Expression of Basement Membrane Molecules by Wharton Jelly Stem Cells (WJSC) in Full-Term Human Umbilical Cords, Cell Cultures and Microtissues
Abstract
1. Introduction
2. Materials and Methods
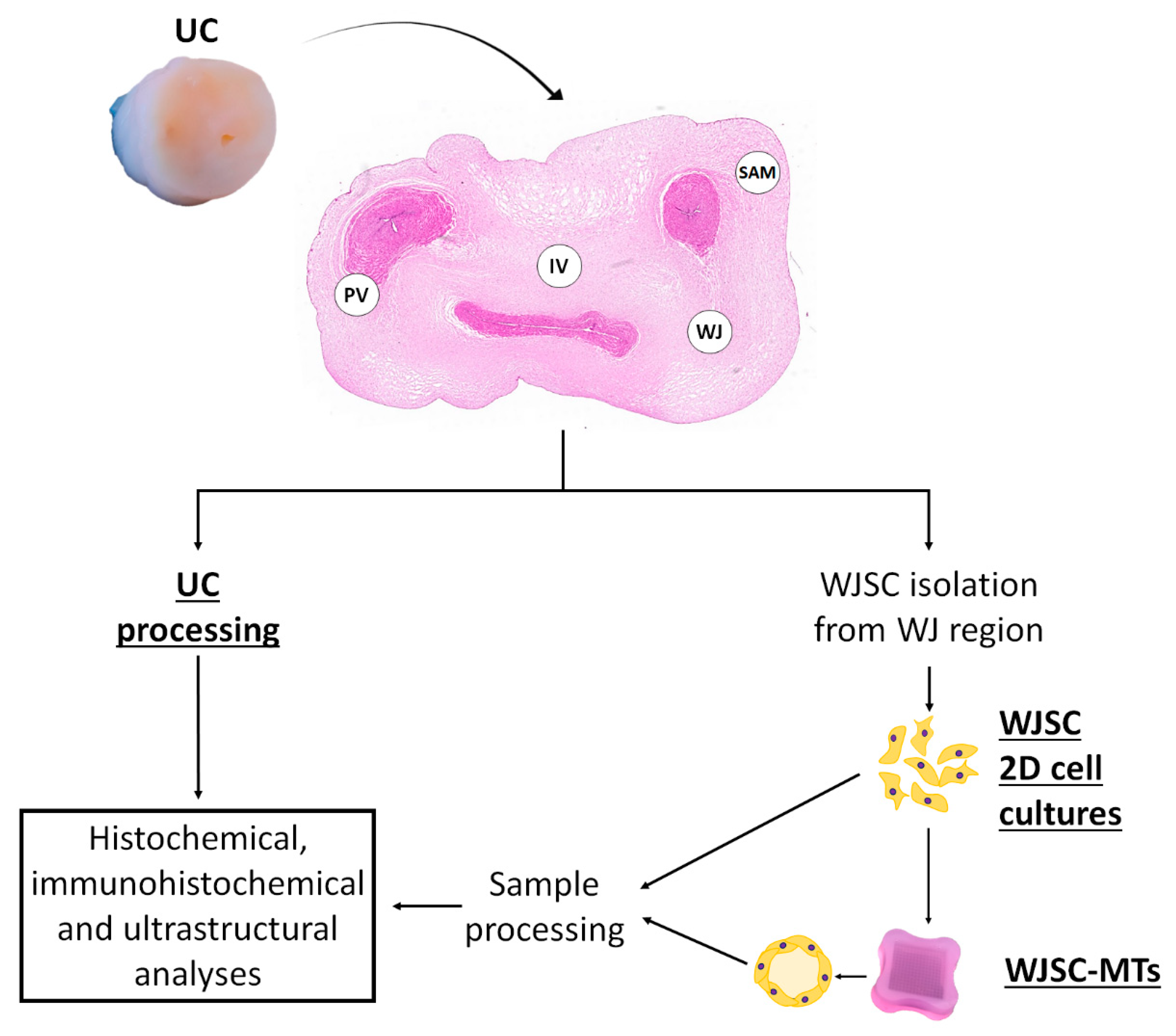
2.1. UC Tissue Samples
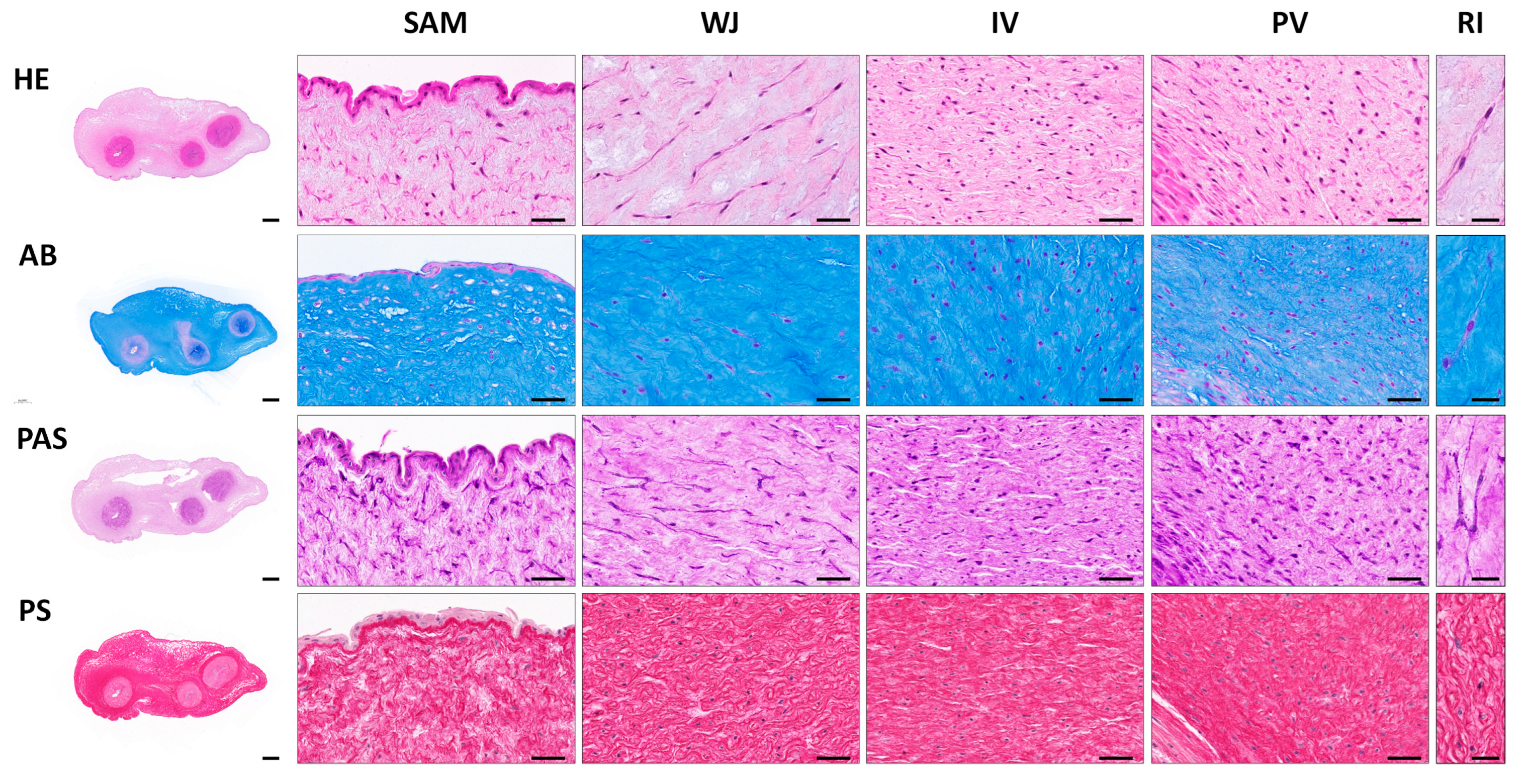
2.2. Histological Analyses
2.3. WJSC Isolation and Culture
2.4. Agarose Microchips and MT Generation
2.5. Ex Vivo 2D Cell Cultures and WJSC Derived MTs Histological Analyses
2.6. Ultrastructural Analyses
2.7. Statistical Analyses
3. Results
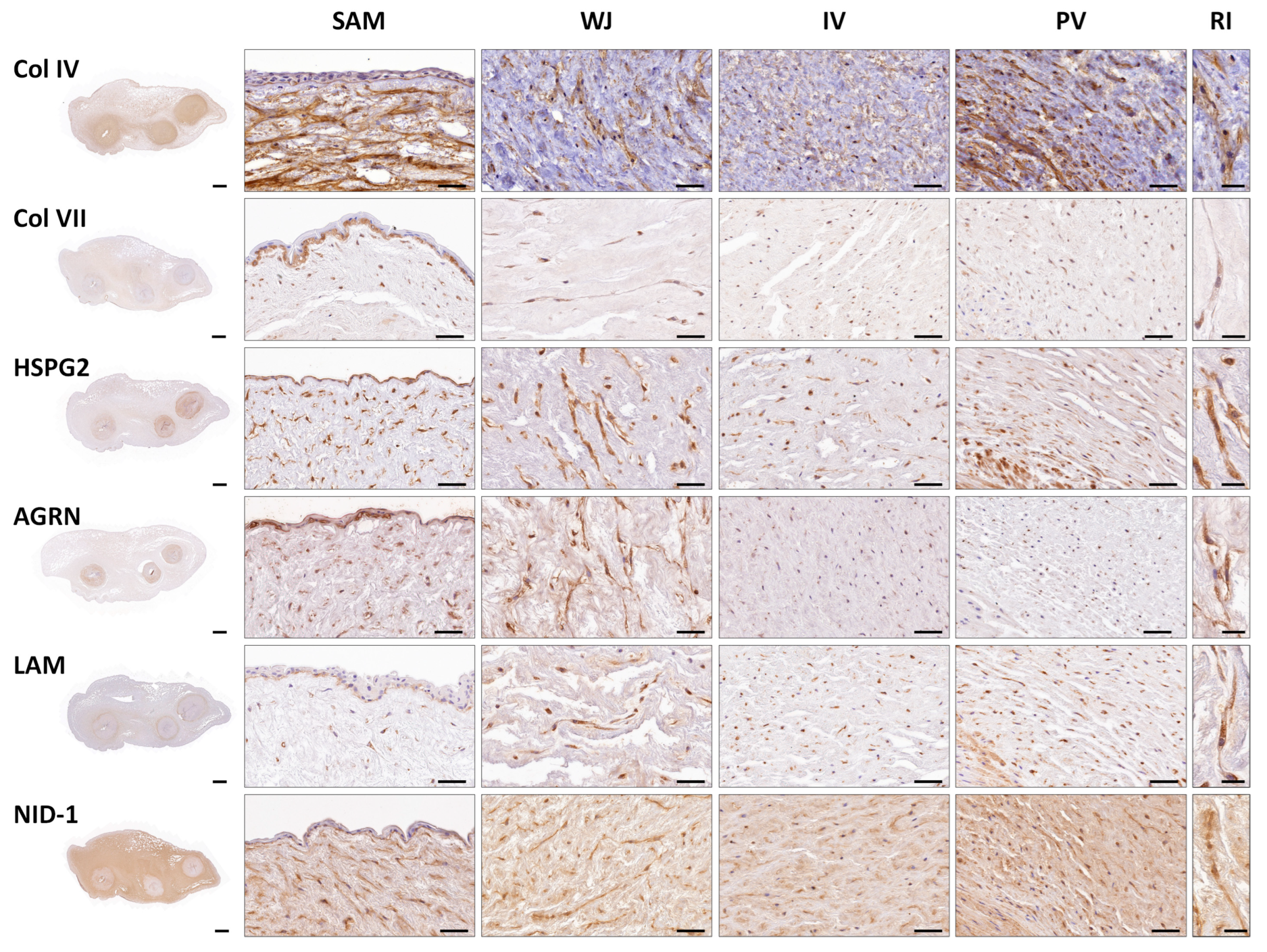
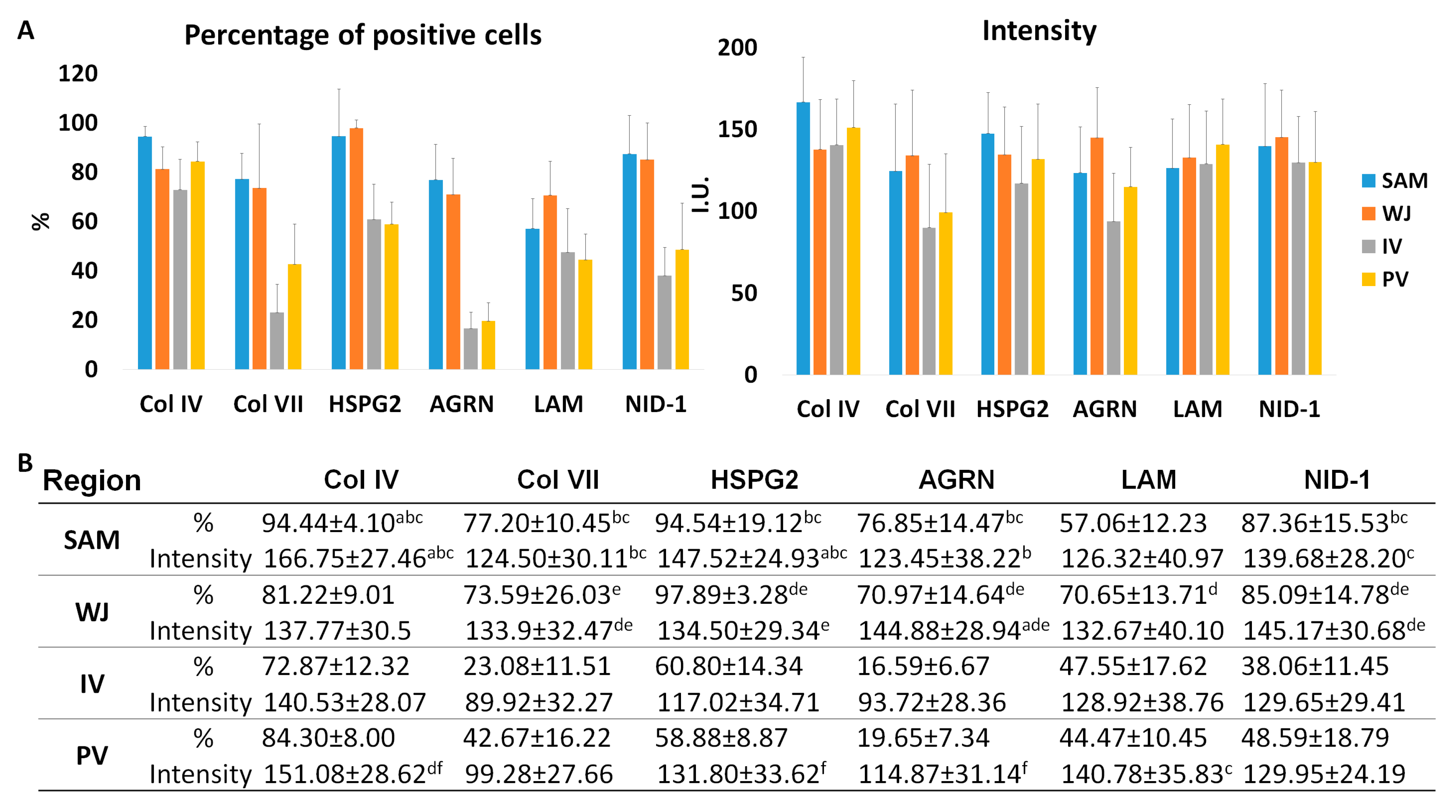
3.1. Histological, Histochemical and Immunohistochemical Results of Full-Term UCs
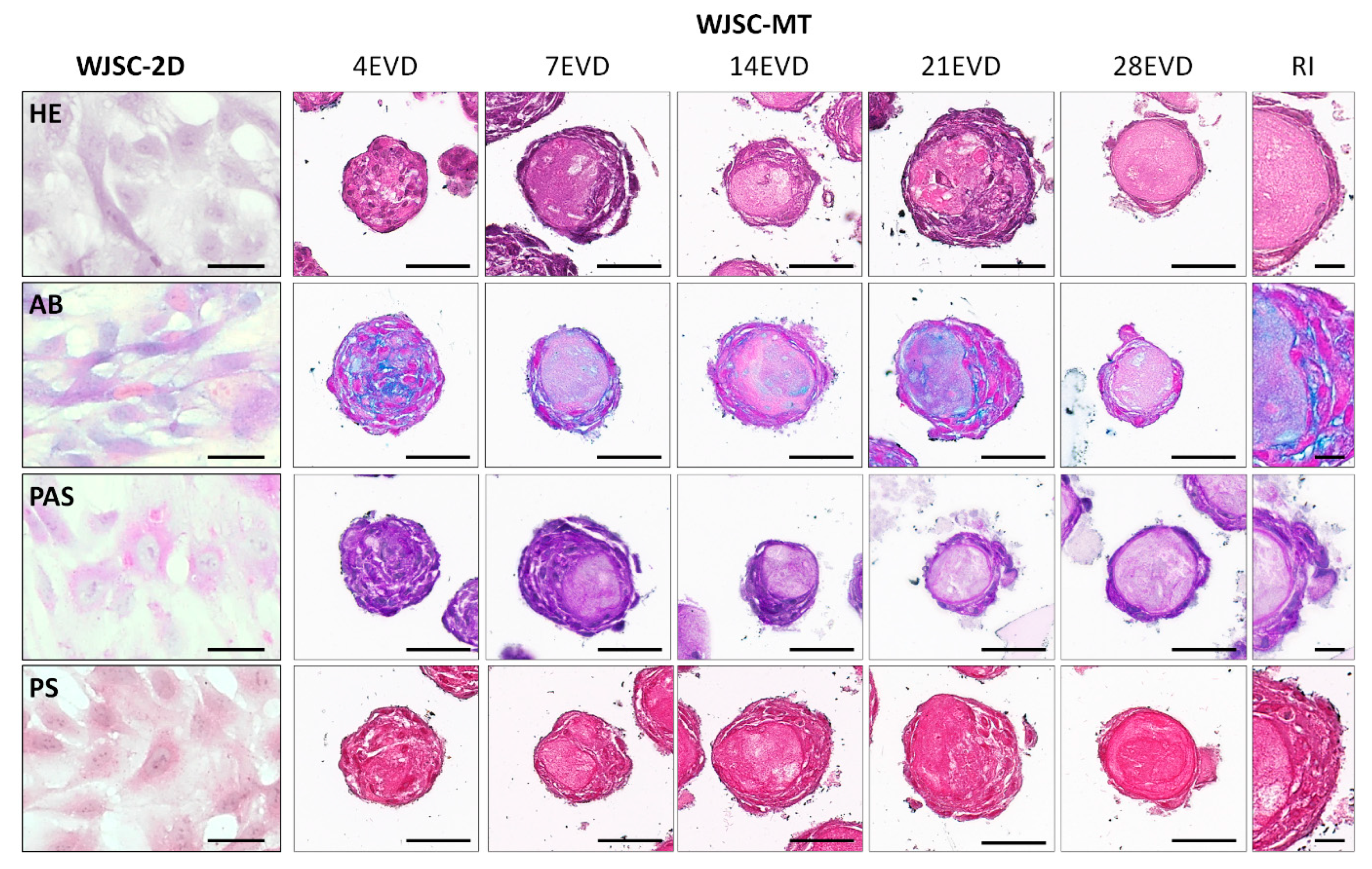
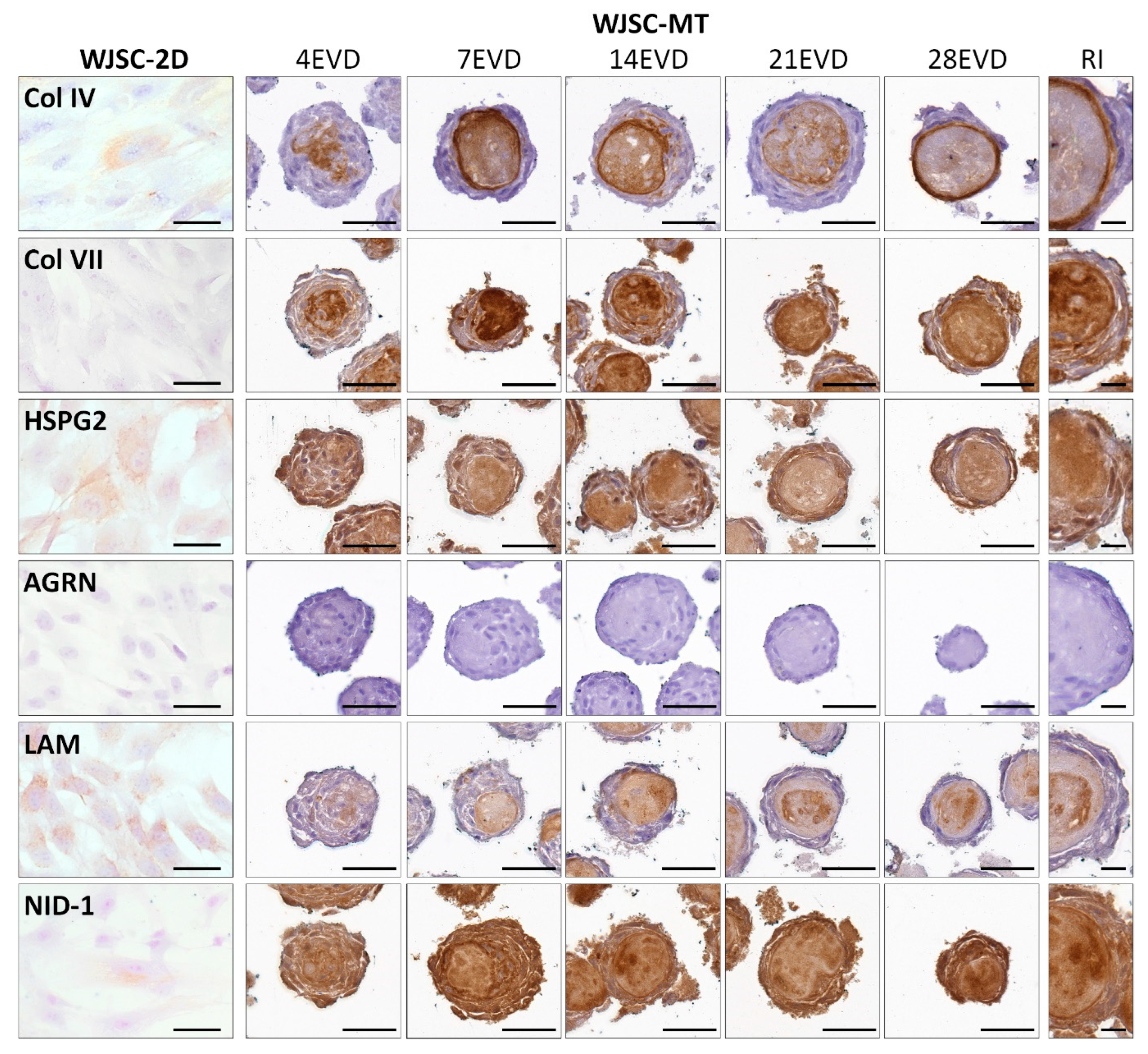
3.2. Ex Vivo 2D Cell Cultures and WJSC Derived MTs Histological Results
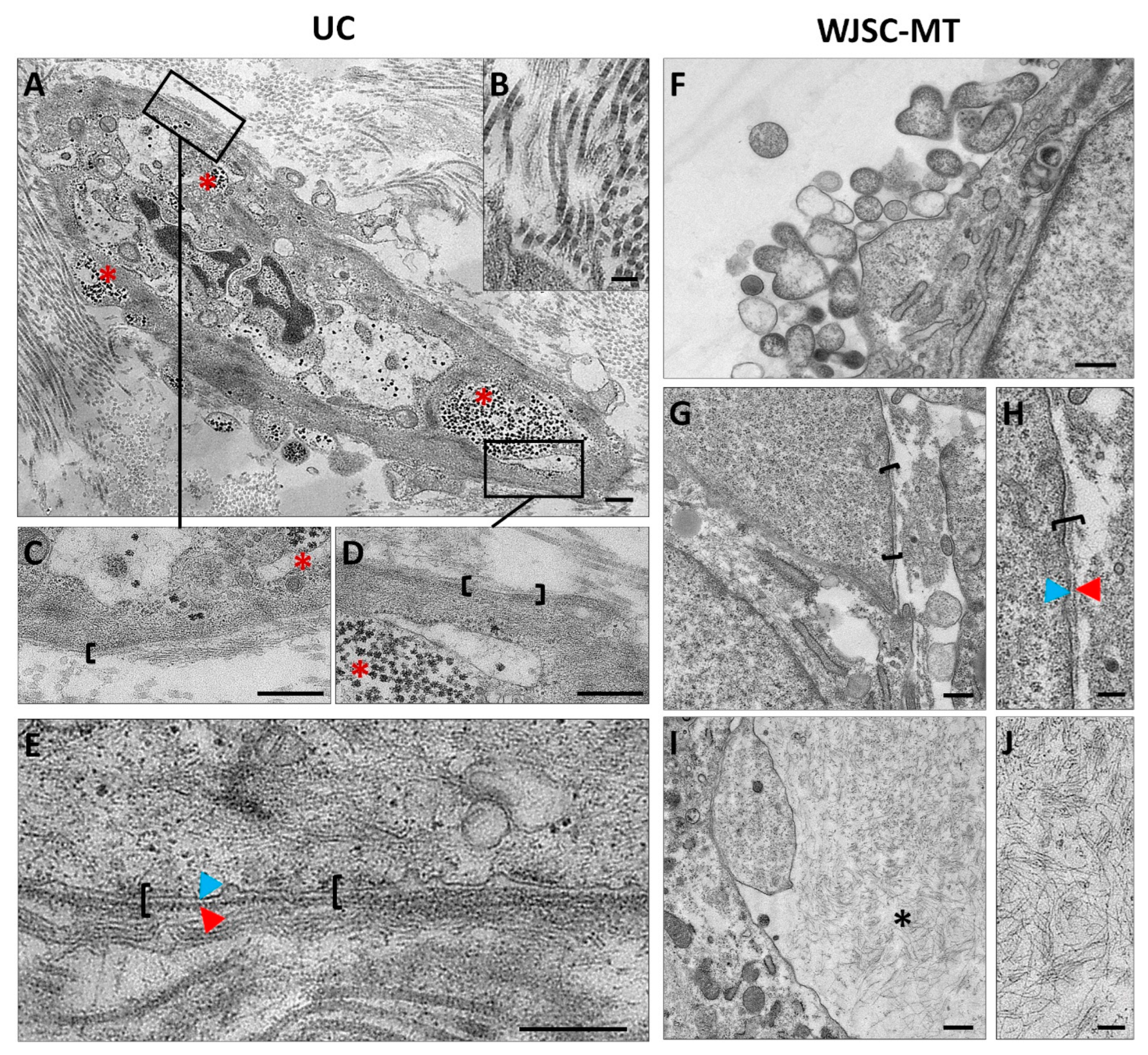
3.3. Ultrastructural Analyses of Full-Term UCs and WJSC-MT by Transmission Electron Microscopy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heil, J.R.; Bordoni, B. Embryology, Umbilical Cord; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Stefanska, K.; Ozegowska, K.; Hutchings, G.; Popis, M.; Moncrieff, L.; Dompe, C.; Janowicz, K.; Pienkowski, W.; Gutaj, P.; Shibli, J.A.; et al. Human Wharton’s Jelly-Cellular Specificity, Stemness Potency, Animal Models, and Current Application in Human Clinical Trials. J. Clin. Med. 2020, 9, 1102. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Russo, C.; Maugeri, G.; Musumeci, G.; Vicario, N.; Tibullo, D.; Giuffrida, R.; Parenti, R.; Lo Furno, D. Adult stem cell niches for tissue homeostasis. J. Cell. Physiol. 2022, 237, 239–257. [Google Scholar] [CrossRef]
- Carriel, V.; Alaminos, M.; Garzon, I.; Campos, A.; Cornelissen, M. Tissue engineering of the peripheral nervous system. Expert Rev. Neurother. 2014, 14, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Porras, D.; Caro-Magdaleno, M.; Gonzalez-Gallardo, C.; Garcia-Garcia, O.D.; Garzon, I.; Carriel, V.; Campos, F.; Alaminos, M. Generation of a Biomimetic Substitute of the Corneal Limbus Using Decellularized Scaffolds. Pharmaceutics 2021, 13, 1718. [Google Scholar] [CrossRef]
