The Multiple Mitotic Roles of the ASPM Orthologous Proteins: Insight into the Etiology of ASPM-Dependent Microcephaly
Abstract
1. Introduction
2. The Structure of the Orthologous Asp/Aspm/ASPM Proteins
3. Subcellular Localization of the Asp/ASPM Proteins
4. The Mitotic Functions of the asp/ASPM Genes
4.1. The Role of Asp/ASPM Orthologs in Mitotic Progression
4.2. The Role of Asp/ASPM Proteins in Chromosome Segregation
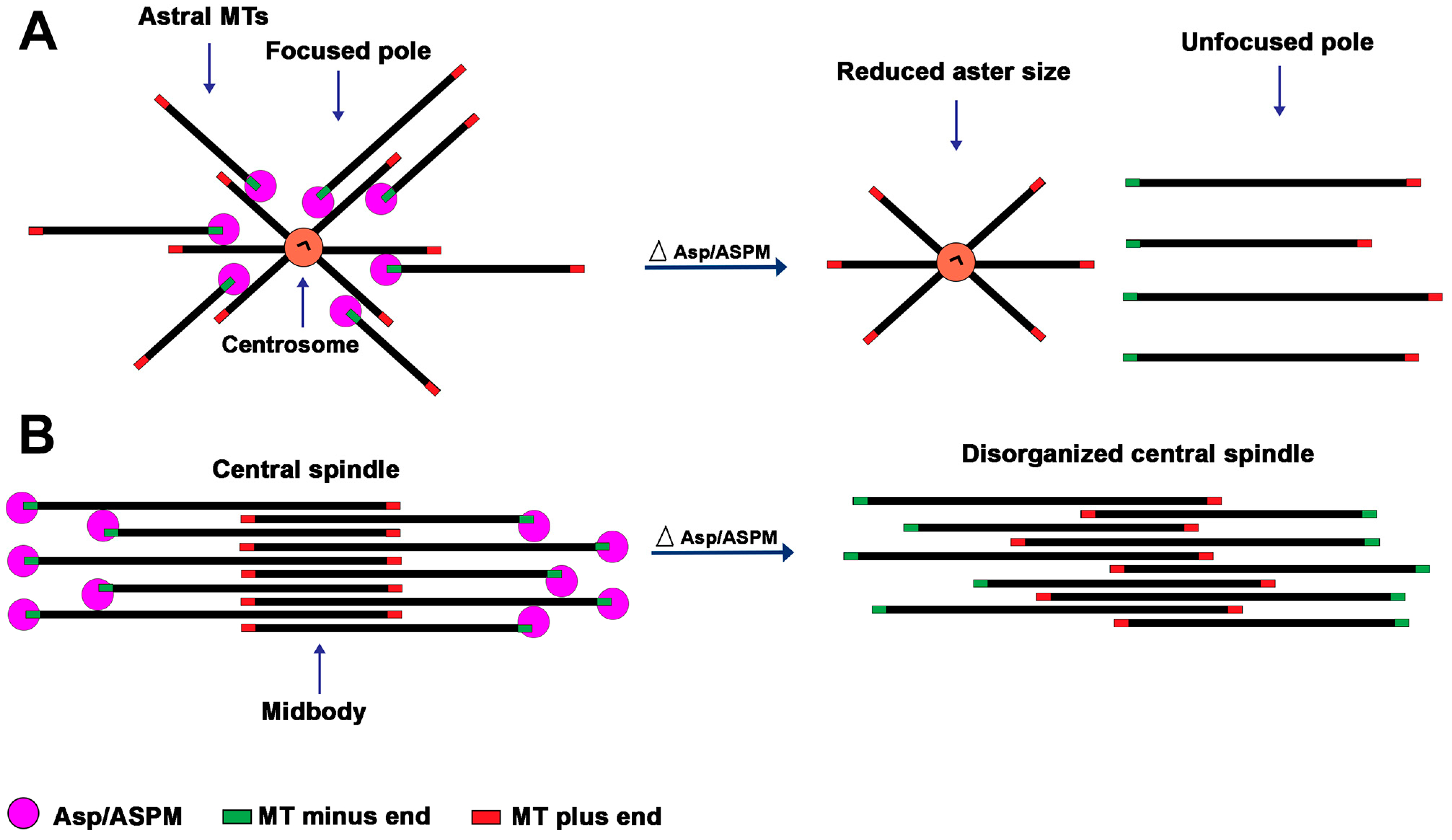
4.3. The Roles of Asp/ASPM Orthologs at the Spindle Poles and Their Interaction with the Centrosome
4.4. The Roles of Asp/ASPM Orthologues in the Orientation of Mitotic Divisions
4.5. The Roles of Asp/ASPM Orthologues in Cytokinesis
4.6. The Role of Asp in Kinetochore-Driven MT Growth
5. Asp/ASPM Interacting Proteins
6. The Functional Meaning of Asp/ASPM Localization along the Spindle MTs
7. Asp/ASPM Functions in Interphase Nuclei
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Ripoll, P.; Pimpinelli, S.; Valdivia, M.M.; Avila, J. A cell division mutant of Drosophila with a functionally abnormal spindle. Cell 1985, 41, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.D.C.; do Carmo Avides, M.; Howard, T.; Gonzalez, C.; Glover, D.M. The Drosophila gene abnormal spindle encodes a novel microtubule-associated protein that associates with the polar regions of the mitotic spindle. J. Cell Biol. 1997, 137, 881–890. [Google Scholar] [CrossRef]
- Bond, J.; Roberts, E.; Mochida, G.H.; Hampshire, D.J.; Scott, S.; Askham, J.M.; Springell, K.; Mahadevan, M.; Crow, Y.J.; Markham, A.F.; et al. ASPM is a major determinant of cerebral cortical size. Nat. Genet. 2002, 32, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.; Scott, S.; Hampshire, D.J.; Springell, K.; Corry, P.; Abramowicz, M.J.; Mochida, G.H.; Hennekam, R.C.M.; Maher, E.R.; Fryns, J.-P.; et al. Protein-truncating mutations in ASPM cause variable reduction in brain size. Am. J. Hum. Genet. 2003, 73, 1170–1177. [Google Scholar] [CrossRef]
- Létard, P.; Drunat, S.; Vial, Y.; Duerinckx, S.; Ernault, A.; Amram, D.; Arpin, S.; Bertoli, M.; Busa, T.; Ceulemans, B.; et al. Autosomal recessive primary microcephaly due to ASPM mutations: An update. Hum. Mutat. 2018, 39, 319–332. [Google Scholar] [CrossRef]
- Jayaraman, D.; Bae, B.-I.; Walsh, C.A. The genetics of primary microcephaly. Annu. Rev. Genom. Hum. Genet. 2018, 19, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Jean, F.; Stuart, A.; Tarailo-Graovac, M. Dissecting the genetic and etiological causes of primary microcephaly. Front. Neurol. 2020, 11, 570830. [Google Scholar] [CrossRef] [PubMed]
