Interleukin-4-Enhanced Oligodendrocyte Differentiation Depends on Extracellular Zinc Uptake via ZIP11
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Transfection
2.2. Immunocytochemistry
2.3. Intracellular Zinc Concentration Measuring by Flow Cytometry
2.4. Quantitative RT-PCR (qRT-PCR)
2.5. Western Blotting
2.6. Statistical Analysis
3. Results
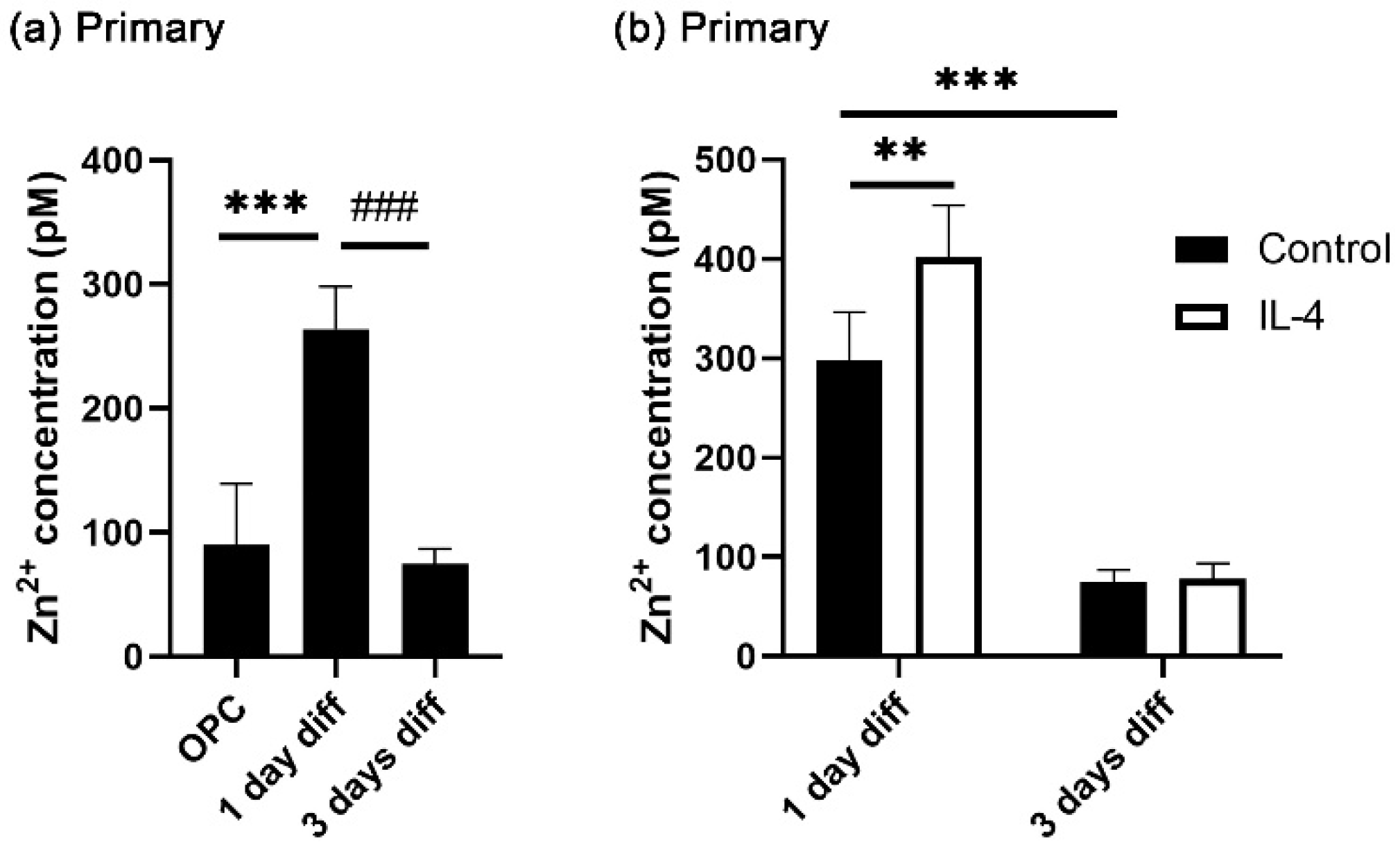
3.1. IL-4 Increases Transiently Intracellular Zinc Concentrations in Differentiating Oligodendroglia
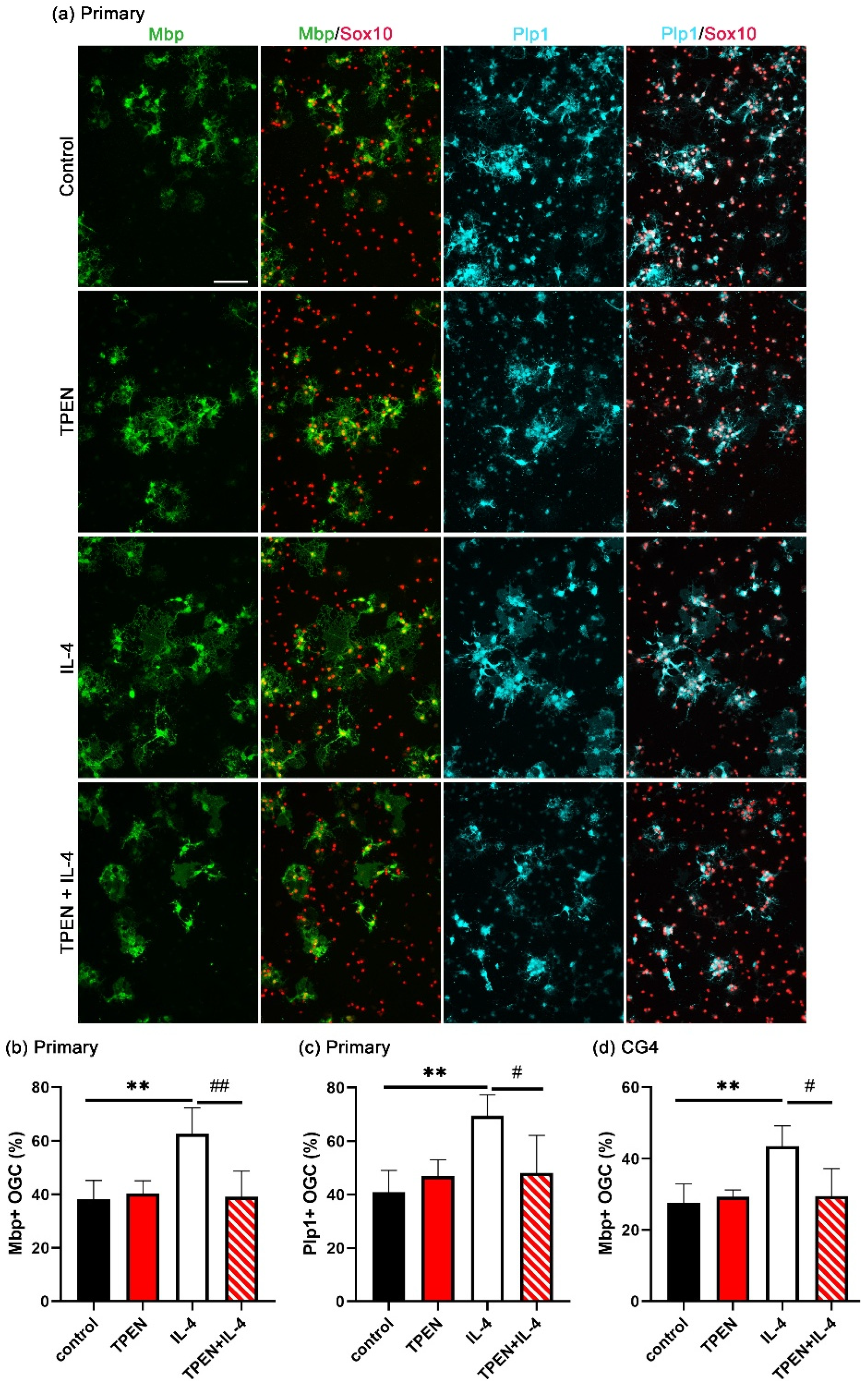
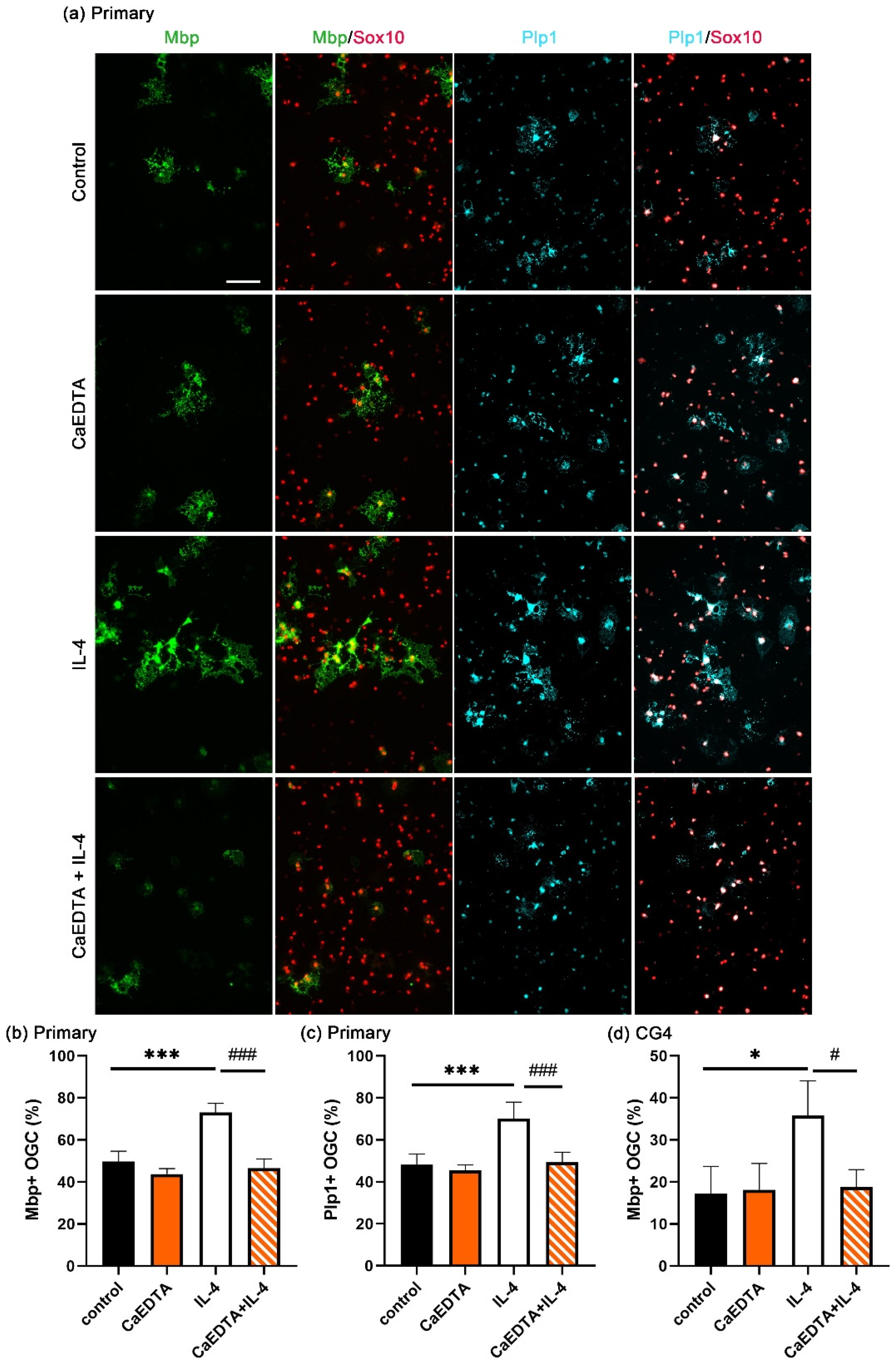
3.2. Increased OL Differentiation by IL-4 Is Zinc-Dependent
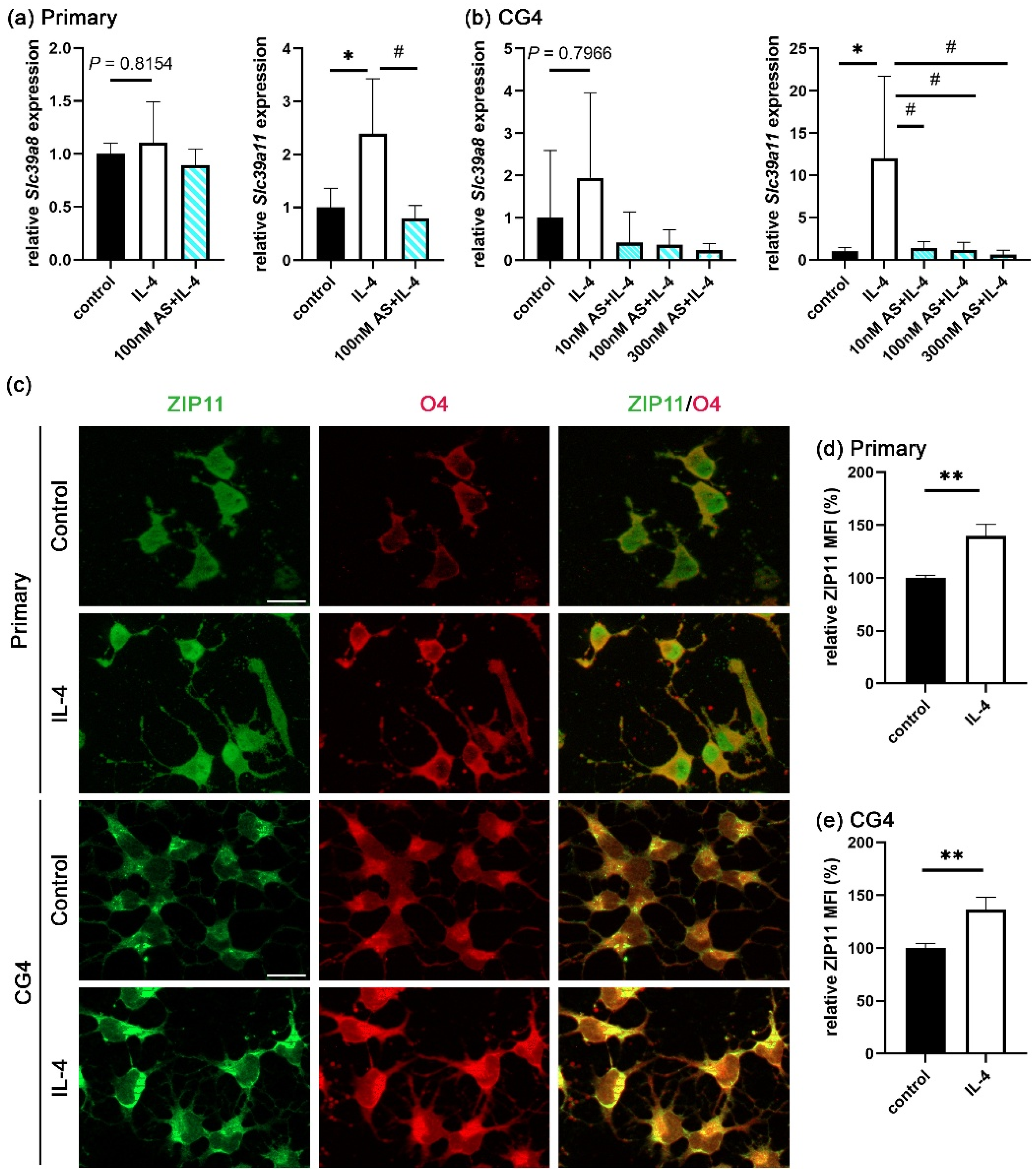
3.3. IL-4 Induces Extracellular Zinc Uptake
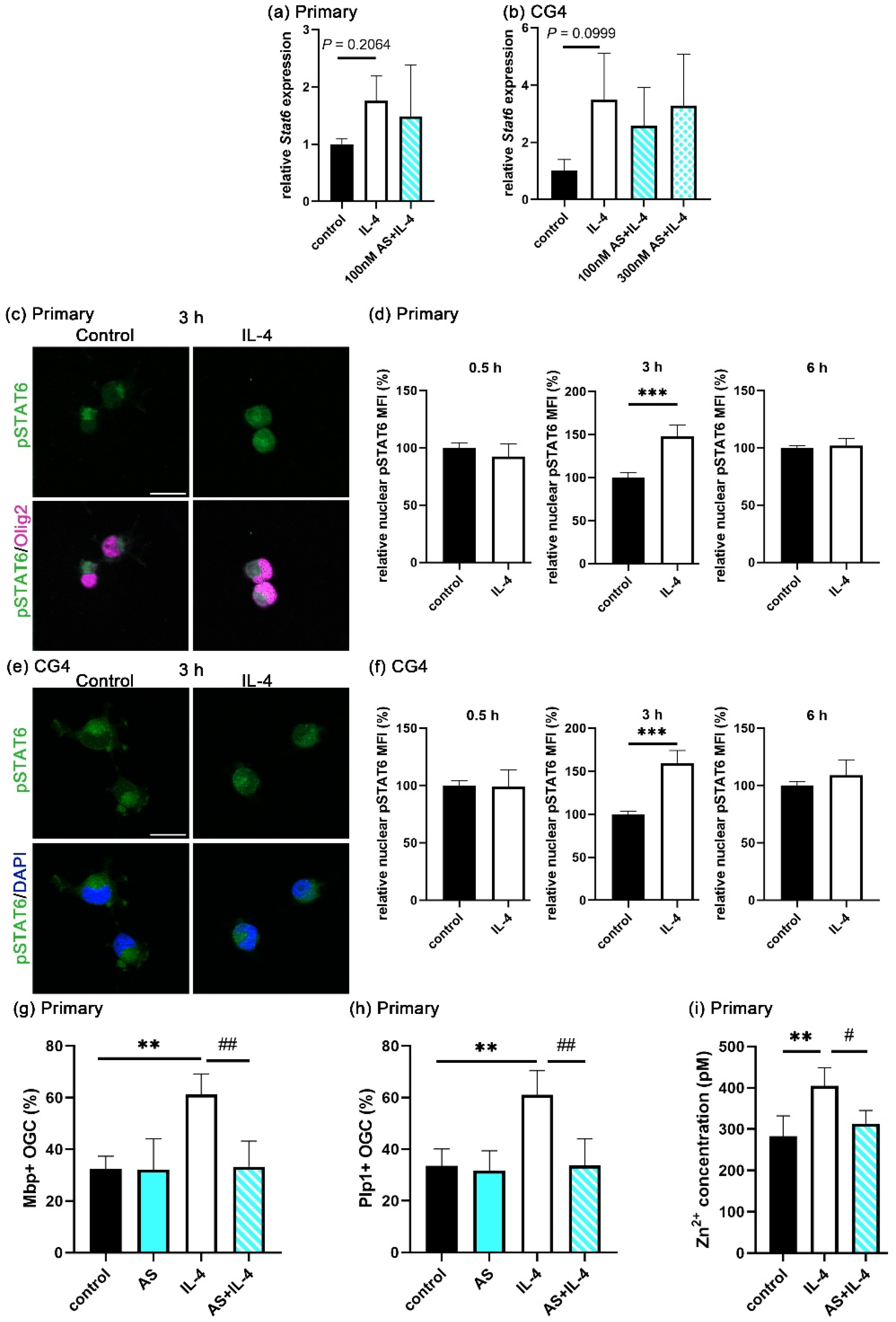
3.4. STAT6 Activation Is Essential for Extracellular Zinc Uptake
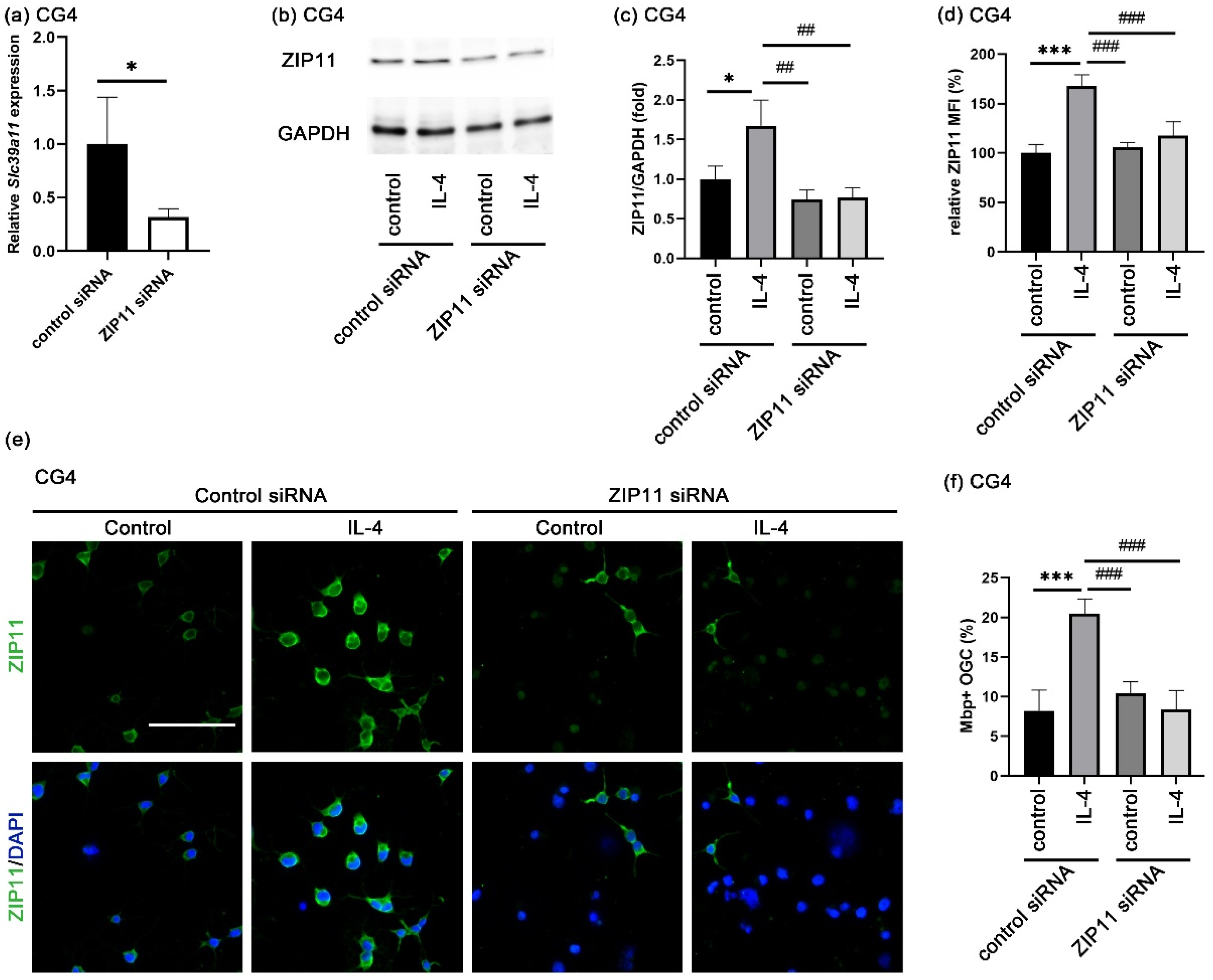
3.5. Zinc Influx via ZIP11 Increases Intracellular Zinc
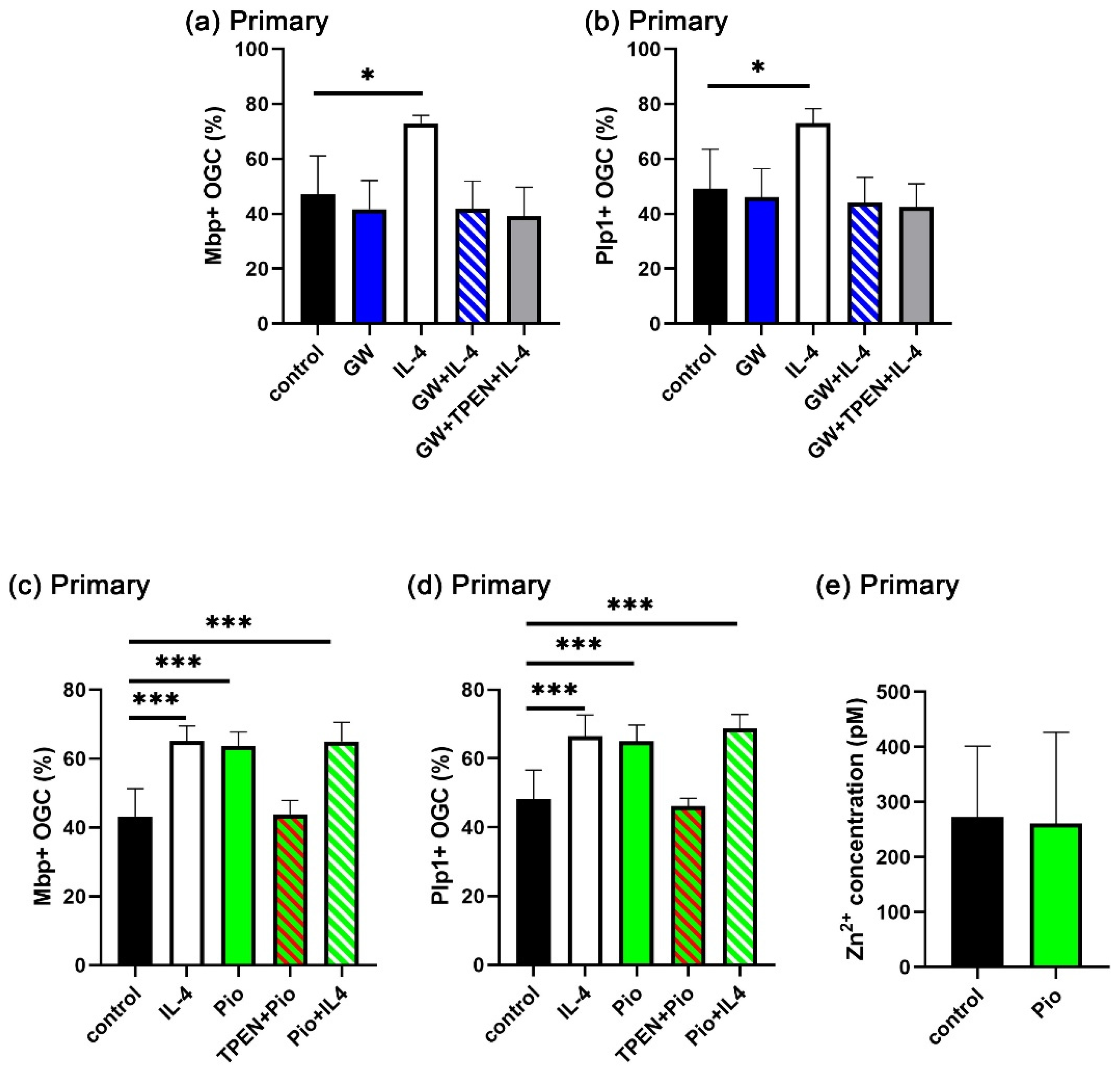
3.6. IL-4-Induced Intracellular Zinc Accumulation Promotes the PPARγ Signaling Pathway
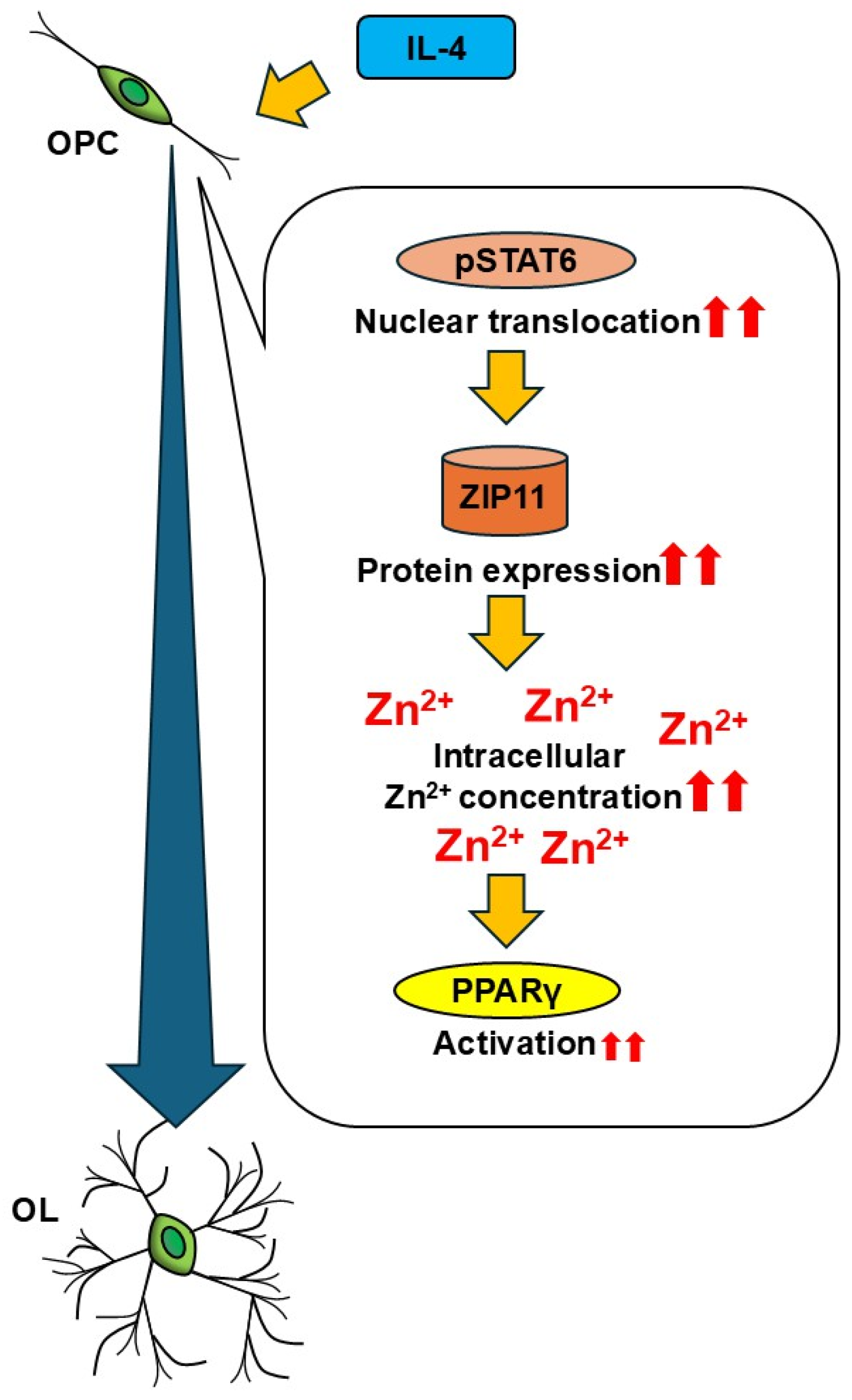
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CaEDTA | Calcium ethylenediaminetetraacetate |
| CNS | Central nervous system |
| DAPI | 4′, 6′-diamidino-2-phenylindole |
| FGF2 | Basic fibroblast growth factor |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| JAK | Janus kinase |
| MAG | Myelin-associated glycoprotein |
| MBP | Myelin basic protein |
| MS | Multiple sclerosis |
| OL | Oligodendrocyte |
| Olig2 | Oligodendrocyte transcription factor 2 |
| OPC | Oligodendrocyte progenitor cells |
| PDGF-AA | Platelet-derived growth factor AA |
| PLP1 | Proteolipid protein 1 |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| pSTAT6 | Phospho-STAT6 |
| STAT6 | Signal transducer and activator of transcription 6 |
| TPEN | N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine |
| ZIP | Zrt, Irt-like protein |
| ZnT | Zinc transporter |
References
- Sock, E.; Wegner, M. Using the Lineage Determinants Olig2 and Sox10 to Explore Transcriptional Regulation of Oligodendrocyte Development. Dev. Neurobiol. 2021, 81, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.J. Neurobiology of Zinc and Zinc-Containing Neurons. Int. Rev. Neurobiol. 1989, 31, 145–238. [Google Scholar] [CrossRef]
