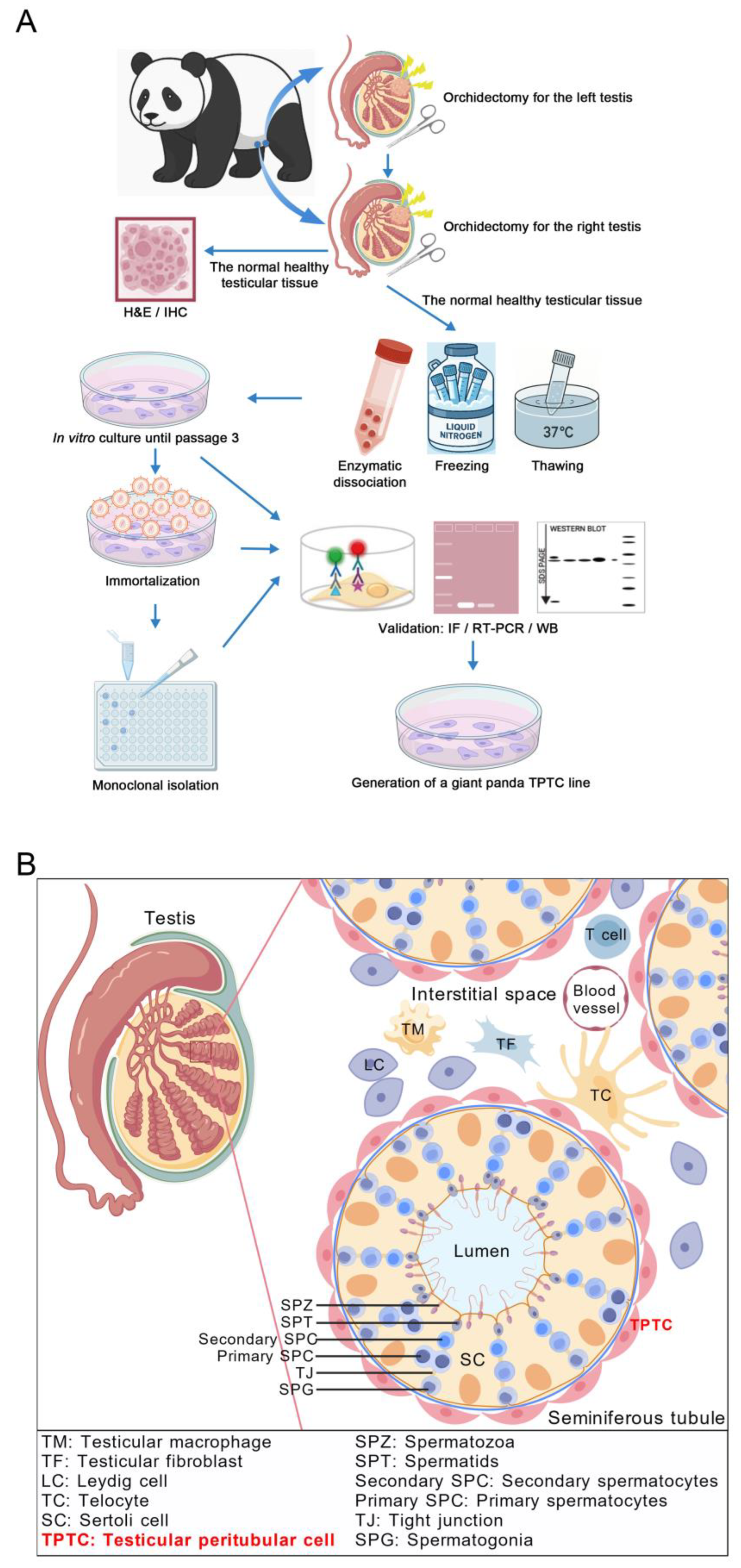
1. Introduction
Spermatogenesis, the process by which mature sperm are produced, is an extraordinarily precise and intricate biological event that safeguards the transmission of genetic information to the next generation. Spermatogenesis occurs in the testis, the male gonad where sex hormones are also synthesized, and maintenance of the testis’ function relies on intricate interactions between germ cells and various types of somatic cells. In addition to the well-characterized Sertoli and Leydig cells, testicular peritubular cells (TPTCs) have increasingly been recognized in recent years as a major somatic cell population that plays indispensable roles in testis function [
1].
TPTCs form the outermost layer surrounding the seminiferous tubules. They exhibit contractile properties, facilitating the transport of sperm towards the epididymis, and display typical smooth muscle-like features, with expression of characteristic markers such as α-smooth muscle actin (ACTA2) and calponin (CNN1) [
2,
3]. TPTCs also possess both endocrine and paracrine functions. For instance, they secrete cytokines such as glial cell line-derived neurotrophic factor (GDNF) and colony-stimulating factor 1 (CSF1) that are important to the self-renewal of spermatogonial stem cells (SSCs) [
4,
5], and they produce a variety of inflammatory factors such as IL-6 and IL-1β [
6], contributing to the testicular niche and immune homeostasis [
7]. Under pathological conditions such as male infertility or testicular fibrosis, TPTCs often undergo morphological alterations, along with changes in cytokine expression profiles and increased extracellular matrix (ECM) deposition, suggesting that TPTCs may play intricate regulatory roles in testicular development and diseases that remain incompletely understood [
8].
To gain more knowledge about TPTCs, both primary and immortalized cell models have been established in mammalian species. For instance, Albrecht and colleagues isolated and cultured TPTCs from human testis tissue [
9], whereas Schmid and coauthors introduced human telomerase reverse transcriptase (hTERT) into TPTCs from the common marmoset (
Callithrix jacchus), generating an immortalized monkey TPTC line with stable morphology and preserved functional properties [
10]. These cells have been demonstrated to contribute to mechanistic studies and drug screening, and extensive data obtained from these in vitro models have disclosed numerous TPTC-related features such as marker expression, cytokine secretion profiles, contractile response, and drug sensitivity. An illustration of this point is that human TPTCs maintained in vitro express classical smooth muscle markers, such as ACTA2 and CNN1 [
11], and exhibit a robust response to stimuli such as ATP and forskolin (FSK) [
10,
12,
13], indicative of their physiological plasticity and relevance.
