Senescent Polarization of Macrophages and Inflammatory Biomarkers in Cardiovascular Disease
Abstract
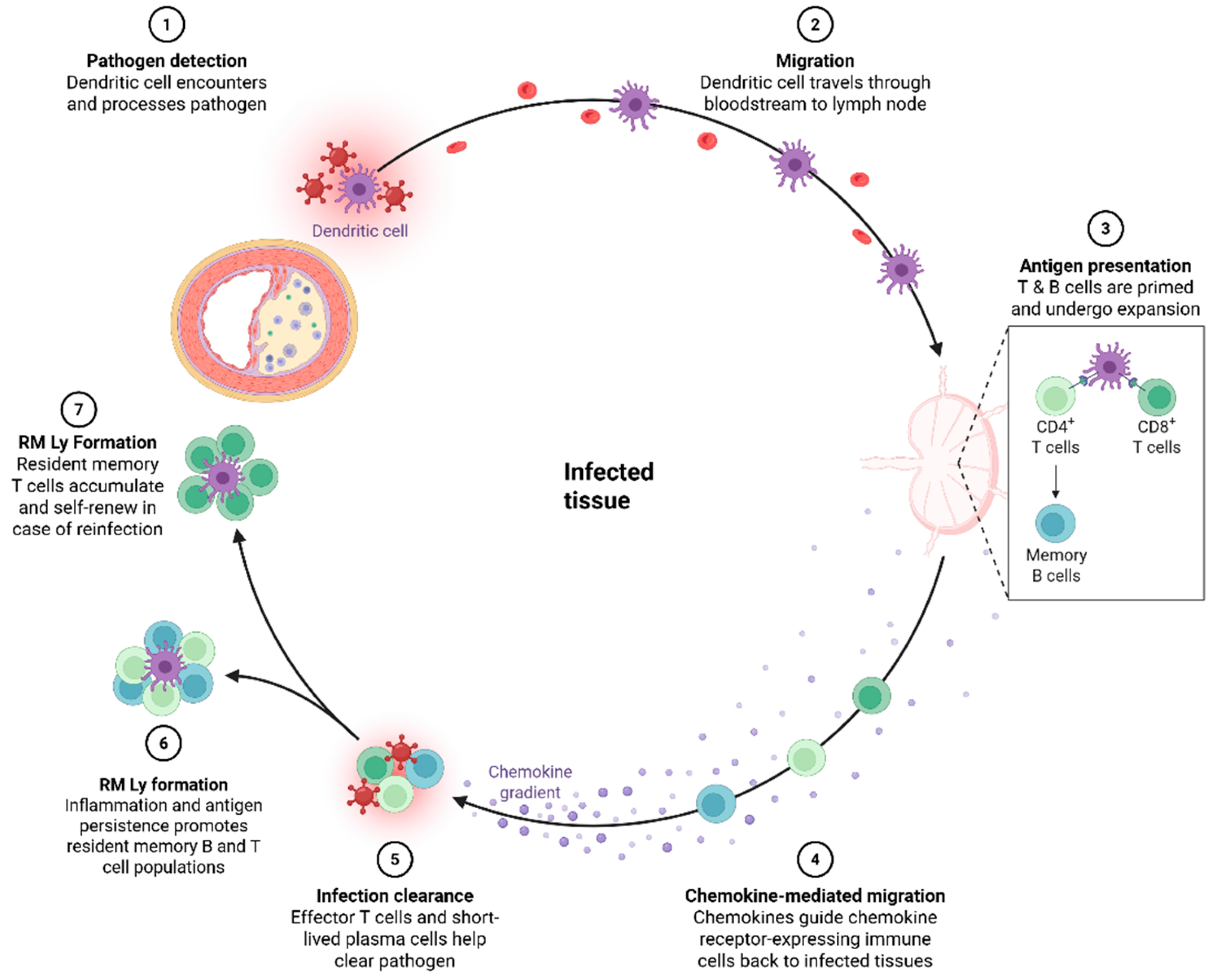
1. Introduction
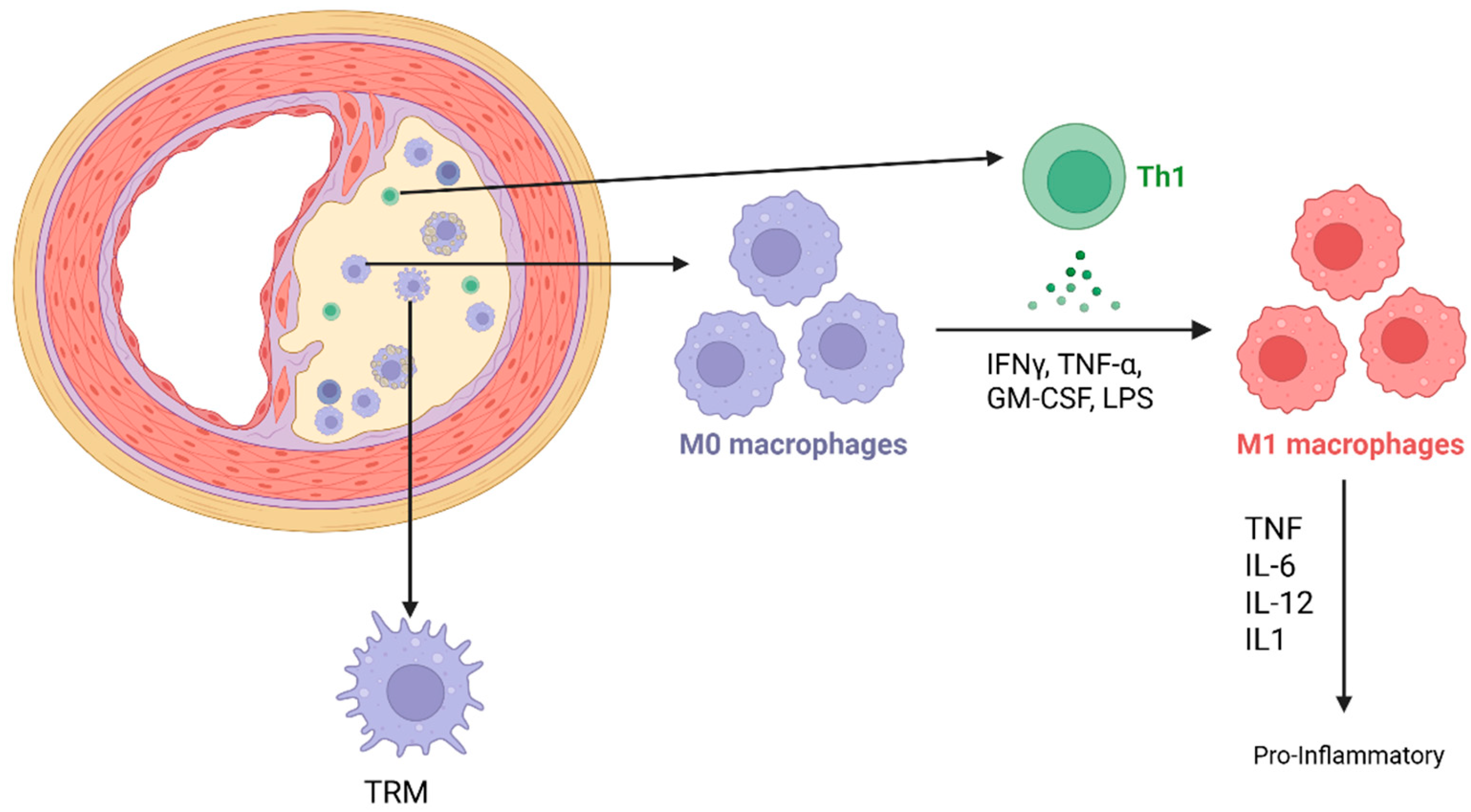
2. Macrophages in Cardiovascular Tissue
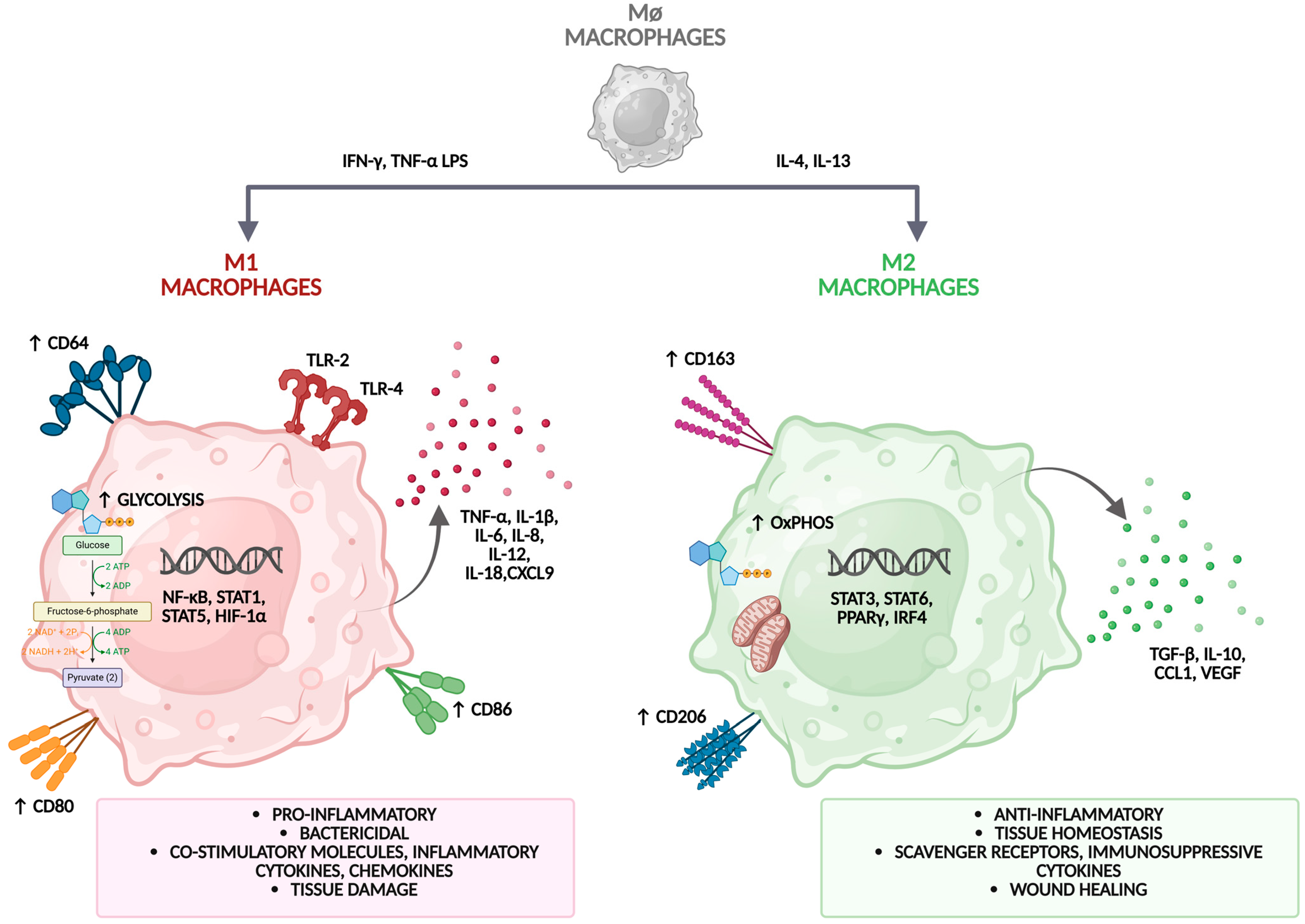
2.1. Macrophage Polarization and Plasticity
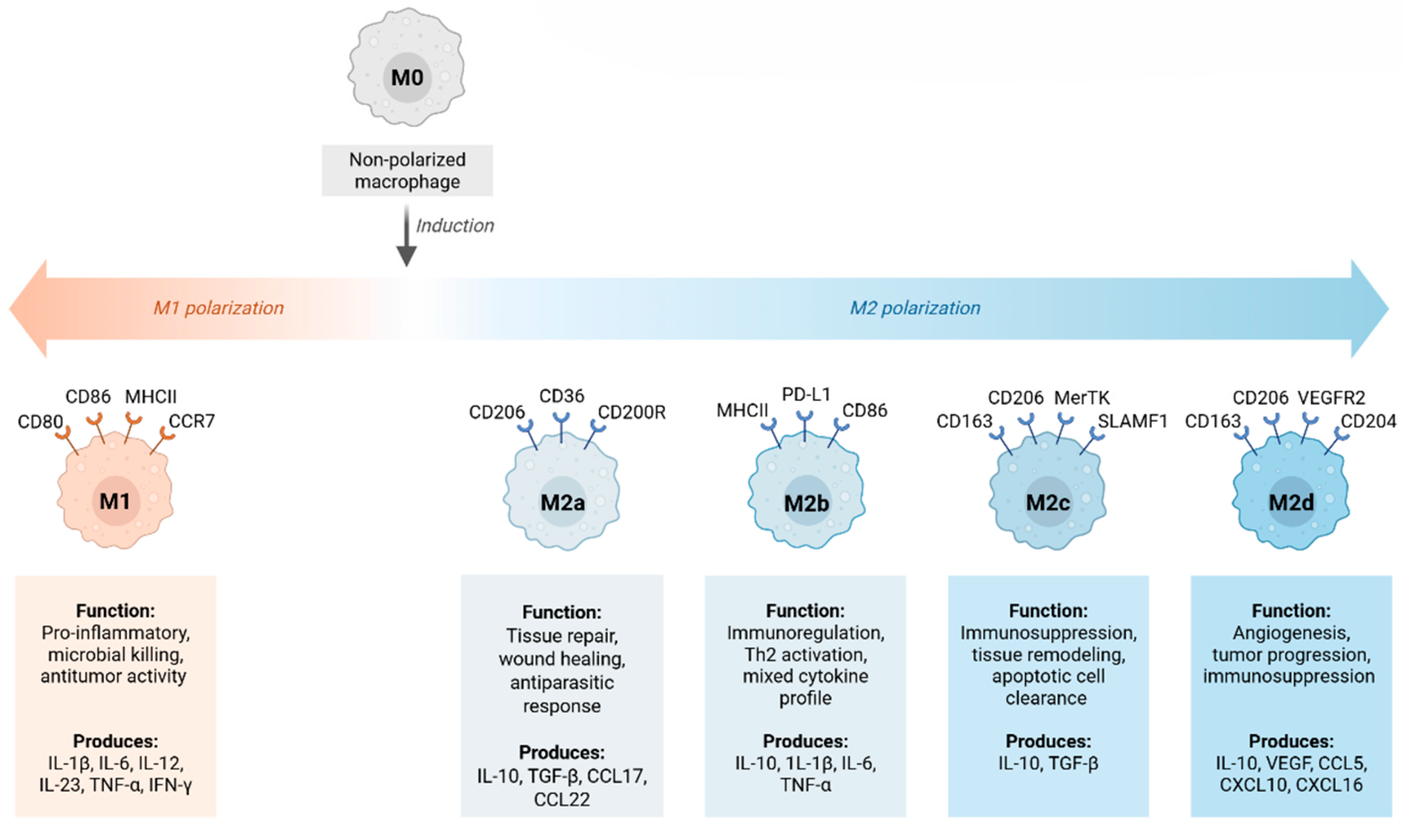
2.2. M1 and M2 Macrophage Phenotypes
| Characteristic | M1 Macrophages (Classically Activated) | M2 Macrophages (Alternatively Activated) |
|---|---|---|
| Activation Stimuli | IFN-γ, LPS (via TLR4), TNF-α [22,23] | IL-4, IL-13, IL-10, TGF-β [21,22] |
| Signaling Pathways | NF-κB, STAT1, IRF5, JAK/STAT1 [22,23,27] | STAT6, PPARγ, KLF4 [27,30] |
| Metabolic Profile | Glycolysis, mitochondrial dysfunction [13,26] | Oxidative phosphorylation, fatty acid oxidation [31] |
| Key Enzymes and Products | iNOS (NO), ROS [21,22] | Arginase-1, VEGF, MMPs [22,32] |
| Surface Markers | CD80, CD86, MHC class II [21,22] | CD206, CD163 [22] |
| Cytokines | TNF-α, IL-1β, IL-6, IL-12, IL-23 [22,23] | IL-10, TGF-β [29] |
| Functions | Antimicrobial defense, Th1 activation, tumor resistance [22,23] | Tissue repair, fibrosis, angiogenesis, immunosuppression [22,29,32] |
| Role in Disease | Chronic inflammation, atherosclerosis, tissue damage [24,26,33] | Fibrotic remodeling, plaque stabilization, immunoregulation [29,30,32] |
| Epigenetic Regulation | Histone acetylation, miR-155, DNA demethylation [28] | H3K27 acetylation, DNA methylation, long-term commitment [31] |
| Subtypes | Not subclassified | M2a (IL-4/IL-13), M2b (ICs + TLR), M2c (IL-10, TGF-β), M2d (IL-6, adenosine) [30,32] |
2.3. Role of Polarized and Senescent Macrophages in Atherosclerosis
3. Biomarkers for the Assessment of Aging Polarization
3.1. Chronic Inflammation in Vascular Injury
3.2. Acute-Phase and Soluble Inflammatory Biomarkers
| Biomarker | Function/Role in Inflammaging and CVD | References |
|---|---|---|
| C-reactive Protein (CRP/hsCRP) | Acute phase reactant; indicates systemic inflammation and predicts CVD risk | [24,43,46,47] |
| Serum Amyloid A (SAA) | Promotes foam cell formation, increases endothelial permeability, predicts plaque instability | [44,46,47] |
| Fibrinogen | Prothrombotic; increases blood viscosity and inflammation, associated with MI and stroke | [43,45,46,47] |
| sVCAM-1, sICAM-1, sE-selectin | Markers of endothelial activation and leukocyte adhesion | [43,46,47] |
| Pentraxin 3 (PTX3) | Reflects local vascular inflammation; elevated in acute coronary syndromes | [46,47] |
| Osteopontin (OPN) | Involved in fibrosis and vascular calcification; predicts arterial stiffness | [41,43,46,47] |
| Soluble CD163 and CD14 | Indicate monocyte/macrophage activation; associated with M2-like phenotype | [46,47] |
| Lipoprotein-associated Phospholipase A2 (Lp-PLA2) | Promotes plaque rupture and ischemic events | [44,45,46,47] |
