Development of Positron Emission Tomography Radiotracers for Imaging α-Synuclein Aggregates
Abstract
1. Introduction
2. Challenges in Developing α-Syn PET Tracers
3. From AD-Radioactive Tracers and New Derivatives
3.1. PIB
3.2. BF227
3.3. PBB3
4. Exploration of Representative Compounds’ Scaffolds and Analogs
4.1. Phenothiazine Derivatives
4.2. Indolinone and Indolinone-Diene Analogs
4.3. Chalcone Analogs
4.4. Quinoline and Bisquinoline Derivatives
4.5. Benzimidazole (BI) Derivatives
4.6. Phenylbenzofuranone (PBF) Derivatives
4.7. Indole Derivatives
4.8. Pyridothiophene Derivatives
4.9. N,N-Dibenzylcinnamamide (DBC) Derivatives
4.10. Arylpyrazolethiazole (APT) Derivatives
4.11. 2,6-Disubstituted Imidazo[2,1-b][1,3,4]thiadiazole (ITA) Derivatives
4.12. 2-Pyridone Analogs
4.13. Benzothiazole-Ethenyl-Phenol Derivative (F0502B)
5. Identifying α-Syn Radioligands Through High-Throughput Screening (HTS)
5.1. [125I]21
5.2. 2FBox and 4FBox
5.3. 4,4′-Disarylbisthiazole (DABTA) Scaffold
5.4. Anle138b Analogs
5.5. N-Phenylbenzamide Analogs
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | β-Amyloid |
| AD | Alzheimer’s disease |
| ARG | Autoradiography |
| α-syn | Alpha-synuclein |
| BBB | Blood–brain barrier |
| CDK5 | Cyclin-dependent kinase 5 |
| CK2 | Casein kinase 2 |
| DLB | Dementia with Lewy bodies |
| GCIs | Glial cytoplasmic inclusions |
| GSK-3β | Glycogen synthase kinase-3β |
| HTS | High-throughput screening |
| LRRK2 | Leucine-rich repeat kinase 2 |
| MRI | Magnetic resonance imaging |
| MSA | Multiple system atrophy |
| NDDs | Neurodegenerative diseases |
| PAF | Pure autonomic failure |
| PD | Parkinson’s disease |
| PDD | Parkinson’s disease dementia |
| PET | Positron emission tomography |
| PLK2 | Polo-like kinase 2 |
| PSP | Progressive supranuclear palsy |
| REM | Rapid eye movement |
| SNpc | Substantia nigra pars compacta |
| SPECT | Single-photon emission computed tomography |
| SUV | Standardized uptake value |
References
- Wilson, D.M., III; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Lamotte, G.; Singer, W. Synucleinopathies. Handb. Clin. Neurol. 2023, 196, 175–202. [Google Scholar] [CrossRef]
- Marsili, L.; Rizzo, G.; Colosimo, C. Diagnostic Criteria for Parkinson’s Disease: From James Parkinson to the Concept of Prodromal Disease. Front. Neurol. 2018, 9, 156. [Google Scholar] [CrossRef]
- Prasad, S.; Katta, M.R.; Abhishek, S.; Sridhar, R.; Valisekka, S.S.; Hameed, M.; Kaur, J.; Walia, N. Recent advances in Lewy body dementia: A comprehensive review. Dis. Mon. 2023, 69, 101441. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abbasi, N.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.-Y.J.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Wanneveich, M.; Moisan, F.; Jacqmin-Gadda, H.; Elbaz, A.; Joly, P. Projections of prevalence, lifetime risk, and life expectancy of Parkinson’s disease (2010–2030) in France. Mov. Disord. 2018, 33, 1449–1455. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef]
- Rossi, A.; Berger, K.; Chen, H.; Leslie, D.; Mailman, R.B.; Huang, X. Projection of the prevalence of Parkinson’s disease in the coming decades: Revisited. Mov. Disord. 2018, 33, 156–159. [Google Scholar] [CrossRef]
- Van Den Eeden, S.K.; Tanner, C.M.; Bernstein, A.L.; Fross, R.D.; Leimpeter, A.; Bloch, D.A.; Nelson, L.M. Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.M.; Vosoughi, K.; Heydarpour, P.; Sepanlou, S.G.; Farzadfar, F.; Tehrani-Banihashemi, A.; Malekzadeh, R.; Sahraian, M.A.; Vollset, S.E.; Naghavi, M.; et al. Burden of neurodegenerative diseases in the Eastern Mediterranean Region, 1990–2016: Findings from the Global Burden of Disease Study 2016. Eur. J. Neurol. 2019, 26, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; San Luciano, M.; Tanner, C. The epidemiology of Parkinson’s disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Okazaki, H.; Lipkin, L.E.; Aronson, S.M. Diffuse intracytoplasmic ganglionic inclusions (Lewy type) associated with progressive dementia and quadriparesis in flexion. J. Neuropathol. Exp. Neurol. 1961, 20, 237–244. [Google Scholar] [CrossRef]
- Fanciulli, A.; Wenning, G.K. Multiple-system atrophy. N. Engl. J. Med. 2015, 372, 249–263. [Google Scholar] [CrossRef]
- Walsh, R.R.; Krismer, F.; Galpern, W.R.; Wenning, G.K.; Low, P.A.; Halliday, G.; Koroshetz, W.J.; Holton, J.; Quinn, N.P.; Rascol, O.; et al. Recommendations of the Global Multiple System Atrophy Research Roadmap Meeting. Neurology 2018, 90, 74–82. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. Jama 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef]
- Tolosa, E.; Wenning, G.; Poewe, W. The diagnosis of Parkinson’s disease. Lancet Neurol. 2006, 5, 75–86. [Google Scholar] [CrossRef]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Dickson, D.W.; Braak, H.; Duda, J.E.; Duyckaerts, C.; Gasser, T.; Halliday, G.M.; Hardy, J.; Leverenz, J.B.; Del Tredici, K.; Wszolek, Z.K.; et al. Neuropathological assessment of Parkinson’s disease: Refining the diagnostic criteria. Lancet Neurol. 2009, 8, 1150–1157. [Google Scholar] [CrossRef]
- Adler, C.H.; Beach, T.G.; Hentz, J.G.; Shill, H.A.; Caviness, J.N.; Driver-Dunckley, E.; Sabbagh, M.N.; Sue, L.I.; Jacobson, S.A.; Belden, C.M.; et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease. Neurology 2014, 83, 406–412. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Lees, A.J. Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology 2001, 57, 1497–1499. [Google Scholar] [CrossRef]
- Lippa, C.F.; Fujiwara, H.; Mann, D.M.A.; Giasson, B.; Baba, M.; Schmidt, M.L.; Nee, L.E.; O’Connell, B.; Pollen, D.A.; St. George-Hyslop, P.; et al. Lewy Bodies Contain Altered α-Synuclein in Brains of Many Familial Alzheimer’s Disease Patients with Mutations in Presenilin and Amyloid Precursor Protein Genes. Am. J. Pathol. 1998, 153, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Serrano, G.E.; Sue, L.I.; Walker, D.G.; Adler, C.H.; Shill, H.A.; Sabbagh, M.N.; Caviness, J.N.; Hidalgo, J.; Saxon-Labelle, M.; et al. Presence of Striatal Amyloid Plaques in Parkinson’s Disease Dementia Predicts Concomitant Alzheimer’s Disease: Usefulness for Amyloid Imaging. J. Park. Dis. 2012, 2, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, M.N.; Adler, C.H.; Lahti, T.J.; Connor, D.J.; Vedders, L.; Peterson, L.K.; Caviness, J.N.; Shill, H.A.; Sue, L.I.; Ziabreva, I.; et al. Parkinson disease with dementia: Comparing patients with and without Alzheimer pathology. Alzheimer Dis. Assoc. Disord. 2009, 23, 295–297. [Google Scholar] [CrossRef]
- Kotzbauer, P.T.; Trojanowsk, J.Q.; Lee, V.M. Lewy body pathology in Alzheimer’s disease. J. Mol. Neurosci. 2001, 17, 225–232. [Google Scholar] [CrossRef]
