1. Introduction
Mycobacterium tuberculosis (Mtb) is one of the leading causes of death worldwide [
1], exacerbated by the emergence of antibiotic-resistant strains, which significantly complicate treatment efforts. Moreover, the global COVID-19 pandemic has contributed to an increase in drug-resistant tuberculosis (DR-TB) strains [
2], thereby making TB eradication more challenging. Traditional antibiotic development has largely focused on directly inhibiting bacterial growth or killing the pathogen by targeting essential processes like cell wall synthesis (e.g., isoniazid), nucleic acid replication (e.g., fluoroquinolones), or metabolic pathways (e.g., rifampicin). While these drugs show effectiveness in initial treatments, Mtb rapidly develops resistance through mechanisms such as gene mutations, drug efflux pump activation, or modifications to drug targets. Additionally, drug-sensitive strains can survive antibiotic pressure in a dormant state (persister cells) or through phenotypic tolerance, which can eventually lead to fully resistant strains [
3,
4]. Unfortunately, the development of new antibiotic classes has not kept pace with the growing need for effective treatments.
A promising alternative to traditional antibiotics is to target functions critical to Mtb’s ability to cause disease, such as virulence factors [
5]. Mtb has evolved complex mechanisms to evade the host’s immune system, making it a highly successful pathogen [
6]. Upon infection, Mtb is primarily detected by macrophages, which engulf the bacteria through phagocytosis. Following this, alveolar macrophages may undergo apoptosis, a form of programmed cell death triggered by the activation of caspase protease systems. This mechanism helps eliminate the pathogen early, presenting apoptotic bodies to other phagocytes to aid in containing Mtb growth and spread [
7]. However, Mtb has evolved multiple strategies to manipulate the host cell machinery to improve its chances of survival. The bacterium resides within the phagosome, from which secreted proteins leak through pores and target various host organelles, including mitochondria. Recent studies have demonstrated that various Mtb proteins, such as Rv1813c and Rv0674, target the host mitochondria [
8,
9]. These proteins modulate mitochondrial signaling, affecting key processes like apoptosis and reactive oxygen species (ROS) production, which allows Mtb to control the host cell and enhance its chances of survival within the host [
10].
In addition to these survival strategies, antibiotic-resistant proteins (ARPs) in Mtb not only contribute to drug resistance but may also regulate virulence and modulate host–pathogen interactions. For example, resistance-related genes such as
katG and
rpoB have been shown to modulate Mtb’s virulence by interfering with the host immune response or inhibiting apoptosis, thereby improving bacterial survival within host cells. This dual function of antibiotic targets suggests that these proteins not only serve as critical sites for drug action, but also act as molecular switches controlling virulence. KatG, the target of isoniazid, mediates drug activation while simultaneously protecting Mtb from oxidative damage by ROS produced by the host [
11]. Therefore, strategies focusing solely on drug resistance mechanisms might overlook the broader roles these targets play in pathogenesis, potentially selecting for more resistant and virulent strains under drug pressure.
Understanding how Mtb uses its resistance-related proteins to modulate host responses unlocks new therapeutic avenues. Targeting both resistance mechanisms and virulence pathways could help mitigate the risks of drug resistance. Combination therapies that inhibit virulence factors (like KatG) to enhance host immune responses while using antibiotics to eradicate residual bacteria [
12] could represent a promising approach. However, much remains unknown about the virulence functions of many resistance-related genes, limiting our ability to fully comprehend Mtb’s adaptive survival strategies and their implications for treatment development.
Fluoroquinolones (FQs) are essential antibiotics for treating drug-resistant tuberculosis (DR-TB), targeting DNA gyrase and topoisomerase IV, which are crucial for bacterial DNA replication [
13,
14]. FQs cause double-strand breaks and replication fork arrest, leading to cell death [
15,
16]. However, mutations in these enzymes, particularly in DNA gyrase, lead to FQ resistance and increase the risk of multidrug-resistant tuberculosis (MDR-TB) [
13]. A key factor in Mtb’s FQ resistance is the mycobacterial fluoroquinolone resistance protein A (MfpA), which inhibits DNA gyrase’s ATP-dependent activity, preventing FQ-induced DNA damage. While MfpA’s role in resistance has been well studied, its impact on host immune responses remains unclear [
17].
This study aimed to address this gap in our understanding by investigating the dual role of MfpA in both the antibiotic resistance of Mtb and its interaction with host cells. Using Mycobacterium bovis Bacillus Calmette-Guérin (M. bovis BCG) as a model organism, we explored the regulatory effects of MfpA on host cell apoptosis and immune signaling pathways. Upon comparing the survival of wild-type (WT) and mfpA-knockout (KO) M. bovis BCG strains within infected host cells, we showed that MfpA functions as a virulent factor, affecting M. bovis BCG survival within the host and causing dysregulated pro-inflammatory cytokine production. MfpA was found to be secreted by the mycobacterium within infected host cells and localized to their mitochondria, where it influences reactive oxygen species (ROS) levels and mitochondrial membrane potential. Additionally, we showed that MfpA is involved in mitochondria-related apoptosis, which plays a crucial role in regulating the host immune response to Mtb infection and significantly affects bacterial survival and persistence. We found that by disrupting mitochondrial membrane potential and modulating ROS production, MfpA affects host cell apoptosis. Further transcriptomic analysis revealed that MfpA promotes Mtb survival by modulating the NF-κB signaling pathway and regulating the expression of Growth arrest and DNA damage-inducible 45 (GADD45B), a critical stress sensor involved in inflammation. These findings highlight the importance of MfpA not only in FQ resistance, but also in regulating host cell responses, providing new insights into Mtb’s ability to persist and evade host immunity by influencing apoptotic pathways.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
In this study, Mycobacterium bovis BCG Pasteur was used to investigate the functional roles of MfpA, a protein that shares 100% genetic identity with Mycobacterium tuberculosis strain H37Rv. The mycobacterial strains were grown in 7H9 medium, which consisted of Middlebrook 7H9 medium (Becton Dickinson, Sparks, MD, USA) supplemented with 10% ADS. The ADS mixture contained 5% (w/v) bovine serum albumin fraction V (Amresco, 0332-100G, Solon, OH, USA ), 2% (w/v) D-dextrose (Sinopharm Chemical Reagent, 6300518, Shanghai, China), and 8.1% (w/v) NaCl (Sinopharm Chemical Reagent, 10019318, Shanghai, China), as well as 0.5% (v/v) glycerol (Innochem, A13031-500ML, Beijing, China) and 0.05% (v/v) TWEEN 80 (Sinopharm Chemical Reagent, 30189828, Shanghai, China). The mfpA-knockout mutant strain was maintained in medium containing 50 mg/L hygromycin B (Roche, Indianapolis, IN, USA). Additionally, recombinant strains such as pMV361-mfpA/BCG::ΔmfpA, pMV261-mfpA::BCG, and pMV261-flag-mfpA::BCG were cultured in medium supplemented with 25 mg/L kanamycin (Amresco, OH, USA).
2.2. Construction of BCG::ΔmfpA, pMV361-mfpA/BCG::ΔmfpA, pMV261-mfpA::BCG, and pMV261-flag-mfpA::BCG
Specialized transduction based on mycobacteriophages was utilized to replace the
mfpA gene and were amplified from
M. bovis BCG genomic DNA using the primer pairs MfpA-LL/MfpA-LR and MfpA-RL/MfpA-RR. These primers are listed in
Table S1. The amplified regions were cloned into the plasmid p0004s [
18], which was previously digested with
Van91I (Thermo Fisher Scientific, Waltham, MA, USA). The resulting plasmid p0004s-
mfpA was then linearized with
PacI (Thermo Fisher Scientific, Waltham, MA, USA) and inserted into
PacI-digested phAE159. The shuttle plasmid was transformed into
E. coli HB101 Electro-Cells (Takara Bio Inc., 9021, Shiga, Japan) through phage packaging using MaxPlax Lambda packaging extract (Epicentre Biotechnologies, MP5120, Madison, WI, USA) [
19]. The plasmids were electroporated into
Mycobacterium smegmatis mc
2155 for phage propagation. The correctness of the transformants were verified by PCR using MfpA-InL/MfpA-InR primers (
Table S1).