| Galectin-3 | Secreted by macrophages; promotes fibrosis and heart failure | [45,46,47] |
| IL-6 | Pro-inflammatory cytokine; induces hepatic acute phase proteins, elevated with age | [45,46,47] |
| TNF-α | Promotes endothelial activation and chronic vascular inflammation | [45,46,47] |
| IL-1β | Key cytokine from NLRP3 inflammasome; drives leukocyte recruitment | [45,46,47] |
| IL-18 | Induces IFN-γ, contributes to plaque instability | [45,46,47] |
| MCP-1/CCL2 | Chemoattractant for monocytes; promotes early atherogenesis | [45,46,47] |
| CXCL8/IL-8 | Neutrophil chemoattractant; linked to vascular remodeling | [45,46,47] |
| IFN-γ | Promotes M1 polarization and plaque vulnerability | [45,46,47,48] |
| CD14++CD16+ Monocytes | Proinflammatory intermediate monocytes enriched in atherosclerotic plaques | [49,50,51,52,53] |
| NK Cells (CD56dimCD16+) | Reduced cytotoxicity; increased proinflammatory cytokine production | [49,51,52,53] |
| Senescent T cells (CD28−CD57+) | Produce TNF-α and IFN-γ; promote inflammaging | [49,51,53] |
| Treg/Th17 Ratio | Imbalance leads to immune dysregulation and plaque rupture | [54,55,56,57,58] |
3.3. Cytokine and Chemokine Biomarkers
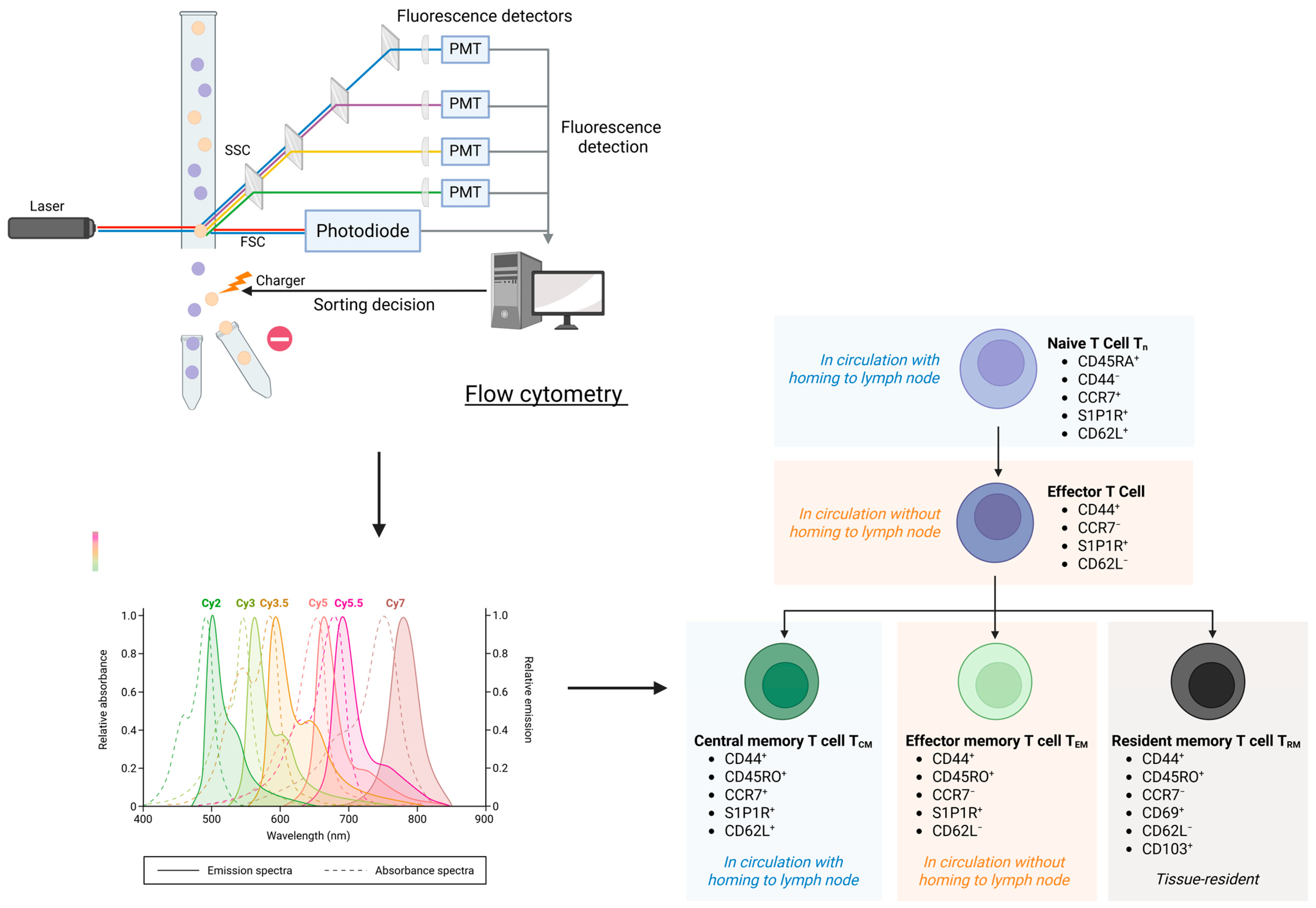
3.4. Immune Cell Biomarkers in Chronic Inflammation and Immunosenescence of Cardiovascular Aging
3.4.1. Innate Immune Cells Biomarkers
3.4.2. Markers of Adaptive Immunosenescence
| Biomarker | Function/Role in Inflammaging and CVD | References |
|---|---|---|
| CD14++CD16+ Monocytes | Proinflammatory intermediate monocytes enriched in atherosclerotic plaques | [49,51,53] |
| NK Cells (CD56dimCD16+) | Reduced cytotoxicity; increased proinflammatory cytokine production | [49,51,53,68,70,71] |
| Senescent T cells (CD28−CD57+) | Produce TNF-α and IFN-γ; promote inflammaging | [49,51,53,68,70,71,72,73] |
| Treg/Th17 Ratio | Imbalance leads to immune dysregulation and plaque rupture | [54,56,57,58] |
4. Therapeutic Interventions Targeting Senescent Macrophage Polarization in Cardiovascular Diseases
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| APPs | Acute Phase Proteins |
| CVD | Cardiovascular Disease |
| TRM | Tissue-Resident Macrophages |
| ROS | Reactive Oxygen Species |
| SASP | Senescence-Associated Secretory Phenotype |
| IFN-γ | Interferon Gamma |
| TNF-α | Tumor Necrosis Factor Alpha |
| IL | Interleukin |
| TLR | Toll-Like Receptor |
| LPS | Lipopolysaccharide |
| MHC | Major Histocompatibility Complex |
| iNOS | Inducible Nitric Oxide Synthase |
| NO | Nitric Oxide |
| VEGF | Vascular Endothelial Growth Factor |
| MMP | Matrix Metalloproteinase |
| Arg1 | Arginase-1 |
| Fizz1 | Found in Inflammatory Zone 1 |
| Ym1 | Chitinase-Like Protein 3 (Chi3l3) |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| STAT | Signal Transducer and Activator of Transcription |
| NF-κB | Nuclear Factor Kappa B |
| JAK | Janus Kinase |