- Garzon, I.; Martin-Piedra, M.A.; Alfonso-Rodriguez, C.; Gonzalez-Andrades, M.; Carriel, V.; Martinez-Gomez, C.; Campos, A.; Alaminos, M. Generation of a biomimetic human artificial cornea model using Wharton’s jelly mesenchymal stem cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4073–4083. [Google Scholar] [CrossRef]
- Subramanian, A.; Fong, C.Y.; Biswas, A.; Bongso, A. Comparative Characterization of Cells from the Various Compartments of the Human Umbilical Cord Shows that the Wharton’s Jelly Compartment Provides the Best Source of Clinically Utilizable Mesenchymal Stem Cells. PLoS ONE 2015, 10, e0127992. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Redondo, P.A.; Pavlou, M.; Loizidou, M.; Cheema, U. Elements of the niche for adult stem cell expansion. J. Tissue Eng. 2017, 8, 2041731417725464. [Google Scholar] [CrossRef] [PubMed]
- Wagers, A.J. The stem cell niche in regenerative medicine. Cell Stem Cell 2012, 10, 362–369. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; Macdonald, B.; Kalluri, R. Structure and function of basement membranes. Exp. Biol. Med. 2007, 232, 1121–1129. [Google Scholar] [CrossRef]
- Kreis, T.; Vale, R. Guidebook to the Extracellular Matrix, Anchor, and Adhesion Proteins, 2nd ed.; Oxford University Press: Oxford, UK, 2011; Volume XIX, 568p. [Google Scholar]
- Yurchenco, P.D. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, A.; Orr, A.W.; Tzima, E.; Schwartz, M.A. Integrins in mechanotransduction. J. Biol. Chem. 2004, 279, 12001–12004. [Google Scholar] [CrossRef]
- Leblond, C.P.; Inoue, S. Structure, composition, and assembly of basement membrane. Am. J. Anat. 1989, 185, 367–390. [Google Scholar] [CrossRef] [PubMed]
