Galectin-3 Is Associated with Cardiac Fibrosis and an Increased Risk of Sudden Death
Abstract
1. Introduction
2. Methods
2.1. Human Post-Mortem Studies
2.1.1. Study Design and Data Abstraction
2.1.2. Postmortem Procedures
2.1.3. Tissue Processing and Storage
2.1.4. Myocardial Tissue Morphometry and Immunohistochemistry
2.2. Porcine Model
2.2.1. Experimental Porcine Model of SCD
2.2.2. Use of Porcine Model for Gradual Coronary Occlusion
2.2.3. Myocardial Periostin Staining
2.2.4. Myocardial Collagen Content
2.2.5. Circulating and Myocardial Galectin-3 Measurements
2.2.6. Statistical Analyses
3. Results
3.1. Clinical Characteristics
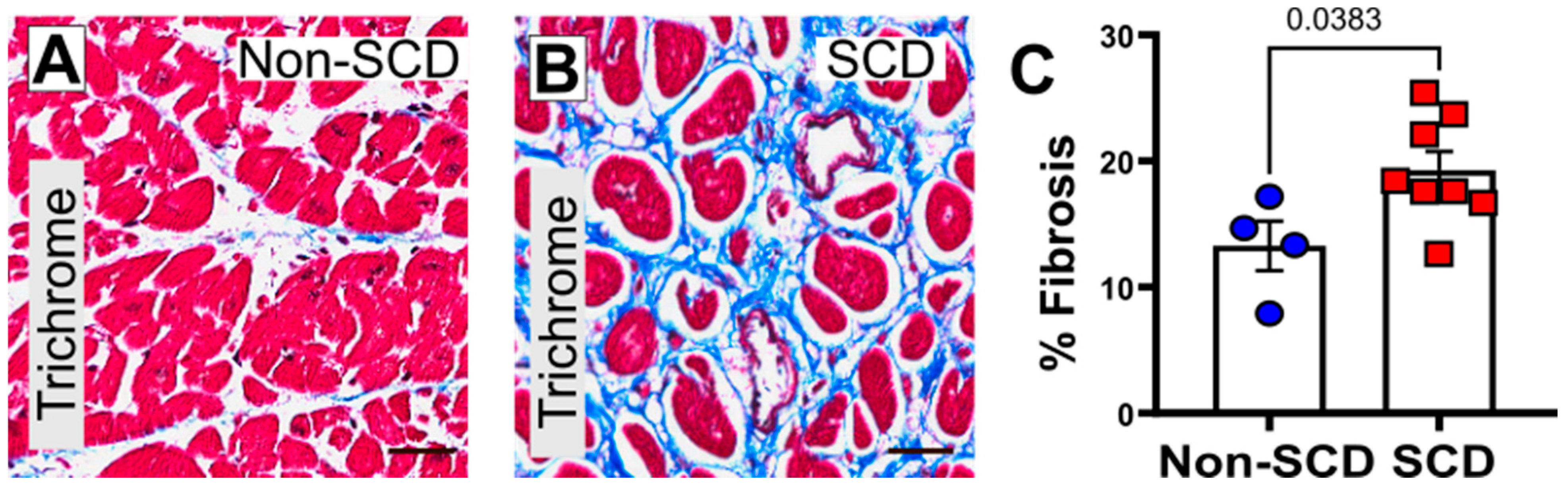
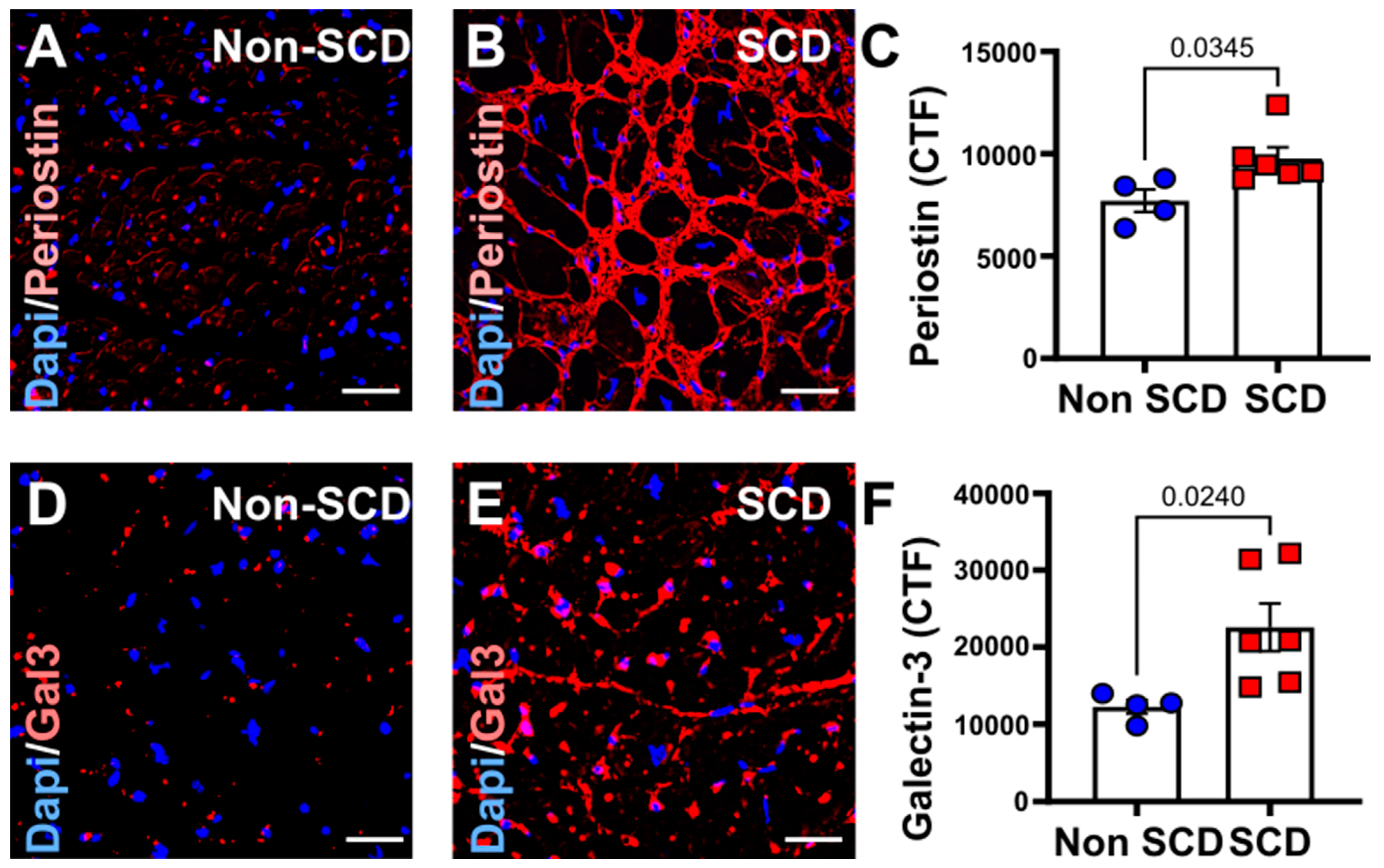
3.2. Increased Myocardial Fibrosis and gal3 Expression in SCD Subjects
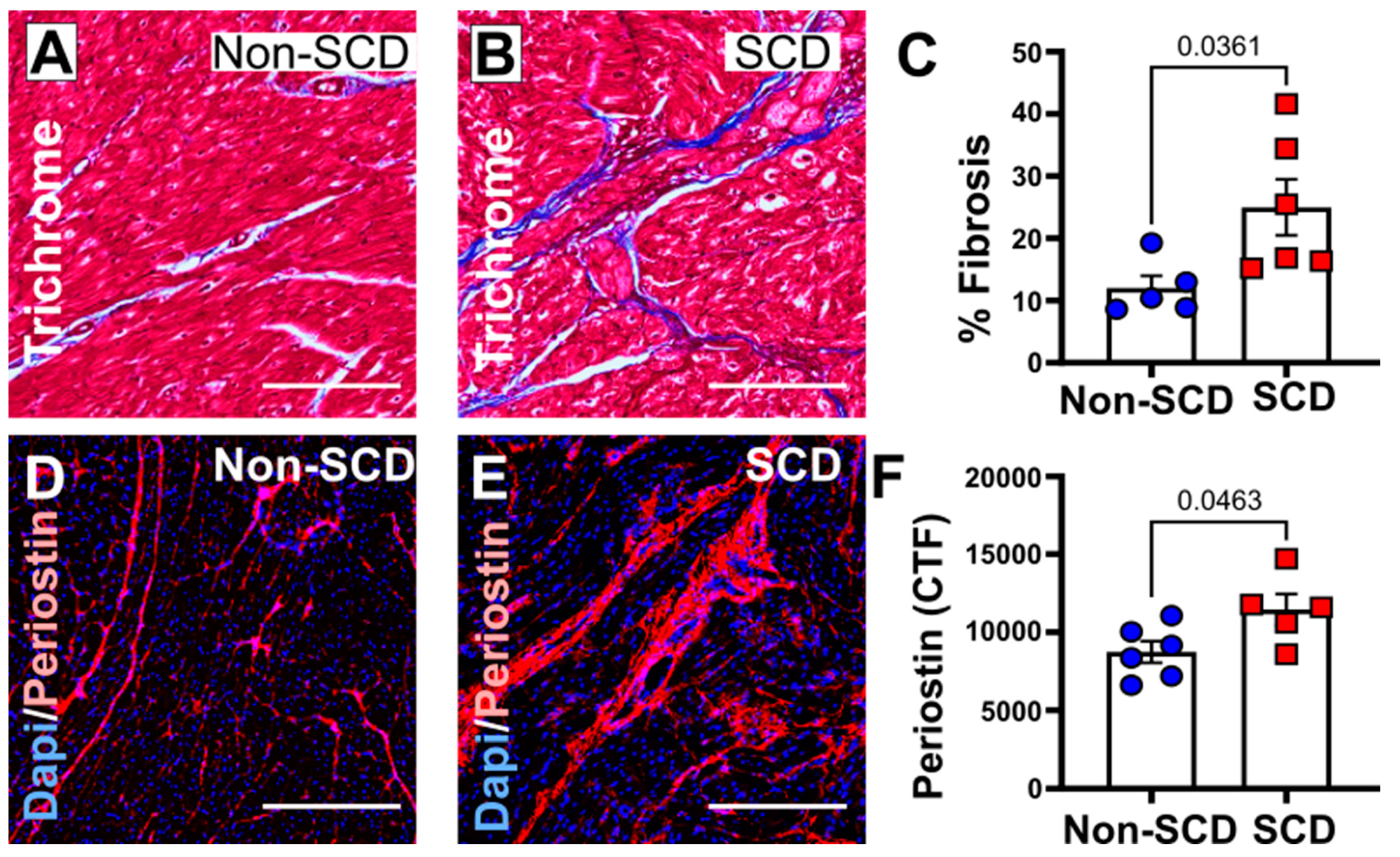
3.3. Increased Myocardial Fibrosis in a Porcine Model with SCD
3.4. Mild Reduction in Cardiac Function in a Porcine Model with SCD
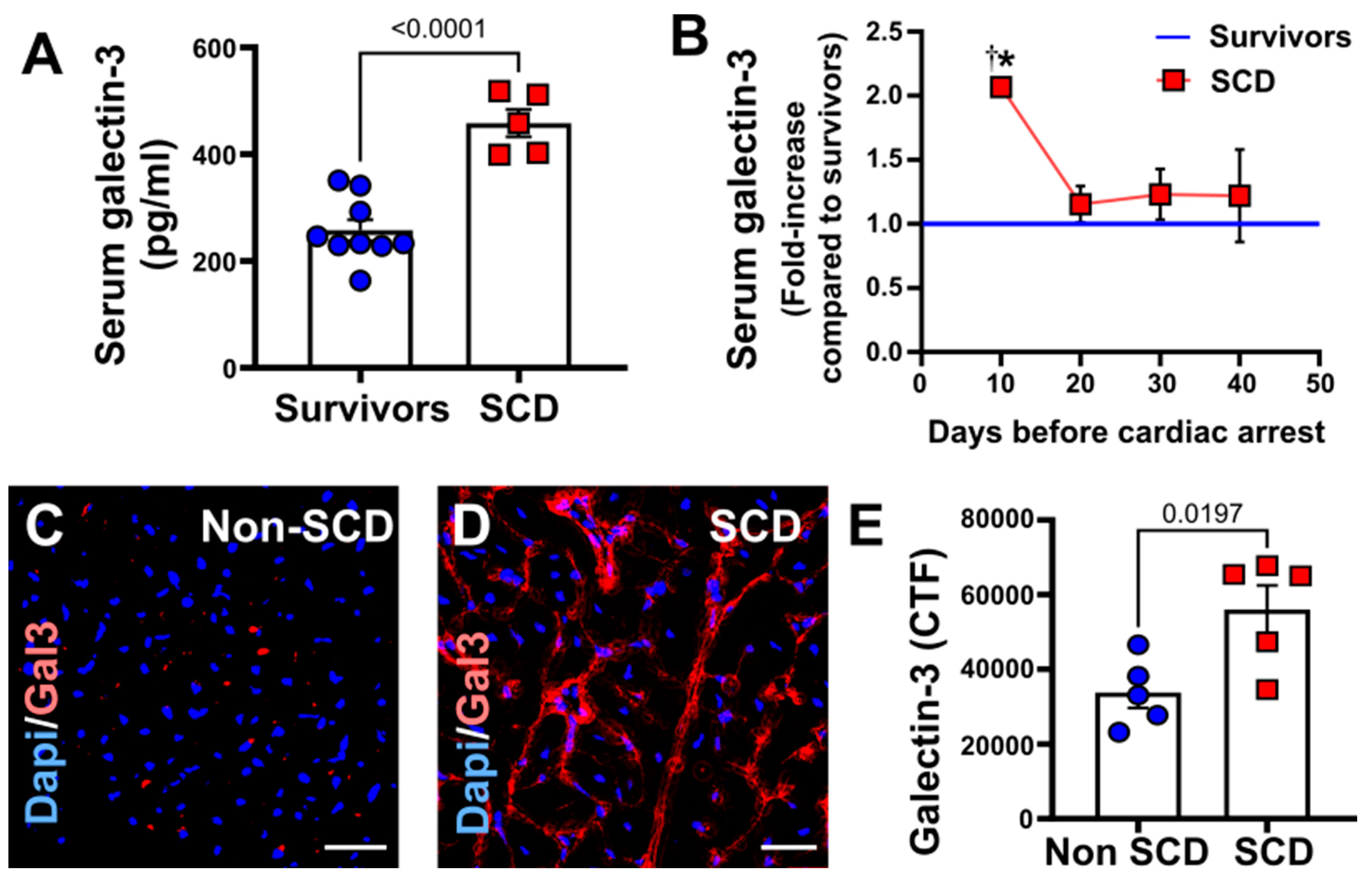
3.5. Increased Circulating and Myocardial Galectin-3 in Porcine Model with SCD
4. Discussion
5. Study Limitations
6. Conclusions and Future Implication
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Myerburg, R.J.; Junttila, M.J. Sudden cardiac death caused by coronary heart disease. Circulation 2012, 125, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Paratz, E.D.; Rowsell, L.; Zentner, D.; Parsons, S.; Morgan, N.; Thompson, T.; James, P.; Pflaumer, A.; Semsarian, C.; Smith, K.; et al. Cardiac arrest and sudden cardiac death registries: A systematic review of global coverage. Open Heart 2020, 7, e001195. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, K.N.; Camp-Rogers, T.R.; Kotini-Shah, P.; Del Rios, M.; Gossip, M.R.; Moitra, V.K.; Haywood, K.L.; Dougherty, C.M.; Lubitz, S.A.; Rabinstein, A.A.; et al. Sudden Cardiac Arrest Survivorship: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e654–e685. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C. Sudden Cardiac Death Substrate Imaged by Magnetic Resonance Imaging: From Investigational Tool to Clinical Applications. Circ. Cardiovasc. Imaging 2017, 10, e005461. [Google Scholar] [CrossRef]