| MAPK | Mitogen-Activated Protein Kinase |
| DC | Dendritic Cell |
| pDC | Plasmacytoid Dendritic Cell |
| mDC | Myeloid Dendritic Cell |
| NK | Natural Killer (cell) |
| Treg | Regulatory T Cell |
| Th1 | T Helper 1 Cell |
| Th17 | T Helper 17 Cell |
| BCL-2 | B-Cell Lymphoma 2 |
| HO-1 | Heme Oxygenase-1 |
| NAD+ | Nicotinamide Adenine Dinucleotide |
| EV | Extracellular Vesicle |
| CRP/hsCRP | (High-Sensitivity) C-Reactive Protein |
| SAA | Serum Amyloid A |
| PTX3 | Pentraxin 3 |
| OPN | Osteopontin |
| sVCAM-1 | Soluble Vascular Cell Adhesion Molecule-1 |
| sICAM-1 | Soluble Intercellular Adhesion Molecule-1 |
| Lp-PLA2 | Lipoprotein-Associated Phospholipase A2 |
| CXCL8 | C-X-C Motif Chemokine Ligand 8 (IL-8) |
| MCP-1/CCL2 | Monocyte Chemoattractant Protein-1/C-C Motif Chemokine Ligand 2 |
| DAMP | Damage-Associated Molecular Pattern |
| PAMP | Pathogen-Associated Molecular Pattern |
References
- World Health Organization. World Health Statistics 2025: Monitoring Health for the SDGs; Sustainable Development Goals; WHO: Geneva, Switzerland, 2025. [Google Scholar]
- Li, Y.; Cao, G.; Jing, W.; Liu, J.; Liu, M. Global trends and regional differences in incidence and mortality of cardiovascular disease, 1990−2019: Findings from 2019 global burden of disease study. Eur. J. Prev. Cardiol. 2023, 30, 276–286. [Google Scholar] [CrossRef]
- Kasal, D.A.; Sena, V.; Huguenin, G.V.B.; De Lorenzo, A.; Tibirica, E. Microvascular endothelial dysfunction in vascular senescence and disease. Front. Cardiovasc. Med. 2025, 12, 1505516. [Google Scholar] [CrossRef] [PubMed]
- Picos, A.; Seoane, N.; Campos-Toimil, M.; Viña, D. Vascular senescence and aging: Mechanisms, clinical implications, and therapeutic prospects. Biogerontology 2025, 26, 118. [Google Scholar] [CrossRef] [PubMed]
- Anwar, I.; Wang, X.; Pratt, R.E.; Dzau, V.J.; Hodgkinson, C.P. The impact of aging on cardiac repair and regeneration. J. Biol. Chem. 2024, 300, 107682. [Google Scholar] [CrossRef] [PubMed]
- Mebratu, Y.A.; Soni, S.; Rosas, L.; Rojas, M.; Horowitz, J.C.; Nho, R. The aged extracellular matrix and the profibrotic role of senescence-associated secretory phenotype. Am. J. Physiol. Cell. Physiol. 2023, 325, C565–C579. [Google Scholar] [CrossRef]
- Suda, M.; Paul, K.H.; Minamino, T.; Miller, J.D.; Lerman, A.; Ellison-Hughes, G.M.; Tchkonia, T.; Kirkland, J.L. Senescent Cells: A Therapeutic Target in Cardiovascular Diseases. Cells 2023, 12, 1296. [Google Scholar] [CrossRef]
- Penna, C.; Pagliaro, P. Endothelial Dysfunction: Redox Imbalance, NLRP3 Inflammasome, and Inflammatory Responses in Cardiovascular Diseases. Antioxidants 2025, 14, 256. [Google Scholar] [CrossRef]
- Anderson, R. Cellular senescence: Implications for metabolism. Mol. Cell 2019, 75, 11–25. [Google Scholar]
- Hall, B.M.; Linehan, E.; Fitzgerald, D.C. Aging of the immune system: Focus on macrophages. Nat. Rev. Immunol. 2017, 17, 299–312. [Google Scholar]
