Cord Blood Plasma and Placental Mesenchymal Stem Cells-Derived Exosomes Increase Ex Vivo Expansion of Human Cord Blood Hematopoietic Stem Cells While Maintaining Their Stemness
Abstract
1. Introduction
2. Methods
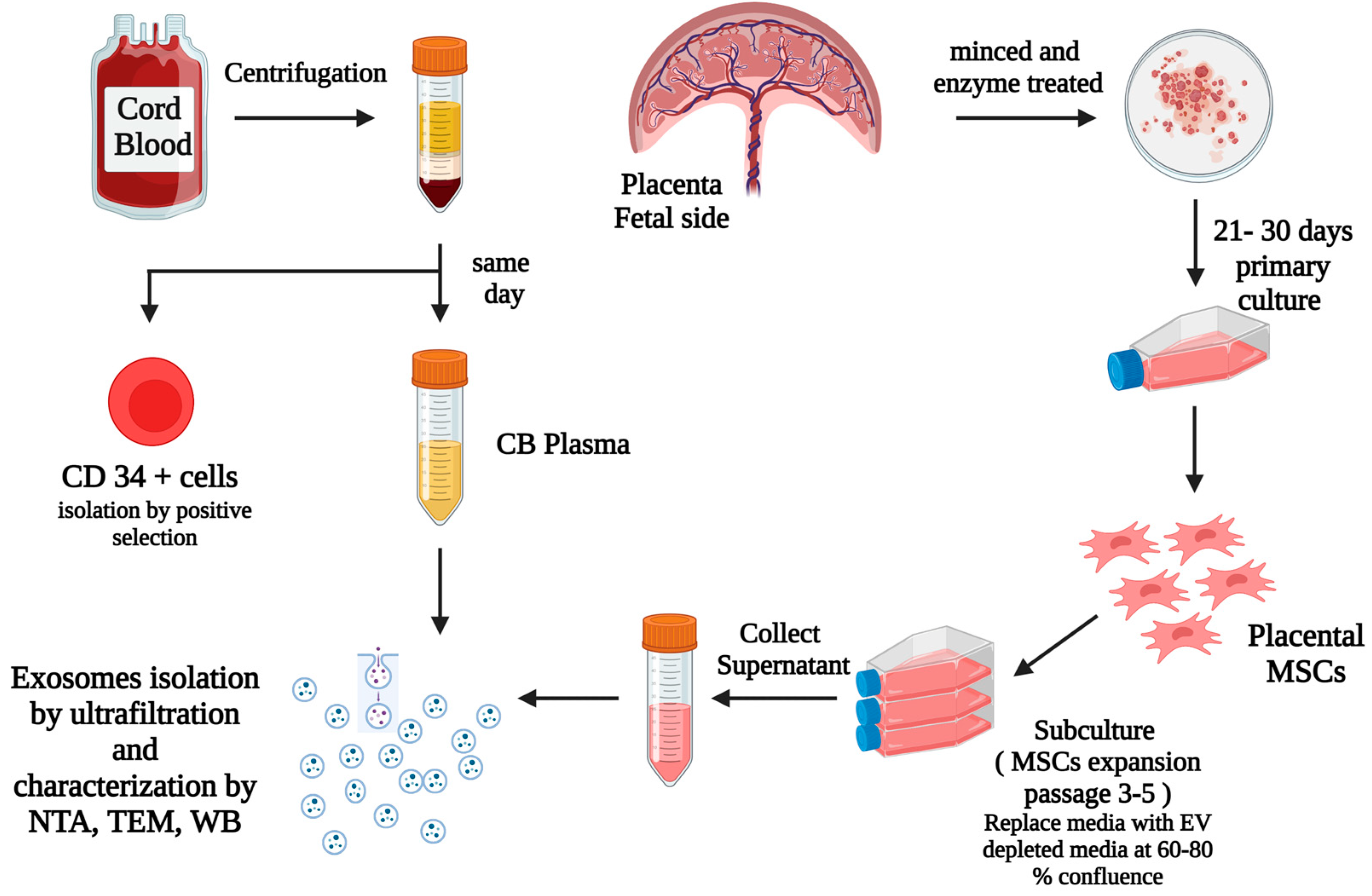
2.1. Sample Collection, Preparation, and Isolation of UCB CD34+ Cells
2.2. Placental Mesenchymal Stem Cells (MSCs) Culture
2.3. Isolation and Identification of Exosomes
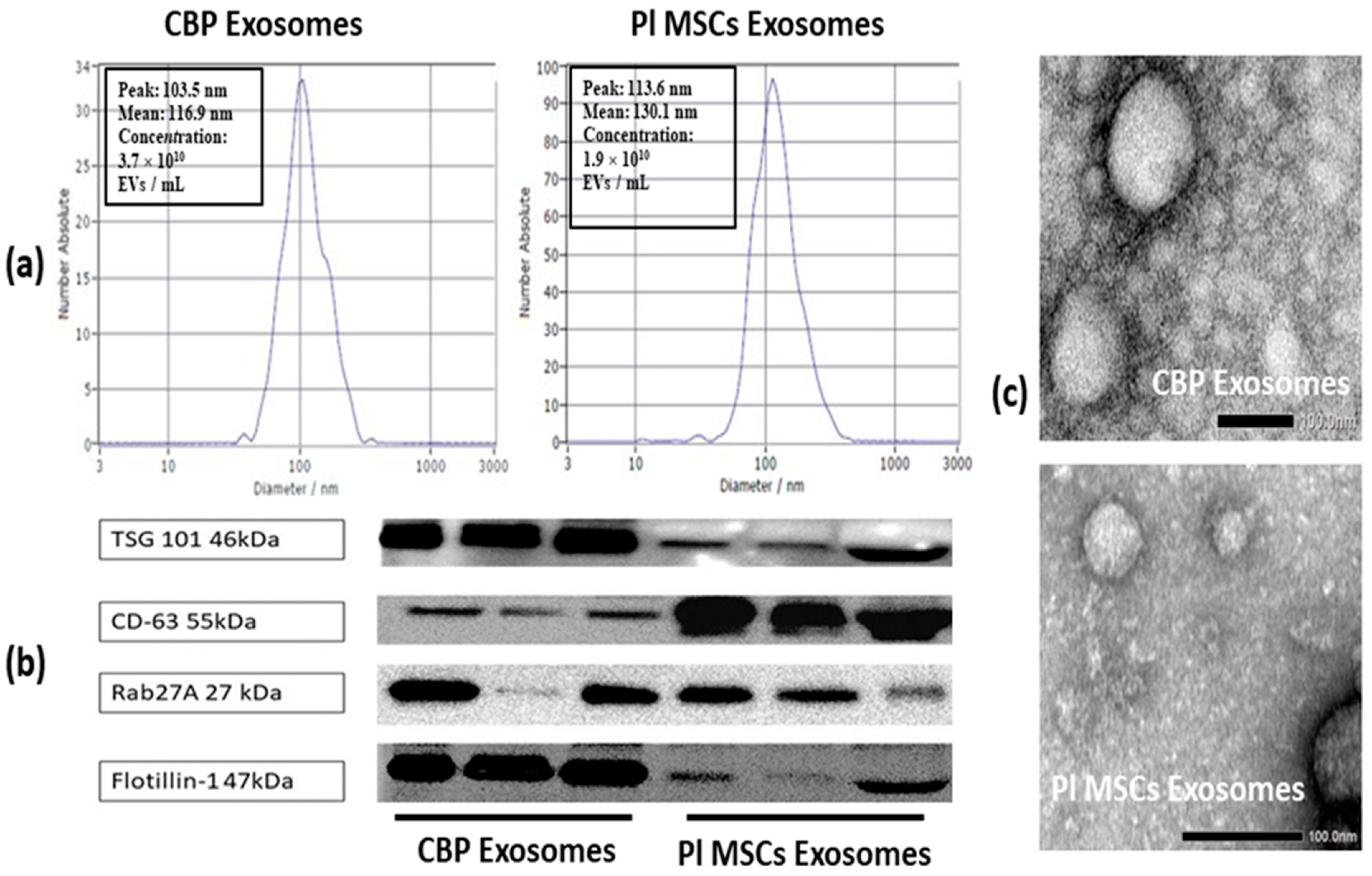
2.3.1. Nanoparticle Tracking Analysis (NTA)
2.3.2. Transmission Electron Microscopy (TEM)
2.3.3. Western Blotting
2.4. Expansion of HSCs
2.5. Immunophenotyping
2.6. Colony Forming Unit (CFU) Assay
3. Statistics
4. Results
4.1. Placental Samples
4.2. MSCs Culture and Identification
4.3. Identification and Analysis of Isolated Exosomes
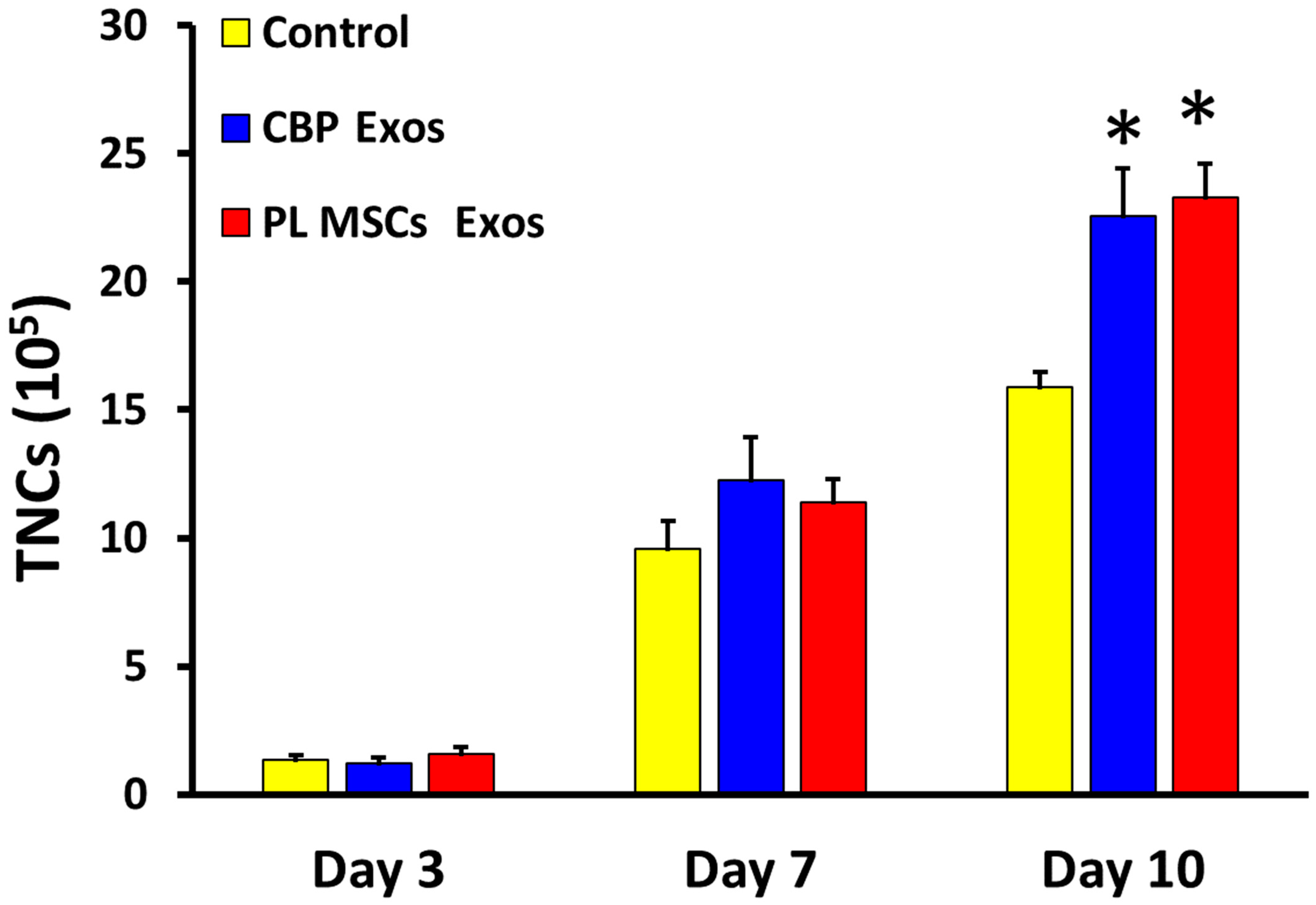
4.4. The Effects of CBP and PL MSCs Exosomes on Ex Vivo Expansion of CD34+ to Total Nucleated Cells (TNCs)
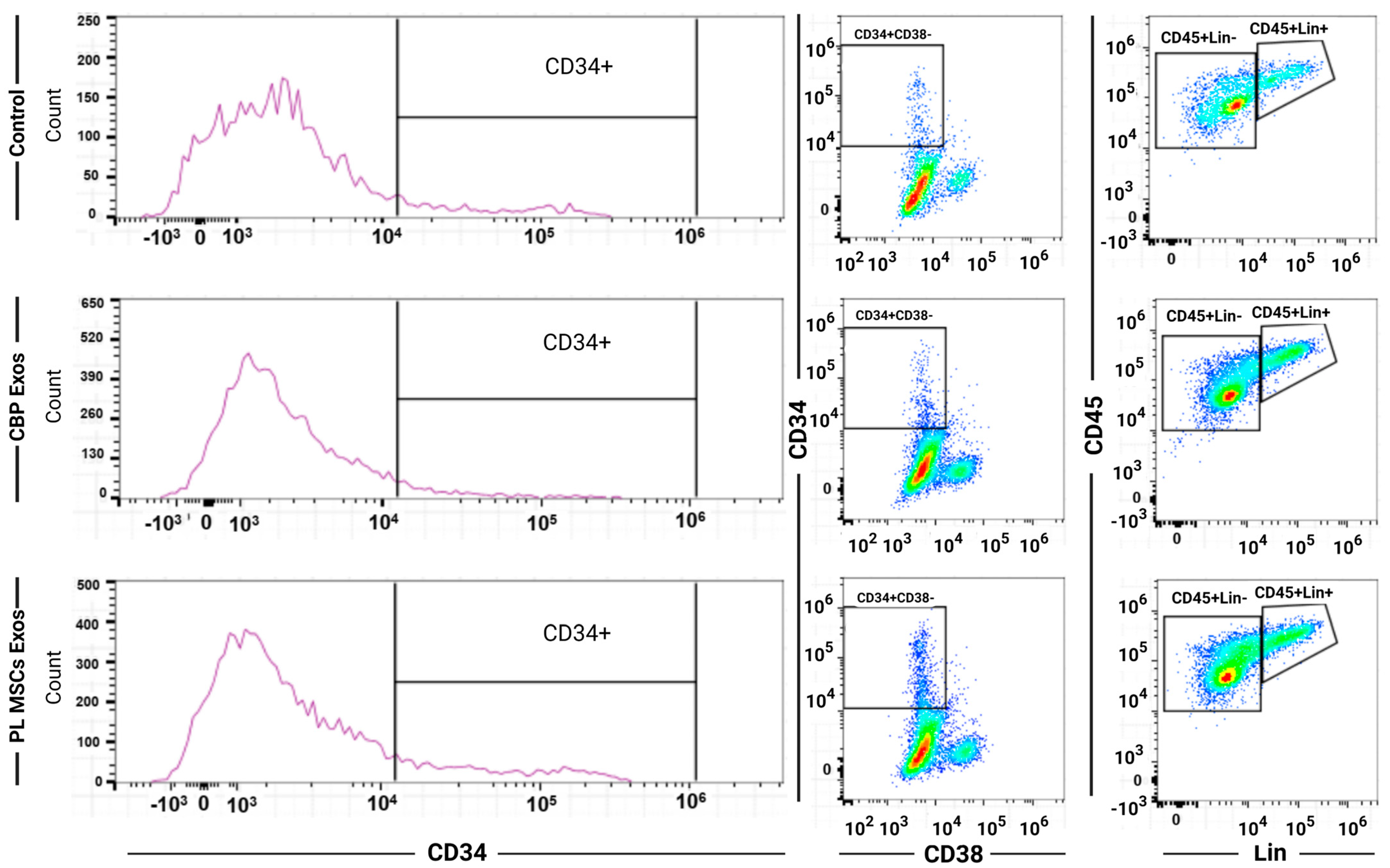
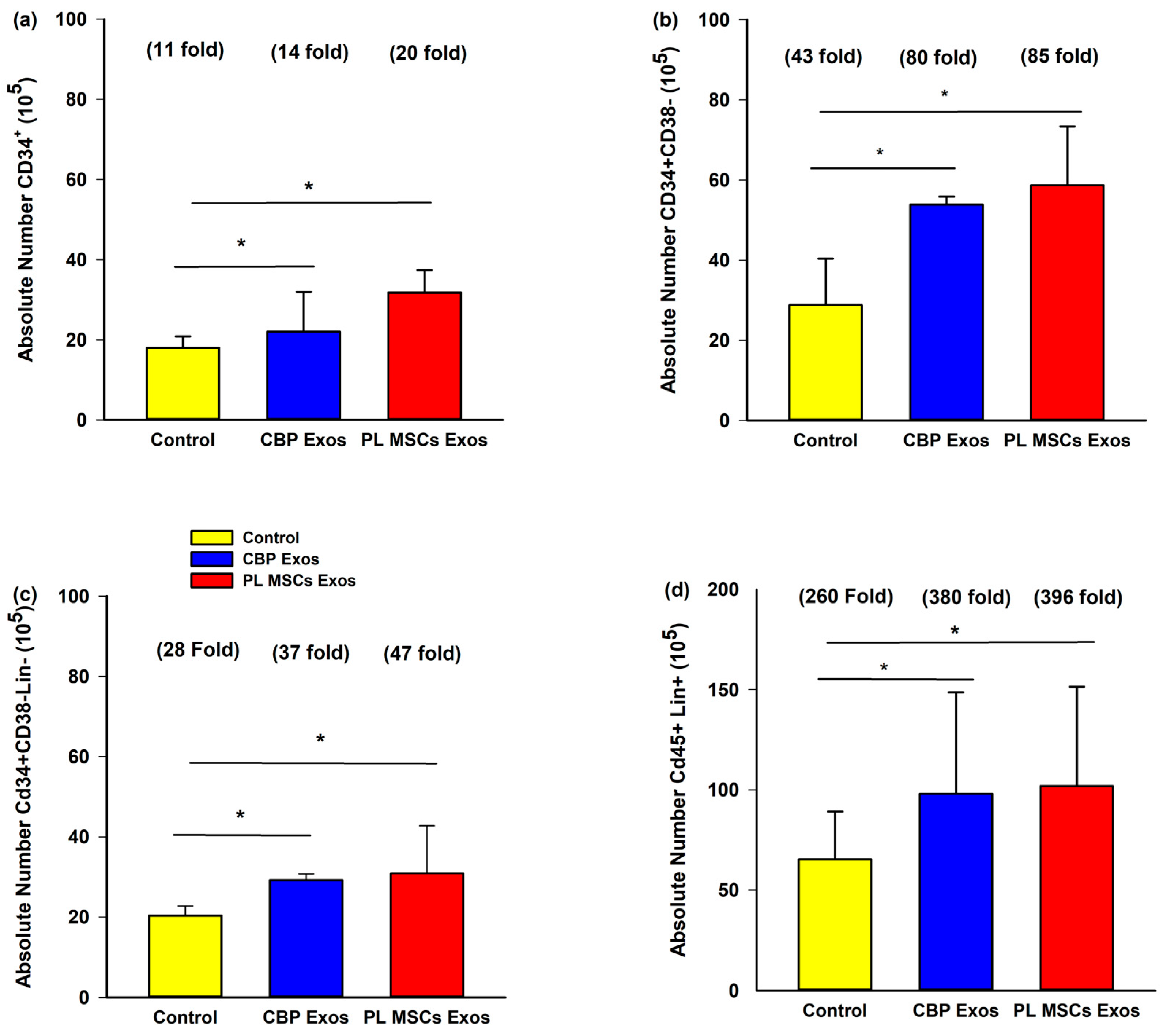
4.5. Flow Cytometry of the Expanded HSCs
4.6. The Effects of CBP and PL MSCs Exosomes on Ex Vivo Expansion of CB CD34+ Cells
4.7. The Effects of CBP and PL MSCs Exosomes on Ex Vivo Expansion of Primitive HCSs Cells
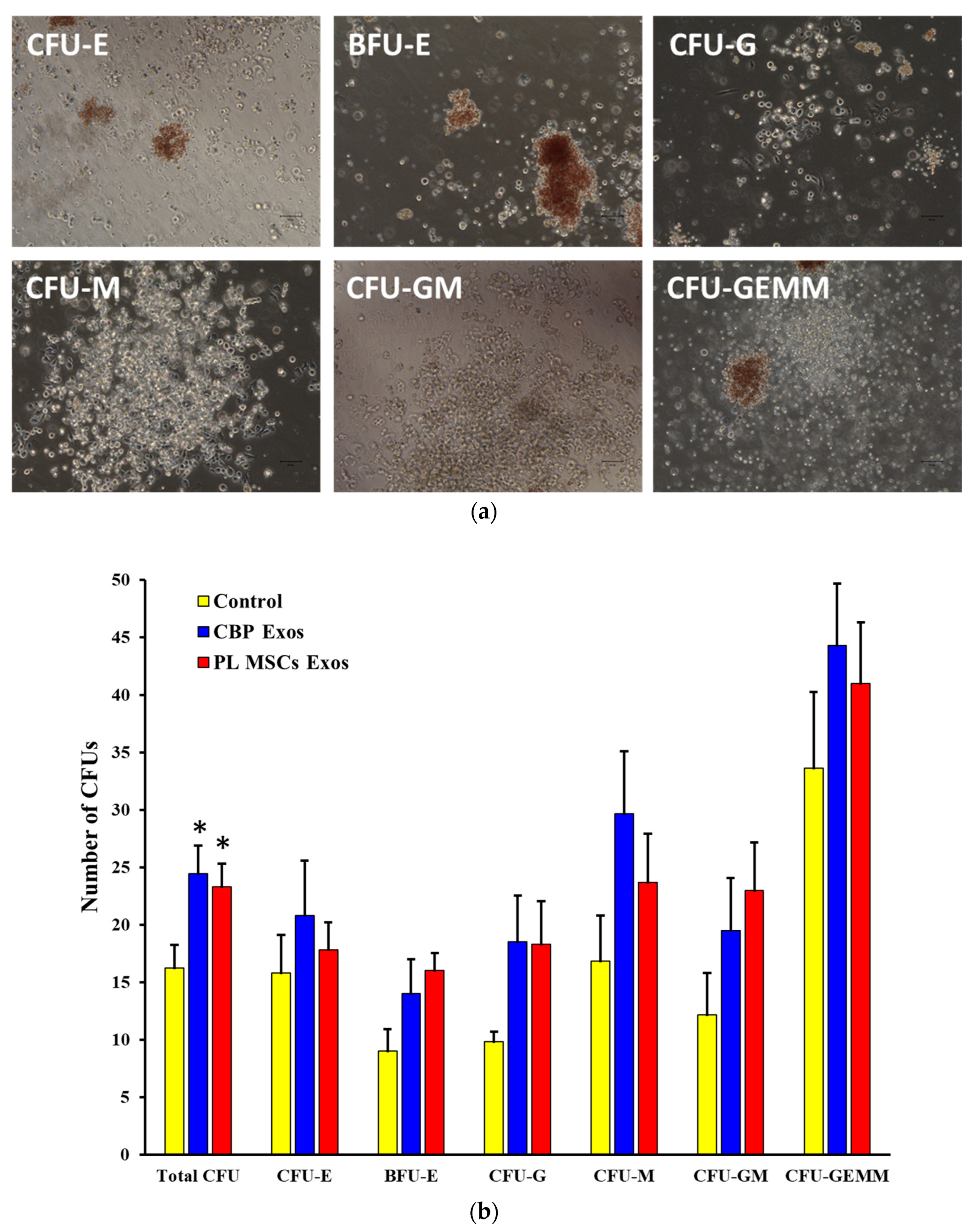
4.8. The Effects of CBP and PL MSCs Exosomes on the Generation of CFUs from the Expanded CD34+ Cells
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gluckman, E.; Rocha, V. Cord Blood Transplantation: State of the Art. Haematologica 2009, 94, 451–454. [Google Scholar] [CrossRef]
- Zhong, X.Y.; Zhang, B.; Asadollahi, R.; Low, S.H.; Holzgreve, W. Umbilical Cord Blood Stem Cells: What to Expect. Ann. N. Y. Acad. Sci. 2010, 1205, 17–22. [Google Scholar] [CrossRef]
- Querol, S.; Mufti, G.J.; Marsh, S.G.E.; Pagliuca, A.; Little, A.M.; Shaw, B.E.; Jeffery, R.; Garcia, J.; Goldman, J.M.; Madrigal, J.A. Cord Blood Stem Cells for Hematopoietic Stem Cell Transplantation in the UK: How Big Should the Bank Be? Haematologica 2009, 94, 536. [Google Scholar] [CrossRef]
- Ruggeri, A. Optimizing Cord Blood Selection. Hematology Am. Soc. Hematol. Educ. Program. 2019, 2019, 522–531. [Google Scholar] [CrossRef]
- Barker, J.N.; Weisdorf, D.J.; Defor, T.E.; Blazar, B.R.; Mcglave, P.B.; Miller, J.S.; Verfaillie, C.M.; Wagner, J.E. Transplantation of 2 Partially HLA-Matched Umbilical Cord Blood Units to Enhance Engraftment in Adults with Hematologic Malignancy. Blood 2005, 105, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Fatobene, G.; Volt, F.; Moreira, F.; Mariano, L.; Chevallier, P.; Furst, S.; Labussi Ére-Wallet, H.; de La Tour, R.P.; Deconinck, E.; Cluzeau, T.; et al. Optimizing Selection of Double Cord Blood Units for Transplantation of Adult Patients with Malignant Diseases. Blood Adv. 2020, 4, 6327. [Google Scholar] [CrossRef] [PubMed]
