1. Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental condition marked by impaired communication, social deficits, and repetitive behaviors [
1]. Symptoms may present differently in different individuals with varying types and severity. Imaging and post-mortem studies of ASD document early aberrations of brain cytoarchitecture, specifically, enlarged brain size and alterations of dendritic spines [
1,
2]. Genetic analysis supports the heritability of ASD, as a significantly higher concordance rate has been found in homozygotic twins compared to heterozygotic siblings [
3]. However, the genetic architecture of ASD seems to be heterogeneous and complex, whereby hundreds to thousands of genetic mutations have been linked to ASD, with no single mutation being responsible for more than 1–2% of cases [
4]. Identified ASD genes are involved in diverse pathways that regulate brain development and synaptic formation and function [
4]. Among the top ASD genetic candidates have been found several mutations in genes associated with calcium (Ca
2+) signaling [
5,
6].
The initial recognition for Ca
2+ dysregulation in ASD stems from Timothy syndrome, a monogenetic disorder with autistic features, caused by a de novo mutation in
CACNA1C, which is a gene that encodes the alpha 1C subunit proteins of an L-type voltage-gated Ca
2+ channel [
7]. Cumulative studies of the idiopathic form of ASD increasingly implicate Ca
2+ dysregulation in the pathogenesis of the disorder. Genetic studies identified a de novo copy number variant in the inositol triphosphate receptor (IP3R) gene in ASD patients [
8]. In addition, functional studies using fibroblasts derived from both monogenetic and sporadic ASD patients showed a shared phenomenon of depressed Ca
2+ release through IP3R [
9,
10]. In parallel, animal studies showed that mice with astrocyte-specific knockout of IP3R displayed autistic symptoms, mainly resulting from compromised Ca
2+ signaling and deficiency in ATP release from astrocytes, which subsequently led to dysfunctional purinergic signaling in neurons [
11]. Given the versatility and diversity of Ca
2+ signaling functions, it has been proposed that Ca
2+ signaling may represent a major hub where genetic abnormalities in ASD converge to produce their deleterious effect, but this requires investigation, especially in the context of human brain development.
The lack of access to living human brains and the limited applicability of animal studies to human brain regions responsible for higher functions are major obstacles to understanding cellular mechanisms that underlie brain disorders, including ASD. Induced pluripotent stem cells (iPSCs) offer a robust complementary tool to study complex human brain disorders in the context of development in vitro [
12,
13]. Several studies have examined idiopathic ASD and/or investigated ASD specific mutations using iPSC technologies and showed aberrant Ca
2+ signaling [
14,
15,
16,
17,
18,
19,
20]. DeRosa et al. (2018) [
14] conducted a time course transcriptomic analysis on neurons derived from idiopathic ASD iPSCs spanning two time points during the process of differentiation to cortical neurons, including day 35 and day 135. Their results yielded Ca
2+ signaling as one of the top pathways dysregulated in ASD, especially early during differentiation at day 35. This was further supported by Ca
2+ imaging, which showed a significant decrease in the number of spontaneous Ca
2+ transients in day 35 differentiated neurons [
14]. Another study performing transcriptomic analyses across different stages of neuronal differentiation in ASD showed that the pathology starts as early as iPSCs start differentiating to neurons and that among the top significant signaling pathways implicated early during the differentiation is Ca
2+ signaling [
15]. Avazzadeh et al. (2019) [
16] reported that ASD iPSC-derived cortical neurons harboring a heterozygous mutation in
NRXN1 alpha displayed altered Ca
2+ release kinetic compared to controls, including higher frequency, longer duration, and bigger amplitude. They also performed transcriptomic analyses and found that among the top differentially expressed genes between ASD and control neurons are genes that encode voltage-gated calcium channels. Teles et al. (2022) [
17] investigated Ca
2+ homeostasis in iPSC-derived neurons harboring a heterozygous missense mutation in the
RELEN gene and a de novo splice site variant in
CACNA1H. ASD cells showed enhanced Ca
2+ influx through Cav3.2 and displayed overactivation of mTORC1 signaling and impaired Reelin signaling. Several recent studies investigating specific ASD risk genes or variants also reported impaired Ca
2+ signaling in neurons derived from iPSCs [
18,
19,
20]. Altogether, these studies implicate aberrant Ca
2+ signaling in the pathogenesis and pathophysiology of ASD early during neuronal differentiation.
Whilst many studies investigated Ca2+ signaling in ASD using iPSC models, the majority focused on studying specific ASD mutations or risk variants and characterizing spontaneous calcium transients. Herein, we sought to conduct stimulus response functional characterizations to investigate receptor-mediated Ca2+ dynamics in idiopathic ASD. We first performed transcriptomic analyses in samples from multiple stages along the course of differentiation, sequentially including the iPSC stage, neural induction stage (NI), neurosphere stage (NSP), and differentiated cortical neurons stage (Diff). Our data revealed that the numbers of Ca2+ signaling-relevant differentially expressed genes (DEGs) between the ASD and control groups were higher in samples from the iPSC and differentiated (Diff) stages. Accordingly, we performed our functional characterization of Ca2+ at the iPSC and Diff stages. Our findings indicated that iPSC-stage ASD samples displayed elevated maximum Ca2+ levels in response to ATP compared to controls. By contrast, differentiated ASD neurons exhibited reduced maximum Ca2+ levels in response to ATP stimulation but showed elevated maximum Ca2+ levels compared to control neurons following stimulation with KCl and (S)-3,5-dihydroxyphenylglycine (DHPG), a selective group I metabotropic glutamate receptor agonist. This may suggest that ASD neurons have higher excitability and/or mature at a faster rate than control neurons. These results provide complementary evidence for Ca2+ signaling dysregulation in ASD and support the use of the iPSC differentiation system as a robust model to study developmental disorders in vitro.
