Blood-Brain Barrier-Associated Proteins Are Elevated in Serum of Epilepsy Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Research Group
3.2. Control Group
3.3. Serum Levels of BBB-Associated Proteins
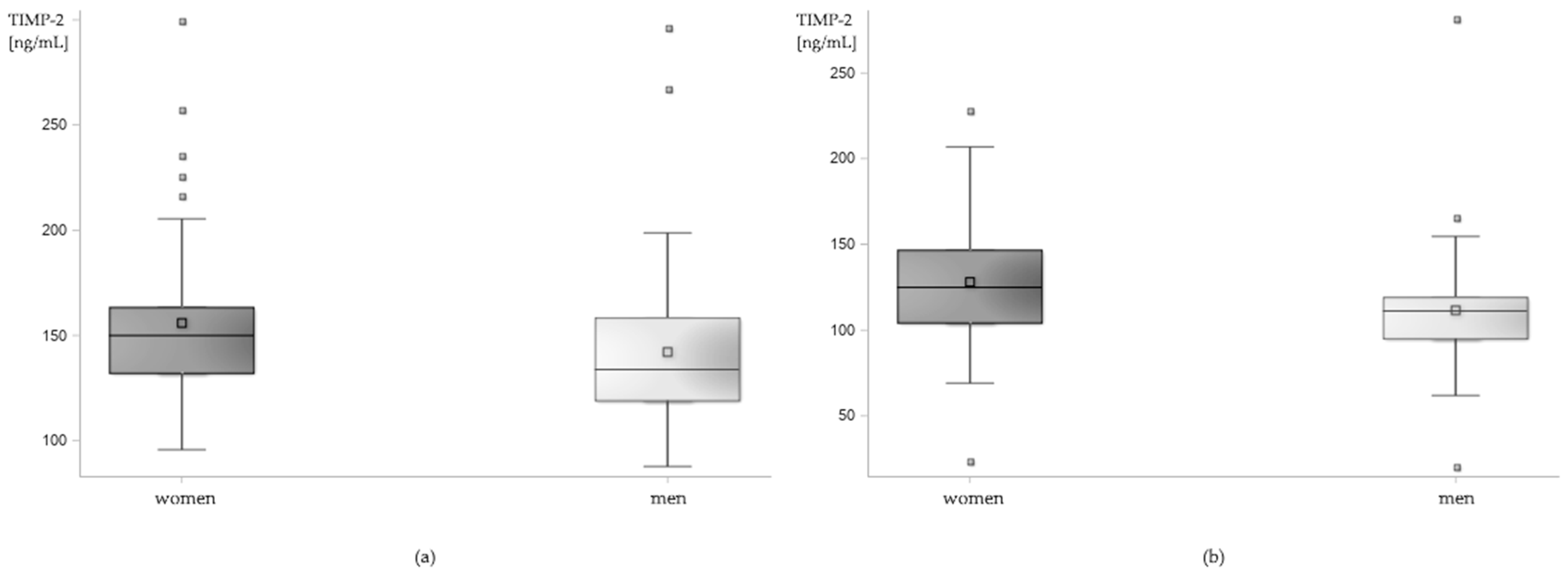
3.4. Influence of Demographic Factors on the Serum Levels of BBB-Associated Proteins
3.5. Correlation between the Serum Levels of BBB-Associated Proteins
4. Discussion
4.1. Proteins Associated with BBB Disruption and Restoration—MMP-9, MMP-2, TIMP-1, TIMP-2, S100B
4.1.1. Short Characteristics of Proteins Associated with BBB Disruption and Restoration
4.1.2. Proteins Associated with BBB Disruption and Restoration in Epilepsy
4.2. Other Proteins Associated with BBB—ICAM-1, P-sel, CCL-2, TSP-2
4.3. Influence of Demographic Factors on the Serum Levels of BBB-Associated Proteins
4.3.1. Age
4.3.2. Sex
4.4. Correlation between the Serum Levels of BBB-Associated Proteins
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Löscher, W.; Klitgaard, H.; Twyman, R.E.; Schmidt, D. New avenues for anti-epileptic drug discovery and development. Nat. Rev. Drug Discov. 2013, 12, 757–776. [Google Scholar] [CrossRef] [PubMed]
- Baeten, K.M.; Akassoglou, K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev. Neurobiol. 2011, 71, 1018–1039. [Google Scholar] [CrossRef] [PubMed]
- Fabene, P.F.; Mora, G.N.; Martinello, M.; Rossi, B.; Ottoboni, L.; Bach, S.; Angiari, S.; Benati, D.; Zanetti, L.; Schio, F.; et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat. Med. 2008, 14, 1377–1383. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef]
- Fabene, P.F.; Laudanna, C.; Constantin, G. Leukocyte trafficking mechanisms in epilepsy. Mol. Immunol. 2013, 55, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, E.; Dreier, J.P.; Ivens, S.; Bechmann, I.; Tomkins, O.; Heinemann, U.; Friedman, A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J. Neurosci. 2004, 24, 7829–7836. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, C.J.D.; LaRivière, C.G.; Young, J.D.; Cass, C.E.; Baldwin, S.A.; Parkinson, F.E. Purine uptake and release in rat C6 glioma cells: Nucleoside transport and purine metabolism under ATP-depleting conditions. J. Neurochem. 2000, 75, 1528–1538. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Keep, R.F.; Kunkel, S.L.; Andjelkovic, A. V Potential role of MCP-1 in endothelial cell tight junction “opening”: Signaling via Rho and Rho kinase. J. Cell Sci. 2003, 116, 4615–4628. [Google Scholar] [CrossRef]
- Haorah, J.; Ramirez, S.H.; Schall, K.; Smith, D.; Pandya, R.; Persidsky, Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J. Neurochem. 2007, 101, 566–576. [Google Scholar] [CrossRef]
- da Fonseca, A.C.C.; Matias, D.; Garcia, C.; Amaral, R.; Geraldo, L.H.; Freitas, C.; Lima, F.R.S. The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell Neurosci. 2014, 8, 1–13. [Google Scholar] [CrossRef]
- Verslegers, M.; Lemmens, K.; Van Hove, I.; Moons, L. Matrix metalloproteinase-2 and -9 as promising benefactors in development, plasticity and repair of the nervous system. Prog. Neurobiol. 2013, 105, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.; Grajkowska, W.; Ziemiańska, K.; Roszkowski, M.; Daszkiewicz, P.; Rysz, A.; Marchel, A.; Koperski, L.; Wilczyński, G.M.; Dzwonek, J. Matrix metalloproteinase-9 (MMP-9) in human intractable epilepsy caused by focal cortical dysplasia. Epilepsy Res. 2013, 104, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, S.; Zhang, C.; Shu, H.; Liu, S.; An, N.; Yang, M.; Yin, Q.; Yang, H. Increased expression of matrix metalloproteinase 9 in cortical lesions from patients with focal cortical dysplasia type IIb and tuberous sclerosis complex. Brain Res. 2012, 1453, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Cudna, A.; Jopowicz, A.; Mierzejewski, P.; Kurkowska-Jastrzębska, I. Serum metalloproteinase 9 levels increase after generalized tonic-clonic seizures. Epilepsy Res. 2017, 129, 33–36. [Google Scholar] [CrossRef]
- Tan, H.K.; Heywood, D.; Ralph, G.S.; Bienemann, A.; Baker, A.H.; Uney, J.B. Tissue inhibitor of metalloproteinase 1 inhibits excitotoxic cell death in neurons. Mol. Cell Neurosci. 2003, 22, 98–106. [Google Scholar] [CrossRef]
- Cudna, A.; Bronisz, E.; Jopowicz, A.; Kurkowska-Jastrzębska, I. Metalloproteinase 2 and its Inhibitors Expression in Serum after Tonic-Clonic Seizures. In Proceedings of the 13th European Congress on Epileptology, Vienna, Austria, 26–30 August 2018. [Google Scholar]
- Cudna, A.; Jopowicz, A.; Mierzejewski, P.; Kurkowska-Jastrzębska, I. Serum MMP-9 and TIMP-1 level increases after generalised tonic-clonic seizures. In Proceedings of the 2nd Congress of the European Academy of Neurology, Copenhagen, Denmark, 28–31 May 2016. [Google Scholar]
- Tian, W.; Sawyer, A.; Kocaoglu, F.B.; Kyriakides, T.R. Astrocyte-derived thrombospondin-2 is critical for the repair of the blood-brain barrier. Am. J. Pathol. 2011, 179, 860–868. [Google Scholar] [CrossRef]
- Alizada, O.; Akgun, M.Y.; Ozdemir, A.F.; Toklu, S.; Kemerdere, R.; Orhan, B.; Inal, B.B.; Yeni, S.N.; Tanriverdi, T. Circulating Levels of Thrombospondin-1 and Thrombospondin-2 in Patients with Temporal Lobe Epilepsy Before and After Surgery. Turk. Neurosurg. 2021, 31, 228–232. [Google Scholar] [CrossRef]
- Marchi, N.; Cavaglia, M.; Fazio, V.; Bhudia, S.; Hallene, K.; Janigro, D. Peripheral markers of blood-brain barrier damage. Clin. Chim. Acta 2004, 342, 1–12. [Google Scholar] [CrossRef]