- Irwin, D.J.; Xie, S.X.; Coughlin, D.; Nevler, N.; Akhtar, R.S.; McMillan, C.T.; Lee, E.B.; Wolk, D.A.; Weintraub, D.; Chen-Plotkin, A.; et al. CSF tau and β-amyloid predict cerebral synucleinopathy in autopsied Lewy body disorders. Neurology 2018, 90, e1038–e1046. [Google Scholar] [CrossRef]
- Kotzbauer, P.T.; Cairns, N.J.; Campbell, M.C.; Willis, A.W.; Racette, B.A.; Tabbal, S.D.; Perlmutter, J.S. Pathologic accumulation of α-synuclein and Aβ in Parkinson disease patients with dementia. Arch. Neurol. 2012, 69, 1326–1331. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Attems, J.; Thal, D.R. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017, 134, 187–205. [Google Scholar] [CrossRef]
- Compta, Y.; Parkkinen, L.; Kempster, P.; Selikhova, M.; Lashley, T.; Holton, J.L.; Lees, A.J.; Revesz, T. The significance of α-synuclein, amyloid-β and tau pathologies in Parkinson’s disease progression and related dementia. Neurodegener. Dis. 2014, 13, 154–156. [Google Scholar] [CrossRef]
- Hamilton, R.L. Lewy bodies in Alzheimer’s disease: A neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000, 10, 378–384. [Google Scholar] [CrossRef]
- Mak, E.; Reid, R.I.; Przybelski, S.A.; Lesnick, T.G.; Schwarz, C.G.; Senjem, M.L.; Raghavan, S.; Vemuri, P.; Jack, C.R., Jr.; Min, H.K.; et al. Influences of amyloid-β and tau on white matter neurite alterations in dementia with Lewy bodies. NPJ Park. Dis. 2024, 10, 76. [Google Scholar] [CrossRef]
- Moussaud, S.; Jones, D.R.; Moussaud-Lamodière, E.L.; Delenclos, M.; Ross, O.A.; McLean, P.J. Alpha-synuclein and tau: Teammates in neurodegeneration? Mol. Neurodegener. 2014, 9, 43. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Prajapati, K.P.; Anand, B.G.; Dubey, K.; Kar, K. Amyloid cross-seeding raises new dimensions to understanding of amyloidogenesis mechanism. Ageing Res. Rev. 2019, 56, 100937. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, Y.; Zhang, M.; Liu, Y.; Zhang, D.; Gong, X.; Feng, Z.; Tang, J.; Chang, Y.; Zheng, J. Fundamentals of cross-seeding of amyloid proteins: An introduction. J. Mater. Chem. B 2019, 7, 7267–7282. [Google Scholar] [CrossRef]
- Gerson, J.E.; Farmer, K.M.; Henson, N.; Castillo-Carranza, D.L.; Murillo, M.C.; Sengupta, U.; Barrett, A.; Kayed, R. Tau oligomers mediate α-synuclein toxicity and can be targeted by immunotherapy. Mol. Neurodegener. 2018, 13, 13. [Google Scholar] [CrossRef]
- Hojjatian, A.; Dasari, A.K.R.; Sengupta, U.; Taylor, D.; Daneshparvar, N.; Yeganeh, F.A.; Dillard, L.; Michael, B.; Griffin, R.G.; Borgnia, M.J.; et al. Tau induces formation of α-synuclein filaments with distinct molecular conformations. Biochem. Biophys. Res. Commun. 2021, 554, 145–150. [Google Scholar] [CrossRef]
- Feng, S.T.; Wang, Z.Z.; Yuan, Y.H.; Sun, H.M.; Chen, N.H.; Zhang, Y. Update on the association between alpha-synuclein and tau with mitochondrial dysfunction: Implications for Parkinson’s disease. Eur. J. Neurosci. 2021, 53, 2946–2959. [Google Scholar] [CrossRef] [PubMed]
- Outeiro, T.F.; Harvey, K.; Dominguez-Meijide, A.; Gerhardt, E. LRRK2, alpha-synuclein, and tau: Partners in crime or unfortunate bystanders? Biochem. Soc. Trans. 2019, 47, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, Y.; Chambers, J.K.; Inoue, H.; Ano, Y.; Takashima, A.; Nakayama, H.; Uchida, K. Phosphorylation and oligomerization of α-synuclein associated with GSK-3β activation in the rTg4510 mouse model of tauopathy. Acta Neuropathol. Commun. 2020, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Wilkaniec, A.; Czapski, G.A.; Adamczyk, A. Cdk5 at crossroads of protein oligomerization in neurodegenerative diseases: Facts and hypotheses. J. Neurochem. 2016, 136, 222–233. [Google Scholar] [CrossRef]
- Candreva, J.; Chau, E.; Rice, M.E.; Kim, J.R. Interactions between Soluble Species of β-Amyloid and α-Synuclein Promote Oligomerization while Inhibiting Fibrillization. Biochemistry 2020, 59, 425–435. [Google Scholar] [CrossRef]
- Marsh, S.E.; Blurton-Jones, M. Examining the mechanisms that link β-amyloid and α-synuclein pathologies. Alzheimer’s Res. Ther. 2012, 4, 11. [Google Scholar] [CrossRef]
- Robinson, J.L.; Lee, E.B.; Xie, S.X.; Rennert, L.; Suh, E.; Bredenberg, C.; Caswell, C.; Van Deerlin, V.M.; Yan, N.; Yousef, A.; et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain J. Neurol. 2018, 141, 2181–2193. [Google Scholar] [CrossRef]
- Beach, T.G.; White, C.L., 3rd; Hladik, C.L.; Sabbagh, M.N.; Connor, D.J.; Shill, H.A.; Sue, L.I.; Sasse, J.; Bachalakuri, J.; Henry-Watson, J.; et al. Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol. 2009, 117, 169–174. [Google Scholar] [CrossRef]
- Boeve, B.F.; Silber, M.H.; Ferman, T.J.; Lin, S.C.; Benarroch, E.E.; Schmeichel, A.M.; Ahlskog, J.E.; Caselli, R.J.; Jacobson, S.; Sabbagh, M.; et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep. Med. 2013, 14, 754–762. [Google Scholar] [CrossRef]
- Tolosa, E.; Gaig, C.; Santamaría, J.; Compta, Y. Diagnosis and the premotor phase of Parkinson disease. Neurology 2009, 72, S12–S20. [Google Scholar] [CrossRef]
- Hawkes, C.H. The prodromal phase of sporadic Parkinson’s disease: Does it exist and if so how long is it? Mov. Disord. 2008, 23, 1799–1807. [Google Scholar] [CrossRef]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain J. Neurol. 1991, 114 Pt 5, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Morrish, P.K.; Rakshi, J.S.; Bailey, D.L.; Sawle, G.V.; Brooks, D.J. Measuring the rate of progression and estimating the preclinical period of Parkinson’s disease with [18F]dopa PET. J. Neurol. Neurosurg. Psychiatry 1998, 64, 314–319. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Kraemmer, J.; Kovacs, G.G.; Perju-Dumbrava, L.; Pirker, S.; Traub-Weidinger, T.; Pirker, W. Correlation of striatal dopamine transporter imaging with post mortem substantia nigra cell counts. Mov. Disord. 2014, 29, 1767–1773. [Google Scholar] [CrossRef]
- Salsone, M.; Labate, A.; Quattrone, A. Cardiac denervation precedes nigrostriatal damage in idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2012, 27, 1068–1069. [Google Scholar] [CrossRef]
- Iranzo, A.; Gelpi, E.; Tolosa, E.; Molinuevo, J.L.; Serradell, M.; Gaig, C.; Santamaria, J. Neuropathology of prodromal Lewy body disease. Mov. Disord. 2014, 29, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Parkinson Study Group. Dopamine transporter brain imaging to assess the effects of pramipexole vs. levodopa on Parkinson disease progression. Jama 2002, 287, 1653–1661. [Google Scholar] [CrossRef]
- Bidesi, N.S.R.; Andersen, I.V.; Windhorst, A.D.; Shalgunov, V.; Herth, M.M. The role of neuroimaging in Parkinson’s disease. J. Neurochem. 2021, 159, 660–689. [Google Scholar] [CrossRef]