- Popescu, B.F.; Frischer, J.M.; Webb, S.M.; Tham, M.; Adiele, R.C.; Robinson, C.A.; Fitz-Gibbon, P.D.; Weigand, S.D.; Metz, I.; Nehzati, S.; et al. Pathogenic Implications of Distinct Patterns of Iron and Zinc in Chronic MS Lesions. Acta. Neuropathol. 2017, 134, 45–64. [Google Scholar] [CrossRef]
- Pawlitzki, M.; Uebelhör, J.; Sweeney-Reed, C.M.; Stephanik, H.; Hoffmann, J.; Lux, A.; Reinhold, D. Lower Serum Zinc Levels in Patients with Multiple Sclerosis Compared to Healthy Controls. Nutrients 2018, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Socha, K.; Karpińska, E.; Kochanowicz, J.; Soroczyńska, J.; Jakoniuk, M.; Wilkiel, M.; Mariak, Z.D.; Borawska, M.H. Dietary Habits; Concentration of Copper, Zinc, and Cu-to-Zn Ratio in Serum and Ability Status of Patients with Relapsing-Remitting Multiple Sclerosis. Nutrition 2017, 39–40, 76–81. [Google Scholar] [CrossRef]
- Bredholt, M.; Frederiksen, J.L. Zinc in Multiple Sclerosis: A Systematic Review and Meta-Analysis. ASN Neuro. 2016, 8, 1759091416651511. [Google Scholar] [CrossRef]
- Nirooei, E.; Kashani, S.M.A.; Owrangi, S.; Malekpour, F.; Niknam, M.; Moazzen, F.; Nowrouzi-Sohrabi, P.; Farzinmehr, S.; Akbari, H. Blood Trace Element Status in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Biol. Trace. Elem. Res. 2022, 200, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Fukada, T. Roles of Zinc Signaling in the Immune System. J. Immunol. Res. 2016, 2016, 1–21. [Google Scholar] [CrossRef]
- Elitt, C.M.; Ross, M.M.; Wang, J.; Fahrni, C.J.; Rosenberg, P.A. Developmental Regulation of Zinc Homeostasis in Differentiating Oligodendrocytes. Neurosci. Lett. 2024, 831, 137727. [Google Scholar] [CrossRef]
- Bourassa, D.; Elitt, C.M.; McCallum, A.M.; Sumalekshmy, S.; McRae, R.L.; Morgan, M.T.; Siegel, N.; Perry, J.W.; Rosenberg, P.A.; Fahrni, C.J. Chromis-1, a Ratiometric Fluorescent Probe Optimized for Two-Photon Microscopy Reveals Dynamic Changes in Labile Zn(II) in Differentiating Oligodendrocytes. ACS Sens. 2018, 3, 458–467. [Google Scholar] [CrossRef]
