miR-451 Is a Driver of Lipotoxic Injury in Patients with Diabetic Cardiomyopathy
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. miR-451 Targeting in Human Cardiomyocytes
2.3. Real-Time PCR
2.4. Expression and In Silico Analysis of MicroRNAs
2.5. RISC(ago2) Immunoprecipitation
2.6. Triacylglycerol Content in Heart Tissue
2.7. Detection of Lipid Oxidation
2.8. MitoBiogenesis Assay
2.9. Mitochondrial Swelling Assay
2.10. Caspase-3 Activity Assay
2.11. Assessment of ATP Content and Fatty Acid Oxidation
2.12. Statistical Analysis
3. Results
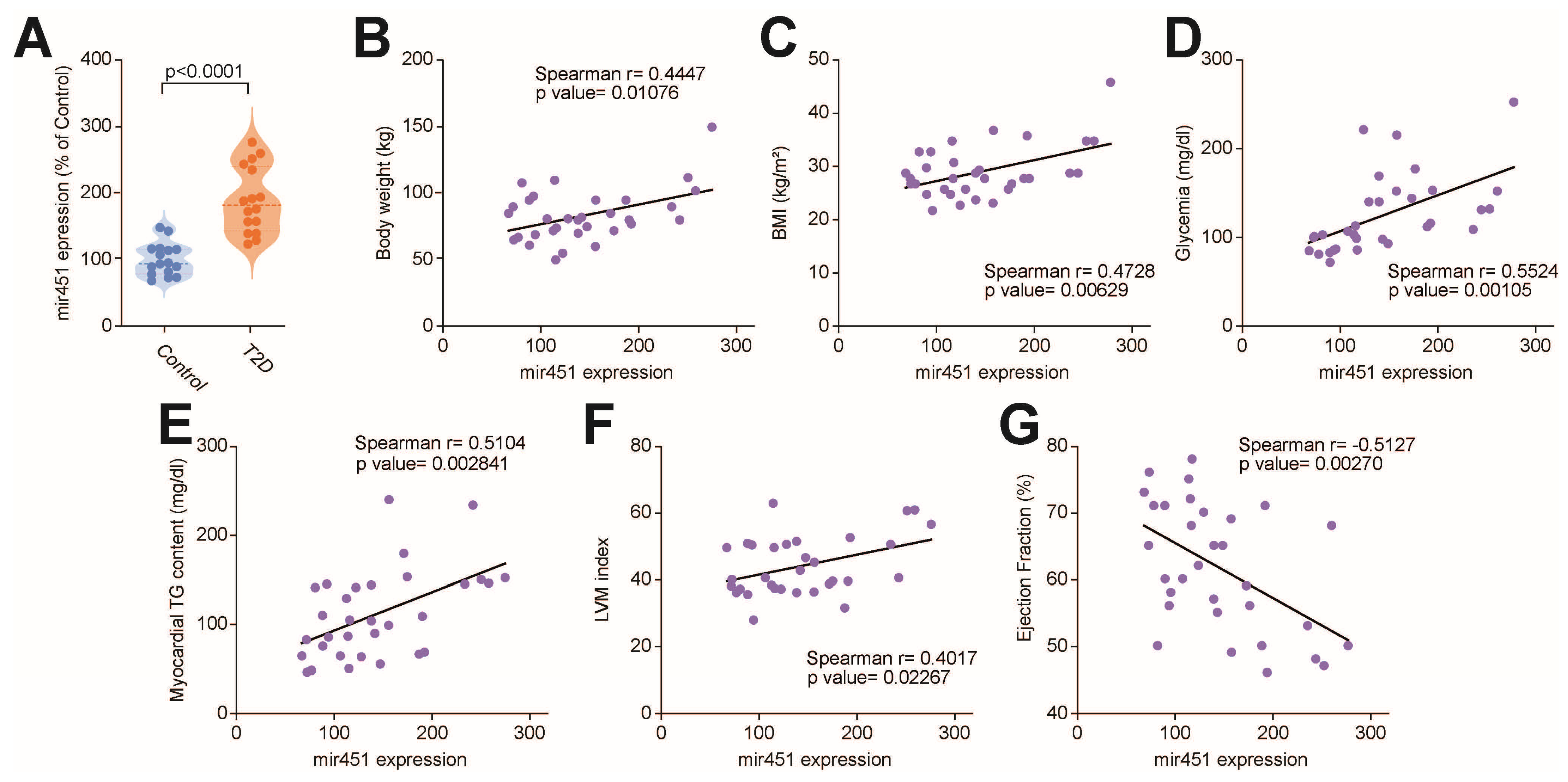
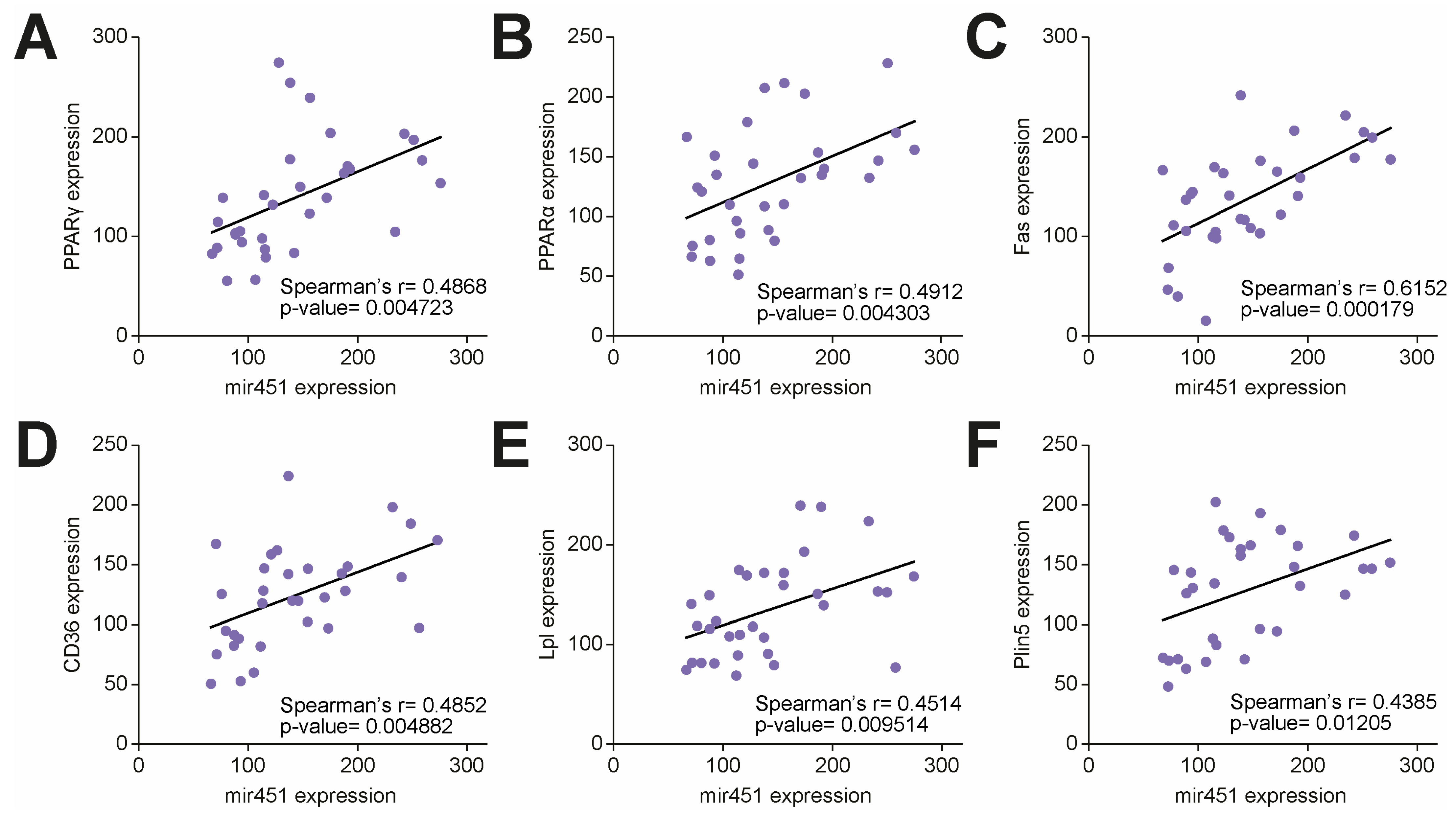
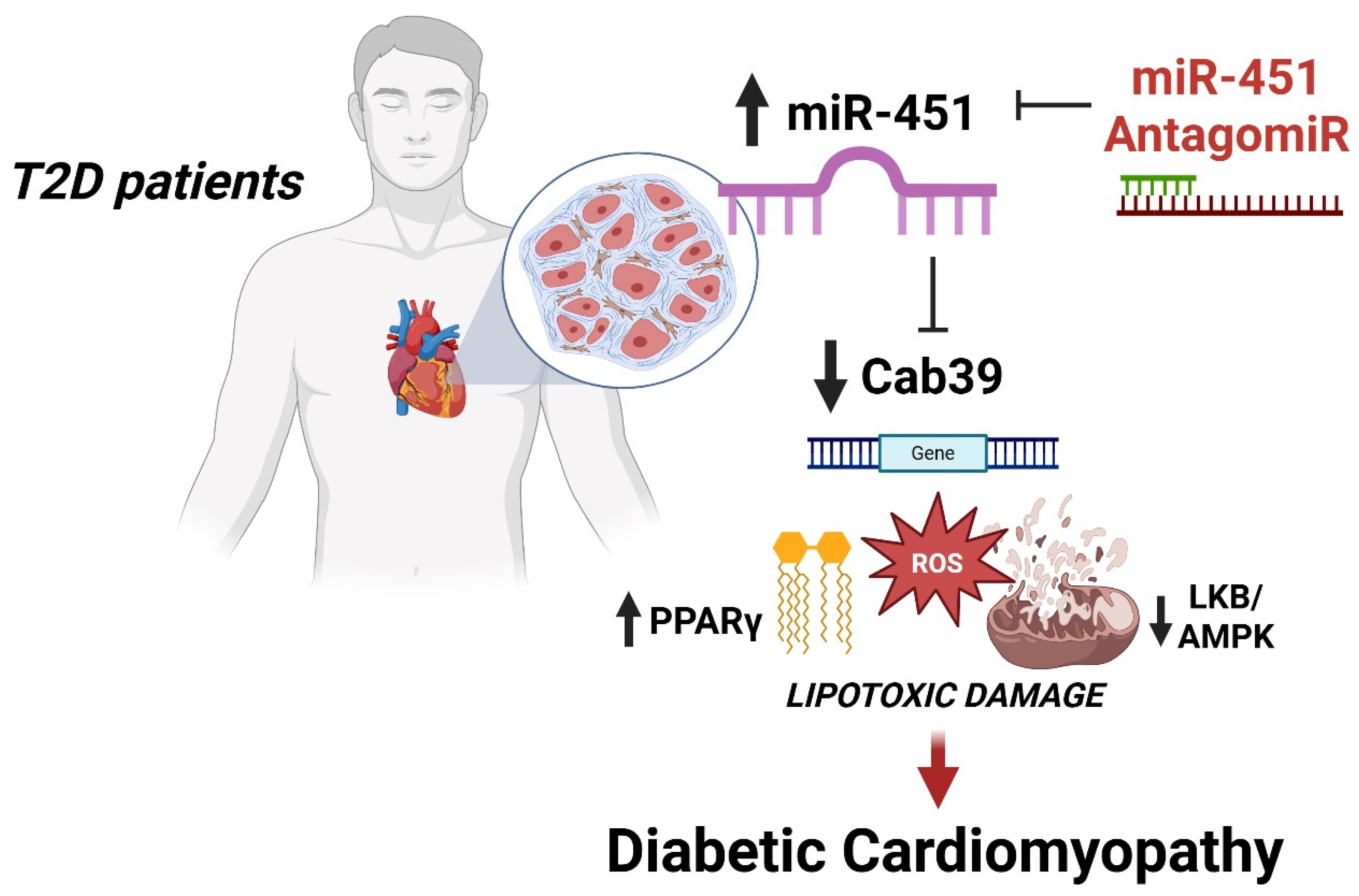
3.1. miR-451 Is Upregulated in the Human Diabetic Myocardium and Positively Correlates with Lipid Accumulation and Genes Involved in Lipotoxic Injury
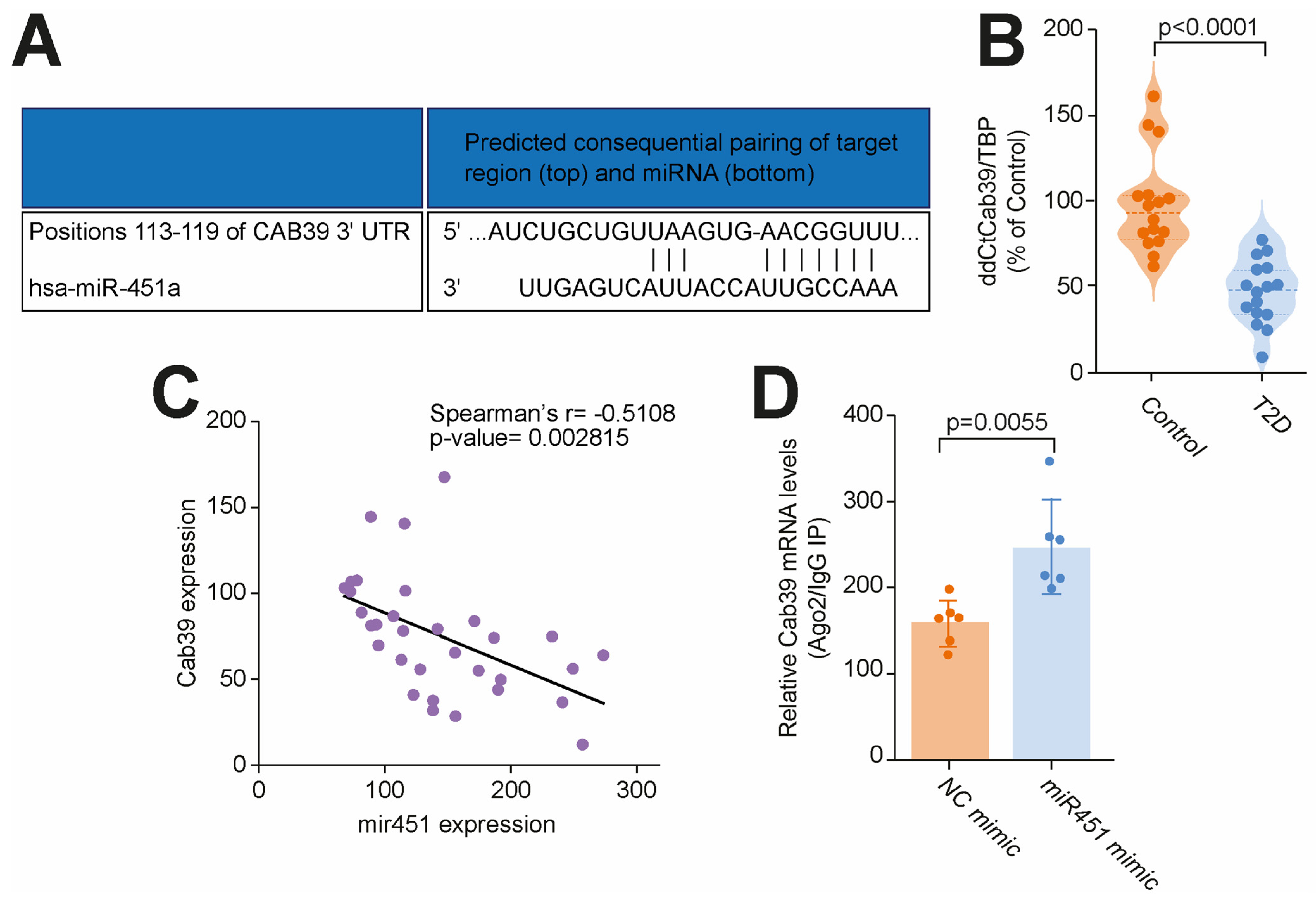
3.2. miR-451 Orchestrates Cab39 Expression
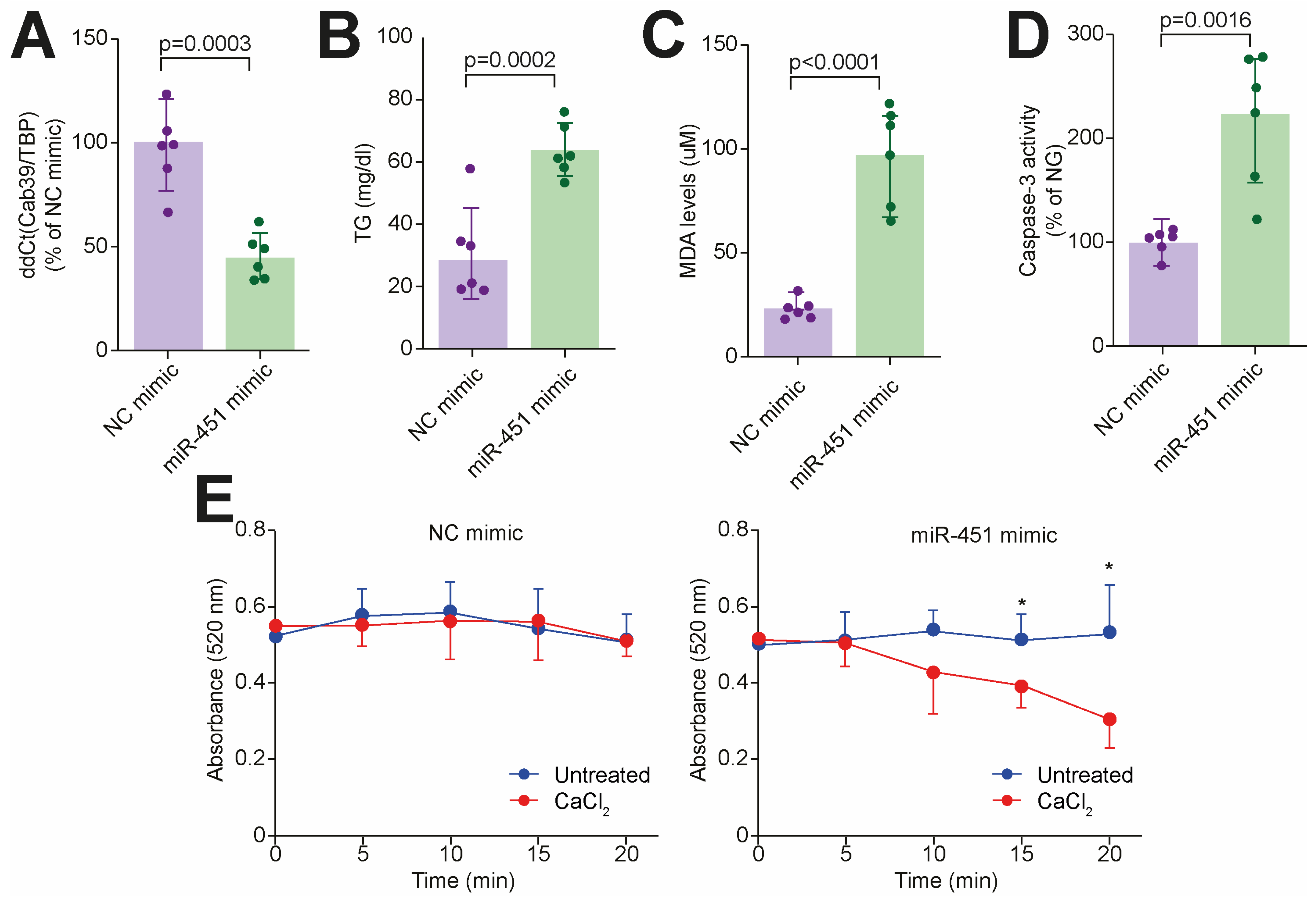
3.3. Targeting miR-451 Rescues Diabetes-Related Lipotoxic Damage and Mitochondrial Dysfunction in Human Cardiomyocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| miR | microRNA |
| T2D | type 2 diabetes |
| HF | heart failure |
| HFpEF | heart failure with a preserved ejection fraction |
| HG | high glucose |
| NG | normal glucose |
| PA | palmitic acid |
| CMs | cardiomyocytes |
| AMPK | adenosine monophosphate-activated protein kinase |
| LKB1 | liver kinase B1 |
| Cab39 | calcium-binding protein 39 |
| TG | triglyceride |
| HFD | high-fat diet |