Giant pandas (
Ailuropoda melanoleuca), a flagship endangered species under priority preservation in China, remain poorly understood in terms of their reproductive biology. One of the underlying reasons is their inherently low reproductive capacity, and because of this, arduous efforts have been made to enhance reproduction in this species. In addition to traditional assisted reproductive technology (ART), some novel approaches such as interspecies pregnancy, i.e., implantation of cloned giant panda embryos into the uterus of a surrogate from a different species, have been attempted, which resulted in fetal development but without live birth [
14]. In a recent study, primary fibroblasts were isolated from giant pandas and reprogrammed into induced pluripotent stem cells (GPiPSCs) using non-integrative episomal vectors [
15], paving the way for in vitro gametogenesis in this endangered species. Despite all these, due to the paucity of samples, the cellular composition of the testes and the mechanisms underlying spermatogenesis remain poorly understood in giant pandas. Recently we have, for the first time to our knowledge, conducted a 10 × genomics-based single-cell RNA-sequencing analysis on an adult giant panda testis, and annotated and characterized the giant panda testicular somatic cell populations including TPTCs at the molecular level [
16]. We also characterized the giant panda TPTCs in situ by conducting ACTA2 immunostaining on the testis’ sections, revealing one single layer of TPTCs surrounding the seminiferous tubules in giant pandas and ACTA2 as a conserved marker for this cell type in mammalian species [
16]. Another study, employing label-free quantitative proteomics, revealed stage-specific protein expression patterns in giant panda testes at distinct developmental stages and identified biomarkers related to reproductive development [
17]. These findings provide novel insights into the reproductive physiology of this endangered species.
Cell immortalization enables cells to proliferate unlimitedly in vitro while retaining the original cellular characteristics, thereby facilitating the relevant functional and mechanistic studies. Concerning TPTCs, they tend to undergo replicative senescence in vitro [
5], which is a hinderance to their in-depth studies, especially in non-model species like giant pandas. To obtain a stable and abundant cellular resource for giant panda TPTCs, thereby bettering the biological understanding of this peculiar cell type, we, for the first time, generated an immortalized monoclonal cell line with TPTC identities from giant pandas. The generated cell line not only represents a promising cellular model to investigate the mechanisms underlying the testicular niche, spermatogenesis, and reproductive disorders of giant pandas, but also lays the foundation for the development of novel ART and preservation strategies in this endangered species.
2. Materials and Methods
2.1. Animals
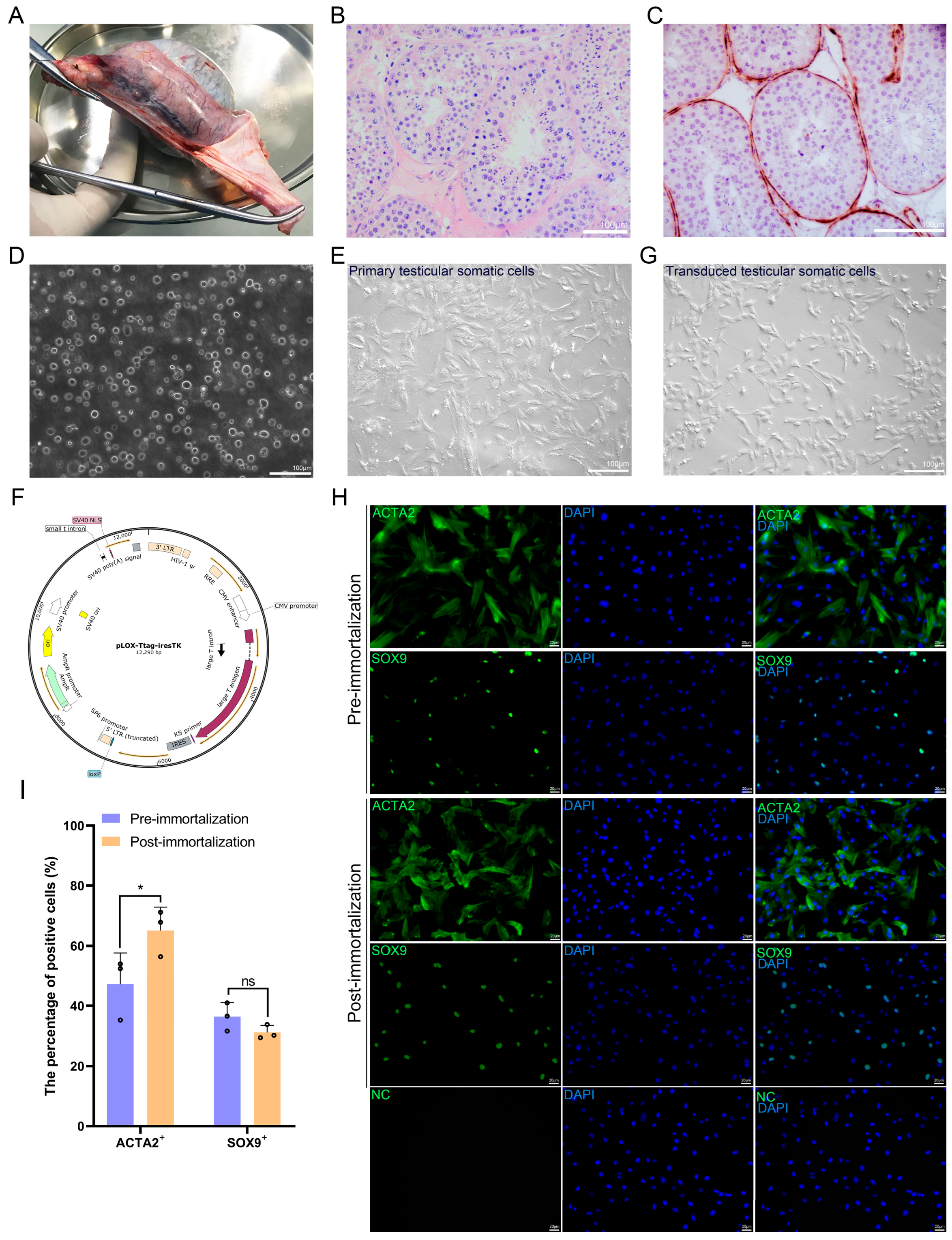
Testis samples were obtained from an adult giant panda raised in Chengdu Research Base of Giant Panda Breeding, Chengdu, China. Specifically, the donor was an 18-year-old male giant panda named Fufu. In January 2019, during a routine physical examination, it was noticed that its left testis was enlarged and had a firmer texture. The subsequent ultrasound examination revealed hypoechoic and hyperechoic masses in the left testis, leading to the preliminary diagnosis of tumors in the left testis. To circumvent metastasis, orchidectomy was performed for the left testis in February 2019. Later, pathological changes were also observed in its right testis, followed by orchidectomy for the right testis in May 2019. Upon removal of the right testis, the tumorous tissue was carefully separated, and only the normal healthy tissue was utilized for a battery of subsequent experiments. Specifically, a small part was used for fixation and staining, and the remainder was all used for enzymatic dissociation. All animal procedures abided with and were approved by the institutional animal ethical committee of Northwest A&F University (Approval Code: DK-20240422; Approval date: 21st March 2024) and the affiliated regulation enforcement agency in Chengdu Research Base of Giant Panda Breeding.