- Do Rosario, M.C.; Bey, G.R.; Nmezi, B.; Liu, F.; Oranburg, T.; Cohen, A.S.A.; Coffman, K.A.; Brown, M.R.; Kiselyov, K.; Waisfisz, Q.; et al. Variants in the Zinc Transporter TMEM163 Cause a Hypomyelinating Leukodystrophy. Brain 2022, 145, 4202–4209. [Google Scholar] [CrossRef]
- Yan, H.; Yang, S.; Hou, Y.; Ali, S.; Escobar, A.; Gao, K.; Duan, R.; Kubisiak, T.; Wang, J.; Zhang, Y.; et al. Functional Study of TMEM163 Gene Variants Associated with Hypomyelination Leukodystrophy. Cells 2022, 11, 1285. [Google Scholar] [CrossRef]
- Keegan, A.D.; Leonard, W.J.; Zhu, J. Recent Advances in Understanding the Role of IL-4 Signaling. Fac. Rev. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, H.; Sun, G.; Zhang, J.; Edwards, N.J.; Aronowski, J. Neuronal Interleukin-4 as a Modulator of Microglial Pathways and Ischemic Brain Damage. J. Neurosci. 2015, 35, 11281–11291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, W.; Xu, F.; Dai, X.; Shi, L.; Cai, W.; Mu, H.; Hitchens, T.K.; Foley, L.M.; Liu, X.; et al. The Interleukin-4/PPARγ Signaling Axis Promotes Oligodendrocyte Differentiation and Remyelination after Brain Injury. PLoS Biol. 2019, 17, e3000330. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Zheng, X.; Jiang, X.; Mu, H.; Xu, F.; Zhu, W.; Ye, Q.; Jizhang, Y.; Hitchens, T.K.; Shi, Y.; et al. Interleukin-4 Improves White Matter Integrity and Functional recovery after Murine Traumatic Brain Injury via Oligodendroglial PPARγ. J. Cereb. Blood Flow Metab. 2020, 41, 511–529. [Google Scholar] [CrossRef]
- Hihi, A.K.; Michalik, L.; Wahli, W. PPARs: Transcriptional Effectors of Fatty Acids and Their Derivatives. Cell. Mol. Life Sci. 2002, 59, 790–798. [Google Scholar] [CrossRef]
- Zeis, T.; Graumann, U.; Reynolds, R.; Schaeren-Wiemers, N. Normal-Appearing White Matter in Multiple Sclerosis Is in a Subtle Balance between Inflammation and Neuroprotection. Brain 2008, 131, 288–303. [Google Scholar] [CrossRef]
- Zeis, T.; Probst, A.; Steck, A.J.; Stadelmann, C.; Brück, W.; Schaeren-Wiemers, N. Molecular Changes in White Matter Adjacent to an Active Demyelinating Lesion in Early Multiple Sclerosis. Brain Pathol. 2009, 19, 459–466. [Google Scholar] [CrossRef]