| CABG | coronary artery bypass grafting |
References
- Collaborators GBDD. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Schulze, P.C.; Drosatos, K.; Goldberg, I.J. Lipid Use and Misuse by the Heart. Circ. Res. 2016, 118, 1736–1751. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Cardiomyopathy in obesity, insulin resistance and diabetes. J. Physiol. 2019, 598, 2977–2993. [Google Scholar] [CrossRef] [PubMed]
- Wenzl, F.A.; Ambrosini, S.; Mohammed, S.A.; Kraler, S.; Luscher, T.F.; Costantino, S.; Paneni, F. Inflammation in Metabolic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 742178. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef]
- Das, S.; Shah, R.; Dimmeler, S.; Freedman, J.E.; Holley, C.; Lee, J.M.; Moore, K.; Musunuru, K.; Wang, D.Z.; Xiao, J.; et al. Noncoding RNAs in Cardiovascular Disease: Current Knowledge, Tools and Technologies for Investigation, and Future Directions: A Scientific Statement From the American Heart Association. Circ. Genom. Precis. Med. 2020, 13, e000062. [Google Scholar] [CrossRef]
- Zarek-Starzewska, A.; Klimczak-Tomaniak, D.; Madrecka, A.; Sygitowicz, G.; Janiszewski, M.; Kuch, M. From Type 2 Diabetes Mellitus To Diabetic Cardiomyopathy—A Systematic Review On The Role Of MicroRNA. Curr. Diab Rep. 2025, 25, 42. [Google Scholar] [CrossRef]