- Hofmann, M.A.; Drury, S.; Fu, C.; Qu, W.; Taguchi, A.; Lu, Y.; Avila, C.; Kambham, N.; Bierhaus, A.; Nawroth, P.; et al. RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell 1999, 97, 889–901. [Google Scholar] [CrossRef]
- Griffin, W.S.; Yeralan, O.; Sheng, J.G.; Boop, F.A.; Mrak, R.E.; Rovnaghi, C.R.; Burnett, B.A.; Feoktistova, A.; Van Eldik, L.J. Overexpression of the neurotrophic cytokine S100 beta in human temporal lobe epilepsy. J. Neurochem. 1995, 65, 228–233. [Google Scholar] [CrossRef]
- Nass, R.D.; Wagner, M.; Surges, R.; Holdenrieder, S. Time courses of HMGB1 and other inflammatory markers after generalized convulsive seizures. Epilepsy Res. 2020, 162, 106301. [Google Scholar] [CrossRef] [PubMed]
- Cudna, A.; Jopowicz, A.; Bronisz, E.; Kurkowska-Jastrzębska, I. Blood-brain barrier markers after tonic-clonic seizures. In Proceedings of the 12th European Congress on Epileptology, Prague, Czech Republic, 11–15 September 2016. [Google Scholar]
- Jiang, Y.; Zhu, J.F.; Luscinskas, F.W.; Graves, D.T. MCP-1-stimulated monocyte attachment to laminin is mediated by beta 2-integrins. Am. J. Physiol. 1994, 267, C1112–C1118. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Kang, S.I.; Lee, S.-Y.; Noh, K.-T.; Kim, E.-C. Involvement of SDF-1 and monocyte chemoattractant protein-1 in hydrogen peroxide-induced extracellular matrix degradation in human dental pulp cells. Int. Endod. J. 2014, 47, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Xu, X.; Luo, Z.; Shen, N.; Wang, F.; Zhao, Y. Pyrrolidine dithiocarbamate (PDTC) inhibits the overexpression of MCP-1 and attenuates microglial activation in the hippocampus of a pilocarpine-induced status epilepticus rat model. Exp. Ther. Med. 2013, 7, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Arisi, G.M.; Foresti, M.L.; Katki, K.; Shapiro, L.A. Increased CCL2, CCL3, CCL5, and IL-1β cytokine concentration in piriform cortex, hippocampus, and neocortex after pilocarpine-induced seizures. J. Neuroinflammation 2015, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.; Wolf, S. ICAM-1 signaling in endothelial cells. Pharmacol. Reports 2009, 61, 22–32. [Google Scholar] [CrossRef]
- Takeshita, Y.; Ransohoff, R.M. Inflammatory cell trafficking across the blood-brain barrier: Chemokine regulation and in vitro models. Immunol. Rev. 2012, 248, 228–239. [Google Scholar] [CrossRef]
- Atmaca, M.M.; Telci, A.; Dirican, A.; Gurses, C. Could sP-Selectin and sICAM-1 be potential biomarkers in status epilepticus? Med. Sci. Discov. 2019, 90, 32–40. [Google Scholar] [CrossRef]
- Cudna, A. Activation of Blood-Brain Barrier in Epilepsy-Dynamics of the Expression of Blood-Brain Barrier Markers after Seizures. Doctoral Thesis, Institute of Psychiatry and Neurology, Warsaw, Poland, 2020. [Google Scholar]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Helen Cross, J.; Elger, C.E.; Engel Jr, J.; Forsgren, L.; French, J.A.; Glynn, M.; et al. A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef]
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W.; et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010, 51, 676–685. [Google Scholar] [CrossRef]
- Konishi, S.; Kitagawa, G. Information Criteria and Statistical Modeling; Springer: New York, NY, USA, 2008. [Google Scholar]
- Burnham, K.P.; Anderson, D. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Acar, G.; Tanriover, G.; Acar, F.; Demir, R. Increased Expression of Matrix Metalloproteinase-9 in Patients with temporal Lobe Epilepsy. Turk. Neurosurg. 2015, 25, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Kaur, M.; Singla, N.; Radotra, B.; Gupta, K.S.; Sahni, D. Mesial Temporal Lobe Epilepsy: Role of Reelin Signalling Pathway. Oral Present. 2019 Annu. Meet. Congr. Neurol. Surg. 2019, 66, 2019. [Google Scholar] [CrossRef]
- Ethell, I.M.; Ethell, D.W. Matrix metalloproteinases in brain development and remodeling: Synaptic functions and targets. J. Neurosci. Res. 2007, 85, 2813–2823. [Google Scholar] [CrossRef]
- Rempe, R.G.; Hartz, A.M.S.; Bauer, B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.L.; Brites, D.; Brito, M.A. Looking at the blood-brain barrier: Molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010, 64, 328–363. [Google Scholar] [CrossRef] [PubMed]
- Bigg, H.F.; Rowan, A.D.; Barker, M.D.; Cawston, T.E. Activity of matrix metalloproteinase-9 against native collagen types I and III. FEBS J. 2007, 274, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Denney, H.; Clench, M.R.; Woodroofe, M.N. Cleavage of chemokines CCL2 and CXCL10 by matrix metalloproteinases-2 and -9: Implications for chemotaxis. Biochem. Biophys. Res. Commun. 2009, 382, 341–347. [Google Scholar] [CrossRef]
- Van Den Steen, P.E.; Proost, P.; Wuyts, A.; Van Damme, J.; Opdenakker, G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-α and leaves RANTES and MCP-2 intact. Blood 2000, 96, 2673–2681. [Google Scholar] [CrossRef]
- Van den Steen, P.E.; Dubois, B.; Nelissen, I.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit. Rev. Biochem. Mol. Biol. 2002, 37, 375–536. [Google Scholar] [CrossRef] [PubMed]
- Asahi, M.; Wang, X.; Mori, T.; Sumii, T.; Jung, J.C.; Moskowitz, M.A.; Fini, M.E.; Lo, E.H. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J. Neurosci. 2001, 21, 7724–7732. [Google Scholar] [CrossRef] [PubMed]