- Chen, J.; Zhu, Z.; Wang, Y.; Yu, J.; Zhang, X.; Xu, Y. Cardiac resident macrophages in cardiovascular disease: From physiology to pathology. Heart 2025, 111, 391–400. [Google Scholar] [CrossRef]
- Wculek, S.K.; Forisch, S.; Miguel, V.; Sancho, D. Metabolic homeostasis of tissue macrophages across the lifespan. Trends Endocrinol. Metab. 2024, 35, 793–808. [Google Scholar] [CrossRef]
- Molawi, K.; Wolf, Y.; Kandalla, P.K.; Favret, J.; Hagemeyer, N.; Frenzel, K.; Pinto, A.R.; Klapproth, K.; Henri, S.; Malissen, B.; et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 2014, 211, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Vellasamy, D.M.; Lee, S.-J.; Goh, K.W.; Goh, B.-H.; Tang, Y.-Q.; Ming, L.C.; YAP, W.H. Targeting Immune Senescence in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 13059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Q.; Wang, T.; Long, Q.; Sun, Y.; Jiao, L.; Gullerova, M. DNA damage response, a double-edged sword for vascular aging. Ageing Res. Rev. 2023, 92, 102137. [Google Scholar] [CrossRef] [PubMed]
- Duong, L.; Pixley, F.J.; Nelson, D.J.; Jackaman, C. Aging leads to increased monocytes and macrophages with altered CSF-1 receptor expression and earlier tumor-associated macrophage expansion in murine mesothelioma. Front. Aging. 2022, 3, 848925. [Google Scholar] [CrossRef]
- Costantini, A.; Viola, N.; Berretta, A.; Galeazzi, R.; Matacchione, G.; Sabbatinelli, J.; Storci, G.; De Matteis, S.; Butini, L.; Rippo, M.R.; et al. Age-related M1/M2 phenotype changes in circulating monocytes from healthy/unhealthy individuals. Aging 2018, 10, 1268–1280. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu. Rev. Pathol. 2020, 15, 493–518. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.; Zhao, Y. The Regulatory Network of Transcription Factors in Macrophage Polarization. Immunotargets. Ther. 2025, 14, 555–575. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Tabas, I.; Bornfeldt, K.E. Macrophage phenotype and function in different stages of atherosclerosis. Circ. Res. 2016, 118, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Velasco, M.; González-Ramos, S.; Boscá, L. Involvement of monocytes/macrophages as key factors in the development and progression of cardiovascular diseases. Biochem. J. 2014, 458, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Frodermann, V.; Nahrendorf, M. Macrophages and cardiovascular health. Physiol Rev. 2018, 98, 2523–2569. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef]
- Czimmerer, Z.; Daniel, B.; Horvath, A.; Rückerl, D.; Nagy, G.; Kiss, M.; Peloquin, M.; Budai, M.M.; Cuaranta-Monroy, I.; Simandi, Z.; et al. The transcription factor STAT6 mediates direct repression of inflammatory enhancers and limits activation of alternatively polarized macrophages. Immunity 2018, 48, 75–90.e6. [Google Scholar] [CrossRef]