- Costantino, S.; Paneni, F.; Luscher, T.F.; Cosentino, F. MicroRNA profiling unveils hyperglycaemic memory in the diabetic heart. Eur. Heart J. 2016, 37, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, P.; Marzano, F.; Salvatore, M.; Basile, C.; Buonocore, D.; Parlati, A.L.M.; Nardi, E.; Asile, G.; Abbate, V.; Colella, A.; et al. MicroRNAs: Diagnostic, prognostic and therapeutic role in heart failure-a review. ESC Heart Fail. 2023, 10, 753–761. [Google Scholar] [CrossRef]
- Landmesser, U.; Poller, W.; Tsimikas, S.; Most, P.; Paneni, F.; Luscher, T.F. From traditional pharmacological towards nucleic acid-based therapies for cardiovascular diseases. Eur. Heart J. 2020, 41, 3884–3899. [Google Scholar] [CrossRef]
- D’Amato, A.; Prosperi, S.; Severino, P.; Myftari, V.; Correale, M.; Perrone Filardi, P.; Badagliacca, R.; Fedele, F.; Vizza, C.D.; Palazzuoli, A.; et al. MicroRNA and Heart Failure: A Novel Promising Diagnostic and Therapeutic Tool. J. Clin. Med. 2024, 13, 7560. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Horie, T.; Baba, O.; Watanabe, S.; Nishiga, M.; Usami, S.; Izuhara, M.; Nakao, T.; Nishino, T.; Otsu, K.; et al. MicroRNA-451 exacerbates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy in mice through suppression of the LKB1/AMPK pathway. Circ. Res. 2015, 116, 279–288. [Google Scholar] [CrossRef]
- Khan, M.; Musazzi, U.M.; Manellari, S.; Mouawad, N.; Damiano, G.; Rinaldi, R.; Raucci, A.; Costantino, S.; Paneni, F.; Minghetti, P.; et al. Messenger RNA (mRNA) based therapeutics in transforming cardiovascular care: Progress, regulatory challenges and future perspectives. Pharmacol. Res. 2025, 218, 107847. [Google Scholar] [CrossRef]