2.2. Hematoxylin and Eosin (H&E) Staining and ACTA2 Immunostaining
H&E staining and ACTA2 immunostaining were carried out as previously depicted [
16]. In brief, the normal healthy tissue from the right testis of the donor was cut into small pieces, followed by fixation with diluted Bouin’s solution and embedding in paraffin. After slicing at a thickness of 5 μm, testis sections were deparaffinized, rehydrated, and stained with H&E. For ACTA2 immunostaining, testis sections were subjected to heat-mediated antigen retrieval in the sodium citrate buffer. After blocking with the blocking buffer (37580, Thermo Fisher Scientific, Waltham, MA, USA) for 2 h at room temperature, testis sections were incubated with mouse anti-ACTA2 (1:50; BM0002, Boster Biological Technology, Pleasanton, CA, USA) primary antibody at 4 °C overnight. For negative controls, the primary antibody was replaced with the isotype mouse immunoglobulin G. After washing on the following day, testis sections were incubated with goat anti-mouse secondary antibody conjugated to HRP (1:50; A0216, Beyotime Biotechnology, Shanghai, China) for 1 h at room temperature. Then, testis sections were stained with DAB (P0202, Beyotime Biotechnology, Shanghai, China) and counterstained with hematoxylin, followed by visualization and image capture under a microscope.
2.3. Isolation of TPTCs
Giant panda testicular single-cell suspension was obtained following previously reported methodology [
16]. Briefly, after removal of the tunica albuginea and the surrounding connective tissue, the normal healthy tissue from the right testis of the donor was minced into small fragments and exposed to Dulbecco’s Modified Eagle Medium (DMEM, high glucose; 10-013-CVRC, Corning, New York, NY, USA) containing 2 mg/mL collagenase type IV (17104019, Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C for 20 min with gentle shaking, followed by centrifugation at 80×
g to remove interstitial cells and erythrocytes, and the precipitate containing seminiferous tubule fragments was collected and incubated with 0.25% trypsin-EDTA (Hyclone, Logan, UT, USA) and 0.5 mg/mL DNase I (Thermo Fisher Scientific, Waltham, MA, USA) at 37 °C for 5 min. Then, fetal bovine serum (FBS; FBS-CE500, Newzerum, Christchurch, New Zealand) was added to terminate the enzymatic dissociation, followed by filtration through a 40 μm mesh. After centrifugation at 450×
g for 5 min, cell pellets containing TPTCs were aliquoted and cryopreserved for future use.
2.4. Cell Culture
An aliquot of testicular somatic cells was thawed and subjected to the standard culture. Both the primary and immortalized testicular cells including PD-TPTCs were cultured with a complete medium consisting of DMEM (high glucose; 10-013-CVRC, Corning, Corning, NY, USA), 5% FBS (FBS-CE500, Newzerum, Christchurch, New Zealand), 1 × GlutaMAX (35050061, Thermo Fisher Scientific, Waltham, MA, USA), 1 × non-essential amino acids (NEAA; 11140050, Thermo Fisher Scientific, Waltham, MA, USA), 1 × penicillin-streptomycin (15140122, Thermo Fisher Scientific, Waltham, MA, USA), and 1 × mycoplasma inhibitor (40607ES08, Yeasen Biotechnology, Shanghai, China). Cells were maintained at 37 °C in an atmosphere of 5% CO2 in air, refreshed every two days, and passaged at a ratio of 1: 3 when arriving at around 80% confluency.
2.5. Immortalization of Giant Panda Testicular Cells
Cell immortalization was conducted as previously depicted [
18]. Briefly, the plox-Ttag-iresTK transfer vector (Addgene No. 12246) coding for the SV40 large T antigen, as well as the second-generation packaging vectors psPAX2 and pMD2.G, were co-transfected into HEK293T cells to generate lentiviral particles. Lentiviruses were then collected, ultracentrifuged for concentration, and titrated. For cell immortalization, the primary testicular somatic cells at passage 3 were seeded into 24-well plates for lentiviral transduction at a multiplicity of infection (MOI) of 5, following an established “spinfection” protocol [
18]. Specifically, cells exposed to viral suspension containing 10 μg/mL polybrene (HY-112735, MCE, Princeton, NJ, USA) were centrifuged at 3000×
g for 1 h at 32 °C. Following centrifugation, cells were incubated at 37 °C and refreshed after 16 h.