- Siskos, N.; Stylianopoulou, E.; Skavdis, G.; Grigoriou, M.E. Molecular genetics of microcephaly primary hereditary: An overview. Brain Sci. 2021, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Zhang, B.; Carlson, M.; Lu, K.V.; Zhu, S.; Felciano, R.M.; Laurance, M.F.; Zhao, W.; Qi, S.; Chen, Z.; et al. Analysis of oncogenic signaling networks in glioblastoma identifies ASPM as a molecular target. Proc. Natl. Acad. Sci. USA 2006, 103, 17402–17407. [Google Scholar] [CrossRef]
- Bikeye, S.-N.N.; Colin, C.; Marie, Y.; Vampouille, R.; Ravassard, P.; Rousseau, A.; Boisselier, B.; Idbaih, A.; Calvo, C.F.; Leuraud, P.; et al. ASPM-associated stem cell proliferation is involved in malignant progression of gliomas and constitutes an attractive therapeutic target. Cancer Cell Int. 2010, 10, 1. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Zhu, L.; Li, Z.; Zuo, P.; Wang, P.; Feng, J.; Mi, Y.; Zhang, C.; Xu, Y.; et al. ASPM promotes hepatocellular carcinoma progression by activating Wnt/β-Catenin signaling through antagonizing autophagy-mediated Dvl2 degradation. FEBS Open Bio 2021, 11, 2784–2799. [Google Scholar] [CrossRef]
- Lang, P.Y.; Gershon, T.R. A new way to treat brain tumors: Targeting proteins coded by microcephaly genes?: Brain tumors and microcephaly arise from opposing derangements regulating progenitor growth. Drivers of microcephaly could be attractive brain tumor t. BioEssays 2018, 40, 1700243. [Google Scholar] [CrossRef]
- Iegiani, G.; Di Cunto, F.; Pallavicini, G. Inhibiting microcephaly genes as alternative to microtubule targeting agents to treat brain tumors. Cell Death Dis. 2021, 12, 956. [Google Scholar] [CrossRef] [PubMed]
- Kouprina, N.; Pavlicek, A.; Mochida, G.H.; Solomon, G.; Gersch, W.; Yoon, Y.-H.; Collura, R.; Ruvolo, M.; Barrett, J.C.; Woods, C.G.; et al. Accelerated evolution of the ASPM gene controlling brain size begins prior to human brain expansion. PLoS Biol. 2004, 2, E126. [Google Scholar] [CrossRef] [PubMed]
- Kouprina, N.; Pavlicek, A.; Collins, N.K.; Nakano, M.; Noskov, V.N.; Ohzeki, J.-I.; Mochida, G.H.; Risinger, J.I.; Goldsmith, P.; Gunsior, M.; et al. The microcephaly ASPM gene is expressed in proliferating tissues and encodes for a mitotic spindle protein. Hum. Mol. Genet. 2005, 14, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Rujano, M.A.; Sanchez-Pulido, L.; Pennetier, C.; le Dez, G.; Basto, R. The microcephaly protein Asp regulates neuroepithelium morphogenesis by controlling the spatial distribution of myosin II. Nat. Cell Biol. 2013, 15, 1294–1306. [Google Scholar] [CrossRef]
- van der Voet, M.; Berends, C.W.H.; Perreault, A.; Nguyen-Ngoc, T.; Gönczy, P.; Vidal, M.; Boxem, M.; van den Heuvel, S. NuMA-Related LIN-5, ASPM-1, calmodulin and dynein promote meiotic spindle rotation independently of cortical LIN-5/GPR/Galpha. Nat. Cell Biol. 2009, 11, 269–277. [Google Scholar] [CrossRef]
- Schoborg, T.; Zajac, A.L.; Fagerstrom, C.J.; Guillen, R.X.; Rusan, N.M. An Asp-CaM complex is required for centrosome-pole cohesion and centrosome inheritance in neural stem cells. J. Cell Biol. 2015, 211, 987–998. [Google Scholar] [CrossRef]