To construct complemented strains, the full-length
mfpA gene was amplified from
M. bovis BCG genomic DNA using the primer pair 361-MfpA-F/361-MfpA-R (
Table S1), and the PCR product was cloned into the integrating vector pMV361. The plasmid was electroporated into the ∆
mfpA-knockout strain, resulting in the construction of pMV361-
mfpA/BCG::Δ
mfpA. A similar method was used to generate pMV261-
mfpA::BCG. The pMV261-
flag-mfpA vector was constructed by amplifying the
mfpA gene with primers 261-FLAG-MfpA-F and 261-FLAG-MfpA-R (
Table S1) and inserting it into the multiple cloning site (MCS) of the pMV261 backbone. The Flag-tag sequence ‘DYKDDDDK’ was fused to the N-terminus of MfpA, and the plasmid was transformed into
M. bovis BCG to generate the pMV261-
flag-
mfpA::BCG.
2.3. In Vitro Growth Kinetics of Recombinant M. bovis BCG Strains
The growth rates of BCG::ΔmfpA, pMV361-mfpA/BCG::ΔmfpA, pMV261-mfpA::BCG, and their parental strains were compared in 7H9 medium supplemented with 10% ADS. The optical density at 600 nm (OD600) was measured every 24 h for each strain. The experiment was repeated three times.
2.4. Cell Culture
Raw264.7 cells were cultured in an incubator at 37 °C with 5% CO2 in Dulbecco’s Modified Eagle Medium (DMEM) (Meilunbio, Dalian, China) supplemented with 10% Fetal Bovine Serum (FBS) (BOBIO, Shanghai, China), 1% penicillin–streptomycin (Biosharp, Hefei, China).
2.5. Intracellular Survival of Recombinant M. bovis BCG Strains
Raw264.7 cells were seeded in 24-well plates at 6 × 105 cells/well (Nest Biotechnology, Wuxi, China) and cultured overnight in DMEM (Meilunbio, Dalian, China) containing 10% FBS (BOBIO, F800-050, China) at 37 °C and 5% CO2 to facilitate cell adherence. The cells were then infected with wild-type BCG, BCG::ΔmfpA, pMV361-mfpA/BCG::ΔmfpA, and pMV261-mfpA::BCG at a multiplicity of infection (MOI) of 5. After 4 h, the bacteria were withdrawn and extracellular bacteria were killed by adding 200 μg/mL gentamicin (Sigma Aldrich, St. Louis, MO, USA). Fresh DMEM containing 10% FBS was added, and the incubation continued for an additional 48 h. After removing extracellular bacteria with 1 × PBS (Meilunbio, Dalian, China), the cells were lysed with pre-cooled 1 × PBST: 1 × PBS containing 0.05% (v/v) Tween 80 (Sinopharm Chemical Reagent, Shanghai, China). Cell lysates were serially diluted and plated onto 7H10 (Becton Dickinson, Sparks, MD, USA) agar plates. The plates were incubated at 37 °C for approximately 3 weeks, and colony-forming units (CFUs) were counted for each infected group. The experiment was repeated three times.
2.6. Identification of MfpA Secretory Protein
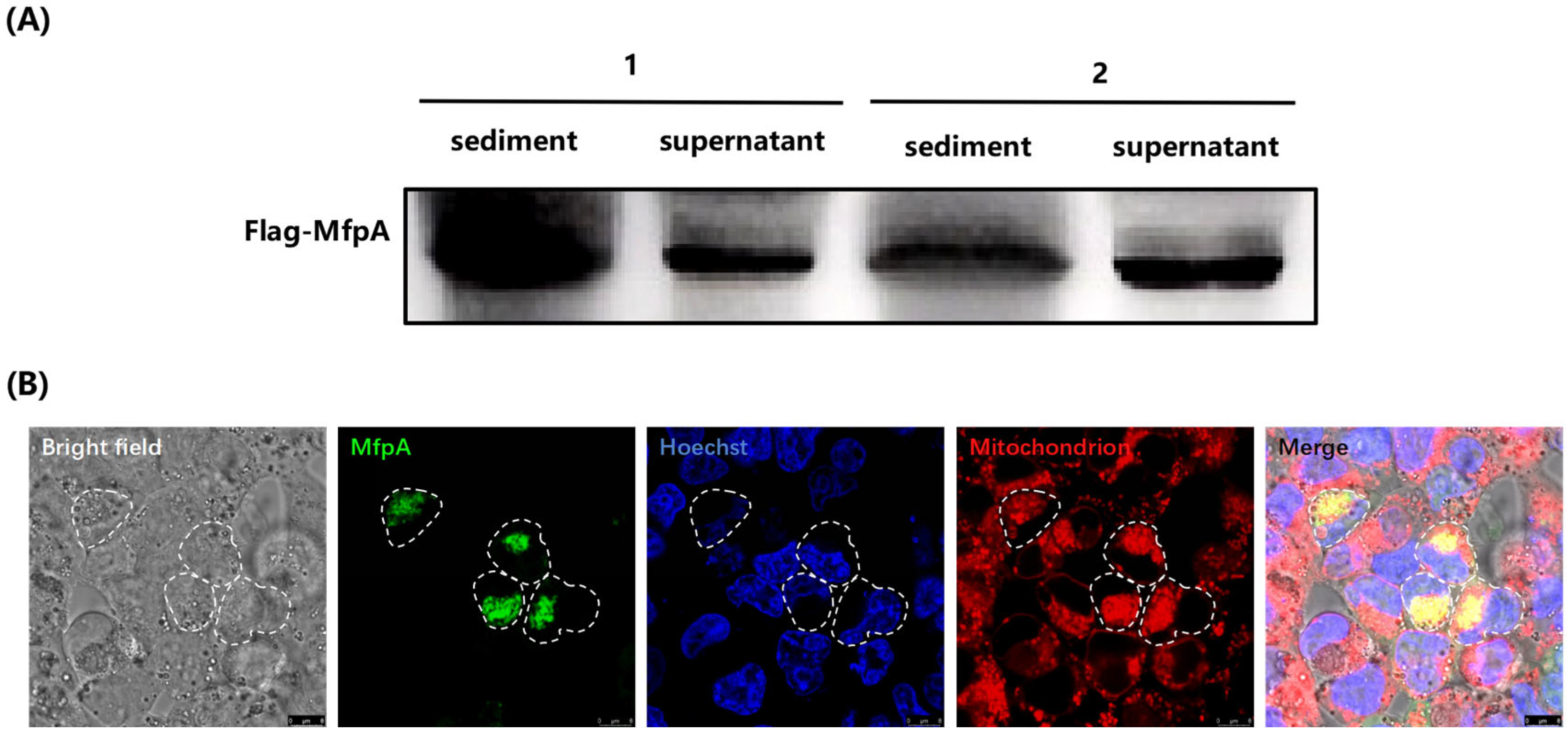
After selecting and verifying positive clones of pMV261-flag-mfpA::BCG, the bacteria were inoculated into fresh 7H9 complete medium containing kanamycin (25 mg/L) at a 1:100 dilution. The cultures were incubated at 37 °C with shaking at 120 rpm until the OD600 reached 1.0. Both bacterial cells and the supernatant were harvested, and proteins were precipitated using acetone precipitation. The expression of the MfpA protein in the bacterial cells and culture supernatant was analyzed by western-blotting using an anti-Flag antibody (1:2500, Abmart, Shanghai, China).
2.7. Analysis of Subcellular Localization of MfpA in Eukaryotic Cells
HEK-293T cells were cultured in DMEM supplemented with 10% FBS at 37 °C with 5% CO2. For transfection, the cells were seeded into glass-bottomed culture dishes (Nest Biotechnology, Wuxi, China) at a density of 5 × 105 cells per well and incubated for 24 h to reach 70–80% confluency. The eukaryotic expression vector pcDNA3.1(+)-egfp-mfpA was diluted in Opti-MEM® Reduced Serum Medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) to a final volume of 125 μL. Lipofectamine 3000 (Invitrogen, Thermo Fisher Scientific, USA) was mixed with Opti-MEM® in a 1:1 (v/v) and incubated at room temperature for 5 min. The plasmid and Lipofectamine 3000 solutions were combined, incubated for 15 min to allow complex formation, and then added dropwise to the cells. After 24 h, the cells were incubated with Mito Tracker Deep Red FM (Beyotime, Shanghai, China) at a concentration of 100 nM in serum-free DMEM for 30 min at 37 °C while protected from light. Cells were washed twice with 1 × PBS, stained with Hoechst 33342 (Lablead, Beijing, China) at 5 μg/mL for 10 min, washed again with 1 × PBS, and immediately imaged using a Leica TCS SP8 STED confocal microscope (Leica Microsystems, Wetzlar, Germany). Fluorescence signals were captured with the following settings: GFP (EGFP-MfpA): Ex/Em = 488–540 nm; Mito Tracker Deep Red FM: Ex/Em = 633–720 nm; Hoechst 33342: Ex/Em = 405–480 nm. Images were processed using Leica LAS X software (v3.7.4).