- Court, F.A.; Wrabetz, L.; Feltri, M.L. Basal lamina: Schwann cells wrap to the rhythm of space-time. Curr. Opin. Neurobiol. 2006, 16, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Nanaev, A.K.; Kohnen, G.; Milovanov, A.P.; Domogatsky, S.P.; Kaufmann, P. Stromal differentiation and architecture of the human umbilical cord. Placenta 1997, 18, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Ryynanen, J.; Tan, E.M.; Hoffren, J.; Woodley, D.T.; Sollberg, S. Type VII collagen gene expression in human umbilical tissue and cells. Lab. Investig. 1993, 69, 300–304. [Google Scholar] [PubMed]
- Blanco-Elices, C.; Chato-Astrain, J.; Gonzalez-Gonzalez, A.; Sanchez-Porras, D.; Carriel, V.; Fernandez-Valades, R.; Sanchez-Quevedo, M.D.C.; Alaminos, M.; Garzon, I. Histological Profiling of the Human Umbilical Cord: A Potential Alternative Cell Source in Tissue Engineering. J. Pers. Med. 2022, 12, 648. [Google Scholar] [CrossRef]
- Liau, L.L.; Ruszymah, B.H.I.; Ng, M.H.; Law, J.X. Characteristics and clinical applications of Wharton’s jelly-derived mesenchymal stromal cells. Curr. Res. Transl. Med. 2020, 68, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.D.; Borlongan, C.V. Wharton’s jelly-derived mesenchymal stem cells: Phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int. J. Mol. Sci. 2013, 14, 11692–11712. [Google Scholar] [CrossRef]
- Abbaszadeh, H.; Ghorbani, F.; Derakhshani, M.; Movassaghpour, A.A.; Yousefi, M.; Talebi, M.; Shamsasenjan, K. Regenerative potential of Wharton’s jelly-derived mesenchymal stem cells: A new horizon of stem cell therapy. J. Cell. Physiol. 2020, 235, 9230–9240. [Google Scholar] [CrossRef]
- Jaimes-Parra, B.D.; Garzon, I.; Carriel, V.; Durand-Herrera, D.; Martin-Piedra, M.A.; Garcia, J.M.; Sanchez-Quevedo, M.C.; Alaminos, M.; Campos, A. Membranes derived from human umbilical cord Wharton’s jelly stem cells as novel bioengineered tissue-like constructs. Histol. Histopathol. 2018, 33, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Durand-Herrera, D.; Campos, F.; Jaimes-Parra, B.D.; Sanchez-Lopez, J.D.; Fernandez-Valades, R.; Alaminos, M.; Campos, A.; Carriel, V. Wharton’s jelly-derived mesenchymal cells as a new source for the generation of microtissues for tissue engineering applications. Histochem. Cell Biol. 2018, 150, 379–393. [Google Scholar] [CrossRef]