- Michaluk, P.; Wawrzyniak, M.; Alot, P.; Szczot, M.; Wyrembek, P.; Mercik, K.; Medvedev, N.; Wilczek, E.; De Roo, M.; Zuschratter, W.; et al. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J. Cell Sci. 2011, 124, 3369–3380. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Anderson, P.; Durbeej, M.; Van Rooijen, N.; Ivars, F.; Opdenakker, G.; Sorokin, L.M. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 2006, 203, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Gorkiewicz, T.; Szczuraszek, K.; Wyrembek, P.; Michaluk, P.; Kaczmarek, L.; Mozrzymas, J.W. Matrix Metalloproteinase-9 reversibly affects the time course of NMDA-induced currents in cultured rat hippocampal neurons. Hippocampus 2010, 20, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Michaluk, P.; Mikasova, L.; Groc, L.; Frischknecht, R.; Choquet, D.; Kaczmarek, L. Matrix Metalloproteinase-9 Controls NMDA Receptor Surface Diffusion through Integrin 1 Signaling. J. Neurosci. 2009, 29, 6007–6012. [Google Scholar] [CrossRef]
- Qin, L.H.; Huang, W.; Mo, X.A.; Chen, Y.L.; Wu, X.H. LPS induces occludin dysregulation in cerebral microvascular endothelial cells via MAPK signaling and augmenting mmp-2 levels. Oxid. Med. Cell Longev. 2015, 2015, 120641. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix Metalloproteinase-Mediated Disruption of Tight Junction Proteins in Cerebral Vessels is Reversed by Synthetic Matrix Metalloproteinase Inhibitor in Focal Ischemia in Rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef]
- Overall, C. Molecular Determinants of Metalloproteinase Substrate Specificity: Matrix Metalloproteinase Substrate Binding Domains, Modules, and Exosites. Mol. Biotechnol. 2002, 22, 51–86. [Google Scholar] [CrossRef]
- Rylski, M.; Amborska, R.; Zybura, K.; Michaluk, P.; Bielinska, B.; Konopacki, F.A.; Wilczynski, G.M.; Kaczmarek, L. JunB is a repressor of MMP-9 transcription in depolarized rat brain neurons. Mol. Cell Neurosci. 2009, 40, 98–110. [Google Scholar] [CrossRef]
- Meighan, S.E.; Meighan, P.C.; Choudhury, P.; Davis, C.J.; Olson, M.L.; Zornes, P.A.; Wright, J.W.; Harding, J.W. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J. Neurochem. 2006, 96, 1227–1241. [Google Scholar] [CrossRef]
- Bartholomé, E.J.; Van Aelst, I.; Koyen, E.; Kiss, R.; Willems, F.; Goldman, M.; Opdenakker, G. Human Monocyte-Derived Dendritic Cells Produce Bioactive Gelatinase B: Inhibition by IFN-β. J. Interf. Cytokine Res. 2001, 21, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Planas, A.M.; Solé, S.; Justicia, C. Expression and Activation of Matrix Metalloproteinase-2 and -9 in Rat Brain after Transient Focal Cerebral Ischemia. Neurobiol. Dis. 2001, 8, 834–846. [Google Scholar] [CrossRef]
- Takacs, E.; Nyilas, R.; Szpesei, Z.; Baracskay, P.; Karlsen, B.; Rosvold, T.; Bjørkum, A.A.; Czurko, A.; Kovacs, Z.; Kekesi, A.K.; et al. Matrix metalloproteinase-9 activity increased by two different types of epileptic seizures that do not induce neuronal death: A possible role in homeostatic synaptic plasticity. Neurochem. Int. 2010, 56, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wu, C.; Korpos, E.; Zhang, X.; Agrawal, S.M.; Wang, Y.; Faber, C.; Schäfers, M.; Körner, H.; Opdenakker, G.; et al. Focal MMP-2 and MMP-9 Activity at the Blood-Brain Barrier Promotes Chemokine-Induced Leukocyte Migration. Cell Rep. 2015, 10, 1040–1054. [Google Scholar] [CrossRef]
- Stetler-Stevenson, W.G. Tissue inhibitors of metalloproteinases in cell signaling: Metalloproteinase-independent biological activities. Sci. Signal. 2008, 1, re6. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-H.; Park, S.-Y.; Rho, S.B.; Lee, J.-H. Tissue inhibitor of metalloproteinases-3 interacts with angiotensin II type 2 receptor and additively inhibits angiogenesis. Cardiovasc. Res. 2008, 79, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, D.M.; Pérez-Martínez, L. Tissue inhibitor of metalloproteinase-2 (TIMP-2) expression is regulated by multiple neural differentiation signals. J. Neurochem. 2006, 98, 234–247. [Google Scholar] [CrossRef]
- Kopitz, C.; Gerg, M.; Bandapalli, O.R.; Ister, D.; Pennington, C.J.; Hauser, S.; Flechsig, C.; Krell, H.-W.; Antolovic, D.; Brew, K.; et al. Tissue Inhibitor of Metalloproteinases-1 Promotes Liver Metastasis by Induction of Hepatocyte Growth Factor Signaling. Cancer Res. 2007, 67, 8615–8623. [Google Scholar] [CrossRef]
- Magnowska, M.; Gorkiewicz, T.; Suska, A.; Wawrzyniak, M.; Rutkowska-Wlodarczyk, I.; Kaczmarek, L.; Wlodarczyk, J. Transient ECM protease activity promotes synaptic plasticity. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Tejima, E.; Guo, S.; Murata, Y.; Arai, K.; Lok, J.; van Leyen, K.; Rosell, A.; Wang, X.; Lo, E.H. Neuroprotective effects of overexpressing tissue inhibitor of metalloproteinase TIMP-1. J. Neurotrauma 2009, 26, 1935–1941. [Google Scholar] [CrossRef]
- Moore, C.S.; Crocker, S.J. An Alternate Perspective on the Roles of TIMPs and MMPs in Pathology. Am. J. Pathol. 2012, 180, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Murcko, R.; Marchi, N.; Bailey, D.; Janigro, D. Diagnostic biomarker kinetics: How brain-derived biomarkers distribute through the human body, and how this affects their diagnostic significance: The case of S100B. Fluids Barriers CNS 2022, 19, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Arcuri, C.; Bianchi, R.; Brozzi, F.; Donato, R. S100B increases proliferation in PC12 neuronal cells and reduces their responsiveness to nerve growth factor via Akt activation. J. Biol. Chem. 2005, 280, 4402–4414. [Google Scholar] [CrossRef] [PubMed]
