Quantitative Phosphoproteomics Reveals the Requirement of DYRK1-Mediated Phosphorylation of Ion Transport- and Cell Junction-Related Proteins for Notochord Lumenogenesis in Ascidian
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animal and Sample Preparation
2.2. Phosphoproteome and Bioinformatic Analysis
2.3. Plasmids Construction and Electroporation
2.4. Immunostaining
2.5. Statistical Analysis
3. Results
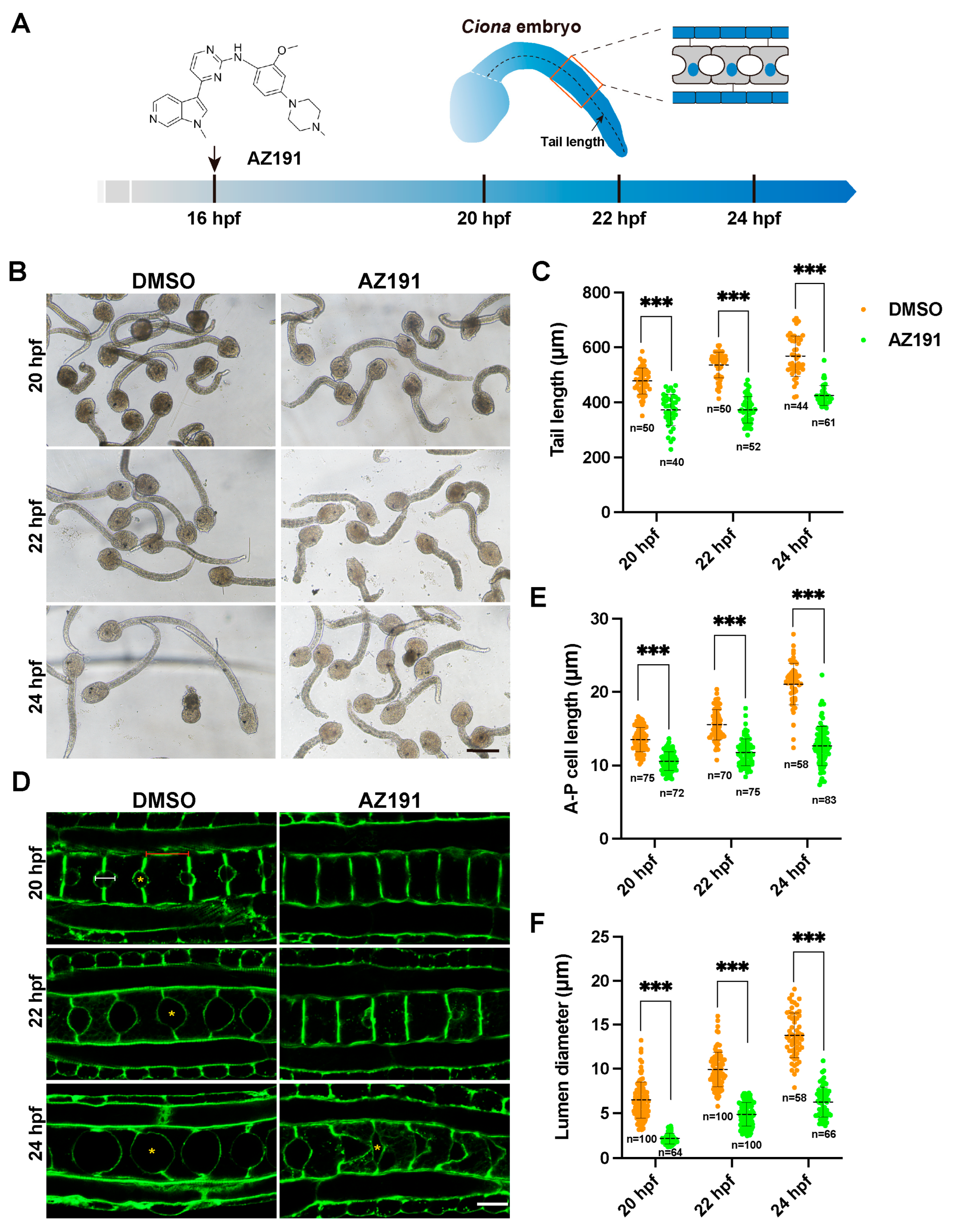
3.1. Kinase Activity of DYRK1 Is Required for Ciona Notochord Development and Lumen Inflation
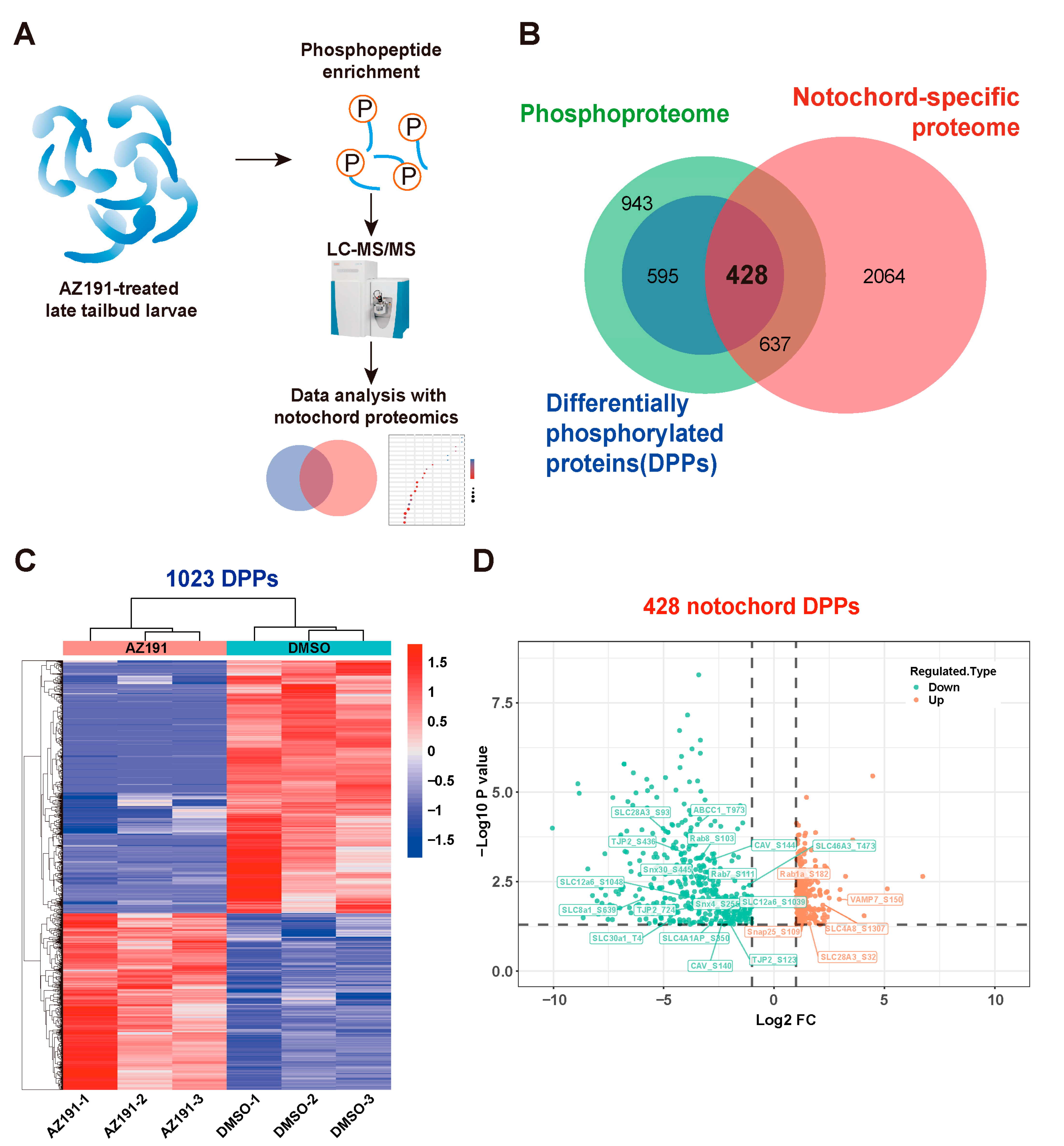
3.2. Phosphoproteomic Analysis of Embryos with DYRK1 Inhibition
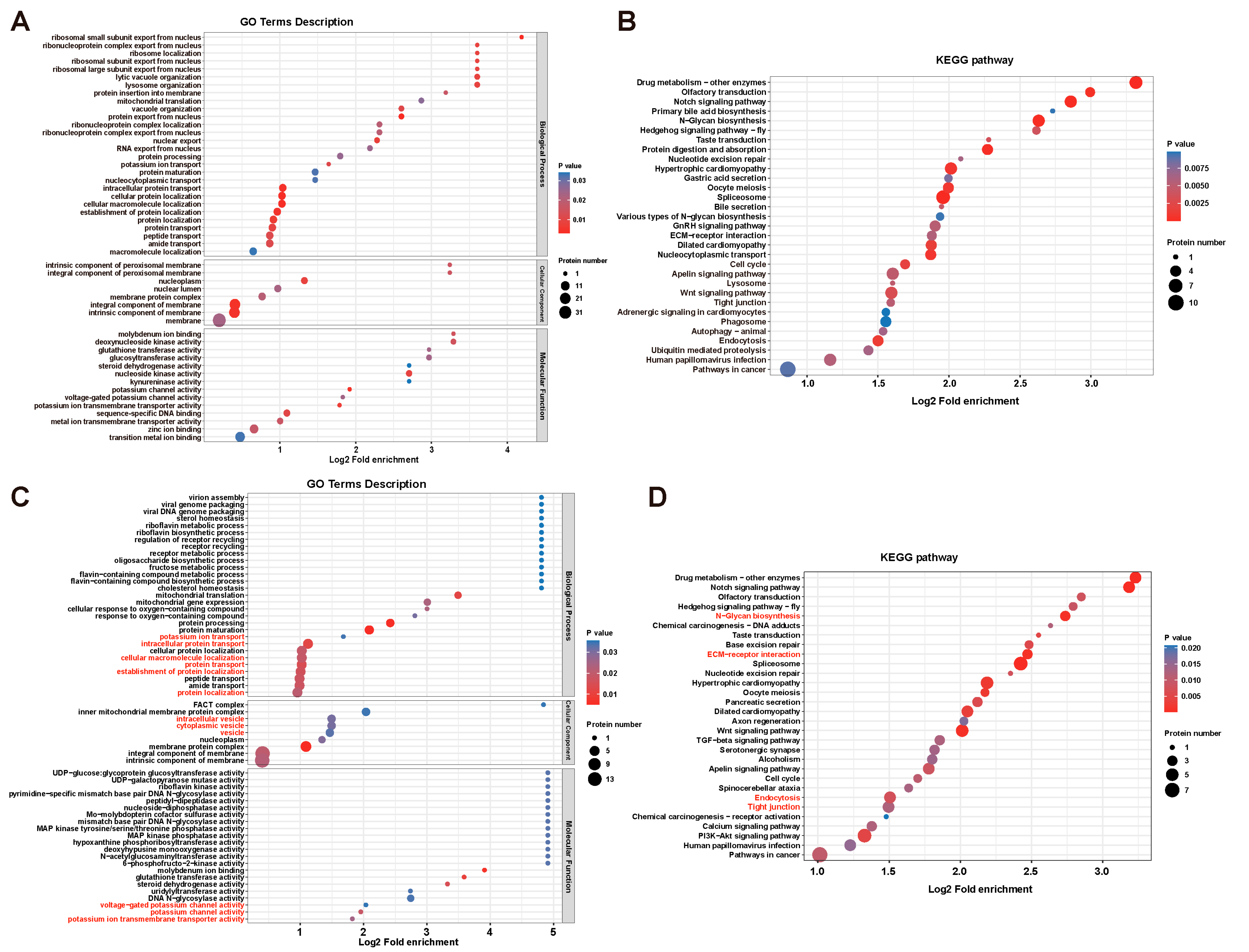
3.3. DYRK1-Mediated Multi-Processes Participated in Ciona Tailbud Larva Notochord Development
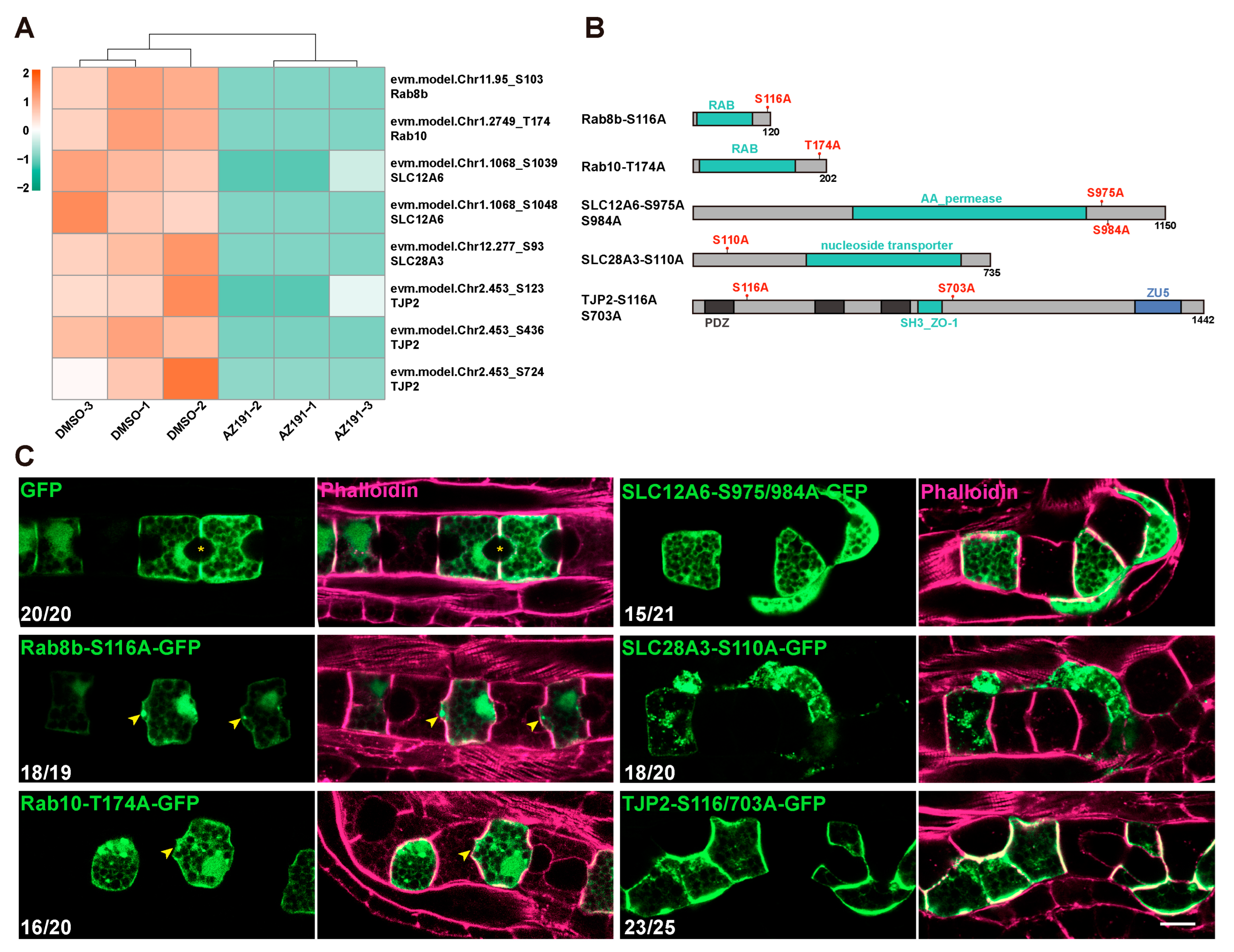
3.4. DYRK1-Mediated Transmembrane Transport and Tight Junction Were Essential for Notochord Lumen Inflation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ouyang, X.; Wang, Z.; Wu, B.; Yang, X.; Dong, B. The Conserved Transcriptional Activation Activity Identified in Dual-Specificity Tyrosine-(Y)-Phosphorylation-Regulated Kinase 1. Biomolecules 2023, 13, 283. [Google Scholar] [CrossRef]
- Aranda, S.; Laguna, A.; de la Luna, S. DYRK family of protein kinases: Evolutionary relationships, biochemical properties, and functional roles. FASEB J. 2011, 25, 449–462. [Google Scholar] [CrossRef]
- Park, J.; Song, W.-J.; Chung, K.C. Function and regulation of Dyrk1A: Towards understanding Down syndrome. Cell. Mol. Life Sci. 2009, 66, 3235–3240. [Google Scholar] [CrossRef]