2. Materials and Methods
2.1. Derivation, Characterization, and Maintenance of Stem Cell Lines
Stem cells were utilized in accordance with the guidelines and regulations of King Faisal Specialist Hospital and Research Centre (KFSH&RC) and with the approval of the Office of Research Affairs Research Ethics Committee and Basic Research Committee (Project no: 2190006 and approval reference no: C380/345/41). Two ASD iPSC lines (SC119 and SC125) and two healthy iPSC controls (007 and FAM) were included in this study (
Table 1). The two idiopathic ASD iPSC lines were generously provided by the University of Melbourne, Melbourne, Australia, under an approved and signed material transfer agreement. Healthy iPSC line 007 was kindly provided by Prof. Alice Pébay (UoM, Melbourne, Australia) [
21,
22], and iPSC line FAM was kindly provided by Dr. Reem Alhejilan (KFSH&RC, KSA, Riyadh, Saudi Arabia) [
23]. Cells were cultured using the feeder free system on vitronectin-coated 60 mm dishes (Stem Cell Technologies, Vancouver, BC, Canada, CAT#: 07180), maintained in TeSR™-E8 defined medium as per the manufacturer’s instructions (Stem Cell Technologies, CAT#: 05990). Colonies were mechanically dissected on a weekly basis and replated on freshly coated plates to maintain their pluripotency, and they were regularly assessed for pluripotency by morphology and expression of OCT4. Cells were maintained at 37 °C under 5% CO
2 and medium changes were performed daily.
Fibroblasts of ASD iPSC lines were originally obtained from Dr. Philip Schwartz (Children’s Hospital of Orange County Research Institute, Orange, CA, USA). ASD Fibroblasts were reprogrammed in the Dottori laboratory using the LONZA Transfection System (Lonza Group AG, Basel, Switzerland, CAT#: VP100) with non-viral episomal vectors provided by the Epi5 Episomal iPSC Reprogramming Kit (Life Technologies, Carlsbad, CA, USA, CAT#: A15960). The generated ASD iPSC lines were validated for pluripotency through the expression of key markers using the immunofluorescence assay (
Figure S1). They were also assessed for their differentiation potential toward endoderm, mesoderm, and ectoderm derivates (
Figure S2).
For endoderm differentiation, embryoid bodies (EBs) were generated from both ASD lines and harvested at day 14 for RT-PCR analysis. Total RNA was extracted using the PureLink RNA Kit according to the manufacturer’s instructions (Life Technologies, CAT#:12183025), and one microgram of RNA was used to synthesize first-strand cDNA using the Sensifast cDNA Synthesis Kit (Bioline, Cincinnati, OH, USA, CAT#; BIO-65054), according to the manufacturer’s instructions, under the following conditions: 25 °C for 10 min (primer annealing), 42 °C for 15 min (reverse transcription), 85 °C for 5 min (inactivation), and 4 °C hold. Each final reaction contained 1 µg cDNA in a volume of 20 µL. The GATA6 transcript—an endoderm marker—was amplified using the OneTaq RT/PCR Kit (BioLabs, Boston, MA, USA, CAT#: E5310S), following the manufacturer’s instructions, and visualized using a FluorChem E blot analyzer (ProteinSimple, San Jose, CA, USA). The following primers sequences were used: GATA6 (F: GCC TCA CTC CAC TCG TGT CT; R: TCA GAT CAG CCA CAC AAT ATG A) and BETA ACTIN (F: CAC CAC ACC TTC TAC AAT GAG C; R: TCG TAG ATG GGC ACA GTG TGG G).
For mesoderm differentiation, both ASD lines were differentiated toward cardiac specification. IPSCs were dissociated into single cells with TrypLE (Thermo Fisher Scientific, Waltham, MA, USA) and seeded onto plates coated with hESC-qualified Matrigel (Corning, Corning, NY, USA) at a density of 1 × 105 cells/cm2 in TeSR-E8 medium supplemented with 10 µM Y-27632 (Tocris Bioscience, Bristol, UK). After 2 days, which is referred to as day 0, the medium was replaced with RPMI 1640 basal medium containing B-27 without insulin supplement (Thermo Fisher Scientific), growth factor-reduced Matrigel (1:60 dilution), and 10 µM CHIR99021 (Cayman Chemical, Ann Arbor, MI, USA). After 24 h, cells were initially treated with 5 ng/mL Activin A (Peprotech, Cranbury, NJ, USA) in RPMI 1640 basal medium containing B-27 without insulin supplement for 24 h. On day 2, the medium was changed to RPMI 1640 basal medium containing B-27 without insulin supplement and 5 µM IWP2 (Tocris Bioscience) for 72 h. Starting from day 5, cultures were maintained in RPMI 1640 supplemented with B-27 (Thermo Fisher Scientific) and 200 µg/mL L-ascorbic acid 2-phosphate sesquimagnesium salt hydrate (Sigma-Aldrich, St. Louis, MO, USA). On day 14, the samples were fixed for immunofluorescence analysis.
