Tritrophic Interactions among Arthropod Natural Enemies, Herbivores and Plants Considering Volatile Blends at Different Scale Levels
Abstract
1. Introduction
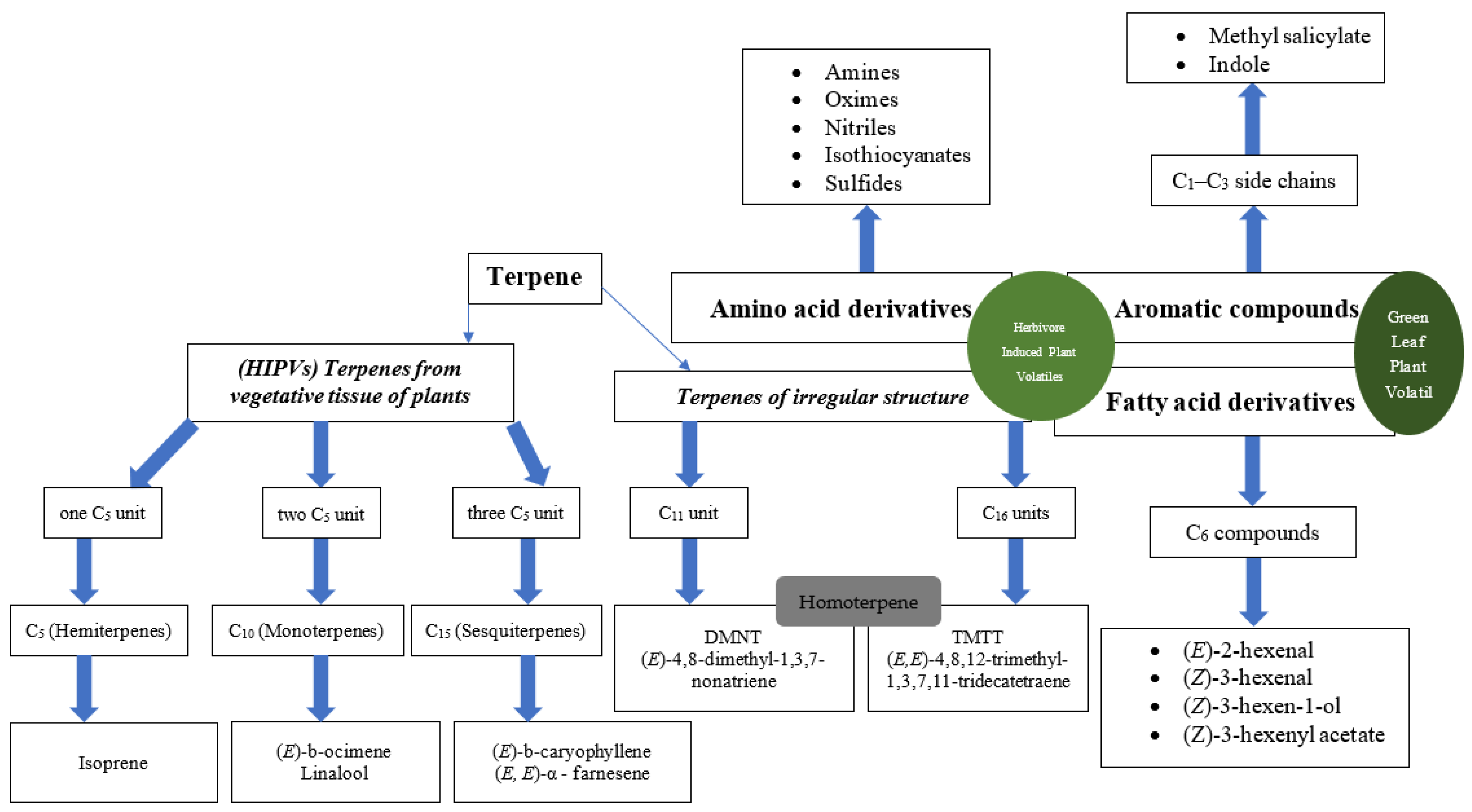
2. Chemical Footprints of Plant Volatiles
3. Different Scales of Interaction
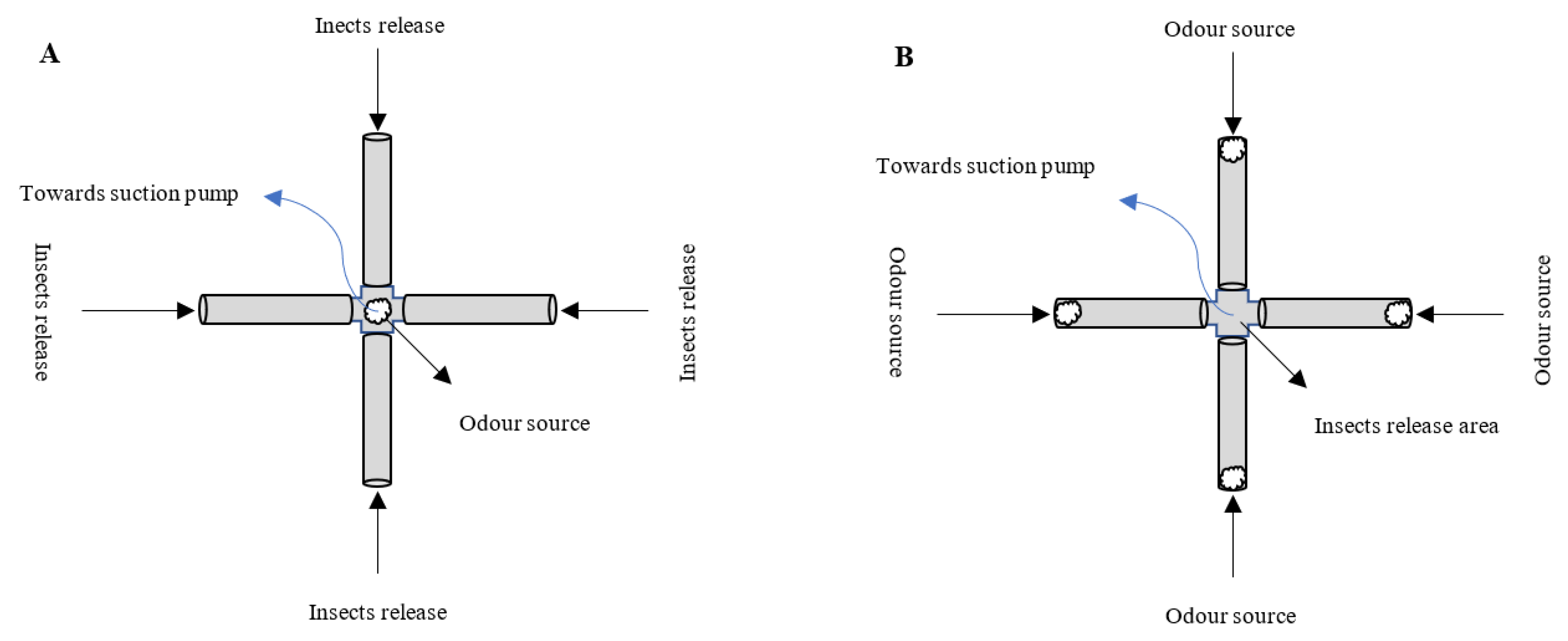
3.1. The Spatial and Temporal Scales of Parasitoid Interactions with Plant
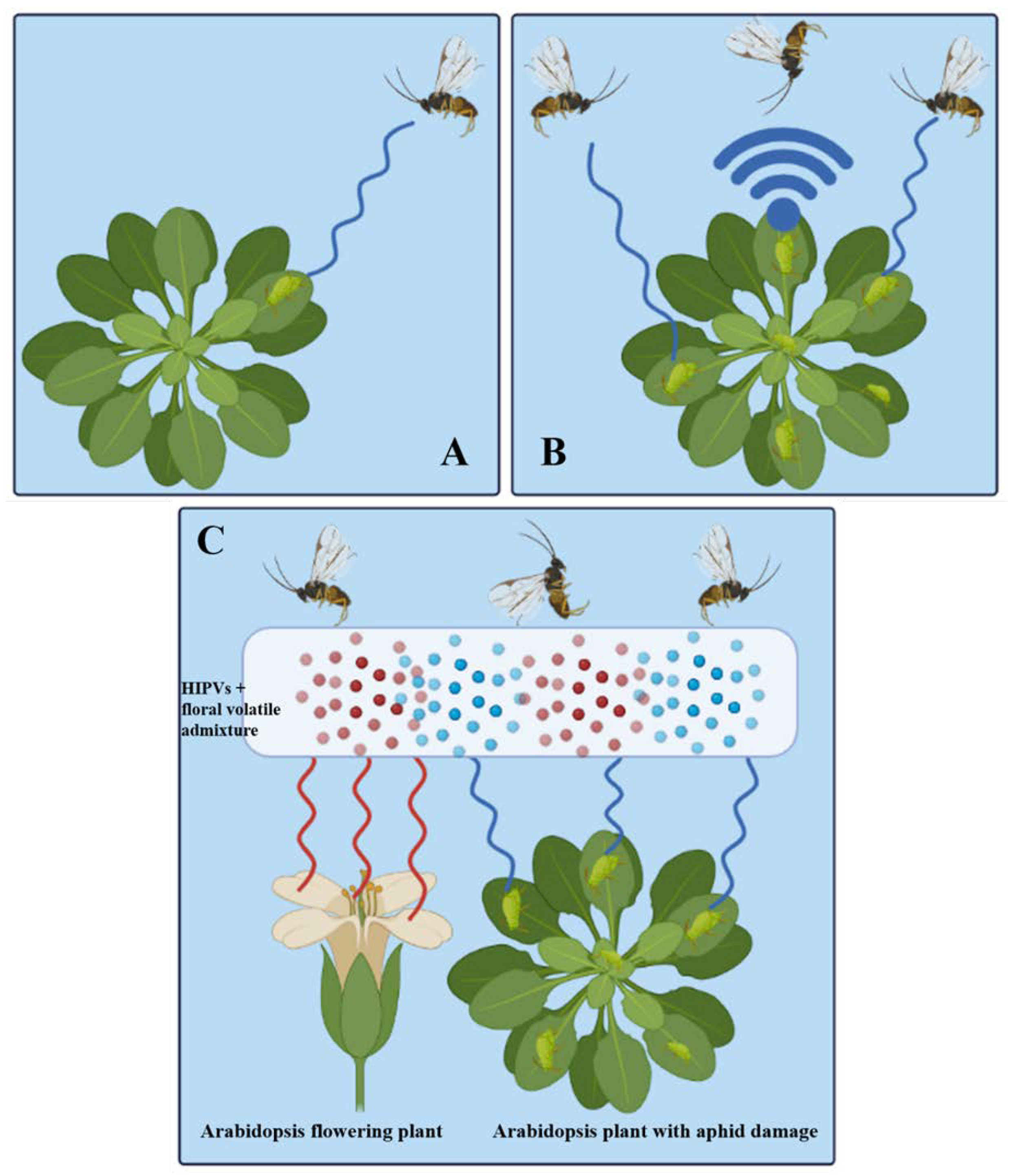
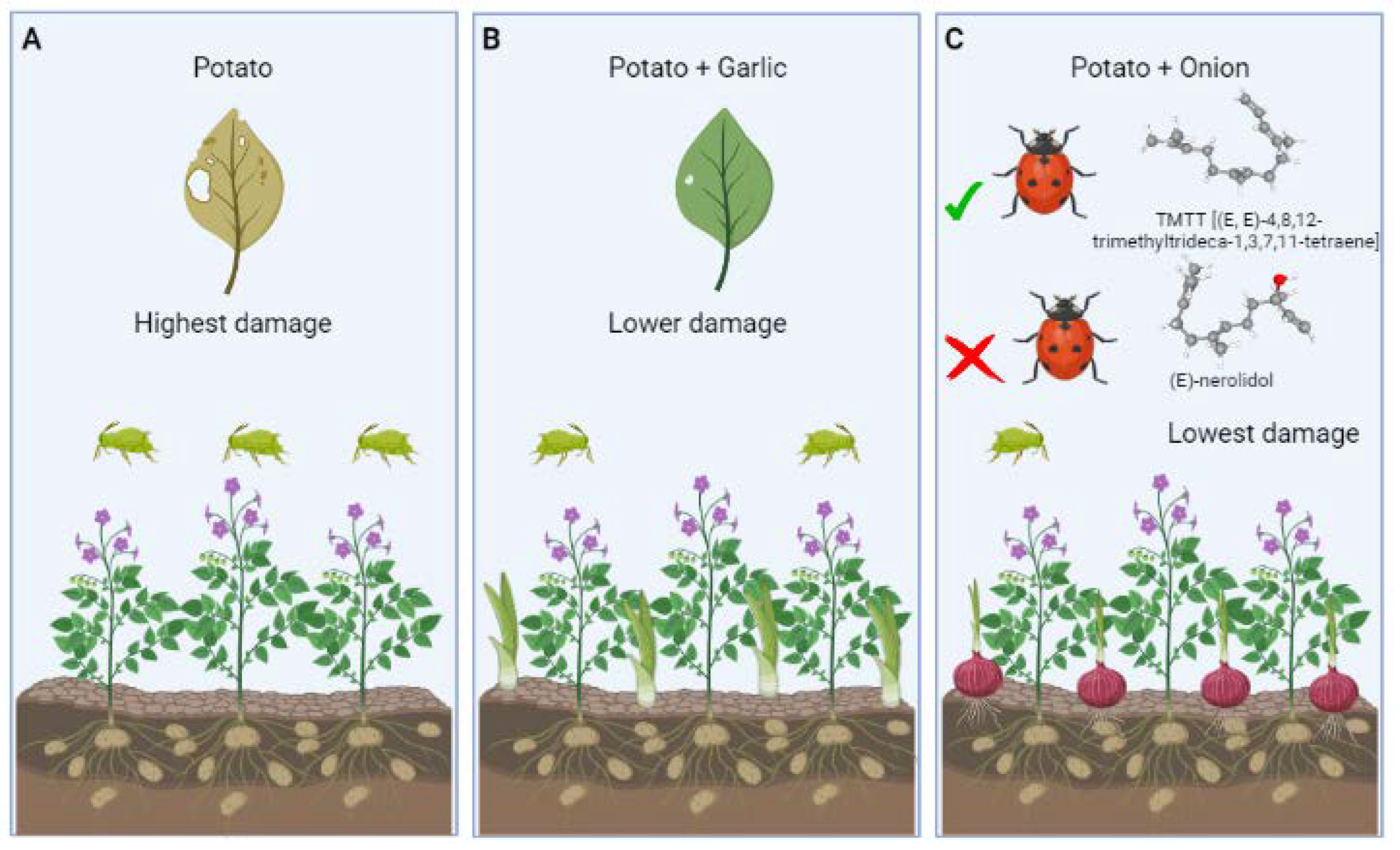
3.2. Tritrophic Interaction in Plant to Plant Signaling
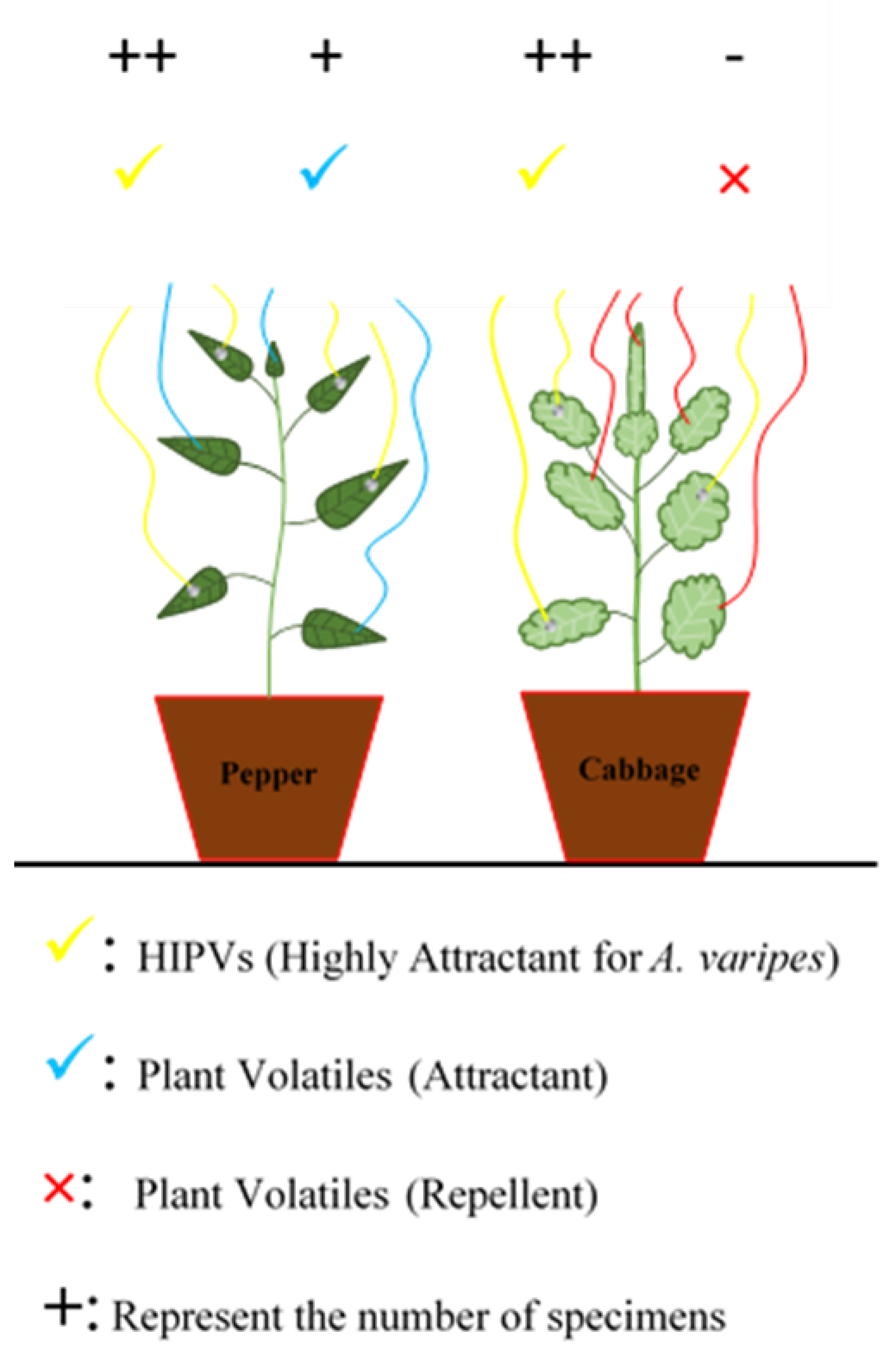
3.3. Tritrophic Interactions at Landscape Scale
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holopainen, J.K.; Gershenzon, J. Multiple Stress Factors and the Emission of Plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef]
- Hare, J.D. Ecological Role of Volatiles Produced by Plants in Response to Damage by Herbivorous Insects. Annu. Rev. Entomol. 2011, 56, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Clavijo McCormick, A.; Unsicker, S.B.; Gershenzon, J. The Specificity of Herbivore-Induced Plant Volatiles in Attracting Herbivore Enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef] [PubMed]
- DICKE, M.; SABELIS, M.W. How plants obtain predatory mites as bodyguards. Neth. J. Zool. 1988, 1, 2–4. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Tumlinson, J.H.; Lewis, W.J. Exploitation of Herbivore-Induced Plant Odors by Host-Seeking Parasitic Wasps. Science 1990, 250, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M. Behavioural and Community Ecology of Plants That Cry for Help. Plant Cell Environ. 2009, 32, 654–665. [Google Scholar] [CrossRef]
- Ali, M.Y.; Naseem, T.; Zhang, J.; Pan, M.; Zhang, F.; Liu, T. Plant Volatiles and Herbivore Induced Plant Volatiles from Chili Pepper Act as Attractant of the Aphid Parasitoid. Plants 2022, 11, 1350. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Naseem, T.; Arshad, M.; Ashraf, I.; Rizwan, M.; Tahir, M.; Rizwan, M.; Sayed, S.; Ullah, M.I.; Khan, R.R.; et al. Host-Plant Variations Affect the Biotic Potential, Survival, and Population Projection of Myzus Persicae (Hemiptera: Aphididae). Insects 2021, 12, 375. [Google Scholar] [CrossRef]
- Ghirardo, A.; Heller, W.; Fladung, M.; Schnitzler, J.P.; Schroeder, H. Function of Defensive Volatiles in Pedunculate Oak (Quercus Robur) Is Tricked by the Moth Tortrix Viridana. Plant Cell Environ. 2012, 35, 2192–2207. [Google Scholar] [CrossRef]
- Dicke, M.; Loon, J.J.A. Van Multitrophic Effects of Herbivore-Induced Plant Volatile in an Evolutionary Context. Entomol. Exp. Appl. 2000, 97, 237–249. [Google Scholar] [CrossRef]
- Mumm, R.; Dicke, M. Variation in Natural Plant Products and the Attraction of Bodyguards Involved in Indirect Plant Defense. Can. J. Zool. 2010, 88, 628–667. [Google Scholar] [CrossRef]
- Hilker, M.; Fatouros, N.E. Plant Responses to Insect Egg Deposition. Annu. Rev. Entomol. 2015, 60, 493–515. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Erb, M. Tritrophic Interactions Mediated by Herbivore-Induced Plant Volatiles: Mechanisms, Ecological Relevance, and Application Potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- Kaplan, I. Attracting Carnivorous Arthropods with Plant Volatiles: The Future of Biocontrol or Playing with Fire? Biol. Control. 2012, 60, 77–89. [Google Scholar] [CrossRef]
- Kelly, J.L.; Hagler, J.R.; Kaplan, I. Semiochemical Lures Reduce Emigration and Enhance Pest Control Services in Open-Field Predator Augmentation. Biol. Control. 2014, 71, 70–77. [Google Scholar] [CrossRef]
- Murali-Baskaran, R.K.; Sharma, K.C.; Kaushal, P.; Kumar, J.; Parthiban, P.; Senthil-Nathan, S.; Mankin, R.W. Role of Kairomone in Biological Control of Crop Pests—A Review. Physiol. Mol. Plant Pathol. 2018, 101, 3–15. [Google Scholar] [CrossRef]
- Peri, E.; Moujahed, R.; Wajnberg, E.; Colazza, S. Applied Chemical Ecology to Enhance Insect Parasitoid Efficacy in the Biological Control of Crop Pests. In Chemical Ecology of Insects; Taylor, F., Ed.; Springer: New York, NY, USA, 2018; pp. 234–267. [Google Scholar]
- Kivimäenpää, M.; Magsarjav, N.; Ghimire, R.; Markkanen, J.M.; Heijari, J.; Vuorinen, M.; Holopainen, J.K. Influence of Tree Provenance on Biogenic VOC Emissions of Scots Pine (Pinus Sylvestris) Stumps. Atmos. Environ. 2012, 60, 477–485. [Google Scholar] [CrossRef]
- Degenhardt, J.; Hiltpold, I.; Köllnera, T.G.; Frey, M.; Gierl, A.; Gershenzon, J.; Hibbard, B.E.; Ellersieck, M.R.; Turlings, T.C.J. Restoring a Maize Root Signal That Attracts Insect-Killing Nematodes to Control a Major Pest. Proc. Natl. Acad. Sci. USA 2009, 106, 17606. [Google Scholar] [CrossRef]
- Van Tol, R.W.H.M.; Van Der Sommen, A.T.C.; Boff, M.I.C.; Van Bezooijen, J.; Sabelis, M.W.; Smits, P.H. Plants Protect Their Roots by Alerting the Enemies of Grubs. Ecol. Lett. 2001, 4, 292–294. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of Entomopathogenic Nematodes by Insect-Damaged Maize Roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Lenk, C.; Degenhardt, J.; Turlings, T.C.J. The Underestimated Role of Roots in Defense against Leaf Attackers. Trends Plant Sci. 2009, 14, 653–659. [Google Scholar] [CrossRef]