- Park, J.; Sung, J.Y.; Park, J.; Song, W.-J.; Chang, S.; Chung, K.C. Dyrk1A negatively regulates the actin cytoskeleton through threonine phosphorylation of N-WASP. J. Cell Sci. 2012, 125 Pt 1, 67–80. [Google Scholar] [CrossRef]
- Ori-McKenney, K.M.; McKenney, R.J.; Huang, H.H.; Li, T.; Meltzer, S.; Jan, L.Y.; Vale, R.D.; Wiita, A.P.; Jan, Y.N. Phosphorylation of beta-Tubulin by the Down Syndrome Kinase, Minibrain/DYRK1a, Regulates Microtubule Dynamics and Dendrite Morphogenesis. Neuron 2016, 90, 551–563. [Google Scholar] [CrossRef]
- Soppa, U.; Becker, W. DYRK protein kinases. Curr. Biol. 2015, 25, R488–R489. [Google Scholar] [CrossRef]
- Huang, Y.; Chen-Hwang, M.-C.; Dolios, G.; Murakami, N.; Padovan, J.C.; Wang, R.; Hwang, Y.-W. Mnb/Dyrk1A Phosphorylation Regulates the Interaction of Dynamin 1 with SH3 Domain-Containing Proteins. Biochemistry 2004, 43, 10173–10185. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Xie, W.; Lu, R.C.; Chen-Hwang, M.-C.; Wieraszko, A.; Hwang, Y.W. Phosphorylation of Amphiphysin I by Minibrain Kinase/Dual-specificity Tyrosine Phosphorylation-regulated Kinase, a Kinase Implicated in Down Syndrome. J. Biol. Chem. 2006, 281, 23712–23724. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Bolton, D.C.; Kida, E.; Xie, W.; Hwang, Y.-W. Phosphorylation by Dyrk1A of Clathrin Coated Vesicle-Associated Proteins: Identification of the Substrate Proteins and the Effects of Phosphorylation. PLoS ONE 2012, 7, e34845. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Smith, W.C. Ascidian notochord morphogenesis. Dev. Dyn. 2007, 236, 1748–1757. [Google Scholar] [CrossRef]