For ectoderm differentiation, both ASD iPSC lines were differentiated toward neuroectoderm specification as described below (see
Section 2.2). Neurospheres (NSPs) at day 28 of differentiation were fixed for the immunofluorescence assay.
2.2. Differentiation of iPSCs
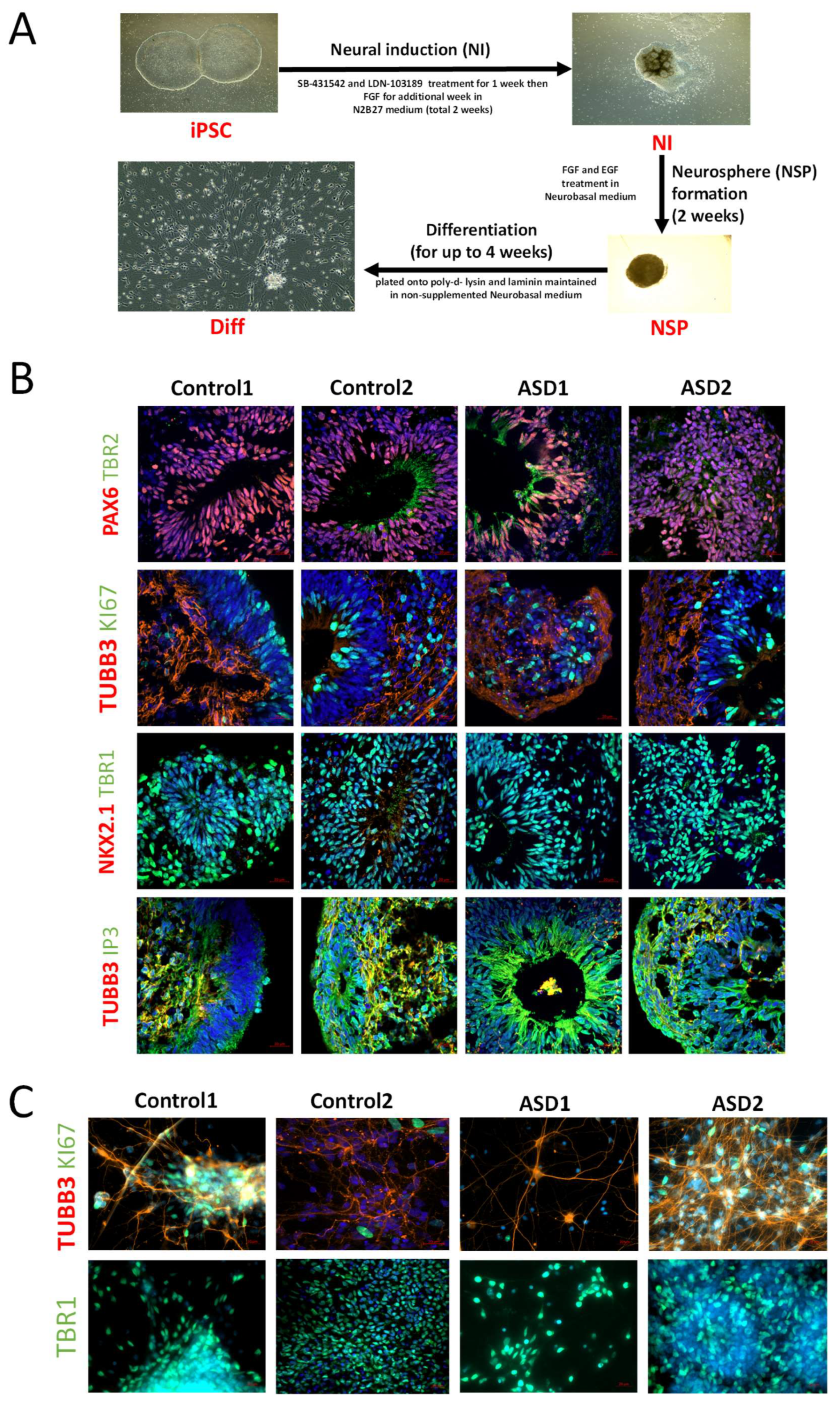
For neural induction and differentiation of iPSC lines, the dual SMAD inhibition protocol was adopted as previously described by Denham and Dottori, with some slight modifications (
Figure 1A) [
24]. Briefly, iPSC lines were mechanically dissected into pieces approximately 0.5 mm in width and transferred onto laminin-coated organ culture plates in N2B27 medium containing 1:1 mix of neurobasal medium with DMEM/F12 medium, supplemented with 1% insulin transferrin selenium, 1% N2, 1% retinol-free B27, 0.3% glucose, 25 U/mL penicillin, and 25 μg/mL streptomycin (Thermo Fisher Scientific, CAT#: 11320033, A3582901, 17502-048, 12587-010, 51300-044, 25030-081, and 15070063, respectively). For induction of cortical neurons, the small molecule inhibitor SB431542 (10 μM, Tocris, CAT#: 1614) and LDN (100 ng/mL, Peprotech, CAT#6053) were added to the medium for the first 7 days, followed by the addition of FGF2 (20 ng/mL, Peprotech, CAT#: 130093841) for the remaining 7 days. Following 2 weeks of neural induction (NI), neural progenitors were mechanically harvested and cultured in suspension in neural basal medium (NBM) supplemented with FGF2 (20 ng/mL, Peprotech, CAT#: 130093841) and EGF (20 ng/mL, Peprotech, CAT#: AF-100-15) to promote NSP formation [
25]. For terminal differentiation (Diff), NSPs were mechanically dissociated, seeded onto 25 mm coverslips coated with poly-D-lysine (SIGMA Aldrich, St. Louis, MO, USA, CAT#: P1024) and laminin (Life Technologies, CAT#: 23017015), and maintained in NBM medium without supplements for up to 4 weeks. Cells were maintained at 37 °C in 5% CO
2 incubators and the medium was changed every second day.