2.8. Detection of Apoptosis
The apoptosis rate of 48 h-infected Raw264.7 macrophages was assessed using the Annexin V/PI method (Annexin V-FITC Apoptosis Detection Kit, Beyotime, Shanghai, China). The control groups included a blank control group (uninfected cells in complete medium), an Annexin V single-staining group (cells stained with Annexin V-FITC only), and a propidium iodide (PI) single-staining group (cells stained with PI only). The cells were washed with PBS and digested with trypsin (Biosharp, Anhui, China) for 2 min, and the digestion was stopped with complete medium. After centrifuging at 1000× g for 5 min, we discarded the supernatant; then, we collected the cells, gently resuspended them in PBS, and counted them. We took ~1 × 104 resuspended cells, centrifuged them at 1000× g for 5 min, discarded the supernatant, and added 195 μL Annexin V-FITC conjugate to gently resuspend the cells. We then added 5 μL Annexin V-FITC and/or 10 μL PI staining solution according to the set grouping and mixed it gently. The solution was incubated at room temperature (25 °C) for 20 min away from light, then placed on ice and analyzed by CytoFLEX (Beckman Coulter, CA, USA) using CytExpert software (v2.6, Beckman Coulter, USA).
2.9. Transmission Electron Microscopy
Monolayers of cells were grown on Aclar Embedding Film (Electron Microscopy Sciences, Hatfield, PA, USA). The cells were fixed with 2.5% (v/v) glutaraldehyde (Sigma-Aldrich, 111-30-8, St. Louis, MO, USA) in phosphate buffer (PB) (0.1 M, pH 7.4) at 4 °C, followed by two washes in PB and two washes in deionized water. Subsequently, the cells were immersed in a 1% (w/v) OsO4 and 1.5% (w/v) potassium ferricyanide aqueous solution (Sigma-Aldrich, St. Louis, MO, USA) at 4 °C for 1 h. After washing, the cells were dehydrated through a graded alcohol series (30%, 50%, 70%, 80%, 90%, 100%, and 100%, 5 min each at 4 °C), and then washed twice in pure acetone. The samples were infiltrated with a graded mixture of acetone and Embed 812 resin—containing 20 mL of 812 (Electron Microscopy Science, USA), 9 mL of DDSA-Dodecenyl Succinic Anhydride Specially Distilled (Electron Microscopy Science, USA), and 10 mL of NMA-Methy-5-Norbornene-2,3-Dicarboxylic Anhydride (Electron Microscopy Science, USA)—in 3:1, 1:1, and 1:3 ratios, followed by pure resin. The samples were embedded in pure resin containing 1.5% BDMA (N, N-Dimethylbenzylamine, SPI-CHEM, West Chester, PA, USA) and polymerized at 45 °C for 12 h and at 60 °C for 48 h. Ultrathin sections (70 nm) were cut using a Leica EM UC6 microtome (Leica Microsystems, Wetzlar, Germany) and double-stained with uranyl acetate and lead citrate. Lastly, the morphological features were observed using a Hitachi HT7800 TEM/Regulus8100 electron microscope (Hitachi LTD; Tokyo, Japan).
2.10. Determination of Mitochondrial Membrane Potential (ΔΨm) in Host Cells
The mitochondrial membrane potential (ΔΨm) of Raw264.7 macrophages infected for 48 h was assayed using a JC-1 fluorescent probe (Enhanced mitochondrial membrane potential assay kit with JC-1, Beyotime, Shanghai, China). Approximately 1 × 104 cells were collected and resuspended in 0.5 mL of cell culture medium, followed by incubation with 0.5 mL of JC-1 staining working solution for 20 min in a cell culture incubator at 37 °C. After incubation, the cells were centrifuged at 600× g for 4 min at 4 °C and then precipitated, and the supernatant was discarded. The cells were washed twice with JC-1 staining buffer at 600× g for 4 min at 4 °C, and the supernatant was discarded. After resuspension in an appropriate amount of JC-1 staining buffer, the cells were analyzed using a CytoFLEX flow cytometer (Beckman Coulter, USA) and CytExpert software (Beckman Coulter, USA).
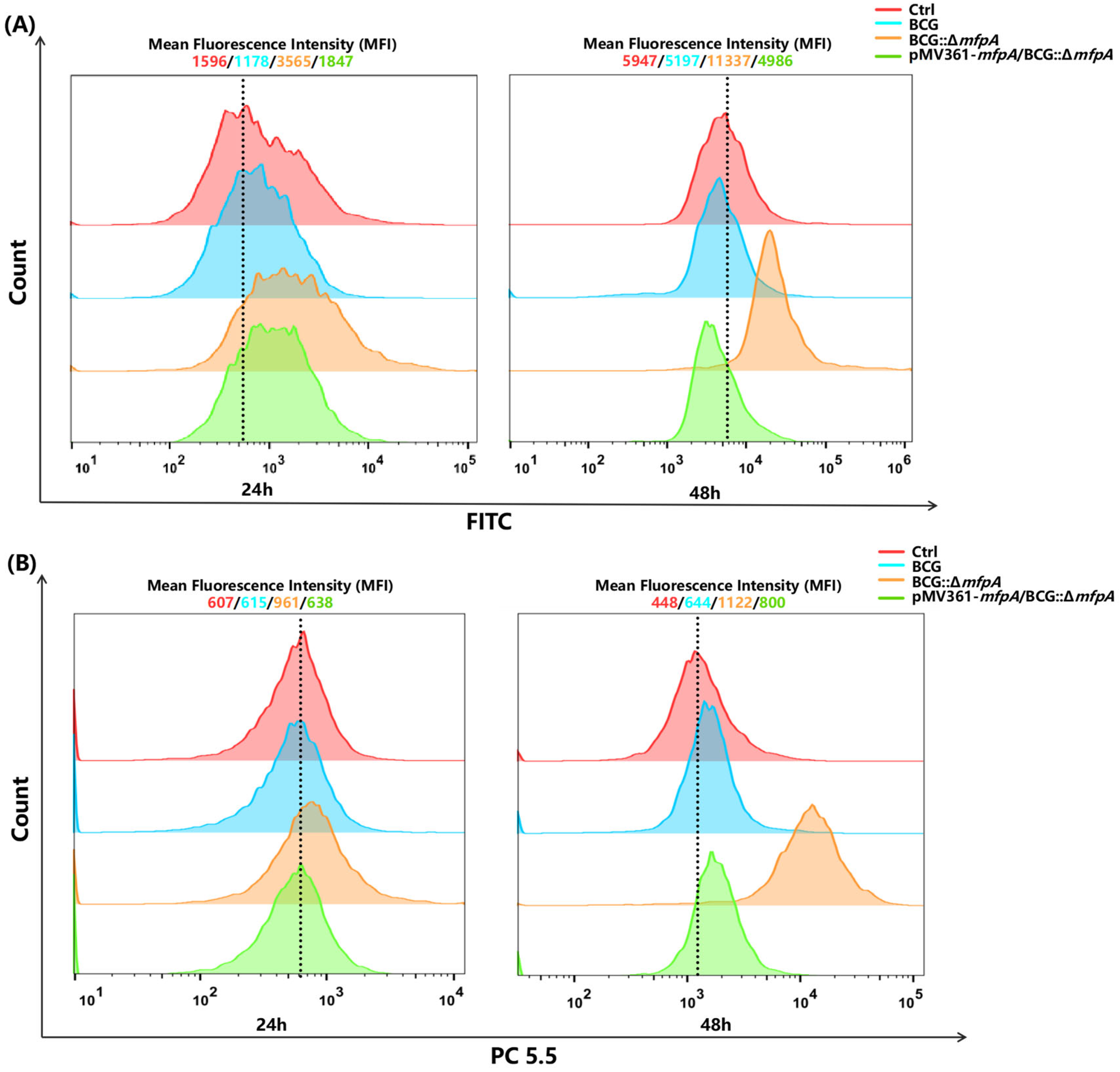
2.11. Detection of Reactive Oxygen Species (ROS) and Mitochondrial ROS in Host Cells
The ROS levels in Raw264.7 macrophages infected for 24 and 48 h were measured using a DCFH-DA fluorescent probe (Reactive Oxygen Detection Kit, Biotronik, Hangzhou, China). Approximately 1 × 104 cells were collected, resuspended in 0.5 mL of ice-cold 1 × PBS, and incubated with 10 μM DCFH-DA for 40 min in the dark at 37 °C.