- Brooks, D.J.; Tambasco, N. Imaging synucleinopathies. Mov. Disord. 2016, 31, 814–829. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Li, H.; Zhang, H. SPECT Molecular Imaging in Parkinson′s Disease. BioMed Res. Int. 2012, 2012, 412486. [Google Scholar] [CrossRef]
- Berg, D.; Lang, A.E.; Postuma, R.B.; Maetzler, W.; Deuschl, G.; Gasser, T.; Siderowf, A.; Schapira, A.H.; Oertel, W.; Obeso, J.A.; et al. Changing the research criteria for the diagnosis of Parkinson’s disease: Obstacles and opportunities. Lancet Neurol. 2013, 12, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Eberling, J.L.; Dave, K.D.; Frasier, M.A. α-synuclein imaging: A critical need for Parkinson’s disease research. J. Park. Dis. 2013, 3, 565–567. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Furumoto, S.; Fodero-Tavoletti, M.; Harada, R.; Mulligan, R.S.; Kudo, Y.; Masters, C.L.; Yanai, K.; Rowe, C.C.; Okamura, N. The Challenges of Tau Imaging. Future Neurol. 2012, 7, 409–421. [Google Scholar] [CrossRef]
- Kallinen, A.; Kassiou, M. Tracer development for PET imaging of proteinopathies. Nucl. Med. Biol. 2022, 114–115, 108–120. [Google Scholar] [CrossRef]
- Kotzbauer, P.T.; Tu, Z.; Mach, R.H. Current status of the development of PET radiotracers for imaging alpha synuclein aggregates in Lewy bodies and Lewy neurites. Clin. Transl. Imaging 2017, 5, 3–14. [Google Scholar] [CrossRef]
- Stefanis, L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Deramecourt, V.; Bombois, S.; Maurage, C.A.; Ghestem, A.; Drobecq, H.; Vanmechelen, E.; Lebert, F.; Pasquier, F.; Delacourte, A. Biochemical staging of synucleinopathy and amyloid deposition in dementia with Lewy bodies. J. Neuropathol. Exp. Neurol. 2006, 65, 278–288. [Google Scholar] [CrossRef]
- Donaghy, P.; Thomas, A.J.; O’Brien, J.T. Amyloid PET Imaging in Lewy body disorders. Am. J. Geriatr. Psychiatry 2015, 23, 23–37. [Google Scholar] [CrossRef]
- Burack, M.A.; Hartlein, J.; Flores, H.P.; Taylor-Reinwald, L.; Perlmutter, J.S.; Cairns, N.J. In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology 2010, 74, 77–84. [Google Scholar] [CrossRef]
- Kalaitzakis, M.E.; Graeber, M.B.; Gentleman, S.M.; Pearce, R.K. Striatal beta-amyloid deposition in Parkinson disease with dementia. J. Neuropathol. Exp. Neurol. 2008, 67, 155–161. [Google Scholar] [CrossRef] [PubMed]
- von Bohlen und Halbach, O.; Schober, A.; Krieglstein, K. Genes, proteins, and neurotoxins involved in Parkinson’s disease. Prog. Neurobiol. 2004, 73, 151–177. [Google Scholar] [CrossRef]
- Mathis, C.A.; Lopresti, B.J.; Ikonomovic, M.D.; Klunk, W.E. Small-molecule PET Tracers for Imaging Proteinopathies. Semin. Nucl. Med. 2017, 47, 553–575. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Seibyl, J.; Cartier, A.; Bhatt, R.; Catafau, A.M. Molecular imaging insights into neurodegeneration: Focus on α-synuclein radiotracers. J. Nucl. Med. 2014, 55, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Korat, Š.; Bidesi, N.S.R.; Bonanno, F.; Di Nanni, A.; Hoàng, A.N.N.; Herfert, K.; Maurer, A.; Battisti, U.M.; Bowden, G.D.; Thonon, D.; et al. Alpha-Synuclein PET Tracer Development-An Overview about Current Efforts. Pharmaceuticals 2021, 14, 847. [Google Scholar] [CrossRef]
- Barrett, P.J.; Greenamyre, J.T. Post-translational modification of α-synuclein in Parkinson’s disease. Brain Res. 2015, 1628, 247–253. [Google Scholar] [CrossRef]
- Schildknecht, S.; Gerding, H.R.; Karreman, C.; Drescher, M.; Lashuel, H.A.; Outeiro, T.F.; Di Monte, D.A.; Leist, M. Oxidative and nitrative alpha-synuclein modifications and proteostatic stress: Implications for disease mechanisms and interventions in synucleinopathies. J. Neurochem. 2013, 125, 491–511. [Google Scholar] [CrossRef]
- Anderson, J.P.; Walker, D.E.; Goldstein, J.M.; de Laat, R.; Banducci, K.; Caccavello, R.J.; Barbour, R.; Huang, J.; Kling, K.; Lee, M.; et al. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 2006, 281, 29739–29752. [Google Scholar] [CrossRef]
- Brembati, V.; Faustini, G.; Longhena, F.; Bellucci, A. Alpha synuclein post translational modifications: Potential targets for Parkinson’s disease therapy? Front. Mol. Neurosci. 2023, 16, 1197853. [Google Scholar] [CrossRef]
- Heiss, W.D.; Herholz, K. Brain receptor imaging. J. Nucl. Med. 2006, 47, 302–312. [Google Scholar]
- Pike, V.W. Considerations in the Development of Reversibly Binding PET Radioligands for Brain Imaging. Curr. Med. Chem. 2016, 23, 1818–1869. [Google Scholar] [CrossRef] [PubMed]
- Derkinderen, P.; Cossais, F.; de Guilhem de Lataillade, A.; Leclair-Visonneau, L.; Neunlist, M.; Paillusson, S.; De Giorgio, R. Gastrointestinal mucosal biopsies in Parkinson’s disease: Beyond alpha-synuclein detection. J. Neural Transm. 2022, 129, 1095–1103. [Google Scholar] [CrossRef]
- Fenyi, A.; Leclair-Visonneau, L.; Clairembault, T.; Coron, E.; Neunlist, M.; Melki, R.; Derkinderen, P.; Bousset, L. Detection of alpha-synuclein aggregates in gastrointestinal biopsies by protein misfolding cyclic amplification. Neurobiol. Dis. 2019, 129, 38–43. [Google Scholar] [CrossRef]
- Shalgunov, V.; Xiong, M.; L’Estrade, E.T.; Raval, N.R.; Andersen, I.V.; Edgar, F.G.; Speth, N.R.; Baerentzen, S.L.; Hansen, H.D.; Donovan, L.L.; et al. Blocking of efflux transporters in rats improves translational validation of brain radioligands. EJNMMI Res. 2020, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Syvänen, S.; Lindhe, O.; Palner, M.; Kornum, B.R.; Rahman, O.; Långström, B.; Knudsen, G.M.; Hammarlund-Udenaes, M. Species differences in blood-brain barrier transport of three positron emission tomography radioligands with emphasis on P-glycoprotein transport. Drug Metab. Dispos. 2009, 37, 635–643. [Google Scholar] [CrossRef]
- Serpell, L.C.; Berriman, J.; Jakes, R.; Goedert, M.; Crowther, R.A. Fiber diffraction of synthetic alpha-synuclein filaments shows amyloid-like cross-beta conformation. Proc. Natl. Acad. Sci. USA 2000, 97, 4897–4902. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.M.; Koulov, A.V.; Balch, W.E.; Kelly, J.W. Functional amyloid—from bacteria to humans. Trends Biochem. Sci. 2007, 32, 217–224. [Google Scholar] [CrossRef]
- Berriman, J.; Serpell, L.C.; Oberg, K.A.; Fink, A.L.; Goedert, M.; Crowther, R.A. Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-beta structure. Proc. Natl. Acad. Sci. USA 2003, 100, 9034–9038. [Google Scholar] [CrossRef]
- Fodero-Tavoletti, M.T.; Smith, D.P.; McLean, C.A.; Adlard, P.A.; Barnham, K.J.; Foster, L.E.; Leone, L.; Perez, K.; Cortés, M.; Culvenor, J.G.; et al. In vitro characterization of Pittsburgh compound-B binding to Lewy bodies. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 10365–10371. [Google Scholar] [CrossRef]
