Development and In Vitro/In Vivo Comparative Characterization of Cryopreserved and Decellularized Tracheal Grafts
Abstract
1. Introduction
2. Materials and Methods
2.1. Tracheal Isolation
2.2. Tracheal Cryopreservation
2.3. Tracheal Decellularization
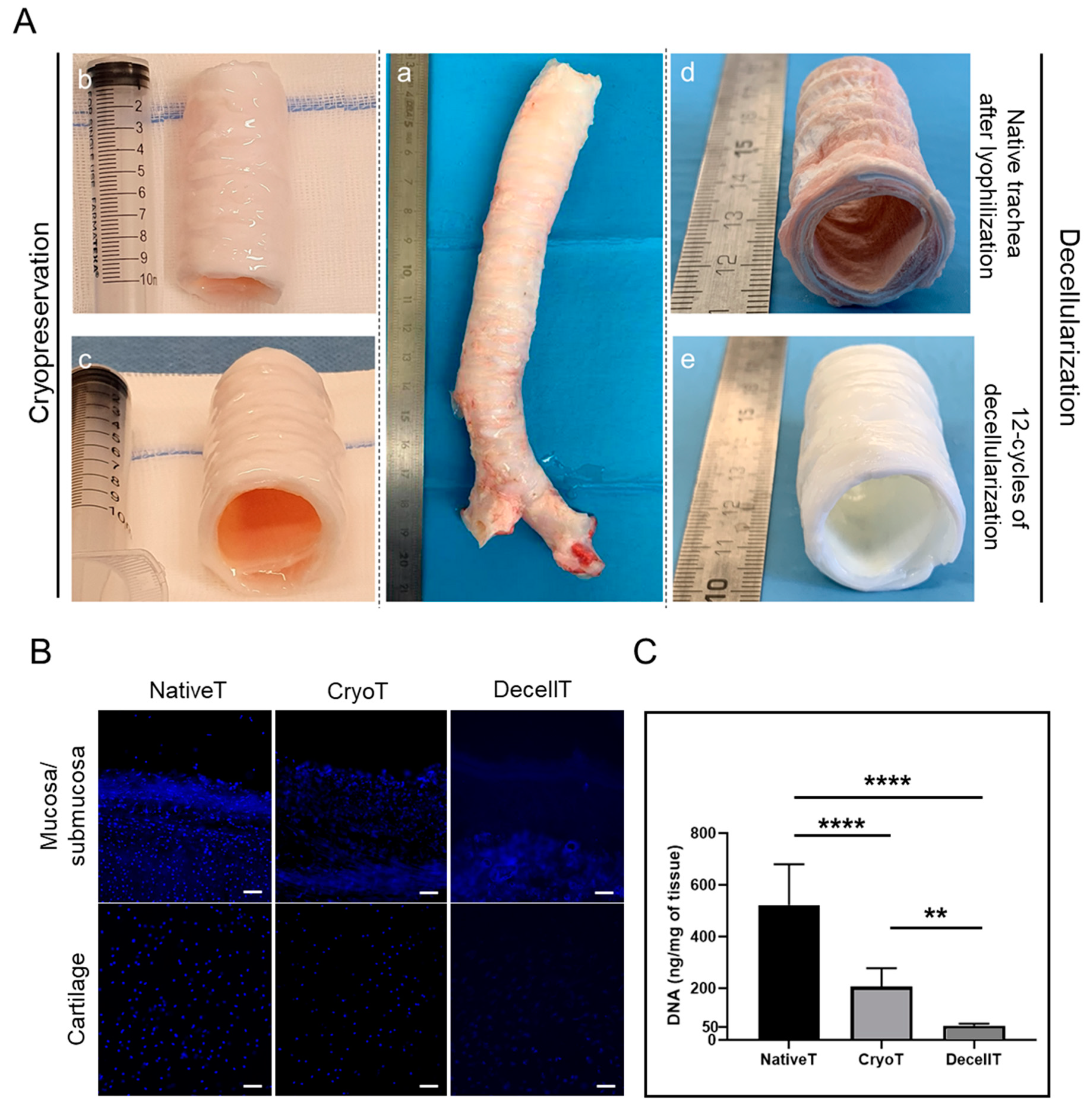
2.4. 4′,6-Diamidino-2-Phenylindole Nuclear Staining
2.5. DNA Extraction and Quantification
2.6. Alpha-Gal Epitope Detection
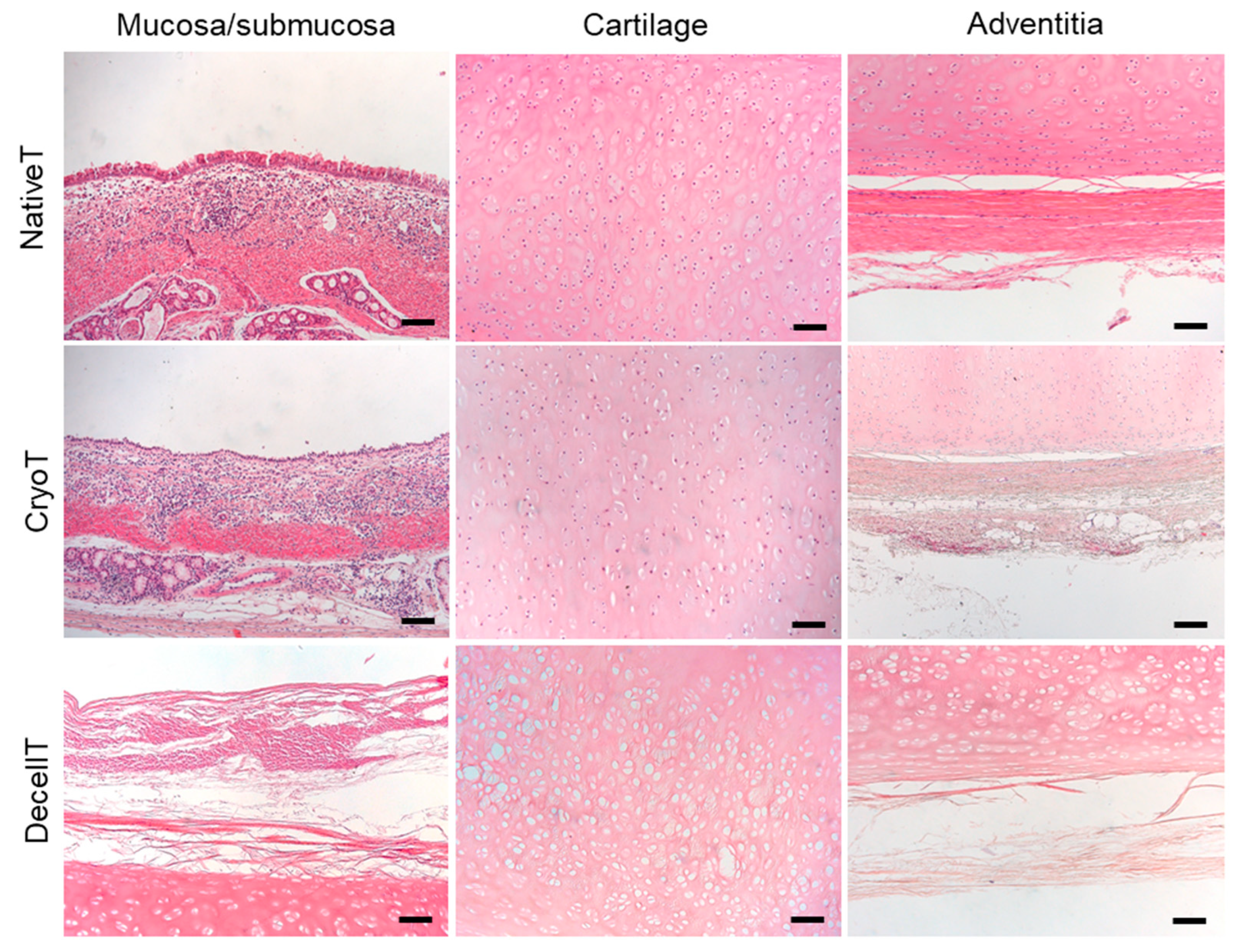
2.7. Histological Analyses
2.7.1. Morphometric Analyses
2.8. Biochemical Assay for Glycosaminoglycans Quantification
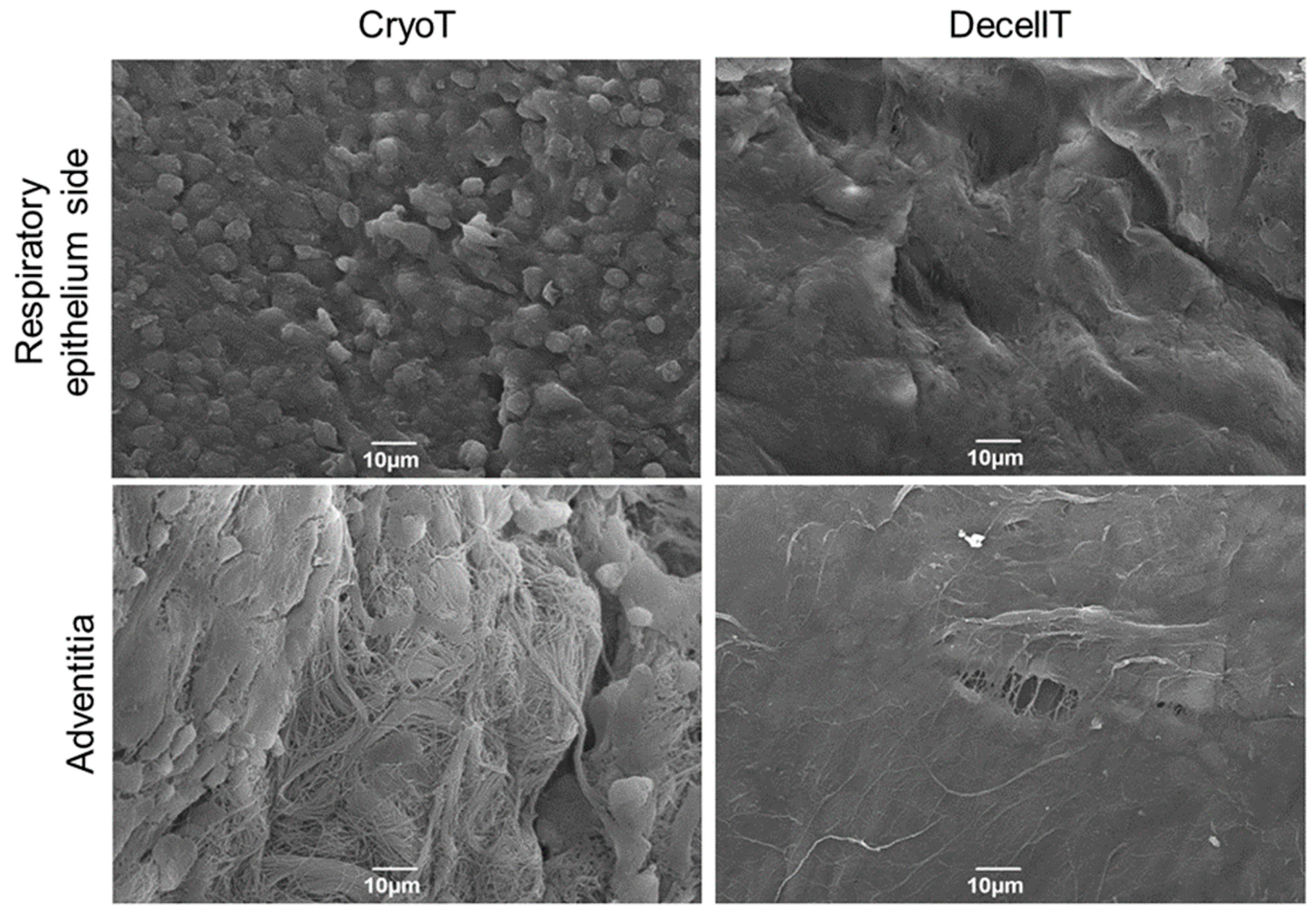
2.9. Scanning Electron Microscopy
2.10. Two Photon Microscopy
2.11. Compressive Mechanical Tests
2.12. Tissue Cytotoxicity and Biocompatibility
2.12.1. In Vitro Cytotoxicity
2.12.2. Cell–Scaffold Interaction
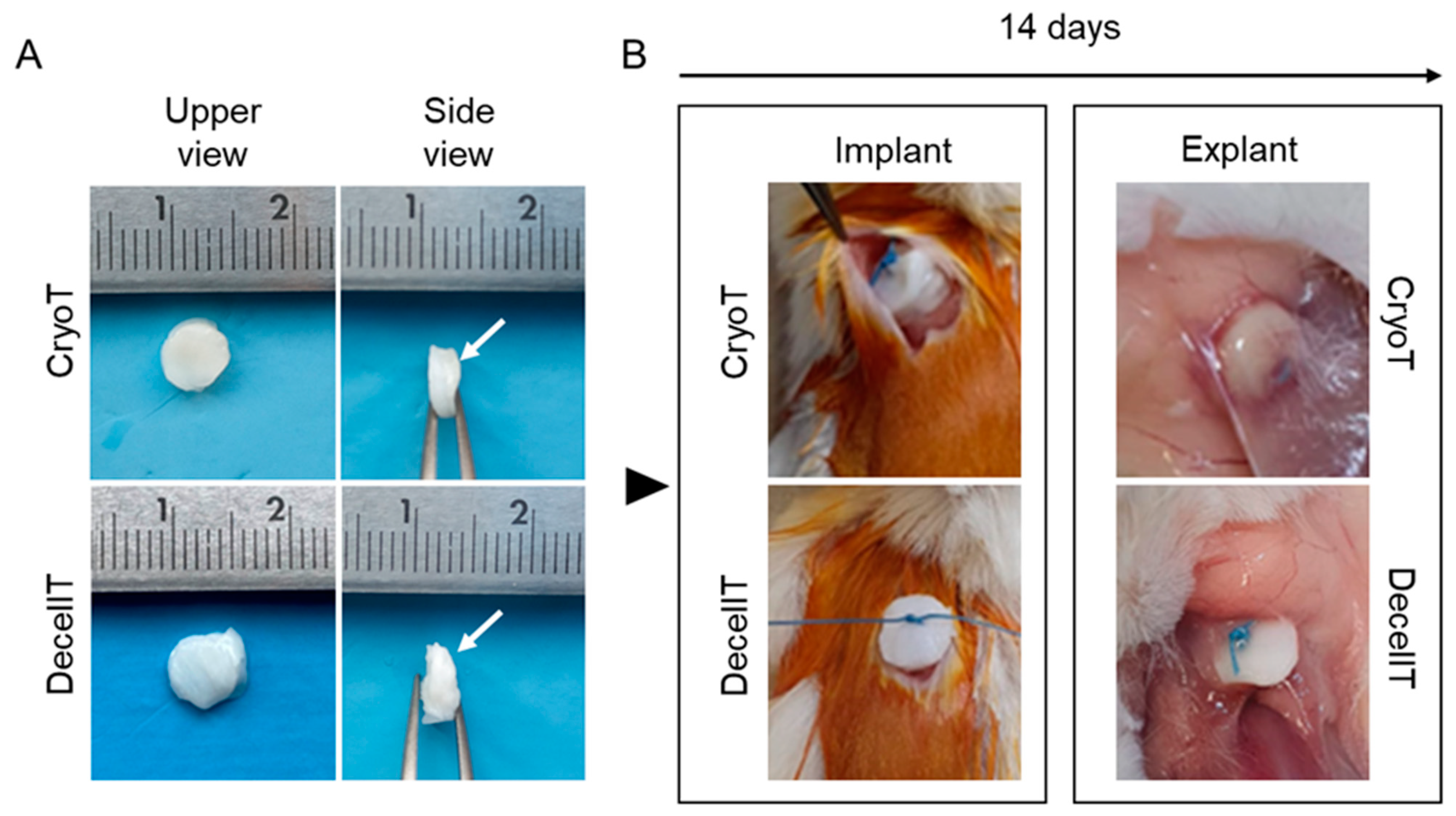
2.12.3. In Vivo Biocompatibility
- Surgery
- Explants characterization
2.13. Statistical Analysis
3. Results
3.1. Gross Appearance, Nuclear Staining, and DNA Content
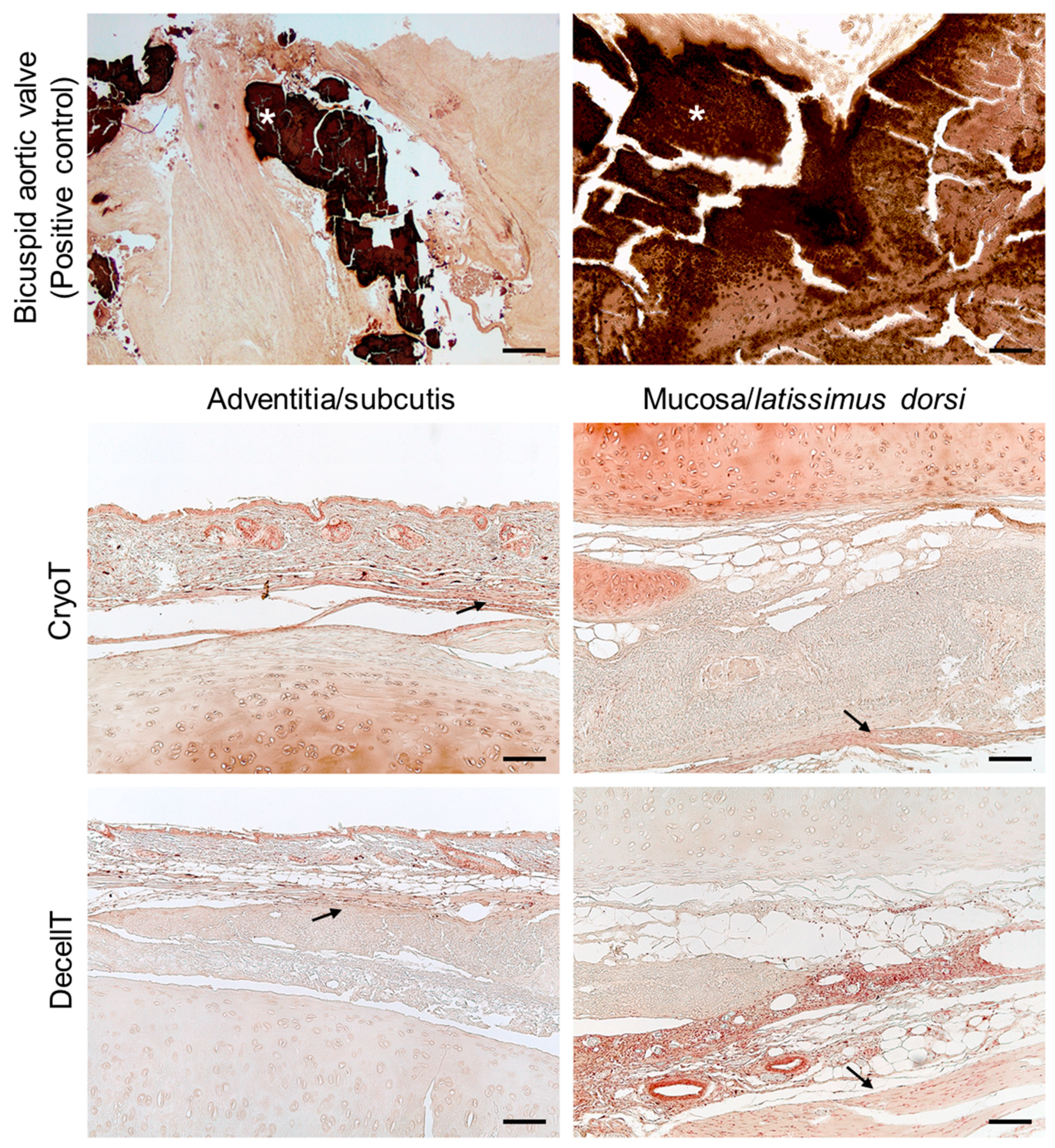
3.2. Immunolocalization of Alpha-Gal Epitopes
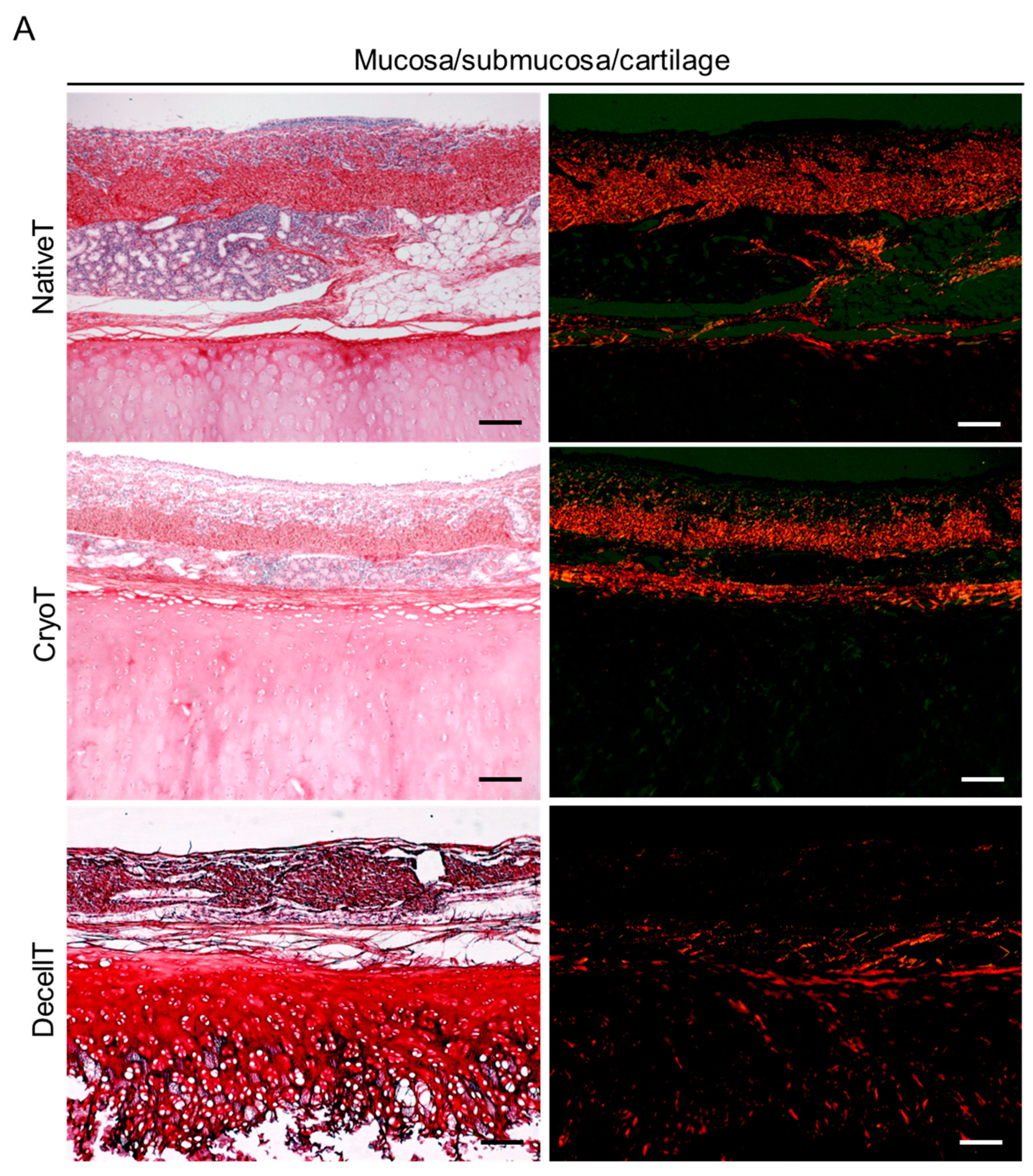
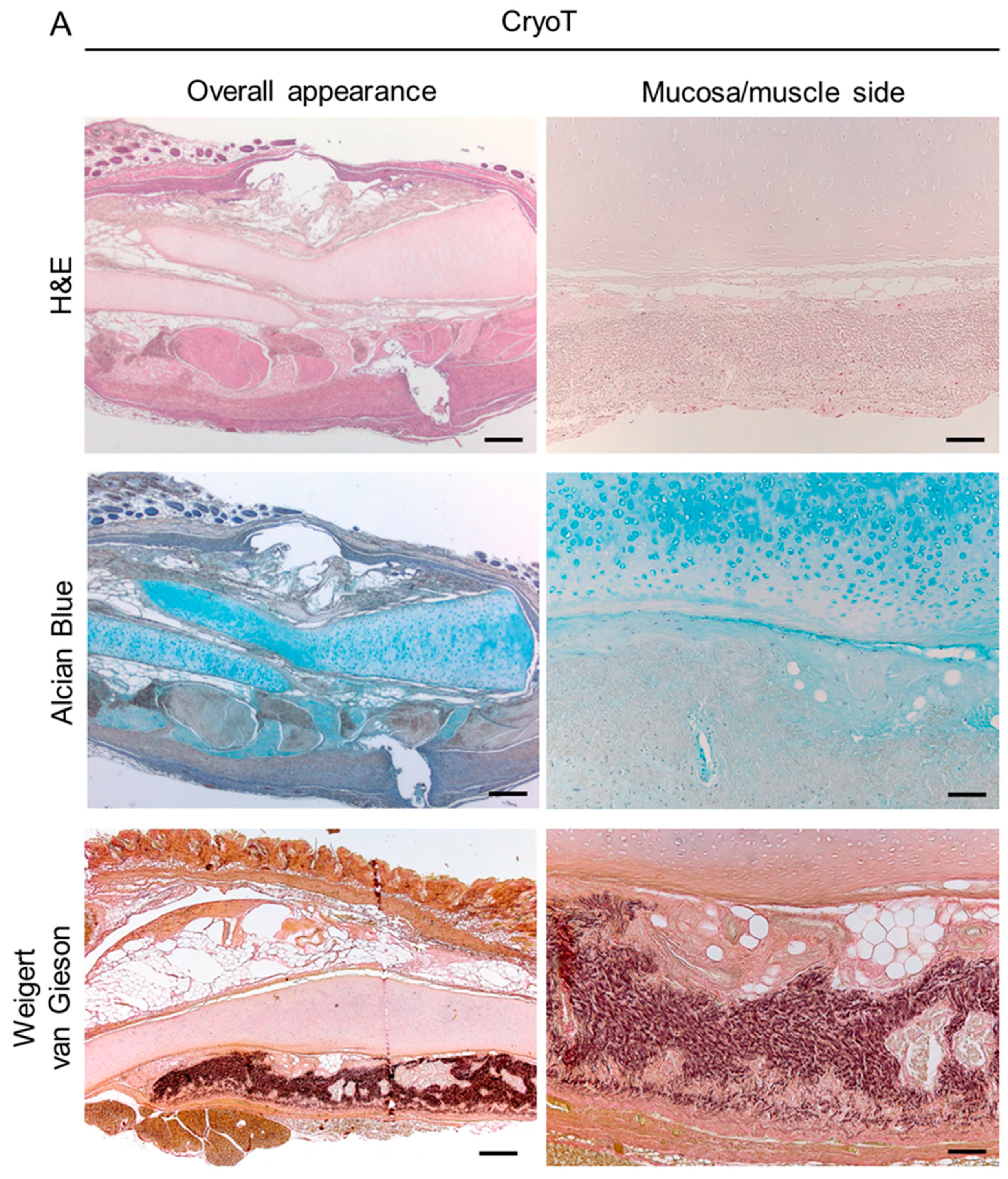
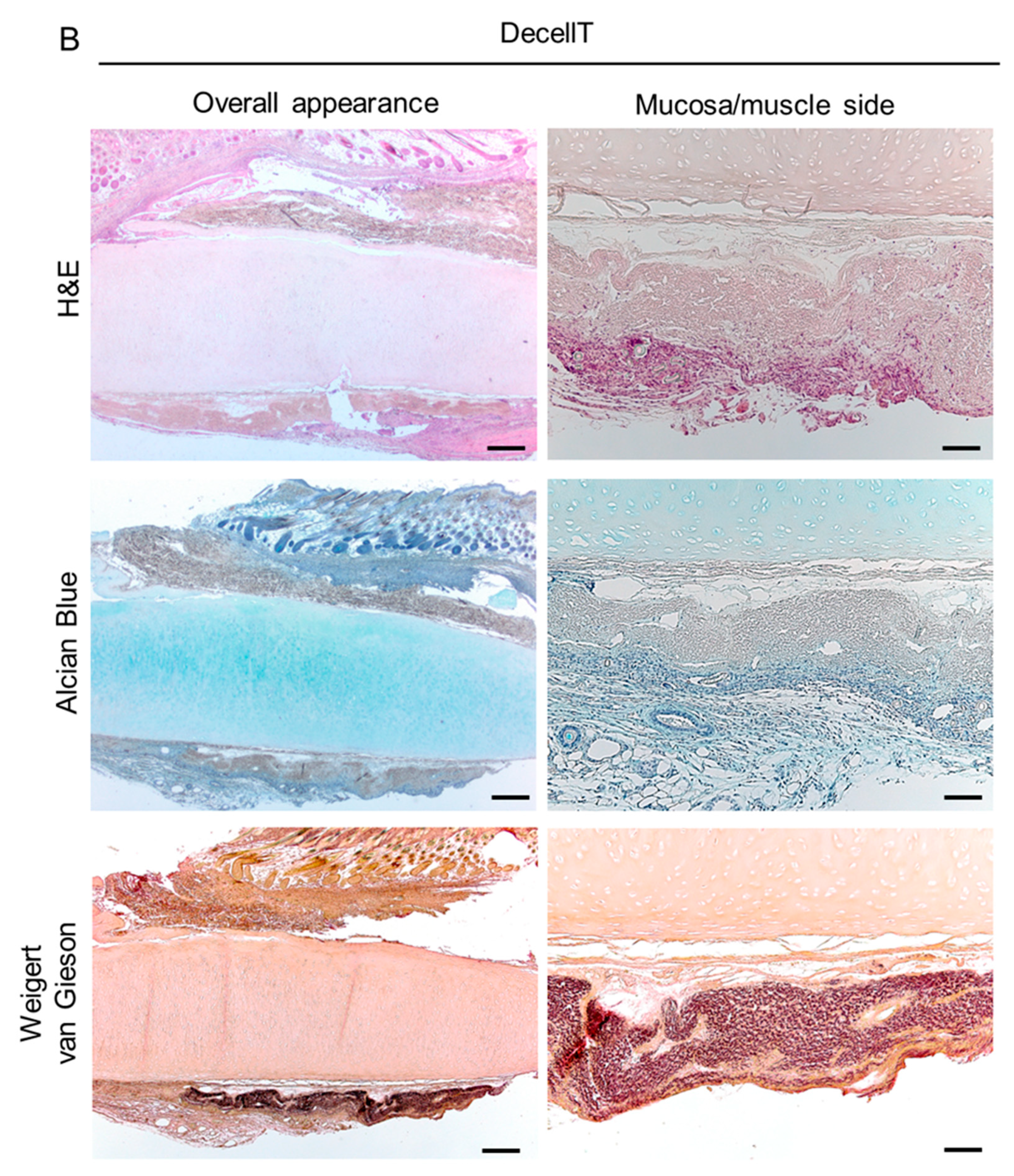
3.3. Histological and Biochemical Analyses
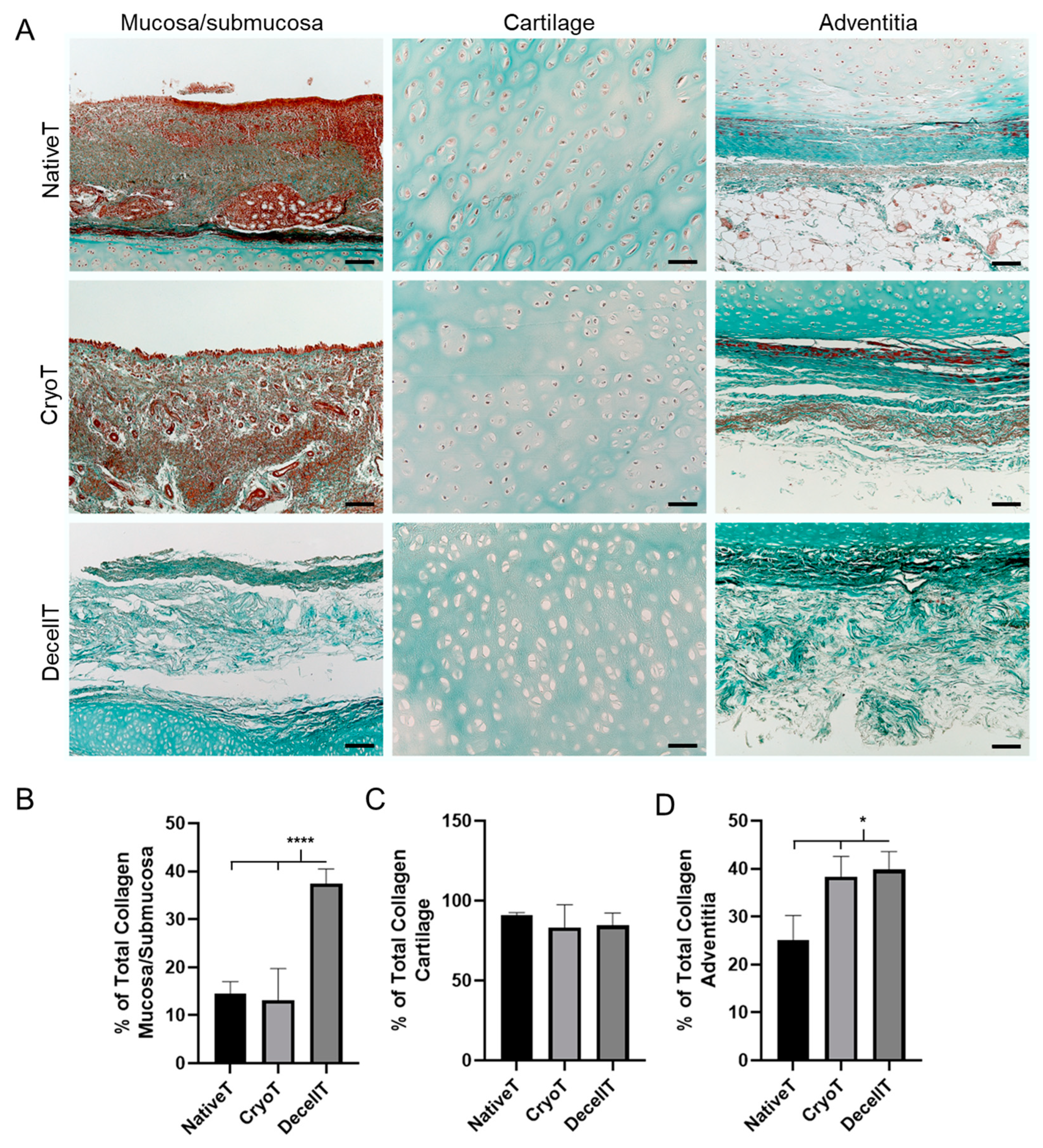
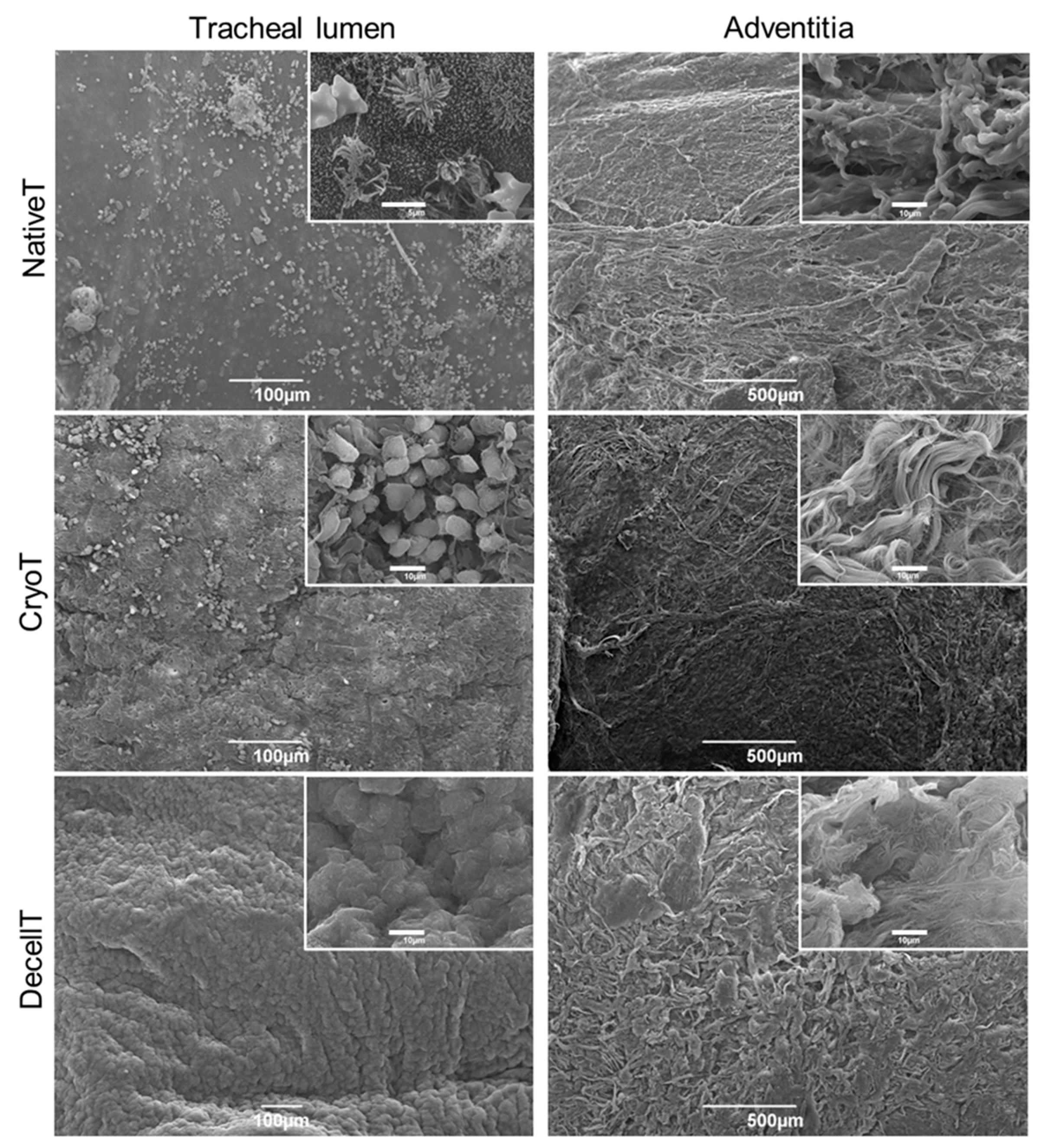
3.4. Ultrastructure and Collagen Fibers Organization
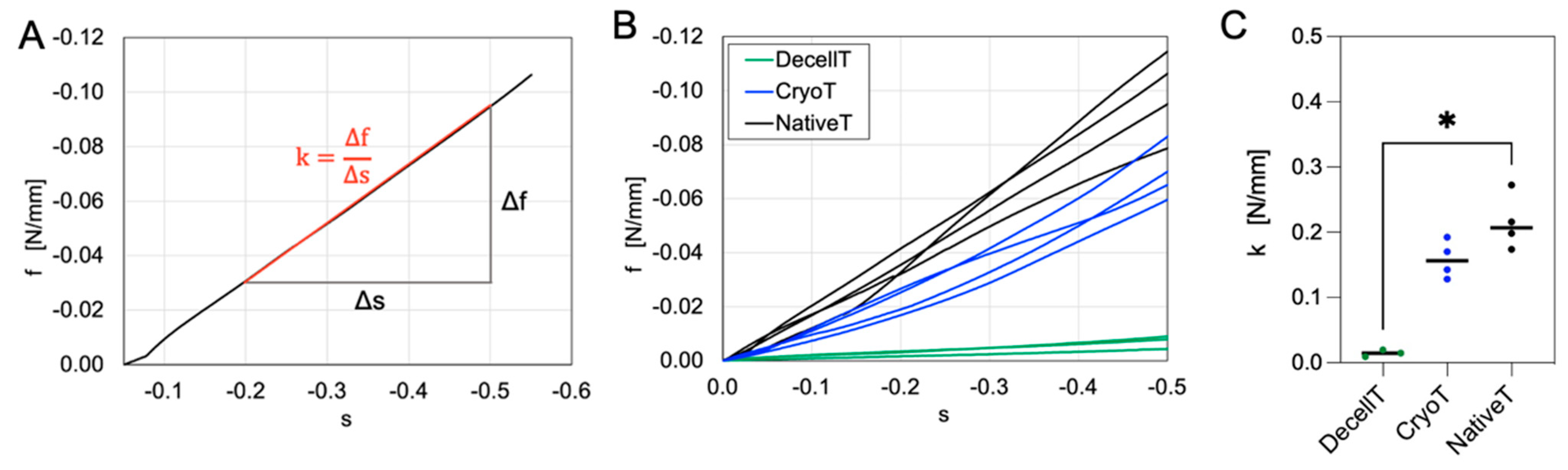
3.5. Compressive Mechanical Behavior
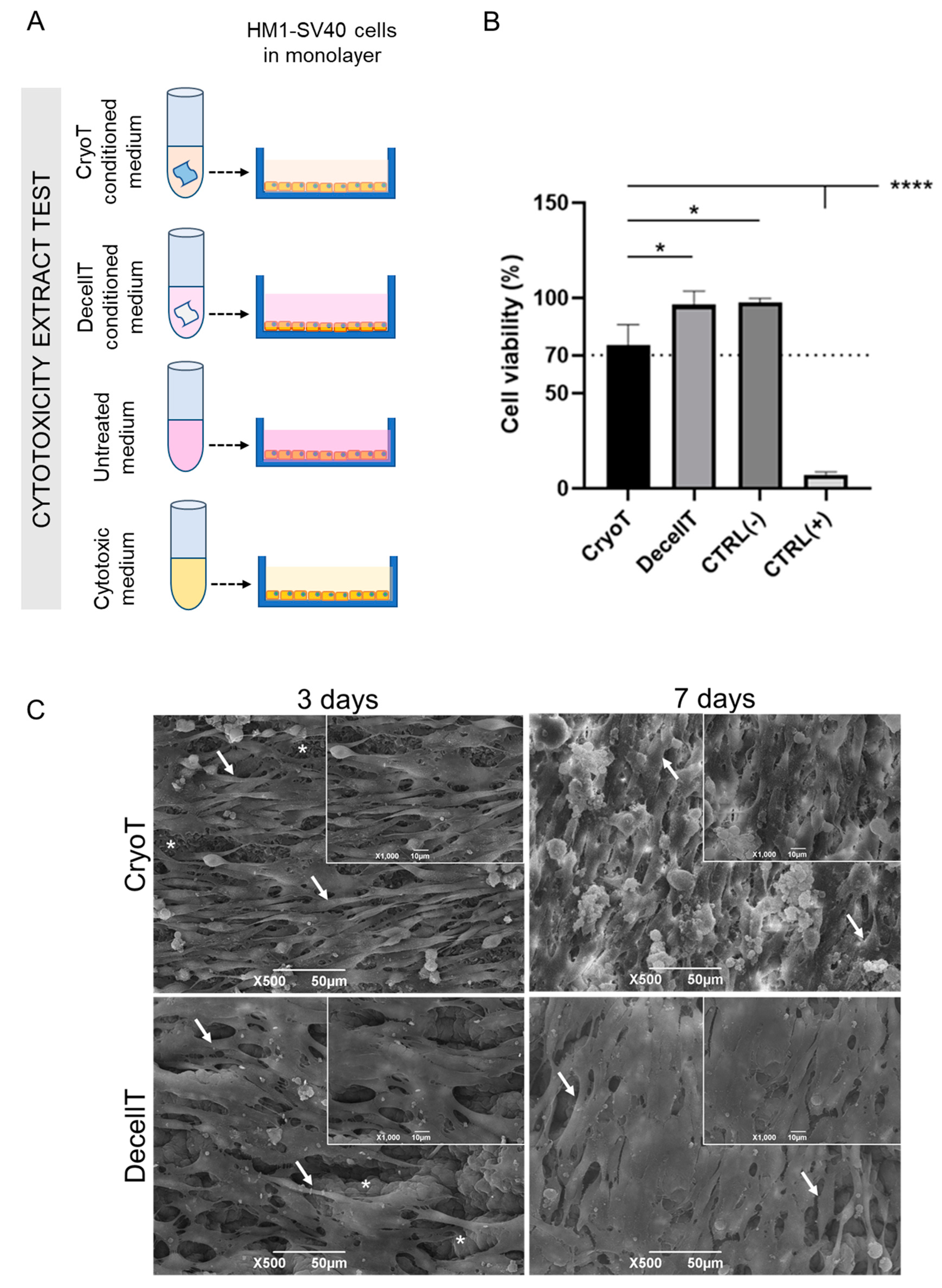
3.6. Cytocompatibility Assessment In Vitro
3.7. Biocompatibility Assessment In Vivo and Explants Characterization
4. Discussion
4.1. Trachea Substitutes Development
4.2. Cryopreservation and Decellularization Decrease Immunogenicity
4.3. ECM and Structural Proteins Content Modification
4.4. Compressive Mechanical Properties
4.5. Confirmation of Tracheal Substitutes Cytocompatibility and Biocompatibility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grillo, H.C. Tracheal replacement: A critical review. Ann. Thorac. Surg. 2002, 73, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, F.; Lu, Y.; Zhang, B.; Zhang, G.; Shi, H. Rapid Preparation Method for Preparing Tracheal Decellularized Scaffolds: Vacuum Assistance and Optimization of DNase I. ACS Omega 2021, 6, 10637–10644. [Google Scholar] [CrossRef]
- Belsey, R. Resection and reconstruction of the intrathoracic trachea. Br. J. Surg. 1950, 38, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sundaram, S.; Le, A.V.; Huang, A.H.; Zhang, J.; Hatachi, G.; Beloiartsev, A.; Caty, M.G.; Yi, T.; Leiby, K.; et al. Engineered Tissue–Stent Biocomposites as Tracheal Replacements. Tissue Eng. Part A 2016, 22, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Lu, Y.; Li, J.; Shi, H. The biological properties of the decellularized tracheal scaffolds and 3D printing biomimetic materials: A comparative study. J. Biomed. Mater. Res. Part A 2022, 110, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, F.; Lu, Y.; Wang, Z.; Shen, Z.; Yuan, L.; Wu, Q.; Wu, C.; Shi, H. A novel decellularized trachea preparation method for the rapid construction of a functional tissue engineered trachea to repair tracheal defects. J. Mater. Chem. B 2022, 10, 4810–4822. [Google Scholar] [CrossRef]