2.3. Immunofluorescence
Cell monolayers (samples from iPSC, NI, and Diff) and NSPs were fixed in 4% PFA for 20 min at 4 °C and then washed briefly in PBS. NSPs were embedded in Tissue-Tek OCT compound (Labtek, Grand Rapids, MI, USA), cut in 15 μm slices on a cryostat, and sections were placed on superfrost slides. After washing in PBS, sections or culture dishes were permeabilized in 0.1% Triton-X-100 in PBS (PBT) for 5 min and then blocked in 10% fetal calf serum in PBT for 60 min at room temperature. Samples were then incubated with primary antibody (diluted in the block buffer) overnight at 4 °C. The following primary antibodies were used: OCT4A (R&D, Minneapolis, MN, USA, CAT# MAB17591), SOX2 (R&D, CAT# MAB2018), NANOG (R&D, CAT#:AF1997), TRA160(R) (R&D, CAT#MAB4770), ACTC1 (Sigma-Aldrich), PAX6 (Santa Cruz, Dallas, TX, USA, CAT#: sc-81649), TBR2 (Abcam, Cambridge, UK, CAT#: ab23345), TBR1 (Abcam, CAT#: ab31940), CTIP2 (Abcam, CAT#: ab18465), SATP2 (Abcam, CAT#: ab51502), TUBB3 (Millipore, Burlington, MA, USA, CAT#: MAB1637), KI67 (Abcam, CAT#: ab15580), NKX2.1 (Abcam, CAT#: ab72876), and IP3R (Abcam, CAT#: ab5804). Following three 5 min washes in PBT, ALEXA-fluor secondary antibodies (Life Technologies/Invitrogen, Carlsbad, CA, USA) (1:1000 diluted in the block buffer) were applied for 1 h at room temperature. All samples were counterstained with 49,6-diamidino-2-phenylindole (Dapi; 1 μg/mL, SIGMA-Aldrich). Samples were then mounted onto glass slides with Mowiol aqueous mountant, followed by viewing and image capturing under Zeiss spinning wheel confocal microscope Axio Observer 7 (Carl Zeiss Microscopy GmbH, Jena, Germany) using ZEN imaging software version 3.4.
2.4. RNA Isolation and Transcriptome Analysis
RNA was isolated from samples using the Qiagen RNeasy Mini Kit, as per the manufacturer’s instructions. In our study, we used two independent iPSC lines reprogrammed from two ASD donors and two independent iPSC lines reprogrammed from two control donors. Each line represented one clone per donor; thus, the biological replicates were donor-derived iPSC lines (n = 2 per group). All four lines underwent the dual SMAD inhibition differentiation protocol on three independent occasions. At each differentiation run, samples were collected at four developmental stages: (i) the iPSC stage (at least three colonies per dish, 3 dishes per line), (ii) the NI stage (day 14 rosettes derived from at least three colonies, 3 dishes per line), (iii) the NSP stage (2-week-old NSPs, 12–18 NSPs per line), and (iv) the Diff stage (neurons differentiated from ~12 NSPs plated into 3 dishes per line). For RNA-seq, RNA was extracted independently from each replicate dish (or NSP batch) per line and time point. Following extraction, RNA samples from replicate dishes within the same line and time point were pooled prior to library preparation. Thus, the unit of biological replication was the iPSC line (one clone per donor), while technical variability between dishes was averaged by pooling post-extraction.
For each of the four iPSC lines (two ASD and two control lines), we collected RNA samples at four time points along the differentiation trajectory: iPSC, 2-week NI, 2-week NSP, and 4-week Diff. This resulted in a total of 16 RNA samples, representing four independent biological replicates (iPSC lines) per time point. The concentration and overall quality of RNA samples were assessed using a Nanodrop ND-100 spectrophotometer and Agilent 2100 Bioanalyzer, with all samples showing an RNA integrity number (RIN) greater than 8 (
Figure S7).