- Francesca Paris, P.M.; Pizzuti, V.; Marchionni, C.; Rossi, M.; Michelotti, M.; Petrovic, B.; Ciani, E.; Simonazzi, G.; Pession, A.; Bonsi, L.; et al. Characterization of Perinatal Stem Cell Spheroids for the Development of Cell Therapy Strategy. Bioengineering 2023, 10, 18. [Google Scholar]
- Chato-Astrain, J.; Philips, C.; Campos, F.; Durand-Herrera, D.; Garcia-Garcia, O.D.; Roosens, A.; Alaminos, M.; Campos, A.; Carriel, V. Detergent-based decellularized peripheral nerve allografts: An in vivo preclinical study in the rat sciatic nerve injury model. J. Tissue Eng. Regen. Med. 2020, 14, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Irastorza-Lorenzo, A.; Sanchez-Porras, D.; Ortiz-Arrabal, O.; de Frutos, M.J.; Esteban, E.; Fernandez, J.; Janer, A.; Campos, A.; Campos, F.; Alaminos, M. Evaluation of Marine Agarose Biomaterials for Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 1923. [Google Scholar] [CrossRef]
- Sanchez-Porras, D.; Bermejo-Casares, F.; Carmona, R.; Weiss, T.; Campos, F.; Carriel, V. Tissue Fixation and Processing for the Histological Identification of Lipids. Methods Mol. Biol. 2023, 2566, 175–186. [Google Scholar] [CrossRef]
- Garcia-Garcia, O.D.; El Soury, M.; Gonzalez-Quevedo, D.; Sanchez-Porras, D.; Chato-Astrain, J.; Campos, F.; Carriel, V. Histological, Biomechanical, and Biological Properties of Genipin-Crosslinked Decellularized Peripheral Nerves. Int. J. Mol. Sci. 2021, 22, 674. [Google Scholar] [CrossRef]
- Sanchez-Porras, D.; Varas, J.; Godoy-Guzman, C.; Bermejo-Casares, F.; San Martin, S.; Carriel, V. Histochemical and Immunohistochemical Methods for the Identification of Proteoglycans. Methods Mol. Biol. 2023, 2566, 85–98. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Garzon, I.; Ionescu, A.M.; Carriel, V.; Cardona Jde, L.; Gonzalez-Andrades, M.; Perez Mdel, M.; Alaminos, M.; Campos, A. Evaluation of small intestine grafts decellularization methods for corneal tissue engineering. PLoS ONE 2013, 8, e66538. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Sanchez-Porras, D.; Durand-Herrera, D.; Paes, A.B.; Chato-Astrain, J.; Verplancke, R.; Vanfleteren, J.; Sanchez-Lopez, J.D.; Garcia-Garcia, O.D.; Campos, F.; Carriel, V. Ex Vivo Generation and Characterization of Human Hyaline and Elastic Cartilaginous Microtissues for Tissue Engineering Applications. Biomedicines 2021, 9, 292. [Google Scholar] [CrossRef]