- Sotres-Vega, A.; Villalba-Caloca, J.; Jasso-Victoria, R.; Olmos-Zúñiga, J.R.; Gaxiola-Gaxiola, M.; Baltazares-Lipp, M.; San-tibañez-Salgado, A.; Santillán-Doherty, P. Cryopreserved Tracheal Grafts: A Review of the Literature. J. Investig. Tive Surg. 2006, 19, 125–135. [Google Scholar] [CrossRef]
- Nakanishi, R. Cryopreservation of the Tracheal Grafts: Review and Perspective. Organogenesis 2009, 5, 113–118. [Google Scholar] [CrossRef]
- Virk, J.S.; Zhang, H.; Nouraei, S.A.R.; Sandhu, G. Prosthetic reconstruction of the trachea: A historical perspective. World J. Clin. Cases 2017, 5, 128–133. [Google Scholar] [CrossRef]
- Etienne, H.; Fabre, D.; Gomez Caro, A.; Kolb, F.; Mussot, S.; Mercier, O.; Mitilian, D.; Stephan, F.; Fadel, E.; Dartevelle, P. Tracheal replacement. Eur. Respir. J. 2018, 51, 1702211. [Google Scholar] [CrossRef]
- Damiano, G.; Palumbo, V.; Fazzotta, S.; Curione, F.; Monte, G.L.; Brucato, V.; Monte, A.L. Current Strategies for Tracheal Replacement: A Review. Life 2021, 11, 618. [Google Scholar] [CrossRef]
- Xu, C.; Ma, Y.; Huang, H.; Ruan, Z.; Li, Y. A Review of Woven Tracheal Stents: Materials, Structures, and Application. J. Funct. Biomater. 2022, 13, 96. [Google Scholar] [CrossRef]
- Gowers, K.H.; Hynds, R.E.; Thakrar, R.M.; Carroll, B.; Birchall, M.A.; Janes, S.M. Optimized isolation and expansion of human airway epithelial basal cells from endobronchial biopsy samples. J. Tissue Eng. Regen. Med. 2018, 12, e313–e317. [Google Scholar] [CrossRef]
- Milian, L.; Sancho-Tello, M.; Roig-Soriano, J.; Foschini, G.; Martínez-Hernández, N.J.; Más-Estellés, J.; Ruiz-Sauri, A.; Zurriaga, J.; Carda, C.; Mata, M. Optimization of a decellularization protocol of porcine tracheas. Long-term effects of cryopreservation. A histological study. Int. J. Artif. Organs 2021, 44, 998–1012. [Google Scholar] [CrossRef]
- De Wolf, J.; Brieu, M.; Zawadzki, C.; Ung, A.; Kipnis, E.; Jashari, R.; Hubert, T.; Fayoux, P.; Mariette, C.; Copin, M.-C.; et al. Successful immunosuppressant-free heterotopic transplantation of tracheal allografts in the pig. Eur. J. Cardio-Thorac. Surg. 2017, 52, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Paolin, A.; Trojan, D.; Leonardi, A.; Mellone, S.; Volpe, A.; Orlandi, A.; Cogliati, E. Cytokine expression and ultrastructural alterations in fresh-frozen, freeze-dried and γ-irradiated human amniotic membranes. Cell Tissue Bank. 2016, 17, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Viscioni, A.; Franco, M.; Paolin, A.; Cogliati, E.; Callegari, M.; Zollino, I.; Sollazzo, V.; Carinci, F. Effectiveness of fresh frozen and cryopreserved homologue iliac crest grafts used in sinus lifting: A comparative study. Cell Tissue Bank. 2011, 12, 263–271. [Google Scholar] [CrossRef]
- Ahmad, S.; Singh, V.A.; Hussein, S.I. Cryopreservation versus Fresh Frozen Meniscal Allograft: A Biomechanical Comparative Analysis. J. Orthop. Surg. 2017, 25, 230949901772794. [Google Scholar] [CrossRef]