- Vahatalo, J.H.; Huikuri, H.V.; Holmstrom, L.T.A.; Kentta, T.V.; Haukilahti, M.A.E.; Pakanen, L.; Kaikkonen, K.S.; Tikkanen, J.; Perkiomaki, J.S.; Myerburg, R.J.; et al. Association of Silent Myocardial Infarction and Sudden Cardiac Death. JAMA Cardiol. 2019, 4, 796–802. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Papadakis, M.; Robertus, J.L.; Dhutia, H.; Steriotis, A.K.; Tome, M.; Mellor, G.; Merghani, A.; Malhotra, A.; Behr, E.; et al. Etiology of Sudden Death in Sports: Insights From a United Kingdom Regional Registry. J. Am. Coll. Cardiol. 2016, 67, 2108–2115. [Google Scholar] [CrossRef]
- Hookana, E.; Junttila, M.J.; Puurunen, V.P.; Tikkanen, J.T.; Kaikkonen, K.S.; Kortelainen, M.L.; Myerburg, R.J.; Huikuri, H.V. Causes of nonischemic sudden cardiac death in the current era. Heart Rhythm. 2011, 8, 1570–1575. [Google Scholar] [CrossRef]
- Sharma, U.C.; Pokharel, S.; van Brakel, T.J.; van Berlo, J.H.; Cleutjens, J.P.; Schroen, B.; Andre, S.; Crijns, H.J.; Gabius, H.J.; Maessen, J.; et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef]
- Wan, L.; Liu, F.-T. Galectin-3 and inflammation. Glycobiol. Insights 2016, 6, 1–9. [Google Scholar]
- Hoja-Łukowicz, D.; Kedracka-Krok, S.; Duda, W.; Lityńska, A. The lectin-binding pattern of nucleolin and its interaction with endogenous galectin-3. Cell. Mol. Biol. Lett. 2014, 19, 461–482. [Google Scholar] [CrossRef]
- Mishra, B.B.; Li, Q.; Steichen, A.L.; Binstock, B.J.; Metzger, D.W.; Teale, J.M.; Sharma, J. Galectin-3 functions as an alarmin: Pathogenic role for sepsis development in murine respiratory tularemia. PLoS ONE 2013, 8, e59616. [Google Scholar] [CrossRef]
- Hsu, D.K.; Chernyavsky, A.I.; Chen, H.Y.; Yu, L.; Grando, S.A.; Liu, F.T. Endogenous galectin-3 is localized in membrane lipid rafts and regulates migration of dendritic cells. J. Investig. Dermatol. 2009, 129, 573–583. [Google Scholar] [CrossRef]