- Denker, E.; Jiang, D. Ciona intestinalis notochord as a new model to investigate the cellular and molecular mechanisms of tubulogenesis. Semin. Cell Dev. Biol. 2012, 23, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Horie, T.; Denker, E.; Kusakabe, T.; Tsuda, M.; Smith, W.C.; Jiang, D. Tube formation by complex cellular processes in Ciona intestinalis notochord. Dev. Biol. 2009, 330, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Bhattachan, P.; Dong, B. Ascidian notochord elongation. Dev. Biol. 2019, 448, 147–153. [Google Scholar] [CrossRef]
- Satoh, N. Developmental Genomics of Ascidians; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Deng, W.; Nies, F.; Feuer, A.; Bočina, I.; Oliver, D.; Jiang, D. Anion translocation through an Slc26 transporter mediates lumen expansion during tubulogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 14972–14977. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.C. Cellular Processes of Notochord Formation. Adv. Exp. Med. Biol. 2018, 1029, 165–177. [Google Scholar] [CrossRef]
- Denker, E.; Bočina, I.; Jiang, D. Tubulogenesis in a simple cell cord requires the formation of bi-apical cells through two discrete Par domains. Development 2013, 140, 2985–2996. [Google Scholar] [CrossRef]
- Bhattachan, P.; Rae, J.; Yu, H.; Jung, W.; Wei, J.; Parton, R.G.; Dong, B. Ascidian caveolin induces membrane curvature and protects tissue integrity and morphology during embryogenesis. FASEB J. 2020, 34, 1345–1361. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, F.; Gao, S.; Liang, Y.; Long, H.; Lv, Z.; Su, Y.; Ye, N.; Zhang, L.; Zhao, C.; et al. Biodiversity-based development and evolution: The emerging research systems in model and non-model organisms. Sci. China Life Sci. 2021, 64, 1236–1280. [Google Scholar] [CrossRef]
- Bryant, D.M.; Mostov, K.E. From cells to organs: Building polarized tissue. Nat. Rev. Mol. Cell Biol. 2008, 9, 887–901. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, Z.; Bi, J.; Liu, A.; Jiang, A.; Dong, B. Proteomic identification of intracellular vesicle trafficking and protein glycosylation requirements for lumen inflation in Ciona notochord. Proteomics 2023, e2200460. [Google Scholar] [CrossRef]
- Denker, E.; Sehring, I.M.; Dong, B.; Audisso, J.; Mathiesen, B.; Jiang, D. Regulation by a TGFbeta-ROCK-actomyosin axis secures a non-linear lumen expansion that is essential for tubulogenesis. Development 2015, 142, 1639–1650. [Google Scholar]
- Christiaen, L.; Wagner, E.; Shi, W.; Levine, M. Isolation of Sea Squirt (Ciona) Gametes, Fertilization, Dechorionation, and Development. Cold Spring Harb. Protoc. 2009, 2009, pdb.prot5344. [Google Scholar] [CrossRef]
- Thingholm, T.E.; Jensen, O.N.; Robinson, P.J.; Larsen, M.R. SIMAC (Sequential Elution from IMAC), a Phosphoproteomics Strategy for the Rapid Separation of Monophosphorylated from Multiply Phosphorylated Peptides. Mol. Cell. Proteom. 2008, 7, 661–671. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Zhang, X.; Dong, B.; Yu, H. Development of a miRNA Sensor by an Inducible CRISPR-Cas9 Construct in Ciona Embryogenesis. Mol. Biotechnol. 