- Teebken, O.; Pichlmaier, M.; Brand, S.; Haverich, A. Cryopreserved Arterial Allografts for In situ Reconstruction of Infected Arterial Vessels. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 597–602. [Google Scholar] [CrossRef]
- Müller-Schweinitzer, E. Cryopreservation of vascular tissues. Organogenesis 2009, 5, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yousif, A.; Ali, K.; Anssar, M.; Harringer, W.; El-Essawi, A.; Brouwer, R. A 20-Year Experience with Cryopreserved Al-lografts as the Valve Replacement of Choice in Aortic Root Reconstruction for Destructive Endocarditis with Abscess Formation. Interact. Cardio Vasc. Thorac. Surg. 2022, 35, ivac188. [Google Scholar] [CrossRef]
- Galeone, A.; Trojan, D.; Gardellini, J.; di Gaetano, R.; Faggian, G.; Luciani, G.B. Cryopreserved aortic homografts for complex aortic valve or root endocarditis: A 28-year experience. Eur. J. Cardio-Thorac. Surg. 2022, 62, ezac193. [Google Scholar] [CrossRef]
- Bakhach, J. The Cryopreservation of Composite Tissues: Principles and Recent Advancement on Cryopreservation of Different Type of Tissues. Organogenesis 2009, 5, 119–126. [Google Scholar] [CrossRef]
- Sotres-Vega, A.; Baltazares-Lipp, M.; Villalba-Caloca, J.; Gaxiola-Gaxiola, M.O.; Santibañez-Salgado, J.A.; Olmos-Zúñiga, J.R.; Jasso-Victoria, R. Tracheal Cryopreservation: Caspase-3 Immunoreactivity in Tracheal Epithelium and in Mixed Glands. Braz. J. Med. Biol. Res. 2009, 42, 1156–1162. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Feng, Y.; Sun, Y.; Ma, R.; Cui, P. Biomechanical changes of freezer-storaged and decellularized pig tracheal scaffoldings. J. Biomater. Appl. 2021, 35, 1208–1217. [Google Scholar] [CrossRef]
- Yokomise, H.; Inui, K.; Wada, H.; Ueda, M.; Hitomi, S. Long-term cryopreservation can prevent rejection of canine tracheal allografts with preservation of graft viability. J. Thorac. Cardiovasc. Surg. 1996, 111, 930–934. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cui, P.; Liu, P.; Li, S.; Ma, R. De-Epithelialized Heterotopic Tracheal Allografts without Immunosuppressants in Dogs: Long-Term Results for Cartilage Viability and Structural Integrity. Ann. Otol. Rhinol. Laryngol. 2021, 130, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Kunachak, S.; Kulapaditharom, B.; Vajaradul, Y.; Rochanawutanon, M. Cryopreserved, Irradiated Tracheal Homograft Transplantation for Laryngotracheal Reconstruction in Human Beings. Otolaryngol. Neck Surg. 2000, 122, 911–916. [Google Scholar] [CrossRef]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; de Caro, R. Tissue-Engineered Grafts from Human Decellu-larized Extracellular Matrices: A Systematic Review and Future Perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef]
- Liu, L.; Dharmadhikari, S.; Spector, B.M.; Tan, Z.H.; E Van Curen, C.; Agarwal, R.; Nyirjesy, S.; Shontz, K.; A Sperber, S.; Breuer, C.K.; et al. Tissue-engineered composite tracheal grafts create mechanically stable and biocompatible airway replacements. J. Tissue Eng. 2022, 13, 20417314221108791. [Google Scholar] [CrossRef] [PubMed]