2.6. Generation of Monoclonal Cell Lines
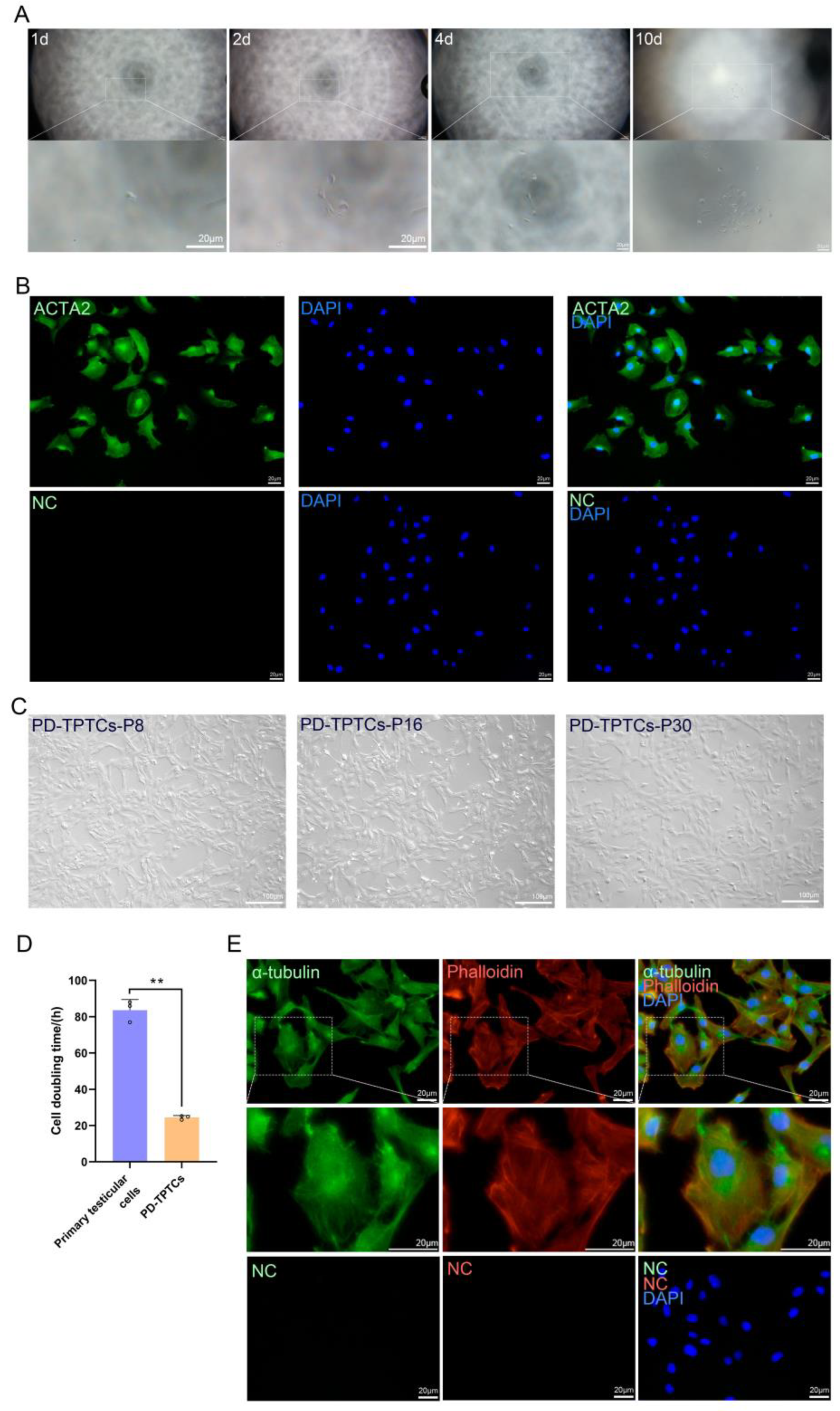
The immortalized giant panda testicular cells at passage 4 were collected and resuspended with the complete medium. The dilution of the cell suspension was limited to a final concentration of 10–100 cells/mL. Then, 100 μL of the diluted cell suspension was seeded into each well of a 96-well plate, ensuring that over half of the wells remained cell-free to maximize the probability of single-cell distribution in each well. Cell growth was monitored daily under a microscope, and wells containing a single cell were marked to facilitate tracking. Upon proliferation to approximately 80% of confluency, the monoclonal cells were dissociated with 0.25% trypsin-EDTA (Hyclone, Logan, UT, USA) and transferred to 24-well plates for further expansion.
2.7. Immunofluorescence
Cells propagated in 96- or 48-well plates were fixed with 4% paraformaldehyde (PFA) for 10 min and permeabilized with 0.25% Triton X-100 for 15 min at room temperature. Subsequently, non-specific binding was blocked by incubating the cells with either blocking buffer (37580, Thermo Fisher Scientific, Waltham, MA, USA) or 3% bovine serum albumin (BSA; 08R81068-CF, MP Biomedicals, Santa Ana, CA, USA) for 2 h at room temperature. After blocking, the cells were incubated with primary antibodies overnight at 4 °C. The primary antibodies utilized were rabbit anti-ACTA2 (1:200; 14395-1-AP, MP Biomedicals, Santa Ana, CA, USA), rabbit anti-SOX9 (1:500; ab185966, Abcam, Cambridge, UK), rabbit anti-SV40 (1:200; 15729S, Cell Signaling Technology, Danvers, MA, USA), rabbit anti-α-tubulin (1:200; 11224-1-AP, MP Biomedicals, Santa Ana, CA, USA), and TRITC-conjugated phalloidin (1:200; 4073ES75, Yeasen Biotechnology, Shanghai, China). For negative controls, the primary antibody was replaced with the isotype rabbit immunoglobulin G. On the following day, after thorough washing, the cells were incubated with donkey anti-rabbit secondary antibody conjugated to Alexa Fluor 488 or 594 (1:400; Yeasen Biotechnology, Shanghai, China) for 2 h at room temperature. After additional washing, cell nuclei were counterstained with DAPI (1:1000; SL7100, Coolaber, Beijing, China) for 5 min at room temperature. Fluorescence images were taken using a Nikon Eclipse 80i fluorescence microscope. To quantify the positive cells, three independent experiments were performed (n = 3), with 200 cells analyzed per group in each experiment.
2.8. Western Blot Analysis
Total proteins were extracted from the cells using RIPA lysis buffer (DY50002, DeeYeebio, Shanghai, China), followed by centrifugation at 1200 rpm for 10 min. Protein concentrations were determined using a BCA Protein Assay Kit (DY30204, DeeYeebio, Shanghai, China). Later, proteins were separated by SDS-PAGE, transferred to PVDF membranes, and blocked, followed by primary antibody incubation overnight at 4 °C. The primary antibodies used for immunoblotting were rabbit anti-SV40 (1:1000; 15729S, Cell Signaling Technology, Danvers, MA, USA), rabbit anti-ACTA2 (1:200; 14395-1-AP, MP Biomedicals, Santa Ana, CA, USA), and rabbit anti-α-tubulin (1:200; 11224-1-AP, MP Biomedicals, Santa Ana, CA, USA). On the following day, after thorough washing, the blots were incubated with the secondary antibody HRP-conjugated goat anti-rabbit IgG (1:2000; DY60202, DeeYeebio, Shanghai, China). After additional washing, the blots were visualized using a Western Bright ECL Kit (DY30208, DeeYeebio, Shanghai, China) and imaged with a Chemi-Doc XRS system (Bio-Rad, Hercules, CA, USA) via chemiluminescence detection.