2021, 63, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Wei, J.; Wang, G.; Li, X.; Ren, P.; Yu, H.; Dong, B. Architectural delineation and molecular identification of extracellular matrix in ascidian embryos and larvae. Biol. Open 2017, 6, 1383–1390. [Google Scholar] [CrossRef]
- Ashford, A.L.; Oxley, D.; Kettle, J.; Hudson, K.; Guichard, S.; Cook, S.J.; Lochhead, P.A. A novel DYRK1B inhibitor AZ191 demonstrates that DYRK1B acts independently of GSK3β to phosphorylate cyclin D1 at Thr286, not Thr288. Biochem. J. 2013, 457, 43–56. [Google Scholar] [CrossRef]
- Sehring, I.M.; Dong, B.; Denker, E.; Bhattachan, P.; Deng, W.; Mathiesen, B.T.; Jiang, D. An Equatorial Contractile Mechanism Drives Cell Elongation but not Cell Division. PLoS Biol. 2014, 12, e1001781. [Google Scholar] [CrossRef]
- Dong, B.; Deng, W.; Jiang, D. Distinct cytoskeleton populations and extensive crosstalk control Ciona notochord tubulogenesis. Development 2011, 138, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Dong, B. Cellular processes and gene regulatory network of notochord development in a marine model animal: Ciona intestinalis. Sci. Bull. 2015, 60, 1167–1179. [Google Scholar] [CrossRef]
- Burikhanov, R.; Hebbar, N.; Noothi, S.K.; Shukla, N.; Sledziona, J.; Araujo, N.; Kudrimoti, M.; Wang, Q.J.; Watt, D.S.; Welch, D.R.; et al. Chloroquine-Inducible Par-4 Secretion Is Essential for Tumor Cell Apoptosis and Inhibition of Metastasis. Cell Rep. 2017, 18, 508–519. [Google Scholar] [CrossRef]