- Jiang, K.; Rezabkova, L.; Hua, S.; Liu, Q.; Capitani, G.; Altelaar, A.F.M.; Heck, A.J.R.; Kammerer, R.A.; Steinmetz, M.O.; Akhmanova, A. Microtubule minus-end regulation at spindle poles by an ASPM-katanin complex. Nat. Cell Biol. 2017, 19, 480–492. [Google Scholar] [CrossRef]
- Xu, X.-L.; Ma, W.; Zhu, Y.-B.; Wang, C.; Wang, B.-Y.; An, N.; An, L.; Liu, Y.; Wu, Z.-H.; Tian, J.-H. The microtubule-associated protein ASPM regulates spindle assembly and meiotic progression in mouse oocytes. PLoS ONE 2012, 7, e49303. [Google Scholar] [CrossRef]
- Ito, A.; Goshima, G. Microcephaly protein Asp focuses the minus ends of spindle microtubules at the pole and within the spindle. J. Cell Biol. 2015, 211, 999–1009. [Google Scholar] [CrossRef]
- Pulvers, J.N.; Bryk, J.; Fish, J.L.; Wilsch-Brauninger, M.; Arai, Y.; Schreier, D.; Naumann, R.; Helppi, J.; Habermann, B.; Vogt, J.; et al. Mutations in mouse Aspm (abnormal spindle-like microcephaly associated) cause not only microcephaly but also major defects in the germline. Proc. Natl. Acad. Sci. USA 2010, 107, 16595–16600. [Google Scholar] [CrossRef]
- do Carmo Avides, M.; Glover, D.M. Abnormal spindle protein, Asp, and the integrity of mitotic centrosomal microtubule organizing centers. Science 1999, 283, 1733–1735. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, J.G.; Bonaccorsi, S.; Gatti, M. The Drosophila protein Asp is involved in microtubule organization during spindle formation and cytokinesis. J. Cell Biol. 2001, 153, 637–648. [Google Scholar] [CrossRef]
- Bosveld, F.; Ainslie, A.; Bellaïche, Y. Sequential activities of dynein, Mud and Asp in centrosome-spindle coupling maintain centrosome number upon mitosis. J. Cell Sci. 2017, 130, 3557–3567. [Google Scholar] [CrossRef] [PubMed]
- Morales-Mulia, S.; Scholey, J.M. Spindle pole organization in Drosophila S2 cells by dynein, abnormal spindle protein (Asp), and KLP10A. Mol. Biol. Cell 2005, 16, 3176–3186. [Google Scholar] [CrossRef]
- Popova, J.V.; Pavlova, G.A.; Razuvaeva, A.V.; Yarinich, L.A.; Andreyeva, E.N.; Anders, A.F.; Galimova, Y.A.; Renda, F.; Somma, M.P.; Pindyurin, A.V.; et al. Genetic control of kinetochore-driven microtubule growth in Drosophila mitosis. Cells 2022, 11, 2127. [Google Scholar] [CrossRef] [PubMed]
- Riparbelli, M.G.; Callaini, G.; Glover, D.M.; do Carmo Avides, M. A Requirement for the abnormal spindle protein to organise microtubules of the central spindle for cytokinesis in Drosophila. J. Cell Sci. 2002, 115, 913–922. [Google Scholar] [CrossRef]
- Riparbelli, M.G.; Massarelli, C.; Robbins, L.G.; Callaini, G. The abnormal spindle protein is required for germ cell mitosis and oocyte differentiation during Drosophila oogenesis. Exp. Cell Res. 2004, 298, 96–106. [Google Scholar] [CrossRef]