- Almkvist, J.; Karlsson, A. Galectins as inflammatory mediators. Glycoconj. J. 2002, 19, 575–581. [Google Scholar] [CrossRef]
- Hashmi, S.; Al-Salam, S. Galectin-3 is expressed in the myocardium very early post-myocardial infarction. Cardiovasc. Pathol. 2015, 24, 213–223. [Google Scholar] [CrossRef]
- Christenson, R.H.; Duh, S.H.; Wu, A.H.; Smith, A.; Abel, G.; deFilippi, C.R.; Wang, S.; Adourian, A.; Adiletto, C.; Gardiner, P. Multi-center determination of galectin-3 assay performance characteristics: Anatomy of a novel assay for use in heart failure. Clin. Biochem. 2010, 43, 683–690. [Google Scholar] [CrossRef]
- de Boer, R.A.; Voors, A.A.; Muntendam, P.; van Gilst, W.H.; van Veldhuisen, D.J. Galectin-3: A novel mediator of heart failure development and progression. Eur. J. Heart Fail. 2009, 11, 811–817. [Google Scholar] [CrossRef]
- van Kimmenade, R.R.; Januzzi, J.L., Jr.; Ellinor, P.T.; Sharma, U.C.; Bakker, J.A.; Low, A.F.; Martinez, A.; Crijns, H.J.; MacRae, C.A.; Menheere, P.P.; et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J. Am. Coll. Cardiol. 2006, 48, 1217–1224. [Google Scholar] [CrossRef]
- Mosleh, W.; Kattel, S.; Bhatt, H.; Al-Jebaje, Z.; Khan, S.; Shah, T.; Dahal, S.; Khalil, C.; Frodey, K.; Elibol, J.; et al. Galectin-3 as a Risk Predictor of Mortality in Survivors of Out-of-Hospital Cardiac Arrest. Circ. Arrhythm. Electrophysiol. 2019, 12, e007519. [Google Scholar] [CrossRef]
- Adrie, C.; Cariou, A.; Mourvillier, B.; Laurent, I.; Dabbane, H.; Hantala, F.; Rhaoui, A.; Thuong, M.; Monchi, M. Predicting survival with good neurological recovery at hospital admission after successful resuscitation of out-of-hospital cardiac arrest: The OHCA score. Eur. Heart J. 2006, 27, 2840–2845. [Google Scholar] [CrossRef]
- Maupain, C.; Bougouin, W.; Lamhaut, L.; Deye, N.; Diehl, J.L.; Geri, G.; Perier, M.C.; Beganton, F.; Marijon, E.; Jouven, X.; et al. The CAHP (Cardiac Arrest Hospital Prognosis) score: A tool for risk stratification after out-of-hospital cardiac arrest. Eur. Heart J. 2016, 37, 3222–3228. [Google Scholar] [CrossRef] [PubMed]