2.9. RT-PCR Analysis
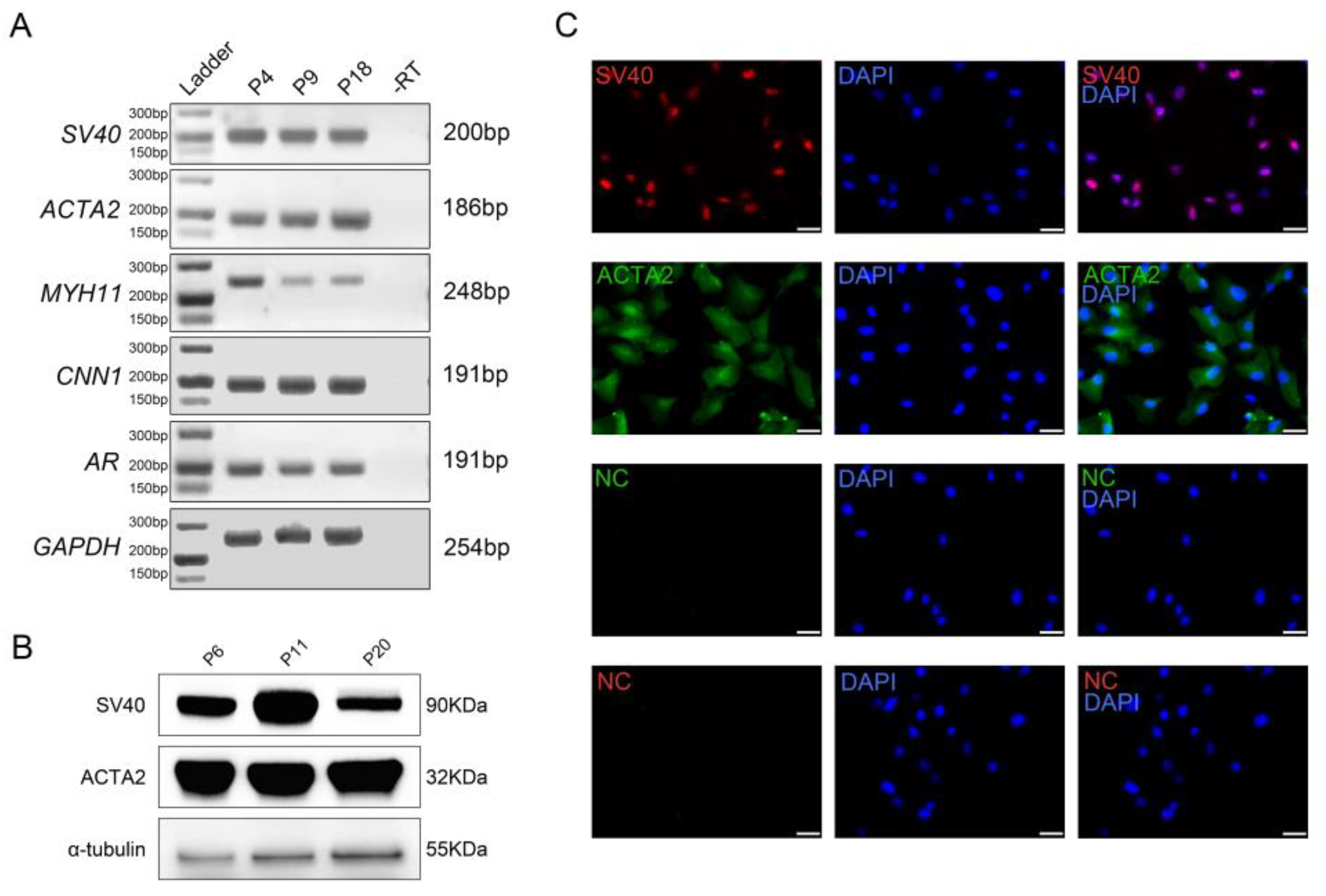
Total RNAs were extracted from the cells using the Trizol reagent (9109, TAKARA, Tokyo, Japan). Following genomic DNA (gDNA) removal by DNase treatment, complementary DNAs (cDNAs) were synthesized using a cDNA synthesis kit (DY90104, DeeYeebio, Shanghai, China). PCRs were then performed using the synthesized cDNAs as templates. The amplified products were separated by electrophoresis on a 2% agarose gel, stained with nucleic acid dye (DY10214, DeeYeebio, Shanghai, China), and visualized under ultraviolet light. Negative controls entailed total RNAs without reverse transcription (-RT) that were subjected to PCR. The primer sequences and expected RT-PCR product sizes are listed in
Table 1.
