physical activities. Eur. Spine J. 2023, 32, 2991–3001. [Google Scholar] [CrossRef]
- Melrose, J.; Roberts, S.; Smith, S.; Menage, J.; Ghosh, P. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine 2002, 27, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.; Buchbinder, R. Back pain: A National Health Priority Area in Australia? Med. J. Aust. 2009, 190, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2010, 380, 2163–2196. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, G. Low back pain. Bull. World Health Organ. 2003, 81, 671–676. [Google Scholar]
- Wu, A.; March, L.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; Hoy, D. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 2020, 8, 299–313. [Google Scholar] [CrossRef]
- Ravindra, V.M.; Senglaub, S.S.; Rattani, A.; Dewan, M.C.; Härtl, R.; Bisson, E.; Park, K.B.; Shrime, M.G. Degenerative Lumbar Spine Disease: Estimating Global Incidence and Worldwide Volume. Glob. Spine J. 2018, 8, 784–794. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]
- Crampon, K.; Giorkallos, A.; Deldossi, M.; Baud, S.; Steffenel, L.A. Machine-learning methods for ligand-protein molecular docking. Drug Discov. Today 2022, 27, 151–164. [Google Scholar] [CrossRef]
- Stanzione, F.; Giangreco, I.; Cole, J.C. Use of molecular docking computational tools in drug discovery. Prog. Med. Chem. 2021, 60, 273–343. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, J.K.; Craft, D.; Weng, J.-K. Toward an integrated omics approach for plant biosynthetic pathway discovery in the age of AI. Trends Biochem. Sci. 2025, 50, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G. Application of Biotechnology in Producing Plant Bio-active Compounds. In Natural Bio-Active Compounds; Akhtar, M., Swamy, M., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Doherty, A.; Wall, A.; Khaldi, N.; Kussmann, M. Artificial Intelligence in Functional Food Ingredient Discovery and Characterisation: A Focus on Bioactive Plant and Food Peptides. Front. Genet. 2021, 12, 768979. [Google Scholar] [CrossRef] [PubMed]
- Bisht, A.; Tewari, D.; Kumar, S.; Chandra, S. Network pharmacology, molecular docking, and molecular dynamics simulation to elucidate the mechanism of anti-aging action of Tinospora cordifolia. Mol. Divers. 2024, 28, 1743–1763. [Google Scholar] [CrossRef]
- Zhao, X.; Xiu, J.; Yang, H.; Han, W.; Jin, Y. Network Pharmacology and Bioinformatics Study of Six Medicinal Food Homologous Plants Against Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 930. [Google Scholar] [CrossRef]
- Hu, X.; Qi, C.; Feng, F.; Wang, Y.; Di, T.; Meng, Y.; Wang, Y.; Zhao, N.; Zhang, X.; Li, P.; et al. Combining network pharmacology, RNA-seq, and metabolomics strategies to reveal the mechanism of Cimicifugae Rhizoma—Smilax glabra Roxb herb pair for the treatment of psoriasis. Phytomedicine 2022, 105, 154384. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, Q.; Wang, Y. Network pharmacology-based investigation of potential targets of astragalus membranaceous-angelica sinensis compound acting on diabetic nephropathy. Sci. Rep. 2021, 11, 19496. [Google Scholar] [CrossRef]
- Zhai, Y.; Liu, L.; Zhang, F.; Chen, X.; Wang, H.; Zhou, J.; Chai, K.; Liu, J.; Lei, H.; Lu, P.; et al. Network pharmacology: A crucial approach in traditional Chinese medicine research. Chin. Med. 2025, 20, 8. [Google Scholar] [CrossRef]
- Orlov, Y.L.; Ivanisenko, V.A.; Dobrovolskaya, O.B.; Chen, M. Plant Biology and Biotechnology: Focus on Genomics and Bioinformatics. Int. J. Mol. Sci. 2022, 23, 6759. [Google Scholar] [CrossRef]
- Kwuegbuenyi, C.A.; Fidai, A.B.; Cardenas, A.; Willett, N.; Robayo, A.; Hamad, M.; Hussain, I.; Bonassar, L.J.; Härtl, R. Bioactive Therapies for Degenerative Disc Disease: Current State of the Art and Clinical Applications. World Neurosurg. 2025, 200, 124107. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, C.; Zhang, X.; Yu, Z.; Zhan, F.; Yu, X.; Wang, S.; He, F.; Han, Y.; Zhao, H. The treatment of intervertebral disc degeneration using traditional Chinese Medicine. J. Ethnopharmacol. 2020, 263, 113117. [Google Scholar] [CrossRef]
- Melrose, J. The Glucosinolates: A Sulphur Glucoside Family of Mustard Anti-Tumour and Antimicrobial Phytochemicals of Potential Therapeutic Application. Biomedicines 2019, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, J.; Luo, W.; Kuang, T. Natural products for intervertebral disc degeneration: Mechanistic insights and therapeutic potentials. Front. Pharmacol. 2025, 16, 1605764. [Google Scholar] [CrossRef] [PubMed]
- Wachtel-Galor, S.; Benzie, I.F.F. Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Xutian, S.; Zhang, J.; Louise, W. New exploration and understanding of traditional Chinese medicine. Am. J. Chin. Med. 2009, 37, 411–426. [Google Scholar] [CrossRef]
- Nestler, G. Traditional Chinese medicine. Med. Clin. N. Am. 2002, 86, 63–73. [Google Scholar] [CrossRef]
- Dincheva, I.; Badjakov, I.; Galunska, B. New Insights into the Research of Bioactive Compounds from Plant Origins with Nutraceutical and Pharmaceutical Potential. Plants 2023, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Dincheva, I.; Badjakov, I.; Galunska, B. New Insights in the Research on Bioactive Compounds from Plant Origins with Nutraceutical and Pharmaceutical Potential II. Plants 2025, 14, 500. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Mohammed, D.M.; Korma, S.A.; Alshahrani, M.Y.; Ahmed, A.E.; Ibrahim, E.H.; Salem, H.M.; Alkafaas, S.S.; Saif, A.M.; et al. Medicinal plants: Bioactive compounds, biological activities, combating multidrug-resistant microorganisms, and human health benefits—A comprehensive review. Front. Immunol. 2025, 16, 1491777. [Google Scholar] [CrossRef]
- Riaz, M.; Khalid, R.; Afzal, M.; Anjum, F.; Fatima, H.; Zia, S.; Rasool, G.; Egbuna, C.; Mtewa, A.G.; Uche, C.Z.; et al. Phytobioactive compounds as therapeutic agents for human diseases: A review. Food Sci. Nutr. 2023, 11, 2500–2529. [Google Scholar] [CrossRef]
- Kang, J.; Georgescu, H.I.; McIntyre-Larkin, L.; Stefanovic-Racic, M.; Donaldson, W.F., 3rd; Evans, C.H. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine 1996, 21, 271–277. [Google Scholar] [CrossRef]
- Kang, J.; Stefanovic-Racic, M.; McIntyre, L.A.; Georgescu, H.I.; Evans, C.H. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine 1997, 22, 1065–1073. [Google Scholar] [CrossRef]
- Weiler, C.; Nerlich, A.G.; Bachmeier, B.E.; Boos, N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: A study in surgical specimen and autopsy controls. Spine 2005, 30, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.G.; Watson, R.W.G.; McCormack, D.; Dowling, F.E.; Walsh, M.G.; Fitzpatrick, J.M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J. Bone Jt. Surg. Br. 2002, 84, 196–201. [Google Scholar] [CrossRef]
- Podichetty, V. The aging spine: The role of inflammatory mediators in intervertebral disc degeneration. Cell. Mol. Biol. 2007, 53, 4–18. [Google Scholar] [PubMed]
- Wong, A.; Karppinen, J.; Samartzis, D. Low back pain in older adults: Risk factors, management options and future directions. Scoliosis Spinal Disord. 2017, 12, 14. [Google Scholar] [CrossRef]
- de Souza, I.; Sakaguchi, T.F.; Yuan, S.L.K.; Matsutani, L.A.; do Espírito-Santo, A.S.; Pereira, C.A.B.; Marques, A.P. Prevalence of low back pain in the elderly population: A systematic review. Clinics 2019, 74, e789. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Deng, Y.; Chen, Y.; Wu, C.; Zhao, X.; Chen, X.; Wang, X.; Zhou, Y.; Zhang, X.; et al. Fisetin suppresses ferroptosis through Nrf2 and attenuates intervertebral disc degeneration in rats. Eur. J. Pharmacol. 2024, 964, 176298. [Google Scholar] [CrossRef]
- Kuai, J.; Zhang, N. Upregulation of SIRT1 by Evodiamine activates PI3K/AKT pathway and blocks intervertebral disc degeneration. Mol. Med. Rep. 2022, 26, 265. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, J.-S.; Hou, S.-X.; Shi, X.-X.; Qin, J.; Zhang, T.-S.; Wang, X.-J. Neuroprotective effects of curcumin alleviate lumbar intervertebral disc degeneration through regulating the expression of iNOS, COX-2, TGF-β1/2, MMP-9 and BDNF in a rat model. Mol. Med. Rep. 2017, 16, 6864–6869. [Google Scholar] [CrossRef]
- Hong, H.; Guo, D.; Xia, T.; Zhang, Y. Dihydromyricetin attenuates intervertebral disc degeneration by inhibiting NLRP3 inflammasome activation via the Keap1/Nrf2/HO-1 pathway. Eur. J. Pharmacol. 2025, 998, 177501. [Google Scholar] [CrossRef]
- Fang, W.; Zhou, X.; Wang, J.; Xu, L.; Zhou, L.; Yu, W.; Tao, Y.; Zhu, J.; Hu, B.; Liang, C.; et al. Wogonin mitigates intervertebral disc degeneration through the Nrf2/ARE and MAPK signaling pathways. Int. Immunopharmacol. 2018, 65, 539–549. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, M.; Chen, H.; Wu, Z.; Gao, Y.; Ma, Z.; He, X.; Kang, X. NF-κB signalling pathways in nucleus pulposus cell function and intervertebral disc degeneration. Cell Prolif. 2021, 54, e13057. [Google Scholar] [CrossRef]
- Navone, S.E.; Marfia, G.; Giannoni, A.; Beretta, M.; Guarnaccia, L.; Gualtierotti, R.; Nicoli, D.; Rampini, P.; Campanella, R. Inflammatory mediators and signalling pathways controlling intervertebral disc degeneration. Histol. Histopathol. 2017, 32, 523–542. [Google Scholar] [CrossRef]
- Melrose, J. The Potential of Flavonoids and Flavonoid Metabolites in the Treatment of Neurodegenerative Pathology in Disorders of Cognitive Decline. Antioxidants 2023, 12, 663. [Google Scholar] [CrossRef]
- You, Q.; Wang, K.; Zhao, Z.; Zhou, H.; Lan, Z.; Liang, H.; Deng, R.; Li, W.; Shen, S.; Wang, R.; et al. Reduction of Bacteroides fragilis in Gut Microbiome of Chronic Hepatitis B Patients Promotes Liver Injury. J. Med. Virol. 2025, 97, e70395. [Google Scholar] [CrossRef] [PubMed]
- Yip, J.; Balasuriya, G.K.; Spencer, S.J.; Hill-Yardin, E.L. The Role of Intestinal Macrophages in Gastrointestinal Homeostasis: Heterogeneity and Implications in Disease. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Cai, Y.; Wang, W.; Deng, J.; Zhao, L.; Han, Z.; Wan, L. The Effect of Gut Microbiota on the Progression of Intervertebral Disc Degeneration. Orthop. Surg. 2023, 15, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Z.; Duan, J.; Liu, E.; Sun, F.; Yang, L.; Chen, L.; Yang, S. Unveiling the Gut-Disc Axis: How Microbiome Dysbiosis Accelerates Intervertebral Disc Degeneration. J. Inflamm. Res. 2024, 17, 8271–8280. [Google Scholar] [CrossRef]
- Dhiman, M.S.; Bader, T.J.; Ponjevic, D.; Salo, P.T.; Hart, D.A.; Swamy, G.; Matyas, J.R.; Duncan, N. A Collagen integrity of the annulus fibrosus in degenerative disc disease individuals quantified with collagen hybridizing peptide. JOR Spine 2024, 7, e1359. [Google Scholar] [CrossRef]