- Fallavollita, J.A.; Heavey, B.M.; Luisi, A.J., Jr.; Michalek, S.M.; Baldwa, S.; Mashtare, T.L., Jr.; Hutson, A.D.; Dekemp, R.A.; Haka, M.S.; Sajjad, M.; et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Fallavollita, J.A.; John, M.; Canty, J. Ischemic cardiomyopathy in pigs with two-vessel occlusion and viable, chronically dysfunctional myocardium. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1370–H1379. [Google Scholar] [CrossRef]
- Fallavollita, J.A.; Canty, J.M., Jr. Differential 18F-2-deoxyglucose uptake in viable dysfunctional myocardium with normal resting perfusion: Evidence for chronic stunning in pigs. Circulation 1999, 99, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Fallavollita, J.A.; Perry, B.J.; Canty, J.M., Jr. 18F-2-deoxyglucose deposition and regional flow in pigs with chronically dysfunctional myocardium. Evidence for transmural variations in chronic hibernating myocardium. Circulation 1997, 95, 1900–1909. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Galié, N.; Murali, S.; Olschewski, H.; Rubenfire, M.; Robbins, I.M.; Farber, H.W.; McLaughlin, V.; Shapiro, S.; Pepke-Zaba, J.; et al. Outcome after cardiopulmonary resuscitation in patients with pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2002, 165, 341–344. [Google Scholar] [CrossRef]
- Sovari, A.A.; Karagueuzian, H.S. Myocardial fibrosis as a risk stratifier for sudden arrhythmic death. Expert Rev. Cardiovasc. Ther. 2011, 9, 951–953. [Google Scholar] [CrossRef]
- Frunza, O.; Russo, I.; Saxena, A.; Shinde, A.V.; Humeres, C.; Hanif, W.; Rai, V.; Su, Y.; Frangogiannis, N.G. Myocardial Galectin-3 Expression Is Associated with Remodeling of the Pressure-Overloaded Heart and May Delay the Hypertrophic Response without Affecting Survival, Dysfunction, and Cardiac Fibrosis. Am. J. Pathol. 2016, 186, 1114–1127. [Google Scholar] [CrossRef]