- Riuzzi, F.; Sorci, G.; Donato, R. S100B protein regulates myoblast proliferation and differentiation by activating FGFR1 in a bFGF-dependent manner. J. Cell Sci. 2011, 124, 2389–2400. [Google Scholar] [CrossRef]
- Xiong, Z.G.; O’Hanlon, D.; Becker, L.E.; Roder, J.; MacDonald, J.F.; Marks, A. Enhanced calcium transients in glial cells in neonatal cerebellar cultures derived from S100B null mice. Exp. Cell Res. 2000, 257, 281–289. [Google Scholar] [CrossRef]
- Heierhorst, J.; Kobe, B.; Feil, S.C.; Parker, M.W.; Benian, G.M.; Weiss, K.R.; Kemp, B.E. Ca2+/S100 regulation of giant protein kinases. Nature 1996, 380, 636–639. [Google Scholar] [CrossRef]
- Hajduková, L.; Sobek, O.; Prchalová, D.; Bílková, Z.; Koudelková, M.; Lukášková, J.; Matuchová, I. Biomarkers of Brain Damage: S100B and NSE Concentrations in Cerebrospinal Fluid-A Normative Study. Biomed Res. Int. 2015, 2015, 379071. [Google Scholar] [CrossRef]
- Kligman, D.; Marshak, D.R. Purification and characterization of a neurite extension factor from bovine brain. Proc. Natl. Acad. Sci. USA 1985, 82, 7136–7139. [Google Scholar] [CrossRef]
- Winningham-Major, F.; Staecker, J.L.; Barger, S.W.; Coats, S.; Van Eldik, L.J. Neurite extension and neuronal survival activities of recombinant S100β proteins that differ in the content and position of cysteine residues. J. Cell Biol. 1989, 109, 3063–3071. [Google Scholar] [CrossRef]
- Nishiyama, H.; Knöpfel, T.; Endo, S.; Itohara, S. Glial protein S100B modulates long-term neuronal synaptic plasticity. Proc. Natl. Acad. Sci. USA 2002, 99, 4037–4042. [Google Scholar] [CrossRef] [PubMed]
- Businaro, R.; Leone, S.; Fabrizi, C.; Sorci, G.; Donato, R.; Lauro, G.M.; Fumagalli, L. S100B protects LAN-5 neuroblastoma cells against Abeta amyloid-induced neurotoxicity via RAGE engagement at low doses but increases Abeta amyloid neurotoxicity at high doses. J. Neurosci. Res. 2006, 83, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Koppal, T.; Lam, A.G.; Guo, L.; Van Eldik, L.J. S100B proteins that lack one or both cysteine residues can induce inflammatory responses in astrocytes and microglia. Neurochem. Int. 2001, 39, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, A.; Seoane, R.; Torres, A.G.; Rosciszewski, G.; Angelo, M.F.; Rossi, A.; Barkert, P.A.; Ramos, A.J. S100B protein activates a RAGE-dependent autocrine loop in astrocytes: Implications for its role in the propagation of reactive gliosis. J. Neurochem. 2014, 131, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, R.; Giambanco, I.; Donato, R. S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 Co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha. Neurobiol. Aging 2010, 31, 665–677. [Google Scholar] [CrossRef]
- Szklarczyk, A.; Lapinska, J.; Rylski, M.; McKay, R.D.G.; Kaczmarek, L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J. Neurosci. 2002, 22, 920–930. [Google Scholar] [CrossRef]
- Gorter, J.A.; van Vliet, E.A.; Aronica, E. Status epilepticus, blood–brain barrier disruption, inflammation, and epileptogenesis. Epilepsy Behav. 2015, 49, 13–16. [Google Scholar] [CrossRef]
- Rankin-Gee, E.K.; McRae, P.A.; Baranov, E.; Rogers, S.; Wandrey, L.; Porter, B.E. Perineuronal net degradation in epilepsy. Epilepsia 2015, 56, 1124–1133. [Google Scholar] [CrossRef]
- Gorter, J.A.; Van Vliet, E.A.; Rauwerda, H.; Breit, T.; Stad, R.; Van Schaik, L.; Vreugdenhil, E.; Redeker, S.; Hendriksen, E.; Aronica, E.; et al. Dynamic changes of proteases and protease inhibitors revealed by microarray analysis in CA3 and entorhinal cortex during epileptogenesis in the rat. Epilepsia 2007, 48, 53–64. [Google Scholar] [CrossRef]
- Li, Y.-J.; Wang, Z.-H.; Zhang, B.; Zhe, X.; Wang, M.-J.; Shi, S.-T.; Bai, J.; Lin, T.; Guo, C.-J.; Zhang, S.-J.; et al. Disruption of the blood-brain barrier after generalized tonic-clonic seizures correlates with cerebrospinal fluid MMP-9 levels. J. Neuroinflammation 2013, 10, 80. [Google Scholar] [CrossRef]
- Tao, H.; Gong, Y.; Yu, Q.; Zhou, H.; Liu, Y. Elevated Serum Matrix Metalloproteinase-9, Interleukin-6, Hypersensitive C-Reactive Protein, and Homocysteine Levels in Patients with Epilepsy. J. Interf. Cytokine Res. 2020, 40, 152–158. [Google Scholar] [CrossRef]
- Wang, R.; Zeng, G.Q.; Liu, X.; Tong, R.Z.; Zhou, D.; Hong, Z. Evaluation of serum matrix metalloproteinase-3 as a biomarker for diagnosis of epilepsy. J. Neurol. Sci. 2016, 367, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Quirico-Santos, T.; Nascimento Mello, A.; Casimiro Gomes, A.; de Carvalho, L.P.; de Souza, J.M.; Alves-Leon, S. Increased metalloprotease activity in the epileptogenic lesion—Lobectomy reduces metalloprotease activity and urokinase-type uPAR circulating levels. Brain Res. 2013, 1538, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zeng, G.Q.; Tong, R.Z.; Zhou, D.; Hong, Z. Serum matrix metalloproteinase-2: A potential biomarker for diagnosis of epilepsy. Epilepsy Res. 2016, 122, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Salih, K.S.; Hamdan, F.B.; Al-Mayah, Q.S. Diagnostic value of matrix metalloproteinase-2 and high mobility group box 1 in patients with refractory epilepsy. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 102. [Google Scholar] [CrossRef]
- Soliman, A.M.; Ebeary, M.E.S.E.; Gouda, T.A.R.; Ebied, A.A. Serum matrix metalloproterinse-2 as a biomarker for diagnosis of idiopathic epilepsy. Zagazig Univ. Med. J. 2019, 25, 155–163. [Google Scholar] [CrossRef]