- Chen, H.-W.; Liu, M.-Q.; Zhang, G.-Z.; Zhang, C.-Y.; Wang, Z.-H.; Lin, A.-X.; Kang, J.-H.; Liu, W.-Z.; Guo, X.-D.; Wang, Y.-D.; et al. Proanthocyanidins inhibit the apoptosis and aging of nucleus pulposus cells through the PI3K/Akt pathway delaying intervertebral disc degeneration. Connect. Tissue Res. 2022, 63, 650–662. [Google Scholar] [CrossRef]
- Du, H.-Y.; Wang, R.; Li, J.-L.; Luo, H.; Xie, X.-Y.; Yan, R.; Jian, Y.-L.; Cai, J.-Y. Ligustrazine induces viability, suppresses apoptosis and autophagy of retinal ganglion cells with ischemia/reperfusion injury through the PI3K/Akt/mTOR signaling pathway. Bioengineered 2021, 12, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Du, H.; Zhang, M.; Xu, H.; Pu, X.; Chen, Q.; Luo, R.; Hu, Y.; Wang, Y.; Tu, H.; et al. Anti-inflammatory effect of Rhein on ulcerative colitis via inhibiting PI3K/Akt/mTOR signaling pathway and regulating gut microbiota. Phytother. Res. 2022, 36, 2081–2094. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wu, W.; Liang, H.; Huang, D.; Jing, D.; Zheng, D.; Shao, Z. Icariin Prevents IL-1β-Induced Apoptosis in Human Nucleus Pulposus via the PI3K/AKT Pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 12. [Google Scholar]
- Deng, X.; Chen, S.; Zheng, D.; Shao, Z.; Liang, H.; Hu, H. Icariin Prevents H2O2-Induced Apoptosis via the PI3K/Akt Pathway in Rat Nucleus Pulposus Intervertebral Disc Cells. Evid. Based Complement. Altern. Med. 2017, 2017, 2694261. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, W.; Chen, Y.; Shang, J.; Wang, W.; Yan, X.; Liu, P.; Zhou, Y. Aloe-emodin ameliorates chronic kidney disease fibrosis by, inhibiting PI3K-mediated signaling pathway. Eur. J. Histochem. 2025, 69, 4228. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, R.; Wang, Z.; Luo, L.; Wu, J.; Zhang, C.; Liu, M.; Shi, C.; Zhou, Y. Kinsenoside ameliorates intervertebral disc degeneration through the activation of AKT-ERK1/2-Nrf2 signaling pathway. Aging 2019, 11, 7961–7977. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Huang, B.; Wang, J.; Shan, Z.; Liu, J.; Chen, Y.; Li, S.; Fan, S.; Zhao, F. Obesity Mediates Apoptosis and Extracellular Matrix Metabolic Imbalances via MAPK Pathway Activation in Intervertebral Disk Degeneration. Front. Physiol. 2019, 10, 1284. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, W.; Abulizi, Y.; Xu, T.; Cao, R.; Xun, C.; Zhang, J.; Sheng, W. Quercetin alleviates intervertebral disc degeneration by modulating p38 MAPK-mediated autophagy. Biomed. Res. Int. 2021, 2021, 6631562. [Google Scholar] [CrossRef]
- Xie, T.; Gu, X.; Pan, R.; Huang, W.; Dong, S. Evodiamine ameliorates intervertebral disc degeneration through the Nrf2 and MAPK pathways. Cytotechnology 2024, 76, 153–166. [Google Scholar] [CrossRef]
- Wu, Y.; Li, F.; Shu, S.; Feng, Z.; Qiu, Y.; Li, S.; Zhu, Z. Baicalin alleviates intervertebral disc degeneration by inhibiting the p38 MAPK signaling pathway. Exp. Gerontol. 2025, 204, 112743. [Google Scholar] [CrossRef]
- Wang, Q.-W.; Su, Y.; Sheng, J.-T.; Gu, L.-M.; Zhao, Y.; Chen, X.-X.; Chen, C.; Li, W.-Z.; Li, K.-S.; Dai, J.-P. Anti-influenza A virus activity of rhein through regulating oxidative stress, TLR4, Akt, MAPK, and NF-κB signal pathways. PLoS ONE 2018, 13, e0191793. [Google Scholar] [CrossRef]
- Shi, Z.; Zhu, L.; Song, Z.; Liu, T.; Hao, D. Roles of p38 MAPK signalling in intervertebral disc degeneration. Cell Prolif. 2023, 56, e13438. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Tao, S.; Guo, F.; Wang, B.; Yang, L.; Ma, L.; Fu, P. Natural flavonol fisetin attenuated hyperuricemic nephropathy via inhibiting IL-6/JAK2/STAT3 and TGF-β/SMAD3 signaling. Phytomedicine 2021, 87, 153552. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, X.; Cui, K.; Chen, Y.; Zhang, C.; Yao, K.; Hao, Y.; Chen, Y. Tanshinone IIA and Astragaloside IV Inhibit miR-223/JAK2/STAT1 Signalling Pathway to Alleviate Lipopolysaccharide-Induced Damage in Nucleus Pulposus Cells. Dis. Markers 2021, 2021, 6554480. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Jiang, M.; Wang, J.; Yang, S.; Liu, Z.; Zhang, H.; Zhu, X. Cyanidin attenuates the apoptosis of rat nucleus pulposus cells and the degeneration of intervertebral disc via the JAK2/STAT3 signal pathway in vitro and in vivo. Pharm. Biol. 2022, 60, 427–436. [Google Scholar] [CrossRef]
- Dai, W.; Hou, Q.; Ye, J. Rhein alleviates hepatic steatosis in NAFLD mice by activating the AMPK/ACC/SREBP1 pathway to enhance lipid metabolism. Mol. Med. 2025, 31, 255. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, J.; Teng, C.; Wang, J.; Yu, J.; Jin, C.; Wang, L.; Wu, L.; Lin, Z.; Yu, Z.; et al. Orientin downregulating oxidative stress-mediated endoplasmic reticulum stress and mitochondrial dysfunction through AMPK/SIRT1 pathway in rat nucleus pulposus cells in vitro and attenuated intervertebral disc degeneration in vivo. Apoptosis 2022, 27, 1031–1048. [Google Scholar] [CrossRef]
- Chen, R.; Gao, S.; Guan, H.; Zhang, X.; Gao, Y.; Su, Y.; Song, Y.; Jiang, Y.; Li, N. Naringin protects human nucleus pulposus cells against TNF-α-induced inflammation, oxidative stress, and loss of cellular homeostasis by enhancing autophagic flux via AMPK/SIRT1 activation. Oxid. Med. Cell Longev. 2022, 2022, 7655142. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, S.; Cao, Z.; Yang, L.; Lu, F.; Li, Y.; Hu, L.; Bai, X. Higenamine mitigates interleukin-1β-induced human nucleus pulposus cell apoptosis by ROS-mediated PI3K/Akt signaling. Mol. Cell Biochem. 2021, 476, 3889–3897. [Google Scholar] [CrossRef]
- Zhang, L.B.; Gao, J.; Li, Z.M.; Zhang, C.M.; Liu, J.M.; Dong, H.M.; Mei, W.M. Astragaloside IV relieves IL-1β-induced human nucleus pulposus cells degeneration through modulating PI3K/Akt signaling pathway. Medicine 2023, 102, e34815. [Google Scholar] [CrossRef]
- Que, Y.; Wong, C.; Qiu, J.; Gao, W.; Lin, Y.; Zhou, H.; Gao, B.; Li, P.; Deng, Z.; Shi, H.; et al. Maslinic acid alleviates intervertebral disc degeneration by inhibiting the PI3K/AKT and NF-κB signaling pathways. Acta Biochim. Biophys. Sin. 2024, 56, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Ren, D.; Wang, P.; Song, Z.; Wu, H.; Yao, S.; Geng, L.; Su, Y.; Bai, X. Upregulation of Sirt1 by tyrosol suppresses apoptosis and inflammation and modulates extracellular matrix remodeling in interleukin-1β-stimulated human nucleus pulposus cells through activation of PI3K/Akt pathway. Int. Immunopharmacol. 2020, 88, 106904. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, D.-K.; Wang, Z.-W.; Zhao, C.; Miao, J. Baicalein alleviates TNF-α-induced apoptosis of human nucleus pulposus cells through PI3K/AKT signaling pathway. J. Orthop. Surg. Res. 2023, 18, 292. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, X.; Chen, X.; Jiang, Y.; Zeng, Y.; Ren, R.; Nie, M.; Zhang, Z.; Bao, Y.; Kang, H. Genkwanin alleviates intervertebral disc degeneration via regulating ITGA2/PI3K/AKT pathway and inhibiting apoptosis and senescence. Int. Immunopharmacol. 2024, 133, 112101. [Google Scholar] [CrossRef]
- Bai, X.; Wang, J.; Ding, S.; Yang, S.; Pei, B.; Yao, M.; Zhu, X.; Jiang, M.; Zhang, M.; Mu, W.; et al. Embelin protects against apoptosis and inflammation by regulating PI3K/Akt signaling in IL-1β-stimulated human nucleus pulposus cells. Tissue Cell 2023, 82, 102089. [Google Scholar] [CrossRef]
- Tu, J.; Li, W.; Zhang, Y.; Wu, X.; Song, Y.; Kang, L.; Liu, W.; Wang, K.; Li, S.; Hua, W.; et al. Simvastatin Inhibits IL-1beta-Induced Apoptosis and Extracellular Matrix Degradation by Suppressing the NF-kB and MAPK Pathways in Nucleus Pulposus Cells. Inflammation 2017, 40, 725–734. [Google Scholar] [CrossRef]
- Tian, Y.; Yuan, W.; Fujita, N.; Wang, J.; Wang, H.; Shapiro, I.M.; Risbud, M.V. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-kappaB. Am. J. Pathol. 2013, 182, 2310–2321. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, R.; Yan, C.; Sun, K.; Gao, L.; Zheng, B.; Shi, J. Hesperidin mitigates oxidative stress-induced ferroptosis in nucleus pulposus cells via Nrf2/NF-κB axis to protect intervertebral disc from degeneration. Cell Cycle 2023, 22, 1196–1214. [Google Scholar] [CrossRef]
- Xie, T.; Yuan, J.; Mei, L.; Li, P.; Pan, R. Hyperoside ameliorates TNF-α-induced inflammation, ECM degradation and ER stress-mediated apoptosis via the SIRT1/NF-κB and Nrf2/ARE signaling pathways in vitro. Mol. Med. Rep. 2022, 26, 260. [Google Scholar] [CrossRef]
- Xie, C.; Ma, H.; Shi, Y.; Li, J.; Wu, H.; Wang, B.; Shao, Z.; Huang, C.; Chen, J.; Sun, L.; et al. Cardamonin protects nucleus pulposus cells against IL-1β-induced inflammation and catabolism via Nrf2/NF-κB axis. Food Funct. 2021, 12, 2703–2714. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, C.; Li, K.; Gao, K.; Fu, M.; Lyu, C.; Quan, Z. Sinomenine Ameliorates IL-1β-Induced Intervertebral Disc Degeneration in Rats Through Suppressing Inflammation and Oxidative Stress via Keap1/Nrf2/NF-κB Signaling Pathways. J. Inflamm. Res. 2023, 16, 4777–4791. [Google Scholar] [CrossRef]
- Xu, Y.; He, J.; He, J. Cyanidin attenuates the high hydrostatic pressure-induced degradation of cellular matrix of nucleus pulposus cell via blocking the Wnt/β-catenin signaling. Tissue Cell 2022, 76, 101798. [Google Scholar] [CrossRef]
- Tao, W.; Ruan, J.; Wu, R.; Zhao, M.; Zhao, T.; Qi, M.; Yau, S.S.; Yao, G.; Zhang, H.; Hu, Y.; et al. A natural carotenoid crocin exerts antidepressant action by promoting adult hippocampal neurogenesis through Wnt/β-catenin signaling. J. Adv. Res. 2023, 43, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, J.; Nisar, M.; Chen, T.; Xu, T.; Zheng, G.; Wang, C.; Jin, H.; Chen, J.; Gao, W.; et al. The SIRT1/P53 Axis in diabetic intervertebral disc degeneration pathogenesis and therapeutics. Oxid. Med. Cell Longev. 2019, 2019, 7959573. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jing, X.; Liu, X.; Chen, F.; Ge, Z.; Liu, X.; Yang, H.; Guo, Y.; Cui, X. Naringin safeguards vertebral endplate chondrocytes from apoptosis and NLRP3 inflammasome activation through SIRT3-mediated mitophagy. Int. Immunopharmacol. 2024, 140, 112801. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Liu, S.-C.; Xu, T.-X.; Liu, Y.; Zhang, T.; Liu, D.-J.; Wang, X.; Wang, J.-Y.; He, Y.-X.; Ma, T. Emodin Inhibits NLRP3 Inflammasome Activation and Protects Against Sepsis via Promoting FUNDC1-Mediated Mitophagy. Int. J. Biol. Sci. 2025, 21, 3631–3648. [Google Scholar] [CrossRef]
- Ding, H.; Li, Y.; Chen, S.; Wen, Y.; Zhang, S.; Luo, E.; Li, X.; Zhong, W.; Zeng, H. Fisetin ameliorates cognitive impairment by activating mitophagy and suppressing neuroinflammation in rats with sepsis-associated encephalopathy. CNS Neurosci. Ther. 2022, 28, 247–258. [Google Scholar] [CrossRef]