- Ghimire, R.P.; Markkanen, J.M.; Kivimäenpää, M.; Lyytikäinen-Saarenmaa, P.; Holopainen, J.K. Emissions and Reduces Below-Ground Emissions of Scots Pine. Environ. Sci. Technol. 2013, 47, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- Schausberger, P.; Peneder, S.; Jürschik, S.; Hoffmann, D. Mycorrhiza Changes Plant Volatiles to Attract Spider Mite Enemies. Funct. Ecol. 2012, 26, 441–449. [Google Scholar] [CrossRef]
- Manninen, A.M.; Holopainen, T.; Holopainen, J.K. Susceptibility of Ectomycorrhizal and Nonmycorrhizal Scots Pine (Pinus sylvestris) Seedlings to a Generalist Insect Herbivore, Lygus Rugulipennis, at Two Nitrogen Availability Levels. New Phytol. 1998, 140, 55–63. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Blande, J.D. Where Do Herbivore-Induced Plant Volatiles Go? Front. Plant Sci. 2013, 4, 185. [Google Scholar] [CrossRef]
- Dicke, M.; Baldwin, I.T. The Evolutionary Context for Herbivore-Induced Plant Volatiles: Beyond the “Cry for Help”. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.J.; Mescher, M.C.; Dervinis, C.; Davis, J.M.; Carlson, J.E.; De Moraes, C.M. Priming Defense Genes and Metabolites in Hybrid Poplar by the Green Leaf Volatile Cis-3-Hexenyl Acetate. New Phytol. 2008, 180, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne Signals Prime Plants against Insect Herbivore Attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef]
- Heil, M.; Bueno, J.C.S. Within-Plant Signaling by Volatiles Leads to Induction and Priming of an Indirect Plant Defense in Nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467–5472. [Google Scholar] [CrossRef]
- Ton, J.; Mauch-Mani, B. β-Amino-Butyric Acid-Induced Resistance against Necrotrophic Pathogens Is Based on ABA-Dependent Priming for Callose. Plant J. 2004, 38, 119–130. [Google Scholar] [CrossRef]
- Heil, M.; Kost, C. Priming of Indirect Defences. Ecol. Lett. 2006, 9, 813–817. [Google Scholar] [CrossRef]
- Hodge, S.; Ward, J.L.; Galster, A.M.; Beale, M.H.; Powell, G. The Effects of a Plant Defence Priming Compound, β-Aminobutyric Acid, on Multitrophic Interactions with an Insect Herbivore and a Hymenopterous Parasitoid. BioControl 2011, 56, 699–711. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and Distribution of Floral Scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Gershenzon, J.; Dudareva, N. The Function of Terpene Natural Products in the Natural World. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.I.; Huber, D.P.W.; Bohlmann, J. Forest Tent Caterpillars (Malacosoma disstria) Induce Local and Systemic Diurnal Emissions of Terpenoid Volatiles in Hybrid Poplar (Populus trichocarpa × Deltoides): CDNA Cloning, Functional Characterization, and Patterns of Gene Expression of (-)-Germacr. Plant J. 2004, 37, 603–616. [Google Scholar] [CrossRef]
- Danner, H.; Boeckler, G.A.; Irmisch, S.; Yuan, J.S.; Chen, F.; Gershenzon, J.; Unsicker, S.B.; Köllner, T.G. Four Terpene Synthases Produce Major Compounds of the Gypsy Moth Feeding-Induced Volatile Blend of Populus Trichocarpa. Phytochemistry 2011, 72, 897–908. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-Induced Nocturnal Plant Volatiles Repel Conspecific Females. Nature 2001, 410, 577–579. [Google Scholar] [CrossRef]
- Kigathi, R.N.; Unsicker, S.B.; Reichelt, M.; Kesselmeier, J.; Gershenzon, J.; Weisser, W.W. Emission of Volatile Organic Compounds after Herbivory from Trifolium pratense (L.) under Laboratory and Field Conditions. J. Chem. Ecol. 2009, 35, 1335–1348. [Google Scholar] [CrossRef]
- Schaub, A.; Blande, J.D.; Graus, M.; Oksanen, E.; Holopainen, J.K.; Hansel, A. Real-Time Monitoring of Herbivore Induced Volatile Emissions in the Field. Physiol. Plant. 2010, 138, 123–133. [Google Scholar] [CrossRef]
- Bellamy, D.E.; Sisterson, M.S.; Walse, S.S. Quantifying Host Potentials: Indexing Postharvest Fresh Fruits for Spotted Wing Drosophila, Drosophila Suzukii. PLoS ONE 2013, 8, e61227. [Google Scholar] [CrossRef]
- Bolton, L.G.; Piñero, J.C.; Barrett, B.A.; Cha, D.H. Electrophysiological and Behavioral Responses of Drosophila Suzukii (Diptera: Drosophilidae) Towards the Leaf Volatile β-Cyclocitral and Selected Fruit-Ripening Volatiles. Environ. Entomol. 2019, 48, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Van Loon, J.J.A.; Soler, R. Chemical Complexity of Volatiles from Plants Induced by Multiple Attack. Nat. Chem. Biol. 2009, 5, 317–324. [Google Scholar] [CrossRef]
- Piesik, D.; Bocianowski, J.; Kotwica, K.; Lema, G.; Piesik, M.; Ruzsanyi, V.; Mayhew, C.A. Responses of Adult Hypera rumicis L. to Synthetic Plant Volatile Blends. Molecules 2022, 27, 6290. [Google Scholar] [CrossRef] [PubMed]
- Piesik, D.; Wenda-Piesik, A.; Krasińska, A.; Wrzesińska, D.; Delaney, K.J. Volatile Organic Compounds Released by Rumex Confertus Following Hypera Rumicis Herbivory and Weevil Responses to Volatiles. J. Appl. Entomol. 2016, 140, 308–316. [Google Scholar] [CrossRef]
- Zhu, X.; Li, L.; Hsiang, T.; Zha, Y.; Zhou, Z.; Chen, R.; Wang, X.; Wu, Q.; Li, J. Chemical Composition and Attractant Activity of Volatiles from Rhus Potaninii to the Spring Aphid Kaburagia Rhusicola. Molecules 2020, 25, 3412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Wang, R.; Yu, L.F.; Lu, P.F.; Luo, Y.Q. Identification of Caragana Plant Volatiles, Overlapping Profiles, and Olfactory Attraction to Chlorophorus Caragana in the Laboratory. J. Plant Interact. 2015, 10, 41–50. [Google Scholar] [CrossRef]