- Solís-Martínez, R.; Cancino-Marentes, M.; Hernández-Flores, G.; Lazareno, P.O.; Mandujano-Álvarez, G.; Cruz-Gálvez, C.; Sierra-Díaz, E.; Rodríguez-Padilla, C.; Jave-Suárez, L.F.; Lemarroy, A.A.; et al. Regulation of immunophenotype modulation of monocytes-macrophages from M1 into M2 by prostate cancer cell-culture supernatant via transcription factor STAT3. Immunol. Lett. 2018, 196, 140–148. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, M.; Lei, W.; Yang, R.; Fu, S.; Fan, Z.; Yang, Y.; Zhang, T. Advances in the role of STAT3 in macrophage polarization. Front. Immunol. 2023, 14, 1160719. [Google Scholar] [CrossRef]
- Zhu, M.; Zhou, H.; Zhou, L.; Yang, Y.; Chen, R. Research progress on macrophages in cardiovascular diseases. J. Cardiothorac. Surg. 2025, 20, 307. [Google Scholar] [CrossRef]
- Wu, K.; Ma, H.X.; Li, Z.Y.; Li, Y.; Wu, D.; Lu, H.Y. Research Progress on the Regulation of Macrophages for Cardiovascular Diseases. FASEB J. 2025, 39, e70745. [Google Scholar] [CrossRef]
- Cui, H.; Wang, N.; Li, H.; Bian, Y.; Wen, W.; Kong, X.; Wang, F. The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: A crosstalk between ancient "Yin-Yang" theory and modern immunology. Cell Commun. Signal. 2024, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int J Mol Sci. 2023, 24, 7910. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, H.; Tang, B.; Luo, Y.; Yang, Y.; Zhong, X.; Chen, S.; Xu, X.; Huang, S.; Liu, C. Macrophages in cardiovascular diseases: Molecular mechanisms and therapeutic targets. Signal Transduct. Target Ther. 2024, 9, 130. [Google Scholar] [CrossRef]
- Suzuki, K.; Susaki, E.A.; Nagaoka, I. Lipopolysaccharides and Cellular Senescence: Involvement in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 11148. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Justice, J.; Belsky, D.W.; Higgins-Chen, A.; Moskalev, A.; Fuellen, G.; Cohen, A.A.; Bautmans, I.; Widschwendter, M.; Ding, J.; et al. Biomarkers of aging: From aging phenotypes to genomic instability. Nat. Rev. Mol. Cell. Biol. 2020, 21, 569–584. [Google Scholar]
- Mensà, E.; Latini, S.; Ramini, D.; Storci, G.; Bonafè, M.; Olivieri, F. The telomere world and aging: A complex network of interactions. Ageing Res. Rev. 2019, 51, 35–49. [Google Scholar]
- Jylhävä, J.; Pedersen, N.L.; Hägg, S. Biological age predictors. EBioMedicine 2017, 21, 29–36. [Google Scholar] [CrossRef]
- Franceschi, C.; Zaikin, A.; Gordleeva, S.; Ivanchenko, M.; Bonifazi, F.; Storci, G.; Bonafè, M. Inflammaging 2021: An update and a new challenge. Nat. Rev. Immunol. 2021, 21, 704–718. [Google Scholar]
- Kuo, C.L.; Moore, A.Z.; Lin, F.R.; Ferrucci, L. Epigenetic aging and cardiovascular disease: Recent insights and future directions. Circ. Res. 2021, 129, 1021–1037. [Google Scholar]
- Zernecke, A.; Tushuizen, M.E.; Diamant, M.; Sturk, A.; Nieuwland, R. Microparticles in cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e1–e7. [Google Scholar]