- Ye, L.; Velasco, A.; Fraser, G.; Beach, T.G.; Sue, L.; Osredkar, T.; Libri, V.; Spillantini, M.G.; Goedert, M.; Lockhart, A. In vitro high affinity alpha-synuclein binding sites for the amyloid imaging agent PIB are not matched by binding to Lewy bodies in postmortem human brain. J. Neurochem. 2008, 105, 1428–1437. [Google Scholar] [CrossRef]
- Di Nanni, A.; Saw, R.S.; Battisti, U.M.; Bowden, G.D.; Boeckermann, A.; Bjerregaard-Andersen, K.; Pichler, B.J.; Herfert, K.; Herth, M.M.; Maurer, A. A Fluorescent Probe as a Lead Compound for a Selective α-Synuclein PET Tracer: Development of a Library of 2-Styrylbenzothiazoles and Biological Evaluation of [(18)F]PFSB and [(18)F]MFSB. ACS Omega 2023, 8, 31450–31467. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Okamura, N.; Furumoto, S.; Tashiro, M.; Furukawa, K.; Maruyama, M.; Itoh, M.; Iwata, R.; Yanai, K.; Arai, H. 2-(2-[2-Dimethylaminothiazol-5-yl]ethenyl)-6-(2-[fluoro]ethoxy)benzoxazole: A novel PET agent for in vivo detection of dense amyloid plaques in Alzheimer’s disease patients. J. Nucl. Med. 2007, 48, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Fodero-Tavoletti, M.T.; Mulligan, R.S.; Okamura, N.; Furumoto, S.; Rowe, C.C.; Kudo, Y.; Masters, C.L.; Cappai, R.; Yanai, K.; Villemagne, V.L. In vitro characterisation of BF227 binding to alpha-synuclein/Lewy bodies. Eur. J. Pharmacol. 2009, 617, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Levigoureux, E.; Lancelot, S.; Bouillot, C.; Chauveau, F.; Verdurand, M.; Verchere, J.; Billard, T.; Baron, T.; Zimmer, L. Binding of the PET radiotracer [¹⁸F]BF227 does not reflect the presence of alpha-synuclein aggregates in transgenic mice. Curr. Alzheimer Res. 2014, 11, 955–960. [Google Scholar] [CrossRef]
- Verdurand, M.; Levigoureux, E.; Lancelot, S.; Zeinyeh, W.; Billard, T.; Quadrio, I.; Perret-Liaudet, A.; Zimmer, L.; Chauveau, F. Amyloid-Beta Radiotracer [(18)F]BF-227 Does Not Bind to Cytoplasmic Glial Inclusions of Postmortem Multiple System Atrophy Brain Tissue. Contrast Media Mol. Imaging 2018, 2018, 9165458. [Google Scholar] [CrossRef]
- Kikuchi, A.; Takeda, A.; Okamura, N.; Tashiro, M.; Hasegawa, T.; Furumoto, S.; Kobayashi, M.; Sugeno, N.; Baba, T.; Miki, Y.; et al. In vivo visualization of alpha-synuclein deposition by carbon-11-labelled 2-[2-(2-dimethylaminothiazol-5-yl)ethenyl]-6-[2-(fluoro)ethoxy]benzoxazole positron emission tomography in multiple system atrophy. Brain J. Neurol. 2010, 133, 1772–1778. [Google Scholar] [CrossRef]
- Josephson, L.; Stratman, N.; Liu, Y.; Qian, F.; Liang, S.H.; Vasdev, N.; Patel, S. The Binding of BF-227-Like Benzoxazoles to Human α-Synuclein and Amyloid β Peptide Fibrils. Mol. Imaging 2018, 17, 1536012118796297. [Google Scholar] [CrossRef]
- Koga, S.; Ono, M.; Sahara, N.; Higuchi, M.; Dickson, D.W. Fluorescence and autoradiographic evaluation of tau PET ligand PBB3 to α-synuclein pathology. Mov. Disord. 2017, 32, 884–892. [Google Scholar] [CrossRef]
- Perez-Soriano, A.; Arena, J.E.; Dinelle, K.; Miao, Q.; McKenzie, J.; Neilson, N.; Puschmann, A.; Schaffer, P.; Shinotoh, H.; Smith-Forrester, J.; et al. PBB3 imaging in Parkinsonian disorders: Evidence for binding to tau and other proteins. Mov. Disord. 2017, 32, 1016–1024. [Google Scholar] [CrossRef]
- Miranda-Azpiazu, P.; Svedberg, M.; Higuchi, M.; Ono, M.; Jia, Z.; Sunnemark, D.; Elmore, C.S.; Schou, M.; Varrone, A. Identification and in vitro characterization of C05-01, a PBB3 derivative with improved affinity for alpha-synuclein. Brain Res. 2020, 1749, 147131. [Google Scholar] [CrossRef]
- Endo, H.; Ono, M.; Takado, Y.; Matsuoka, K.; Takahashi, M.; Tagai, K.; Kataoka, Y.; Hirata, K.; Takahata, K.; Seki, C.; et al. Imaging α-synuclein pathologies in animal models and patients with Parkinson’s and related diseases. Neuron 2024, 112, 2540–2557.e2548. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Ono, M.; Takado, Y.; Hirata, K.; Endo, H.; Ohfusa, T.; Kojima, T.; Yamamoto, T.; Onishi, T.; Orihara, A.; et al. High-Contrast Imaging of α-Synuclein Pathologies in Living Patients with Multiple System Atrophy. Mov. Disord. 2022, 37, 2159–2161. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Capotosti, F.; Schain, M.; Ohlsson, T.; Vokali, E.; Molette, J.; Touilloux, T.; Hliva, V.; Dimitrakopoulos, I.K.; Puschmann, A.; et al. The α-synuclein PET tracer [18F] ACI-12589 distinguishes multiple system atrophy from other neurodegenerative diseases. Nat. Commun. 2023, 14, 6750. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Capotosti, F.; Schain, M.; Ohlsson, T.; Touilloux, T.; Hliva, V.; Vokali, E.; Luthi-Carter, R.; Molette, J.; Dimitrakopoulos, I.K.; et al. Initial clinical scans using [18F]ACI-12589, a novel α-synuclein PET-tracer. Alzheimer’s Dement. 2022, 18, e065394. [Google Scholar] [CrossRef]
- Bagchi, D.P.; Yu, L.; Perlmutter, J.S.; Xu, J.; Mach, R.H.; Tu, Z.; Kotzbauer, P.T. Binding of the radioligand SIL23 to α-synuclein fibrils in Parkinson disease brain tissue establishes feasibility and screening approaches for developing a Parkinson disease imaging agent. PLoS ONE 2013, 8, e55031. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, H.; Padakanti, P.K.; Li, J.; Yang, H.; Fan, J.; Mach, R.H.; Kotzbauer, P.; Tu, Z. Radiosynthesis and in Vivo Evaluation of Two PET Radioligands for Imaging α-Synuclein. Appl. Sci. 2014, 4, 66–78. [Google Scholar] [CrossRef]
- Chu, W.; Zhou, D.; Gaba, V.; Liu, J.; Li, S.; Peng, X.; Xu, J.; Dhavale, D.; Bagchi, D.P.; d’Avignon, A.; et al. Design, Synthesis, and Characterization of 3-(Benzylidene)indolin-2-one Derivatives as Ligands for α-Synuclein Fibrils. J. Med. Chem. 2015, 58, 6002–6017. [Google Scholar] [CrossRef]
- Ono, M.; Maya, Y.; Haratake, M.; Ito, K.; Mori, H.; Nakayama, M. Aurones serve as probes of beta-amyloid plaques in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2007, 361, 116–121. [Google Scholar] [CrossRef]
- Ono, M.; Haratake, M.; Mori, H.; Nakayama, M. Novel chalcones as probes for in vivo imaging of beta-amyloid plaques in Alzheimer’s brains. Bioorg. Med. Chem. 2007, 15, 6802–6809. [Google Scholar] [CrossRef]
- Ono, M.; Yoshida, N.; Ishibashi, K.; Haratake, M.; Arano, Y.; Mori, H.; Nakayama, M. Radioiodinated flavones for in vivo imaging of beta-amyloid plaques in the brain. J. Med. Chem. 2005, 48, 7253–7260. [Google Scholar] [CrossRef]
- Meng, X.; Munishkina, L.A.; Fink, A.L.; Uversky, V.N. Effects of Various Flavonoids on the α-Synuclein Fibrillation Process. Park. Dis. 2010, 2010, 650794. [Google Scholar] [CrossRef]
- Zhu, M.; Han, S.; Fink, A.L. Oxidized quercetin inhibits α-synuclein fibrillization. Biochim. Biophys. Acta 2013, 1830, 2872–2881. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Doi, Y.; Watanabe, H.; Ihara, M.; Ozaki, A.; Saji, H. Structure–activity relationships of radioiodinated diphenyl derivatives with different conjugated double bonds as ligands for α-synuclein aggregates. RSC Adv. 2016, 6, 44305–44312. [Google Scholar] [CrossRef]