- Zeis, T.; Howell, O.W.; Reynolds, R.; Schaeren-Wiemers, N. Molecular Pathology of Multiple Sclerosis Lesions Reveals a Heterogeneous Expression Pattern of Genes Involved in Oligodendrogliogenesis. Exp. Neurol. 2018, 305, 76–88. [Google Scholar] [CrossRef]
- Aratake, T.; Higashi, Y.; Ueba, Y.; Hamada, T.; Shimizu, T.; Shimizu, S.; Yawata, T.; Ueba, T.; Saito, M. The Inhibitory Role of Intracellular Free Zinc in the Regulation of Arg-1 Expression in Interleukin-4-Induced Activation of M2 Microglia. Metallomics 2018, 10, 1501–1509. [Google Scholar] [CrossRef]
- Vignesh, K.S.; Figueroa, J.A.L.; Porollo, A.; Divanovic, S.; Caruso, J.A.; Deepe, G.S. IL-4 Induces Metallothionein 3- and SLC30A4-Dependent Increase in Intracellular Zn 2+ that Promotes Pathogen Persistence in Macrophages. Cell Rep. 2016, 16, 3232–3246. [Google Scholar] [CrossRef]
- McCarthy, K.D.; De Vellis, J. Preparation of Separate Astroglial and Oligodendroglial Cell Cultures from Rat Cerebral Tissue. J. Cell Biol. 1980, 85, 890–902. [Google Scholar] [CrossRef]
- Louis, J.C.; Magal, E.; Muir, D.; Manthorpe, M.; Varon, S. CG-4, A New Bipotential Glial Cell Line from Rat Brain, Is Capable of Differentiating in Vitro into Either Mature Oligodendrocytes or Type-2 Astrocytes. J. Neurosci. Res. 1992, 31, 193–204. [Google Scholar] [CrossRef]
- Aberle, T.; Piefke, S.; Hillgärtner, S.; Tamm, E.R.; Wegner, M.; Küspert, M. Transcription Factor Zfp276 Drives Oligodendroglial Differentiation and Myelination by Switching off the Progenitor Cell Program. Nucleic Acids Res. 2022, 50, 1951–1968. [Google Scholar] [CrossRef] [PubMed]
- Wüst, H.M.; Wegener, A.; Fröb, F.; Hartwig, A.C.; Wegwitz, F.; Kari, V.; Schimmel, M.; Tamm, E.R.; Johnsen, S.A.; Wegner, M.; et al. Egr2-Guided Histone H2B Monoubiquitination Is Required for Peripheral Nervous System Myelination. Nucleic Acids Res. 2020, 48, 8959–8976. [Google Scholar] [CrossRef] [PubMed]