The mitochondrial ROS levels in Raw264.7 macrophages infected for 24 and 48 h were detected using a MitoSOX Red probe (Thermo Fisher Scientific, M36007, Waltham, MA, USA). Approximately 1 × 104 cells were gently resuspended in a pre-warmed (37 °C) staining solution containing 250 nM MitoSOX Red probe and incubated in a cell culture incubator, protected from light, for 40 min. The ROS and mitochondrial ROS levels were analyzed using a CytoFLEX flow cytometer (Beckman Coulter, Brea, CA, USA) and FlowJo® (version 10.8.1; FlowJo LLC., Ashland, OR, USA).
2.12. RNA Sequencing (RNA-Seq) Analysis and Validation of Recombinant M. bovis BCG Strains Following Infection of Host Cells and RT-qPCR
After infecting Raw264.7 macrophages for 48 h, the total RNA was extracted using TRIzol reagent (Invitrogen, CN, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA quality was assessed by agarose gel electrophoresis (OD260/280 ≥ 1.9), and RNA concentration and purity were measured using a Nanodrop Spectrophotometer (TIENGEN, Beijing, China). mRNA was then purified using oligo dT microbeads, fragmented, and reverse-transcribed to synthesize double-stranded cDNA. The cDNA was subjected to end repair, 3’ end ‘A’ addition, and adapter ligation for the construction of a sequencing library. High-fidelity polymerase amplification was performed to generate the sequencing library. The library was denatured with sodium hydroxide to form single-stranded DNA fragments, which were fixed on a microarray for bridge PCR amplification. DNA clusters were generated, linearized into single strands, and sequenced using the Illumina platform (San Diego, CA, USA). Three biological replicates were used for each sample, resulting in the sequencing of 12 transcriptome samples, generating 83.55 G of clean data. The effective data volume for each sample ranged from 6.88 to 7.06 G, with the Q30 base percentages ranging from 95.75 to 96.27%. The average GC content was 47.66% and the genome alignment rates ranged from 92.40% to 93.39%.
cDNA synthesis was performed using 5 × All-In One RT MasterMix (Applied Biological Materials, G592, Richmond, BC, Canada). Quantitative real-time PCR was performed using BlasTaqTM 2 × qPCR MasterMix (Applied Biological Materials, G891, Richmond, BC, Canada) on a Bio-Rad CFX Connect Real-Time SystemTM (Bio-Rad Laboratories, Hercules, CA, USA).
β-
actin was used as the housekeeping gene, and the relative gene expression was calculated using the 2
−∆∆CT method [
20]. The RT-qPCR primers used are listed in
Table S1.
2.13. Cytokine Production Induced by Recombinant M. bovis BCG Strains in Raw264.7 Cells
The concentrations of TNF-α and IL-6 in the supernatants of Raw264.7 macrophages infected for 48 h were measured using sandwich enzyme-linked immunosorbent assay (ELISA) kits (UpingBio, Shanghai, China; TNF-α: SYP-M0036; IL-6: SYP-M0031). After 48 h of infection, cell culture supernatants were harvested and centrifuged at 2000× g for 10 min at 4 °C to remove cell debris. The supernatants were aliquoted and stored at −80 °C until analysis. Lyophilized TNF-α and IL-6 standards were reconstituted in assay diluent (provided in the kit) to generate a 7-point standard curve. Serial dilutions were prepared in sterile PBS, and pre-coated 96-well plates were equilibrated to room temperature (25 °C) for 30 min.
For the ELISA procedure, the following wells were set up: calibrator, sample dilution, blank, and sample wells. Fifty microliters of calibrator, at various concentrations, was added to the calibrator wells, while the blank well was left empty. Fifty microliters of sample dilution and 50 μL of the supernatant were added to the sample dilution and sample wells, respectively. In all wells except the blank, 100 μL of biotin-labeled antibody was added, and the plates were incubated in the dark at 37 °C for 60 min. The plates were then washed 5 times with wash buffer. HRP-labeled avidin (100 μL) was added to each well (except the blank), and the plates were incubated for 20 min at 37 °C in the dark. After washing the plates 5 times, 100 μL of TMB substrate solution was added to each well, and the optical density (OD) was measured at 450 nm using a SpectraMax® Paradigm Multi-Mode Microplate Reader (Molecular Devices, San Jose, CA, USA). Standard curves were generated using 4-parameter logistic (4PL) regression in ELISA Calc v0.2 (Molecular Devices).
2.14. Western-Blotting
After 48 h of infection, Raw264.7 macrophages were washed with 1 x PBS, then centrifuged at 12,000× g for 5 min at 4 °C, and the supernatant was discarded. Cells were lysed with cell lysis buffer (Lablead, Beijing, China) containing protease inhibitor (1 mM PMSF (Lablead, Beijing, China) and a 1% phosphatase inhibitor cocktail (Lablead, Beijing, China), followed by 30 min of incubation on ice. The lysates were centrifugated at 12,000× g for 5 min at 4 °C, and the supernatant was collected. The protein concentration was measured, and the samples were mixed with protein loading buffer and boiled at 95 °C for 5 min. SDS-PAGE was performed to separate the proteins, which were then transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore immobilon-P, Billerica, MA, USA).
The membrane was blocked with 5% skim milk (Becton Dickinson, Sparks, MD, USA) for 2 h at room temperature, and was then incubated with primary antibodies against Bax (1:1000, Abmart, Shanghai, China), Bcl2 (1:1000, Abmart, T40056 China), β-actin (1:8000, Abmart, P30002, China), β-tublin (1:8000, Abmart, M20005, China), Gadd45B (1:1000, Bioss, bs-5904R, China), p65 (1:1000, Abmart, T55034, China), phospho-p65 (1:1000, Abmart, TP56372, China), IκBα (1:1000, Abmart, T55026, China), and phospho-IκBα (1:1000, Abmart, TA2002, China). After washing with PBST (1 × PBS containing 0.05% (v/v) Tween 80 (Sinopharm Chemical Reagent, Shanghai, China), the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody goat anti-rabbit IgG (1:10,000) or goat anti-mouse IgG (1:10,000) (Cell Signaling Technology, Danvers, MA, USA). The protein bands were visualized using enhanced chemiluminescence (ECL) substrate (Thermo Fisher Scientific), and the images were analyzed using ImageJ (v1.54p, National Institutes of Health, Bethesda, MD, USA).
2.15. Statistical Analysis
All experiments were independently repeated at least three times. The data are expressed as the mean ± standard error of the mean (SEM). For comparisons between two groups, the normality of data distribution was first assessed using the Shapiro–Wilk test (for small sample sizes, n ≤ 50) or the D’Agostino–Pearson test (for large sample sizes, n > 50). If data passed the normality test (p > 0.05), parametric tests (unpaired Student’s t-test for equal variances or Welch’s t-test for unequal variances) were applied. If data did not pass the normality test, the non-parametric Mann–Whitney U test was used. For comparisons involving three or more groups, normality was verified via the Shapiro–Wilk test (p > 0.05), and homogeneity of variances was confirmed by the Brown–Forsythe test (p > 0.05). One-way ANOVA followed by Tukey’s post hoc test was performed for parametric data. Statistical analyses were performed using GraphPad Prism v9.0 (GraphPad Software, San Diego, CA, USA). The significance of the p values was determined as follows: **** p < 0.0001; *** p < 0.001; ** p < 0.01; * p < 0.05; and ns, not significant.