- Barbosa, V.; Yamamoto, R.R.; Henderson, D.S.; Glover, D.M. Mutation of a Drosophila gamma tubulin ring complex subunit encoded by discs degenerate-4 differentially disrupts centrosomal protein localization. Genes Dev. 2000, 14, 3126–3139. [Google Scholar] [CrossRef]
- D’Avino, P.P.; Giansanti, M.G.; Petronczki, M. Cytokinesis in animal cells. Cold Spring Harb. Perspect. Biol. 2015, 7, a015834. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.L.; Kosodo, Y.; Enard, W.; Pääbo, S.; Huttner, W.B. Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc. Natl. Acad. Sci. USA 2006, 103, 10438–10443. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Liu, L.; Zhao, A.; Pfeifer, G.P.; Xu, X. The abnormal spindle-like, microcephaly-associated (ASPM) gene encodes a centrosomal protein. Cell Cycle Georget. Tex 2005, 4, 1227–1229. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, M.; Chang, Y.J.; LoTurco, J.J. ASPM and Citron kinase co-localize to the midbody ring during cytokinesis. Cell Cycle Georget. Tex 2007, 6, 1605–1612. [Google Scholar] [CrossRef]
- Higgins, J.; Midgley, C.; Bergh, A.-M.; Bell, S.M.; Askham, J.M.; Roberts, E.; Binns, R.K.; Sharif, S.M.; Bennett, C.; Glover, D.M.; et al. Human ASPM participates in spindle organisation, spindle orientation and cytokinesis. BMC Cell Biol. 2010, 11, 85. [Google Scholar] [CrossRef]
- Jayaraman, D.; Kodani, A.; Gonzalez, D.M.; Mancias, J.D.; Mochida, G.H.; Vagnoni, C.; Johnson, J.; Krogan, N.; Harper, J.W.; Reiter, J.F.; et al. Microcephaly proteins Wdr62 and Aspm define a mother centriole complex regulating centriole biogenesis, apical complex, and cell fate. Neuron 2016, 92, 813–828. [Google Scholar] [CrossRef]
- Mastronarde, D.N.; McDonald, K.L.; Ding, R.; McIntosh, J.R. Interpolar spindle microtubules in PTK Cells. J. Cell Biol. 1993, 123, 1475–1489. [Google Scholar] [CrossRef]
- Borgal, L.; Wakefield, J.G. Context-dependent spindle pole focusing. Essays Biochem. 2018, 62, 803–813. [Google Scholar] [CrossRef]
- Eggert, U.S.; Mitchison, T.J.; Field, C.M. Animal cytokinesis: From parts list to mechanisms. Annu. Rev. Biochem. 2006, 75, 543–566. [Google Scholar] [CrossRef]
- She, Z.-Y.; Wei, Y.-L.; Lin, Y.; Li, Y.-L.; Lu, M.-H. Mechanisms of the Ase1/PRC1/MAP65 family in central spindle assembly. Biol. Rev. Camb. Philos. Soc. 2019, 94, 2033–2048. [Google Scholar] [CrossRef]
- Chen, C.-T.; Ettinger, A.W.; Huttner, W.B.; Doxsey, S.J. Resurrecting remnants: The lives of post-mitotic midbodies. Trends Cell Biol. 2013, 23, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.; Gonzalez, C.; Wandosell, F.; Avila, J.; Ripoll, P. Abnormal meiotic spindles cause a cascade of defects during spermatogenesis in Asp males of Drosophila. Dev. Camb. Engl. 1990, 108, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, A.; Itoh, K.; Goto, S.; Hirakawa, H.; Wang, B.; Kokubo, T.; Kito, S.; Tsukamoto, S.; Fushiki, S. Disruption of Aspm causes microcephaly with abnormal neuronal differentiation. Brain Dev. 2014, 36, 661–669. [Google Scholar] [CrossRef]