- Kutten, J.C.; McGovern, D.; Hobson, C.M.; Luffy, S.A.; Nieponice, A.; Tobita, K.; Francis, R.; Reynolds, S.D.; Isenberg, J.S.; Gilbert, T.W. Decellularized Tracheal Extracellular Matrix Supports Epithelial Migration, Differentiation, and Function. Tissue Eng. Part A 2015, 21, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Aoki, F.G.; Varma, R.; Marin-Araujo, A.E.; Lee, H.; Soleas, J.P.; Li, A.H.; Soon, K.; Romero, D.; Moriya, H.T.; Haykal, S.; et al. De-epithelialization of porcine tracheal allografts as an approach for tracheal tissue engineering. Sci. Rep. 2019, 9, 1023. [Google Scholar] [CrossRef]
- Liu, L.; Dharmadhikari, S.; Shontz, K.M.; Tan, Z.H.; Spector, B.M.; Stephens, B.; Bergman, M.; Manning, A.; Zhao, K.; Reynolds, S.D.; et al. Regeneration of partially decellularized tracheal scaffolds in a mouse model of orthotopic tracheal replacement. J. Tissue Eng. 2021, 12, 20417314211017417. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.J.; De Coppi, P.; Speggiorin, S.; Roebuck, D.; Butler, C.R.; Samuel, E.; Crowley, C.; McLaren, C.; Fierens, A.; Vondrys, D.; et al. Stem-cell-based, tissue engineered tracheal replacement in a child: A 2-year follow-up study. Lancet 2012, 380, 994–1000. [Google Scholar] [CrossRef]
- Hamilton, N.J.; Kanani, M.; Roebuck, D.J.; Hewitt, R.J.; Cetto, R.; Culme-Seymour, E.J.; Toll, E.; Bates, A.J.; Comerford, A.P.; McLaren, C.A.; et al. Tissue-Engineered Tracheal Replacement in a Child: A 4-Year Follow-Up Study. Am. J. Transplant. 2015, 15, 2750–2757. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ahmed, S.; Varghese, K.S.; Mathew, D.M.; Pandey, R.; Rogando, D.O.; Salazar, S.A.; Fusco, P.J.; Levy, K.H. Decellularized versus cryopreserved pulmonary allografts for right ventricular outflow tract reconstruction during the Ross procedure: A meta-analysis of short- and long-term outcomes. Egypt. Heart J. 2021, 73, 100. [Google Scholar] [CrossRef]
- Lauk-Dubitskiy, S.E.; Pushkarev, A.V.; Korovin, I.A.; Shakurov, A.V.; Burkov, I.A.; Severgina, L.O.; Zherdev, A.A.; Tsiganov, D.I.; Novikov, I.A. Porcine heart valve, aorta and trachea cryopreservation and thawing using polydime-thylsiloxane. Cryobiology 2020, 93, 91–101. [Google Scholar] [CrossRef]
- Silan, F.; Consiglio, F.; Dell’Antonia, F.; Montagner, G.; Trojan, D.; Berna, G. Cryopreserved fascia lata allograft use in surgical facial reanimation: A retrospective study of seven cases. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 1–6. [Google Scholar] [CrossRef]
- Paolin, A.; Spagnol, L.; Battistella, G.; Trojan, D. Evaluation of allograft decontamination with two different antibiotic cocktails at the Treviso Tissue Bank Foundation. PLoS ONE 2018, 13, e0201792. [Google Scholar] [CrossRef]
- Montagner, G.; Trojan, D.; Cogliati, E.; Manea, F.; Vantini, A.; Paolin, A. Stability analysis of the antibiotic cocktail used by Treviso Tissue Bank Foundation for tissues decontamination. Cell Tissue Bank. 2018, 19, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Iop, L.; Paolin, A.; Aguiari, P.; Trojan, D.; Cogliati, E.; Gerosa, G. Decellularized Cryopreserved Allografts as Off-the-Shelf Allogeneic Alternative for Heart Valve Replacement: In Vitro Assessment Before Clinical Translation. J. Cardiovasc. Transl. Res. 2017, 10, 93–103. [Google Scholar] [CrossRef]