- Berglund, S.; Magalhaes, I.; Gaballa, A.; Vanherberghen, B.; Uhlin, M. Advances in Umbilical Cord Blood Cell Therapy: The Present and the Future. Expert Opin. Biol. Ther. 2017, 17, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.N.; Scaradavou, A.; Stevens, C.E. Combined Effect of Total Nucleated Cell Dose and HLA Match on Transplantation Outcome in 1061 Cord Blood Recipients with Hematologic Malignancies. Blood 2010, 115, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Guo, B. Update on Preclinical and Clinical Efforts on Ex-Vivo Expansion of Hematopoietic Stem and Progenitor Cells. Curr. Opin. Hematol. 2022, 29, 167–173. [Google Scholar] [CrossRef]
- Hofmeister, C.C.; Zhang, J.; Knight, K.L.; Le, P.; Stiff, P.J. Ex Vivo Expansion of Umbilical Cord Blood Stem Cells for Transplantation: Growing Knowledge from the Hematopoietic Niche. Bone Marrow. Transplant. 2007, 39, 11–23. [Google Scholar] [CrossRef]
- Fajardo-Orduña, G.R.; Mayani, H.; Montesinos, J.J. Hematopoietic Support Capacity of Mesenchymal Stem Cells: Biology and Clinical Potential. Arch. Med. Res. 2015, 46, 589–596. [Google Scholar] [CrossRef]
- Frenette, P.S.; Pinho, S.; Lucas, D.; Scheiermann, C. Mesenchymal Stem Cell: Keystone of the Hematopoietic Stem Cell Niche and a Stepping-Stone for Regenerative Medicine. Annu. Rev. Immunol. 2013, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Niu, C.; Ye, L.; Huang, H.; He, X.; Tong, W.G.; Ross, J.; Haug, J.; Johnson, T.; Feng, J.Q.; et al. Identification of the Haematopoietic Stem Cell Niche and Control of the Niche Size. Nature 2003, 425, 836–841. [Google Scholar] [CrossRef]
- Bruschi, M.; Vanzolini, T.; Sahu, N.; Balduini, A.; Magnani, M.; Fraternale, A. Functionalized 3D Scaffolds for Engineering the Hematopoietic Niche. Front. Bioeng. Biotechnol. 2022, 10, 968086. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.N.; Ng, J.; Niu, T.; Yang, H.; McMannis, J.D.; Karandish, S.; Kaur, I.; Fu, P.; del Angel, M.; Messinger, R.; et al. Superior Ex Vivo Cord Blood Expansion Following Co-Culture with Bone Marrow-Derived Mesenchymal Stem Cells. Bone Marrow Transpl. 2006, 37, 359–366. [Google Scholar] [CrossRef]