- Upschulte, E.; Harmeling, S.; Amunts, K.; Dickscheid, T. Contour Proposal Networks for Biomedical Instance Segmentation. Med Image Anal. 2022, 77, 102371. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Hebel, S.; Engelhardt, G.; Rink, L. Flow Cytometric Measurement of Labile Zinc in Peripheral Blood Mononuclear Cells. Anal. Biochem. 2006, 352, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.L.; Kremp, M.; Aratake, T.; Cui, S.; Lin, Y.; Zhong, X.; Lu, Q.R.; Zhang, C.; Qiu, M.; Aberle, T.; et al. The Myelination-Associated G Protein-Coupled Receptor 37 Is Regulated by Zfp488, Nkx2.2, and Sox10 during Oligodendrocyte Differentiation. Glia 2024, 72, 1304–1318. [Google Scholar] [CrossRef]
- Diamant, I.; Clarke, D.J.B.; Evangelista, J.E.; Lingam, N.; Ma’ayan, A. Harmonizome 3.0: Integrated Knowledge about Genes and Proteins from Diverse Multi-Omics Resources. Nucleic. Acids. Res. 2025, 53, D1016–D1028. [Google Scholar] [CrossRef] [PubMed]
- Cousins, R.J.; Martin, A.B.; Aydemir, T.B.; Guthrie, G.J.; Samuelson, D.A.; Chang, S.M. Gastric and Colonic Zinc Transporter ZIP11 (Slc39a11) in Mice Responds to Dietary Zinc and Exhibits Nuclear Localization. J. Nutr. 2013, 143, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.L.; Velasquez, V.; Croxford, T.P.; McCormick, N.H.; Lopez, V.; Macdavid, J. Mapping the Zinc-Transporting System in Mammary Cells: Molecular Analysis Reveals a Phenotype-Dependent Zinc-Transporting Network during Lactation. J. Cell. Physiol. 2012, 227, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Olea-Flores, M.; Kan, J.; Carlson, A.; Syed, S.A.; McCann, C.; Mondal, V.; Szady, C.; Ricker, H.M.; McQueen, A.; Navea, J.G.; et al. ZIP11 Regulates Nuclear Zinc Homeostasis in HeLa Cells and Is Required for Proliferation and Establishment of the Carcinogenic Phenotype. Front. Cell Dev. Biol. 2022, 10, 895433. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, A.; Zhang, Z.; Yan, G.; Zhang, F.; Zhang, L.; Shen, X.; Hu, R.; Zhang, Y.; Zhang, K.; et al. Characterization of the GufA Subfamily Member SLC39A11/Zip11 as a Zinc Transporter. J. Nutr. Biochem. 2013, 24, 1697–1708. [Google Scholar] [CrossRef]
- Elitt, C.M.; Fahrni, C.J.; Rosenberg, P.A. Zinc Homeostasis and Zinc Signaling in White Matter Development and Injury. Neurosci. Lett. 2019, 707, 134247. [Google Scholar] [CrossRef]