2.10. Functional Validation by Quantitative Real-Time PCR (qPCR)
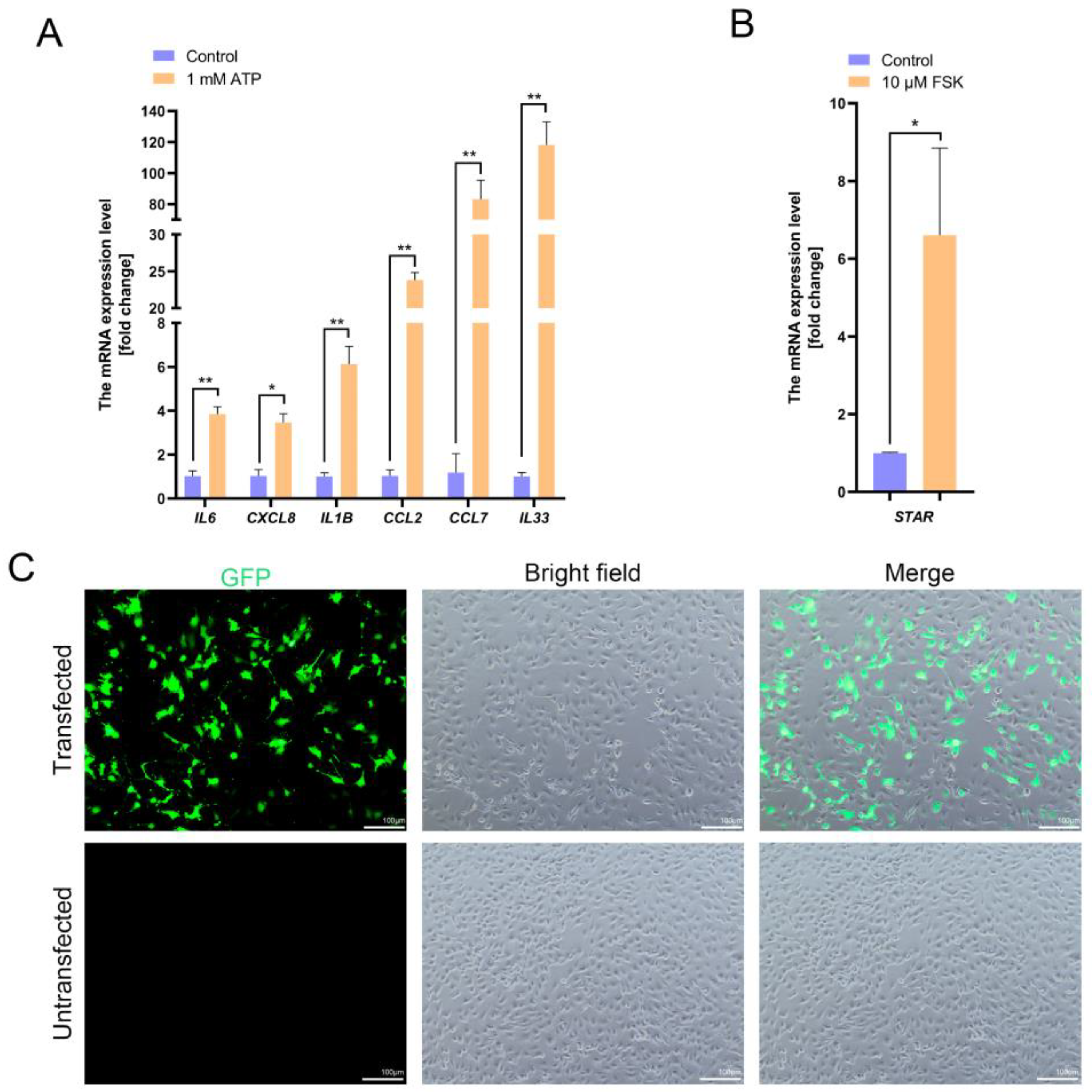
Functional validation of giant panda TPTCs was performed as previously described [
10]. Briefly, the immortalized monoclonal TPTCs at passage 25 were exposed to a serum-free medium harboring ATP (1 mM; Y38247, Shanghai Yuanye Bio-Technology, Shanghai, China), FSK (10 µM; F70001, Psaitong, Beijing, China), or 0.1% ethanol (control) for 24 h. Then, total RNAs were extracted and reversely transcribed using a cDNA synthesis kit (DY90104, DeeYeebio, Shanghai, China). The resultant cDNAs underwent a 30-fold dilution, followed by qPCR using the ChamA Universal SYBR qPCR Master Mix (Q711-02, Vazyme, Nanjing, China) and running on an FQD-96A platform (Bioer, Hangzhou, China). The thermal cycling conditions were 95 °C for 5 min, 40 cycles of 98 °C for 10 s and 60 °C for 30 s, and a final extension at 72 °C for 10 min.
GAPDH was used as the reference gene, and the relative gene expression level was calculated using the 2
−ΔΔCT method. The results were normalized to those in the control. The primer sequences and qPCR product sizes are listed in
Table 1.
2.11. Transfection
For transfection, the immortalized monoclonal TPTCs at passage 25 were seeded into 24-well plates, and when reaching 50–70% of confluency, they were transfected with GFP plasmids (pGreenPuro; System Biosciences, Palo Alto, CA, USA) using the Hieff Trans™ Liposomal Transfection Reagent (40802ES, Yeasen Biotechnology, Shanghai, China), following the manufacturer’s instructions. Cells were refreshed and fluorescence images were taken 24 and 48 h post-transfection. To quantify the transfection efficiency, three independent experiments were performed (n = 3), with 200 cells analyzed in each experiment.
2.12. Karyotyping
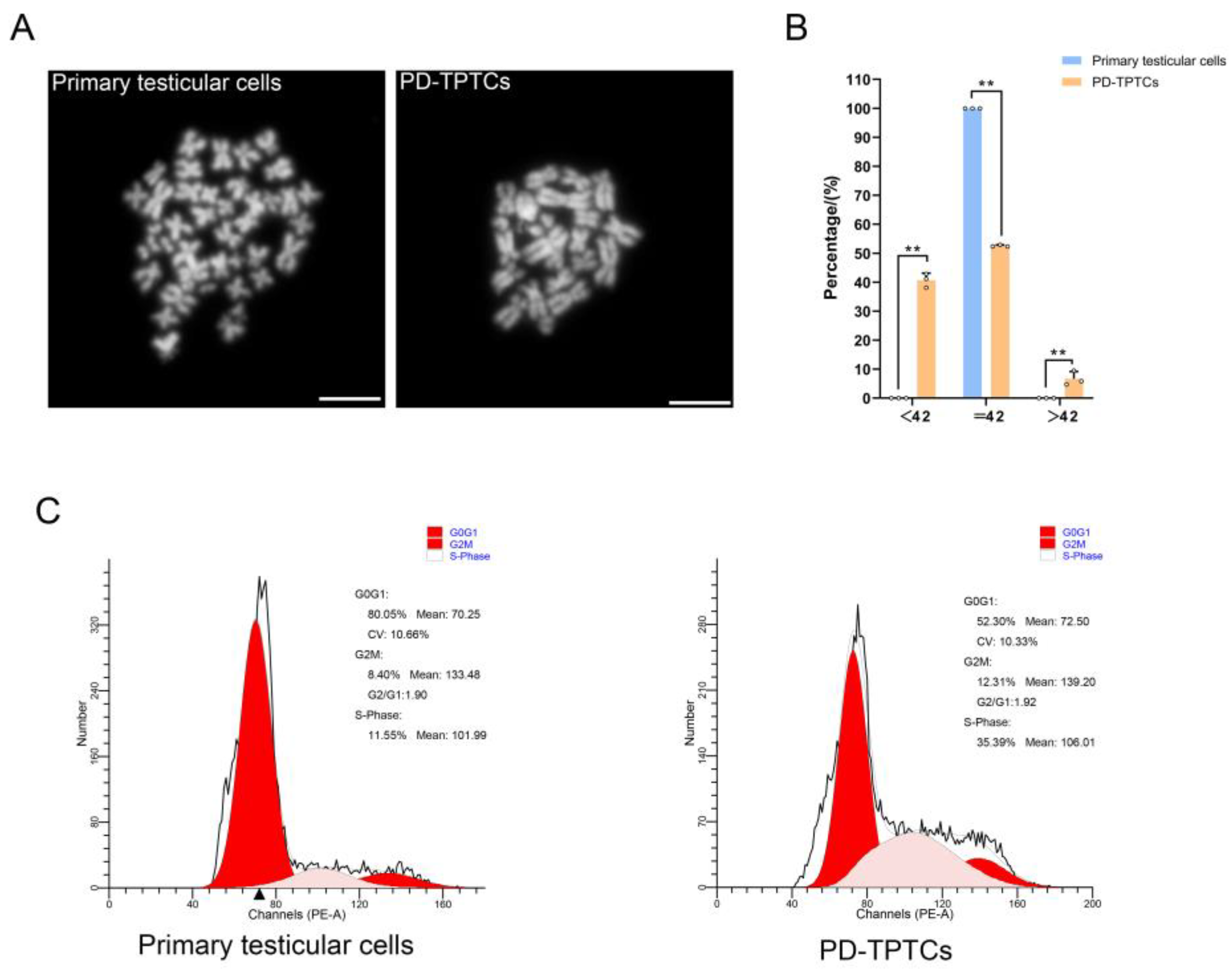
Karyotyping was carried out on both primary giant panda testicular cells (passages 3–5) and the immortalized monoclonal TPTCs (passage 26). Briefly, cells at roughly 70% of confluency were treated with 0.4 μg/mL colchicine (ST1173, Beyotime Biotechnology, Shanghai, China) for 4 h, swollen with 75 mmol/L KCl, and fixed with methanol–glacial acetic acid (3: 1). The cell suspension was then dropped onto cold slides. After air drying, the slides were stained with DAPI (1:1000; SL7100, Coolaber, Beijing, China) and visualized using a Nikon Eclipse 80i fluorescence microscope. Three independent experiments were performed (n = 3), with 100 cells analyzed per group in each experiment.
2.13. DNA Content Analysis
A DNA content analysis was conducted on both primary giant panda testicular cells (passages 3–5) and the immortalized monoclonal TPTCs (passage 28). The DNA content was analyzed by flow cytometry as previously described [
19], using a FACS analyzer (BD Biosciences, San Jose, CA, USA) and ModFit LT 32 software version 4.0 (Verity Software House, Topsham, Maine, USA).
2.14. Statistics
Unless otherwise specified, all data are presented as the mean ± standard error of the mean (SEM) from three independent experiments. The statistical significance between the two groups was assessed using a two-tailed Student’s t-test. Differences were considered statistically significant at p < 0.05 (*) and highly significant at p < 0.01 (**).