- Walenda, T.; Bork, S.; Horn, P.; Wein, F.; Saffrich, R.; Diehlmann, A.; Eckstein, V.; Ho, A.D.; Wagner, W. Co-Culture with Mesenchymal Stromal Cells Increases Proliferation and Maintenance of Haematopoietic Progenitor Cells. J. Cell Mol. Med. 2010, 14, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Magin, A.S.; Körfer, N.R.; Partenheimer, H.; Lange, C.; Zander, A.; Noll, T. Primary Cells as Feeder Cells for Coculture Expansion of Human Hematopoietic Stem Cells from Umbilical Cord Blood—A Comparative Study. Stem. Cells Dev. 2009, 18, 173–186. [Google Scholar] [CrossRef] [PubMed]
- McNiece, I.K.; Harrington, J.; Turney, J.; Kellner, J.; Shpall, E.J. Ex Vivo Expansion of Cord Blood Mononuclear Cells on Mesenchymal Stem Cells. Cytotherapy 2004, 6, 311–317. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Novelli, E.; Grove, J.E.; Rinder, H.M.; Civin, C.; Cheng, L.; Krause, D.S. Cotransplantation of Human Mesenchymal Stem Cells Enhances Human Myelopoiesis and Megakaryocytopoiesis in NOD/SCID Mice. Exp. Hematol. 2003, 31, 413–420. [Google Scholar] [CrossRef] [PubMed]
- De Lima, M.; McNiece, I.; Robinson, S.N.; Munsell, M.; Eapen, M.; Horowitz, M.; Alousi, A.; Saliba, R.; McMannis, J.D.; Kaur, I.; et al. Cord-Blood Engraftment with Ex Vivo Mesenchymal-Cell Coculture From the Departments of Stem Cell Transplantation and Cellular Therapy A Bs t r Ac t. N. Engl. J. Med. 2012, 367, 2305–2320. [Google Scholar] [CrossRef]
- McNiece, I.; Jones, R.; Bearman, S.I.; Cagnoni, P.; Nieto, Y.; Franklin, W.; Ryder, J.; Steele, A.; Stoltz, J.; Russell, P.; et al. Ex Vivo Expanded Peripheral Blood Progenitor Cells Provide Rapid Neutrophil Recovery after High-Dose Chemotherapy in Patients with Breast Cancer. Blood 2000, 96, 3001–3007. [Google Scholar] [CrossRef]
- Maitra, B.; Szekely, E.; Gjini, K.; Laughlin, M.J.; Dennis, J.; Haynesworth, S.E.; Koç, O.N. Human Mesenchymal Stem Cells Support Unrelated Donor Hematopoietic Stem Cells and Suppress T-Cell Activation. Bone Marrow Transpl. 2004, 33, 597–604. [Google Scholar] [CrossRef]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Götherström, C.; Hassan, M.; Uzunel, M.; Ringdén, O. Treatment of Severe Acute Graft-versus-Host Disease with Third Party Haploidentical Mesenchymal Stem Cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Chen, G.; Yue, A.; Ruan, Z.; Yin, Y.; Wang, R.; Ren, Y.; Zhu, L. Comparison of Biological Characteristics of Mesenchymal Stem Cells Derived from Maternal-Origin Placenta and Wharton’s Jelly. Stem Cell Res. Ther. 2015, 6, 228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, L.; Chen, Q.; Wang, F.; Li, Q.; Zeng, Q.; Huang, J.; Luo, M.; Li, W.; Zheng, Y.; et al. Hypoxia with Wharton’s Jelly Mesenchymal Stem Cell Coculture Maintains Stemness of Umbilical Cord Blood-Derived CD34+ Cells. Stem. Cell. Res. Ther. 2018, 9, 158. [Google Scholar] [CrossRef]