- Shang, P.; Tang, Q.; Hu, Z.; Huang, S.; Hu, Y.; Zhu, J.; Liu, H. Procyanidin B3 alleviates intervertebral disc degeneration via interaction with the TLR4/MD-2 complex. J. Cell Mol. Med. 2020, 24, 3701–3711. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, B.; Ni, F.; Han, Y.; Shu, S.; Xiong, L.; Shao, Z.; Wei, Y. Kongensin a attenuates intervertebral disc degeneration by inhibiting TAK1-mediated PANoptosis of nucleus pulposus cells. Int. Immunopharmacol. 2024, 129, 111661. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Z.; Wang, J.; Tang, C.; Khor, S.; Chen, J.; Chen, X.; Zhang, Z.; Tang, Q.; Wang, C.; et al. Berberine suppresses apoptosis and extracellular matrix (ECM) degradation in nucleus pulposus cells and ameliorates disc degeneration in a rodent model. Int. J. Biol. Sci. 2018, 14, 682–692. [Google Scholar] [CrossRef]
- Chen, T.; Li, P.; Qiu, J.; Hu, W.; Li, S.; Shi, H.; Qiu, X.; Huang, D.; Gao, W.; Liang, A. Aloin Regulates Matrix Metabolism and Apoptosis in Human Nucleus Pulposus Cells via the TAK1/NF-κB/NLRP3 Signaling Pathway. Stem Cells Int. 2022, 2022, 5865011. [Google Scholar] [CrossRef]
- Wang, L.; Gu, Y.; Zhao, H.; Chen, R.; Chen, W.; Qi, H.; Gao, W. Dioscin Attenuates Interleukin 1β (IL-1β)-Induced Catabolism and Apoptosis via Modulating the Toll-Like Receptor 4 (TLR4)/Nuclear Factor kappa B (NF-κB) Signaling in Human Nucleus Pulposus Cells. Med. Sci. Monit. 2020, 26, e923386. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xu, G.; Lin, Z.; Song, J.; Zhang, Y.; Wang, H.; Lu, F.; Xia, X.; Ma, X.; Zou, F.; et al. Sulforaphane Delays Intervertebral Disc Degeneration by Alleviating Endoplasmic Reticulum Stress in Nucleus Pulposus Cells via Activating Nrf-2/HO-1. Oxidative Med. Cell. Longev. 2023, 2023, 3626091. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Lian, Y.; Hu, C.; Yang, S.; Pei, B.; Yao, M.; Zhu, X.; Shang, L.; Li, Z. Cyanidin-3-glucoside protects against high glucose-induced injury in human nucleus pulposus cells by regulating the Nrf2/HO-1 signaling. J. Appl. Toxicol. 2022, 24, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Aleksandra Kozłowska, D.-W. Flavonoids-food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 65. [Google Scholar]
- Zhao, L.; Yuan, X.; Wang, J.; Feng, Y.; Ji, F.; Li, Z.; Bian, J. A review on flavones targeting serine/threonine protein kinases for potential anticancer drugs. Bioorg. Med. Chem. 2019, 27, 677–685. [Google Scholar] [CrossRef]
- Zhao, K.; Yuan, Y.; Lin, B.; Miao, Z.; Li, Z.; Guo, Q.; Lu, N. LW-215, a newly synthesized flavonoid, exhibits potent anti-angiogenic activity in vitro and in vivo. Gene 2018, 642, 533–541. [Google Scholar] [CrossRef]
- Camero, C.M.; Germanò, M.P.; Rapisarda, A.; D’aNgelo, V.; Amira, S.; Benchikh, F.; Braca, A.; De Leo, M. Anti-angiogenic activity of iridoids from Galium tunetanum. Rev. Bras. De. Farm. 2018, 28, 374–377. [Google Scholar] [CrossRef]
- Patel, K.; Kumar, V.; Rahman, M.; Verma, A.; Patel, D.K. New insights into the medicinal importance, physiological functions and bioanalytical aspects of an important bioactive compound of foods ‘Hyperin’: Health benefits of the past, the present, the future. Beni-Suef Univ. J. Basic. Appl. Sci. 2018, 7, 31–42. [Google Scholar] [CrossRef]
- Costăchescu, B.; Niculescu, A.-G.; Teleanu, R.I.; Iliescu, B.F.; Rădulescu, M.; Grumezescu, A.M.; Dabija, M.G. Recent Advances in Managing Spinal Intervertebral Discs Degeneration. Int. J. Mol. Sci. 2022, 23, 6460. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.-S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.-Y.; Xie, T.; Bai, R. Natural terpenoids with anti-inflammatory activities: Potential leads for anti-inflammatory drug discovery. Bioorg. Chem. 2022, 124, 105817. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qian, Q.; Xu, H.; Zhou, H.; Chen, L.; Shao, N.; Zhang, K.; Chen, T.; Tian, H.; Zhang, Z.; et al. Mitochondrial-Targeted Metal-Phenolic Nanoparticles to Attenuate Intervertebral Disc Degeneration: Alleviating Oxidative Stress and Mitochondrial Dysfunction. ACS Nano 2024, 18, 8885–8905. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity-An Update and Forward Look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef]
- Amirkia, V.; Heinrich, M. Alkaloids as drug leads—A predictive structural and biodiversity-based analysis. Phytochem. Lett. 2014, 2014, 10. [Google Scholar]
- Ren, J.; Barton, C.D.; Zhan, J. Engineered production of bioactive polyphenolic O-glycosides. Biotechnol. Adv. 2023, 65, 108146. [Google Scholar] [CrossRef]
- Johnson, J.B.; Mani, J.S.; Broszczak, D.; Prasad, S.S.; Ekanayake, C.P.; Strappe, P.; Valeris, P.; Naiker, M. Hitting the sweet spot: A systematic review of the bioactivity and health benefits of phenolic glycosides from medicinally used plants. Phytother. Res. 2021, 35, 3484–3508. [Google Scholar] [CrossRef]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Siddiqui, S.; Aftab, K.; Gilani, A.-U. Fully acetylated carbamate and hypotensive thiocarbamate glycosides from Moringa oleifera. Phytochemistry 1995, 38, 957–963. [Google Scholar] [CrossRef]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. The Anti-Inflammatory Properties of Terpenoids from Cannabis. Cannabis Cannabinoid Res. 2018, 3, 282–290. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Cortés, H.; Caballero-Florán, I.H.; González-Torres, M.; Escutia-Guadarrama, L.; Bernal-Chávez, S.A.; Giraldo-Gomez, D.M.; Magaña, J.J.; Leyva-Gómez, G. Therapeutic Applications of Terpenes on Inflammatory Diseases. Front. Pharmacol. 2021, 12, 704197. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, J.; Liu, H.; Fu, C. Natural products can modulate inflammation in intervertebral disc degeneration. Front. Pharmacol. 2023, 14, 1150835. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.-Y.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Xia, C.; Yang, C.; Chen, J.; Hai, L.; Wu, Y.; Yang, Z. Synthesis and evaluation of glycosylated quercetin to enhance neuroprotective effects on cerebral ischemia-reperfusion. Bioorg. Med. Chem. 2022, 73, 117008. [Google Scholar] [CrossRef] [PubMed]
- Gurung, R.B.; Gong, S.Y.; Dhakal, D.; Le, T.T.; Jung, N.R.; Jung, H.J.; Oh, T.J.; Sohng, J.K. Synthesis of Curcumin Glycosides with Enhanced Anticancer Properties Using One-Pot Multienzyme Glycosylation Technique. J. Microbiol. Biotechnol. 2017, 27, 1639–1648, Erratum in J. Microbiol. Biotechnol. 2017, 28, 347. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.E.; Mwesige, B.; Yi, Y.-S.; Yoo, B.C. Ginsenosides: The need to move forward from bench to clinical trials. J. Ginseng Res. 2019, 43, 361–367. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Ma, Z.; Zhao, J. Crocin exerts anti-inflammatory and anti-catabolic effects on rat intervertebral discs by suppressing the activation of JNK. Int. J. Mol. Med. 2015, 36, 1291–1299. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Mamoulakis, C.; Tsarouhas, K.; Georgiadis, G.; Lazopoulos, G.; Tsatsakis, A.; Asrami, E.S.; Rezaee, R. Crocin: A fighter against inflammation and pain. Food Chem. Toxicol. 2020, 143, 111521. [Google Scholar] [CrossRef]
- Boozari, M.; Hosseinzadeh, H. Crocin molecular signaling pathways at a glance: A comprehensive review. Phytother. Res. 2022, 36, 3859–3884. [Google Scholar] [CrossRef]
- Faisal, S.; Badshah, S.L.; Kubra, B.; Emwas, A.-H.; Jaremko, M. Alkaloids as potential antivirals. A comprehensive review. Nat. Prod. Bioprospect. 2023, 13, 4. [Google Scholar] [CrossRef]
- Adeosun, W.B.; Loots, D.T. Medicinal Plants against Viral Infections: A Review of Metabolomics Evidence for the Antiviral Properties and Potentials in Plant Sources. Viruses 2024, 16, 218. [Google Scholar] [CrossRef]
- Stepp, J.R.; Moerman, D.E. The importance of weeds in ethnopharmacology. J. Ethnopharmacol. 2001, 75, 19–23. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Alam Khan, S.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Kasolo, J.; Bimenya, G.S.; Ojok, L.; Ochieng, J.; Ogwal-Okeng, J. Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J. Med. Plants Res. 2010, 16, 753–757. [Google Scholar]
- Panda, S.; Kar, A.; Sharma, P.; Sharma, A. Cardioprotective potential of N,α-l-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: In vivo and in vitro studies. Bioorg. Med. Chem. Lett. 2013, 23, 959–962. [Google Scholar] [CrossRef]
- Liang, Q.-Q.; Ding, D.-F.; Xi, Z.-J.; Chen, Y.; Li, C.-G.; Liu, S.-F.; Lu, S.; Zhao, Y.-J.; Shi, Q.; Wang, Y.-J. Protective effect of ligustrazine on lumbar intervertebral disc degeneration of rats induced by prolonged upright posture. Evid. Based Complement. Altern. Med. 2014, 2014, 508461. [Google Scholar] [CrossRef]
- Zeng, P.; Yi, Y.; Su, H.-F.; Ye, C.-Y.; Sun, Y.-W.; Zhou, X.-W.; Lu, Y.; Shi, A.; Tian, Q. Key Phytochemicals and Biological Functions of Chuanxiong Rhizoma Against Ischemic Stroke: A Network Pharmacology and Experimental Assessment. Front. Pharmacol. 2021, 12, 758049. [Google Scholar] [CrossRef]
- Che, X.-W.; Zhang, Y.; Wang, H.; Wang, W. Effect of ligustrazine injection on levels of interleukin-4 and interferon-γ in patients with bronchial asthma. Chin. J. Integr. Med. 2008, 14, 217–220. [Google Scholar] [CrossRef]
- Hu, J.; Luo, C.Y.; Kang, M.; Lü, H.B.; Lei, G.H.; Dai, Z. Therapeutic effects of intraarticular injection of ligustrazine on knee osteoarthritis. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2006, 31, 591–594. [Google Scholar]
- Zhang, X.-P.; Liu, D.-R.; Shi, Y. Study progress in therapeutic effects of traditional Chinese medicine monomer in severe acute pancreatitis. J. Zhejiang Univ. Sci. B 2007, 8, 147–152. [Google Scholar] [CrossRef]
- Leng, Y.-F.; Gao, X.-M.; Wang, S.-X.; Xing, Y.-H. Effects of tetramethylpyrazine on neuronal apoptosis in the superficial dorsal horn in a rat model of neuropathic pain. Am. J. Chin. Med. 2012, 40, 1229–1239. [Google Scholar] [CrossRef]
- Ju, X.-D.; Deng, M.; Ao, Y.-F.; Yu, C.-L.; Wang, J.-Q.; Yu, J.-K.; Cui, G.-Q.; Hu, Y.-L. The protective effect of tetramethylpyrazine on cartilage explants and chondrocytes. J. Ethnopharmacol. 2010, 132, 414–420. [Google Scholar] [CrossRef]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet Flavonoids”: Glycosidase-Catalyzed Modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef]
- Wang, D.; He, X.; Wang, D.; Peng, P.; Xu, X.; Gao, B.; Zheng, C.; Wang, H.; Jia, H.; Shang, Q.; et al. Quercetin suppresses apoptosis and attenuates intervertebral disc degeneration via the SIRT1-autophagy pathway. Front. Cell Dev. Biol. 2020, 8, 613006. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, B.; Shi, Y.; Xie, C.; Huang, C.; Chen, B.; Zhang, H.; Zeng, G.; Liang, H.; Wu, Y.; et al. Senolytic agent quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-κB axis. Osteoarthr. Cart. 2021, 29, 413–422. [Google Scholar] [CrossRef]