RNA sequencing and bioinformatics analysis were outsourced to BGI Genomics (Hong Kong) using the DNBSEQ platform (MGI Tech Co., Ltd., Shenzhen, China), averagely generating about 6.62G Gb bases per sample. The sequencing data were filtered using SOAPnuke. All of the samples had greater than 80% clean reads after filtering out reads of low quality (base quality ≤ 15), reads with adaptor sequences, and reads with high levels of unknown base N. The clean reads were mapped to human genome reference hg19 GRCh37 using HISAT. The average total mapping ratio with the reference genome was 95.92%, and the average total mapping ratio with genes was 74.28%. A total of 18,119 genes were identified.
For gene expression analysis, the clean reads were mapped to reference transcripts using Bowtie2. Analysis was carried out to compare ASD and control samples at each of the 4 stages of differentiation, including iPSC, NI, NSP and Diff. Gene expression level was determined using RSEM (v1.2.28), which provided read count, FPKM, and TPM values. Differential expression gene (DEG) analysis was carried out through DESeq2 with a Q value of ≤0.05. The resulting DEG analysis findings were visualized in a heatmap using pheatmap. To gain insight into changes in the phenotype, Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were performed using the phyper function in R software (R Foundation for Statistical Computing, Vienna, Austria). The significance levels of both terms and pathways were subjected to correction by Q values, applying a stringent threshold (Q value ≤ 0.05).
2.5. Calcium Imaging