- Carriel, V.; Garzon, I.; Campos, A.; Cornelissen, M.; Alaminos, M. Differential expression of GAP-43 and neurofilament during peripheral nerve regeneration through bio-artificial conduits. J. Tissue Eng. Regen. Med. 2017, 11, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Gogiel, T.; Bankowski, E.; Jaworski, S. Proteoglycans of Wharton’s jelly. Int. J. Biochem. Cell Biol. 2003, 35, 1461–1469. [Google Scholar] [CrossRef]
- Mills, S.E. Histology for Pathologists, 5th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2020; Volume XVI, 1320p. [Google Scholar]
- Brunelli, R.; De Spirito, M.; Giancotti, A.; Palmieri, V.; Parasassi, T.; Di Mascio, D.; Flammini, G.; D’Ambrosio, V.; Monti, M.; Boccaccio, A.; et al. The biomechanics of the umbilical cord Wharton Jelly: Roles in hemodynamic proficiency and resistance to compression. J. Mech. Behav. Biomed. Mater. 2019, 100, 103377. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Ruan, X.Z.; Zhang, H.M.; Zeng, Y.J. Biomechanical properties of different segments of human umbilical cord vein and its value for clinical application. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Jimenez, F.J.; Carriel, V.; Santos-Mateo, J.J.; Fernandez, A.; Garcia-Hernandez, S.; Ramos, K.A.; Piqueras-Flores, J.; Cabrera-Romero, E.; Barriales-Villa, R.; de la Higuera Romero, L.; et al. ROD2 domain filamin C missense mutations exhibit a distinctive cardiac phenotype with restrictive/hypertrophic cardiomyopathy and saw-tooth myocardium. Rev. Esp. Cardiol. 2022, in press. [Google Scholar] [CrossRef]
- Gervaso, F.; Boschetti, F.; Pennati, G. Evaluation of the Wharton’s jelly poroelastic parameters through compressive tests on placental and foetal ends of human umbilical cords. J. Mech. Behav. Biomed. Mater. 2014, 35, 51–58. [Google Scholar] [CrossRef]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef]