- Rivera, S.; Tremblay, E.; Timsit, S.; Canals, O.; Ben-Ari, Y.; Khrestchatisky, M. Tissue inhibitor of metalloproteinases-1 (TIMP-1) is differentially induced in neurons and astrocytes after seizures: Evidence for developmental, immediate early gene, and lesion response. J. Neurosci. 1997, 17, 4223–4235. [Google Scholar] [CrossRef]
- Stein, V.M.; Genini, S.; Puff, C.; Baumgärtner, W.; Tipold, A. Seizure activity in dogs is associated with enhanced TIMP-2 expression of microglia. Vet. Immunol. Immunopathol. 2012, 146, 101–105. [Google Scholar] [CrossRef]
- Kittaka, S.; Hasegawa, S.; Ito, Y.; Ohbuchi, N.; Suzuki, E.; Kawano, S.; Aoki, Y.; Nakatsuka, K.; Kudo, K.; Wakiguchi, H.; et al. Serum levels of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinases-1 in human herpesvirus-6–infected infants with or without febrile seizures. J. Infect. Chemother. 2014, 20, 716–721. [Google Scholar] [CrossRef]
- Suenaga, N.; Ichiyama, T.; Kubota, M.; Isumi, H.; Tohyama, J.; Furukawa, S. Roles of matrix metalloproteinase-9 and tissue inhibitors of metalloproteinases 1 in acute encephalopathy following prolonged febrile seizures. J. Neurol. Sci. 2008, 266, 126–130. [Google Scholar] [CrossRef]
- Leppert, D.; Leib, S.L.; Grygar, C.; Miller, K.M.; Schaad, U.B.; Holländer, G. a Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: Association with blood-brain barrier damage and neurological sequelae. Clin. Infect. Dis. 2000, 31, 80–84. [Google Scholar] [CrossRef]
- Haberlandt, E.; Rauchenzauner, M.; Morass, M.; Wondrak, P.; Bürgi, S.S.; Rostásy, K.; Karall, D. Matrix-metalloproteinases and proinflammatory cytokines in children with febrile convulsions and epilepsy-Cause or consequence? Epilepsy Res. 2013, 105, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Filibian, M.; Frasca, A.; Maggioni, D.; Micotti, E.; Vezzani, A.; Ravizza, T. In vivo imaging of glia activation using 1H-magnetic resonance spectroscopy to detect putative biomarkers of tissue epileptogenicity. Epilepsia 2012, 53, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Oses, J.P.; Leke, R.; Portela, L.V.; Lara, D.R.; Schmidt, A.P.; Casali, E.A.; Wofchuk, S.; Souza, D.O.; Sarkis, J.J.F. Biochemical brain markers and purinergic parameters in rat CSF after seizure induced by pentylenetetrazol. Brain Res. Bull. 2004, 64, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Blyth, B.J.; Farhavar, A.; Gee, C.; Hawthorn, B.; He, H.; Nayak, A.; Stöcklein, V.; Bazarian, J.J. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J. Neurotrauma 2009, 26, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Marchi, N.; Angelov, L.; Masaryk, T.; Fazio, V.; Granata, T.; Hernandez, N.; Hallene, K.; Diglaw, T.; Franic, L.; Najm, I.; et al. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia 2007, 48, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, J.; Sun, W.; Feng, L.; Li, L.; Liu, A.; Li, J.; Mao, W.; Wei, H.; Gao, L.; et al. Elevated plasma S100B concentration is associated with mesial temporal lobe epilepsy in Han Chinese: A case-control study. Neurosci. Lett. 2010, 484, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Calik, M.; Abuhandan, M.; Sonmezler, A.; Kandemir, H.; Oz, I.; Taskin, A.; Selek, S.; Iscan, A. Elevated serum S-100B levels in children with temporal lobe epilepsy. Seizure 2013, 22, 99–102. [Google Scholar] [CrossRef]
- Portela, L.V.C.; Tort, A.B.L.; Walz, R.; Bianchin, M.; Trevisol-Bittencourt, P.C.; Wille, P.R.; Cardoso, R.C.; Ishida, M.M.I.; VonWangenheim, A.; Grisard, E.C.; et al. Interictal serum S100B levels in chronic neurocysticercosis and idiopathic epilepsy. Acta Neurol. Scand. 2003, 108, 424–427. [Google Scholar] [CrossRef]
- Palmio, J.; Peltola, J.; Vuorinen, P.; Laine, S.; Suhonen, J.; Keränen, T. Normal CSF neuron-specific enolase and S-100 protein levels in patients with recent non-complicated tonic-clonic seizures. J. Neurol. Sci. 2001, 183, 27–31. [Google Scholar] [CrossRef]
- Liang, K.G.; Mu, R.Z.; Liu, Y.; Jiang, D.; Jia, T.T.; Huang, Y.J. Increased serum S100B levels in patients with epilepsy: A systematic review and meta-analysis study. Front. Neurosci. 2019, 13, 456. [Google Scholar] [CrossRef]
- Librizzi, L.; Regondi, M.C.; Pastori, C.; Frigerio, S.; Frassoni, C.; De Curtis, M. Expression of adhesion factors induced by epileptiform activity in the endothelium of the isolated guinea pig brain in vitro. Epilepsia 2007, 48, 743–751. [Google Scholar] [CrossRef]
- Kan, A.A.; de Jager, W.; de Wit, M.; Heijnen, C.; van Zuiden, M.; Ferrier, C.; van Rijen, P.; Gosselaar, P.; Hessel, E.; van Nieuwenhuizen, O.; et al. Protein expression profiling of inflammatory mediators in human temporal lobe epilepsy reveals co-activation of multiple chemokines and cytokines. J. Neuroinflammation 2012, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.I.; Elisevich, K.V. Brain region and epilepsy-associated differences in inflammatory mediator levels in medically refractory mesial temporal lobe epilepsy. J. Neuroinflammation 2016, 13, 270. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Baybis, M.; Newman, D.; Kolson, D.L.; Chen, W.; McKhann, G.; Gutmann, D.H.; Crino, P.B. Expression of ICAM-1, TNF-α, NFκB, and MAP kinase in tubers of the tuberous sclerosis complex. Neurobiol. Dis. 2003, 14, 279–290. [Google Scholar] [CrossRef]
- Luo, J.; Wang, W.; Xi, Z.; Dan, C.; Wang, L.; Xiao, Z.; Wang, X. Concentration of Soluble Adhesion Molecules in Cerebrospinal Fluid and Serum of Epilepsy Patients. J. Mol. Neurosci. 2014, 54, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Cudna, A.; Kurkowska-Jastrzebska, I. Blood–brain barrier markers after acute epileptic seizures. J. Neuroimmunol. 2014, 275, 28. [Google Scholar] [CrossRef]