- Gruber, M.Y.; Xu, N.; Grenkow, L.; Li, X.; Onyilagha, J.; Soroka, J.J.; Westcott, N.D.; Hegedus, D.D. Responses of the Crucifer Flea Beetle to Brassica Volatiles in an Olfactometer. Environ. Entomol. 2009, 38, 1467–1479. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive Chemical Signals Induced by a Plant Virus Attract Insect Vectors to Inferior Hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef]
- Ali, J.; Covaci, A.D.; Roberts, J.M.; Sobhy, I.S.; Kirk, W.D.J.; Bruce, T.J.A. Effects of Cis-Jasmone Treatment of Brassicas on Interactions With Myzus Persicae Aphids and Their Parasitoid Diaeretiella Rapae. Front. Plant Sci. 2021, 12, 711896. [Google Scholar] [CrossRef]
- Pagadala Damodaram, K.J.; Gadad, H.S.; Parepally, S.K.; Vaddi, S.; Ramanna Hunashikatti, L.; Bhat, R.M. Low Moisture Stress Influences Plant Volatile Emissions Affecting Herbivore Interactions in Tomato, Solanum Lycopersicum. Ecol. Entomol. 2021, 46, 637–650. [Google Scholar] [CrossRef]
- Du, X.; Witzgall, P.; Wu, K.; Yan, F.; Ma, C.; Zheng, H.; Xu, F.; Ji, G.; Wu, X. Volatiles from Prunus Persica Flowers and Their Correlation with Flower-Visiting Insect Community in Wanbailin Ecological Garden, China. Adv. Entomol. 2018, 6, 116–133. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, N.; Zhou, L.L.; Si, S.Y.; Lei, C.L.; Ai, H.; Wang, X.P. Antennal and Behavioral Responses of Female Maruca Vitrata to the Floral Volatiles of Vigna Unguiculata and Lablab Purpureus. Entomol. Exp. Appl. 2014, 152, 248–257. [Google Scholar] [CrossRef]
- Coppola, M.; Cascone, P.; Madonna, V.; Di Lelio, I.; Esposito, F.; Avitabile, C.; Romanelli, A.; Guerrieri, E.; Vitiello, A.; Pennacchio, F.; et al. Plant-To-Plant Communication Triggered by Systemin Primes Anti-Herbivore Resistance in Tomato. Sci. Rep. 2017, 7, 15522. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martínez, E.S.; Bosque-Pérez, N.A.; Berger, P.H.; Zemetra, R.S.; Ding, H.; Eigenbrode, S.D. Volatile Cues Influence the Response of Rhopalosiphum Padi (Homoptera: Aphididae) to Barley Yellow Dwarf Virus-Infected Transgenic and Untransformed Wheat. Environ. Entomol. 2004, 33, 1207–1216. [Google Scholar] [CrossRef]
- Qiu, X.; Cao, L.; Han, R. Analysis of Volatile Components in Different Ophiocordyceps Sinensis and Insect Host Products. Molecules 2020, 25, 1603. [Google Scholar] [CrossRef]
- Ganassi, S.; Grazioso, P.; De Cristofaro, A.; Fiorentini, F.; Sabatini, M.A.; Evidente, A.; Altomare, C. Long Chain Alcohols Produced by Trichoderma Citrinoviride Have Phagodeterrent Activity against the Bird Cherry-Oat Aphid Rhopalosiphum Padi. Front. Microbiol. 2016, 7, 297. [Google Scholar] [CrossRef]
- Adebisi, O.; Dolma, S.K.; Verma, P.K.; Singh, B.; Reddy, S.G.E. Volatile, Non-Volatile Composition and Insecticidal Activity of Eupatorium Adenophorum Spreng against Diamondback Moth, Plutella xylostella (L.), and Aphid, Aphis Craccivora Koch. Toxin Rev. 2019, 38, 143–150. [Google Scholar] [CrossRef]
- Huang, K.; Shang, H.; Zhou, Q.; Wang, Y.; Shen, H.; Yan, Y. Volatiles Induced from Hypolepis punctata (Dennstaedtiaceae) by Herbivores Attract Sclomina erinacea (Hemiptera: Reduviidae): Clear Evidence of Indirect Defense in Fern. Insects 2021, 12, 978. [Google Scholar] [CrossRef]
- Badra, Z.; Larsson Herrera, S.; Cappellin, L.; Biasioli, F.; Dekker, T.; Angeli, S.; Tasin, M. Species-Specific Induction of Plant Volatiles by Two Aphid Species in Apple: Real Time Measurement of Plant Emission and Attraction of Lacewings in the Wind Tunnel. J. Chem. Ecol. 2021, 47, 653–663. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, X.; Zhou, S.; Cheng, Z.; Shi, K.; Zhang, C.; Shao, H. Phthalic Acid Esters: Natural Sources and Biological Activities. Toxins 2021, 13, 495. [Google Scholar] [CrossRef]
- Vidal, D.M.; Moreira, M.A.B.; Coracini, M.D.A.; Zarbin, P.H.G. Isophorone Derivatives as a New Structural Motif of Aggregation Pheromones in Curculionidae. Sci. Rep. 2019, 9, 776. [Google Scholar] [CrossRef]
- Zhang, P.J.; Zheng, S.J.; Van Loon, J.J.A.; Boland, W.; David, A.; Mumm, R.; Dicke, M. Whiteflies Interfere with Indirect Plant Defense against Spider Mites in Lima Bean. Proc. Natl. Acad. Sci. USA 2009, 106, 21202–21207. [Google Scholar] [CrossRef]
- Maffei, M.; Gertsch, J.; Appendino, G. Plant Volatiles: Production, Function and Pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of Plant Volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Harvey, J.A.; Kamp, A.F.D.; Vet, L.E.M.; Van Der Putten, W.H.; Van Dam, N.M.; Stuefer, J.F.; Gols, R.; Hordijk, C.A.; Bezemer, T.M. Root Herbivores Influence the Behaviour of an Aboveground Parasitoid through Changes in Plant-Volatile Signals. Oikos 2007, 116, 367–376. [Google Scholar] [CrossRef]
- Delphia, C.M.; Mescher, M.C.; De Moraes, C.M. Induction of Plant Volatiles by Herbivores with Different Feeding Habits and the Effects of Induced Defenses on Host-Plant Selection by Thrips. J. Chem. Ecol. 2007, 33, 997–1012. [Google Scholar] [CrossRef] [PubMed]
- Piesik, D.; Pańka, D.; Jeske, M.; Wenda-Piesik, A.; Delaney, K.J.; Weaver, D.K. Volatile Induction of Infected and Neighbouring Uninfected Plants Potentially Influence Attraction/Repellence of a Cereal Herbivore. J. Appl. Entomol. 2013, 137, 296–309. [Google Scholar] [CrossRef]