4. Discussion
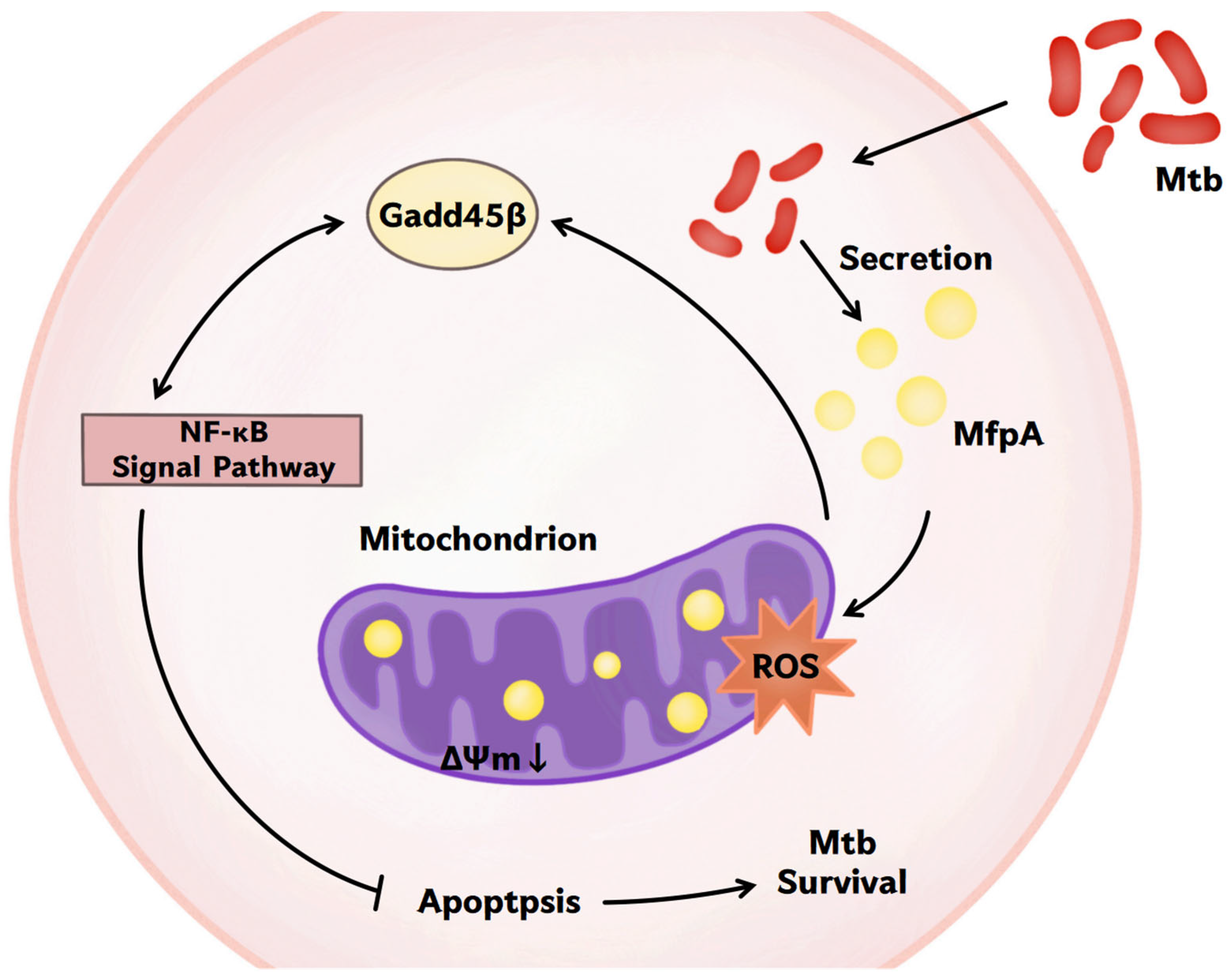
In this study, we revealed a previously unrecognized dual role of the mycobacterial antibiotic resistance protein (ARP) MfpA; we achieved this using Mycobacterium bovis Bacillus Calmette-Guérin (M. bovis BCG), a widely used vaccine strain related to Mycobacterium tuberculosis (Mtb), which is a model organism for studying mycobacterial survival mechanisms. While MfpA is well known for its role as a resistance factor, we show for the first time that it also functions as a virulence factor that enhances mycobacterial survival. It achieves this by modulating host mitochondrial function and suppressing apoptosis via the NF-κB signaling pathway. This novel mechanism contributes to M. bovis BCG’s ability to evade host immune responses and persist within host cells. Our finding provides the first direct evidence of MfpA’s interaction with host mitochondria, affecting key cellular processes such as reactive oxygen species (ROS) generation and mitochondrial membrane potential (ΔΨm), both crucial for apoptosis induction.
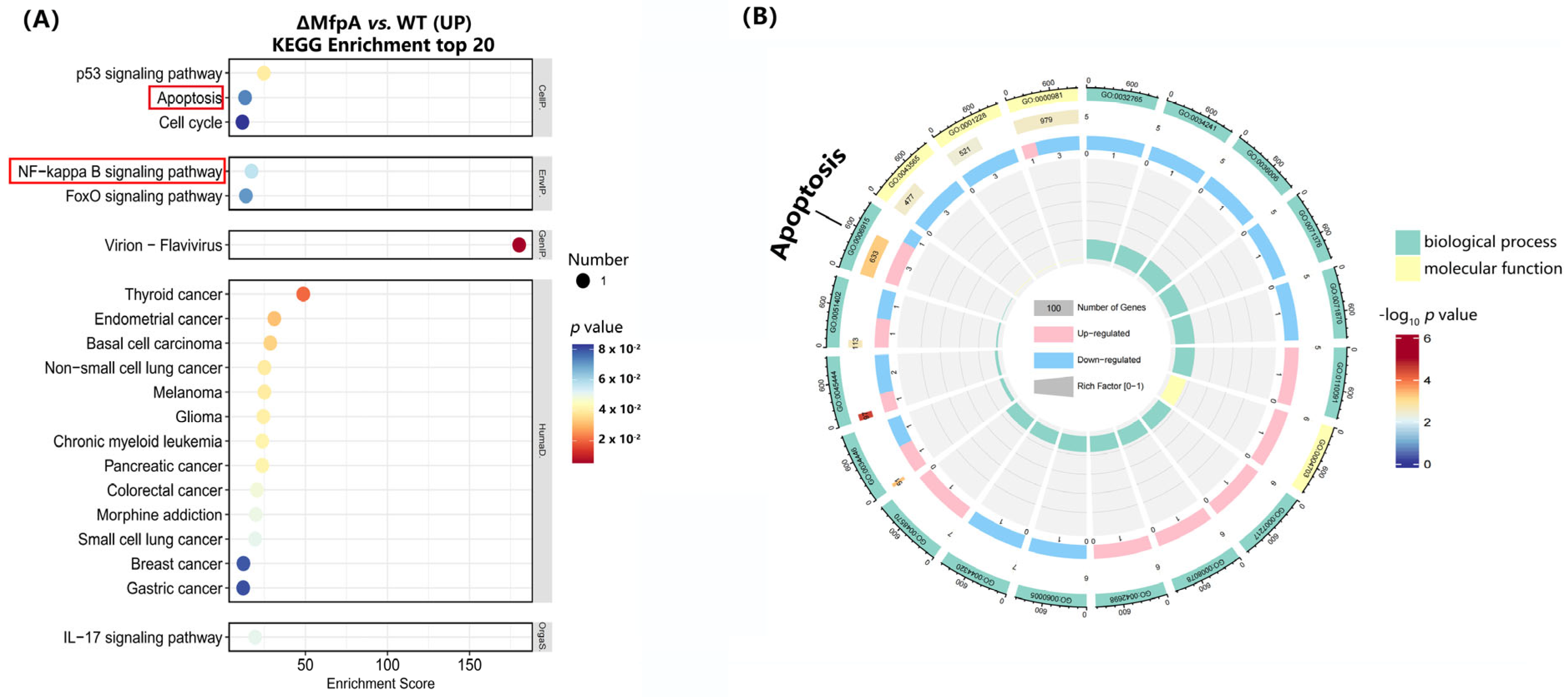
The link between antibiotic resistance and virulence in mycobacteria has been sporadically reported [