For calcium imaging, we first recorded timelapse images from iPSC colonies. For these experiments, three colonies per line were plated on coverslips, and recordings were obtained from at least three coverslips for each line and condition (ATP and KCl stimulation). This procedure was repeated at three independent times. We then recorded timelapse images from differentiated neurons. Two-week-old NSPs (3–6 per coverslip) were plated on three coverslips per line, and recordings were obtained at both 1 week and 4 weeks post-differentiation. For each time point and condition (ATP, KCl, and DHPG stimulation), at least three coverslips were analyzed per line. This workflow was also repeated across three independent differentiation runs. In both cases, the unit of biological replication was the iPSC line, while multiple coverslips and cells within each line provided technical replication.
IPSC colonies and 1-week-old and 4-week-old cultured differentiated neurons were loaded with Ca
2+ indicator Fluo-4 AM (Thermo Fisher Scientific) in freshly prepared regular Krebs-HEPES buffer with a PH of 7.4 for a duration of 30 min and then were processed for imaging, as previously described [
26]. Krebs-HEPES buffer contained 120 mM NaCl, 1.3 mM CaCl
2, 1.2 mM MgSO
4, 4.8 mM KCl, 1.2 mM KH
2PO
4, 25 mM HEPES, and 0.1% BSA. All salts, HEPES, and BSA were prepared from respective powders dissolved in ultrapure (deionized-distilled) water produced by the Milli-Q
® Integral Water Purification System.
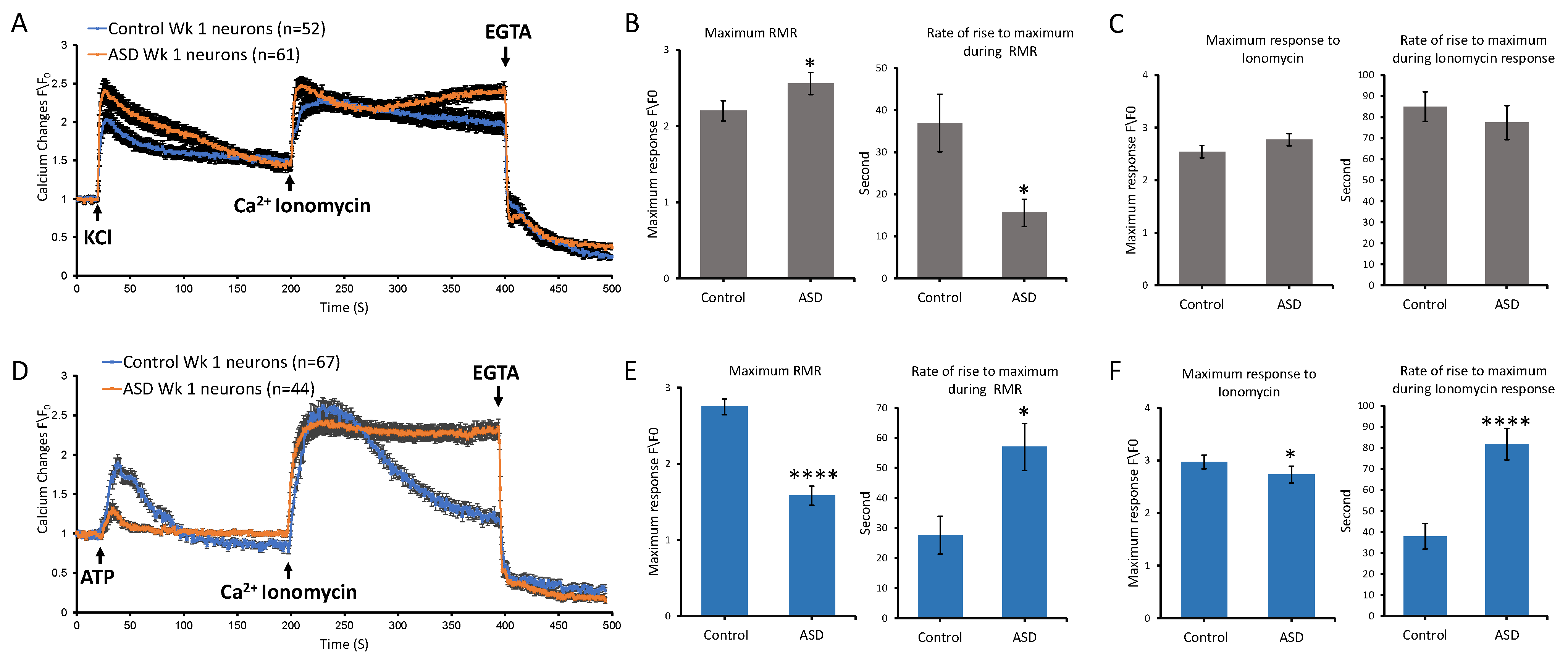
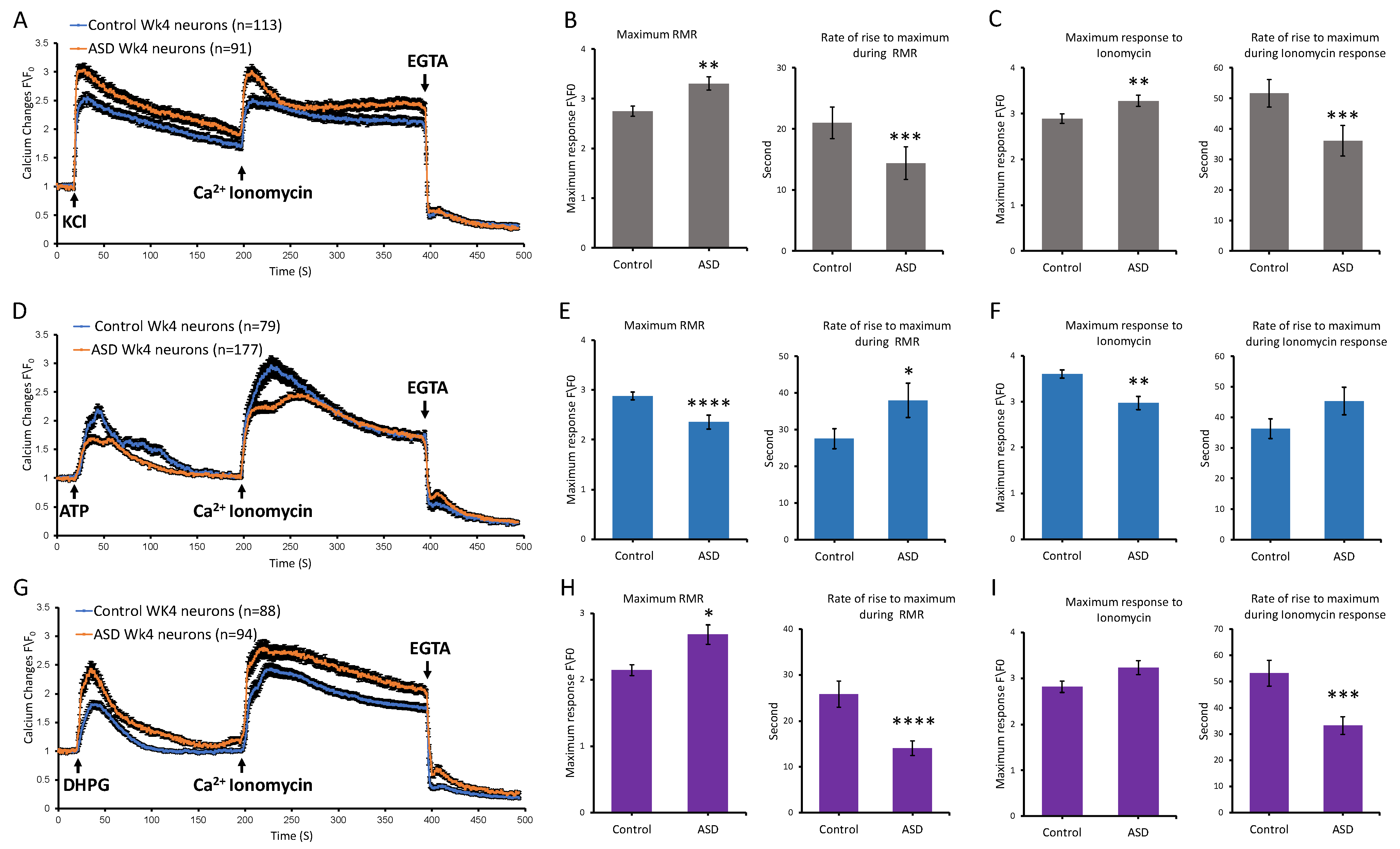
Image acquisition and analyses were performed using Zeiss LSM META 510 laser scanning confocal microscope and CLSM software version 3.2. Serial images were acquired at approximately 1 s intervals. A baseline recording was first taken, then the stimulus was delivered at second 20, whereby cells were either stimulated with 25 mM KCl, 100 µM ATP (Tocris, CAT#3245), or 200 µM DHPG (SIGMA Aldrich, CAT#: D368), followed by 4 μM ionomycin at second 200 and EGTA at second 400. Imaging of cells was performed at 37 °C.