- Zhao, W.-J.; Liu, X.; Hu, M.; Zhang, Y.; Shi, P.-Z.; Wang, J.-W.; Lu, X.-H.; Cheng, X.-F.; Tao, Y.-P.; Feng, X.-M.; et al. Quercetin ameliorates oxidative stress-induced senescence in rat nucleus pulposus-derived mesenchymal stem cells via the miR-34a-5p/SIRT1 axis. World J. Stem Cells 2023, 15, 842–865. [Google Scholar] [CrossRef]
- Ding, F.; Li, X. Apigenin mitigates intervertebral disc degeneration through the amelioration of tumor necrosis factor α (TNF-α) signaling pathway. Med. Sci. Monit. 2020, 26, 924587. [Google Scholar] [CrossRef]
- Padmavathi, G.; Roy, N.K.; Bordoloi, D.; Arfuso, F.; Mishra, S.; Sethi, G.; Bishayee, A.; Kunnumakkara, A.B. Butein in health and disease: A comprehensive review. Phytomedicine 2017, 25, 118–127. [Google Scholar] [CrossRef]
- Song, Z.; Shanmugam, M.K.; Yu, H.; Sethi, G. Butein and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 928, 419–433. [Google Scholar] [CrossRef]
- Jin, H.; Wang, Q.; Wu, J.; Han, X.; Qian, T.; Zhang, Z.; Wang, J.; Pan, X.; Wu, A.; Wang, X. Baicalein inhibits the IL-1β-induced inflammatory response in nucleus pulposus cells and attenuates disc degeneration in vivo. Inflammation 2019, 42, 1032–1044. [Google Scholar] [CrossRef]
- Wang, X.; Tan, Y.; Liu, F.; Wang, J.; Liu, F.; Zhang, Q.; Li, J. Pharmacological network analysis of the functions and mechanism of kaempferol from Du Zhong in intervertebral disc degeneration (IDD). J. Orthop. Transl. 2023, 39, 135–146. [Google Scholar] [CrossRef]
- Gao, W.; Bao, J.; Zhang, Y.; He, D.; Zhang, L.; Zhang, J.; Pan, H.; Wang, D. Injectable kaempferol-loaded fibrin glue regulates the metabolic balance and inhibits inflammation in intervertebral disc degeneration. Sci. Rep. 2023, 13, 20001. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, H.; Zhang, Z.; Zhang, Y.; Qiu, C.; Zhang, L.; Huang, P.; Li, F. Kaempferol slows intervertebral disc degeneration by modifying LPS-induced osteogenesis/adipogenesis imbalance and inflammation response in BMSCs. Int. Immunopharmacol. 2017, 43, 236–242. [Google Scholar] [CrossRef]
- Nepal, M.; Li, L.; Cho, H.K.; Park, J.K.; Soh, Y. Kaempferol induces chondrogenesis in ATDC5 cells through activation of ERK/BMP-2 signaling pathway. Food Chem. Toxicol. 2013, 62, 238. [Google Scholar] [CrossRef]
- Long, L.; Tang, X.; Wang, Y.; Gu, J.; Xiong, J.; Luo, H.; Lv, H.; Zhou, F.; Cao, K.; Lin, S. Network pharmacology and experimental validation to elucidate the pharmacological mechanisms of luteolin against chondrocyte senescence. Comb. Chem. High. Throughput Screen. 2025, 86, 163–170. [Google Scholar] [CrossRef]
- Xie, T.; Yuan, J.; Mei, L.; Li, P.; Pan, R. Luteolin suppresses TNF-α-induced inflammatory injury and senescence of nucleus pulposus cells via the Sirt6/NF-κB pathway. Exp. Ther. Med. 2022, 24, 469. [Google Scholar] [CrossRef]
- Lin, J.; Chen, J.; Zhang, Z.; Xu, T.; Shao, Z.; Wang, X.; Ding, Y.; Tian, N.; Jin, H.; Sheng, S.; et al. Luteoloside Inhibits IL-1β-Induced Apoptosis and Catabolism in Nucleus Pulposus Cells and Ameliorates Intervertebral Disk Degeneration. Front. Pharmacol. 2019, 10, 868. [Google Scholar] [CrossRef]
- Devraj, V.M.; Vemuri, S.K.; Banala, R.R.; Gunda, S.K.; Av, G.R.; Gpv, S. Evaluation of anti-inflammatory and regenerative efficiency of naringin and naringenin in degenerated human nucleus pulposus cells: Biological and molecular modeling studies. Asian Spine J. 2019, 13, 875–889. [Google Scholar] [CrossRef]
- Li, N.; Whitaker, C.; Xu, Z.; Heggeness, M.; Yang, S.-Y. Therapeutic effects of naringin on degenerative human nucleus pulposus cells for discogenic low back pain. Spine J. 2016, 16, 1231–1237. [Google Scholar] [CrossRef]
- Gao, G.; Chang, F.; Zhang, T.; Huang, X.; Yu, C.; Hu, Z.; Ji, M.; Duan, Y. Naringin protects against interleukin 1β (IL-1β)-induced human nucleus pulposus cells degeneration via downregulation nuclear factor kappa B (NF-κB) pathway and p53 expression. Med. Sci. Monit. 2019, 25, 9963–9972. [Google Scholar] [CrossRef]
- Chao-Yang, G.; Peng, C.; Hai-Hong, Z. Roles of NLRP3 inflammasome in intervertebral disc degeneration. Osteoarthr. Cart. 2021, 29, 793–801. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Shangguan, W.-J.; Zhao, Z.-J.; Zhou, F.-C.; Liu, H.-T.; Liang, Z.-H.; Song, J.; Shao, J. Naringin Inhibits Apoptosis Induced by Cyclic Stretch in Rat Annular Cells and Partially Attenuates Disc Degeneration by Inhibiting the ROS/NF-κB Pathway. Oxid. Med. Cell Longev. 2022, 2022, 6179444. [Google Scholar] [CrossRef]
- Tang, X.; Zhong, J.; Luo, H.; Zhou, F.; Wang, L.; Lin, S.; Xiong, J.; Lv, H.; Zhou, Z.; Yu, H.; et al. Efficacy of Naringenin against aging and degeneration of nucleus pulposus cells through IGFBP3 inhibition. Sci. Rep. 2025, 15, 6780. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Lin, J.; Jin, H.; Wang, K.; Yan, Y.; Wang, J.; Wu, C.; Nisar, M.; Tian, N.; et al. Therapeutic Potential of Naringin for Intervertebral Disc Degeneration: Involvement of Autophagy Against Oxidative Stress-Induced Apoptosis in Nucleus Pulposus Cells. Am. J. Chin. Med. 2018, 46, 1561–1580, Erratum in Am. J. Chin. Med. 2021, 49, 2049–2052. [Google Scholar] [CrossRef]
- Szabó, R.; Rácz, C.P.; Dulf, F.V. Bioavailability improvement strategies for icariin and its derivates: A review. Int. J. Mol. Sci. 2022, 23, 7519. [Google Scholar] [CrossRef]
- Wang, S.; Ma, J.; Zeng, Y.; Zhou, G.; Wang, Y.; Zhou, W.; Sun, X.; Wu, M. Icariin, an up-and-coming bioactive compound against neurological diseases: Network pharmacology-based study and literature review. Drug Des. Devel Ther. 2021, 15, 3619–3641. [Google Scholar] [CrossRef]
- Song, L.; Chen, X.; Mi, L.; Liu, C.; Zhu, S.; Yang, T.; Luo, X.; Zhang, Q.; Lu, H.; Liang, X. Icariin-induced inhibition of SIRT6/NF-κB triggers redox mediated apoptosis and enhances anti-tumor immunity in triple-negative breast cancer. Cancer Sci. 2020, 111, 4242–4256. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiong, Y.; Yang, T.; Wang, Y.; Zeng, J.; Zhou, S.; Luo, Y.; Li, L. Icariin and its metabolites as potential protective phytochemicals against cardiovascular disease: From effects to molecular mechanisms. Biomed. Pharmacother. 2022, 147, 112642. [Google Scholar] [CrossRef]
- Si, Y.; Li, Y.; Gu, K.; Yin, H.; Ma, Y. Icariin ameliorates osteoporosis in ovariectomized rats by targeting Cullin 3/Nrf2/OH pathway for osteoclast inhibition. Biomed. Pharmacother. 2024, 173, 116422. [Google Scholar] [CrossRef]
- Hua, W.; Zhang, Y.; Wu, X.; Kang, L.; Tu, J.; Zhao, K.; Li, S.; Wang, K.; Song, Y.; Luo, R.; et al. Icariin attenuates interleukin-1β-induced inflammatory response in human nucleus pulposus cells. Curr. Pharm. Des. 2018, 23, 6071–6078. [Google Scholar] [CrossRef]
- Hua, W.; Li, S.; Luo, R.; Wu, X.; Zhang, Y.; Liao, Z.; Song, Y.; Wang, K.; Zhao, K.; Yang, S.; et al. Icariin protects human nucleus pulposus cells from hydrogen peroxide-induced mitochondria-mediated apoptosis by activating nuclear factor erythroid 2-related factor 2. BBA-Mol. Basis Dis. 2020, 1866, 165575, Erratum in BBA-Mol. Basis Dis. 2020, 1866, 165718. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, L.; Yang, G.; Wang, W.; Liu, X.; Du, T.; Chen, F.; Jing, X.; Cui, X. Icariin protects vertebral endplate chondrocytes against apoptosis and degeneration via activating Nrf-2/HO-1 pathway. Front. Pharmacol. 2022, 13, 937502. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef]
- Wang, B.; Yang, L.-N.; Yang, L.-T.; Liang, Y.; Guo, F.; Fu, P.; Ma, L. Fisetin ameliorates fibrotic kidney disease in mice via inhibiting ACSL4-mediated tubular ferroptosis. ACTA Pharmacol. SIN 2024, 45, 150–165. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Y.; Nie, S.; Zhou, S.; Gao, X.; Chen, G. Biological effects and mechanisms of fisetin in cancer: A promising anti-cancer agent. Eur. J. Med. Res. 2023, 28, 297. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhu, C.; Xuan, A.; Zhang, J.; Zhu, Z.; Tang, L.; Ruan, D. Fisetin regulates the biological effects of rat nucleus pulposus mesenchymal stem cells under oxidative stress by sirtuin-1 pathway. Immun. Inflamm. Dis. 2023, 11, e865. [Google Scholar] [CrossRef]
- Wu, C.; Yan, J.; Li, W. Acacetin as a potential protective compound against cardiovascular diseases. Evid-Based Compl Alt. 2022, 2022, 6265198. [Google Scholar] [CrossRef]
- Zhang, G.; Dong, J.; Lu, L.; Liu, Y.; Hu, D.; Wu, Y.; Zhao, A.; Xu, H. Acacetin exerts antitumor effects on gastric cancer by targeting EGFR. Front. Pharmacol. 2023, 14, 1121643. [Google Scholar] [CrossRef]
- Bu, J.; Mahan, Y.; Zhang, S.; Wu, X.; Zhang, X.; Zhou, L.; Zhang, Y. Acacetin inhibits inflammation by blocking MAPK/NF-κB pathways and NLRP3 inflammasome activation. Front. Pharmacol. 2024, 15, 1286546. [Google Scholar] [CrossRef]
- Mu, J.; Chen, H.; Ye, M.; Zhang, X.; Ma, H. Acacetin resists UVA photoaging by mediating the SIRT3/ROS/MAPKs pathway. J. Cell Mol. Med. 2022, 26, 4624–4628. [Google Scholar] [CrossRef]
- Liou, C.-J.; Wu, S.-J.; Shen, S.-C.; Chen, L.-C.; Chen, Y.-L.; Huang, W.-C. Acacetin protects against non-alcoholic fatty liver disease by regulating lipid accumulation and inflammation in mice. Int. J. Mol. Sci. 2022, 23, 4687. [Google Scholar] [CrossRef]
- Li, S.; Lv, Q.; Sun, X.; Tang, T.; Deng, X.; Yin, Y.; Li, L. Acacetin inhibits Streptococcus pneumoniae virulence by targeting pneumolysin. J. Pharm. Pharmacol. 2020, 72, 1092–1100. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Z.; Pang, Z.; Zhou, T.; Gu, Y. Acacetin alleviates inflammation and matrix degradation in nucleus pulposus cells and ameliorates intervertebral disc degeneration in vivo. Drug Des. Devel Ther. 2020, 14, 4801–4813. [Google Scholar] [CrossRef]
- Pan, C.; Hou, W.; Deng, X.; Liu, J.; Chi, R.; Shang, X.; Xu, T.; Hao, X. The pivotal role of Nrf2 signal Axis in intervertebral disc degeneration. J. Inflamm. Res. 2023, 16, 5819–5833. [Google Scholar] [CrossRef]
- Fahmy, M.I.; Sadek, M.A.; Abdou, K.; El-Dessouki, A.M.; El-Shiekh, R.A.; Khalaf, S.S. Orientin: A comprehensive review of a promising bioactive flavonoid. Inflammopharmacology 2025, 33, 1713–1728. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z.; Yang, X.; Cao, X.; Liang, Z.; Ma, H.; Zhao, J. Morin attenuates pyroptosis of nucleus pulposus cells and ameliorates intervertebral disc degeneration via inhibition of the TXNIP/NLRP3/Caspase-1/IL-1β signaling pathway. Biochem. Biophys. Res. Commun. 2021, 559, 106–112. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Cheng, X.; Wei, H.; Li, P.; Fan, L.; Liu, K.; Zhang, S.; Wang, H. The antioxidant Glycitin protects against intervertebral disc degeneration through antagonizing inflammation and oxidative stress in nucleus pulposus cells. Aging 2023, 15, 13693–13709. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, J.; Li, S.; Shi, Y.; Yu, L.; Wu, A.; Wang, X. Isoliquiritigenin mitigates intervertebral disc degeneration induced by oxidative stress and mitochondrial impairment through a PPARγ-dependent pathway. Free Radic. Biol. Med. 2024, 225, 98–111. [Google Scholar] [CrossRef]