- Carr, M.W.; Roth, S.J.; Luther, E.; Rose, S.S.; Springer, T.A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc. Natl. Acad. Sci. USA 1994, 91, 3652–3656. [Google Scholar] [CrossRef]
- Allavena, P.; Bianchi, G.; Zhou, D.; van Damme, J.; Jílek, P.; Sozzani, S.; Mantovani, A. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. Eur. J. Immunol. 1994, 24, 3233–3236. [Google Scholar] [CrossRef]
- Park, J.; Ryu, D.R.; Li, J.J.; Jung, D.S.; Kwak, S.J.; Lee, S.H.; Yoo, T.H.; Han, S.H.; Lee, J.E.; Kim, D.K.; et al. MCP-1/CCR2 system is involved in high glucose-induced fibronectin and type IV collagen expression in cultured mesangial cells. Am. J. Physiol. Ren. Physiol. 2008, 295, F749–F757. [Google Scholar] [CrossRef]
- Song, L.; Pachter, J.S. Monocyte chemoattractant protein-1 alters expression of tight junction-associated proteins in brain microvascular endothelial cells. Microvasc. Res. 2004, 67, 78–89. [Google Scholar] [CrossRef]
- Nelson, T.E.; Hao, C.; Manos, J.; Ransohoff, R.M.; Gruol, D.L. Altered hippocampal synaptic transmission in transgenic mice with astrocyte-targeted enhanced CCL2 expression. Brain. Behav. Immun. 2011, 25 (Suppl. 1), S106–S119. [Google Scholar] [CrossRef] [PubMed]
- Kalehua, A.N.; Nagel, J.E.; Whelchel, L.M.; Gides, J.J.; Pyle, R.S.; Smith, R.J.; Kusiak, J.W.; Taub, D.D. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-2 are involved in both excitotoxin-induced neurodegeneration and regeneration. Exp. Cell Res. 2004, 297, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Cerri, C.; Genovesi, S.; Allegra, M.; Pistillo, F.; Püntener, U.; Guglielmotti, A.; Perry, V.H.; Bozzi, Y.; Caleo, M. The Chemokine CCL2 Mediates the Seizure-enhancing Effects of Systemic Inflammation. J. Neurosci. 2016, 37, 1–5. [Google Scholar] [CrossRef]
- Lee, T.-S.; Mane, S.; Eid, T.; Zhao, H.; Lin, A.; Guan, Z.; Kim, J.H.; Schweitzer, J.; King-Stevens, D.; Weber, P.; et al. Gene Expression in Temporal Lobe Epilepsy is Consistent with Increased Release of Glutamate by Astrocytes. Mol. Med. 2007, 13, 1–13. [Google Scholar] [CrossRef]
- Iyer, A.; Zurolo, E.; Spliet, W.G.M.; Van Rijen, P.C.; Baayen, J.C.; Gorter, J.A.; Aronica, E. Evaluation of the innate and adaptive immunity in type I and type II focal cortical dysplasias. Epilepsia 2010, 51, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Aalbers, M.W.; Rijkers, K.; Majoie, H.J.M.; Dings, J.T.; Schijns, O.E.M.G.; Schipper, S.; De Baets, M.H.; Kessels, A.; Vles, J.S.H.; Hoogland, G. The influence of neuropathology on brain inflammation in human and experimental temporal lobe epilepsy. J. Neuroimmunol. 2014, 271, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kothur, K.; Bandodkar, S.; Wienholt, L.; Chu, S.; Pope, A.; Gill, D.; Dale, R.C. Etiology is the key determinant of neuroinflammation in epilepsy: Elevation of cerebrospinal fluid cytokines and chemokines in febrile infection-related epilepsy syndrome and febrile status epilepticus. Epilepsia 2019, 1678–1688. [Google Scholar] [CrossRef]
- Kawamura, Y.; Yamazaki, Y.; Ohashi, M.; Ihira, M.; Yoshikawa, T. Monocyte chemoattractant protein-1 alters expression of tight junction-associated proteins in brain microvascular endothelial cells. J. Med. Virol. 2014, 86, 512–518. [Google Scholar] [CrossRef]
- Yamanaka, G.; Morishita, N.; Morichi, S.; Takeshita, M.; Tomomi, U.; Ishida, Y.; Tomoko, T.; Oana, S.; Watanabe, Y.; Go, S.; et al. Serial Analysis of Multiple Serum Cytokine Responses to Adrenocorticotropic Hormone Therapy in Patients With West Syndrome. J. Child Neurol. 2018, 33, 528–533. [Google Scholar] [CrossRef]
- Shiihara, T.; Miyashita, M.; Yoshizumi, M.; Watanabe, M.; Yamada, Y.; Kato, M. Peripheral lymphocyte subset and serum cytokine profiles of patients with West syndrome. Brain Dev. 2010, 32, 695–702. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Apeksha, A.; Norenberg, M.D. Role of Matricellular Proteins in Disorders of the Central Nervous System. Neurochem. Res. 2017, 42, 858–875. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.A.; Noubary, F.; Wang, D.; Dulla, C.G. α2δ-1 signaling drives cell death, synaptogenesis, circuit reorganization, and gabapentin-mediated neuroprotection in a model of insult-induced cortical malformation. eNeuro 2017, 4, ENEURO.0316-17.2017. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kyriakides, T.R.; Bornstein, P. Matricellular proteins as modulators of cell-matrix interactions: Adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol. Biol. Cell 2000, 11, 3353–3364. [Google Scholar] [CrossRef] [PubMed]
- Andresen, L.; Hampton, D.; Taylor, A.; Morel, L.; Yang, Y.; Maguire, J.; Dulla, C.G.; Dulla, C. Gabapentin attenuates hyperexcitability in the freeze-lesion model of developmental cortical malformation. Neurobiol. Dis. 2014, 71, 305–316. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Schafer, M.J.; Hooten, N.N.; Atkinson, E.J.; Evans, M.K.; Baker, D.J.; Quarles, E.K.; Robbins, P.D.; Ladiges, W.C.; Lebrasseur, N.K.; et al. Circulating levels of monocyte chemoattractant protein-1 as a potential measure of biological age in mice and frailty in humans. Aging Cell 2017, 17, e12706. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Lee, H.J.; Heo, J.; Lim, J.; Kim, M.; Kim, M.K.; Nam, H.Y.; Hong, G.H.; Cho, Y.S.; Choi, S.J.; et al. Senescence-Associated MCP-1 Secretion Is Dependent on a Decline in BMI1 in Human Mesenchymal Stromal Cells. Antioxid. Redox Signal. 2016, 24, 471–485. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A: Biomed. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- Inadera, H.; Egashira, K.; Takemoto, M.; Ouchi, Y.; Matsushima, K. Increase in circulating levels of monocyte chemoattractant protein-1 with aging. J. Interf. Cytokine Res. 1999, 19, 1179–1182. [Google Scholar] [CrossRef]