- Hayes, A.J.; Farrugia, B.L.; Biose, I.J.; Bix, G.J.; Melrose, J. Perlecan, a Multi-Functional, Cell-Instructive, Matrix-Stabilizing Proteoglycan with Roles in Tissue Development Has Relevance to Connective Tissue Repair and Regeneration. Front. Cell Dev. Biol. 2022, 10, 856261. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. J. Int. Soc. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Carriel, V.; Garzon, I.; Jimenez, J.M.; Oliveira, A.C.; Arias-Santiago, S.; Campos, A.; Sanchez-Quevedo, M.C.; Alaminos, M. Epithelial and stromal developmental patterns in a novel substitute of the human skin generated with fibrin-agarose biomaterials. Cells Tissues Organs 2012, 196, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Linares-Gonzalez, L.; Rodenas-Herranz, T.; Campos, F.; Ruiz-Villaverde, R.; Carriel, V. Basic Quality Controls Used in Skin Tissue Engineering. Life 2021, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Bonhome-Espinosa, A.B.; Campos, F.; Durand-Herrera, D.; Sanchez-Lopez, J.D.; Schaub, S.; Duran, J.D.G.; Lopez-Lopez, M.T.; Carriel, V. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103619. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, L.; Campos, F.; Godoy-Guzman, C.; Del Carmen Sanchez-Quevedo, M.; Garzon, I.; Alaminos, M.; Campos, A.; Carriel, V. Encapsulation of human elastic cartilage-derived chondrocytes in nanostructured fibrin-agarose hydrogels. Histochem. Cell Biol. 2017, 147, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, A.E.; Bening, M.R.; Pherribo, G.; Dauer, E.A.; Oudega, M. Laminin polymer treatment accelerates repair of the crushed peripheral nerve in adult rats. Acta Biomater. 2019, 86, 185–193. [Google Scholar] [CrossRef]
- Carriel, V.; Garrido-Gomez, J.; Hernandez-Cortes, P.; Garzon, I.; Garcia-Garcia, S.; Saez-Moreno, J.A.; Del Carmen Sanchez-Quevedo, M.; Campos, A.; Alaminos, M. Combination of fibrin-agarose hydrogels and adipose-derived mesenchymal stem cells for peripheral nerve regeneration. J. Neural Eng. 2013, 10, 026022. [Google Scholar] [CrossRef]