- Pierre, P.S.; Jansen, J.J.; Hordijk, C.A.; van Dam, N.M.; Cortesero, A.M.; Dugravot, S. Differences in Volatile Profiles of Turnip Plants Subjected to Single and Dual Herbivory Above- and Belowground. J. Chem. Ecol. 2011, 37, 368–377. [Google Scholar] [CrossRef]
- Pasteels, J.M.; Gregoire, J.C. Selective Predation on Chemically Defended Chrysomelid Larvae—A Conditioning Process. J. Chem. Ecol. 1984, 10, 1693–1700. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kruglova, O.Y.; Kozlov, M.V. Drivers of Host Plant Shifts in the Leaf Beetle Chrysomela Lapponica: Natural Enemies or Competition? Ecol. Entomol. 2010, 35, 611–622. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Hogg, D.B.; Gratton, C. Methyl Salicylate Attracts Natural Enemies and Reduces Populations of Soybean Aphids (Hemiptera: Aphididae) in Soybean Agroecosystems. J. Econ. Entomol. 2011, 104, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; LeClere, S.; Carroll, M.J.; Alborn, H.T.; Teal, P.E.A. Cowpea Chloroplastic ATP Synthase Is the Source of Multiple Plant Defense Elicitors during Insect Herbivory. Plant Physiol. 2007, 144, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Carroll, M.J.; LeClere, S.; Phipps, S.M.; Meredith, J.; Chourey, P.S.; Alborn, H.T.; Teal, P.E.A. Fragments of ATP Synthase Mediate Plant Perception of Insect Attack. Proc. Natl. Acad. Sci. USA 2006, 103, 8894–8899. [Google Scholar] [CrossRef]
- Hilker, M.; Stein, C.; Schröder, R.; Varama, M.; Mumm, R. Insect Egg Deposition Induces Defence Responses in Pinus Sylvestris: Characterisation of the Elicitor. J. Exp. Biol. 2005, 208, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Alborn, H.T.; Hansen, T.V.; Jones, T.H.; Bennett, D.C.; Tumlinson, J.H.; Schmelz, E.A.; Teal, P.E.A. Disulfooxy Fatty Acids from the American Bird Grasshopper Schistocerca Americana, Elicitors of Plant Volatiles. Proc. Natl. Acad. Sci. USA 2007, 104, 12976–12981. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Engelberth, J.; Alborn, H.T.; Tumlinson, J.H.; Teal, P.E.A. Phytohormone-Based Activity Mapping of Insect Herbivore-Produced Elicitors. Proc. Natl. Acad. Sci. USA 2009, 106, 653–657. [Google Scholar] [CrossRef]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf Whitefly Induces Salicylic Acid Defenses and Suppresses Effectual Jasmonic Acid Defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, Function and Metabolic Engineering of Plant Volatile Organic Compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Schiestl, F.P. Ecology and Evolution of Floral Volatile-Mediated Information Transfer in Plants. New Phytol. 2015, 206, 571–577. [Google Scholar] [CrossRef]
- Yoneya, K.; Kugimiya, S.; Takabayashi, J. Can Herbivore-Induced Plant Volatiles Inform Predatory Insect about the Most Suitable Stage of Its Prey? Physiol. Entomol. 2009, 34, 379–386. [Google Scholar] [CrossRef]
- Takabayashi, J.; Takahashi, S.; Dicke, M.; Posthumus, M.A. Developmental stage of herbivore Pseudaletia induced synomone by corn plants. J. Chem. Ecol. 1995, 21, 273–287. [Google Scholar] [CrossRef]
- Köpke, D.; Schröder, R.; Fischer, H.M.; Gershenzon, J.; Hilker, M.; Schmidt, A. Does Egg Deposition by Herbivorous Pine Sawflies Affect Transcription of Sesquiterpene Synthases in Pine? Planta 2008, 228, 427–438. [Google Scholar] [CrossRef]
- Mumm, R.; Schrank, K.; Wegener, R.; Schulz, S.; Hilker, M. Chemical Analysis of Volatiles Emitted by Pinus Sylvestris after Induction by Insect Oviposition. J. Chem. Ecol. 2003, 29, 1235–1252. [Google Scholar] [CrossRef]
- Horiuchi, J.-I.; Arimura, G.-I.; Ozawa, R.; Shimoda, T.; Takabayashi, J.; Nishioka, T. A Comparison of the Response of Tetranychus urticae (Acari: Tetranychidae) and Phytoseiulus persimilis (Acari: Phytoseiidae) to Volatiles Emitted from Lima Bean Leaves with Different Levels of Damage Made by T. urticae or Spodoptera exigua (Lepidoptera: N. Appl. Entomol. Zool. 2003, 38, 109–116. [Google Scholar] [CrossRef]
- Girling, R.D.; Stewart-Jones, A.; Dherbecourt, J.; Staley, J.T.; Wright, D.J.; Poppy, G.M. Parasitoids Select Plants More Heavily Infested with Their Caterpillar Hosts: A New Approach to Aid Interpretation of Plant Headspace Volatiles. Proc. R. Soc. B Biol. Sci. 2011, 278, 2646–2653. [Google Scholar] [CrossRef]
- Poelman, E.H.; Bruinsma, M.; Zhu, F.; Weldegergis, B.T.; Boursault, A.E.; Jongema, Y.; van Loon, J.J.A.; Vet, L.E.M.; Harvey, J.A.; Dicke, M. Hyperparasitoids Use Herbivore-Induced Plant Volatiles to Locate Their Parasitoid Host. PLoS Biol. 2012, 10, e1001435. [Google Scholar] [CrossRef]
- McIndoo, N.E. An Insect Olfactometer. J. Econ. Entomol. 1926, 19, 545–571. [Google Scholar] [CrossRef]
- Snapp, O.I.; Swingle, H.S. Further Results with the McIndoo Olfactometer. J. Econ. Entomol. 1929, 22, 984–985. [Google Scholar]
- Sakuma, M.; Fukami, H. The Linear Track Olfactometer: An Assay Device for Taxes of the German Cockroach, Blattella Germanica (L.) (Dictyoptera: Blattellidae) Toward Their Aggregation Pheromone. Appl. Entomol. Zool. 1985, 20, 387–402. [Google Scholar] [CrossRef]
- SABELIS, M.W.; VAN DE BAAN, H.E. Location of Distant Spider Mite Colonies By Phytoseiid Predators: Demonstration of Specific Kairomones Emitted By Tetranychus Urticae and Panonychus Ulmi. Entomol. Exp. Appl. 1983, 33, 303–314. [Google Scholar] [CrossRef]
- Steinberg, S.; Dicke, M.; Vet, L.E.M.; Wanningen, R. Response of the Braconid Parasitoid Cotesia (=Apanteles) Glomerata to Volatile Infochemicals: Effects of Bioassay Set-up, Parasitoid Age and Experience and Barometric Flux. Entomol. Exp. Appl. 1992, 63, 163–175. [Google Scholar] [CrossRef]