- Seidler, S.; Zimmermann, H.W.; Bartneck, M.; Trautwein, C.; Tacke, F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010, 11, 30. [Google Scholar] [CrossRef]
- Tayebjee, M.H.; Lip, G.Y.H.; Blann, A.D.; MacFadyen, R.J. Effects of age, gender, ethnicity, diurnal variation and exercise on circulating levels of matrix metalloproteinases (MMP)-2 and -9, and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMP)-1 and -2. Thromb. Res. 2005, 115, 205–210. [Google Scholar] [CrossRef]
- Cancemi, P.; Aiello, A.; Accardi, G.; Caldarella, R.; Candore, G.; Caruso, C.; Ciaccio, M.; Cristaldi, L.; Di Gaudio, F.; Siino, V.; et al. The Role of Matrix Metalloproteinases (MMP-2 and MMP-9) in Ageing and Longevity: Focus on Sicilian Long-Living Individuals (LLIs). Mediators Inflamm. 2020, 2020, 8635158. [Google Scholar] [CrossRef] [PubMed]
- Beaudeux, J.L.; Giral, P.; Bruckert, E.; Bernard, M.; Foglietti, M.J.; Chapman, M.J. Serum matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 as potential markers of carotid atherosclerosis in infraclinical hyperlipidemia. Atherosclerosis 2003, 169, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.L.; Mcdonald, V.M.; Baines, K.J.; Oreo, K.M.; Wang, F.; Hansbro, P.M.; Gibson, P.G. Influence of Age, Past Smoking, and Disease Severity on TLR2, Neutrophilic Inflammation, and MMP-9 Levels in COPD. Mediators Inflamm. 2013, 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Bonnema, D.D.; Webb, C.S.; Pennington, W.R.; Stroud, R.E.; Leonardi, A.E.; Clark, L.L.; McClure, C.D.; Finklea, L.; Spinale, F.G.; Zile, M.R. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). J Card. Fail 2007, 13, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Komosinska-Vassev, K.; Olczyk, P.; Winsz-Szczotka, K.; Kuznik-Trocha, K.; Klimek, K.; Olczyk, K. Age- and gender-dependent changes in connective tissue remodeling: Physiological differences in circulating MMP-3, MMP-10, TIMP-1 and TIMP-2 level. Gerontology 2010, 57, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Portela, L.V.C.; Tort, A.B.L.; Schaf, D.V.; Ribeiro, L.; Nora, D.B.; Walz, R.; Rotta, L.N.; Silva, C.T.; Busnello, J.V.; Kapczinski, F.; et al. The serum S100B concentration is age dependent. Clin. Chem. 2002, 48, 950–952. [Google Scholar] [CrossRef]
- Deneva-Koycheva, T.I.; Vladimirova-Kitova, L.G.; Angelova, E.A.; Tsvetkova, T.Z. Serum levels of siCAM-1, sVCAM-1, sE-selectin, sP-selectin in healthy Bulgarian people. Folia Med. (Plovdiv) 2011, 53, 22–28. [Google Scholar] [CrossRef]
- Grothey, A.; Heistermann, P.; Philippou, S.; Voigtmann, R. Serum levels of soluble intercellular adhesion molecule-1 (ICAM-1, CD54) in patients with non-small cell lung cancer: Correlation with histological expression of ICAM-1 and tumour stage. Br. J. Cancer 1998, 77, 801–807. [Google Scholar] [CrossRef]
- Morikawa, N.; Adachi, H.; Enomoto, M.; Fukami, A.; Kumagai, E.; Nakamura, S.; Nohara, Y.; Nakao, E.; Kono, S.; Tsuru, T.; et al. Thrombospondin-2 as a potential risk factor in a general population. Int. Heart J. 2019, 60, 310–317. [Google Scholar] [CrossRef]
- Hanatani, S.; Izumiya, Y.; Takashio, S.; Kimura, Y.; Araki, S.; Rokutanda, T.; Tsujita, K.; Yamamoto, E.; Tanaka, T.; Yamamuro, M.; et al. Circulating thrombospondin-2 reflects disease severity and predicts outcome of heart failure with reduced ejection fraction. Circ. J. 2014, 78, 903–910. [Google Scholar] [CrossRef]
- Young-Min, S.A.; Beeton, C.; Laughton, R.; Plumpton, T.; Bartram, S.; Murphy, G.; Black, C.; Cawston, T.E. Serum TIMP-1, TIMP-2, and MMP-1 in patients with systemic sclerosis, primary Raynaud’s phenomenon, and in normal controls. Ann. Rheum. Dis. 2001, 60, 846–851. [Google Scholar] [PubMed]
- Wiesmann, M.; Missler, U.; Gottmann, D.; Gehring, S. Plasma S-100b protein concentration in healthy adults is age- and sex- independent. Clin. Chem. 1998, 44, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Lara, D.R.; Gama, C.S.; Belmonte-De-Abreu, P.; Portela, L.V.C.; Gonçalves, C.A.; Fonseca, M.; Hauck, S.; Souza, D.O. Increased serum S100B protein in schizophrenia: A study in medication-free patients. J. Psychiatr. Res. 2001, 35, 11–14. [Google Scholar] [CrossRef]
- Calik, M.; Abuhandan, M.; Kandemir, H.; Güzel, B.; Solmaz, A.; Celik, H.; Taskin, A.; Iscan, A. Interictal serum S-100B protein levels in intractable epilepsy: A case-control study. Neurosci. Lett. 2014, 558, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Irani, S.R.; Bera, K.; Waters, P.; Zuliani, L.; Maxwell, S.; Zandi, M.S.; Friese, M.A.; Galea, I.; Kullmann, D.M.; Beeson, D.; et al. N-methyl-d-aspartate antibody encephalitis: Temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010, 133, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Murea, M.; Register, T.C.; Divers, J.; Bowden, D.W.; Carr, J.J.; Hightower, C.R.; Xu, J.; Smith, S.C.; Hruska, K.A.; Langefeld, C.D.; et al. Relationships between serum MCP-1 and subclinical kidney disease: African American-Diabetes Heart Study. BMC Nephrol. 2012, 13, 6–8. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Nevala, W.K.; Dronca, R.S.; Leontovich, A.A.; Shuster, L.; Markovic, S.N. Normal ageing is associated with an increase in Th2 cells, MCP-1 (CCL1) and RANTES (CCL5), with differences in sCD40L and PDGF-AA between sexes. Clin. Exp. Immunol. 2012, 170, 186–193. [Google Scholar] [CrossRef]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Van Lint, P.; Libert, C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J. Leukoc. Biol. 2007, 82, 1375–1381. [Google Scholar] [CrossRef]