- Ho, J.E.; Liu, C.; Lyass, A.; Courchesne, P.; Pencina, M.J.; Vasan, R.S.; Larson, M.G.; Levy, D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J. Am. Coll. Cardiol. 2012, 60, 1249–1256. [Google Scholar] [CrossRef]
- Basso, C.; Aguilera, B.; Aguilera, B.; Banner, J.; Cohle, S.; d’Amati, G.; de Gouveia, R.H.; di Gioia, C.; Fabre, A.; Gallagher, P.J.; et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017, 471, 691–705. [Google Scholar] [CrossRef]
- Sonkawade, S.D.; Pokharel, S.; Karthikeyan, B.; Kim, M.; Xu, S.; Kc, K.; Sexton, S.; Catalfamo, K.; Spernyak, J.A.; Sharma, U.C. Small Endogeneous Peptide Mitigates Myocardial Remodeling in a Mouse Model of Cardioselective Galectin-3 Overexpression. Circ. Heart Fail. 2021, 14, e008510. [Google Scholar] [CrossRef]
- Sharma, U.C.; Mosleh, W.; Chaudhari, M.R.; Katkar, R.; Weil, B.; Evelo, C.; Cimato, T.R.; Pokharel, S.; Blankesteijn, W.M.; Suzuki, G. Myocardial and Serum Galectin-3 Expression Dynamics Marks Post-Myocardial Infarction Cardiac Remodelling. Heart Lung Circ. 2017, 26, 736–745. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Scholz, D.G.; Hagen, P.T.; Ilstrup, D.M.; Edwards, W.D. Age-related changes in normal human hearts during the first 10 decades of life. Part II (Maturity): A quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin. Proc. 1988, 63, 137–146. [Google Scholar] [CrossRef]
- Leyva, F.; Zegard, A.; Okafor, O.; Foley, P.; Umar, F.; Taylor, R.J.; Marshall, H.; Stegemann, B.; Moody, W.; Steeds, R.P.; et al. Myocardial Fibrosis Predicts Ventricular Arrhythmias and Sudden Death After Cardiac Electronic Device Implantation. J. Am. Coll. Cardiol. 2022, 79, 665–678. [Google Scholar] [CrossRef]
- MacKinnon, A.C.; Liu, X.; Hadoke, P.W.F.; Miller, M.R.; Newby, D.E.; Sethi, T. Inhibition of galectin-3 reduces atherosclerosis in apolipoprotein E-deficient mice. Glycobiology 2013, 23, 654–663. [Google Scholar] [CrossRef]
- Wiersma, V.R.; de Bruyn, M.; Helfrich, W.; Bremer, E. Therapeutic potential of Galectin-9 in human disease. Med. Res. Rev. 2013, 33, E102–E126. [Google Scholar] [CrossRef]