- Robin, C.; Bollerot, K.; Mendes, S.; Haak, E.; Crisan, M.; Cerisoli, F.; Lauw, I.; Kaimakis, P.; Jorna, R.; Vermeulen, M.; et al. Human Placenta Is a Potent Hematopoietic Niche Containing Hematopoietic Stem and Progenitor Cells throughout Development. Cell Stem Cell 2009, 5, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine Mechanisms of Mesenchymal Stem Cell-Based Therapy: Current Status and Perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef]
- Weil, B.R.; Markel, T.A.; Herrmann, J.L.; Abarbanell, A.M.; Meldrum, D.R. Mesenchymal Stem Cells Enhance the Viability and Proliferation of Human Fetal Intestinal Epithelial Cells Following Hypoxic Injury via Paracrine Mechanisms. Surgery 2009, 146, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of Mesenchymal Stem/Stromal Cell Function. Stem Cell Res. Ther. 2016, 7, 125. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Khan, Y.S. Histology, Extracellular Vesicles; Stat Pearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-Derived Microvesicles: Important and Underappreciated Mediators of Cell-to-Cell Communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.T.; Abdelhamed, S.; Kurre, P. Extracellular Vesicles in the Hematopoietic Microenvironment. Haematologica 2018, 103, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic Stem Cell-Derived Microvesicles Reprogram Hematopoietic Progenitors: Evidence for Horizontal Transfer of MRNA and Protein Delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Morhayim, J.; van de Peppel, J.; Braakman, E.; Rombouts, E.W.J.C.; ter Borg, M.N.D.; Dudakovic, A.; Chiba, H.; van der Eerden, B.C.J.; Raaijmakers, M.H.; van Wijnen, A.J.; et al. Osteoblasts Secrete MiRNA-Containing Extracellular Vesicles That Enhance Expansion of Human Umbilical Cord Blood Cells. Sci. Rep. 2016, 6, 32034. [Google Scholar] [CrossRef]
- Xie, H.; Sun, L.; Zhang, L.; Liu, T.; Chen, L.; Zhao, A.; Lei, Q.; Gao, F.; Zou, P.; Li, Q.; et al. Mesenchymal Stem Cell-Derived Microvesicles Support Ex Vivo Expansion of Cord Blood-Derived CD34(+) Cells. Stem Cells Int. 2016, 2016, 6493241. [Google Scholar] [CrossRef] [PubMed]
- Damien, P.; Allan, D.S. Regenerative Therapy and Immune Modulation Using Umbilical Cord Blood-Derived Cells. Biol. Blood Marrow Transplant. 2015, 21, 1545–1554. [Google Scholar] [CrossRef]