- Hsieh, C.J.; Xu, K.; Lee, I.; Graham, T.J.A.; Tu, Z.; Dhavale, D.; Kotzbauer, P.; Mach, R.H. Chalcones and Five-Membered Heterocyclic Isosteres Bind to Alpha Synuclein Fibrils In Vitro. ACS Omega 2018, 3, 4486–4493. [Google Scholar] [CrossRef]
- Kaide, S.; Watanabe, H.; Iikuni, S.; Hasegawa, M.; Itoh, K.; Ono, M. Chalcone Analogue as New Candidate for Selective Detection of α-Synuclein Pathology. ACS Chem. Neurosci. 2022, 13, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Kaide, S.; Watanabe, H.; Iikuni, S.; Hasegawa, M.; Ono, M. Synthesis and Evaluation of (18)F-Labeled Chalcone Analogue for Detection of α-Synuclein Aggregates in the Brain Using the Mouse Model. ACS Chem. Neurosci. 2022, 13, 2982–2990. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, H.M.; Liu, B.L. (E)-5-styryl-1H-indole and (E)-6-styrylquinoline derivatives serve as probes for β-amyloid plaques. Molecules 2012, 17, 4252–4265. [Google Scholar] [CrossRef]
- Yoshimura, M.; Ono, M.; Matsumura, K.; Watanabe, H.; Kimura, H.; Cui, M.; Nakamoto, Y.; Togashi, K.; Okamoto, Y.; Ihara, M.; et al. Structure-Activity Relationships and in Vivo Evaluation of Quinoxaline Derivatives for PET Imaging of β-Amyloid Plaques. ACS Med. Chem. Lett. 2013, 4, 596–600. [Google Scholar] [CrossRef]
- Tago, T.; Furumoto, S.; Okamura, N.; Harada, R.; Adachi, H.; Ishikawa, Y.; Yanai, K.; Iwata, R.; Kudo, Y. Structure-Activity Relationship of 2-Arylquinolines as PET Imaging Tracers for Tau Pathology in Alzheimer Disease. J. Nucl. Med. 2016, 57, 608–614. [Google Scholar] [CrossRef]
- Yue, X.; Dhavale, D.D.; Li, J.; Luo, Z.; Liu, J.; Yang, H.; Mach, R.H.; Kotzbauer, P.T.; Tu, Z. Design, synthesis, and in vitro evaluation of quinolinyl analogues for α-synuclein aggregation. Bioorg. Med. Chem. Lett. 2018, 28, 1011–1019. [Google Scholar] [CrossRef]
- Kaide, S.; Watanabe, H.; Shimizu, Y.; Iikuni, S.; Nakamoto, Y.; Hasegawa, M.; Itoh, K.; Ono, M. Identification and Evaluation of Bisquinoline Scaffold as a New Candidate for α-Synuclein-PET Imaging. ACS Chem. Neurosci. 2020, 11, 4254–4261. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Watanabe, H.; Kaide, S.; Ono, M. Structure-Activity Relationships of Styrylquinoline and Styrylquinoxaline Derivatives as α-Synuclein Imaging Probes. ACS Med. Chem. Lett. 2022, 13, 1598–1605. [Google Scholar] [CrossRef]
- Watanabe, H.; Ariyoshi, T.; Ozaki, A.; Ihara, M.; Ono, M.; Saji, H. Synthesis and biological evaluation of novel radioiodinated benzimidazole derivatives for imaging α-synuclein aggregates. Bioorg. Med. Chem. 2017, 25, 6398–6403. [Google Scholar] [CrossRef] [PubMed]
- Kuebler, L.; Buss, S.; Leonov, A.; Ryazanov, S.; Schmidt, F.; Maurer, A.; Weckbecker, D.; Landau, A.M.; Lillethorup, T.P.; Bleher, D.; et al. [(11)C]MODAG-001-towards a PET tracer targeting α-synuclein aggregates. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1759–1772. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Bian, J.; Zhang, P.; Bu, L.L.; Shen, Y.; Yu, W.B.; Lu, X.H.; Lin, X.; Ye, D.Y.; Wang, J.; et al. Design, synthesis and identification of N, N-dibenzylcinnamamide (DBC) derivatives as novel ligands for α-synuclein fibrils by SPR evaluation system. Bioorg. Med. Chem. 2020, 28, 115358. [Google Scholar] [CrossRef]
- Akasaka, T.; Watanabe, H.; Kaide, S.; Iikuni, S.; Hasegawa, M.; Ono, M. Synthesis and evaluation of novel radioiodinated phenylbenzofuranone derivatives as α-synuclein imaging probes. Bioorg. Med. Chem. Lett. 2022, 64, 128679. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, S.; Cui, M. Structure-Activity Relationships of Cyano-substituted Indole Derivatives as Ligands for α-Synuclein Aggregates. ACS Med. Chem. Lett. 2023, 14, 1467–1471. [Google Scholar] [CrossRef]
- Pees, A.; Tong, J.; Birudaraju, S.; Munot, Y.S.; Liang, S.H.; Guarino, D.S.; Mach, R.H.; Mathis, C.A.; Vasdev, N. Development of Pyridothiophene Compounds for PET Imaging of α-Synuclein. Chemistry 2024, 30, e202303921. [Google Scholar] [CrossRef]
- Bonanno, F.; Saw, R.S.; Bleher, D.; Papadopoulos, I.; Bowden, G.D.; Bjerregaard-Andersen, K.; Windhorst, A.D.; Pichler, B.J.; Herfert, K.; Maurer, A. Advancing Parkinson’s Disease Diagnostics: The Potential of Arylpyrazolethiazole Derivatives for Imaging α-Synuclein Aggregates. ACS Omega 2024, 9, 24774–24788. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, X.; Li, Y.; Zhang, Q.; Dai, J.; Yan, X.X.; Liu, Y.; Zhang, J.; Liu, S.; Cui, M. Discovery and Evaluation of Imidazo[2,1-b][1,3,4]thiadiazole Derivatives as New Candidates for α-Synuclein PET Imaging. J. Med. Chem. 2024, 67, 12695–12710. [Google Scholar] [CrossRef]
- Cegelski, L.; Pinkner, J.S.; Hammer, N.D.; Cusumano, C.K.; Hung, C.S.; Chorell, E.; Aberg, V.; Walker, J.N.; Seed, P.C.; Almqvist, F.; et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat. Chem. Biol. 2009, 5, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Horvath, I.; Weise, C.F.; Andersson, E.K.; Chorell, E.; Sellstedt, M.; Bengtsson, C.; Olofsson, A.; Hultgren, S.J.; Chapman, M.; Wolf-Watz, M.; et al. Mechanisms of protein oligomerization: Inhibitor of functional amyloids templates α-synuclein fibrillation. J. Am. Chem. Soc. 2012, 134, 3439–3444. [Google Scholar] [CrossRef] [PubMed]
- Pokrzywa, M.; Pawełek, K.; Kucia, W.E.; Sarbak, S.; Chorell, E.; Almqvist, F.; Wittung-Stafshede, P. Effects of small-molecule amyloid modulators on a Drosophila model of Parkinson’s disease. PLoS ONE 2017, 12, e0184117. [Google Scholar] [CrossRef] [PubMed]
- Chermenina, M.; Chorell, E.; Pokrzywa, M.; Antti, H.; Almqvist, F.; Strömberg, I.; Wittung-Stafshede, P. Single injection of small-molecule amyloid accelerator results in cell death of nigral dopamine neurons in mice. NPJ Park. Dis. 2015, 1, 15024. [Google Scholar] [CrossRef]
- Aberg, V.; Norman, F.; Chorell, E.; Westermark, A.; Olofsson, A.; Sauer-Eriksson, A.E.; Almqvist, F. Microwave-assisted decarboxylation of bicyclic 2-pyridone scaffolds and identification of Abeta-peptide aggregation inhibitors. Org. Biomol. Chem. 2005, 3, 2817–2823. [Google Scholar] [CrossRef]
- Cairns, A.G.; Vazquez-Romero, A.; Mahdi Moein, M.; Ådén, J.; Elmore, C.S.; Takano, A.; Arakawa, R.; Varrone, A.; Almqvist, F.; Schou, M. Increased Brain Exposure of an Alpha-Synuclein Fibrillization Modulator by Utilization of an Activated Ester Prodrug Strategy. ACS Chem. Neurosci. 2018, 9, 2542–2547. [Google Scholar] [CrossRef]
- Xiang, J.; Tao, Y.; Xia, Y.; Luo, S.; Zhao, Q.; Li, B.; Zhang, X.; Sun, Y.; Xia, W.; Zhang, M.; et al. Development of an α-synuclein positron emission tomography tracer for imaging synucleinopathies. Cell 2023, 186, 3350–3367.e3319. [Google Scholar] [CrossRef]