- Taubel, J.; Hauke, W.; Rump, S.; Viereck, J.; Batkai, S.; Poetzsch, J.; Rode, L.; Weigt, H.; Genschel, C.; Lorch, U.; et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: Results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur. Heart J. 2021, 42, 178–188. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schutt, K.; Muller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Akhmedov, A.; Melina, G.; Mohammed, S.A.; Othman, A.; Ambrosini, S.; Wijnen, W.J.; Sada, L.; Ciavarella, G.M.; Liberale, L.; et al. Obesity-induced activation of JunD promotes myocardial lipid accumulation and metabolic cardiomyopathy. Eur. Heart J. 2019, 40, 997–1008. [Google Scholar] [CrossRef]
- Paneni, F.; Costantino, S.; Castello, L.; Battista, R.; Capretti, G.; Chiandotto, S.; D’Amario, D.; Scavone, G.; Villano, A.; Rustighi, A.; et al. Targeting prolyl-isomerase Pin1 prevents mitochondrial oxidative stress and vascular dysfunction: Insights in patients with diabetes. Eur. Heart J. 2015, 36, 817–828. [Google Scholar] [CrossRef]
- Fang, X.; Shen, F.; Lechauve, C.; Xu, P.; Zhao, G.; Itkow, J.; Wu, F.; Hou, Y.; Wu, X.; Yu, L.; et al. miR-144/451 represses the LKB1/AMPK/mTOR pathway to promote red cell precursor survival during recovery from acute anemia. Haematologica 2018, 103, 406–416. [Google Scholar] [CrossRef]
- Rizza, V.; Tondi, L.; Patti, A.M.; Cecchi, D.; Lombardi, M.; Perone, F.; Ambrosetti, M.; Rizzo, M.; Cianflone, D.; Maranta, F. Diabetic cardiomyopathy: Pathophysiology, imaging assessment and therapeutical strategies. Int. J. Cardiol. Cardiovasc. Risk Prev. 2024, 23, 200338. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.; DeVore, A.D.; Wu, J.; Matsouaka, R.A.; Fonarow, G.C.; Heidenreich, P.A.; Yancy, C.W.; Green, J.B.; Altman, N.; Hernandez, A.F. Heart Failure with Preserved Ejection Fraction and Diabetes: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Delalat, S.; Sultana, I.; Osman, H.; Sieme, M.; Zhazykbayeva, S.; Herwig, M.; Budde, H.; Kovacs, A.; Kacmaz, M.; Goztepe, E.; et al. Dysregulated inflammation, oxidative stress, and protein quality control in diabetic HFpEF: Unraveling mechanisms and therapeutic targets. Cardiovasc. Diabetol. 2025, 24, 211. [Google Scholar] [CrossRef]
- Herwig, M.; Sieme, M.; Kovacs, A.; Khan, M.; Mugge, A.; Schmidt, W.E.; Elci, F.; Sasidharan, S.; Haldenwang, P.; Wintrich, J.; et al. Diabetes mellitus aggravates myocardial inflammation and oxidative stress in aortic stenosis: A mechanistic link to HFpEF features. Cardiovasc. Diabetol. 2025, 24, 203. [Google Scholar] [CrossRef]
- Costantino, S.; Mohammed, S.A.; Ambrosini, S.; Telesca, M.; Mengozzi, A.; Walavalkar, K.; Gorica, E.; Herwig, M.; van Heerebeek, L.; Xia, J.; et al. Chromatin Rewiring by SETD2 Drives Lipotoxic Injury in Cardiometabolic HFpEF. Circ. Res. 2025, 136, 1079–1095. [Google Scholar] [CrossRef]
- Bernardo, B.C.; Yildiz, G.S.; Kiriazis, H.; Harmawan, C.A.; Tai, C.M.K.; Ritchie, R.H.; McMullen, J.R. In Vivo Inhibition of miR-34a Modestly Limits Cardiac Enlargement and Fibrosis in a Mouse Model with Established Type 1 Diabetes-Induced Cardiomyopathy, but Does Not Improve Diastolic Function. Cells 2022, 11, 3117. [Google Scholar] [CrossRef]