- Brunstein, C.G.; Miller, J.S.; McKenna, D.H.; Hippen, K.L.; DeFor, T.E.; Sumstad, D.; Curtsinger, J.; Verneris, M.R.; MacMillan, M.L.; Levine, B.L.; et al. Umbilical Cord Blood-Derived T Regulatory Cells to Prevent GVHD: Kinetics, Toxicity Profile, and Clinical Effect. Blood 2016, 127, 1044–1051. [Google Scholar] [CrossRef]
- Gervassi, A.; Lejarcegui, N.; Dross, S.; Jacobson, A.; Itaya, G.; Kidzeru, E.; Gantt, S.; Jaspan, H.; Horton, H. Myeloid Derived Suppressor Cells Are Present at High Frequency in Neonates and Suppress in Vitro t Cell Responses. PLoS ONE 2014, 9, e107816. [Google Scholar] [CrossRef]
- Chen, S.J.; Liu, Y.L.; Sytwu, H.K. Immunologic Regulation in Pregnancy: From Mechanism to Therapeutic Strategy for Immunomodulation. Clin. Dev. Immunol. 2012, 2012, 258391. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Roura, S.; Gálvez-Montón, C.; Pujal, J.M.; Aran, G.; Sanjurjo, L.; Franquesa, M.; Sarrias, M.R.; Bayes-Genis, A.; Borràs, F.E. Nanosized UCMSC-Derived Extracellular Vesicles but Not Conditioned Medium Exclusively Inhibit the Inflammatory Response of Stimulated T Cells: Implications for Nanomedicine. Theranostics 2017, 7, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Akyurekli, C.; Le, Y.; Richardson, R.B.; Fergusson, D.; Tay, J.; Allan, D.S. A Systematic Review of Preclinical Studies on the Therapeutic Potential of Mesenchymal Stromal Cell-Derived Microvesicles. Stem Cell Rev. Rep. 2015, 11, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Li, J.; Rui, C.; Ji, H.; Ding, H.; Lu, Y.; De, W.; Sun, L. Comparative Proteomic Profile of the Human Umbilical Cord Blood Exosomes between Normal and Preeclampsia Pregnancies with High-Resolution Mass Spectrometry. Cell Physiol. Biochem. 2015, 36, 2299–2306. [Google Scholar] [CrossRef]
- Huang, S.; Tang, Z.; Wang, Y.; Chen, D.; Li, J.; Zhou, C.; Lu, X.; Yuan, Y. Comparative Profiling of Exosomal MiRNAs in Human Adult Peripheral and Umbilical Cord Blood Plasma by Deep Sequencing. Epigenomics 2020, 12, 825–842. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhou, X.; Huang, X.; Xu, X.; Jia, Y.; Wu, Y.; Yao, J.; Wu, Y.; Wang, K. Maternal and Umbilical Cord Serum-Derived Exosomes Enhance Endothelial Cell Proliferation and Migration. FASEB J. 2018, 32, 4534–4543. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from Human Umbilical Cord Blood Accelerate Cutaneous Wound Healing through MiR-21-3p-Mediated Promotion of Angiogenesis and Fibroblast Function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef]
- Kim, S.; Maeng, J.Y.; Hyun, S.J.; Sohn, H.J.; Kim, S.Y.; Hong, C.H.; Kim, T.G. Extracellular Vesicles from Human Umbilical Cord Blood Plasma Modulate Interleukin-2 Signaling of T Cells to Ameliorate Experimental Autoimmune Encephalomyelitis. Theranostics 2020, 10, 5011–5028. [Google Scholar] [CrossRef]