- Schönbeck, U.; Mach, F.; Libby, P. Generation of biologically active IL-1 beta by matrix metalloproteinases: A novel caspase-1-independent pathway of IL-1 beta processing. J. Immunol. 1998, 161, 3340–3346. [Google Scholar] [CrossRef]
- Munaut, C.; Noël, A.; Hougrand, O.; Foidart, J.M.; Boniver, J.; Deprez, M. Vascular endothelial growth factor expression correlates with matrix metalloproteinases MT1-MMP, MMP-2 and MMP-9 in human glioblastomas. Int. J. Cancer 2003, 106, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Juttermann, R.; Soloway, P.D. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J. Biol. Chem. 2000, 275, 26411–26415. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; McEver, R.P. The cytoplasmic domain of P-selectin is phosphorylated on serine and threonine residues. Blood 1993, 82, 1758–1766. [Google Scholar] [CrossRef] [PubMed]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| diagnosis of epilepsy * | malignant tumor |
| minimum time of seizure freedom of seven days | inflammatory disease |
| severe ongoing neurological disease with vascular damage(e.g., acute stroke within last six months) | |
| neuroimmunological disease | |
| immunosuppressive or immunomodulatory treatment during last six months | |
| surgery within last two weeks | |
| significant trauma within last two weeks | |
| hepatic, renal, or cardiac insufficiency | |
| severe psychiatric disease | |
| symptoms of infection | |
| CRP above the laboratory norm | |
| pregnancy |
| Research Group | Control Group | |
|---|---|---|
| age (mean ± SEM) | 43.01 ± 1.53 | 42.16 ± 1.58 |
| age (median) | 39 | 39 |
| age (min–max) | 19–82 | 17–84 |
| women | 52 | 52 |
| men | 48 | 48 |
| women: age (mean ± SEM) | 43.89 ± 2.17 | 42.65 ± 2.20 |
| women: age (median) | 39 | 39.5 |
| women: age (min–max) | 19–78 | 17–76 |
| men: age (mean ± SEM) | 42.06 ± 2.16 | 42.63 ± 2.30 |
| men: age (median) | 39 | 38.5 |
| men: age (min–max) | 22–82 | 20–84 |
| Research Group | Control Group | ||
|---|---|---|---|
| Mean ± SEM | Mean ± SEM | p | |
| MMP-9 | 846.66 | 533.35 | <0.0001 |
| [ng/mL] | ±56.35 | ±32.89 | |
| TIMP-1 | 217.6 | 166.12 | 0.001 |
| [ng/mL] | ±11.99 | ±11.83 | |
| MMP-2 | 294.18 | 200.29 | <0.0001 |
| [ng/mL] | ±11.84 | ±7.00 | |
| TIMP-2 | 149.29 | 120.12 | <0.0001 |
| [ng/mL] | ±3.93 | ±3.60 | |
| MMP-9 | 10.21 | 5.31 | 0.71 |
| /TIMP-1 | ±2.64 | ±0.58 | |
| MMP-2 /TIMP-2 | 1.98 | 1.86 | 0.0087 |
| ±0.07 | ±0.14 | ||
| S100B [pg/mL] | 58.93 | 23.62 | <0.0001 |
| ±9.36 | ±3.13 | ||
| TSP-2 [ng/mL] | 29.37 | 33.25 | 0.2 |
| ±1.75 | ±2.46 | ||
| ICAM-1 | 169.5 | 187.34 | 0.09 |
| [ng/mL] | ±6.46 | ±11.03 | |
| CCL-2 | 333.85 | 310.65 | 0.4 |
| [pg/mL] | ±16.49 | ±14.09 | |
| P-sel [ng/mL] | 104.72 ± 6.62 | 126.82 ± 9.38 | 0.19 |
| MMP−9 | MMP−2 | TIMP−1 | TIMP−2 | S100B | ICAM−1 | P−sel | CCL−2 | TSP−2 | MMP−9/ TIMP−1 | MMP−2/ TIMP−2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MMP−9 | 0.278; 0.005 | −0.239; 0.016 | 0.408; <0.0001 | −0.209; 0.037 | 0.722; <0.0001 | 0.259; 0.009 | |||||
| MMP−2 | 0.278; 0.005 | 0.523; <0.0001 | 0.254; 0.011 | −0.501; <0.0001 | 0.218; 0.029 | 0.813; <0.0001 | |||||
| TIMP−1 | −0.239; 0.016 | −0.364; 0.0002 | −0.797; <0.0001 | ||||||||
| TIMP−2 | 0.523; <0.0001 | −0.288; 0.004 | |||||||||
| S100B | |||||||||||
| ICAM−1 | |||||||||||
| P−sel | 0.408; <0.0001 | 0.254; 0.011 | −0.206; 0.040 | 0.312; 0.002 | 0.228; 0.023 | ||||||
| CCL−2 | −0.364; 0.0002 | 0.280; 0.005 | |||||||||
| TSP−2 | −0.209; 0.037 | −0.501; <0.0001 | −0.288; 0.004 | −0.206; 0.040 | −0.377; 0.0001 | ||||||
| MMP−9/ TIMP−1 | 0.722; <0.0001 | 0.218; 0.029 | −0.797; <0.0001 | 0.312; 0.002 | 0.280; 0.005 | ||||||
| MMP−2/ TIMP−2 | 0.259; 0.009 | 0.813; <0.0001 | 0.228; 0.023 | −0.377; 0.0001 | |||||||
| correlation coefficient | positive correlation | negative correlation | |||||||||
| 0.0–0.2 | |||||||||||
| 0.2–0.4 | |||||||||||
| 0.4–0.6 | |||||||||||
| 0.6–0.8 | |||||||||||
| 0.8–1.0 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bronisz, E.; Cudna, A.; Wierzbicka, A.; Kurkowska-Jastrzębska, I. Blood-Brain Barrier-Associated Proteins Are Elevated in Serum of Epilepsy Patients. Cells 2023, 12, 368. https://doi.org/10.3390/cells12030368
Bronisz E, Cudna A, Wierzbicka A, Kurkowska-Jastrzębska I. Blood-Brain Barrier-Associated Proteins Are Elevated in Serum of Epilepsy Patients. Cells. 2023; 12(3):368. https://doi.org/10.3390/cells12030368
Chicago/Turabian StyleBronisz, Elżbieta, Agnieszka Cudna, Aleksandra Wierzbicka, and Iwona Kurkowska-Jastrzębska. 2023. "Blood-Brain Barrier-Associated Proteins Are Elevated in Serum of Epilepsy Patients" Cells 12, no. 3: 368. https://doi.org/10.3390/cells12030368
APA StyleBronisz, E., Cudna, A., Wierzbicka, A., & Kurkowska-Jastrzębska, I. (2023). Blood-Brain Barrier-Associated Proteins Are Elevated in Serum of Epilepsy Patients. Cells, 12(3), 368. https://doi.org/10.3390/cells12030368