- Bartlet, E.; Blight, M.M.; Hick, A.J.; Williams, I.H. The Responses of the Cabbage Seed Weevil (Ceutorhynchus assimilis) to the Odour of Oilseed Rape (Brassica napus) and to Some Volatile Isothiocyanates. Entomol. Exp. Appl. 1993, 68, 295–302. [Google Scholar] [CrossRef]
- Pallini, A.; Janssen, A.; Sabelis, M.W. Odour-Mediated Responses of Phytophagous Mites to Conspecific and Heterospecific Competitors. Oecologia 1997, 110, 179–185. [Google Scholar] [CrossRef]
- Bernasconi, M.L.; Turlings, T.C.J.; Ambrosetti, L.; Bassetti, P.; Dorn, S. Herbivore-Induced Emissions of Maize Volatiles Repel the Corn Leaf Aphid, Rhopalosiphum Maidis. Entomol. Exp. Appl. 1998, 87, 133–142. [Google Scholar] [CrossRef]
- Sullivan, B.T.; Pettersson, E.M.; Seltmann, K.C.; Berisford, C.W. Attraction of the Bark Beetle Parasitoid Roptrocerus Xylophagorum (Hymenoptera: Pteromalidae) to Host-Associated Olfactory Cues. Environ. Entomol. 2000, 29, 1138–1151. [Google Scholar] [CrossRef]
- Conchou, L.; Lucas, P.; Meslin, C.; Proffit, M.; Staudt, M.; Renou, M. Insect Odorscapes: From Plant Volatiles to Natural Olfactory Scenes. Front. Physiol. 2019, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Iovinella, I.; Zhu, J.; Wang, G.; Dani, F.R. Beyond Chemoreception: Diverse Tasks of Soluble Olfactory Proteins in Insects. Biol. Rev. 2018, 93, 184–200. [Google Scholar] [CrossRef]
- Hare, J.D.; Sun, J.J. Production of Induced Volatiles by Datura Wrightii in Response to Damage by Insects: Effect of Herbivore Species and Time. J. Chem. Ecol. 2011, 37, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Defensive Function of Herbivore-Induced Plant Volatile Emissions in Nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef]
- Van Dam, N.M.; Poppy, G.M. Why Plant Volatile Analysis Needs Bioinformatics—Detecting Signal from Noise in Increasingly Complex Profiles. Plant Biol. 2008, 10, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Aartsma, Y.; Bianchi, F.J.J.A.; van der Werf, W.; Poelman, E.H.; Dicke, M. Herbivore-Induced Plant Volatiles and Tritrophic Interactions across Spatial Scales. New Phytol. 2017, 216, 1054–1063. [Google Scholar] [CrossRef]
- Aartsma, Y.; Pappagallo, S.; van der Werf, W.; Dicke, M.; Bianchi, F.J.J.A.; Poelman, E.H. Spatial Scale, Neighbouring Plants and Variation in Plant Volatiles Interactively Determine the Strength of Host–Parasitoid Relationships. Oikos 2020, 129, 1429–1439. [Google Scholar] [CrossRef]
- Andersson, P.; Löfstedt, C.; Hambäck, P.A. How Insects Sense Olfactory Patches—The Spatial Scaling of Olfactory Information. Oikos 2013, 122, 1009–1016. [Google Scholar] [CrossRef]
- Voskamp, K.E.; Den Otter, C.J.; Noorman, N. Electroantennogram Responses of Tsetse Flies (Glossina pallidipes) to Host Odours in an Open Field and Riverine Woodland. Physiol. Entomol. 1998, 23, 176–183. [Google Scholar] [CrossRef]
- Hilker, M.; McNeil, J. Behavioral Ecology of Insect Parasitoids. In Behavioral Ecology of Insect Parasitoids; Wajnberg, É., Bernstein, C., van Alphen, J.J.M., Eds.; Blackwell Publishing: Oxford, UK, 2008; pp. 92–112. [Google Scholar]
- Wäschke, N.; Meiners, T.; Rostás, M. Foraging Strategies of Parasitoids in Complex Chemical Environments. In Chemical Ecology of Insect Parasitoids; Wajnberg, E., Colazza, S., Eds.; Wiley: Chichester, UK, 2013; pp. 37–63. ISBN 9781118409527. [Google Scholar]
- Desurmont, G.A.; von Arx, M.; Turlings, T.C.J.; Schiestl, F.P. Floral Odors Can Interfere With the Foraging Behavior of Parasitoids Searching for Hosts. Front. Ecol. Evol. 2020, 8, 148. [Google Scholar] [CrossRef]
- Knauer, A.C.; Schiestl, F.P. Bees Use Honest Floral Signals as Indicators of Reward When Visiting Flowers. Ecol. Lett. 2015, 18, 135–143. [Google Scholar] [CrossRef]
- Yuan, J.S.; Himanen, S.J.; Holopainen, J.K.; Chen, F.; Stewart, C.N. Smelling Global Climate Change: Mitigation of Function for Plant Volatile Organic Compounds. Trends Ecol. Evol. 2009, 24, 323–331. [Google Scholar] [CrossRef]
- Ali, M.Y.; Lu, Z.; Ali, A.; Amir, M.B.; Ahmed, M.A.; Shahid, S.; Liu, T.; Pan, M. Effects of Plant-Mediated Differences in Aphid Size on Suitability of Its Parasitoid, Aphelinus Varipes (Hymenoptera: Aphelinidae). J. Econ. Entomol. 2021, 10, 74–80. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Nerg, A.-M.; Blande, J.D. Multitrophic Signalling in Polluted Atmospheres; Springer: Dordrecht, The Netherlands, 2013; Volume 5, ISBN 978-94-007-6605-1. [Google Scholar]
- Vet, L.E.M.; Dicke, M. Ecology of Infochemical Use by Natural Enemies in a Tritrophic Context. Annu. Rev. Entomol. 1992, 37, 141–172. [Google Scholar] [CrossRef]
- Puente, M.; Magori, K.; Kennedy, G.G.; Gould, F. Impact of Herbivore-Induced Plant Volatiles on Parasitoid Foraging Success: A Spatial Simulation of the Cotesia Rubecula, Pieris Rapae, and Brassica Oleracea System. J. Chem. Ecol. 2008, 34, 959–970. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Gas-Phase Tropospheric Chemistry of Biogenic Volatile Organic Compounds: A Review. Atmos. Environ. 2003, 37, 197–219. [Google Scholar] [CrossRef]
- Mentel, T.F.; Wildt, J.; Kiendler-Scharr, A.; Kleist, E.; Tillmann, R.; Dal Maso, M.; Fisseha, R.; Hohaus, T.; Spahn, H.; Uerlings, R.; et al. Photochemical Production of Aerosols from Real Plant Emissions. Atmos. Chem. Phys. 2009, 9, 4387–4406. [Google Scholar] [CrossRef]