- Wang, B.; Fang, T.; Chen, H. Zinc and Central Nervous System Disorders. Nutrients 2023, 15, 2140. [Google Scholar] [CrossRef]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef]
- Mnatsakanyan, H.; Serra, R.S.I.; Salmeron-Sanchez, M.; Rico, P. Zinc Maintains Embryonic Stem Cell Pluripotency and Multilineage Differentiation Potential via AKT Activation. Front. Cell Dev. Biol. 2019, 7, 180. [Google Scholar] [CrossRef]
- Morris, D.R.; Levenson, C.W. Zinc Regulation of Transcriptional Activity during Retinoic Acid-Induced Neuronal Differentiation. J. Nutr. Biochem. 2013, 24, 1940–1944. [Google Scholar] [CrossRef]
- Pfaender, S.; Föhr, K.; Lutz, A.K.; Putz, S.; Achberger, K.; Linta, L.; Liebau, S.; Boeckers, T.M.; Grabrucker, A.M. Cellular Zinc Homeostasis Contributes to Neuronal Differentiation in Human Induced Pluripotent Stem Cells. Neural. Plast. 2016, 2016, 3760702. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sanford, L.; Simpson, D.M.; Dowell, R.D.; Palmer, A.E. Remodeling of Zn2+ Homeostasis upon Differentiation of Mammary Epithelial Cells. Metallomics 2020, 12, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Kursula, P.; Meriläinen, G.; Lehto, V.P.; Heape, A.M. The Small Myelin-Associated Glycoprotein Is a Zinc-Binding Protein. J. Neurochem. 1999, 73, 2110–2118. [Google Scholar] [CrossRef] [PubMed]
- Tsang, D.; Tsang, Y.S.; Ho, W.K.K.; Wong, R.N.S. Myelin Basic Protein Is a Zinc-Binding Protein in Brain: Possible Role in Myelin Compaction. Neurochem. Res. 1997, 22, 811–819. [Google Scholar] [CrossRef]
- Riccio, P.; Giovannelli, S.; Bobba, A.; Romito, E.; Fasano, A.; Bleve-Zacheo, T.; Favilla, R.; Quagliariello, E.; Cavatorta, P. Specificity of Zinc Binding to Myelin Basic Protein. Neurochem. Res. 1995, 20, 1107–1113. [Google Scholar] [CrossRef]
- Bernardo, A.; Plumitallo, C.; De Nuccio, C.; Visentin, S.; Minghetti, L. Curcumin Promotes Oligodendrocyte Differentiation and Their Protection against TNF-α through the Activation of the Nuclear Receptor PPAR-γ. Sci. Rep. 2021, 11, 4952. [Google Scholar] [CrossRef]