- Huang, Y.J.; Cao, J.; Lee, C.Y.; Wu, Y.M. Umbilical Cord Blood Plasma-Derived Exosomes as a Novel Therapy to Reverse Liver Fibrosis. Stem Cell Res. Ther. 2021, 12, 568. [Google Scholar] [CrossRef]
- Beeravolu, N.; McKee, C.; Alamri, A.; Mikhael, S.; Brown, C.; Perez-Cruet, M.; Chaudhry, G.R. Isolation and Characterization of Mesenchymal Stromal Cells from Human Umbilical Cord and Fetal Placenta. J. Vis. Exp. 2017, 122, 55224. [Google Scholar] [CrossRef]
- Shelke, G.V.; Lässer, C.; Gho, Y.S.; Lötvall, J. Importance of Exosome Depletion Protocols to Eliminate Functional and RNA-Containing Extracellular Vesicles from Fetal Bovine Serum. J. Extracell. Vesicles 2014, 3, 24783. [Google Scholar] [CrossRef]
- Kanada, M.; Kim, B.D.; Hardy, J.W.; Ronald, J.A.; Bachmann, M.H.; Bernard, M.P.; Perez, G.I.; Zarea, A.A.; Ge, T.J.; Withrow, A.; et al. Microvesicle-Mediated Delivery of Minicircle DNA Results in Effective Gene-Directed Enzyme Prodrug Cancer Therapy. Mol. Cancer Ther. 2019, 18, 2331–2342. [Google Scholar] [CrossRef]
- Jung, M.K.; Mun, J.Y. Sample Preparation and Imaging of Exosomes by Transmission Electron Microscopy. J. Vis. Exp. 2018, 131, e56482. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b Control Different Steps of the Exosome Secretion Pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef]
- Barker, J.N.; Kempenich, J.; Kurtzberg, J.; Brunstein, C.G.; Delaney, C.; Milano, F.; Politikos, I.; Shpall, E.J.; Scaradavou, A.; Dehn, J. CD34+ Cell Content of 126 341 Cord Blood Units in the US Inventory: Implications for Transplantation and Banking. Blood Adv. 2019, 3, 1267–1271. [Google Scholar] [CrossRef]
- Robinson, S.N.; Simmons, P.J.; Yang, H.; Alousi, A.M.; Marcos De Lima, J.; Shpall, E.J. Mesenchymal Stem Cells in Ex Vivo Cord Blood Expansion. Best Pract. Res. Clin. Haematol. 2011, 24, 83–92. [Google Scholar] [CrossRef]
- Eliasson, P.; Jönsson, J.I. The Hematopoietic Stem Cell Niche: Low in Oxygen but a Nice Place to Be. J. Cell Physiol. 2010, 222, 17–22. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Sokic, S.; Schardt, J.S.; Raiker, R.S.; Lin, J.W.; Jay, S.M. Emerging Roles for Extracellular Vesicles in Tissue Engineering and Regenerative Medicine. Tissue Eng. Part B Rev. 2015, 21, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Seshareddy, K.; Troyer, D.; Weiss, M.L. Method to Isolate Mesenchymal-like Cells from Wharton’s Jelly of Umbilical Cord. Methods Cell Biol. 2008, 86, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Mennan, C.; Wright, K.; Bhattacharjee, A.; Balain, B.; Richardson, J.; Roberts, S. Isolation and Characterisation of Mesenchymal Stem Cells from Different Regions of the Human Umbilical Cord. Biomed. Res. Int. 2013, 2013, 916136. [Google Scholar] [CrossRef] [PubMed]