- Pratt, K.A.; Mielke, L.H.; Shepson, P.B.; Bryan, A.M.; Steiner, A.L.; Ortega, J.; Daly, R.; Helmig, D.; Vogel, C.S.; Griffith, S.; et al. Contributions of Individual Reactive Biogenic Volatile Organic Compounds to Organic Nitrates above a Mixed Forest. Atmos. Chem. Phys. 2012, 12, 10125–10143. [Google Scholar] [CrossRef]
- Joutsensaari, J.; Loivamäki, M.; Vuorinen, T.; Miettinen, P.; Nerg, A.M.; Holopainen, J.K.; Laaksonen, A. Nanoparticle Formation by Ozonolysis of Inducible Plant Volatiles. Atmos. Chem. Phys. 2005, 5, 1489–1495. [Google Scholar] [CrossRef]
- Kiendler-Scharr, A.; Wildt, J.; Maso, M.D.; Hohaus, T.; Kleist, E.; Mentel, T.F.; Tillmann, R.; Uerlings, R.; Schurr, U.; Wahner, A. New Particle Formation in Forests Inhibited by Isoprene Emissions. Nature 2009, 461, 381–384. [Google Scholar] [CrossRef]
- Arneth, A.; Niinemets, Ü. Induced BVOCs: How to Bug Our Models? Trends Plant Sci. 2010, 15, 118–125. [Google Scholar] [CrossRef]
- Ponzio, C.; Cascone, P.; Cusumano, A.; Weldegergis, B.T.; Fatouros, N.E.; Guerrieri, E.; Dicke, M.; Gols, R. Volatile-Mediated Foraging Behaviour of Three Parasitoid Species under Conditions of Dual Insect Herbivore Attack. Anim. Behav. 2016, 111, 197–206. [Google Scholar] [CrossRef]
- Geervliet, J.B.F.; Vet, L.E.M.; Dicke, M. Innate Responses of the Parasitoids Cotesia Glomerata and C. Rubecula (Hymenoptera: Braconidae) to Volatiles from Different Plant-Herbivore Complexes. J. Insect Behav. 1996, 9, 525–538. [Google Scholar] [CrossRef]
- Potting, R.P.J.; Vet, L.E.M.; Dicke, M. Host microhabitat location by stem-borer parasitoid Cotesia Flavipes: The role of herbivore volatiles and locally and systemically induced plant volatiles. J. Chem. Ecol. 1995, 21, 525–539. [Google Scholar] [CrossRef]
- Shiojiri, K.; Ozawa, R.; Kugimiya, S.; Uefune, M.; Van Wijk, M.; Sabelis, M.W.; Takabayashi, J. Herbivore-Specific, Density-Dependent Induction of Plant Volatiles: Honest or “Cry Wolf” Signals? PLoS ONE 2010, 5, e12161. [Google Scholar] [CrossRef]
- Rowen, E.; Kaplan, I. Eco-Evolutionary Factors Drive Induced Plant Volatiles: A Meta-Analysis. New Phytol. 2016, 210, 284–294. [Google Scholar] [CrossRef]
- Peñaflor, M.F.G.V.; Bento, J.M.S. Herbivore-Induced Plant Volatiles to Enhance Biological Control in Agriculture. Neotrop. Entomol. 2013, 42, 331–343. [Google Scholar] [CrossRef]
- Arimura, G.I.; Köpke, S.; Kunert, M.; Volpe, V.; David, A.; Brand, P.; Dabrowska, P.; Maffei, M.E.; Boland, W. Effects of Feeding Spodoptera Littoralis on Lima Bean Leaves: IV. Diurnal and Nocturnal Damage Differentially Initiate Plant Volatile Emission. Plant Physiol. 2008, 146, 965–973. [Google Scholar] [CrossRef]
- Van Den Boom, C.E.M.; Van Beek, T.A.; Posthumus, M.A.; De Groot, A.; Dicke, M. Qualitative and Quantitative Variation among Volatile Profiles Induced by Tetranychus Urticae Feeding on Plants from Various Families. J. Chem. Ecol. 2004, 30, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Gols, R.; Bullock, J.M.; Dicke, M.; Bukovinszky, T.; Harvey, J.A. Smelling the Wood from the Trees: Non-Linear Parasitoid Responses to Volatile Attractants Produced by Wild and Cultivated Cabbage. J. Chem. Ecol. 2011, 37, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Poelman, E.H.; Oduor, A.M.O.; Broekgaarden, C.; Hordijk, C.A.; Jansen, J.J.; Van Loon, J.J.A.; Van Dam, N.M.; Vet, L.E.M.; Dicke, M. Field Parasitism Rates of Caterpillars on Brassica Oleracea Plants Are Reliably Predicted by Differential Attraction of Cotesia Parasitoids. Funct. Ecol. 2009, 23, 951–962. [Google Scholar] [CrossRef]
- Rijk, M. Foraging Behaviour of Parasitoids in Multi-Herbivore Communities. Wagening. Univ. Res. 2013, 85, 1517–1528. [Google Scholar]
- Afsheen, S.; Xia, W.; Ran, L.; Zhu, C.S.; Lou, Y.G. Differential Attraction of Parasitoids in Relation to Specificity of Kairomones from Herbivores and Their By-Products. Insect Sci. 2008, 15, 381–397. [Google Scholar] [CrossRef]
- Gross, H.R.; Lewis, W.J.; Jones, R.L.; Nordlund, D.A. Kairomones and Their Use for Management of Entomophagous Insects: III. Stimulation of Trichogramma Achaeae, T. Pretiosum, and Microplitis Croceipes with Host-Seeking Stimuli at Time of Release to Improve Their Efficiency. J. Chem. Ecol. 1975, 1, 431–438. [Google Scholar] [CrossRef]
- Lewis, W.J.; Jones, R.L.; Nordlund, D.A.; Sparks, A.N. Kairomones and Their Use for Management of Entomophagous Insects: I. Evaluation for Increasing Rates of Parasitization by Trichogramma spp. in the Field. J. Chem. Ecol. 1975, 1, 343–347. [Google Scholar] [CrossRef]
- Rasmann, S.; Turlings, T.C.J. Simultaneous Feeding by Aboveground and Belowground Herbivores Attenuates Plant-Mediated Attraction of Their Respective Natural Enemies. Ecol. Lett. 2007, 10, 926–936. [Google Scholar] [CrossRef]
- Heil, M.; Karban, R. Explaining Evolution of Plant Communication by Airborne Signals. Trends Ecol. Evol. 2010, 25, 137–144. [Google Scholar] [CrossRef]
- De Rijk, M.; Dicke, M.; Poelman, E.H. Foraging Behaviour by Parasitoids in Multiherbivore Communities. Anim. Behav. 2013, 85, 1517–1528. [Google Scholar] [CrossRef]