- Li, X.; Meng, C.; Han, F.; Yang, J.; Wang, J.; Zhu, Y.; Cui, X.; Zuo, M.; Xu, J.; Chang, B. Vildagliptin Attenuates Myocardial Dysfunction and Restores Autophagy via miR-21/SPRY1/ERK in Diabetic Mice Heart. Front. Pharmacol. 2021, 12, 634365. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Zhao, X.D.; Shi, K.H.; Ding, X.S.; Tao, H. MiR-21-3p triggers cardiac fibroblasts pyroptosis in diabetic cardiac fibrosis via inhibiting androgen receptor. Exp. Cell Res. 2021, 399, 112464. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Gao, L.; Liu, Y.; Liu, Y.; Yao, R.; Li, Y.; Xiao, L.; Wu, L.; Du, B.; Huang, Z.; et al. MiR-451 antagonist protects against cardiac fibrosis in streptozotocin-induced diabetic mouse heart. Life Sci. 2019, 224, 12–22. [Google Scholar] [CrossRef]
- Ikeda, Y.; Sato, K.; Pimentel, D.R.; Sam, F.; Shaw, R.J.; Dyck, J.R.; Walsh, K. Cardiac-specific deletion of LKB1 leads to hypertrophy and dysfunction. J. Biol. Chem. 2009, 284, 35839–35849. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, S.C.; Parajuli, N.; Dyck, J.R. The role of AMPK in cardiomyocyte health and survival. Biochim. Biophys. Acta 2016, 1862, 2199–2210. [Google Scholar] [CrossRef]
- Turdi, S.; Kandadi, M.R.; Zhao, J.; Huff, A.F.; Du, M.; Ren, J. Deficiency in AMP-activated protein kinase exaggerates high fat diet-induced cardiac hypertrophy and contractile dysfunction. J. Mol. Cell Cardiol. 2011, 50, 712–722. [Google Scholar] [CrossRef]