- Ferrucci, L.; Tanaka, T.; Polidori, M.C. Biomarkers of aging: The proxies of resilience. Science 2020, 370, eabc8051. [Google Scholar]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and ’garb-aging’. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The role of inflammation in cardiovascular disease. Lancet 2023, 401, 248–264. [Google Scholar]
- Bugibayeva, A.; Kurmanova, A.; Abzaliyev, K.; Abzaliyeva, S.; Kurmanova, G.; Sundetova, D.; Abdykassymova, M.; Bitemirova, R.; Sagalbayeva, U.; Absatarova, K.; et al. Immunological Markers of Cardiovascular Pathology in Older Patients. Biomedicines 2025, 13, 1392. [Google Scholar] [CrossRef] [PubMed]
- Netala, V.R.; Hou, T.; Wang, Y.; Zhang, Z.; Teertam, S.K. Cardiovascular Biomarkers: Tools for Precision Diagnosis and Prognosis. Int. J. Mol. Sci. 2025, 26, 3218. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, J.; Liu, J.; Ye, J.; Xu, Y.; Wang, Z.; Yu, J.; Ye, D.; Zhao, M.; Feng, Y.; et al. The role of interleukin-10 family members in cardiovascular diseases. Int. Immunopharmacol. 2021, 94, 107475. [Google Scholar] [CrossRef] [PubMed]
- Suleimenova, M.; Abzaliyev, K.; Mansurova, M.; Abzaliyeva, S.; Kurmanova, A.; Tokhtakulinova, G.; Bugibayeva, A.; Sundetova, D.; Abdykassymova, M.; Sagalbayeva, U.; et al. A Predictive Model of Cardiovascular Aging by Clinical and Immunological Markers Using Machine Learning. Diagnostics 2025, 15, 850. [Google Scholar] [CrossRef]
- Nyugen, J.; Agrawal, S.; Gollapudi, S.; Gupta, S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J. Clin. Immunol. 2010, 30, 806–813. [Google Scholar] [CrossRef]
- Carrasco, E.; Gómez de las Heras, M.M.; Gabandé-Rodríguez, E.; Desdín-Micó, G.; Aranda, J.F.; Mittelbrunn, M. The role of T cells in age-related diseases. Nat. Rev. Immunol. 2022, 22, 97–111. [Google Scholar] [CrossRef]
- Vardaman, D.; Ali, M.A.; Bolding, C.; Tidwell, H.; Stephens, H.; Tyrrell, D.J. Development of a Spectral Flow Cytometry Analysis Pipeline for High-Dimensional Immune Cell Characterization. J. Immunol. 2024, 213, 1713–1724. [Google Scholar] [CrossRef]
- Kong, W.T.; Bied, M.; Ginhoux, F. Spectral Flow Cytometry Analysis of Resident Tissue Macrophages. Methods Mol. Biol. 2024, 2713, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Pulko, V.; Davies, J.S.; Martinez, C.; Lanteri, M.C.; Busch, M.P.; Diamond, M.S.; Knox, K.; Bush, E.; Sims, P.; Sinari, S.; et al. Human memory T cells with a naive phenotype accumulate with aging and respond to persistent viruses. Nat. Immunol. 2016, 17, 966–975. [Google Scholar] [CrossRef]
- Ye, J.; Huang, X.; Hsueh, E.C.; Zhang, Q.; Ma, C.; Zhang, Y.; Varvares, M.A.; Hoft, D.F.; Peng, G. Human regulatory T cells induce T-lymphocyte senescence. Blood 2012, 120, 2021–2031. [Google Scholar] [CrossRef]