- González, C.; Casal, J.; Ripoll, P. Functional monopolar spindles caused by mutation in Mgr, a cell division gene of Drosophila melanogaster. J. Cell Sci. 1988, 89 Pt 1, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Sunkel, C.E.; Glover, D.M. Interactions between Mgr, Asp, and Polo: Asp function modulated by Polo and needed to maintain the poles of monopolar and bipolar spindles. Chromosoma 1998, 107, 452–460. [Google Scholar] [CrossRef]
- Buffin, E.; Emre, D.; Karess, R.E. Flies without a spindle checkpoint. Nat. Cell Biol. 2007, 9, 565–572. [Google Scholar] [CrossRef]
- Rieder, C.L.; Maiato, H. Stuck in division or passing through: What happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell 2004, 7, 637–651. [Google Scholar] [CrossRef]
- Pavlova, G.A.; Razuvaeva, A.V.; Popova, J.V.; Andreyeva, E.N.; Yarinich, L.A.; Lebedev, M.O.; Pellacani, C.; Bonaccorsi, S.; Somma, M.P.; Gatti, M.; et al. The role of patronin in Drosophila mitosis. BMC Mol. Cell Biol. 2019, 20, 7. [Google Scholar] [CrossRef]
- Williams, S.E.; Garcia, I.; Crowther, A.J.; Li, S.; Stewart, A.; Liu, H.; Lough, K.J.; O’Neill, S.; Veleta, K.; Oyarzabal, E.A.; et al. Aspm sustains postnatal cerebellar neurogenesis and medulloblastoma growth in mice. Dev. Camb. Engl. 2015, 142, 3921–3932. [Google Scholar] [CrossRef]
- Tungadi, E.A.; Ito, A.; Kiyomitsu, T.; Goshima, G. Human microcephaly ASPM protein is a spindle pole-focusing factor that functions redundantly with CDK5RAP2. J. Cell Sci. 2017, 130, 3676–3684. [Google Scholar] [CrossRef]
- Carmena, M.; Gonzalez, C.; Casal, J.; Ripoll, P. Dosage dependence of maternal contribution to somatic cell division in Drosophila melanogaster. Dev. Camb. Engl. 1991, 113, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Saunders, R.D.; Casal, J.; Molina, I.; Carmena, M.; Ripoll, P.; Glover, D.M. Mutations at the Asp locus of Drosophila lead to multiple free centrosomes in syncytial embryos, but restrict centrosome duplication in larval neuroblasts. J. Cell Sci. 1990, 96 Pt 4, 605–616. [Google Scholar] [CrossRef]
- Trammell, M.A.; Mahoney, N.M.; Agard, D.A.; Vale, R.D. Mob4 plays a role in spindle focusing in Drosophila S2 cells. J. Cell Sci. 2008, 121, 1284–1292. [Google Scholar] [CrossRef]
- Gai, M.; Bianchi, F.T.; Vagnoni, C.; Vernì, F.; Bonaccorsi, S.; Pasquero, S.; Berto, G.E.; Sgrò, F.; Chiotto, A.M.; Annaratone, L.; et al. ASPM and CITK regulate spindle orientation by affecting the dynamics of astral microtubules. EMBO Rep. 2016, 17, 1396–1409. [Google Scholar] [CrossRef]
- Bond, J.; Roberts, E.; Springell, K.; Lizarraga, S.B.; Scott, S.; Higgins, J.; Hampshire, D.J.; Morrison, E.E.; Leal, G.F.; Silva, E.O.; et al. A Centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat. Genet. 2005, 37, 353–355. [Google Scholar] [CrossRef]