- Bieback, K.; Kern, S.; Klüter, H.; Eichler, H. Critical Parameters for the Isolation of Mesenchymal Stem Cells from Umbilical Cord Blood. Stem Cells 2004, 22, 625–634. [Google Scholar] [CrossRef]
- Andrade-Zaldívar, H.; Santos, L.; de León Rodríguez, A. Expansion of Human Hematopoietic Stem Cells for Transplantation: Trends and Perspectives. Cytotechnology 2008, 56, 151–160. [Google Scholar] [CrossRef]
- Seita, J.; Weissman, I.L. Hematopoietic Stem Cell: Self-Renewal versus Differentiation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 640–653. [Google Scholar] [CrossRef]
- Jiang, J.; Woulfe, D.S.; Papoutsakis, E.T. Shear Enhances Thrombopoiesis and Formation of Microparticles That Induce Megakaryocytic Differentiation of Stem Cells. Blood 2014, 124, 2094–2103. [Google Scholar] [CrossRef]
- Sarvar, D.P.; Karimi, M.H.; Movassaghpour, A.; Akbarzadehlaleh, P.; Aqmasheh, S.; Timari, H.; Shamsasenjan, K. The Effect of Mesenchymal Stem Cell-Derived Microvesicles on Erythroid Differentiation of Umbilical Cord Blood-Derived CD34+ Cells. Adv. Pharm. Bull. 2018, 8, 291–296. [Google Scholar] [CrossRef]
- Angulski, A.B.B.; Capriglione, L.G.; Batista, M.; Marcon, B.H.; Senegaglia, A.C.; Stimamiglio, M.A.; Correa, A. The Protein Content of Extracellular Vesicles Derived from Expanded Human Umbilical Cord Blood-Derived CD133+ and Human Bone Marrow-Derived Mesenchymal Stem Cells Partially Explains Why Both Sources Are Advantageous for Regenerative Medicine. Stem Cell Rev. Rep. 2017, 13, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Van Os, R.P.; Dethmers-Ausema, B.; de Haan, G. In Vitro Assays for Cobblestone Area-Forming Cells, LTC-IC, and CFU-C. Methods Mol. Biol. 2008, 430, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Ringden, O.; Baygan, A.; Remberger, M.; Gustafsson, B.; Winiarski, J.; Khoein, B.; Moll, G.; Klingspor, L.; Westgren, M.; Sadeghi, B. Placenta-Derived Decidua Stromal Cells for Treatment of Severe Acute Graft-Versus-Host Disease. Stem. Cells Transl. Med. 2018, 7, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jin, N.; Wang, F.; Chen, B. Mesenchymal Stem Cells: A Promising Way in Therapies of Graft-versus-Host Disease. Cancer Cell Int. 