- Kanakasabai, S.; Pestereva, E.; Chearwae, W.; Gupta, S.K.; Ansari, S.; Bright, J.J. PPARγ Agonists Promote Oligodendrocyte Differentiation of Neural Stem Cells by Modulating Stemness and Differentiation Genes. PLoS ONE 2012, 7, e50500. [Google Scholar] [CrossRef]
- Krishna, S.; Cheng, B.; Sharma, D.R.; Yadav, S.; Stempinski, E.S.; Mamtani, S.; Shah, E.; Deo, A.; Acherjee, T.; Thomas, T.; et al. PPAR-γ Activation Enhances Myelination and Neurological Recovery in Premature Rabbits with Intraventricular Hemorrhage. Proc. Natl. Acad. Sci. USA 2021, 118, e2103084118. [Google Scholar] [CrossRef]
- Han, L.; Cai, W.; Mao, L.; Liu, J.; Li, P.; Leak, R.K.; Xu, Y.; Hu, X.; Chen, J. Rosiglitazone Promotes White Matter Integrity and Long-Term Functional Recovery after Focal Cerebral Ischemia. Stroke 2015, 46, 2628–2636. [Google Scholar] [CrossRef]
- Szanto, A.; Balint, B.L.; Nagy, Z.S.; Barta, E.; Dezso, B.; Pap, A.; Szeles, L.; Poliska, S.; Oros, M.; Evans, R.M.; et al. STAT6 Transcription Factor Is a Facilitator of the Nuclear Receptor PPARγ-Regulated Gene Expression in Macrophages and Dendritic Cells. Immunity 2010, 33, 699–712. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, B.M.; Ahn, Y.H.; Choi, J.H.; Choi, Y.H.; Kang, J.L. STAT6 Signaling Mediates PPARγ Activation and Resolution of Acute Sterile Inflammation in Mice. Cells 2021, 10, 501. [Google Scholar] [CrossRef]
- Kim, E.Y.; Verdejo-Torres, O.; Diaz-Rodriguez, K.; Hasanain, F.; Caromile, L.; Padilla-Benavides, T. Single Nucleotide Polymorphisms and Zn Transport by ZIP11 Shape Functional Phenotypes of HeLa Cells. Metallomics 2024, 16, 6. [Google Scholar] [CrossRef]
- Xia, Z.; Tang, B.; Li, X.; Li, X.; Jia, Y.; Jiang, J.; Chen, J.; Song, J.; Liu, S.; Min, J.; et al. A Novel Role for the Longevity-Associated Protein SLC39A11 as a Manganese Transporter. Research 2024, 7, 0440. [Google Scholar] [CrossRef] [PubMed]
- Andregg, G.; Hubmann, E.; Podder, N.G.; Wenk, F. Pyridinderivate als Komplexbildner. XI†. Die Thermodynamik der Metallkomplexbildung mit Bis-, Tris- und Tetrakis[(2-pyridyl)methyl]-aminen. Helv. Chim. Acta 1977, 60, 123–140. [Google Scholar] [CrossRef]