- Mao, T.; Fan, J. Myricetin Restores Autophagy to Attenuate Lumbar Intervertebral Disk Degeneration Via Negative Regulation of the JAK2/STAT3 Pathway. Biochem Genet 2025, 63, 2743–2759. [Google Scholar] [CrossRef]
- Hua, W.; Xie, L.; Dong, C.; Yang, G.; Chi, S.; Xu, Z.; Yang, C.; Wang, H.; Wu, X. Procyanidin C1 ameliorates acidic pH stress induced nucleus pulposus degeneration through SIRT3/FOXO3-mediated mitochondrial dynamics. J. Transl. Med. 2024, 22, 1071. [Google Scholar] [CrossRef]
- Krupkova, O.; Sekiguchi, M.; Klasen, J.; Hausmann, O.; Konno, S.; Ferguson, S.; Wuertz-Kozak, K. Epigallocatechin 3-gallate suppresses interleukin-1β-induced inflammatory responses in intervertebral disc cells in vitro and reduces radiculopathic pain in rats. Eur. Cell Mater. 2014, 28, 372–386. [Google Scholar] [CrossRef]
- Tian, Y.; Bao, Z.; Ji, Y.; Mei, X.; Yang, H. Epigallocatechin-3-Gallate Protects H2O2-Induced Nucleus Pulposus Cell Apoptosis and Inflammation by Inhibiting cGAS/Sting/NLRP3 Activation. Drug Des. Devel Ther. 2020, 14, 2113–2122. [Google Scholar] [CrossRef]
- Krupkova, O.; Handa, J.; Hlavna, M.; Klasen, J.; Ospelt, C.; Ferguson, S.J.; Wuertz-Kozak, K. The Natural Polyphenol Epigallocatechin Gallate Protects Intervertebral Disc Cells from Oxidative Stress. Oxid. Med. Cell Longev. 2016, 2016, 7031397. [Google Scholar] [CrossRef]
- Ishimoto, H.; Shibata, M.; Myojin, Y.; Ito, H.; Sugimoto, Y.; Tai, A.; Hatano, T. In vivo anti-inflammatory and antioxidant properties of ellagitannin metabolite urolithin A. Bioorg. Med. Chem. Lett. 2011, 21, 5901–5904. [Google Scholar] [CrossRef]
- Gimenez-Bastida, J.; Gonzalez-Sarrias, A.; Larrosa, M.; Tomas-Barberan, F.; Espin, J.C.; Garcia-Conesa, M.T. Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-alpha-induced inflammation and associated molecular markers in human aortic endothelial cells. Mol. Nutr. Food Res. 2012, 56, 784–796. [Google Scholar] [CrossRef]
- Liu, H.; Kang, H.; Song, C.; Lei, Z.; Li, L.; Guo, J.; Xu, Y.; Guan, H.; Fang, Z.; Li, F. Urolithin A Inhibits the Catabolic Effect of TNFalpha on Nucleus Pulposus Cell and Alleviates Intervertebral Disc Degeneration in vivo. Front. Pharmacol. 2018, 9, 1043. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Xu, B.; Mao, L.; Zhao, J. Sesamin inhibits lipopolysaccharide-induced inflammation and extracellular matrix catabolism in rat intervertebral disc. Connect. Tissue Res. 2016, 57, 347–359. [Google Scholar] [CrossRef]
- Li, K.; Lv, C. Intradiscal injection of sesamin protects from lesion-induced degeneration. Connect. Tissue Res. 2020, 61, 594–603. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Z.; Wu, Z.; Wu, Y. Therapeutic targeting of Nrf2/HO-1/NF-κB signaling axis with casticin mitigates intervertebral disc degeneration: In vitro and in vivo investigations. Vitr. Cell. Dev. Biol. Anim. 2025, 1–17. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Silwal, P.; Nguyen-Thai, A.M.; Mohammad, H.A.; Wang, Y.; Robbins, P.D.; Lee, J.Y.; Vo, N.V. Cellular Senescence in Intervertebral Disc Aging and Degeneration: Molecular Mechanisms and Potential Therapeutic Opportunities. Biomolecules 2023, 13, 686. [Google Scholar] [CrossRef]
- Kim, K.; Chung, H.N.; Ha, K.Y.; Lee, J.S.; Kim, Y.Y. Senescence mechanisms of nucleus pulposus chondrocytes in human intervertebral discs. Spine J. 2009, 9, 658–666. [Google Scholar] [CrossRef]
- Kim, K.-W.; Ha, K.-Y.; Lee, J.-S.; Na, K.-H.; Kim, Y.-Y.; Woo, Y.-K. Senescence of nucleus pulposus chondrocytes in human intervertebral discs. Asian Spine J. 2008, 2, 1–8. [Google Scholar] [CrossRef]
- Roberts, S.; Evans, E.H.; Kletsas, D.; Jaffray, D.C.; Eisenstein, S.M. Senescence in human intervertebral discs. Eur. Spine J. 2006, 15, S312–S316. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Y. Icariin, an Anti-atherosclerotic Drug from Chinese Medicinal Herb Horny Goat Weed. Front. Pharmacol. 2017, 8, 734. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.M.; Castilho, P.C. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules 2024, 29, 3861. [Google Scholar] [CrossRef]
- Zou, K.; Ying, J.; Xu, H.; Zeng, Q.; Huang, H.; Chen, W.; Li, X.; Wang, P.; Jin, H.; Li, J.; et al. Aucubin Alleviates Intervertebral Disc Degeneration by Repressing NF-κB-NLRP3 Inflammasome Activation in Endplate Chondrocytes. J. Inflamm. Res. 2023, 16, 5899–5913. [Google Scholar] [CrossRef]
- Zeng, X.; Guo, F.; Ouyang, D. A review of the pharmacology and toxicology of aucubin. Fitoterapia 2020, 140, 104443. [Google Scholar] [CrossRef]
- Li, B.; Lei, S.; Xiong, S.; Chen, S.; Zhang, Z. Pharmacokinetics and Pharmacodynamics of Morroniside: A Review. Nat. Prod. Commun. 2019, 14, 1934578X19856526. [Google Scholar] [CrossRef]
- Zhou, C.; Yao, S.; Fu, F.; Bian, Y.; Zhang, Z.; Zhang, H.; Luo, H.; Ge, Y.; Chen, Y.; Ji, W.; et al. Morroniside attenuates nucleus pulposus cell senescence to alleviate intervertebral disc degeneration via inhibiting ROS-Hippo-p53 pathway. Front. Pharmacol. 2022, 13, 942435. [Google Scholar] [CrossRef]
- Venkatesha, S.H.; Moudgil, K.D. Celastrol and Its Role in Controlling Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 267–289. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Li, L.; Chen, S.; Wang, L.; Li, Q.; Wang, X.; Lei, X.; Shen, Z. Natural Product Kongensin A is a Non-Canonical HSP90 Inhibitor that Blocks RIP3-dependent Necroptosis. Cell Chem. Biol. 2016, 23, 257–266. [Google Scholar] [CrossRef]
- Moriwaki, K.; Chan, F.K.-M. RIP3: A molecular switch for necrosis and inflammation. Genes. Dev. 2013, 27, 1640–1649. [Google Scholar] [CrossRef]
- Chen, J.; Xuan, J.; Gu, Y.-T.; Shi, K.-S.; Xie, J.-J.; Chen, J.-X.; Zheng, Z.-M.; Chen, Y.; Chen, X.-B.; Wu, Y.-S.; et al. Celastrol reduces IL-1β induced matrix catabolism, oxidative stress and inflammation in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration in vivo. Biomed. Pharmacother. 2017, 91, 208–219, Erratum in Biomed. Pharmacother. 2024, 181, 117631. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Ma, T.; Guo, C.-J.; Zhao, X.; Wu, L.; Sun, S.-X.; Jin, Q.-H. The effect of curcumin on NF-κB expression in rat with lumbar intervertebral disc degeneration. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1305–1314. [Google Scholar]
- Klawitter, M.; Quero, L.; Klasen, J.; Gloess, A.N.; Klopprogge, B.; Hausmann, O.; Boos, N.; Wuertz, K. Curcuma DMSO extracts and curcumin exhibit an anti-inflammatory and anti-catabolic effect on human intervertebral disc cells, possibly by influencing TLR2 expression and JNK activity. J. Inflamm. 2012, 9, 29. [Google Scholar] [CrossRef]
- Wan, C.; Liu, S.; Zhao, L.; Chang, C.; Li, H.; Li, R.; Chen, B. Curcumin protects rat endplate chondrocytes against IL-1β-induced apoptosis via Bcl-2/Bax regulation. J. Mol. Histol. 2025, 56, 111. [Google Scholar] [CrossRef]
- Mao, T.; Fan, J. Myricetin Protects Against Rat Intervertebral Disc Degeneration Partly Through the Nrf2/HO-1/NF-κB Signaling Pathway. Biochem. Genet. 2024, 62, 950–967. [Google Scholar] [CrossRef]
- Zhang, B.; He, Z.; Guo, J.; Li, F.; Huang, Z.; Zheng, W.; Xing, W.; Li, M.; Zhu, Y.; Yang, X. Sesamin-mediated high expression of BECN2 ameliorates cartilage endplate degeneration by reducing autophagy and inflammation. Aging 2024, 16, 1145–1160. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef]
- Du, G.-J.; Zhang, Z.; Wen, X.-D.; Yu, C.; Calway, T.; Yuan, C.-S.; Wang, C.-Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Basile, A.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin Gallate (EGCG): Pharmacological Properties, Biological Activities and Therapeutic Potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Luan, Y.; Li, X.; Meng, X.; Liao, W.; Tang, J.; Wang, Z. cGAS-STING, inflammasomes and pyroptosis: An overview of crosstalk mechanism of activation and regulation. Cell Commun. Signal. 2024, 22, 22. [Google Scholar] [CrossRef]
- de Morais Junior, A.; Junior, A.C.d.M.; Schincaglia, R.M.; Passarelli, M.; Pimentel, G.D.; Mota, J.F. Acute Epigallocatechin-3-Gallate Supplementation Alters Postprandial Lipids after a Fast-Food Meal in Healthy Young Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2020, 12, 2533. [Google Scholar] [CrossRef]
- Choi, E.; Rha, C.S.; Balusamy, S.R.; Kim, D.O.; Shim, S.M. Impact of Bioconversion of Gallated Catechins and Flavonol Glycosides on Bioaccessibility and Intestinal Cellular Uptake of Catechins. J. Agric. Food Chem. 2019, 67, 2331–2339. [Google Scholar] [CrossRef]
- Chen, H.; Sang, S. Biotransformation of tea polyphenols by gutmicrobiota. J. Funct. Foods 2014, 7, 26–42. [Google Scholar] [CrossRef]
- Guo, T.; Song, D.; Cheng, L.; Zhang, X. Interactions of tea catechins with intestinal microbiota and their implication for human health. Food Sci. Biotechnol. 2019, 28, 1617–1625. [Google Scholar] [CrossRef]
- Su, Y.; Hu, K.; Li, D.; Guo, H.; Sun, L.; Xie, Z. Microbial-Transferred Metabolites and Improvement of Biological Activities of Green Tea Catechins by Human Gut Microbiota. Foods 2024, 13, 792. [Google Scholar] [CrossRef]
- Xie, H.-F.; Kong, Y.-S.; Li, R.-Z.; Nothias, L.-F.; Melnik, A.V.; Zhang, H.; Liu, L.-L.; An, T.-T.; Liu, R.; Yang, Z.; et al. Feature-Based Molecular Networking Analysis of the Metabolites Produced by In Vitro Solid-State Fermentation Reveals Pathways for the Bioconversion of Epigallocatechin Gallate. J. Agric. Food Chem. 2020, 68, 7995–8007. [Google Scholar] [CrossRef]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, e2101019. [Google Scholar] [CrossRef]
- Kohri, T.; Matsumoto, N.; Yamakawa, M.; Suzuki, M.; Nanjo, F.; Hara, Y.; Oku, N. Metabolic fate of (-)-[4-3H]epigallocatechin gallate in rats after oral administration. J. Agric. Food Chem. 2001, 49, 4102–4112. [Google Scholar] [CrossRef]
- Huo, Y.; Yang, D.; Lai, K.; Tu, J.; Zhu, Y.; Ding, W.; Yang, S. Antioxidant Effects of Resveratrol in Intervertebral Disk. J. Invest. Surg. 2022, 35, 1135–1144. [Google Scholar] [CrossRef]
- Li, X.; Phillips, F.M.; An, H.S.; Ellman, M.; Thonar, E.J.; Wu, W.; Park, D.; Im, H.-J. The action of resveratrol, a phytoestrogen found in grapes, on the intervertebral disc. Spine 2008, 33, 2586–2595. [Google Scholar] [CrossRef]
- Kwon, Y. Resveratrol has anabolic effects on disc degeneration in a rabbit model. J. Korean Med. Sci. 2013, 28, 939–945. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Zang, W.; Zhang, K.; Li, H.; Gao, Y. Pharmacological Effects of Resveratrol in Intervertebral Disc Degeneration: A Literature Review. Orthop. Surg. 2022, 14, 3141–3149. [Google Scholar] [CrossRef]