- Kantarci, K. Toward imaging of alpha-synuclein with PET. Cell 2023, 186, 3327–3329. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, Z.; Wu, S.; Ye, K. Positron emission tomography tracers for synucleinopathies. Mol. Neurodegener. 2025, 20, 1. [Google Scholar] [CrossRef]
- Ferrie, J.J.; Lengyel-Zhand, Z.; Janssen, B.; Lougee, M.G.; Giannakoulias, S.; Hsieh, C.J.; Pagar, V.V.; Weng, C.C.; Xu, H.; Graham, T.J.A.; et al. Identification of a nanomolar affinity α-synuclein fibril imaging probe by ultra-high throughput in silico screening. Chem. Sci. 2020, 11, 12746–12754. [Google Scholar] [CrossRef]
- Janssen, B.; Tian, G.; Lengyel-Zhand, Z.; Hsieh, C.J.; Lougee, M.G.; Riad, A.; Xu, K.; Hou, C.; Weng, C.C.; Lopresti, B.J.; et al. Identification of a Putative α-synuclein Radioligand Using an in silico Similarity Search. Mol. Imaging Biol. 2023, 25, 704–719. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J.; Lee, V.M.; Trojanowski, J.Q. Parkinson’s disease dementia: Convergence of α-synuclein, tau and amyloid-β pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Verdurand, M.; Levigoureux, E.; Zeinyeh, W.; Berthier, L.; Mendjel-Herda, M.; Cadarossanesaib, F.; Bouillot, C.; Iecker, T.; Terreux, R.; Lancelot, S.; et al. In Silico, in Vitro, and in Vivo Evaluation of New Candidates for α-Synuclein PET Imaging. Mol. Pharm. 2018, 15, 3153–3166. [Google Scholar] [CrossRef]
- Uzuegbunam, B.C.; Li, J.; Paslawski, W.; Weber, W.; Svenningsson, P.; Ågren, H.; Yousefi, B.H. In Silico and In Vitro Study towards the Rational Design of 4,4’-Disarylbisthiazoles as a Selective α-Synucleinopathy Biomarker. Int. J. Mol. Sci. 2023, 24, 16445. [Google Scholar] [CrossRef] [PubMed]
- Uzuegbunam, B.C.; Li, J.; Paslawski, W.; Weber, W.; Svenningsson, P.; Ågren, H.; Yousefi, B.H. Toward Novel [(18)F]Fluorine-Labeled Radiotracers for the Imaging of α-Synuclein Fibrils. Front. Aging Neurosci. 2022, 14, 830704. [Google Scholar] [CrossRef]
- Wagner, J.; Ryazanov, S.; Leonov, A.; Levin, J.; Shi, S.; Schmidt, F.; Prix, C.; Pan-Montojo, F.; Bertsch, U.; Mitteregger-Kretzschmar, G.; et al. Anle138b: A novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease. Acta Neuropathol. 2013, 125, 795–813. [Google Scholar] [CrossRef]
- Heras-Garvin, A.; Weckbecker, D.; Ryazanov, S.; Leonov, A.; Griesinger, C.; Giese, A.; Wenning, G.K.; Stefanova, N. Anle138b modulates α-synuclein oligomerization and prevents motor decline and neurodegeneration in a mouse model of multiple system atrophy. Mov. Disord. 2019, 34, 255–263. [Google Scholar] [CrossRef]
- Wegrzynowicz, M.; Bar-On, D.; Calo, L.; Anichtchik, O.; Iovino, M.; Xia, J.; Ryazanov, S.; Leonov, A.; Giese, A.; Dalley, J.W.; et al. Depopulation of dense α-synuclein aggregates is associated with rescue of dopamine neuron dysfunction and death in a new Parkinson’s disease model. Acta Neuropathol. 2019, 138, 575–595. [Google Scholar] [CrossRef] [PubMed]
- Deeg, A.A.; Reiner, A.M.; Schmidt, F.; Schueder, F.; Ryazanov, S.; Ruf, V.C.; Giller, K.; Becker, S.; Leonov, A.; Griesinger, C.; et al. Anle138b and related compounds are aggregation specific fluorescence markers and reveal high affinity binding to α-synuclein aggregates. Biochim. Biophys. Acta 2015, 1850, 1884–1890. [Google Scholar] [CrossRef]
- Levin, J.; Sing, N.; Melbourne, S.; Morgan, A.; Mariner, C.; Spillantini, M.G.; Wegrzynowicz, M.; Dalley, J.W.; Langer, S.; Ryazanov, S.; et al. Safety, tolerability and pharmacokinetics of the oligomer modulator anle138b with exposure levels sufficient for therapeutic efficacy in a murine Parkinson model: A randomised, double-blind, placebo-controlled phase 1a trial. EBioMedicine 2022, 80, 104021. [Google Scholar] [CrossRef]
- Orlovskaya, V.V.; Fedorova, O.S.; Viktorov, N.B.; Vaulina, D.D.; Krasikova, R.N. One-Pot Radiosynthesis of [(18)F]Anle138b-5-(3-Bromophenyl)-3-(6-[(18)F]fluorobenzo[d][1,3]dioxol-5-yl)-1H-pyrazole-A Potential PET Radiotracer Targeting α-Synuclein Aggregates. Molecules 2023, 28, 2732. [Google Scholar] [CrossRef] [PubMed]
- Maurer, A.; Leonov, A.; Ryazanov, S.; Herfert, K.; Kuebler, L.; Buss, S.; Schmidt, F.; Weckbecker, D.; Linder, R.; Bender, D.; et al. (11) C Radiolabeling of anle253b: A Putative PET Tracer for Parkinson’s Disease That Binds to α-Synuclein Fibrils in vitro and Crosses the Blood-Brain Barrier. ChemMedChem 2020, 15, 411–415. [Google Scholar] [CrossRef]
- Raval, N.R.; Madsen, C.A.; Shalgunov, V.; Nasser, A.; Battisti, U.M.; Beaman, E.E.; Juhl, M.; Jørgensen, L.M.; Herth, M.M.; Hansen, H.D.; et al. Evaluation of the α-synuclein PET radiotracer (d(3))-[(11)C]MODAG-001 in pigs. Nucl. Med. Biol. 2022, 114–115, 42–48. [Google Scholar] [CrossRef]
- Tiepolt, S.; Patt, M.; Aghakhanyan, G.; Meyer, P.M.; Hesse, S.; Barthel, H.; Sabri, O. Current radiotracers to image neurodegenerative diseases. EJNMMI Radiopharm. Chem. 2019, 4, 17. [Google Scholar] [CrossRef]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.J.; Ferrie, J.J.; Xu, K.; Lee, I.; Graham, T.J.A.; Tu, Z.; Yu, J.; Dhavale, D.; Kotzbauer, P.; Petersson, E.J.; et al. Alpha Synuclein Fibrils Contain Multiple Binding Sites for Small Molecules. ACS Chem. Neurosci. 2018, 9, 2521–2527. [Google Scholar] [CrossRef]
- Kim, H.Y.; Chia, W.K.; Hsieh, C.-J.; Graham, T.; Schneider, M.; Lee, H.; Petersson, E.J.; O’shea, J.; Kotzbauer, P.; Mathis, C.; et al. A Novel PET Radiotracer for Imaging Alpha Synuclein Fibrils in Multiple System Atrophy (MSA). J. Nucl. Med. 2023, 64, P1402. [Google Scholar]
- Kim, H.Y.; Chia, W.K.; Hsieh, C.J.; Saturnino Guarino, D.; Graham, T.J.A.; Lengyel-Zhand, Z.; Schneider, M.; Tomita, C.; Lougee, M.G.; Kim, H.J.; et al. A Novel Brain PET Radiotracer for Imaging Alpha Synuclein Fibrils in Multiple System Atrophy. J. Med. Chem. 2023, 66, 12185–12202. [Google Scholar] [CrossRef] [PubMed]
- Poduslo, J.F.; Curran, G.L.; Berg, C.T. Macromolecular permeability across the blood-nerve and blood-brain barriers. Proc. Natl. Acad. Sci. USA 1994, 91, 5705–5709. [Google Scholar] [CrossRef]
- Bard, F.; Cannon, C.; Barbour, R.; Burke, R.L.; Games, D.; Grajeda, H.; Guido, T.; Hu, K.; Huang, J.; Johnson-Wood, K.; et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000, 6, 916–919. [Google Scholar] [CrossRef]
- Alzghool, O.M.; van Dongen, G.; van de Giessen, E.; Schoonmade, L.; Beaino, W. α-Synuclein Radiotracer Development and In Vivo Imaging: Recent Advancements and New Perspectives. Mov. Disord. 2022, 37, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Saeed, U.; Lang, A.E.; Masellis, M. Neuroimaging Advances in Parkinson’s Disease and Atypical Parkinsonian Syndromes. Front. Neurol. 2020, 11, 572976. [Google Scholar] [CrossRef] [PubMed]