- Martelloni, M.; Boccaletto, P.; Montagner, G.; Trojan, D.; Abate, R. Bilaminar Technique with Coronally Advanced Flap and Cryopreserved Human Amniotic Membrane in the Treatment of Gingival Recessions. Case Rep. Dent. 2020, 2020, 1–4. [Google Scholar] [CrossRef]
- Barbon, S.; Biccari, A.; Stocco, E.; Capovilla, G.; D’Angelo, E.; Todesco, M.; Sandrin, D.; Bagno, A.; Romanato, F.; Macchi, V.; et al. Bio-Engineered Scaffolds Derived from Decellularized Human Esophagus for Functional Organ Reconstruction. Cells 2022, 11, 2945. [Google Scholar] [CrossRef]
- Barbon, S.; Stocco, E.; Contran, M.; Facchin, F.; Boscolo-Berto, R.; Todros, S.; Sandrin, D.; Romanato, F.; Pavan, P.; Macchi, V.; et al. Preclinical Development of Bioengineered Allografts Derived from Decellularized Human Diaphragm. Biomedicines 2022, 10, 739. [Google Scholar] [CrossRef] [PubMed]
- Naso, F.; Gandaglia, A.; Iop, L.; Spina, M.; Gerosa, G. Alpha-Gal detectors in xenotransplantation research: A word of caution. Xenotransplantation 2012, 19, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Belluzzi, E.; Contran, M.; Boscolo-Berto, R.; Picardi, E.; Guidolin, D.; Fontanella, C.G.; Olivotto, E.; Filardo, G.; Borile, G.; et al. Age-Dependent Remodeling in Infrapatellar Fat Pad Adipocytes and Extracellular Matrix: A Comparative Study. Front. Med. 2021, 8, 661403. [Google Scholar] [CrossRef]
- Emmi, A.; Antonini, A.; Sandre, M.; Baldo, A.; Contran, M.; Macchi, V.; Guidolin, D.; Porzionato, A.; De Caro, R. To-pography and distribution of adenosine A2A and dopamine D2 receptors in the human Subthalamic Nucleus. Front. Neurosci. 2022, 16, 945574. [Google Scholar] [CrossRef]
- Emmi, A.; Stocco, E.; Boscolo-Berto, R.; Contran, M.; Belluzzi, E.; Favero, M.; Ramonda, R.; Porzionato, A.; Ruggieri, P.; De Caro, R.; et al. Infrapatellar Fat Pad-Synovial Membrane Anatomo-Fuctional Unit: Microscopic Basis for Piezo1/2 Mechanosensors Involvement in Osteoarthritis Pain. Front. Cell Dev. Biol. 2022, 10, 886604. [Google Scholar] [CrossRef]
- Filippi, A.; Sasso, E.D.; Iop, L.; Armani, A.; Gintoli, M.; Sandri, M.; Gerosa, G.; Romanato, F.; Borile, G. Multimodal label-free ex vivo imaging using a dual-wavelength microscope with axial chromatic aberration compensation. J. Biomed. Opt. 2018, 23, 091403. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Yang, H.; Zhang, X.; Li, Z.; Xu, S. Quantitative analysis on collagen morphology in aging skin based on multiphoton microscopy. J. Biomed. Opt. 2011, 16, 040502. [Google Scholar] [CrossRef] [PubMed]
- Borile, G.; Sandrin, D.; Filippi, A.; Anderson, K.; Romanato, F. Label-Free Multiphoton Microscopy: Much More Than Fancy Images. Int. J. Mol. Sci. 2021, 22, 2657. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Barbon, S.; Mammana, M.; Zambello, G.; Contran, M.; Parnigotto, P.P.; Macchi, V.; Conconi, M.T.; Rea, F.; De Caro, R.; et al. Preclinical and clinical orthotopic transplantation of decellularized/engineered tracheal scaffolds: A systematic literature review. J. Tissue Eng. 2023, 14, 20417314231151826. [Google Scholar] [CrossRef]
- ISO 10993-6 2009; Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects after Implantation. Organization for Standardization: Geneva, Switzerland, 2009.