- Sato, T.; Mushiake, S.; Kato, Y.; Sato, K.; Sato, M.; Takeda, N.; Ozono, K.; Miki, K.; Kubo, Y.; Tsuji, A.; et al. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature 2007, 448, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Iwano, T.; Kunii, M.; Matsuda, S.; Mizuguchi, R.; Jung, Y.; Hagiwara, H.; Yoshihara, Y.; Yuzaki, M.; Harada, R.; et al. Rab8a and Rab8b are essential for several apical transport pathways but insufficient for ciliogenesis. J. Cell. Sci. 2014, 127 Pt 2, 422–431. [Google Scholar] [CrossRef]
- Heidrych, P.; Zimmermann, U.; Breß, A.; Pusch, C.M.; Ruth, P.; Pfister, M.; Knipper, M.; Blin, N. Rab8b GTPase, a protein transport regulator, is an interacting partner of otoferlin, defective in a human autosomal recessive deafness form. Hum. Mol. Genet. 2008, 17, 3814–3821. [Google Scholar] [CrossRef]
- Kobayashi, S.; Suzuki, T.; Kawaguchi, A.; Phongphaew, W.; Yoshii, K.; Iwano, T.; Harada, A.; Kariwa, H.; Orba, Y.; Sawa, H. Rab8b Regulates Transport of West Nile Virus Particles from Recycling Endosomes. J. Biol. Chem. 2016, 291, 6559–6568. [Google Scholar] [CrossRef]
- Hutagalung, A.H.; Novick, P.J. Role of Rab GTPases in Membrane Traffic and Cell Physiology. Physiol. Rev. 2011, 91, 119–149. [Google Scholar] [CrossRef] [PubMed]
- Stöckli, J.; Fazakerley, D.J.; James, D.E. GLUT4 exocytosis. J. Cell Sci. 2011, 124 Pt 24, 4147–4159. [Google Scholar] [CrossRef] [PubMed]
- Babbey, C.M.; Ahktar, N.; Wang, E.; Chen, C.C.-H.; Grant, B.D.; Dunn, K.W. Rab10 Regulates Membrane Transport through Early Endosomes of Polarized Madin-Darby Canine Kidney Cells. Mol. Biol. Cell 2006, 17, 3156–3175. [Google Scholar] [CrossRef]