- Goel, S.; Singh, R.; Singh, V.; Singh, H.; Kumari, P.; Chopra, H.; Sharma, R.; Nepovimova, E.; Valis, M.; Kuca, K.; et al. Metformin: Activation of 5′ AMP-activated protein kinase and its emerging potential beyond anti-hyperglycemic action. Front. Genet. 2022, 13, 1022739. [Google Scholar] [CrossRef] [PubMed]
- Pieri, M.; Vayianos, P.; Nicolaidou, V.; Felekkis, K.; Papaneophytou, C. Alterations in Circulating miRNA Levels after Infection with SARS-CoV-2 Could Contribute to the Development of Cardiovascular Diseases: What We Know So Far. Int. J. Mol. Sci. 2023, 24, 2380. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Jin, X.; Xu, Z.; Zeng, M.; Chen, D.; He, Y.; Zhang, J.; Jiang, T.; Chen, J. Circulating miR-451a levels as a potential biomarker to predict the prognosis of patients with multiple myeloma. Oncol. Lett. 2020, 20, 263. [Google Scholar] [CrossRef] [PubMed]

| Controls (n = 16) | T2D (n = 16) | p-Value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 59.8 ± 8.1 | 61.1 ± 9.1 | 0.65 |
| Gender (F:M) | 10:5 | 13:4 | 0.54 |
| BMI (kg/m2) | 28.5 ± 3.4 | 30.1 ± 6.2 | 0.38 |
| Systolic blood pressure (mmHg) | 122 ± 10 | 119 ± 31 | 0.68 |
| Diastolic blood pressure (mmHg) | 72.3 ± 10.2 | 73.1 ± 7.9 | 0.8 |
| Laboratory parameters | |||
| FPG (mg/dL) | 93 ± 11 | 157 ± 40 | <0.01 |
| HbA1c (%) | 5.6 ± 0.37 | 7.0 ± 0.82 | <0.01 |
| Duration of diabetes (years) | 0.0 ± 0.0 | 5.8 ± 4.1 | NA |
| Triglycerides (mg/dL) | 87 ± 31 | 138 ± 52 | 0.002 |