- Parshad, S.; Gogna, S.; Saroha, V.; Lohchab, S.S.; Karwasra, R.K. Tracheal Resection and Reconstruction for Malignant Tumor. Indian J. Surg. Oncol. 2020, 11, 199–203. [Google Scholar] [CrossRef]
- He, J.; Yang, C.; Yang, H.; Chen, H.; He, J.; Li, S. Resection and reconstruction via median sternotomy incision for tracheal tumors. Transl. Lung Cancer Res. 2022, 11, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Du, X.; Zhu, L.; Wang, S.; Xu, Z.; Lu, Z. Surgical management of long-segment congenital tracheal stenosis with tracheobronchial malacia. Eur. J. Cardio-Thorac. Surg. 2022, 61, 1001–1010. [Google Scholar] [CrossRef]
- Brand-Saberi, B.E.M.; Schäfer, T. Trachea: Anatomy and Physiology. Thorac. Surg. Clin. 2014, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mabrut, J.; Adham, M.; Bourgeot, J.; Eljaafari, A.; DelaRoche, E.; Ducerf, C.; Baulieux, J.; Rigal, D. Mechanical and histological characteristics of human trachea before and after cryopreservation: An opportunity for tracheal tissue banking. Transplant. Proc. 2001, 33, 609–611. [Google Scholar] [CrossRef]
- Candas, F.; Gorur, R.; Haholu, A.; Yildizhan, A.; Yucel, O.; Ay, H.; Memis, A.; Isitmangil, T. Is Tracheal Transplantation Possible with Cryopreserved Tracheal Allograft and Hyperbaric Oxygen Therapy? An Experimental Study. Ann. Thorac. Surg. 2016, 101, 1139–1144. [Google Scholar] [CrossRef]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef]
- Godehardt, A.W.; Tönjes, R.R. Xenotransplantation of decellularized pig heart valves—Regulatory aspects in Europe. Xenotransplantation 2020, 27, e12609. [Google Scholar] [CrossRef]
- Deschamps, C.; Trastek, V.F.; Ferguson, J.L.; Martin, W.J.; Colby, T.V.; Pairolero, P.C.; Payne, W. Cryopreservation of canine trachea: Functional and histological changes. Ann. Thorac. Surg. 1989, 47, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nakamura, T.; Yamamoto, Y.; Matsumoto, K.; Sekine, T.; Ueda, H.; Shimizu, Y. A New Tracheal Bioartificial Organ: Evaluation of a Tracheal Allograft with Minimal Antigenicity after Treatment by Detergent. ASAIO J. 2000, 46, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nakamura, T.; Sekine, T.; Matsumoto, K.; Ueda, H.; Yoshitani, M.; Toba, T.; Shimizu, Y. New Type of Tracheal Bioartificial Organ Treated with Detergent: Maintaining Cartilage Viability Is Necessary for Successful Immunosuppressant Free Allotransplantation. ASAIO J. 2002, 48, 21–25. [Google Scholar] [CrossRef]
- Sotres-Vega, A.; Santibañez-Salgado, J.A.; Villalba-Caloca, J.; Gaxiola-Gaxiola, M.; Ramos-Abraham, C.; Rosales-Torres, A.M.; Jiménez-García, L.F. Canine Tracheal Cartilage Cryopreservation: Freezing Injury Is Not Related to Caspase-3 Ex-pression. Biopreserv. Biobank. 2013, 11, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Lenot, B.; Macchiarini, P.; Dulmet, E.; Weiss, M.; Dartevelle, P. Tracheal allograft replacement *1An unsuccessful method. Eur. J. Cardio-Thorac. Surg. 1993, 7, 648–652. [Google Scholar] [CrossRef]
- Messineo, A.; Filler, R.M.; Bahoric, A.; Smith, C.R. Repair of long tracheal defects with cryopreserved cartilaginous allografts. J. Pediatr. Surg. 1992, 27, 1131–1135. [Google Scholar] [CrossRef]
- Messineo, A.; Filler, R.M.; Joseph, T.; Bahoric, A.; Smith, C.R. Tracheoplasty without stent, using preshaped cryopreserved cartilage allografts in neonatal pigs. J. Pediatr. Surg. 1994, 29, 697–700. [Google Scholar] [CrossRef]
- Yokomise, H.; Inui, K.; Wada, H.; Hasegawa, S.; Ohno, N.; Hitomi, S. Reliable cryopreservation of trachea for one month in a new trehalose solution. J. Thorac. Cardiovasc. Surg. 1995, 110, 382–385. [Google Scholar] [CrossRef][Green Version]
- Inutsuka, K.; Kawahara, K.; Takachi, T.; Okabayashi, K.; Shiraishi, T.; Shirakusa, T. Reconstruction of trachea and carina with immediate or cryopreserved allografts in dogs. Ann. Thorac. Surg. 1996, 62, 1480–1484. [Google Scholar] [CrossRef]
- Tojo, T.; Niwaya, K.; Sawabata, N.; Nezu, K.; Kawachi, K.; Kitamura, S. Tracheal allogenic immunoresponse is reduced by cryopreservation: Canine experiment. Transplant. Proc. 1996, 28, 1814–1815. [Google Scholar] [PubMed]
- Ueda, M.; Yokomise, H.; Wada, H.; Hitomi, S. Experimental tracheal transplantation for possible clinical application. Transplant. Proc. 1997, 29, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hua, T.-C.; Zhou, Y.-Z.; Wang, Q.-F.; Yang, Y.; Bao, L.-L. Cryopreservation and Transplantation of Dog Tra-cheaa. Ann. N. Y. Acad. Sci. 1998, 858, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Mukaida, T.; Shimizu, N.; Aoe, M.; Andou, A.; Date, H.; Okabe, K.; Yamashita, M.; Ichiba, S. Experimental study of tracheal allotransplantation with cryopreserved grafts. J. Thorac. Cardiovasc. Surg. 1998, 116, 262–266. [Google Scholar] [CrossRef][Green Version]
- Tojo, T.; Kitamura, S.; Gojo, S.; Kushibe, K.; Nezu, K.; Taniguchi, S. Epithelial regeneration and preservation of tracheal cartilage after tracheal replacement with cryopreserved allograft in the rat. J. Thorac. Cardiovasc. Surg. 1998, 116, 624–627. [Google Scholar] [CrossRef]
- Kawahara, K.; Inutsuka, K.; Hiratsuka, M.; Makihata, S.; Okabayashi, K.; Shiraishi, T.; Shirakusa, T. Tracheal Trans-plantation for Carinal Reconstruction in Dogs. J. Thorac. Cardiovasc. Surg. 1998, 116, 397–401. [Google Scholar] [CrossRef]
- Mukaida, T.; Shimizu, N.; Aoe, M.; Andou, A.; Date, H. Tracheal Allotransplantation after Varying Terms of Cryopreservation. Transpl. Proc. 1998, 30, 3397–3400. [Google Scholar] [CrossRef]
- Tojo, T.; Niwaya, K.; Sawabata, N.; Kushibe, K.; Nezu, K.; Taniguchi, S.; Kitamura, S. Tracheal Replacement with Cryo-preserved Tracheal Allograft: Experiment in Dogs. Ann. Thorac. Surg. 1998, 66, 209–213. [Google Scholar] [CrossRef]
- Aoki, T.; Yamato, Y.; Tsuchida, M.; Souma, T.; Yoshiya, K.; Watanabe, T.; Hayashi, J.-I. Successful tracheal transplantation using cryopreserved allografts in a rat model. Eur. J. Cardio-Thorac. Surg. 1999, 16, 169–173. [Google Scholar] [CrossRef][Green Version]
- Moriyama, H.; Sasajima, T.; Hirata, S.; Yamazaki, K.; Yatsuyanagi, E.; Kubo, Y. Revascularization of canine cryopreserved tracheal allografts. Ann. Thorac. Surg. 2000, 69, 1701–1706. [Google Scholar] [CrossRef]
- Nakanishi, R.; Hashimoto, M.; Muranaka, H.; Yasumoto, K. Effect of cryopreservation period on rat tracheal allografts. J. Heart Lung Transplant. 2001, 20, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Kushibe, K.; Nezu, K.; Nishizaki, K.; Takahama, M.; Taniguchi, S. Tracheal allotransplantation maintaining cartilage viability with long-term cryopreserved allografts. Ann. Thorac. Surg. 2001, 71, 1666–1669. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, K.; Hiratsuka, M.; Mikami, K.; Makihata, S.; Yoneda, S.; Shiraishi, T.; Okabayashi, K.; Shirakusa, T. Obliterative airway disease and graft stenting in pig-to-dog tracheal xenotransplantation. Jpn. J. Thorac. Cardiovasc. Surg. 2001, 49, 53–57. [Google Scholar] [CrossRef]
- Hashimoto, M.; Nakanishi, R.; Umesue, M.; Muranaka, H.; Hachida, M.; Yasumoto, K. Feasibility of Cryopreserved Tra-cheal Xenotransplants with the Use of Short-Course Immunosuppression. J. Thorac. Cardiovasc. Surg. 2001, 121, 241–248. [Google Scholar] [CrossRef]
- Nakanishi, R.; Onitsuka, T.; Shigematsu, Y.; Hashimoto, M.; Muranaka, H.; Yasumoto, K. The immunomodulatory effect of cryopreservation in rat tracheal allotransplantation. J. Heart Lung Transplant. 2002, 21, 890–898. [Google Scholar] [CrossRef]
- Murakawa, T.; Nakajima, J.; Motomura, N.; Murakami, A.; Takamoto, S. Successful allotransplantation of cryopreserved tracheal grafts with preservation of the pars membranacea in nonhuman primates. J. Thorac. Cardiovasc. Surg. 2002, 123, 153–160. [Google Scholar] [CrossRef][Green Version]
- Tanaka, H.; Maeda, K.; Okita, Y. Transplantation of the cryopreserved tracheal allograft in growing rabbits. J. Pediatr. Surg. 2003, 38, 1707–1711. [Google Scholar] [CrossRef]
- Hisamatsu, C.; Maeda, K.; Tanaka, H.; Okita, Y. Transplantation of the cryopreserved tracheal allograft in growing rabbits: Effect of immunosuppressant. Pediatr. Surg. Int. 2006, 22, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, R.; Ding, J.; Qiao, Y.; Wang, Q. Histological Examination of Cryopreserved Rat Tracheal Grafts. ASAIO J. 2007, 53, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Ding, J.; Wang, H.; Zheng, R.; Wang, Q. Ultrastructural Changes in Cryopreserved Tracheal Grafts of Sprague-Dawley Rats. ASAIO J. 2009, 55, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Iyikesici, T.; Tuncozgur, B.; Sanli, M.; Isik, A.F.; Meteroglu, F.; Elbeyli, L. Two-Piece Cryopreserved Tracheal Allotrans-plantation: An Experimental Study. Eur. J. Cardio-Thorac. Surg. 2009, 36, 722–726. [Google Scholar] [CrossRef][Green Version]