11,
32,
33]; however, our work is the first to show that MfpA—an ARP associated with fluoroquinolone resistance through DNA gyrase protection—directly manipulates host mitochondrial functions to suppress apoptosis (
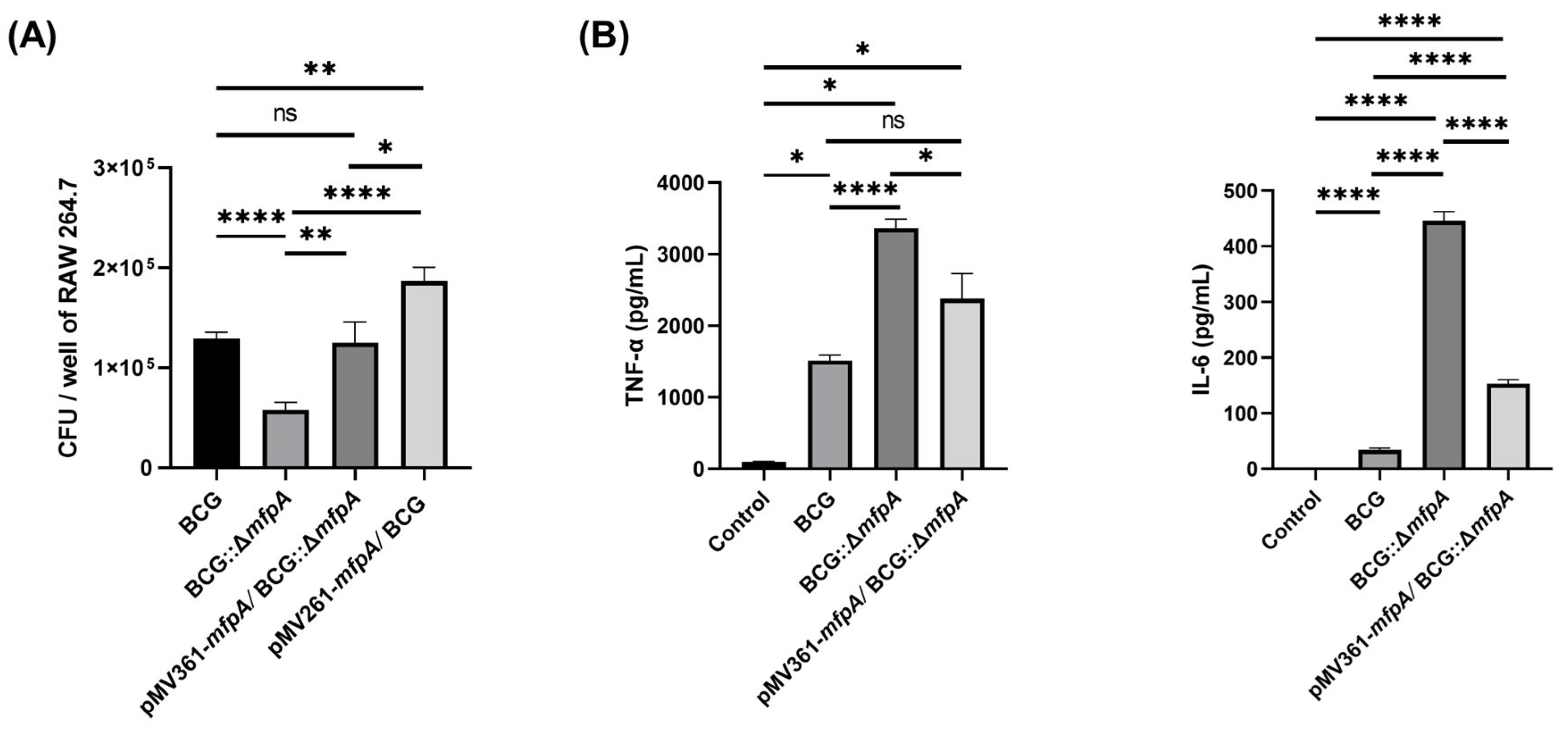
Figure 11). This shifts the perspective on antibiotic resistance genes, positioning them as active participants in immune evasion rather than survival tools against antibiotics. The reduced survival of the Δ
mfpA::BCG strain in host cells (
Figure 2A), coupled with elevated TNF-α/IL-6 levels and increased apoptosis (
Figure 2B and
Figure 3), emphasizes the crucial role of MfpA in both virulence and resistance.
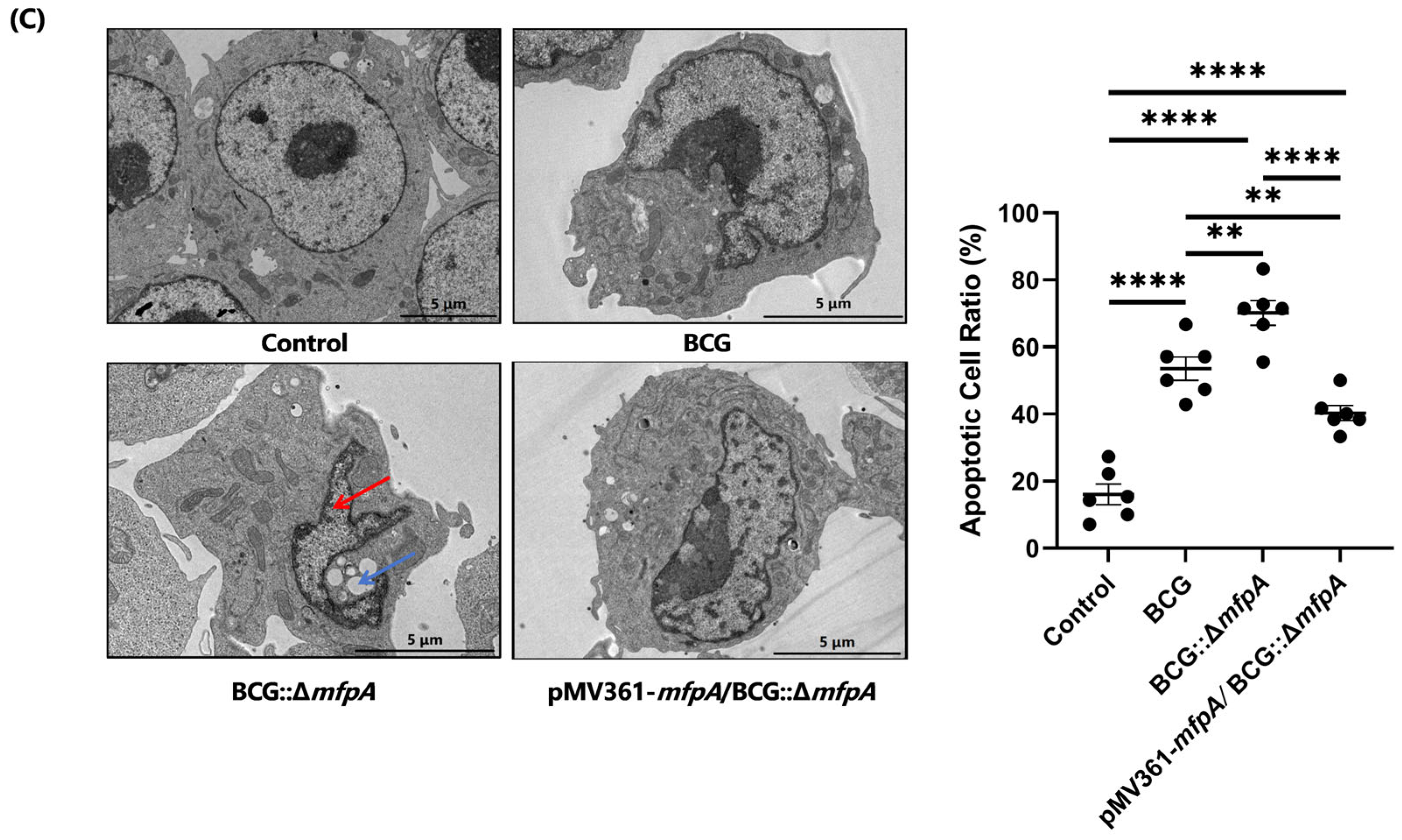
Mitochondria play a crucial role in regulating apoptosis, a process that eliminates infected or damaged cells for immune defense. However, pathogens have evolved mechanisms to evade apoptosis and prolong their survival within host cells. In this study, we showed that MfpA co-localized with host mitochondria (
Figure 4B), affecting ROS production and mitochondrial membrane potential (ΔΨm)—key triggers for apoptosis [
34]—indicating that MfpA targets mitochondria to block apoptosis (
Figure 3C).