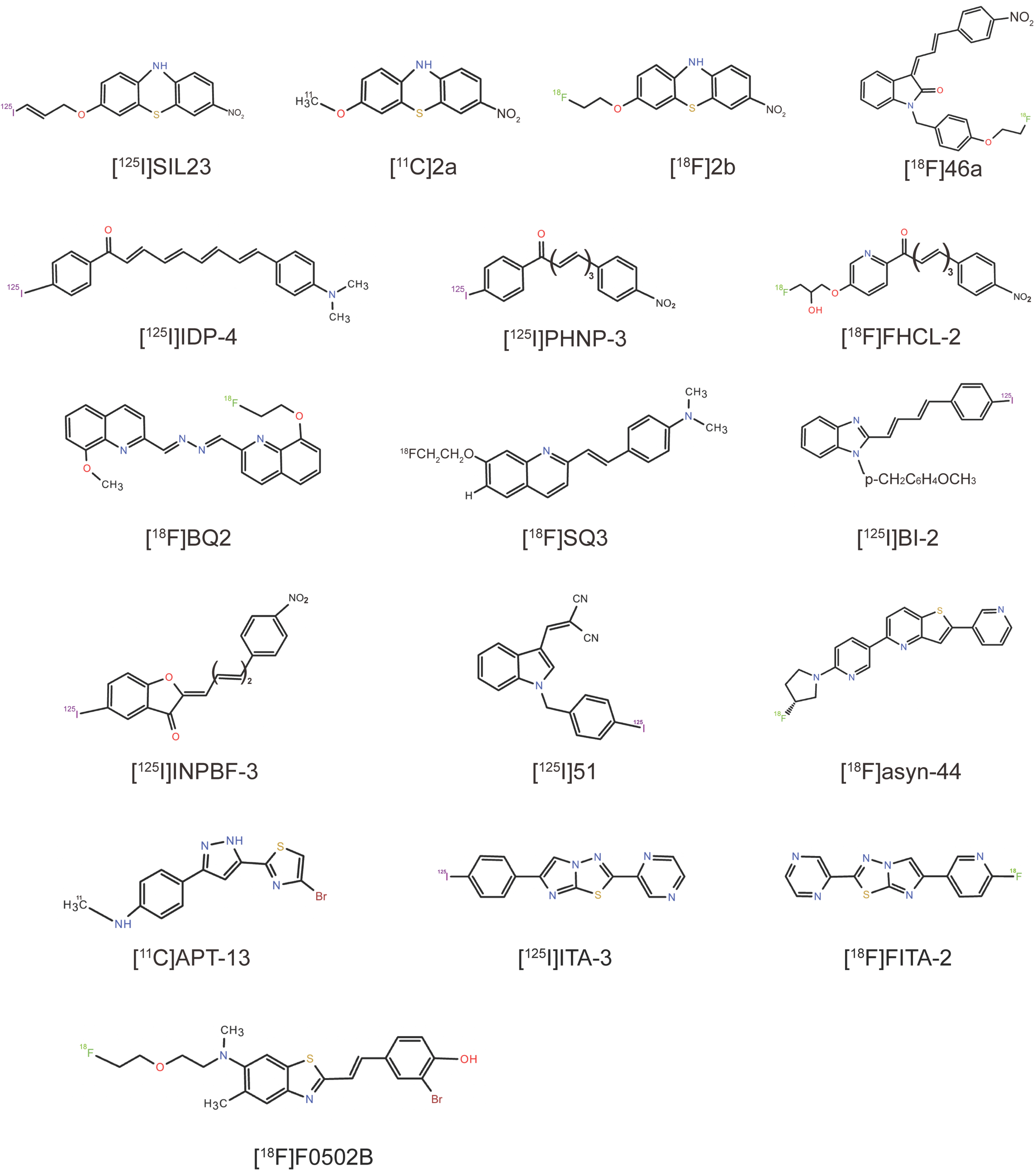
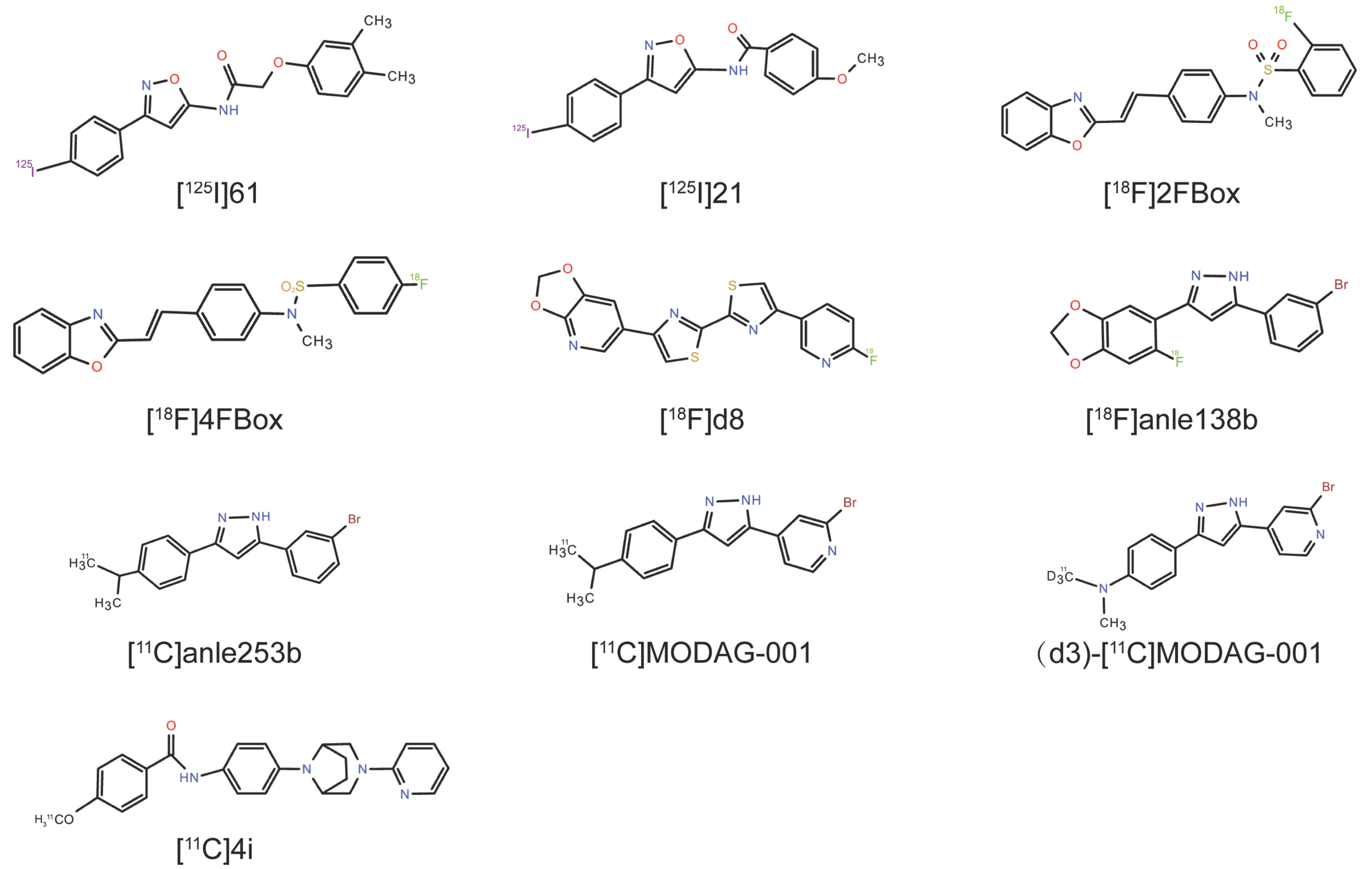
| Radiolabeled Compounds | In Vitro Assays | Preclinical Trial | Clinical Trial | Advantages | Limitations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki or kd (α-Syn Fibrils) (nm) | Ki or kd (aβ Fibrils) (nm) | Ki or kd (tau Fibrils) (nm) | High Selectivity (α-Syn/aβ) | Rcy [%] | Am [gbq/μmol] | Bbb Permeability with Early Peak Uptake of Suv | ≥0.4% id/g (Rat Brain) or ≥4.0% id/g (Mouse Brain) [%] | Clearance Rate | Pet Imaging in Animals | Pet Imaging in Human Subjects | Tested In Vitro Autoradiography (Gold Standard) | |||
| [11C]-PIB | 10.07 | 0.71 | N.T. | No | N.T. | 2.679 | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | Fails to bind to pure DLB (Aβ-free) brain homogenates |
| [18F]PFSB | 25.4 ± 2.3 | N.T. | N.T. | Yes | 5.8 ± 1.3 | 36.5 ± 8.5 | 4.78 | N.T. | N.T. | N.T. | N.T. | High binding in MSA and PD brain tissues; no binding in Aβ plaques and tau pathology | Selective binding to α-syn pathology on human brain slices | Highly lipophilic |
| [18F]MFSB | 10.3 ± 4.7 | N.T. | N.T. | Yes | 11.6 ± 2.9 | 41.2 ± 12.0 | Passing BBB, SUV = 1.79 ± 0.02 | N.T. | Fast to moderate clearance | N.T. | N.T. | High specific binding in MSA brain slices and no increased binding in AD sample | High affinity and selectivity to α-syn over Aβ, less lipophilic | Pharmacokinetic profile requires some improvement to determine and reduce nonspecific binding |
| [18F]BF-227 | 9.63 | 1.31–80 | N.T. | No | N.T. | 40–840 | Passing BBB, SUVpeak = 2.7 | 0.65 | N.T. | N.T. | N.T. | Autoradiography does not support significant binding to GCI | High affinity for α-syn | No binding towards α-syn aggregates |
| [11C]BF-227 | N.T. | N.T. | N.T. | No | >50, based on [11C]-CH3OTf) | 119–138 | Passing BBB, SUVpeak > 1.5 | N.T. | More gradual clearance than in HC brains | N.T. | HCs (8); probable MSA (8) | N.T. | Binding to GCI-rich brain regions by PET study on patients | Contradictory results among different research groups concerning binding towards α-syn aggregates |
| [11C]PBB3 | N.T. | N.T. | N.T. | No | N.T. | 133 | N.T. | N.T. | N.T. | N.T. | HCs (3); PSP-P (4); PSP-RS (1); DCTN1 mutation with PSP-P phenotype (1); MSA-P (1) | No significant binding in LB disease cases and significant binding to MSA cases | Usefulness of differentiating tauopathies from α-synucleinopathies | Low affinity for α-syn; cannot detect α-syn in LBD |
| [3H]-C05-01 | 25 | N.T. | N.T. | No | N.T. | 0.81 | N.T. | N.T. | N.T. | N.T. | N.T. | Specific binding in cases with α-syn pathology | High affinity for α-syn from DLB patient | Relatively high nonspecific and off-target binding |
| 18F-C05-05 | N.T. | N.T. | N.T. | Yes | N.T. | 63–557 | Passing BBB, SUV = 1.11 − 2.5 | N.T. | Slightly slower than PM-PBB3 and control | α-syn marmoset (1) | HCs (8); PD (8); DLB (2); MSA-P (3) | Intense binding to GCIs and DLB and PDD cases | High affinity for α-syn aggregates (IC50 = 8.0 nM); not highly binding with Aβ and tau aggregates in AD tissues (IC50 of 12.9 nM) | Not markedly penetrant through BBB; not sensitive to early-stage Lewy pathologies |
| 18F-SPAL-T-06 | 2.49 | N.T. | N.T. | Yes | N.T. | 237.5 ± 53.9 | N.T. | N.T. | Rapid clearance | N.T. | MSA-P (2); MSA-C (1); HC (1) | High binding with GCIs | High reactivity with MSA-type α-syn; negligible cross-reactivity with off-target components | Fails to capture PD and DLB pathologies |
| [18F]ACI-12589 | 33.5 ± 17.4 | No binding | No binding | Yes | 25.3 ± 4.5 | 11.1 | Passing BBB with rapid brain uptake | N.T. | Rapid washout | N.T. | α-syn related disorders (23); other neurodegenerative disorders (11) and HCs (8) | Specific binding in cases with MSA, PD, PDD, and LBV-AD | Specific to α-syn and good selectivity | Fails to capture PD and DLB pathologies |
| Radiolabeled Compounds | In Vitro Assays | Preclinical Trial | Clinical Trial | Advantages | Limitations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki or Kd (α-Syn Fibrils) (nM) | Ki or Kd (Aβ Fibrils) (nM) | Ki or Kd (tau Fibrils) (nM) | High Selectivity (α-Syn/Aβ) | RCY [%] | AM [GBq/μmol] | BBB Permeability with Early Peak Uptake of SUV | ≥0.4% ID/g (Rat Brain) or ≥4.0% ID/g (Mouse Brain) [%] | Clearance Rate | PET Imaging in Animals | PET Imaging in Human Subjects | Tested In Vitro Autoradiography (Gold Standard) | |||
| [125I]SIL23 | 120–180 | 635 | 230 | Yes | 43 | 81.4 | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | High affinity and relative selectivity for α-syn, specific binding to insoluble protein from human PD brain samples | High lipophilicity (calculated logP = 5.7) |
| [11C]2a | 32.1 ± 1.3 | Ki-α-syn/Ki-Aβ > 3 times | Ki-α-syn/Ki-tau > 4 times | Yes | 35–45 | >363 | Passing BBB with high initial uptake | 0.953 ± 0.115 | Rapid washout kinetics, faster washout kinetics than [18F]2b | Cynomolgus macaque (1) | N.T. | N.T. | High initial uptake and rapid washout | Moderate affinity |
| [18F]2b | 49.0 ± 4.9 | Ki-α-syn/Ki-Aβ = 2.1 times | Ki-α-syn/Ki-tau = 2.5 times | Yes | 55–65 | >200 | Passing BBB with high initial uptake | 0.758 ± 0.013 | Rapid washout kinetics | N.T. | N.T. | N.T. | High initial uptake and rapid washout | Moderate affinity |
| [18F]46a | 8.9 | 271 | 50 | Yes | N.T. | 29.6–185 | N.T. | N.T. | N.T. | N.T. | N.T. | N.T. | - | High logP value (4.18) |
| [125I]IDP-4 | 5.4 ± 1.5 | 12.9–37.1 | N.T. | No | 19–60 | 81.4 | N.T. | 0.45% | Slow washout | N.T. | N.T. | N.T. | Selective binding affinity for α-syn aggregates | Low brain uptake |
| [125I]PHNP-3 | 6.9 | 102 ± 21 | N.T. | Yes | 25 | N.T. | Poor BBB penetration | 0.78 | N.T. | N.T. | N.T. | N.T. | Modest uptake; high affinity and selectivity | Low brain uptake; high lipophilicity; high molecular weight (431 Da) |
| [18F]FHCL-2 | 3.4 | N.T. | N.T. | Yes | 36 | 5.3 | Passing BBB with high brain uptake | 2.4 | Gradual clearance | N.T. | N.T. | N.T. | High binding affinity for α-syn aggregates | Low 2/60 min ratios of radioactivity in brain (1.1–1.5) |
| [18F]BQ2 | 11.6 | 7.3 | No | No | 1.2 | 8.9 | Passing BBB, moderate brain uptake | 1.59 | Does not satisfy criteria of brain kinetics | N.T. | N.T. | N.T. | High affinity for α-syn | Nonspecific binding to myelin sheaths |
| [18F]SQ3 | 39.3 | 230 | N.T. | Yes, moderate selectivity | 20 | 0.00426 | Moderate brain permeability | 2.08 | Slow clearance rate | N.T. | N.T. | N.T. | Favorable pharmacokinetics in terms of brain permeability and stability against defluorination | Good binding affinity for α-syn aggregates and moderate selectivity |
| [125I]BI-2 | 99.5 ± 20.8 | 727 ± 227 | N.T. | No | 52 | 81.4 | Passing BBB, low initial brain uptake | 0.56 | Slow clearance | N.T. | N.T. | N.T. | High selective binding affinity for α-syn aggregates | Low brain uptake and clearance |