- Schmid, R.W.; Reilley, C.N. A Rapid Electrochemical Method for the Determination of Metal Chelate Stability Constants. J. Am. Chem. Soc. 1956, 78, 5513–5518. [Google Scholar] [CrossRef]
- Kallaur, A.P.; Oliveira, S.R.; Simao, A.N.C.; De Almeida, E.R.D.; Morimoto, H.K.; Lopes, J.; De Carvalho Jennings Pereira, W.L.; Andrade, R.M.; Pelegrino, L.M.; Borelli, S.D.; et al. Cytokine Profile in Relapsing-Remitting Multiple Sclerosis Patients and the Association between Progression and Activity of the Disease. Mol. Med. Rep. 2013, 7, 1010–1020. [Google Scholar] [CrossRef]
- Lu, C.Z.; Jensen, M.A.; Arnason, B.G.W. Interferon γ- and Interleukin-4-Secreting Cells in Multiple Sclerosis. J. Neuroimmunol. 1993, 46, 123–128. [Google Scholar] [CrossRef]
- Hohnoki, K.; Inoue, A.; Koh, C.S. Elevated Serum Levels of IFN-γ, IL-4 and TNF-α/Unelevated Serum Levels of IL-10 in Patients with Demyelinating Diseases during the Acute Stage. J. Neuroimmunol. 1998, 87, 27–32. [Google Scholar] [CrossRef]
| Transcript | Primers |
|---|---|
| Gapdh | 5′-TCCAGTATGACTCTACCCACG-3′ |
| 5′-CACGACATACTCAGCACCAG-3′ | |
| Stat6 | 5′-GCATCTATCAGAGGGACCCC-3′ |
| 5′-GGGAAGTGGGGTAGCACAAT-3′ | |
| Slc39a8 | 5′-TGCCCAGCATACTTTGTTCC-3′ |
| 5′-CTTTTGGGTTCCTTGGGAGT-3′ | |
| Slc39a11 | 5′-TCGGCTAGCTCTGAGAACCT-3′ |
| 5′-GACCCTGTTACGCTGGTTCA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aratake, T.; Kurita, S.; Wegner, M. Interleukin-4-Enhanced Oligodendrocyte Differentiation Depends on Extracellular Zinc Uptake via ZIP11. Cells 2025, 14, 1756. https://doi.org/10.3390/cells14221756
Aratake T, Kurita S, Wegner M. Interleukin-4-Enhanced Oligodendrocyte Differentiation Depends on Extracellular Zinc Uptake via ZIP11. Cells. 2025; 14(22):1756. https://doi.org/10.3390/cells14221756
Chicago/Turabian StyleAratake, Takaaki, Serika Kurita, and Michael Wegner. 2025. "Interleukin-4-Enhanced Oligodendrocyte Differentiation Depends on Extracellular Zinc Uptake via ZIP11" Cells 14, no. 22: 1756. https://doi.org/10.3390/cells14221756
APA StyleAratake, T., Kurita, S., & Wegner, M. (2025). Interleukin-4-Enhanced Oligodendrocyte Differentiation Depends on Extracellular Zinc Uptake via ZIP11. Cells, 14(22), 1756. https://doi.org/10.3390/cells14221756