For analysis, we performed background subtraction, then we calculated ratio changes by normalizing intensity changes to the averaged intensity of the first three images before delivering the stimulus. Further analysis was performed to compare ASD and control neurons in terms of rate of rise, maximum receptor mediated response, and maximum ionomycin response. For both statistical analysis and visualization, data from the two ASD cell lines were combined into a single ASD group, and data from the two control cell lines were combined into a single control group for each condition: iPSC, 1-week Diff, and 4-week Diff. Statistical analysis was performed to compare between ASD and control datasets using the two-tailed Mann–Whitney U test in GraphPad Prism version 5.04 (GraphPad Software, San Diego, CA, USA).
4. Discussion
This study investigated receptor-mediated Ca2+ release of idiopathic ASD iPSC-derived cortical neurons at different stages along their differentiation, including the iPSC, NI, NSP, and Diff stages. Our transcriptomic analyses showed that the numbers of Ca2+ signaling-relevant DEGs between the ASD and control groups were higher in samples from the iPSC and Diff stages, with the majority of DEGs being upregulated rather than downregulated. Accordingly, Ca2+ imaging studies were carried out in samples from the iPSC and Diff stages spanning two time points at the Diff stage, including 1-week and 4-week Diff neurons. Our characterizations included testing receptor-mediated Ca2+ release upon activation of purinergic receptors, voltage-gated calcium channels, and mGLURs receptors, using ATP, KCl, and DHPG, respectively. Our results suggested that ASD samples had altered Ca2+ transient kinetics in response to these stimuli compared to controls. At the iPSC stage, ASD samples displayed elevated maximum Ca2+ levels in response to ATP compared to controls. By contrast, ASD neurons from 1- and 4-week time points at the Diff stage exhibited notably reduced maximum Ca2+ responses to ATP and higher maximum responses to KCl and DHPG in comparison to controls. Our functional data showed that ionomycin-releasable stores were also affected differently in control versus ASD cells, pointing to multiple alterations in Ca2+ signaling, including the L-type Ca2+ channels (CACNA1S), which affects calcium influx magnitude and kinetics, SERCA pump (ATP2A1), which controls calcium clearance and store refiling, SERCA regulation (SLN) which fine-tunes calcium reuptake kinetics, and ryanodine receptors (RYR1 and RYI3), which control calcium release from internal stores and the calcium storage protein calsequestrin (CASQ2). Taken together, these results indicate aberrant calcium homeostasis as a result of multiple effects on calcium transients.
While our findings support the notion of early Ca
2+ dynamics alteration during neurogenesis in ASD, it is important to acknowledge that the relatively small number of iPSC lines analyzed (two idiopathic ASD lines and two controls), along with the use of a single clone per line, limits the generalizability of our conclusions. Validating these findings across multiple clones and added individuals will be essential to account for inter-clonal variability and to capture the heterogeneity of ASD more accurately. In this context, the observed differences in the distribution and number of neuronal cells across samples may reflect primary differences in the differentiation process among lines, which could underlie subsequent genetic alterations due to the heterogeneous nature of the derived neurons. Line variation is a recognized limitation in the use of stem cells, both embryonic stem (hES) cell lines and iPSC lines [
30,
31,
32,
33,
34,
35]. The field has developed various strategies to address it, including reference cell lines and improved characterization methods to account for this inherent variability. The source of this variability may be attributed to many factors, including genetic background differences, culture passage effects, reprogramming artifacts, and perhaps inherent variability in how efficiently each line responds to differentiation activators. Neuronal heterogeneity seems to amplify this. Cortical neurons are one of the most diverse cell populations in the body. Moreover, recent advances in single-cell technologies suggest that there may be hundreds of distinct neuronal subtypes in the cortex, far more than the traditional handful of categories [
36]. This heterogeneity appears to be fundamental to how cortical circuits process information, with different neuron types contributing specialized computational functions to overall network behavior.
The literature on iPSC-based models of ASD repeatedly documents aberrant Ca
2+ signaling as a potential contributor to the atypical developmental processes in ASD. Many of these studies, however, were directed toward examining spontaneous Ca
2+ transients and/or studying specific ASD mutations or risk variants. Avazzadeh et al. (2019) [
16] investigated spontaneous Ca
2+ signaling of cortical neurons derived from iPSCs that harbor ASD-relevant mutations in
NRXN1. They showed that ASD iPSC-derived cortical neurons had higher frequency of Ca
2+ transients, with longer duration and bigger amplitude. Teles et al. (2022) [
17] investigated iPSCs harboring ASD risk variants in the
RELN and
CACNA1H genes. They showed that ASD-associated variants in the
CACNA1 and
RELN genes caused an increased influx of Ca
2+ into the neural progenitors and an abnormal migration phenotype. Using the organoid system, Paulsen et al. (2022) [
20] investigated three haploinsufficiencies in three ASD risk genes,
SUV420H1,
ARID1B, and
CHD8, and reported asynchronous development of the organoids, accelerated neuronal differentiation, and reduced spontaneous Ca
2+ transients. Most recently, Shin et al. (2023) [
18] showed that knockout of ASD risk gene
TRPC6 in iPSC-derived neurons caused dysregulated Ca
2+ homeostasis, leading to hyperexcitability of neurons.