| Total cholesterol (mg/dL) | 177 ± 33 | 169. ± 45 | 0.54 |
| Medications | |||
| Statins (%) | 31.3 | 43.8 | NS |
| ACE-is/ARBs (%) | 50.0 | 62.5 | NS |
| Beta-blockers (%) | 50.0 | 68.8 | NS |
| Diuretics (%) | 25.0 | 37.5 | NS |
| Metformin (%) | 12.5 | 37.5 | <0.01 |
| Insulin (%) | 0.0 | 43.8 | <0.01 |
| GLP1RA (%) | 0.0 | 6.25 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantino, S.; Mohammed, S.A.; Ranocchi, F.; Zito, F.; Delfine, V.; Hamdani, N.; Vinci, M.C.; Melina, G.; Paneni, F. miR-451 Is a Driver of Lipotoxic Injury in Patients with Diabetic Cardiomyopathy. Cells 2025, 14, 1401. https://doi.org/10.3390/cells14171401
Costantino S, Mohammed SA, Ranocchi F, Zito F, Delfine V, Hamdani N, Vinci MC, Melina G, Paneni F. miR-451 Is a Driver of Lipotoxic Injury in Patients with Diabetic Cardiomyopathy. Cells. 2025; 14(17):1401. https://doi.org/10.3390/cells14171401
Chicago/Turabian StyleCostantino, Sarah, Shafeeq A. Mohammed, Federico Ranocchi, Francesco Zito, Valentina Delfine, Nazha Hamdani, Maria Cristina Vinci, Giovanni Melina, and Francesco Paneni. 2025. "miR-451 Is a Driver of Lipotoxic Injury in Patients with Diabetic Cardiomyopathy" Cells 14, no. 17: 1401. https://doi.org/10.3390/cells14171401
APA StyleCostantino, S., Mohammed, S. A., Ranocchi, F., Zito, F., Delfine, V., Hamdani, N., Vinci, M. C., Melina, G., & Paneni, F. (2025). miR-451 Is a Driver of Lipotoxic Injury in Patients with Diabetic Cardiomyopathy. Cells, 14(17), 1401. https://doi.org/10.3390/cells14171401