- Karban, R.; Shiojiri, K.; Huntzinger, M.; McCall, A.C. Damage-Induced Resistance in Sagebrush: Volatiles Are Key to Intra- and Interplant Communication. Ecology 2006, 87, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Halitschke, R.; Stenberg, J.A.; Kessler, D.; Kessler, A.; Baldwin, I.T. Shared Signals—“Alarm Calls” from Plants Increase Apparency to Herbivores and Their Enemies in Nature. Ecol. Lett. 2008, 11, 24–34. [Google Scholar] [CrossRef]
- Mäntylä, E.; Alessio, G.A.; Blande, J.D.; Heijari, J.; Holopainen, J.K.; Laaksonen, T.; Piirtola, P.; Klemola, T. From Plants to Birds: Higher Avian Predation Rates in Trees Responding to Insect Herbivory. PLoS ONE 2008, 3, e2832. [Google Scholar] [CrossRef]
- Kessler, A.; Halitschke, R.; Diezel, C.; Baldwin, I.T. Priming of Plant Defense Responses in Nature by Airborne Signaling between Artemisia Tridentata and Nicotiana Attenuata. Oecologia 2006, 148, 280–292. [Google Scholar] [CrossRef]
- Himanen, S.J.; Blande, J.D.; Klemola, T.; Pulkkinen, J.; Heijari, J.; Holopainen, J.K. Birch (Betula Spp.) Leaves Adsorb and Re-Release Volatiles Specific to Neighbouring Plants—A Mechanism for Associational Herbivore Resistance? New Phytol. 2010, 186, 722–732. [Google Scholar] [CrossRef]
- Karban, R. Associational Resistance for Mule’s Ears with Sagebrush Neighbors. Plant Ecol. 2007, 191, 295–303. [Google Scholar] [CrossRef]
- Himanen, S.J.; Bui, T.N.T.; Maja, M.M.; Holopainen, J.K. Utilizing Associational Resistance for Biocontrol: Impacted by Temperature, Supported by Indirect Defence. BMC Ecol. 2015, 15, 16. [Google Scholar] [CrossRef]
- Erb, M.; Veyrat, N.; Robert, C.A.M.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C.J. Indole Is an Essential Herbivore-Induced Volatile Priming Signal in Maize. Nat. Commun. 2015, 6, 6273. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.J.; Appel, H.M.; Carlson, J.E.; De Moraes, C.M.; Mescher, M.C.; Schultz, J.C. Within-Plant Signalling via Volatiles Overcomes Vascular Constraints on Systemic Signalling and Primes Responses against Herbivores. Ecol. Lett. 2007, 10, 490–498. [Google Scholar] [CrossRef]
- Karban, R.; Shiojiri, K.; Ishizaki, S.; Wetzel, W.C.; Evans, R.Y. Kin Recognition Affects Plant Communication and Defence. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123062. [Google Scholar] [CrossRef] [PubMed]
- Ninkovic, V.; Dahlin, I.; Vucetic, A.; Petrovic-Obradovic, O.; Glinwood, R.; Webster, B. Volatile Exchange between Undamaged Plants—A New Mechanism Affecting Insect Orientation in Intercropping. PLoS ONE 2013, 8, e69431. [Google Scholar] [CrossRef] [PubMed]
- Randlkofer, B.; Obermaier, E.; Casas, J.; Meiners, T. Connectivity Counts: Disentangling Effects of Vegetation Structure Elements on the Searching Movement of a Parasitoid. Ecol. Entomol. 2010, 35, 446–455. [Google Scholar] [CrossRef]
- Atema, J. Eddy Chemotaxis and Odor Landscapes: Exploration of Nature with Animal Sensors. Biol. Bull. 1996, 191, 129–138. [Google Scholar] [CrossRef]
- Beyaert, I.; Hilker, M. Plant Odour Plumes as Mediators of Plant-Insect Interactions. Biol. Rev. 2014, 89, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.; Gurr, G.M.; Simmons, A.T.; Wratten, S.D.; James, D.G.; Leeson, G.; Nicol, H.I.; Orre-Gordon, G.U.S. Attract and Reward: Combining Chemical Ecology and Habitat Manipulation to Enhance Biological Control in Field Crops. J. Appl. Ecol. 2011, 48, 580–590. [Google Scholar] [CrossRef]
- Schellhorn, N.A.; Bianchi, F.J.J.A.; Hsu, C.L. Movement of Entomophagous Arthropods in Agricultural Landscapes: Links to Pest Suppression. Annu. Rev. Entomol. 2014, 59, 559–581. [Google Scholar] [CrossRef]
- James, D.G. Synthetic Herbivore-Induced Plant Volatiles as Field Attractants for Beneficial Insects. Environ. Entomol. 2003, 32, 977–982. [Google Scholar] [CrossRef]
- James, D.G.; Price, T.S. Field-Testing of Methyl Salicylate for Recruitment and Retention of Beneficial Insects in Grapes and Hops. J. Chem. Ecol. 2004, 30, 1613–1628. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.P.; Horton, D.R.; Mills, N.J.; Unruh, T.R.; Baker, C.C.; Melton, T.D.; Milickzy, E.; Steffan, S.A.; Shearer, P.W.; Amarasekare, K.G. Evaluating Plant Volatiles for Monitoring Natural Enemies in Apple, Pear and Walnut Orchards. Biol. Control. 2016, 102, 53–65. [Google Scholar] [CrossRef]
- Lucchi, A.; Loni, A.; Gandini, L.M.; Scaramozzino, P.; Ioriatti, C.; Ricciardi, R.; Shearer, P.W. Using Herbivore-Induced Plant Volatiles to Attract Lacewings, Hoverflies and Parasitoid Wasps in Vineyards: Achievements and Constraints. Bull. Insectol. 2017, 70, 273–282. [Google Scholar]
- Fatouros, N.E.; Lucas-Barbosa, D.; Weldegergis, B.T.; Pashalidou, F.G.; van Loon, J.J.A.; Dicke, M.; Harvey, J.A.; Gols, R.; Huigens, M.E. Plant Volatiles Induced by Herbivore Egg Deposition Affect Insects of Different Trophic Levels. PLoS ONE 2012, 7, e43607. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Alborn, H.T.; Loughrin, J.H.; Tumlinson, J.H. Volicitin, an Elicitor of Maize Volatiles in Oral Secretion of Spodoptera Exigua: Isolation and Bioactivity. J. Chem. Ecol. 2000, 26, 189–202. [Google Scholar] [CrossRef]
- Ayelo, P.M.; Pirk, C.W.W.; Yusuf, A.A.; Chailleux, A.; Mohamed, S.A.; Deletre, E. Exploring the Kairomone-Based Foraging Behaviour of Natural Enemies to Enhance Biological Control: A Review. Front. Ecol. Evol. 2021, 9, 641974. [Google Scholar] [CrossRef]