2020, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, J.; Hu, Z.; Yang, Y.G.; Zhou, Q.; Sun, L.; Wu, J. Current Status of Clinical Trials Assessing Mesenchymal Stem Cell Therapy for Graft versus Host Disease: A Systematic Review. Stem Cell Res. Ther. 2022, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Tieu, A.; Slobodian, M.; Shorr, R.; Burger, D.; Lalu, M.M.; Allan, D.S. Preclinical Studies of MSC-Derived Extracellular Vesicles to Treat or Prevent Graft Versus Host Disease: A Systematic Review of the Literature. Stem Cell Rev. Rep. 2021, 17, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.A.; Abdul-Hafez, A.; Ibrahim, S.; Pillai, N.; Abdulmageed, M.; Thiruvenkataramani, R.P.; Mohamed, T.; Madhukar, B.V.; Uhal, B.D. Stem-Cell Therapy for Bronchopulmonary Dysplasia (BPD) in Newborns. Cells 2022, 11, 1275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teleb, R.S.; Abdul-Hafez, A.; Othman, A.; Ahmed, A.E.-A.; Elsaid, A.A.; Arif, H.; Zarea, A.A.; Abdulmageed, M.; Mohamed, H.; Ibrahim, S.A.; et al. Cord Blood Plasma and Placental Mesenchymal Stem Cells-Derived Exosomes Increase Ex Vivo Expansion of Human Cord Blood Hematopoietic Stem Cells While Maintaining Their Stemness. Cells 2023, 12, 250. https://doi.org/10.3390/cells12020250
Teleb RS, Abdul-Hafez A, Othman A, Ahmed AE-A, Elsaid AA, Arif H, Zarea AA, Abdulmageed M, Mohamed H, Ibrahim SA, et al. Cord Blood Plasma and Placental Mesenchymal Stem Cells-Derived Exosomes Increase Ex Vivo Expansion of Human Cord Blood Hematopoietic Stem Cells While Maintaining Their Stemness. Cells. 2023; 12(2):250. https://doi.org/10.3390/cells12020250
Chicago/Turabian StyleTeleb, Rasha S., Amal Abdul-Hafez, Amira Othman, Ahmed El-Abd Ahmed, Abdelrahman A. Elsaid, Hattan Arif, Ahmed A. Zarea, Mohammed Abdulmageed, Hend Mohamed, Sherif Abdelfattah Ibrahim, and et al. 2023. "Cord Blood Plasma and Placental Mesenchymal Stem Cells-Derived Exosomes Increase Ex Vivo Expansion of Human Cord Blood Hematopoietic Stem Cells While Maintaining Their Stemness" Cells 12, no. 2: 250. https://doi.org/10.3390/cells12020250
APA StyleTeleb, R. S., Abdul-Hafez, A., Othman, A., Ahmed, A. E.-A., Elsaid, A. A., Arif, H., Zarea, A. A., Abdulmageed, M., Mohamed, H., Ibrahim, S. A., Thiruvenkataramani, R. P., Mohamed, T., Kanada, M., Madhukar, B. V., Arellano, M. G., Sayed, M. M., Qubaisy, H. M., & Omar, S. A. (2023). Cord Blood Plasma and Placental Mesenchymal Stem Cells-Derived Exosomes Increase Ex Vivo Expansion of Human Cord Blood Hematopoietic Stem Cells While Maintaining Their Stemness. Cells, 12(2), 250. https://doi.org/10.3390/cells12020250