- Ye, J.; Ma, C.; Hsueh, E.C.; Eickhoff, C.S.; Zhang, Y.; Varvares, M.A.; Hoft, D.F.; Peng, G. Tumor-derived gammadelta regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J. Immunol. 2013, 190, 2403–2414. [Google Scholar] [CrossRef]
- Vakka, A.; Warren, J.S.; Drosatos, K. Cardiovascular aging: From cellular and molecular changes to therapeutic interventions. J. Cardiovasc. Aging 2023, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, M.; Wang, G.; Cui, X. Senotherapeutics: An emerging approach to the treatment of viral infectious diseases in the elderly. Front. Cell. Infect. Microbiol. 2023, 13, 1098712. [Google Scholar] [CrossRef] [PubMed]
- Hazeldine, J.; Lord, J.M. Innate immunesenescence: Underlying mechanisms and clinical relevance. Biogerontology 2015, 16, 187–201. [Google Scholar] [CrossRef]
- Van Beek, A.A.; Van den Bossche, J.; Mastroberardino, P.G.; de Winther, M.P.J.; Leenen, P.J.M. Metabolic Alterations in Aging Macrophages: Ingredients for Inflammaging? Trends Immunol. 2019, 40, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Schultze, J.L.; Murray, P.J.; Ochando, J.; Biswas, S.K. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat. Immunol. 2016, 17, 34–40. [Google Scholar] [CrossRef]
- Plackett, T.P.; Boehmer, E.D.; Faunce, D.E.; Kovacs, E.J. Aging and innate immune cells. J. Leukoc. Biol. 2004, 76, 291–299. [Google Scholar] [CrossRef]
- Wenisch, C.; Patruta, S.; Daxbock, F.; Krause, R.; Horl, W. Effect of age on human neutrophil function. J. Leukoc. Biol. 2000, 67, 40–45. [Google Scholar] [CrossRef]
- Jing, Y.; Shaheen, E.; Drake, R.R.; Chen, N.; Gravenstein, S.; Deng, Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum. Immunol. 2009, 70, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Zacca, E.R.; Crespo, M.I.; Acland, R.P.; Roselli, E.; Nunez, N.G.; Maccioni, M.; Maccioni, M.; Maletto, B.A.; Pistoresi, M.C.; Morón, G. Aging Impairs the Ability of Conventional Dendritic Cells to Cross-Prime CD8+ T Cells upon Stimulation with a TLR7 Ligand. PLoS ONE 2015, 10, e0140672. [Google Scholar] [CrossRef] [PubMed]
- Alpert, A.; Pickman, Y.; Leipold, M.; Rosenberg-Hasson, Y.; Ji, X.; Gaujoux, R.; Rabani, H.; Starosvetsky, E.; Kveler, K.; Schaffert, S.; et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 2019, 5, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Bronikowski, A.; Cunnane, S.C.; Ferrucci, L.; Franceschi, C.; Fulop, T. The conundrum of human immune system “senescence”. Mech. Ageing Dev. 2020, 192, 111357. [Google Scholar] [CrossRef]
- Manser, A.R.; Uhrberg, M. Age-related changes in natural killer cell repertoires: Impact on NK cell function and immune surveillance. Cancer Immunol. Immunother. 2016, 65, 417–426. [Google Scholar] [CrossRef]
- Tarazona, R.; Sanchez-Correa, B.; Casas-Aviles, I.; Campos, C.; Pera, A.; Morgado, S.; Sejas, N.L.; Hassouneh, F.; Bergua, J.M.; Arcos, M.J.; et al. Immunosenescence: Limitations of natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother. 2017, 66, 233–245. [Google Scholar] [CrossRef]