- Flint, H.M.; Salter, S.S.; Walters, S. Caryophyllene: An Attractant for the Green Lacewingl. Environ. Entomol. 1979, 8, 1123–1125. [Google Scholar] [CrossRef]
- Dean, G.J.; Satasook, C. Response of Chrysoperla Carnea (Stephens) (Neuroptera: Chrysopidae) to Some Potential Attractants. Bull. Entomol. Res. 1983, 73, 619–624. [Google Scholar] [CrossRef]
- Szendrei, Z.; Rodriguez-Saona, C. A Meta-Analysis of Insect Pest Behavioral Manipulation with Plant Volatiles. Entomol. Exp. Appl. 2010, 134, 201–210. [Google Scholar] [CrossRef]
- Xu, X.; Cai, X.; Bian, L.; Luo, Z.; Li, Z.; Chen, Z. Does Background Odor in Tea Gardens Mask Attractants? Screening and Application of Attractants for Empoasca Onukii Matsuda. J. Econ. Entomol. 2017, 110, 2357–2363. [Google Scholar] [CrossRef]
- Tóth, M.; Bozsik, A.; Szentkirályi, F.; Letardi, A.; Tabilio, M.R.; Verdinelli, M.; Zandigiacomo, P.; Jekisa, J.; Szarukán, I. Phenylacetaldehyde: A Chemical Attractant for Common Green Lacewings (Chrysoperla Carnea s.l., Neuroptera: Chrysopidae). Eur. J. Entomol. 2006, 103, 267–271. [Google Scholar] [CrossRef]
- Ye, L.; Yang, C.; Li, W.; Hao, J.; Sun, M.; Zhang, J.; Zhang, Z. Evaluation of Volatile Compounds from Chinese Dwarf Cherry (Cerasus Humilis (Bge.) Sok.) Germplasms by Headspace Solid-Phase Microextraction and Gas Chromatography–Mass Spectrometry. Food Chem. 2017, 217, 389–397. [Google Scholar] [CrossRef]
- Maatallah, S.; Dabbou, S.; Castagna, A.; Guizani, M.; Hajlaoui, H.; Ranieri, A.M.; Flamini, G. Prunus Persica By-Products: A Source of Minerals, Phenols and Volatile Compounds. Sci. Hortic. 2020, 261, 109016. [Google Scholar] [CrossRef]
- Najar-Rodriguez, A.; Orschel, B.; Dorn, S. Season-Long Volatile Emissions from Peach and Pear Trees In Situ, Overlapping Profiles, and Olfactory Attraction of an Oligophagous Fruit Moth in the Laboratory. J. Chem. Ecol. 2013, 39, 418–429. [Google Scholar] [CrossRef]
- Thompson, A.C.; Baker, D.N.; Gueldner, R.C.; Hedin, P.A. Identification and Quantitative Analysis of the Volatile Substances Emitted by Maturing Cotton in the Field. Plant Physiol. 1971, 48, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.; Crafts-Brandner, S.J.; Paré, P.W.; Henneberry, T.J. Exogenous Methyl Jasmonate Induces Volatile Emissions in Cotton Plants. J. Chem. Ecol. 2001, 27, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Starr, G.; Petersen, M.A.; Jespersen, B.M.; Hansen, A.S. Variation of Volatile Compounds among Wheat Varieties and Landraces. Food Chem. 2015, 174, 527–537. [Google Scholar] [CrossRef]
- Pålsson, J.; Porcel, M.; Dekker, T.; Tasin, M. Attract, Reward and Disrupt: Responses of Pests and Natural Enemies to Combinations of Habitat Manipulation and Semiochemicals in Organic Apple. J. Pest Sci. 2022, 95, 619–631. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The Use of Push-Pull Strategies in Integrated Pest Management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- Niu, Y.; Han, S.; Wu, Z.; Pan, C.; Wang, M.; Tang, Y.; Zhang, Q.-H.; Tan, G.; Han, B. A Push–Pull Strategy for Controlling the Tea Green Leafhopper (Empoasca Flavescens F.) Using Semiochemicals from Tagetes Erecta and Flemingia Macrophylla. Pest Manag. Sci. 2022, 78, 2161–2172. [Google Scholar] [CrossRef] [PubMed]



| BVOC | Lifetimes for Reaction with Oxidants | ||||
|---|---|---|---|---|---|
| HIPVs Compounds | Class | OH a | O3 b | NO3 c | Reference |
| cis-/trans-Ocimene | Monoterpene | 33 min | 44 min | 3 min | [116] |
| β-Phellandrene | Monoterpene | 50 min | 8.4 h | 8 min | [116] |
| Linalool | Monoterpene | 52 min | 55 min | 6 min | [116] |
| β-Caryophyllene | Sesquiterpene | 42 min | 2 min | 3 min | [116] |
| β-Farnesene | Sesquiterpene | 52 min | 26 min | – | [121] |
| DMNT (4,8-dimethyl- ,3,7 nonatriene) | Homoterpene | 40 min | 60 min | 3 min | I |
| TMTT (4,8,12-trimethyl- 1,3,7,11-tridecatetraene) | Homoterpene | 30 min | 30 min | 2 min | I |
| cis-3-Hexenyl acetate | Green leaf volatile | 1.8 h | 7.3 h | 4.5 h | [116] |
| cis-3-Hexen-1-ol | Green leaf volatile | 1.3 h | 6.2 h | 4.1 h | [116] |
| cis-3-Hexenal | Green leaf volatile | 11.2 day | 3.0 h | – | [121] |
| Methyl salicylate | Aromatics | 73.5 h | >9.8 year | – | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.Y.; Naseem, T.; Holopainen, J.K.; Liu, T.; Zhang, J.; Zhang, F. Tritrophic Interactions among Arthropod Natural Enemies, Herbivores and Plants Considering Volatile Blends at Different Scale Levels. Cells 2023, 12, 251. https://doi.org/10.3390/cells12020251
Ali MY, Naseem T, Holopainen JK, Liu T, Zhang J, Zhang F. Tritrophic Interactions among Arthropod Natural Enemies, Herbivores and Plants Considering Volatile Blends at Different Scale Levels. Cells. 2023; 12(2):251. https://doi.org/10.3390/cells12020251
Chicago/Turabian StyleAli, Muhammad Yasir, Tayyaba Naseem, Jarmo K. Holopainen, Tongxian Liu, Jinping Zhang, and Feng Zhang. 2023. "Tritrophic Interactions among Arthropod Natural Enemies, Herbivores and Plants Considering Volatile Blends at Different Scale Levels" Cells 12, no. 2: 251. https://doi.org/10.3390/cells12020251
APA StyleAli, M. Y., Naseem, T., Holopainen, J. K., Liu, T., Zhang, J., & Zhang, F. (2023). Tritrophic Interactions among Arthropod Natural Enemies, Herbivores and Plants Considering Volatile Blends at Different Scale Levels. Cells, 12(2), 251. https://doi.org/10.3390/cells12020251