- Xu, L.; Nagai, Y.; Kajihara, Y.; Ito, G.; Tomita, T. The Regulation of Rab GTPases by Phosphorylation. Biomolecules 2021, 11, 1340. [Google Scholar] [CrossRef] [PubMed]
- Waschbüsch, D.; Khan, A.R. Phosphorylation of Rab GTPases in the regulation of membrane trafficking. Traffic 2020, 21, 712–719. [Google Scholar] [CrossRef]
- Hebert, S.C.; Mount, D.B.; Gamba, G. Molecular physiology of cation-coupled Cl? cotransport: The SLC12 family. Pflugers Arch. 2004, 447, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Kahle, K.T.; Flores, B.; Bharucha-Goebel, D.; Zhang, J.; Donkervoort, S.; Hegde, M.; Begum, G.; Duran, D.; Liang, B.; Sun, D.; et al. Peripheral motor neuropathy is associated with defective kinase regulation of the KCC3 cotransporter. Sci. Signal. 2016, 9, ra77. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, M.W.; Ng, A.M.; Yao, S.Y.; Graham, K.; Loewen, S.K.; Smith, K.M.; Ritzel, R.G.; Mowles, D.A.; Carpenter, P.; Chen, X.-Z.; et al. Molecular Identification and Characterization of Novel Human and Mouse Concentrative Na+-Nucleoside Cotransporter Proteins (hCNT3 and mCNT3) Broadly Selective for Purine and Pyrimidine Nucleosides (System cib). J. Biol. Chem. 2001, 276, 2914–2927. [Google Scholar] [CrossRef] [PubMed]
- Martí, E.; Altafaj, X.; Dierssen, M.; de la Luna, S.; Fotaki, V.; Alvarez, M.; Perez-Riba, M.; Ferrer, I.; Estivill, X. Dyrk1A expression pattern supports specific roles of this kinase in the adult central nervous system. Brain Res. 2003, 964, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Arbones, M.L.; Thomazeau, A.; Nakano-Kobayashi, A.; Hagiwara, M.; Delabar, J.M. DYRK1A