- Taverna, E.; Götz, M.; Huttner, W.B. The cell biology of neurogenesis: Toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell Dev. Biol. 2014, 30, 465–502. [Google Scholar] [CrossRef]
- Gonzalez, C. Spindle orientation, asymmetric division and tumour suppression in Drosophila stem cells. Nat. Rev. Genet. 2007, 8, 462–472. [Google Scholar] [CrossRef]
- Morin, X.; Bellaïche, Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev. Cell 2011, 21, 102–119. [Google Scholar] [CrossRef]
- di Pietro, F.; Echard, A.; Morin, X. Regulation of mitotic spindle orientation: An integrated view. EMBO Rep. 2016, 17, 1106–1130. [Google Scholar] [CrossRef] [PubMed]
- Capecchi, M.R.; Pozner, A. ASPM regulates symmetric stem cell division by tuning cyclin E ubiquitination. Nat. Commun. 2015, 6, 8763. [Google Scholar] [CrossRef] [PubMed]
- Rebollo, E.; González, C. Visualizing the spindle checkpoint in Drosophila spermatocytes. EMBO Rep. 2000, 1, 65–70. [Google Scholar] [CrossRef]
- Giansanti, M.G.; Bonaccorsi, S.; Williams, B.; Williams, E.V.; Santolamazza, C.; Goldberg, M.L.; Gatti, M. Cooperative interactions between the central spindle and the contractile ring during Drosophila cytokinesis. Genes Dev. 1998, 12, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Sikirzhytski, V.; Renda, F.; Tikhonenko, I.; Magidson, V.; McEwen, B.F.; Khodjakov, A. Microtubules assemble near most kinetochores during early prometaphase in human cells. J. Cell Biol. 2018, 217, 2647–2659. [Google Scholar] [CrossRef]
- Franke, J.D.; Boury, A.L.; Gerald, N.J.; Kiehart, D.P. Native nonmuscle myosin II stability and light chain binding in Drosophila melanogaster. Cell Motil. Cytoskelet. 2006, 63, 604–622. [Google Scholar] [CrossRef] [PubMed]
- Goshima, G.; Wollman, R.; Goodwin, S.S.; Zhang, N.; Scholey, J.M.; Vale, R.D.; Stuurman, N. Genes required for mitotic spindle assembly in Drosophila S2 cells. Science 2007, 316, 417–421. [Google Scholar] [CrossRef]
- Kiyomitsu, T.; Boerner, S. The nuclear mitotic apparatus (NuMA) protein: A key player for nuclear formation, spindle assembly, and spindle positioning. Front. Cell Dev. Biol. 2021, 9, 653801. [Google Scholar] [CrossRef]
- Bowman, S.K.; Neumüller, R.A.; Novatchkova, M.; Du, Q.; Knoblich, J.A. The Drosophila NuMA homolog Mud regulates spindle orientation in asymmetric cell division. Dev. Cell 2006, 10, 731–742. [Google Scholar] [CrossRef]
- Capalbo, L.; D’Avino, P.P.; Archambault, V.; Glover, D.M. Rab5 GTPase controls chromosome alignment through lamin disassembly and relocation of the NuMA-like protein Mud to the poles during mitosis. Proc. Natl. Acad. Sci. USA 2011, 108, 17343–17348. [Google Scholar] [CrossRef] [PubMed]