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Dębski, H. Anthocyanins of fruits and vegetables-their occurrence, analysis and role in human nutrition. Veg. Crops Res. Bull. 2008, 68, 5–22. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Plotnikova, T.M. Tyrosol as a Neuroprotector: Strong Effects of a “Weak” Antioxidant. Curr. Neuropharmacol. 2021, 19, 434–448. [Google Scholar] [CrossRef]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Mohd Yusof, Y. Gingerol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 177–207. [Google Scholar] [CrossRef]
- Xi, H.; Weng, Y.; Zheng, Y.; Wu, L.; Han, D. Diacetoxy-6-gingerdiol protects the extracellular matrix of nucleus pulposus cells and ameliorates intervertebral disc degeneration by inhibiting the IL-1β-mediated NLRP3 pathway. Heliyon 2024, 10, e37877. [Google Scholar] [CrossRef]
- Jiang, X.; Li, S.; Wang, N.; Li, J. Ligustrazine as a multitarget scaffold in drug design and discovery. Bioorg. Med. Chem. 2025, 121, 118110. [Google Scholar] [CrossRef]
- Zou, J.; Gao, P.; Hao, X.; Xu, H.; Zhan, P.; Liu, X. Recent progress in the structural modification and pharmacological activities of ligustrazine derivatives. Eur. J. Med. Chem. 2018, 147, 150–162. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, Y.; Tan, Y.; Dong, J.; Bian, Q. Ligustrazine Prevents Intervertebral Disc Degeneration via Suppression of Aberrant TGFβ Activation in Nucleus Pulposus Cells. Biomed. Res. Int. 2019, 2019, 5601734. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, B.; Shi, H.; Liang, Q.; Fu, Y.; Yang, Z.; Xu, L.; Wang, Y.; Bian, Q. Ligustrazine Inhibits Cartilage Endplate Hypertrophy via Suppression of TGF-β1. Evid. Based Complement. Altern. Med. 2016, 2016, 1042489. [Google Scholar] [CrossRef]
- Wang, K.; Yin, J.; Chen, J.; Ma, J.; Si, H.; Xia, D. Inhibition of inflammation by berberine: Molecular mechanism and network pharmacology analysis. Phytomedicine 2024, 128, 155258. [Google Scholar] [CrossRef]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef]
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Barrea, L.; Hassan, S.T.S.; Šmejkal, K.; Malaník, M.; et al. Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics. Theranostics 2019, 9, 1923–1951. [Google Scholar] [CrossRef]
- Gasmi, A.; Asghar, F.; Zafar, S.; Oliinyk, P.; Khavrona, O.; Lysiuk, R.; Peana, M.; Piscopo, S.; Antonyak, H.; Pen, J.J.; et al. Berberine: Pharmacological Features in Health, Disease and Aging. Curr. Med. Chem. 2024, 31, 1214–1234. [Google Scholar] [CrossRef]
- Cicero, A.; Baggioni, A. Berberine and Its Role in Chronic Disease. Adv. Exp. Med. Biol. 2016, 928, 27–45. [Google Scholar] [CrossRef]
- Luo, R.; Liao, Z.; Song, Y.; Yin, H.; Zhan, S.; Li, G.; Ma, L.; Lu, S.; Wang, K.; Li, S.; et al. Berberine ameliorates oxidative stress-induced apoptosis by modulating ER stress and autophagy in human nucleus pulposus cells. Life Sci. 2019, 228, 85–97. [Google Scholar] [CrossRef]
- Lu, L.; Hu, J.; Wu, Q.; An, Y.; Cui, W.; Wang, J.; Ye, Z. Berberine prevents human nucleus pulposus cells from IL-1β induced extracellular matrix degradation and apoptosis by inhibiting the NFκB pathway. Int. J. Mol. Med. 2019, 43, 1679–1686. [Google Scholar] [CrossRef]
- Jiang, S.; Li, S.; Pang, S.; Liu, M.; Sun, H.; Zhang, N.; Liu, J. A systematic review: Sinomenine. Heliyon 2024, 10, e29976. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, W.; Gao, T.; Li, T.; Yin, Z.; Guo, H.; Wang, L.; Han, Y.; Jiang, J.-D. Analgesic Mechanism of Sinomenine against Chronic Pain. Pain Res. Manag. 2020, 2020, 1876862. [Google Scholar] [CrossRef]
- Gao, Z.; Lin, Y.; Zhang, P.; Cheng, Q.; Ye, L.; Wu, F.; Chen, Y.; Fu, M.; Cheng, C.; Gao, Y. Sinomenine ameliorates intervertebral disc degeneration via inhibition of apoptosis and autophagy in vitro and in vivo. Am. J. Transl. Res. 2019, 11, 5956–5966. [Google Scholar]
- Zhang, N.; Lian, Z.; Peng, X.; Li, Z.; Zhu, H. Applications of Higenamine in pharmacology and medicine. J. Ethnopharmacol. 2017, 196, 242–252. [Google Scholar] [CrossRef]
- Bai, X.; Ding, W.; Yang, S.; Guo, X. Higenamine inhibits IL-1β-induced inflammation in human nucleus pulposus cells. Biosci Rep. 2019, 39, BSR20190857. [Google Scholar] [CrossRef]
- Ju, Y.; Wang, C.-M.; Yu, J.-J.; Li, X.; Qi, M.-X.; Ren, J.; Wang, Y.; Liu, P.; Zhou, Y.; Ma, Y.-X.; et al. Higenamine inhibits acute and chronic inflammatory pain through modulation of TRPV4 channels. Eur. J. Pharmacol. 2024, 964, 176295. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, Q.; Li, D.; Li, Y.; Jiang, X. Evodiamine: A Privileged Structure with Broad-ranging Biological Activities. Mini Rev. Med. Chem. 2022, 22, 2680–2701. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Song, J.; Zhong, L.; Liao, Y.; Liu, L.; Li, X. Palmatine: A review of its pharmacology, toxicity and pharmacokinetics. Biochimie 2019, 162, 176–184. [Google Scholar] [CrossRef]
- Napolitano, G.; Ballabio, A. TFEB at a glance. J. Cell Sci. 2016, 129, 2475–2481. [Google Scholar] [CrossRef]
- Yu, H.; Chen, K.; Li, X.; Liang, J.; Jin, Y.; Bao, Y.; Chen, H.; Gou, Y.; Lu, K.; Wu, L.; et al. Palmatine activation of TFEB enhances autophagy and alleviates endoplasmic reticulum stress in intervertebral disc degeneration. Phytomedicine 2025, 139, 156431. [Google Scholar] [CrossRef]
- Chen, M.; Dai, Y.; Liu, S.; Fan, Y.; Ding, Z.; Li, D. TFEB Biology and Agonists at a Glance. Cells 2021, 10, 333. [Google Scholar] [CrossRef]
- Shi, Z.; Zeng, J.; Wong, A. Chemical structures and pharmacological profiles of ginseng saponins. Molecules 2019, 24, 2443. [Google Scholar] [CrossRef]
- Metwaly, A.M.; Lianlian, Z.; Luqi, H.; Deqiang, D. Black ginseng and its saponins: Preparation, phytochemistry and pharmacological effects. Molecules 2019, 24, 1856. [Google Scholar] [CrossRef]
- Yu, L.; Hao, Y.; Peng, C.; Zhang, P.; Zhu, J.; Cai, Y.; Zhu, G. Effect of Ginsenoside Rg1 on the intervertebral disc degeneration rats and the degenerative pulposus cells and its mechanism. Biomed. Pharmacother. 2020, 123, 109738. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Gu, X.-P.; Hu, H.; Hu, B.; Wan, X.-L.; Gu, Z.-P.; Zhong, S.-J. Ginsenoside Rg1 inhibits nucleus pulposus cell apoptosis, inflammation and extracellular matrix degradation via the YAP1/TAZ pathway in rats with intervertebral disc degeneration. J. Orthop. Surg. Res. 2022, 17, 555. [Google Scholar] [CrossRef]
- Yu, L.; Hao, Y.-J.; Ren, Z.-N.; Zhu, G.-D.; Zhou, W.-W.; Lian, X.; Wu, X.-J. Ginsenoside Rg1 relieves rat intervertebral disc degeneration and inhibits IL-1β-induced nucleus pulposus cell apoptosis and inflammation via NF-κB signaling pathway. Vitr. Cell. Dev. Biol. Anim. 2024, 60, 287–299. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, B.; Wu, L.; Xu, J. Ginsenoside Rg3 exhibits anti-catabolic and anti-apoptotic effects in IL-1β treated human disc nucleus pulposus cells and in a rat model of disc degeneration by inactivating the MAPK pathway. Cell Mol. Biol. 2024, 70, 233–238. [Google Scholar] [CrossRef]
- Tang, K.; Su, W.; Huang, C.; Wu, Y.; Wu, X.; Lu, H. Notoginsenoside R1 suppresses inflammatory response and the pyroptosis of nucleus pulposus cells via inactivating NF-κB/NLRP3 pathways. Int. Immunopharmacol. 2021, 101, 107866. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xi, X.; Li, J. Notoginsenoside R1: A systematic review of its pharmacological properties. Die Pharm. Int. J. Pharm. Sci. 2019, 74, 641–647. [Google Scholar] [CrossRef]
- Chen, D.; Fan, T.; Sun, K.; Rao, W.; Sheng, X.; Wan, Z.; Shu, B.; Chen, L. Network pharmacology and experimental validation to reveal the pharmacological mechanisms of Astragaloside IV in treating intervertebral disc degeneration. Eur. J. Pharmacol. 2024, 982, 176951. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, J.; Sun, Y.; Liu, G.; Wei, X. ANXA5: Related mechanisms of osteogenesis and additional biological functions. Front. Cell Dev. Biol. 2025, 13, 1553683. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Anand, U.; Gadekar, V.S.; Jha, N.K.; Gupta, P.K.; Behl, T.; Kumar, M.; Radha; Shekhawat, M.S.; Dey, A. Dioscin: A review on pharmacological properties and therapeutic values. Biofactors 2022, 48, 22–55. [Google Scholar] [CrossRef]
- Heydari, M.; Zare, M.; Badie, M.R.; Watson, R.R.; Talebnejad, M.R.; Afarid, M. Crocin as a vision supplement. Clin. Exp. Optom. 2023, 106, 249–256. [Google Scholar] [CrossRef]
- Pourmousavi, L.; Asadi, R.H.; Zehsaz, F.; Jadidi, R.P. Potential therapeutic effects of crocin. Naunyn-Schmiedeberg Arch. Pharmacol. 2024, 397, 7395–7420. [Google Scholar] [CrossRef]
- Chen, J.; Liu, G.-Z.; Sun, Q.; Zhang, F.; Liu, C.-Y.; Yuan, L.; Zhao, X.-Q.; Wang, Y.-J.; Jia, Y.-S. Protective effects of ginsenoside Rg3 on TNF-α-induced human nucleus pulposus cells through inhibiting NF-κB signaling pathway. Life Sci. 2019, 216, 1–9. [Google Scholar] [CrossRef]
- Tian, Y.; Chu, X.; Huang, Q.; Guo, X.; Xue, Y.; Deng, W. Astragaloside IV attenuates IL-1β-induced intervertebral disc degeneration through inhibition of the NF-κB pathway. J. Orthop. Surg. Res. 2022, 17, 545. [Google Scholar] [CrossRef]
- Hong, H.; Xiao, J.; Guo, Q.; Du, J.; Jiang, Z.; Lu, S.; Zhang, H.; Zhang, X.; Wang, X. Cycloastragenol and Astragaloside IV activate telomerase and protect nucleus pulposus cells against high glucose-induced senescence and apoptosis. Exp. Ther. Med. 2021, 22, 1326. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Li, M.; Wang, K.; Jin, S.; Zeng, W.; Liao, Z.; Chen, E.; Liang, Y.; Xing, T.; Wen, G.; et al. Emodin ameliorates matrix degradation and apoptosis in nucleus pulposus cells and attenuates intervertebral disc degeneration through LRP1 in vitro and in vivo. Exp. Cell Res. 2023, 432, 113794. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Guo, S.; Zhang, M.; Bai, X. Emodin protects against apoptosis and inflammation by regulating reactive oxygen species-mediated NF-κB signaling in interleukin-1β-stimulated human nucleus pulposus cells. Hum. Exp. Toxicol. 2023, 42, 9603271221138552. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, C.; Chen, Q.; Yang, Z. Rhein: A potential biological therapeutic drug for intervertebral disc degeneration. Med. Hypotheses 2011, 77, 1105–1107. [Google Scholar] [CrossRef]
- Sun, H.; Luo, G.; Chen, D.; Xiang, Z. A Comprehensive and System Review for the Pharmacological Mechanism of Action of Rhein, an Active Anthraquinone Ingredient. Front. Pharmacol. 2016, 7, 247. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Xia, W.; Yue, W.; Peng, C.; Rahman, K.; Zhang, H. Rhein: A Review of Pharmacological Activities. Evid. Based Complement. Altern. Med. 2015, 2015, 578107. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, L.; Liu, L.; Kong, L.; Sun, H.; Sun, Y.; Yin, F.; Yan, G.; Wang, X. Rhein: An Updated Review Concerning Its Biological Activity, Pharmacokinetics, Structure Optimization, and Future Pharmaceutical Applications. Pharmaceuticals 2024, 17, 1665. [Google Scholar] [CrossRef]