- Han, Y.; Lan, N.; Pang, C.; Tong, X. Bone Marrow-Derived Mesenchymal Stem Cells Enhance Cryopreserved Trachea Allograft Epithelium Regeneration and Vascular Endothelial Growth Factor Expression. Transplantation 2011, 92, 620–626. [Google Scholar] [CrossRef]
- Hysi, I.; Wurtz, A.; Zawadzki, C.; Kipnis, E.; Jashari, R.; Hubert, T.; Ung, A.; Copin, M.-C.; Jude, B. Immune tolerance of epithelium-denuded-cryopreserved tracheal allograft. Eur. J. Cardio-Thorac. Surg. 2014, 45, e180–e186. [Google Scholar] [CrossRef] [PubMed]
- Hysi, I.; Kipnis, E.; Fayoux, P.; Copin, M.-C.; Zawadzki, C.; Jashari, R.; Hubert, T.; Ung, A.; Ramon, P.; Jude, B.; et al. Successful orthotopic transplantation of short tracheal segments without immunosuppressive therapy. Eur. J. Cardio-Thorac. Surg. 2015, 47, e54–e61. [Google Scholar] [CrossRef][Green Version]
- Lu, T.; Huang, Y.; Liu, Y.; Shen, Y.; Qiao, Y.; Zhang, Y. Effects of cryopreservation on tracheal allograft antigenicity in dogs. J. Thorac. Dis. 2017, 9, 2038–2047. [Google Scholar] [CrossRef]
- Ratto, C.; Parolini, O.; Marra, A.A.; Orticelli, V.; Parello, A.; Campennì, P.; de Simone, V.; Trojan, D.; Litta, F. Human Amniotic Membrane for the Treatment of Cryptoglandular Anal. Fistulas. J. Clin. Med. 2022, 11, 1350. [Google Scholar] [CrossRef]
- Wood, M.W.; Murphy, S.V.; Feng, X.; Wright, J.S.C. Tracheal Reconstruction in a Canine Model. Otolaryngol. Neck Surg. 2014, 150, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-H.; Su, C.-H.; Lin, S.-E.; Tseng, H. Preliminary experiences in trachea scaffold tissue engineering with segmental organ decellularization. Laryngoscope 2016, 126, 2520–2527. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.H.; Hung, S.-H.; Tseng, Y.; Quang, L.X.; Le, N.T.N.; Fang, C.-L.; Tseng, H. Partial Decellularized Scaffold Com-bined with Autologous Nasal Epithelial Cell Sheet for Tracheal Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 10322. [Google Scholar] [CrossRef]
- Dang, L.; Tseng, Y.; Tseng, H.; Hung, S.-H. Partial Decellularization for Segmental Tracheal Scaffold Tissue Engineering: A Preliminary Study in Rabbits. Biomolecules 2021, 11, 866. [Google Scholar] [CrossRef]
- Tan, Z.H.; Dharmadhikari, S.; Liu, L.; Wolter, G.; Shontz, K.M.; Reynolds, S.D.; Johnson, J.; Breuer, C.K.; Chiang, T. Tra-cheal Macrophages During Regeneration and Repair of Long-Segment Airway Defects. Laryngoscope 2022, 132, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Go, T.; Jungebluth, P.; Baiguero, S.; Asnaghi, A.; Martorell, J.; Ostertag, H.; Mantero, S.; Birchall, M.; Bader, A.; Macchiarini, P. Both epithelial cells and mesenchymal stem cell–derived chondrocytes contribute to the survival of tissue-engineered airway transplants in pigs. J. Thorac. Cardiovasc. Surg. 2010, 139, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Gray, F.L.; Turner, C.G.; Ahmed, A.; Calvert, C.E.; Zurakowski, D.; Fauza, D.O. Prenatal tracheal reconstruction with a hybrid amniotic mesenchymal stem cells–engineered construct derived from decellularized airway. J. Pediatr. Surg. 2012, 47, 1072–1079. [Google Scholar] [CrossRef]
- Batioglu-Karaaltin, A.; Karaaltin, M.V.; Ovali, E.; Yigit, O.; Kongur, M.; Inan, O.; Bozkurt, E.; Cansiz, H. In Vivo Tis-sue-Engineered Allogenic Trachea Transplantation in Rabbits: A Preliminary Report. Stem. Cell Rev. Rep. 2015, 11, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Maughan, E.F.; Butler, C.R.; Crowley, C.; Teoh, G.Z.; den Hondt, M.; Hamilton, N.J.; Hynds, R.E.; Lange, P.; Ansari, T.; Urbani, L.; et al. A comparison of tracheal scaffold strategies for pediatric transplantation in a rabbit model. Laryngoscope 2017, 127, E449–E457. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Fuchimoto, Y.; Hsu, H.-C.; Higuchi, M.; Komura, M.; Yamaoka, T.; Umezawa, A.; Enosawa, S.; Kuroda, T. Airway reconstruction using decellularized tracheal allografts in a porcine model. Pediatr. Surg. Int. 2017, 33, 1065–1071. [Google Scholar] [CrossRef]
- Ershadi, R.; Rahim, M.; Jahany, S.; Rakei, S. Transplantation of the decellularized tracheal allograft in animal model (rabbit). Asian J. Surg. 2018, 41, 328–332. [Google Scholar] [CrossRef]
- Jang, S.J.; Park, M.-H.; Lee, T.-K.; Choi, S.H. Healing Effect of Platelet-Rich Plasma on Decellularized Tracheal Allotrans-plantation in Rabbits. Vivo 2018, 32, 1443–1447. [Google Scholar] [CrossRef]
- Zhou, Q.; Ye, X.; Ran, Q.; Kitahara, A.; Matsumoto, Y.; Moriyama, M.; Ajioka, Y.; Saijo, Y. Trachea Engineering Using a Centrifugation Method and Mouse-Induced Pluripotent Stem Cells. Tissue Eng. Part C Methods 2018, 24, 524–533. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, W.; Pan, Z.Y.; Pan, S.; Zhang, S.Q.; Wang, Z.H.; Gu, S.; Shi, H. In Vivo transplantation of stem cells with a genipin linked scaffold for tracheal construction. J. Biomater. Appl. 2019, 34, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Lu, Y.; Wang, Z.; Zhang, B.; Shen, Z.; Yuan, L.; Wu, C.; Wu, Q.; Yang, W.; Zhang, G.; et al. Directly construct microvascularization of tissue engineering trachea in orthotopic transplantation. Mater. Sci. Eng. C 2021, 128, 112201. [Google Scholar] [CrossRef] [PubMed]
- Villalba-Caloca, J.; Sotres-Vega, A.; Giraldo-Gómez, D.M.; O Gaxiola-Gaxiola, M.; Piña-Barba, M.C.; A García-Montes, J.; Martínez-Fonseca, S.; Alonso-Gómez, M.; Santibáñez-Salgado, J.A. In Vivo performance of decellularized tracheal grafts in the reconstruction of long length tracheal defects: Experimental study. Int. J. Artif. Organs 2021, 44, 718–726. [Google Scholar] [CrossRef]
- Nakamura, N.; Kimura, T.; Kishida, A. Overview of the Development, Applications, and Future Perspectives of Decellularized Tissues and Organs. ACS Biomater. Sci. Eng. 2017, 3, 1236–1244. [Google Scholar] [CrossRef]
- Han, M.-N.; Kim, J.-H.; Choi, S.H. Evaluation of Biomechanical Properties and Morphometric Structures of the Trachea in Pigs and Rabbits. Vivo 2022, 36, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.F.; Rehmani, S.S.; Baig, M.Z.; Jadoon, Y.; Bhora, F.Y. Successes and Failures in Tracheal Bioengineering: Lessons Learned. Ann. Thorac. Surg. 2021, 112, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, C.-Z.; Wang, X.-N.; Zhu, Y.-B.; Gu, Y.J. Favorable effects of the detergent and enzyme extraction method for preparing decellularized bovine pericardium scaffold for tissue engineered heart valves. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91B, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, A.J.; Beck, E.C.; Dennis, S.C.; Converse, G.L.; Hopkins, R.A.; Berkland, C.J.; Detamore, M.S. Decellularized Cartilage May Be a Chondroinductive Material for Osteochondral Tissue Engineering. PLoS ONE 2015, 10, e0121966. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Dalzoppo, D.; Lora, S.; Sartore, L.; Folin, M.; Parnigotto, P.P.; Grandi, C. Tailored PVA/ECM Scaffolds for Cartilage Regeneration. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Radossi, P.; Rajendran, S.; Dalzoppo, D.; Bortolami, M.; Bagno, A.; Grandi, F.; Gamba, P.G.; Parnigotto, P.P.; et al. Autologous chondrocytes as a novel source for neo-chondrogenesis in haemophiliacs. Cell Tissue Res. 2016, 366, 51–61. [Google Scholar] [CrossRef]
- Hung, S.-H.; Su, C.-H.; Lee, F.-P.; Tseng, H. Larynx Decellularization: Combining Freeze-Drying and Sonication as an Effective Method. J. Voice 2013, 27, 289–294. [Google Scholar] [CrossRef]
- Marrangoni, A.G. Homotransplantation of tracheal segments preserved by lyophilization; an experimental study. J. Thorac. Surg. 1951, 21, 398–401. [Google Scholar] [CrossRef]
- Batioglu-Karaaltin, A.; Ovali, E.; Karaaltin, M.V.; Yener, M.; Yılmaz, M.; Eyüpoğlu, F.; Yılmaz, Y.Z.; Bozkurt, E.R.; Demir, N.; Konuk, E.; et al. Decellularization of Trachea With Combined Techniques for Tissue-Engineered Trachea Transplantation. Clin. Exp. Otorhinolaryngol. 2019, 12, 86–94. [Google Scholar] [CrossRef]
- Keane, T.J.; Londono, R.; Turner, N.J.; Badylak, S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012, 33, 1771–1781. [Google Scholar] [CrossRef]
- Giraldo-Gomez, D.M.; Garcia-Lopez, J.; Tamay-De-Dios, L.; Sánchez-Sánchez, R.; Villalba-Caloca, J.; Sotres-Vega, A.; Del Prado-Audelo, M.L.; Gómez-Lizárraga, K.K.; Garciadiego-Cázares, D.; Piña-Barba, M.C. Fast cyclical-decellularized trachea as a natural 3D scaffold for organ engineering. Mater. Sci. Eng. C 2019, 105, 110142. [Google Scholar] [CrossRef]
- Bujia, J.; Wilmes, E.; Hammer, C.; Kastenbauer, E. Tracheal Transplantation: Demonstration of HLA Class II Subregion Gene Products on Human Trachea. Acta Oto-Laryngol. 1990, 110, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Yang, B.; Wang, R.; Qin, C. Xenotransplantation: Current Status in Preclinical Research. Front. Immunol. 2020, 10, 3060. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, W.; Liu, X.; Wang, H.; Shen, J.; Xiao, H.; Mei, J.; Chai, Y.; Wen, G. Extracellular matrix scaffold-immune microenvironment modulates tissue regeneration. Compos. Part B Eng. 2022, 230, 109524. [Google Scholar] [CrossRef]