- Zhang, Z. Chondrons and the pericellular matrix of chondrocytes. Tissue Eng. Part B Rev. 2015, 21, 267–277. [Google Scholar] [CrossRef]
- Chu, W.C.; Zhang, S.; Sng, T.J.; Ong, Y.J.; Tan, W.L.; Ang, V.Y.; Foldager, C.B.; Toh, W.S. Distribution of pericellular matrix molecules in the temporomandibular joint and their chondroprotective effects against inflammation. Int. J. Oral Sci. 2017, 9, 43–52. [Google Scholar] [CrossRef]
- Wilusz, R.E.; Sanchez-Adams, J.; Guilak, F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. J. Int. Soc. Matrix Biol. 2014, 39, 25–32. [Google Scholar] [CrossRef]
- Foldager, C.B.; Toh, W.S.; Christensen, B.B.; Lind, M.; Gomoll, A.H.; Spector, M. Collagen Type IV and Laminin Expressions during Cartilage Repair and in Late Clinically Failed Repair Tissues from Human Subjects. Cartilage 2016, 7, 52–61. [Google Scholar] [CrossRef]
- SundarRaj, N.; Fite, D.; Ledbetter, S.; Chakravarti, S.; Hassell, J.R. Perlecan is a component of cartilage matrix and promotes chondrocyte attachment. J. Cell Sci. 1995, 108 Pt 7, 2663–2672. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Dilution/Incubation | Pretreatment | Reference |
|---|---|---|---|
| Mouse monoclonal anti-collagen type IV | Prediluted Overnight at 4 °C | EDTA buffer pH 8 20 min at 95 °C | Master Diagnóstica (Granada, Spain) (Cat. nº: MAD001060QD) |
| Rabbit polyclonal anti-collagen type VII | 1:50 Overnight at 4 °C | EDTA buffer pH 8 20 min at 95 °C | Novus Biological (Englewood, CO, USA) (Cat. nº: NBP2-37900) |
| Rabbit polyclonal anti-HSPG2 | 1:250 Overnight at 4 °C | EDTA buffer pH 8 20 min at 95 °C | Abbexa (Cambridge Science Park, UK) (Cat. nº: abx103270) |
| Rabbit polyclonal anti-agrin | 1:500 Overnight at 4 °C | Citrate buffer pH 6 20 min at 95 °C | Abbexa (Minneapolis, MN, USA) (Cat. nº: abx037897) |
| Rabbit polyclonal anti-laminin | 1:250 Overnight at 4 °C | Citrate buffer pH 6 20 min at 95 °C | Abcam (Bristol, UK) (Cat. nº: ab11575) |
| Goat polyclonal anti-nidogen/entactin-1 | 1:30 Overnight at 4 °C | EDTA buffer pH 8 20 min at 95 °C | R&D Systems (Minneapolis, MN, USA) (Cat. nº: AF2570) |