- Corianò, M.; Tona, F. Strategies for Sudden Cardiac Death Prevention. Biomedicines 2022, 10, 639. [Google Scholar] [CrossRef]
- Alba, A.C.; Gaztañaga, J.; Foroutan, F.; Thavendiranathan, P.; Merlo, M.; Alonso-Rodriguez, D.; Vallejo-García, V.; Vidal-Perez, R.; Corros-Vicente, C.; Barreiro-Pérez, M.; et al. Prognostic Value of Late Gadolinium Enhancement for the Prediction of Cardiovascular Outcomes in Dilated Cardiomyopathy: An International, Multi-Institutional Study of the MINICOR Group. Circ. Cardiovasc. Imaging 2020, 13, e010105. [Google Scholar] [CrossRef]
- Assomull, R.G.; Prasad, S.K.; Lyne, J.; Smith, G.; Burman, E.D.; Khan, M.; Sheppard, M.N.; Poole-Wilson, P.A.; Pennell, D.J. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 1977–1985. [Google Scholar] [CrossRef]
- Keil, L.; Chevalier, C.; Kirchhof, P.; Blankenberg, S.; Lund, G.; Müllerleile, K.; Magnussen, C. CMR-Based Risk Stratification of Sudden Cardiac Death and Use of Implantable Cardioverter-Defibrillator in Non-Ischemic Cardiomyopathy. Int. J. Mol. Sci. 2021, 22, 7115. [Google Scholar] [CrossRef]
| Categorical Variables | Non-Cardiac Cause of Death (n = 54) | Cardiac Cause of Death (n = 167) | Total | p-Value | |
|---|---|---|---|---|---|
| (n = 221) | |||||
| Sex | Female | 27 (33%) | 55 (67%) | 82 | 0.024 * |
| Male | 27 (19%) | 112 (81%) | 139 | ||
| Family History of SCA | 1 (5%) | 21 (95%) | 22 | 0.068 | |
| Chest pain before Arrest | 6 (12%) | 44 (88%) | 50 | 0.001 * | |
| NYHA Class | 0 | 45 (28%) | 113 (72%) | 158 | 0.169 |
| 1 | 2 (14%) | 12 (86%) | 14 | ||
| 2 | 1 (11%) | 8 (89%) | 9 | ||
| 3 | 1 (10%) | 9 (90%) | 10 | ||
| 4 | 0 (0%) | 11 (100%) | 11 | ||
| Revascularization | 5 (10%) | 43 (90%) | 48 | 0.025 * | |
| LBBB | 1 (20%) | 4 (80%) | 5 | 0.480 | |
| RBBB | 7 (37%) | 12 (63%) | 19 | 0.268 | |
| LV Fibrosis | 20 (16%) | 108 (84%) | 128 | 0.001 * | |
| RV Fibrosis | 3 (19%) | 13 (81%) | 16 | 0.583 | |
| Septal Fibrosis | 3 (5%) | 55 (95%) | 58 | 0.000 * | |