- Feng, W.; Li, D.; Zang, J.; Fu, L. Biomechanical comparison of xenogeneic bone material treated with different methods. Xenotransplantation 2017, 24, e12343. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shao, A.; Shan, Y.; Zhao, H.; Leiguo, M.; Zhang, Y.; Tang, Y.; zhang, W.; Jin, Y.; Xu, L. A Standardized Quantitative Method for Detecting Remnant Alpha-Gal Antigen in Animal Tissues or Animal Tissue-Derived Biomaterials and Its Ap-plication. Sci. Rep. 2018, 8, 15424. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Tsuchiya, T.; Doi, R.; Matsumoto, K.; Higami, Y.; Kobayashi, E.; Nagayasu, T. Alteration of the extracellular matrix and alpha-gal antigens in the rat lung scaffold reseeded using human vascular and adipogenic stromal cells. J. Tissue Eng. Regen. Med. 2019, 13, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Shaddy, R.E.; Hunter, D.D.; Osborn, K.A.; Lambert, L.M.; Minich, L.L.; Hawkins, J.A.; McGough, E.C.; Fuller, T.C. Prospective Analysis of HLA Immunogenicity of Cryopreserved Valved Allografts Used in Pediatric Heart Surgery. Circulation 1996, 94, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Breinholt, J.P., 3rd; Hawkins, J.A.; Lambert, L.M.; Fuller, T.C.; Profaizer, T.; Shaddy, R.E. A prospective analysis of the immunogenicity of cryopreserved nonvalved allografts used in pediatric heart surgery. Circulation 2000, 102, III179–III182. [Google Scholar] [CrossRef]
- Hooper, D.K.; Hawkins, J.A.; Fuller, T.C.; Profaizer, T.; Shaddy, R.E. Panel-Reactive Antibodies Late After Allograft Implantation in Children. Ann. Thorac. Surg. 2005, 79, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Mirelli, M.; Buzzi, M.; Pasquinelli, G.; Tazzari, P.; Testi, G.; Ricchi, E.; Conte, R.; Stella, A. Fresh and Cryopreserved Arterial Homografts: Immunological and Clinical Results. Transplant. Proc. 2005, 37, 2688–2691. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Iyer, S.; Subramaniam, S.; Ramu, J.; Sharma, M.; Nambiar, A.; Unni, A.; Sivanarayanan, S. Evaluation of Antigenicity of Components of Tracheal Allotransplant and Effect of Immunosuppressant Regime in a Rodent Model. Indian J. Plast. Surg. 2020, 53, 357–362. [Google Scholar] [CrossRef]
- García-Gareta, E.; Abduldaiem, Y.; Sawadkar, P.; Kyriakidis, C.; Lali, F.; Greco, K.V. Decellularised scaffolds: Just a framework? Current knowledge and future directions. J. Tissue Eng. 2020, 11, 2041731420942903. [Google Scholar] [CrossRef]
- Jena, S.; Aksan, A. Effect of high DMSO concentration on albumin during freezing and vitrification. RSC Adv. 2017, 7, 43611–43620. [Google Scholar] [CrossRef]
- Butler, C.R.; Hynds, R.E.; Crowley, C.; Gowers, K.H.; Partington, L.; Hamilton, N.J.; Carvalho, C.; Platé, M.; Samuel, E.R.; Burns, A.J.; et al. Vacuum-assisted decellularization: An accelerated protocol to generate tissue-engineered human tracheal scaffolds. Biomaterials 2017, 124, 95–105. [Google Scholar] [CrossRef]
- Jeong, W.; Kim, M.K.; Kang, H.-W. Effect of detergent type on the performance of liver decellularized extracellular matrix-based bio-inks. J. Tissue Eng. 2021, 12, 2041731421997091. [Google Scholar] [CrossRef]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef]
- Frejo, L.; Goldstein, T.; Swami, P.; Patel, N.A.; Grande, D.A.; Zeltsman, D.; Smith, L.P. A two-stage in vivo approach for implanting a 3D printed tissue-engineered tracheal replacement graft: A proof of concept. Int. J. Pediatr. Otorhinolaryngol. 2022, 155, 111066. [Google Scholar] [CrossRef]
- Faulk, D.; Carruthers, C.; Warner, H.; Kramer, C.; Reing, J.; Zhang, L.; D’Amore, A.; Badylak, S. The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater. 2014, 10, 183–193. [Google Scholar] [CrossRef]
- Burdick, J.A.; Mauck, R.L.; Gorman, J.H.; Gorman, R.C. Acellular Biomaterials: An Evolving Alternative to Cell-Based Therapies. Sci. Transl. Med. 2013, 5, 176ps4. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Macchi, V.; Tiengo, C.; Petrelli, L.; Rambaldo, A.; Borean, A.; Capelli, S.; Filippi, A.; Romanato, F.; et al. New bioresorbable wraps based on oxidized polyvinyl alcohol and leukocyte-fibrin-platelet membrane to support peripheral nerve neurorrhaphy: Preclinical comparison versus NeuraWrap. Sci. Rep. 2019, 9, 17193. [Google Scholar] [CrossRef] [PubMed]
- Ayyalasomayajula, V.; Skallerud, B. Microstructure and mechanics of the bovine trachea: Layer specific investigations through SHG imaging and biaxial testing. J. Mech. Behav. Biomed. Mater. 2022, 134, 105371. [Google Scholar] [CrossRef]
- Stocco, E.; Porzionato, A.; de Rose, E.; Barbon, S.; de Caro, R.; Macchi, V. Meniscus Regeneration by 3D Printing Tech-nologies: Current Advances and Future Perspectives. J. Tissue Eng. 2022, 13, 204173142110658. [Google Scholar] [CrossRef] [PubMed]
- Kamel, K.S.; Beckert, L.E.; Stringer, M.D. Novel insights into the elastic and muscular components of the human trachea. Clin. Anat. 2009, 22, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.-J.; Park, J.; Shin, D.-A.; Ryu, Y.-J.; Kim, H.C.; Lee, J.C.; Kwon, S.K. Characterization of the biomechanical properties of canine trachea using a customized 3D-printed apparatus. Auris Nasus Larynx 2019, 46, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, N.J.; Mas-Estellés, J.; Milián-Medina, L.; Martínez-Ramos, C.; Cerón-Navarro, J.; Galbis-Caravajal, J.; Roig-Bataller, A.; Mata-Roig, M. A Standardised Approach to the Biomechanical Evaluation of Tracheal Grafts. Biomolecules 2021, 11, 1461. [Google Scholar] [CrossRef] [PubMed]
- Marzi, J.; Biermann, A.C.; Brauchle, E.M.; Brockbank, K.G.M.; Stock, U.A.; Schenke-Layland, K. Marker-Independent In Situ Quantitative Assessment of Residual Cryoprotectants in Cardiac Tissues. Anal. Chem. 2019, 91, 2266–2272. [Google Scholar] [CrossRef] [PubMed]
- Sugishita, Y.; Meng, L.; Suzuki-Takahashi, Y.; Nishimura, S.; Furuyama, S.; Uekawa, A.; Tozawa-Ono, A.; Migitaka-Igarashi, J.; Koizumi, T.; Seino, H.; et al. Quantification of residual cryoprotectants and cytotoxicity in thawed bovine ovarian tissues after slow freezing or vitrification. Hum. Reprod. 2022, 37, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Sotnichenko, A.S.; Nakokhov, R.Z.; Gubareva, E.A.; Kuevda, E.V.; Gumenyuk, I.S. Morphological Evaluation of the Tissue Reaction to Subcutaneous Implantation of Decellularized Matrices. Bull. Exp. Biol. Med. 2018, 166, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Methe, M.K.N.; Nayakawde, N.B.; Banerjee, D.; Sihlbom, C.; Agbajogu, C.; Travnikova, M.G.; Olausson, M. Differential Activation of Immune Cells for Genetically Different Decellularized Cardiac Tissues. Tissue Eng. Part A 2020, 26, 1180–1198. [Google Scholar] [CrossRef]
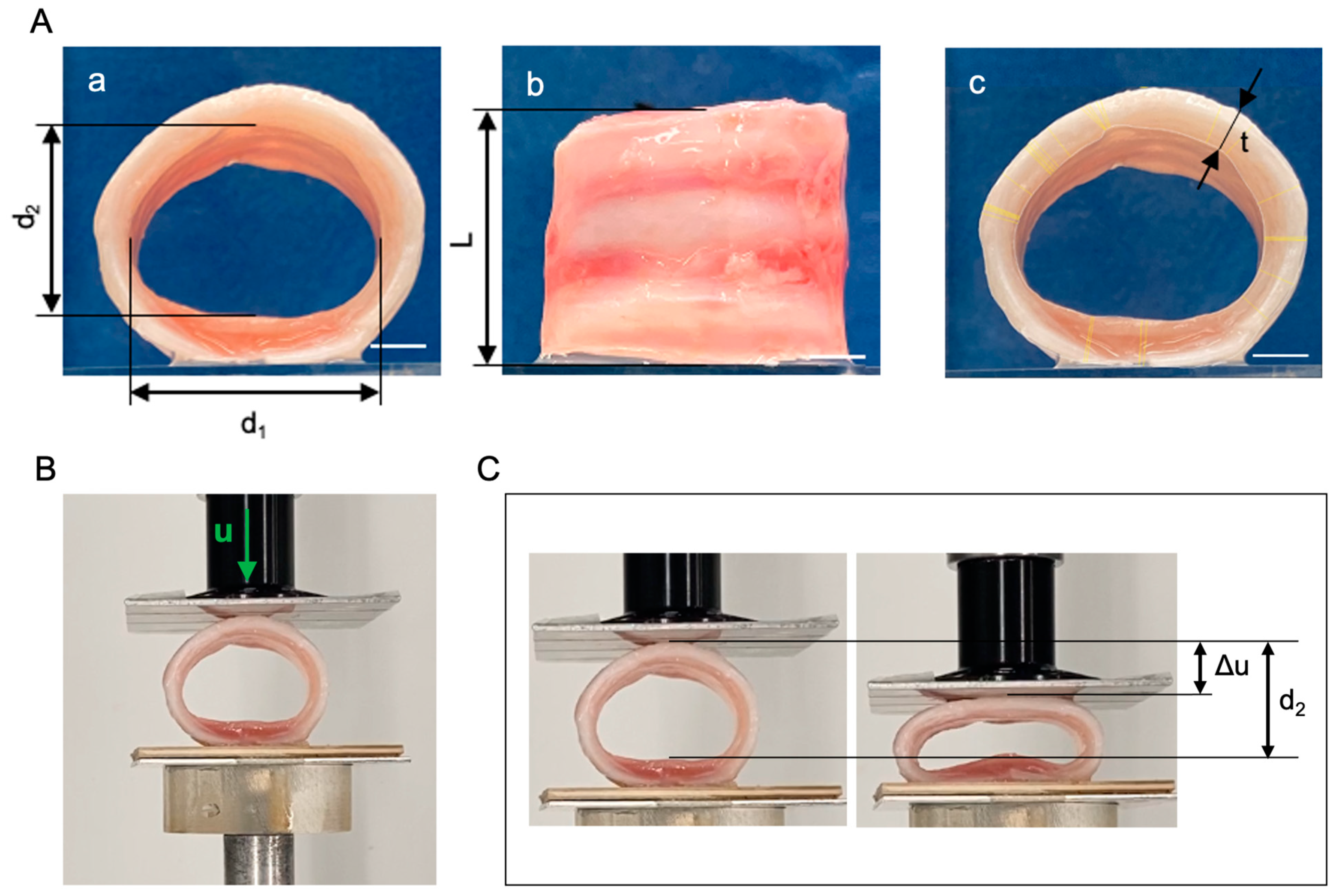






| d1 [mm] | d2 [mm] | t [mm] | L [mm] | d1 [mm] | |
|---|---|---|---|---|---|
| NativeT | 19.6 | 14.9 | 2.4 | 15.9 | 19.6 |
| 19.8 | 14.5 | 2.6 | 17.3 | 19.8 | |
| 19.7 | 15.7 | 2.7 | 18.5 | 19.7 | |
| 21.6 | 16.9 | 2.7 | 14.7 | 21.6 | |
| CryoT | 20.0 | 15.4 | 1.9 | 12.6 | 20 |
| 21.4 | 16.3 | 2.7 | 15.3 | 21.4 | |
| 20.7 | 16.3 | 2.3 | 16.5 | 20.7 | |
| 20.8 | 15.2 | 2.1 | 14.7 | 20.8 | |
| DecellT | 27.9 | 13.7 | 2.4 | 17.7 | 27.9 |
| 26.6 | 11.8 | 2.8 | 13.2 | 26.6 | |
| 27.9 | 13.9 | 2.8 | 15.7 | 27.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stocco, E.; Barbon, S.; Mammana, M.; Trojan, D.; Bianchin, A.; Favaretto, F.; Contran, M.; Zambello, G.; Vogliardi, A.; Confalonieri, M.; et al. Development and In Vitro/In Vivo Comparative Characterization of Cryopreserved and Decellularized Tracheal Grafts. Cells 2023, 12, 888. https://doi.org/10.3390/cells12060888
Stocco E, Barbon S, Mammana M, Trojan D, Bianchin A, Favaretto F, Contran M, Zambello G, Vogliardi A, Confalonieri M, et al. Development and In Vitro/In Vivo Comparative Characterization of Cryopreserved and Decellularized Tracheal Grafts. Cells. 2023; 12(6):888. https://doi.org/10.3390/cells12060888
Chicago/Turabian StyleStocco, Elena, Silvia Barbon, Marco Mammana, Diletta Trojan, Alice Bianchin, Francesca Favaretto, Martina Contran, Giovanni Zambello, Andrea Vogliardi, Marta Confalonieri, and et al. 2023. "Development and In Vitro/In Vivo Comparative Characterization of Cryopreserved and Decellularized Tracheal Grafts" Cells 12, no. 6: 888. https://doi.org/10.3390/cells12060888
APA StyleStocco, E., Barbon, S., Mammana, M., Trojan, D., Bianchin, A., Favaretto, F., Contran, M., Zambello, G., Vogliardi, A., Confalonieri, M., Todros, S., Pavan, P. G., Romanato, F., Conconi, M. T., Macchi, V., De Caro, R., Rea, F., & Porzionato, A. (2023). Development and In Vitro/In Vivo Comparative Characterization of Cryopreserved and Decellularized Tracheal Grafts. Cells, 12(6), 888. https://doi.org/10.3390/cells12060888
