4. Discussion
TPTCs, a major somatic cell type in the testis, were first observed through electron microscopy in the mid-20th century and described as contractile cells arranged around the seminiferous tubules that exhibit the morphological characteristics of smooth muscle cells [
20]. In our previous study, cells located in the giant panda peritubular compartment were found to express ACTA2 [
16], suggesting the presence of TPTCs with contractile features in this species as well. For a long time, their role was simply perceived as purely mechanical, assisting in the transport of sperm towards the epididymis. Yet, recent studies have revealed that TPTCs possess a wide array of functions beyond mechanical support, including immune modulation, maintenance of the testicular niche, ECM remodeling, and SSC fate determination [
1]. In mice, TPTCs have been demonstrated to secrete essential regulatory factors such as GDNF and CSF1 that influence the SSC behavior [
21], and by conditional knockout using TPTC-specific Cre drivers, their indispensable roles in spermatogenesis have been studied in detail [
22]. In humans, TPTCs can be isolated and cultivated in vitro [
9], and the primary TPTCs display the classic smooth muscle-like phenotype, express markers such as ACTA2, CNN1, and CALD1, and are capable of secreting key factors like GDNF, IL-6, and CXCL8 that are important to SSC maintenance and immune homeostasis [
11]; thus, they have been extensively harnessed to investigate the effects of various compounds and hormones (e.g., FSK and ATP) on TPTC functions. Nonetheless, the primary culture of TPTCs is associated with issues such as replicative senescence and donor-derived variability. In non-human primates, an immortalized TPTC line from the common marmoset (
Callithrix jacchus) has been successfully established through delivering hTERT into the cells. This immortalized cell line closely resembles its human counterpart in terms of marker expression and functional response [
10], making it one of the most physiologically relevant TPTC models suitable for comparative studies.
While there are studies on TPTCs in mice, humans, and non-human primates that have significantly advanced the understanding of their functions, corresponding research in specific endangered species such as giant pandas remains extremely limited, which is largely ascribed to the paucity of samples and the lack of robust in vitro cell models. Indeed, TPTCs tend to undergo replicative senescence under in vitro conditions, gradually losing proliferative capacity with time [
5]. In this study we have, for the first time to the best of our knowledge, established an immortalized monoclonal cell line with TPTC identities from giant pandas (PD-TPTCs). The generated cell line was demonstrated to express key TPTC markers such as ACTA2, MYH11, CNN1, and AR. Further functional assays revealed that they could respond to ATP stimulation with a pro-inflammatory gene expression profile and to FSK with increased steroidogenic activity. Moreover, they were shown to be amenable to lipofection. Hence, the established PD-TPTC line represents a promising cellular model to investigate the mechanisms underlying the testicular niche, spermatogenesis, and reproductive disorders in giant pandas.