- Moutinho-Pereira, S.; Stuurman, N.; Afonso, O.; Hornsveld, M.; Aguiar, P.; Goshima, G.; Vale, R.D.; Maiato, H. Genes involved in centrosome-independent mitotic spindle assembly in Drosophila S2 Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 19808–19813. [Google Scholar] [CrossRef]
- Goshima, G.; Nédélec, F.; Vale, R.D. Mechanisms for focusing mitotic spindle poles by minus end-directed motor proteins. J. Cell Biol. 2005, 171, 229–240. [Google Scholar] [CrossRef]
- Merdes, A.; Heald, R.; Samejima, K.; Earnshaw, W.C.; Cleveland, D.W. Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J. Cell Biol. 2000, 149, 851–862. [Google Scholar] [CrossRef]
- Rogers, G.C.; Rogers, S.L.; Sharp, D.J. Spindle microtubules in flux. J. Cell Sci. 2005, 118, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Bogoyevitch, M.A.; Yeap, Y.Y.C.; Qu, Z.; Ngoei, K.R.; Yip, Y.Y.; Zhao, T.T.; Heng, J.I.; Ng, D.C.H. WD40-repeat protein 62 is a JNK-phosphorylated spindle pole protein required for spindle maintenance and timely mitotic progression. J. Cell Sci. 2012, 125, 5096–5109. [Google Scholar] [CrossRef]
- Mishra-Gorur, K.; Çağlayan, A.O.; Schaffer, A.E.; Chabu, C.; Henegariu, O.; Vonhoff, F.; Akgümüş, G.T.; Nishimura, S.; Han, W.; Tu, S.; et al. Mutations in KATNB1 cause complex cerebral malformations by disrupting asymmetrically dividing neural progenitors. Neuron 2014, 84, 1226–1239. [Google Scholar] [CrossRef] [PubMed]
- Goshima, G.; Kimura, A. New look inside the spindle: Microtubule-dependent microtubule generation within the spindle. Curr. Opin. Cell Biol. 2010, 22, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, X.; Wang, P.; Cao, S.-L.; Peng, B.; Xu, X. ASPM promotes homologous recombination-mediated DNA repair by safeguarding BRCA1 stability. iScience 2021, 24, 102534. [Google Scholar] [CrossRef]
- Bae, I.; Rih, J.K.; Kim, H.J.; Kang, H.J.; Haddad, B.; Kirilyuk, A.; Fan, S.; Avantaggiati, M.L.; Rosen, E.M. BRCA1 regulates gene expression for orderly mitotic progression. Cell Cycle Georget. Tex 2005, 4, 1641–1666. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, A.; Yaoi, T.; Ogi, H.; Wang, B.; Suetomi, K.; Sekine, E.; Yu, D.; Kato, T.; Takahashi, S.; Okayasu, R.; et al. Ionizing radiation downregulates ASPM, a gene responsible for microcephaly in humans. Biochem. Biophys. Res. Commun. 2008, 369, 953–957. [Google Scholar] [CrossRef]
- Kato, T.A.; Okayasu, R.; Jeggo, P.A.; Fujimori, A. ASPM influences DNA double-strand break repair and represents a potential target for radiotherapy. Int. J. Radiat. Biol. 2011, 87, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, S.; Wang, P.; Wang, Z.-Q.; Chen, H.; Xu, X.; Peng, B. ASPM promotes ATR-CHK1 activation and stabilizes stalled replication forks in response to replication stress. Proc. Natl. Acad. Sci. USA 2022, 119, e2203783119. [Google Scholar] [CrossRef]