| Coronary Atherosclerosis | 37 (20%) | 150 (80%) | 187 | 0.000 * | |
| Minimal/Mild CAD | 15 (45%) | 18 (55%) | 33 | 0.000 * | |
| Acute MI | 15 (14%) | 93 (86%) | 108 | 0.000 * | |
| Continuous Variables | Non-Cardiac Cause of Death | Cardiac Cause of Death (n = 167) | Total | p-Value |
|---|---|---|---|---|
| (n = 54) | (n = 221) | |||
| Age | 62.4 ± 17.8 | 62.3 ± 14.9 | 62.4 ± 15.6 | 0.972 |
| Weight | 100.2 ± 41.5 | 87.5 ± 24 | 90.6 ± 29.6 | 0.006 * |
| Height | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 0.386 |
| BMI | 36.2 ± 15 | 31 ± 8 | 32.3 ± 10.4 | 0.001 * |
| BSA | 2.1 ± 0.4 | 2 ± 0.3 | 2 ± 0.4 | 0.034 |
| Ejection Fraction | 57.3 ± 9.8 | 40.5 ± 18 | 42.9 ± 18.1 | 0.004 * |
| Systolic Blood Pressure | 103.5 ± 40.3 | 95.5 ± 44.1 | 97.4 ± 43.2 | 0.392 |
| Diastolic Blood Pressure | 62.2 ± 33.6 | 55.3 ± 28.7 | 56.9 ± 29.9 | 0.292 |
| Heart Rate | 101.2 ± 25.5 | 84.6 ± 31.2 | 88.4 ± 30.7 | 0.013 * |
| Creatinine | 2.4 ± 2.1 | 2.4 ± 2.2 | 2.4 ± 2.2 | 0.982 |
| Troponin | 0.9 ± 2 | 34.6 ± 153.1 | 26.3 ± 133.6 | 0.224 |
| Myoglobin | 1242.4 ± 2527 | 1342.3 ± 4151.2 | 1327.8 ± 3942.7 | 0.942 |
| CK-MB | 283 ± 753.2 | 100.5 ± 188.3 | 135.7 ± 367.5 | 0.140 |
| LV Thickness | 1.6 ± 0.4 | 1.8 ± 0.4 | 1.7 ± 0.4 | 0.005 * |
| RV Thickness | 0.6 ± 0.7 | 0.6 ± 0.3 | 0.6 ± 0.4 | 0.668 |
| Septal Thickness | 1.5 ± 0.4 | 1.7 ± 0.5 | 1.7 ± 0.4 | 0.291 |
| Mitral Valve | 10.2 ± 1.2 | 10.3 ± 1.4 | 10.2 ± 1.4 | 0.681 |
| Aortic Valve | 7.1 ± 1.2 | 7.5 ± 1 | 7.4 ± 1 | 0.060 |
| Tricuspid Valve | 12.1 ± 1.5 | 11.9 ± 2.2 | 12 ± 2.1 | 0.642 |
| Pulmonic Valve | 8.2 ± 1.1 | 8.1 ± 1.1 | 8.1 ± 1.1 | 0.869 |
| Heart Weight | 493.2 ± 103.8 | 549.2 ± 150.3 | 535.9 ± 142.4 | 0.013 |
| Categorical Variables | Non-Cardiac Cause of Death (n = 54) | Cardiac Cause of Death (n = 167) | Total (n = 221) | p-Value | Odds Ratio (Via Baptista-–Pike) | 95% CI |
|---|---|---|---|---|---|---|
| Septal Fibrosis | 3 (5%) | 55 (95%) | 58 | 0.000 * | 8.35 | 2.706, 26.370 |