| ImmPRESS® HRPAnti-Mouse IgG (Peroxidase) | 1 h at RT | - | Vector Laboratories (Burlingame, CA, USA) (Cat. nº: MP-7401) |
| ImmPRESS® HRP Anti-Rabbit IgG (Peroxidase) | 1 h at RT | - | Vector Laboratories. (Burlingame, CA, USA) (Cat. nº: MP-7402) |
| ImmPRESS® HRP Anti-Goat IgG (Peroxidase) | 1 h at RT | - | Vector Laboratories. (Burlingame, CA, USA) (Cat. nº: MP-7405) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Porras, D.; Durand-Herrera, D.; Carmona, R.; Blanco-Elices, C.; Garzón, I.; Pozzobon, M.; San Martín, S.; Alaminos, M.; García-García, Ó.D.; Chato-Astrain, J.; et al. Expression of Basement Membrane Molecules by Wharton Jelly Stem Cells (WJSC) in Full-Term Human Umbilical Cords, Cell Cultures and Microtissues. Cells 2023, 12, 629. https://doi.org/10.3390/cells12040629
Sánchez-Porras D, Durand-Herrera D, Carmona R, Blanco-Elices C, Garzón I, Pozzobon M, San Martín S, Alaminos M, García-García ÓD, Chato-Astrain J, et al. Expression of Basement Membrane Molecules by Wharton Jelly Stem Cells (WJSC) in Full-Term Human Umbilical Cords, Cell Cultures and Microtissues. Cells. 2023; 12(4):629. https://doi.org/10.3390/cells12040629
Chicago/Turabian StyleSánchez-Porras, David, Daniel Durand-Herrera, Ramón Carmona, Cristina Blanco-Elices, Ingrid Garzón, Michela Pozzobon, Sebastián San Martín, Miguel Alaminos, Óscar Darío García-García, Jesús Chato-Astrain, and et al. 2023. "Expression of Basement Membrane Molecules by Wharton Jelly Stem Cells (WJSC) in Full-Term Human Umbilical Cords, Cell Cultures and Microtissues" Cells 12, no. 4: 629. https://doi.org/10.3390/cells12040629
APA StyleSánchez-Porras, D., Durand-Herrera, D., Carmona, R., Blanco-Elices, C., Garzón, I., Pozzobon, M., San Martín, S., Alaminos, M., García-García, Ó. D., Chato-Astrain, J., & Carriel, V. (2023). Expression of Basement Membrane Molecules by Wharton Jelly Stem Cells (WJSC) in Full-Term Human Umbilical Cords, Cell Cultures and Microtissues. Cells, 12(4), 629. https://doi.org/10.3390/cells12040629