| [125I]INPBF-3 | 0.28 ± 0.17 | 1.2 ± 0.55 | N.T. | Yes, ∼4.4-fold | 19.6 | 81,400 | Passing BBB, low brain uptake, SUV < 1 | N.T. | N.T. | N.T. | N.T. | N.T. | High affinity for α-syn | High logP value = 6.17 |
| [125I]51 | 17.4 ± 5.6 | 73 | N.T. | Yes, moderate selectivity | 55.8 | N.T. | Passing BBB, moderate brain uptake | 3.57 ± 0.28 | Good washout rate | N.T. | N.T. | N.T. | High affinity and good selectivity | High lipophilicity (cLogP = 5.15) |
| [18F]asyn-44 | 1.85 ± 0.38 | 170 ± 60 | 4600, or >10,000 | Yes | 6 ± 2 | 263 ± 121 | Good brain permeability, SUV > 1.5 | N.T. | Moderate washout | N.T. | N.T. | High binding to MSA and PD donors, weak binding in AD, no binding with tau | Favorable in vitro and in vivo characteristics for neuroimaging | Presence of radiometabolites in rat brain |
| [11C]APT-13 | 27.8 ± 9.7 | 92.6 ± 48.8 | N.T. | Yes | 13.5 ± 1.8 | 98.7 ± 12.7 | Excellent brain penetration; SUV = 1.94 ± 0.29 | N.T. | Fast washout (t1/2 = 9 ± 1 min) | N.T. | N.T. | - | Highest affinity for α-syn with good selectivity and favorable pharmacokinetic properties | Lack of studies using brain tissues and rodent models |
| [125I]ITA-3 | >1000 | 3.7 ± 1.3 | N.T. | No | 16–75 | N.T. | Satisfactory BBB permeation | 4.9 ± 0.9 | N.T. | N.T. | N.T. | - | Moderate affinity for α-syn (IC50 = 55 nM) in human PD brain sections | Slow clearance, high logP values |
| [18F]FITA-2 | >1000 | 106.6 ± 9.8 | N.T. | No | >25 | >110 | Passing BBB, good brain uptake, SUVpeak = 2.80 ± 0.45 | 5.4 ± 0.6 | Fast clearance rate | N.T. | N.T. | High specific binding to α-syn pathologies in postmortem PD brain tissues | Moderate binding affinity to α-syn pathologies (IC50 = 245 nM) | Binding affinity to α-syn pathologies and selectivity was not optimal |
| [18F]F0502B | 10.97 | 109.2 | 120.5 | Yes | ~10 | 74 | High BBB permeability | N.T. | Rapid clearance | 8 rhesus macaques, injection of PBS (2), AAVs encoding A53T mutated human α-syn (3) and α-syn PFFs (3), respectively | N.T. | High specific binding to α-syn pathologies | High binding affinity and selectivity to α-syn | Imaging characteristics in patients still need further investigation |
| Radiolabeled Compounds | In Vitro Assays | Preclinical Trial | Clinical Trial | Advantages | Limitations | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki or Kd (α-Syn Fibrils) (nM) | Ki or Kd (Aβ Fibrils) (nM) | Ki or Kd (tau Fibrils) (nM) | High Selectivity (α-Syn/Aβ) | RCY [%] | AM [GBq/μmol] | BBB Permeability with Early Peak Uptake of SUV | ≥0.4% ID/g (Rat Brain) or ≥4.0% ID/g (Mouse Brain) [%] | Clearance Rate | PET Imaging in Animals | PET Imaging in Human Subjects | Tested In Vitro Autoradiography (Gold Standard) | |||
| [125I]61 | 1.06 | 4.56 | N.T. | Yes, ∼5-fold | 57 | 81 | N.T. | N.T. | N.T. | N.T. | N.T. | [125I]61 binds to sarkosyl-insoluble fraction in A53T mouse brain | High affinity with α-syn fibrils | Relatively high nonspecific binding |
| [125I]21 | 0.48 ± 0.08 | 2.47 ± 1.30 | N.T. | Yes, ∼5.2-fold, suboptimal selectivity | 12 ± 2 | 344 ± 235 | Passing BBB with peak SUV of ~2.3 | N.T. | Fast washout | Rhesus macaques (2) | PD (1); AD (1); HC (1); homogenate | N.T. | Rapid metabolic rate | High nonspecific binding |
| [18F]2FBox | 3.3 ± 2.8 | 145.3 ± 114.5 | N.T. | Yes, ∼44-fold | 10–19 | 68–543 | Good BBB permeation with peak SUV of 1.6 | 0.47 | Moderate washout kinetics | N.T. | PD (1); MSA (1); HC (1); AD (1) | Cannot image Lewy bodies or neurites | High affinity with α-syn fibrils | No binding to α-syn aggregates confirmed by postmortem tissue investigation |
| [18F]4FBox | 155.4 ± 96.5 | 7.7 ± 2.6 | N.T. | Yes, ∼20-fold | 10–19 | 68–543 | Good BBB permeation with peak SUV of 1.6 | 0.47 | Moderate washout kinetics | N.T. | PD (1); MSA (1); HC (1); AD (1) | Cannot image Lewy bodies or neurites | High affinity with α-syn fibrils | No binding to α-syn aggregates confirmed by postmortem tissue investigation |
| [18F]d8 | 0.1 | 386.3 | >1000 | Yes, 1/3863 | ≥25 | 40–104 | Good BBB permeation | >4 | Fast washout | N.T. | N.T. | N.T. | High binding affinity to α-syn; low lipophilicity | - |
| [18F]anle138b | 190 ± 120 | N.T. | N.T. | N.T. | N.T. | N.T. | Good BBB permeation | N.T. | N.T. | N.T. | N.T. | N.T. | - | High lipophilicity (logP = 4.34) |
| [11C]anle253b | N.T. | N.T. | N.T. | N.T. | 47 | 15.1 ± 3.4 | Clear penetration | 0.25–0.3 | Slow washout | N.T. | N.T. | N.T. | Direct binding to α-syn fibrils (IC50 = 1.6 nM) | High lipophilicity (logP = 5.21), lack of information about affinity using human-derived brain tissue |
| [11C]MODAG-001 | 0.6 ± 0.1 | 20 ± 10 | 19 ± 6.4 | Yes, ∼30-fold | 3.6 ± 1.1 | 98.6 ± 24.7 | Good BBB permeation, SUV = 1.4 | N.T. | Fast washout | N.T. | LBD (2); AD (1); PSP (1); HC (1) | Negative binding in in vitro ARG using DLB brain sections | High binding affinity to α-syn, no binding to tau in PSP and AD brain tissues | Detection of radiometabolites in mouse brain, high nonspecific binding in human LBD brain tissue |
| (d3)-[11C]MODAG-001 | 0.6 | N.T. | N.T. | Yes | N.T. | N.T. | Passing BBB with rapid brain uptake, SUV = 1.7 | N.T. | Fast washout | Female domestic pigs (4) | N.T. | N.T. | High binding affinity to α-syn fibrils and succeeded in detecting α-syn in fibril-inoculated rat model | - |
| [11C]4i | 3.0 ± 1.4 | N.T. | N.T. | Yes | 8.0 ± 2.9 | 106 ± 56 | Good brain permeability | 1.68 ± 0.54 | Rapid washout | Male rhesus macaque (1) | N.T. | Binding to α-syn in MSA and not PD and controls; binding affinity in PD tissue sections by ARG not as high as in vitro binding assays conducted in PD brain homogenates | Nanomolar binding affinity for α-syn, lower binding affinity for Aβ; high signal-to-background ratio | High affinity to aggregated tau proteins |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Xiang, J.; Ye, K.; Zhang, Z. Development of Positron Emission Tomography Radiotracers for Imaging α-Synuclein Aggregates. Cells 2025, 14, 907. https://doi.org/10.3390/cells14120907
Guo X, Xiang J, Ye K, Zhang Z. Development of Positron Emission Tomography Radiotracers for Imaging α-Synuclein Aggregates. Cells. 2025; 14(12):907. https://doi.org/10.3390/cells14120907
Chicago/Turabian StyleGuo, Xiaodi, Jie Xiang, Keqiang Ye, and Zhentao Zhang. 2025. "Development of Positron Emission Tomography Radiotracers for Imaging α-Synuclein Aggregates" Cells 14, no. 12: 907. https://doi.org/10.3390/cells14120907
APA StyleGuo, X., Xiang, J., Ye, K., & Zhang, Z. (2025). Development of Positron Emission Tomography Radiotracers for Imaging α-Synuclein Aggregates. Cells, 14(12), 907. https://doi.org/10.3390/cells14120907