- Möröy, T.; Geisen, C. Cyclin E. Int. J. Biochem. Cell Biol. 2004, 36, 1424–1439. [Google Scholar] [CrossRef] [PubMed]
- Degrassi, F.; Damizia, M.; Lavia, P. The mitotic apparatus and kinetochores in microcephaly and neurodevelopmental diseases. Cells 2019, 9, 49. [Google Scholar] [CrossRef] [PubMed]

| Organism/Cell Type with Asp/ASPM Depletion | Process Affected | ||||||
|---|---|---|---|---|---|---|---|
| Aster Formation | Spindle Poles Focusing | Centrosome Attachment | Spindle Orientation | Metaphase Delay/ Arrest | Chromosome Segregation | Cytokinesis | |
| D.m. central brain neuroblasts (1) | Yes | Yes | Yes | NR | Yes | Yes | N/A |
| D.m. S2 cells (2) | NR | Yes | Yes | N/A | Yes | NR | NR |
| D.m. optic lobe neuroepithelium (3) | NR | Yes | NR | Yes | No | Yes | No |
| D.m. epithelial cells notum (4) | No | Yes | Yes | No | NR | NR | NR |
| D.m. cuticular cells (5) | N/A | N/A | N/A | N/A | N/A | Yes | N/A |
| D.m. embryo divisions (6) | NR | NR | Yes | N/A | Yes | Yes | N/A |
| D.m. male meiotic cells (7) | Yes | Yes | Yes | N/A | N/A | Yes | Yes |
| Mouse neuroepithelial (NE) cells (8) | NR | NR | Yes | Yes/No | No | NR | NR |
| Mouse cerebellum cells (9) | NR | NR | NR | Yes | Yes | NR | NR |
| Human U2OS cells (10) | NR | NR | NR | Yes | No | NR | Yes |
| Human HeLa cells (11) | Yes | NR | NR | Yes | NR | NR | NR |
| Human HCT116 cells (12) | NR | No | NR | NR | No | NR | No |
| Human HCT116 depleted of CDK4RAP2 (12) | NR | Yes | NR | NR | Yes | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razuvaeva, A.V.; Graziadio, L.; Palumbo, V.; Pavlova, G.A.; Popova, J.V.; Pindyurin, A.V.; Bonaccorsi, S.; Somma, M.P.; Gatti, M. The Multiple Mitotic Roles of the ASPM Orthologous Proteins: Insight into the Etiology of ASPM-Dependent Microcephaly. Cells 2023, 12, 922. https://doi.org/10.3390/cells12060922
Razuvaeva AV, Graziadio L, Palumbo V, Pavlova GA, Popova JV, Pindyurin AV, Bonaccorsi S, Somma MP, Gatti M. The Multiple Mitotic Roles of the ASPM Orthologous Proteins: Insight into the Etiology of ASPM-Dependent Microcephaly. Cells. 2023; 12(6):922. https://doi.org/10.3390/cells12060922
Chicago/Turabian StyleRazuvaeva, Alyona V., Lucia Graziadio, Valeria Palumbo, Gera A. Pavlova, Julia V. Popova, Alexey V. Pindyurin, Silvia Bonaccorsi, Maria Patrizia Somma, and Maurizio Gatti. 2023. "The Multiple Mitotic Roles of the ASPM Orthologous Proteins: Insight into the Etiology of ASPM-Dependent Microcephaly" Cells 12, no. 6: 922. https://doi.org/10.3390/cells12060922
APA StyleRazuvaeva, A. V., Graziadio, L., Palumbo, V., Pavlova, G. A., Popova, J. V., Pindyurin, A. V., Bonaccorsi, S., Somma, M. P., & Gatti, M. (2023). The Multiple Mitotic Roles of the ASPM Orthologous Proteins: Insight into the Etiology of ASPM-Dependent Microcephaly. Cells, 12(6), 922. https://doi.org/10.3390/cells12060922