- Trybus, W.; Król, T.; Trybus, E.; Stachurska, A. Physcion Induces Potential Anticancer Effects in Cervical Cancer Cells. Cells 2021, 10, 2029. [Google Scholar] [CrossRef]
- Adnan, M.; Rasul, A.; Hussain, G.; Shah, M.A.; Sarfraz, I.; Nageen, B.; Riaz, A.; Khalid, R.; Asrar, M.; Selamoglu, Z.; et al. Physcion and Physcion 8-O-β-D-glucopyranoside: Natural Anthraquinones with Potential Anticancer Activities. Curr. Drug Targets 2021, 22, 488–504. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, S.; Yang, S.; Peng, Y.; Ren, S.; Wen, B.; Chen, N. Physcion and physcion 8-O-β-glucopyranoside: A review of their pharmacology, toxicities and pharmacokinetics. Chem. Biol. Interact. 2019, 310, 108722. [Google Scholar] [CrossRef]
- Endo, A. The origin of the statins. Atheroscler. Suppl. 2004, 5, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C. The pharmacology of statins. Pharmacol. Res. 2014, 88, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, A.; Osiński, P.; Roztocka, A.; Kaczmarz-Chojnacka, K.; Zapora, E.; Sawicka, D.; Car, H. Statins-From Fungi to Pharmacy. Int. J. Mol. Sci. 2023, 25, 466. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, R.; Sahai, V. Compactin-a review. Appl. Microbiol. Biotechnol. 2004, 64, 618–624. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Liu, X.; Ma, Z.; Zhang, Q.; Zhang, Y.; An, J.; Luo, Z. The protective effect and experimental research progress of pleotropic statins in intervertebral disc degeneration. J. Orthop. Surg. Res. 2025, 20, 122. [Google Scholar] [CrossRef]
- Gologorsky, Y.; Chi, J. Statins for disc degeneration. Neurosurgery 2014, 74, N18–N19. [Google Scholar] [CrossRef]
- Hu, M.-H.; Yang, K.-C.; Chen, Y.-J.; Sun, Y.-H.; Yang, S.-H. Lovastatin prevents discography-associated degeneration and maintains the functional morphology of intervertebral discs. Spine J. 2014, 14, 2459–2466. [Google Scholar] [CrossRef]
- Than, K.D.; Rahman, S.U.; Wang, L.; Khan, A.; Kyere, K.A.; Than, T.T.; Miyata, Y.; Park, Y.-S.; La Marca, F.; Kim, H.M.; et al. Intradiscal injection of simvastatin results in radiologic, histologic, and genetic evidence of disc regeneration in a rat model of degenerative disc disease. Spine J. 2014, 14, 1017–1028. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.-Y. Simvastatin stimulates chondrogenic phenotype of intervertebral disc cells partially through BMP-2 pathway. Spine 2008, 33, E525–E531. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Q.; Li, K.; Fu, M.; Gao, K.; Lv, C. Rosuvastatin: A Potential Therapeutic Agent for Inhibition of Mechanical Pressure-Induced Intervertebral Disc Degeneration. J. Inflamm. Res. 2024, 17, 3825–3838. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Xia, P.; Cheng, K.; Wang, Q.; Wang, X.; Lin, Q.; Song, J.; Chen, A.; Li, X. Ultrasound-targeted simvastatin-loaded microbubble destruction promotes OA cartilage repair by modulating the cholesterol efflux pathway mediated by PPARgamma in rabbits. Bone Jt. Res. 2021, 10, 693–703, Erratum in Bone Jt. Res. 2022, 11, 251. [Google Scholar] [CrossRef]
- Wamer, W.G.; Vath, P.; Falvey, D.E. In vitro studies on the photobiological properties of aloe emodin and aloin A. Free Rad. Biol. Med. 2003, 34, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, S.; Chen, Y.; Su, J. The potential health benefits of aloin from genus Aloe. Phytother. Res. 2022, 36, 873–890. [Google Scholar] [CrossRef] [PubMed]
- Ussia, S.; Ritorto, G.; Mollace, R.; Serra, M.; Tavernese, A.; Altomare, C.; Muscoli, C.; Fini, M.; Barillà, F.; Indolfi, C.; et al. Exploring the Benefits of Extra Virgin Olive Oil on Cardiovascular Health Enhancement and Disease Prevention: A Systematic Review. Nutrients 2025, 17, 1843. [Google Scholar] [CrossRef]
- Chen, J.; Hou, C.; Chen, X.; Wang, D.; Yang, P.; He, X.; Zhou, J.; Li, H. Protective effect of cannabidiol on hydrogen peroxide induced apoptosis, inflammation and oxidative stress in nucleus pulposus cells. Mol. Med. Rep. 2016, 14, 2321–2327. [Google Scholar] [CrossRef]
- Silveira, J.W.; Issy, A.C.; Castania, V.A.; Salmon, C.E.G.; Nogueira-Barbosa, M.H.; Guimarães, F.S.; Defino, H.L.A.; Del Bel, E. Protective effects of cannabidiol on lesion-induced intervertebral disc degeneration. PLoS ONE 2014, 9, e113161. [Google Scholar] [CrossRef]
- Dmytriv, T.; Lushchak, O.; Lushchak, V.I. Glucoraphanin conversion into sulforaphane and related compounds by gut microbiota. Front. Physiol. 2025, 16, 1497566. [Google Scholar] [CrossRef]
- Qin, T.; Shi, M.; Xie, Y.; Feng, N.; Liu, C.; Chen, K.; Chen, Y.; Zheng, W.; Zhu, M.; Peng, S.; et al. Activation of LAMP1-mediated lipophagy by sulforaphane inhibits cellular senescence and intervertebral disc degeneration. J. Orthop. Transl. 2025, 53, 12–25. [Google Scholar] [CrossRef]
- Ruiz-Fernández, C.; Francisco, V.; Pino, J.; Mera, A.; González-Gay, M.A.; Gómez, R.; Lago, F.; Gualillo, O. Molecular relationships among obesity, inflammation and intervertebral disc degeneration: Are adipokines the common link? Int. J. Mol. Sci. 2019, 20, 2030. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; González-Gay, M.Á.; Lago, F.; Karppinen, J.; Tervonen, O.; Mobasheri, A.; Gualillo, O. A new immunometabolic perspective of intervertebral disc degeneration. Nat. Rev. Rheumatol. 2022, 18, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Bing, T.; Shanlin, X.; Jisheng, W.; Jie, H.; Ruichao, C.; Zhiwei, Z.; Bin, Y.; Zhaoxin, M.; Zhenming, H.; Nian, Z. Dysregulated lipid metabolism and intervertebral disc degeneration: The important role of ox-LDL/LOX-1 in endplate chondrocyte senescence and calcification. Mol. Med. 2024, 30, 117. [Google Scholar] [CrossRef] [PubMed]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxid. Med. Cell Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Panahi, Y.; Johnston, T.P.; Sahebkar, A. Antidiabetic drugs and oxidized low-density lipoprotein: A review of anti-atherosclerotic mechanisms. Pharmacol. Res. 2021, 172, 105819. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Hu, Z.; Chen, Z.; Li, H.; Liu, X.; Yong, Z.Y.; Wang, S.; Wei, Z.; Han, Y.; et al. Possible involvement of the oxLDL/LOX-1 system in the pathogenesis and progression of human intervertebral disc degeneration or herniation. Sci. Rep. 2017, 7, 7403. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Yan, M.; Yang, M.; Wang, S.; Pan, J.; Li, L.; Tan, J. Oxidative damage induces apoptosis and promotes calcification in disc cartilage endplate cell through ROS/MAPK/NF-κB pathway: Implications for disc degeneration. Biochem. Biophys. Res. Commun. 2019, 516, 1026–1032. [Google Scholar] [CrossRef]
- Zhang, Y.; Si, M.; Li, C.; Liu, Y.; Han, Y.; Nie, L.; Wang, M. Effect of hyperlipidaemia to accelerate intervertebral disc degeneration in the injured rat caudal disc model. J. Orthop. Sci. 2019, 24, 42–49. [Google Scholar] [CrossRef]
- Poorani, R.; Bhatt, A.N.; Dwarakanath, B.; Das, U.N. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur. J. Pharmacol. 2016, 785, 116–132. [Google Scholar] [CrossRef]
- Kumar, R.; Clerc, A.C.; Gori, I.; Russell, R.; Pellegrini, C.; Govender, L.; Wyss, J.C.; Golshayan, D.; O Canny, G. Lipoxin A4 Prevents the Progression of De Novo and Established Endometriosis in a Mouse Model by Attenuating Prostaglandin E2 Production and Estrogen Signaling. R6. J. Inflamm. Res. 2014, 8, 181–192. [Google Scholar] [CrossRef]
- Chandrasekharan, J.; Sharma-Walia, N. Lipoxins: Nature’s way to resolve inflammation. J. Inflamm. Res. 2015, 8, 181–192. [Google Scholar] [CrossRef]
- Miao, G.-S.; Liu, Z.-H.; Wei, S.-X.; Luo, J.-G.; Fu, Z.-J.; Sun, T. Lipoxin A4 attenuates radicular pain possibly by inhibiting spinal ERK, JNK and NF-κB/p65 and cytokine signals, but not p38, in a rat model of non-compressive lumbar disc herniation. Neuroscience 2015, 300, 10–18. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Miao, G.-S.; Wang, J.-N.; Yang, C.-X.; Fu, Z.-J.; Sun, T. Resolvin D1 Inhibits Mechanical Hypersensitivity in Sciatica by Modulating the Expression of Nuclear Factor-κB, Phospho-extracellular Signal-regulated Kinase, and Pro- and Antiinflammatory Cytokines in the Spinal Cord and Dorsal Root Ganglion. Anesthesiology 2016, 124, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-C.; Zhang, Y.; Gao, X.; Li, L.-X.; Tang, Y.-R.; Wang, Y.-H. Protectin D1 ameliorates non-compressive lumbar disc herniation through SIRT1-mediated CGRP signaling. Mol. Pain 2024, 20, 17448069241232349. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Li, Y.; Wang, J.-N.; Zhao, Q.-X.; Jin, J.; Wen, S.; Wang, S.-C.; Sun, T. Maresin 1 Attenuates Radicular Pain Through the Inhibition of NLRP3 Inflammasome-Induced Pyroptosis via NF-κB Signaling. Front. Neurosci. 2020, 14, 831. [Google Scholar] [CrossRef] [PubMed]
- Lopategi, A.; Flores-Costa, R.; Rius, B.; López-Vicario, C.; Alcaraz-Quiles, J.; Titos, E.; Clària, J. Frontline Science: Specialized proresolving lipid mediators inhibit the priming and activation of the macrophage NLRP3 inflammasome. J. Leukoc. Biol. 2019, 105, 25–36. [Google Scholar] [CrossRef]
- Li, J.; Guan, H.; Liu, H.; Zhao, L.; Li, L.; Zhang, Y.; Tan, P.; Mi, B.; Li, F. Epoxyeicosanoids prevent intervertebral disc degeneration in vitro and in vivo. Oncotarget 2017, 8, 3781–3797. [Google Scholar] [CrossRef]
- Das, U. Lipoxins, resolvins, protectins, maresins and nitrolipids and their clinical implications with specific reference to cancer: Part I. Clin. Lipidol. 2013, 8, 437–463. [Google Scholar] [CrossRef]
- Das, U. Lipoxins, resolvins, protectins, maresins and nitrolipids and their clinical implications with specific reference to diabetes mellitus and other diseases: Part II. Clin. Lipidol. 2013, 8, 465–480. [Google Scholar] [CrossRef]
- Dai, Z.; Xia, C.; Zhao, T.; Wang, H.; Tian, H.; Xu, O.; Zhu, X.; Zhang, J.; Chen, P. Platelet-derived extracellular vesicles ameliorate intervertebral disc degeneration by alleviating mitochondrial dysfunction. Mater. Today Bio 2023, 18, 100512. [Google Scholar] [CrossRef]
- Kang, L.; Liu, S.; Li, J.; Tian, Y.; Xue, Y.; Liu, X. The mitochondria-targeted anti-oxidant MitoQ protects against intervertebral disc degeneration by ameliorating mitochondrial dysfunction and redox imbalance. Cell Prolif. 2020, 53, e12779. [Google Scholar] [CrossRef]
- Lin, J.; Zhuge, J.; Zheng, X.; Wu, Y.; Zhang, Z.; Xu, T.; Meftah, Z.; Xu, H.; Wu, Y.; Tian, N.; et al. Urolithin A-induced mitophagy suppresses apoptosis and attenuates intervertebral disc degeneration via the AMPK signaling pathway. Free Radic. Biol. Med. 2020, 150, 109–119. [Google Scholar] [CrossRef]