The manipulation of mitochondrial ROS is closely linked to pro-inflammatory signaling through the activation of the NF-κB signaling pathway, which drives cytokine production [
35,
36]. This immune evasion strategy is similar to mechanisms employed by other pathogens. For example,
Legionella pneumophila stabilized mitochondrial membranes through its MitF protein to inhibit apoptosis, and
Salmonella used its SseL protein to degrade mitochondrial ubiquitin chains, suppressing ROS-driven NF-κB signaling pathway activation [
35,
37]. Similarly, Mtb proteins like Rv1813c [
38] and Rv0674 [
8] also target host mitochondria to induce apoptosis. These studies highlight mitochondria as a crucial site for both pathogens and hosts, and suggest that host-directed therapy holds promise for TB management. For example, metformin, a hypoglycemic drug that inhibits mitochondrial complex I, has been shown to reduce the risk of TB [
39]. In our study, the drug-resistance-associated gene
mfpA was found to target host mitochondria in
M. bovis BCG, suggesting that mitochondria-targeting drugs hold promise for addressing the emerging issue of drug resistance in infectious diseases. Additional research is necessary to fully understand these interactions and to identify new therapeutic targets for combating Mtb and similar pathogens.
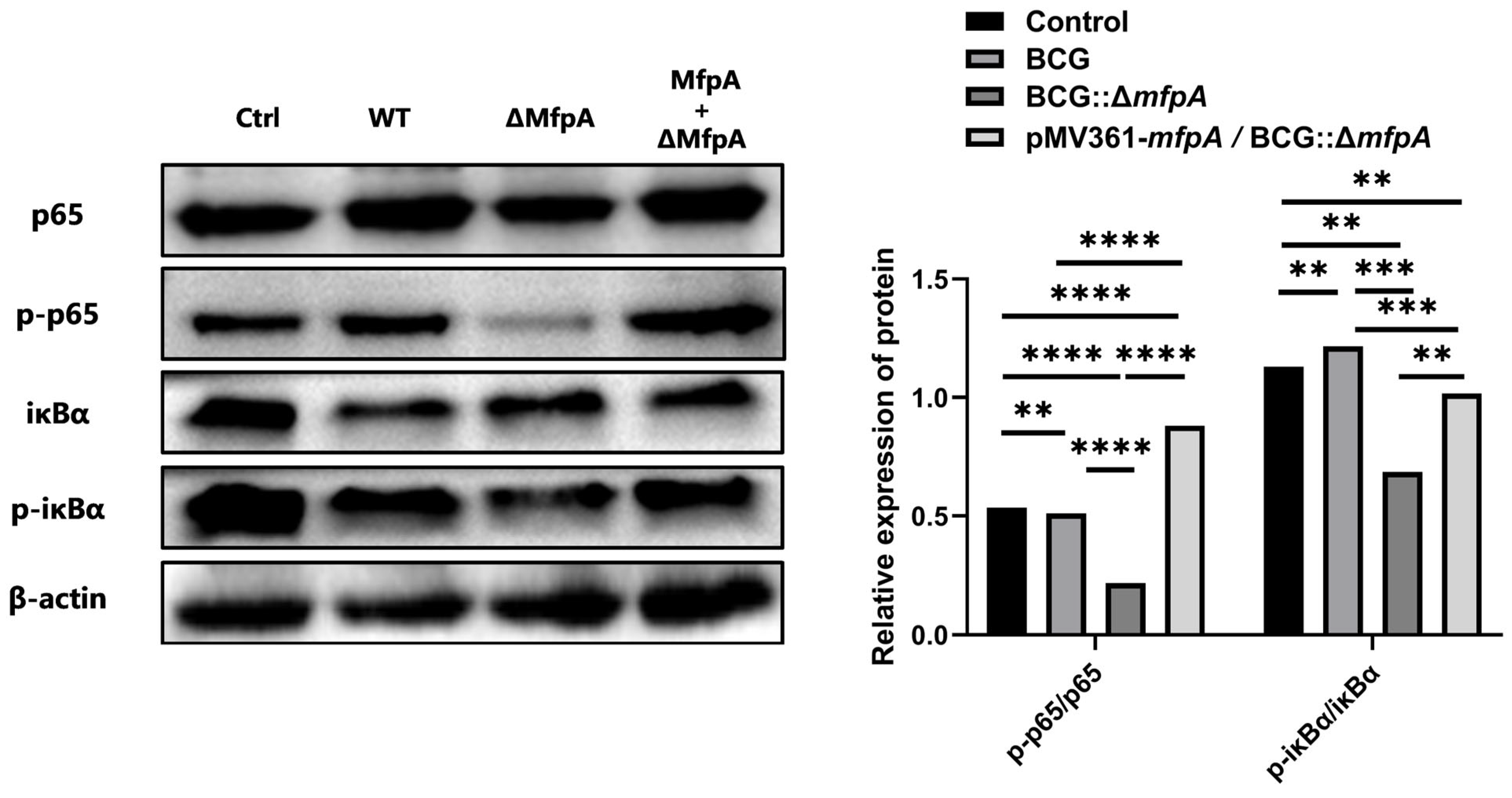
The NF-κB signaling pathway plays a dual role in tuberculosis (TB) by promoting antibacterial defense while also potentially causing harm to the host through excessive inflammation [
40,
41]. MfpA was found to modulate NF-κB activity, paradoxically reducing apoptosis despite NF-κB’s canonical role in promoting anti-apoptotic responses [
42,
43]. This paradox may arise because, in the context of BCG infection, suppression of the NF-κB pathway mitigates excessive TNF-α-driven inflammation, thereby preventing inflammation-induced apoptosis. Additionally, by stabilizing IκB, MfpA may counteract JNK-mediated apoptosis, contributing to the survival of infected cells and promoting bacterial persistence. In addition, we also observed that inhibiting NF-κB signaling affects the expression of GADD45B, a key protein involved in DNA damage repair, cell cycle regulation, and apoptosis [
44,
45]. While
gadd45β expression was upregulated at the transcriptional level (
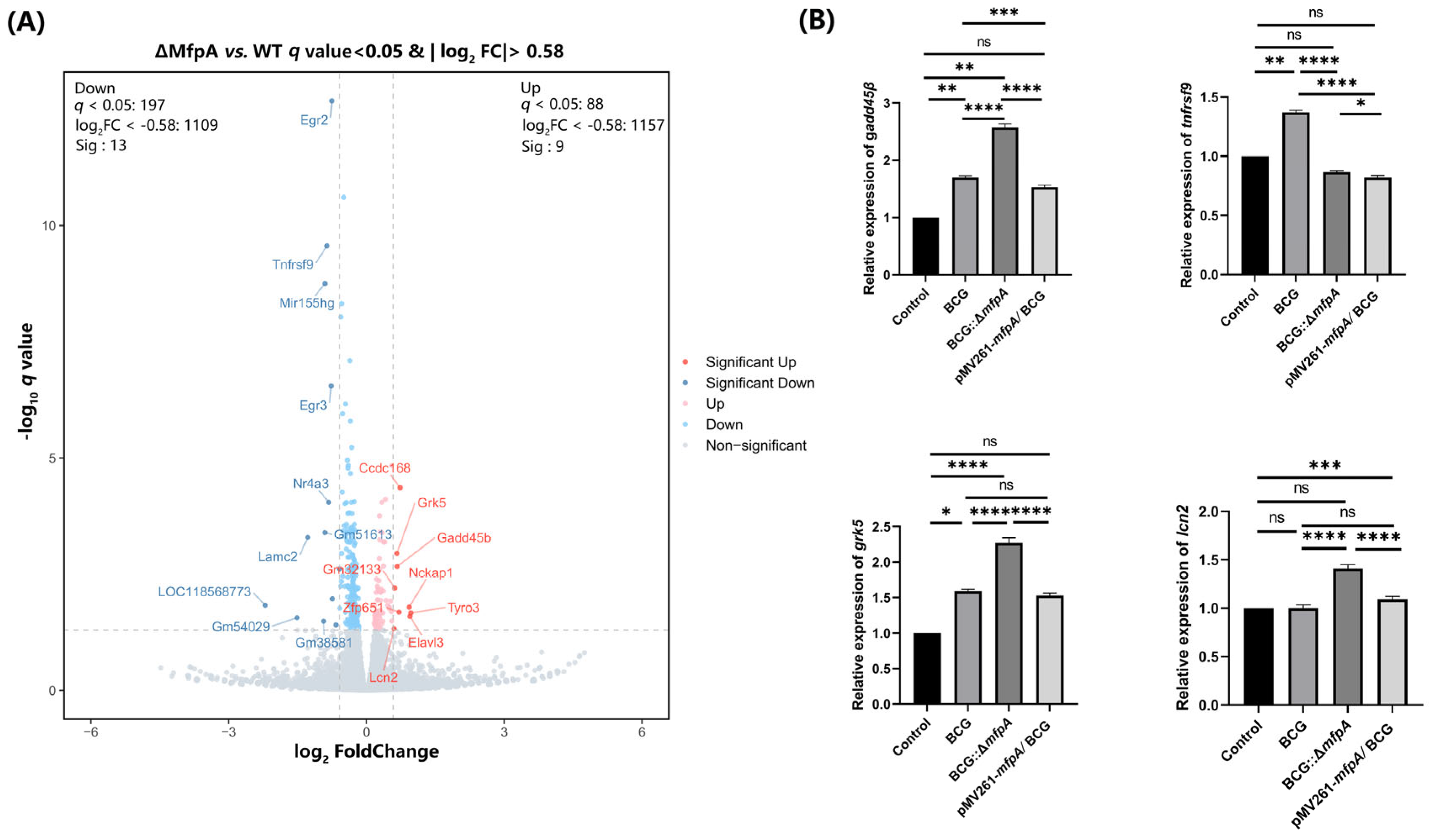
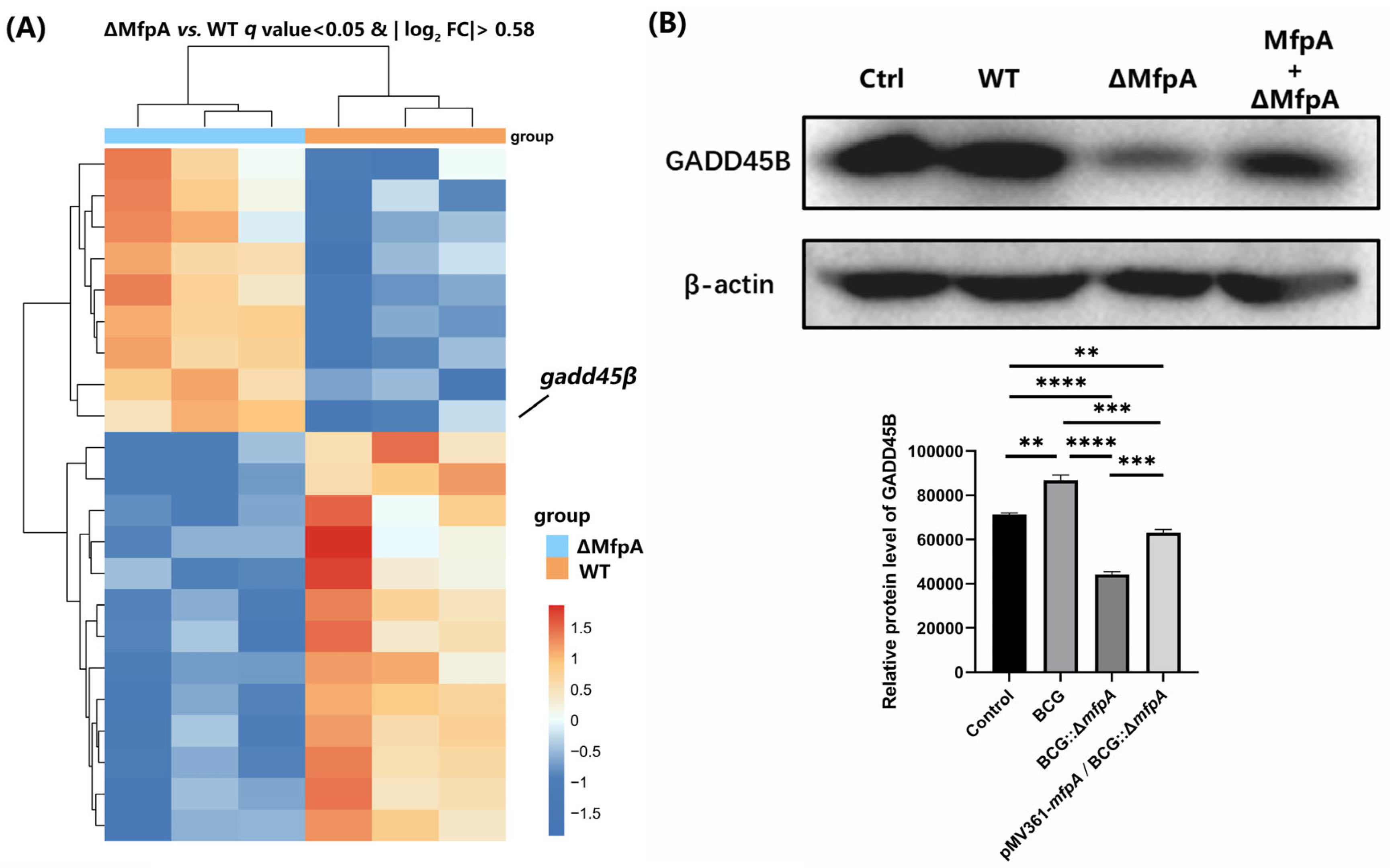
Figure 8A), its protein levels were significantly reduced in the BCG::Δ
mfpA-infected group (