and cognition: A lifelong relationship. Pharmacol. Ther. 2019, 194, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Demuro, S.; Di Martino, R.M.C.; Ortega, J.A.; Cavalli, A. GSK-3β, FYN, and DYRK1A: Master Regulators in Neurodegenerative Pathways. Int. J. Mol. Sci. 2021, 22, 9098. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Ouyang, X.; Tan, Z.; Yang, L.; Dong, B. Quantitative Phosphoproteomics Reveals the Requirement of DYRK1-Mediated Phosphorylation of Ion Transport- and Cell Junction-Related Proteins for Notochord Lumenogenesis in Ascidian. Cells 2023, 12, 921. https://doi.org/10.3390/cells12060921
Wang Z, Ouyang X, Tan Z, Yang L, Dong B. Quantitative Phosphoproteomics Reveals the Requirement of DYRK1-Mediated Phosphorylation of Ion Transport- and Cell Junction-Related Proteins for Notochord Lumenogenesis in Ascidian. Cells. 2023; 12(6):921. https://doi.org/10.3390/cells12060921
Chicago/Turabian StyleWang, Zhuqing, Xiuke Ouyang, Zicheng Tan, Likun Yang, and Bo Dong. 2023. "Quantitative Phosphoproteomics Reveals the Requirement of DYRK1-Mediated Phosphorylation of Ion Transport- and Cell Junction-Related Proteins for Notochord Lumenogenesis in Ascidian" Cells 12, no. 6: 921. https://doi.org/10.3390/cells12060921
APA StyleWang, Z., Ouyang, X., Tan, Z., Yang, L., & Dong, B. (2023). Quantitative Phosphoproteomics Reveals the Requirement of DYRK1-Mediated Phosphorylation of Ion Transport- and Cell Junction-Related Proteins for Notochord Lumenogenesis in Ascidian. Cells, 12(6), 921. https://doi.org/10.3390/cells12060921