- Carlsten, M.; Bjorkstrom, N.K.; Norell, H.; Bryceson, Y.; van Hall, T.; Baumann, B.C.; Hanson, M.; Schedvins, K.; Kiessling, R.; Ljunggren, H.G.; et al. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer Res. 2007, 67, 1317–1325. [Google Scholar] [CrossRef]
- Lakshmikanth, T.; Burke, S.; Ali, T.H.; Kimpfler, S.; Ursini, F.; Ruggeri, L.; Capanni, M.; Umansky, V.; Paschen, A.; Sucker, A.; et al. NCRs and DNAM-1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J. Clin. Investig. 2009, 119, 1251–1263. [Google Scholar] [CrossRef]
- Padgett, L.E.; Dinh, H.Q.; Wu, R. Naive CD8+ T Cells Expressing CD95 Increase Human Cardiovascular Disease Severity. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e383. [Google Scholar] [CrossRef]
- Palmer, S.; Albergante, L.; Blackburn, C.C.; Newman, T.J. Thymic involution and rising disease incidence with age. Proc. Natl. Acad. Sci. USA 2018, 115, 1883–1888. [Google Scholar] [CrossRef]
- Hale, J.S.; Boursalian, T.E.; Turk, G.L.; Fink, P.J. Thymic output in aged mice. Proc. Natl. Acad. Sci. USA 2006, 103, 8447–8452. [Google Scholar] [CrossRef]
- Rezzani, R.; Nardo, L.; Favero, G.; Peroni, M.; Rodella, L.F. Thymus and aging: Morphological, radiological, and functional overview. Age 2014, 36, 313–351. [Google Scholar] [CrossRef]
- Chinn, I.K.; Blackburn, C.C.; Manley, N.R.; Sempowski, G.D. Changes in primary lymphoid organs with aging. Semin. Immunol. 2012, 24, 309–320. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Nat. Med. 2015, 21, 1321–1330. [Google Scholar] [CrossRef]
- Roos, C.M.; Zhang, B.; Palmer, A.K.; Shao, L.; Laberge, R.M.; Demaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 2016, 15, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.M.; Demaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W.; et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016, 22, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y.; Ding, H.; Wang, Y.; Xie, Y.; Zhang, X. Cellular Senescence in Cardiovascular Diseases: From Pathogenesis to Therapeutic Challenges. J. Cardiovasc. Dev. Dis. 2023, 10, 439. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ihan, A. Senescent Polarization of Macrophages and Inflammatory Biomarkers in Cardiovascular Disease. Cells 2025, 14, 1374. https://doi.org/10.3390/cells14171374
Ihan A. Senescent Polarization of Macrophages and Inflammatory Biomarkers in Cardiovascular Disease. Cells. 2025; 14(17):1374. https://doi.org/10.3390/cells14171374
Chicago/Turabian StyleIhan, Alojz. 2025. "Senescent Polarization of Macrophages and Inflammatory Biomarkers in Cardiovascular Disease" Cells 14, no. 17: 1374. https://doi.org/10.3390/cells14171374
APA StyleIhan, A. (2025). Senescent Polarization of Macrophages and Inflammatory Biomarkers in Cardiovascular Disease. Cells, 14(17), 1374. https://doi.org/10.3390/cells14171374