Calcium signaling follows a precise developmental timeline that is critical for normal neural development, where specific calcium kinetics are required for normal development [
37,
38,
39]. Disruptions at any stage cause lasting consequences. Components like parvalbumin, which is a slow calcium buffer [
40], undergo developmental changes that affect calcium kinetics. In addition,
Ip3r2 knockout mice exhibit ASD-like behaviors, creating developmentally inappropriate calcium kinetics. Our transcriptomic data and others [
14,
15] revealed differential expression of calcium-handling genes in ASD patients. DeRosa et al. (2018) [
14] examined transcriptional differences between iPSC-derived cortical neurons of idiopathic ASD and controls at two time points along the course of differentiation, including day 35 and day 135. Their findings revealed that calcium signaling pathways were dysregulated, and this dysregulation was particularly evident in day 35 of the developmental time course. They also provided functional evidence whereby ASD neurons displayed reduced spontaneous Ca
2+ transients and less activity on microelectrode array recordings compared controls [
14]. Li et al. (2021) [
15] conducted transcriptomic analyses using non-syndromic ASD iPSCs and performed their studies in samples from different stages along the differentiation process. Their analysis revealed that many ASD target genes are enriched in the Ca
2+ signaling pathway, and this was detected across both neural progenitor and mature neurons. Altogether, these studies support the notion that disrupted Ca
2+ homeostasis emerges early during neurogenesis and may act as a contributing factor in the abnormal developmental processes in ASD.
Our pharmacological characterization of receptor-mediated Ca
2+ release showed that differentiated cortical neurons had a reduced maximum Ca
2+ response to ATP in comparison to iPSCs and displayed strong responses to KCl and DHPG. We used ATP at the iPSC stage during initial scouting to ascertain whether the difference in calcium DEG transcriptomics was associated with a fundamental difference in real-time calcium signaling. Calcium transients are clear examples of biological computation, involving triggers, dynamic amplification, intracellular store release, extracellular influx, store recharging, intracellular buffering, and mitochondrial buffering, which in turn integrate calcium signals with the cell’s metabolic states. This interconnected network involves more players because cells express different receptors as they differentiate and mature. Neurons are excitable cells, hence, during their differentiation and maturation, they upregulate voltage-gated channels, ligand-gated channels, and all associated machinery required for their specialization. It is therefore likely that the different Ca
2+ responses to specific stimuli in Diff neurons compared to iPSC Cells reflect their cellular specialization. It is well established that KCl depolarizes neurons and induces Ca
2+ influx via voltage-gated Ca
2+ channels [
41], while DHPG activates mGLURs and induces mobilization of Ca
2+ from internal stores via the phospholipase C beta/IP3 pathway [
42]. These signaling pathways are vital for neuronal excitability, synaptic neurotransmission, and modulation of synaptic functions.
We observed reduced Ca
2+ responses to ATP at the Diff stage compared to the iPSC stage, which may reflect developmental changes during neurogenesis. ATP activates purinergic receptors, including ionotropic P2X and metabotropic P2Y subtypes, leading to Ca
2+ influx either directly through channel receptors or indirectly via internal stores through the phospholipase C beta/IP
3 pathway, depending on the receptor subtype. Purinergic signaling has been shown to play a critical role in regulating the proliferation, survival, and differentiation of various cell types, including stem cells and neural progenitors [
43,
44,
45]. During early neurodevelopment, ATP and purinergic signaling are known to influence the migration of neural precursors and intermediate progenitor cells [
44,
46]. However, as these cells mature into postmitotic neurons, their responsiveness to ATP diminishes [
44], which may explain the attenuated Ca
2+ responses observed in our differentiated neurons.
Our findings indicated that the differential Ca
2+ kinetic profiles in response to ATP, KCl, and DHPG across iPSC and Diff neurons were more notable in ASD samples, whereby the ATP-induced maximum Ca
2+ level at the Diff stage was significantly lower and the responses to KCl and DHPG were significantly higher compared to controls. Adhya et al. (2021) [
47] investigated early-stage neurogenesis in heterogeneous ASD iPSCs, revealing atypical differentiation patterns, including abnormal rosette formation, premature maturation, and imbalances in cell fate specification toward excitatory and inhibitory neuronal populations. Paulsen et al. (2022) [
20] also showed an accelerated differentiation phenotype specifically in deep layer cortical neurons and asynchronous formation of excitatory and inhibitory neurons in their model organoids harboring mutations in ASD risk genes. Using a whole-cell patch clamp, Hussein et al. (2023) [
19] reported early maturation and hyperexcitability of neurons derived from iPSCs harboring mutations in ASD risk genes. In addition, several reports of hyperexcitable phenotypes have been documented in many other studies investigating ASD-specific risk genes and or chromosomal changes [
16,
18,
19]. Future studies should include electrophysiological characterization (patch-clamp/MEA), comprehensive gene expression analysis, and detailed morphometric assessments, including synaptogenesis metrics, all of which would provide important functional validation of our findings.
In conclusion, our findings support the notion that calcium dysregulation emerges early during neurogenesis and may act as a contributing factor to abnormal developmental processes in ASD. Future studies may include a larger number of idiopathic ASD iPSC lines to replicate these findings and identify specific molecular targets within calcium signaling pathways that may confer therapeutic potential to restore optimal calcium levels and neuronal excitability.