Indeed, testis samples from giant pandas are extremely rare. Typically, they are only available postmortem, and in some exceptional cases, surgically excised tissue can be collected during medical interventions. In this study, testicular tumors were, fortunately, identified at an early stage, and the testis was only partially compromised, with three clearly different sections, i.e., normal tissue, tumorous tissue, and necrotic tissue, macroscopically discerned. Notably, all testicular tissue used in this study was taken from the central portion of macroscopically normal tissue located away from the lesion margin, and H&E staining revealed the normal histology of the collected healthy tissue, showing the typical seminiferous tubule architecture and the harbored various stages of germ cells. Moreover, the karyotyping analysis disclosed the normal karyotype of primary testicular cells that consisted of 21 pairs of chromosomes characteristic of the giant panda, together minimizing the possibility of contamination with transformed cells. Despite all these, we acknowledge the limited representativeness of the findings, since the cell line was derived from a single giant panda testis, which is chiefly due to the paucity of testis samples from this endangered species. In this sense, future studies are needed to corroborate the utility and physiological relevance of the generated cell line.
SV40 large T antigen is a well-acknowledged potent exogenous agent needed to attain cell immortalization [
23]. By binding to the tumor suppressor p53 and the retinoblastoma susceptibility protein Rb and inhibiting their functions, SV40 large T antigen enables cells to evade apoptosis and sustain continuous proliferation [
24]. Given the lack of approaches to efficiently enriching TPTCs at the moment, we first introduced SV40 large T antigen into primary testicular somatic cells derived from giant pandas by employing an optimized lentiviral transduction strategy termed “spinfection” [
18], followed by monoclonal isolation, leading to the ultimate generation of an immortalized monoclonal cell line with TPTC identities (PD-TPTCs). This cell line exhibited robust proliferative capacity and propagated in vitro for over five months, thus providing a reliable cellular resource for downstream functional assays. Notably, no manifest morphological alterations were observed throughout the culture process, and the cells consistently showed the spindle or elongated fibroblast-like morphology with relatively compact arrangement. Nevertheless, karyotyping and DNA content assays revealed that the PD-TPTC line exhibited chromosomal structural alterations or aneuploidy, which is not surprising, as SV40 large T antigen-mediated cell immortalization is typically associated with cell transformation characterized by chromosomal structural or numerical anomalies [
25].
ACTA2 has extensively been utilized as a reliable marker for characterization of TPTCs. Recently, by conducting ACTA2 immunostaining on testis sections from an adult giant panda, we characterized the giant panda TPTCs in situ, revealing one single layer of TPTCs surrounding the seminiferous tubules in giant pandas [
16] and suggesting that these cells may also exhibit smooth muscle-like phenotypes in this endangered species. Here, to characterize the generated cell line, we performed RT-PCR, immunofluorescence, and Western blot analyses, and the results showed that the PD-TPTC line expressed ACTA2, MYH11, CNN1, and AR. This marker expression pattern highly resembles that in human TPTCs [
9], confirming that the generated cell line retains the TPTC characteristics. Among these markers, ACTA2 and CNN1 are key components of the contractile apparatus [
11], and their expression suggests that the PD-TPTC line retains the contractile property that is characteristic of myoid cells. CNN1 has also been widely acknowledged as a TPTC marker in non-human primates. It plays a critical role in the fine-tuning of smooth muscle contractility, with its expression level commonly used to assess the functional state of TPTCs [
26]. The expression of CNN1 in the generated cell line lends further support to its utility as a reliable marker for TPTCs in giant pandas. In addition, AR was shown to be expressed in the PD-TPTC line, suggesting that these cells may be capable of responding to androgen signaling [
27] and, therefore, performing a potential role in testicular paracrine and endocrine regulation.
To functionally validate the generated PD-TPTC line, we applied a previously depicted strategy by Schmid and coauthors. As they reported, treatment of immortalized monkey TPTCs with 1 mM ATP markedly upregulated inflammatory mediators such as IL1B, CCL2, and CCL7, indicative of immune response to local inflammatory stimuli [
10]. ATP, as an exogenous purinergic signaling molecule, is massively released upon tissue injury or under stress conditions, acting as a “danger-associated molecular pattern (DAMP)” through the activation of P2X/P2Y receptors and mediating intracellular Ca
2+ signaling, leading to activation of the NF-κB pathway and the inflammatory factor cascade [
28,
29]. Here, treatment of PD-TPTCs with 1 mM ATP for 24 h consistently resulted in significant upregulation of inflammatory factors such as IL6, CXCL8, IL1B, CCL2, CCL7, and IL33, indicating a robust response to inflammatory cues, which highly resembles that in immortalized monkey TPTCs, suggesting that the PD-TPTC line generated in this study retains the core biological features of TPTCs that are related to inflammation regulation. In addition, FSK, a well-known adenylyl cyclase activator, is commonly used to stimulate steroidogenic activity by activating the cAMP/PKA signaling pathway and inducing the expression of steroidogenesis-related genes [
30,
31]. It has been reported that FSK stimulation elevated the expression of STAR in immortalized monkey TPTCs [
10], suggesting a supportive role of TPTCs in local steroid homeostasis in testes. Consistently, our results showed that treatment of PD-TPTCs with 1 mM FSK for 24 h also significantly increased the
STAR mRNA level, pinpointing the steroidogenic response of the generated PD-TPTC line.
To date, no direct experimental evidence has linked TPTC dysfunction to fertility impairment in giant pandas. Despite this, analogous findings from other species suggest that TPTC abnormalities may have a detrimental influence on reproductive health in this endangered species as well. In humans and mice, TPTC dysfunction has been associated with male infertility, testicular dysgenesis, and testicular fibrosis, all hallmarked by disorganized TPTC architecture, dysregulated cytokine secretion, and increased ECM deposition, collectively undermining the SSC niche, impeding SSC maintenance, and reducing sperm output [
7,
32].
In addition, there is currently no direct evidence that TPTC disfunction causes fertilization defects. But on the other hand, TPTCs’ critical roles in orchestrating the SSC niche and sperm quality represent a vital avenue for future research. With the advent of single-cell omics, TPTC-Sertoli cell co-culture systems, and in vitro organ-on-chip testicular models, future studies are expected to elucidate how TPTCs influence SSC fate, sperm maturation, and ultimate fertilization competence, thereby providing potential strategies to support reproductive preservation in endangered species such as giant pandas.