Figure 8B). This suggest that MfpA may modulate GADD45B protein levels through the NF-κB suppression pathway, potentially affecting its translation or stability. These changes could affect the host’s immune response and alter the outcome of infection.
MfpA’s multifunctional role both in antibiotic resistance and as a virulence factor offers promising opportunities for combination therapies targeting both pathogen resistance and virulence mechanisms. Such a dual-target approach could reduce “single-pressure selection”, delay drug resistance, and improve treatment effectiveness. Inhibiting MfpA’s virulence factors may restore immune functions suppressed by MfpA, such as ROS production and apoptosis inhibition, thereby strengthening the immune response and improving the efficacy of existing antibiotics. This could ultimately lead to faster bacterial clearance, reduced relapse, and delayed resistance development, offering a more sustainable treatment for tuberculosis.
Our study also unlocks exciting possibilities for therapeutic interventions aimed at reducing pathogen virulence while simultaneously restoring normal immune function. For example, compounds designed to reverse the effects of MfpA on mitochondrial ROS production or apoptotic inhibition could enhance host immune responses and improve clinical outcomes in Mtb infections. Combination therapies targeting both mitochondrial dynamics and immune modulation could harness the full potential of the host immune system, offering a more holistic and effective approach to treatment.
Furthermore, the involvement of GADD45B in modulating pro-inflammatory cytokines and immune cell activation suggests that it could serve as a valuable therapeutic target. Understanding the precise mechanisms underlying GADD45B’s role and its interactions with other immune pathways will be crucial for optimizing drug development.
In conclusion, this work highlights the importance of considering antibiotic resistance proteins as multifunctional virulence factors. By revealing how MfpA actively modulates host cellular mechanisms, we unlock new avenues for therapeutic intervention. Targeting MfpA’s interactions with host mitochondria or its effects on NF-kB signaling could provide strategies to enhance immune responses while combating antibiotic resistance in Mtb. Further research should aim to explore additional ARPs that share similar functions and refine therapeutic strategies to disrupt these pathways, ultimately improving treatment outcomes for tuberculosis and similar infections.