- Song, Y.; Li, S.; Geng, W.; Luo, R.; Liu, W.; Tu, J.; Wang, K.; Kang, L.; Yin, H.; Wu, X.; et al. Sirtuin 3-dependent mitochondrial redox homeostasis protects against AGEs-induced intervertebral disc degeneration. Redox Biol. 2018, 19, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Li, J.; Qin, R.; Li, M.; Li, Y.; Zhang, J.; Wang, R.; Goltzman, D.; Miao, D.; Yang, R. Nrf2 activation by pyrroloquinoline quinone inhibits natural aging-related intervertebral disk degeneration in mice. Aging Cell 2024, 23, e14202. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jiang, G.; Liu, H.; Li, Z.; Pei, Y.; Wang, H.; Pan, H.; Cui, H.; Long, J.; Wang, J.; et al. Melatonin alleviates intervertebral disc degeneration by disrupting the IL-1beta/NF-kappaB-NLRP3 inflammasome positive feedback loop. Bone Res. 2020, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Zhao, Y.; Lin, J.; Jiang, S.; Li, W. The Nrf2 antioxidant defense system in intervertebral disc degeneration: Molecular insights. Exp. Mol. Med. 2022, 54, 1067–1075. [Google Scholar] [CrossRef]
- Tang, Z.; Hu, B.; Zang, F.; Wang, J.; Zhang, X.; Chen, H. Nrf2 drives oxidative stress-induced autophagy in nucleus pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect intervertebral disc from degeneration. Cell Death Dis. 2019, 10, 510. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Hu, X.C.; Zhang, G.Z.; Liu, M.Q.; Chen, H.W.; Kang, X.W. Role of Nrf2 and HO-1 in intervertebral disc degeneration. Connect. Tissue Res. 2022, 63, 559–576. [Google Scholar] [CrossRef]
- Kang, L.; Zhang, H.; Jia, C.; Zhang, R.; Shen, C. Targeting Oxidative Stress and Inflammation in Intervertebral Disc Degeneration: Therapeutic Perspectives of Phytochemicals. Front. Pharmacol. 2022, 13, 956355. [Google Scholar] [CrossRef]
- Qin, S.; Hou, D.X. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol. Nutr. Food Res. 2016, 60, 1731–1755. [Google Scholar] [CrossRef]
- He, W.-J.; Lv, C.-H.; Chen, Z.; Shi, M.; Zeng, C.-X.; Hou, D.-X.; Qin, S. The Regulatory Effect of Phytochemicals on Chronic Diseases by Targeting Nrf2-ARE Signaling Pathway. Antioxidants 2023, 12, 236. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation. Antioxidants 2021, 10, 1859. [Google Scholar] [CrossRef]
- Ouyang, Z.-H.; Wang, W.-J.; Yan, Y.-G.; Wang, B.; Lv, G.-H. The PI3K/Akt pathway: A critical player in intervertebral disc degeneration. Oncotarget 2017, 8, 57870–57881. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Burt, K.G.; Kim, M.K.M.; Viola, D.C.; Abraham, A.C.; Chahine, N.O. Nuclear factor κB overactivation in the intervertebral disc leads to macrophage recruitment and severe disc degeneration. Sci. Adv. 2024, 10, eadj3194. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Zhang, Y.; Liu, Y.; Sun, X.; Liu, X.; Li, B.; Yang, Q. Role of macrophage in intervertebral disc degeneration. Bone Res. 2025, 13, 15. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Israël, A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb. Perspect. Biol. 2010, 2, a000158. [Google Scholar] [CrossRef]
- Häcker, H.; Karin, M. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006, 357, re13. [Google Scholar] [CrossRef]
- Kannan, G.; Paul, B.M.; Thangaraj, P. Stimulation, regulation, and inflammaging interventions of natural compounds on nuclear factor kappa B (NF-kB) pathway: A comprehensive review. Inflammopharmacology 2025, 33, 145–162. [Google Scholar] [CrossRef]
- Liang, H.; Luo, R.; Li, G.; Zhang, W.; Song, Y.; Yang, C. The Proteolysis of ECM in Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2022, 23, 1715. [Google Scholar] [CrossRef]
- Vo, N.V.; Hartman, R.A.; Yurube, T.; Jacobs, L.J.; Sowa, G.A.; Kang, J.D. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013, 13, 331–341. [Google Scholar] [CrossRef]
- Wuertz, K.; Vo, N.; Kletsas, D.; Boos, N. Inflammatory and catabolic signalling in intervertebral discs: The roles of NF-κB and MAP kinases. Eur. Cell Mater. 2012, 23, 103–119. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, M.; Zhang, Y.-Q.; Xu, Z.-H. JNK pathway: Diseases and therapeutic potential. Acta Pharmacol. Sin. 2007, 28, 601–608. [Google Scholar] [CrossRef]
- Liu, G.; Gao, L.; Wang, Y.; Xie, X.; Gao, X.; Wu, X. The JNK signaling pathway in intervertebral disc degeneration. Front. Cell Dev. Biol. 2024, 12, 1423665. [Google Scholar] [CrossRef]
- Li, S.-T.; Dai, Q.; Zhang, S.-X.; Liu, Y.-J.; Yu, Q.-Q.; Tan, F.; Lu, S.-H.; Wang, Q.; Chen, J.-W.; Huang, H.-Q.; et al. Ulinastatin attenuates LPS-induced inflammation in mouse macrophage RAW264.7 cells by inhibiting the JNK/NF-κB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol. 2018, 39, 1294–1304. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, Y.; Zhu, L.; Li, T.; Guan, Y.; Zhao, S.; Wang, Q.; Wang, J.; Li, L. Hua-tan-sheng-jing decoction treats obesity with oligoasthenozoospermia by up-regulating the PI3K-AKT and down-regulating the JNK MAPK signaling pathways: At the crossroad of obesity and oligoasthenozoospermia. Front. Pharmacol. 2022, 13, 896434. [Google Scholar] [CrossRef]
- Kriehuber, E.; Bauer, W.; Charbonnier, A.-S.; Winter, D.; Amatschek, S.; Tamandl, D.; Schweifer, N.; Stingl, G.; Maurer, D. Balance between NF-kappaB and JNK/AP-1 activity controls dendritic cell life and death. Blood 2005, 106, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yao, M.; Zhu, X.; Lian, Y.; Zhang, M. Baicalin suppresses interleukin-1β-induced apoptosis, inflammatory response, oxidative stress, and extracellular matrix degradation in human nucleus pulposus cells. Immunopharmacol. Immunotoxicol. 2023, 45, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhuang, J.; Wang, D.; Lv, L.; Zhu, F.; Yao, A.; Xu, T. Glycyrrhizin suppresses inflammation and cell apoptosis by inhibition of HMGB1 via p38/p-JUK signaling pathway in attenuating intervertebral disc degeneration. Am. J. Transl. 2019, 11, 5105–5113. [Google Scholar]
- Ahmed, A.S.; Berg, S.; Alkass, K.; Druid, H.; Hart, D.A.; Svensson, C.I.; Kosek, E. NF-κB-Associated Pain-Related Neuropeptide Expression in Patients with Degenerative Disc Disease. Int. J. Mol. Sci. 2019, 20, 658. [Google Scholar] [CrossRef]
- Zàaba, N.F.; Ogaili, R.H.; Ahmad, F.; Isa, I.L.M. Neuroinflammation and nociception in intervertebral disc degeneration: A review of precision medicine perspective. Spine J. 2025, 25, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.J.; Cui, H.; Pan, H.; Mc Cheung, K.; Cao, X.; Iatridis, J.C.; Zheng, Z. Painful intervertebral disc degeneration and inflammation: From laboratory evidence to clinical interventions. Bone Res. 2021, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.H.; Peng, Y.J.; Tsou, H.K.; Salter, D.M.; Lee, H.S. Nerve growth factor promotes expression of novel genes in intervertebral disc cells that regulate tissue degradation. J. Neurosurg. Spine 2014, 21, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Arai, F.; Hiyama, A.; Sakai, D.; Yokoyama, K.; Mochida, J. The expression and role of non-canonical (PKC) signaling in nucleus pulposus cell metabolism. J. Orthop. Res. 2012, 30, 1478–1485. [Google Scholar] [CrossRef]
- Aatif, M. Current Understanding of Polyphenols to Enhance Bioavailability for Better Therapies. Biomedicine 2023, 11, 2078. [Google Scholar] [CrossRef]
- Albin, K.J.; Noble, P.N.N.; Kumar, N.P.; Imran, K. The Nexus Between Polyphenols and Gut Microbiota and Their Interplay in Human Health: A Brief Review. J. Nat. Remedies 2024, 24, 1895–1908. [Google Scholar] [CrossRef]
- Na, G.; He, C.; Zhang, S.; Tian, S.; Bao, Y.; Shan, Y. Dietary Isothiocyanates: Novel Insights Into the Potential for Cancer Prevention and Therapy. Int. J. Mol. Sci. 2023, 24, 1962. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, J.; Oh, J. Taming Neuroinflammation in Alzheimer’s Disease: The Protective Role of Phytochemicals Through the Gut−Brain Axis. Biomed. Pharmacother. 2024, 178, 117277. [Google Scholar] [CrossRef]
- Ayaz, M.; Mosa, O.F.; Nawaz, A.; Hamdoon, A.A.E.; Elkhalifa, M.E.M.; Sadiq, A.; Ullah, F.; Ahmed, A.; Kabra, A.; Khan, H.; et al. Neuroprotective Potentials of Lead Phytochemicals Against Alzheimer’s Disease with Focus on Oxidative Stress-Mediated Signaling Pathways: Pharmacokinetic Challenges, Target Specificity, Clinical Trials and Future Perspectives. Phytomedicine 2024, 124, 155272. [Google Scholar] [CrossRef]
- Zenjanab, M.K.; Hashemzadeh, N.; Alimohammadvand, S.; Sharifi-Azad, M.; Abdolahinia, E.D.; Jahanban-Esfahlan, R.; Mohamadvand, S. Notch Signaling Suppression by Golden Phytochemicals: Potential for Cancer Therapy. Adv. Pharm. Bull. 2024, 14, 302–313. [Google Scholar] [CrossRef]
- Murai, H.; Kuboniwa, M.; Kakiuchi, M.; Matsumura, R.; Hirata, Y.; Amano, A. Curcumin Inhibits Growth of Porphyromonas gingivalis by Arrest of Bacterial Dipeptidyl Peptidase Activity. J. Oral. Microbiol. 2024, 15, 2373040. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lai, K.; Chopra, N.; Zheng, Z.; Das, A.; Diwan, A.D. Gut-disc axis: A cause of intervertebral disc degeneration and low back pain? Eur. Spine J. 2022, 31, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ma, Z.; Wang, Y.; Yang, J. Multiple nano-drug delivery systems for intervertebral disc degeneration: Current status and future perspectives. Bioact. Mater. 2022, 23, 274–299. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Ou, X.; Liu, C.; Li, R.; Zheng, Q.; Hu, L. Nanotechnology-Enhanced Pharmacotherapy for Intervertebral Disc Degeneration Treatment. Int. J. Nanomed. 2024, 19, 14043–14058. [Google Scholar] [CrossRef]
- Kamali, A.; Ziadlou, R.; Lang, G.; Pfannkuche, J.; Cui, S.; Li, Z.; Richards, R.G.; Alini, M.; Grad, S. Small molecule-based treatment approaches for intervertebral disc degeneration: Current options and future directions. Theranostics 2021, 11, 27–47. [Google Scholar] [CrossRef]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef]
- Smith, M.; Melrose, J. Pentosan Polysulfate, An Anti-Viral Heparinoid, Prevents Severe Acute Respiratory Syndrome Corona Virus-2 Infection and Treats Symptoms of Long Coronavirus Disease. Med. Res. Arch. 2025, 13. [Google Scholar]
- Smith, M.M.; Melrose, J. Pentosan Polysulfate Affords Pleotropic Protection to Multiple Cells and Tissues. Pharmaceuticals 2023, 16, 437. [Google Scholar] [CrossRef]
- Lin, Z.; Mai, L.; Luo, H.; Wang, Z.; Lin, Y.; Wang, H.; Chen, S.; Su, G.; Hu, X.; Chen, B.; et al. Smart responsive injectable hydrogel loaded with icariin inhibits ferroptosis and promotes nucleus pulposus repair in intervertebral disc degeneration. Biomater. Adv. 2026, 179, 214513. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).