| Coronary Atherosclerosis | 37 (20%) | 150 (80%) | 187 | 0.000 * | 4.05 | 1.869, 8.815 |
| Minimal/Mild CAD | 15 (45%) | 18 (55%) | 33 | 0.000 * | 0.21 | 0.096, 0.470 |
| Acute MI | 15 (14%) | 93 (86%) | 108 | 0.000 * | 3.27 | 1.707, 6.242 |
| LV Fibrosis | 20 (16%) | 108 (84%) | 128 | 0.001 * | 3.02 | 1.626, 5.606 |
| Aspirin | 9 (11%) | 75 (89%) | 84 | 0.000 * | 4.56 | 2.114, 10.200 |
| Chest pain before Arrest | 6 (12%) | 44 (88%) | 50 | 0.001 * | 3.98 | 1.566, 9.743 |
| Statin | 11 (13%) | 74 (87%) | 85 | 0.002 * | 3.50 | 1.632, 7.551 |
| HTN | 29 (19%) | 124 (81%) | 153 | 0.013 * | 2.69 | 1.394, 5.095 |
| Revascularization | 5 (10%) | 43 (90%) | 48 | 0.025 * | 3.45 | 1.326, 8.439 |
| DM | 12 (14%) | 71 (86%) | 83 | 0.028 * | 2.59 | 1.257, 5.200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherpa, M.D.; Sonkawade, S.D.; Jonnala, V.; Pokharel, S.; Khazaeli, M.; Yatsynovich, Y.; Kalot, M.A.; Weil, B.R.; Canty, J.M., Jr.; Sharma, U.C. Galectin-3 Is Associated with Cardiac Fibrosis and an Increased Risk of Sudden Death. Cells 2023, 12, 1218. https://doi.org/10.3390/cells12091218
Sherpa MD, Sonkawade SD, Jonnala V, Pokharel S, Khazaeli M, Yatsynovich Y, Kalot MA, Weil BR, Canty JM Jr., Sharma UC. Galectin-3 Is Associated with Cardiac Fibrosis and an Increased Risk of Sudden Death. Cells. 2023; 12(9):1218. https://doi.org/10.3390/cells12091218
Chicago/Turabian StyleSherpa, Mingma D., Swati D. Sonkawade, Vinesh Jonnala, Saraswati Pokharel, Mahyar Khazaeli, Yan Yatsynovich, Mohamad A. Kalot, Brian R. Weil, John M. Canty, Jr., and Umesh C. Sharma. 2023. "Galectin-3 Is Associated with Cardiac Fibrosis and an Increased Risk of Sudden Death" Cells 12, no. 9: 1218. https://doi.org/10.3390/cells12091218
APA StyleSherpa, M. D., Sonkawade, S. D., Jonnala, V., Pokharel, S., Khazaeli, M., Yatsynovich, Y., Kalot, M. A., Weil, B. R., Canty, J. M., Jr., & Sharma, U. C. (2023). Galectin-3 Is Associated with Cardiac Fibrosis and an Increased Risk of Sudden Death. Cells, 12(9), 1218. https://doi.org/10.3390/cells12091218







