Calcein-Modified CeO2 for Intracellular ROS Detection: Mechanisms of Action and Cytotoxicity Analysis In Vitro
Abstract
:1. Introduction
2. Materials and Methods
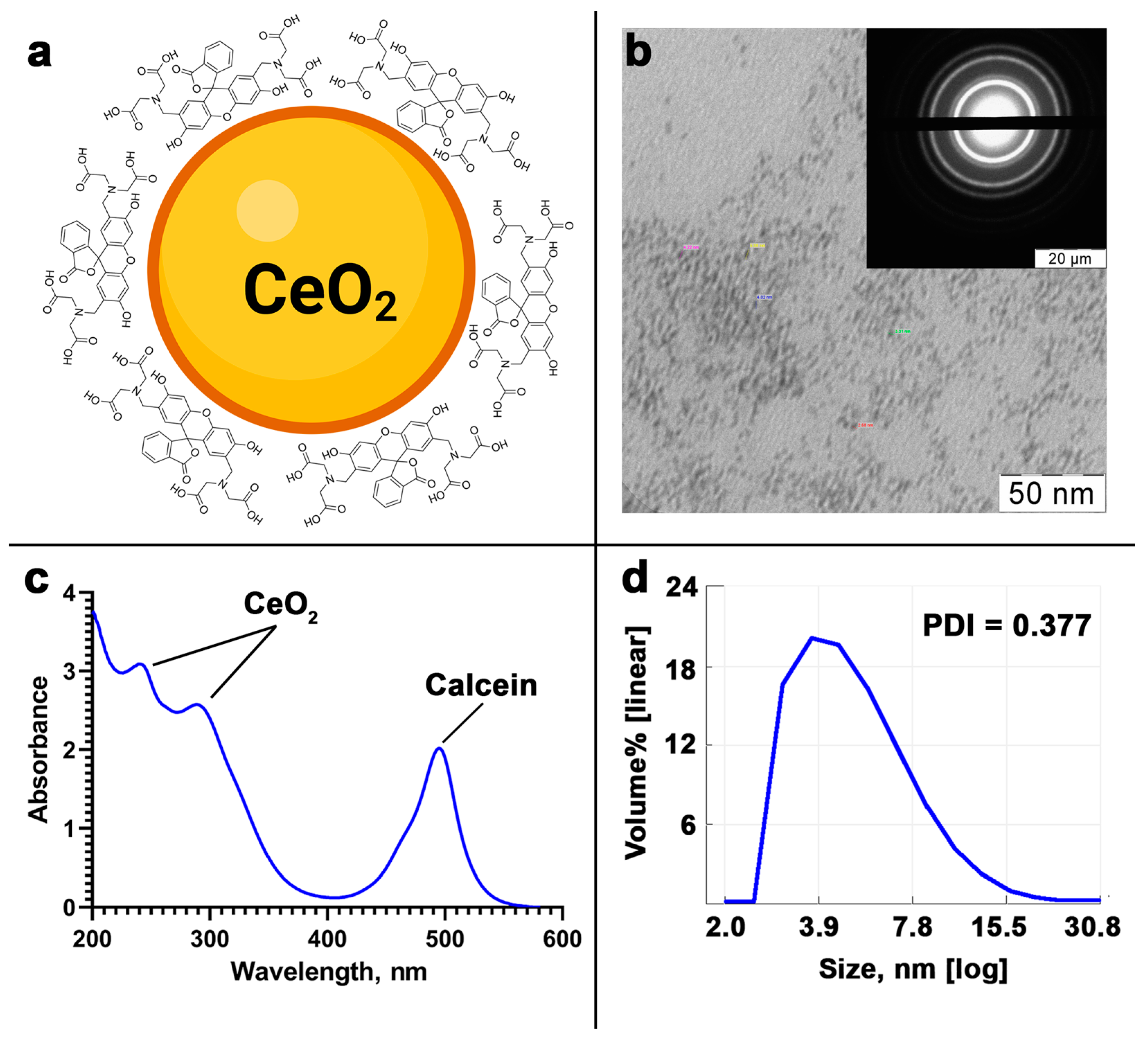
2.1. CeO2-Calcein Synthesis and Analysis
2.2. Cell Culture
2.3. MTT Assay
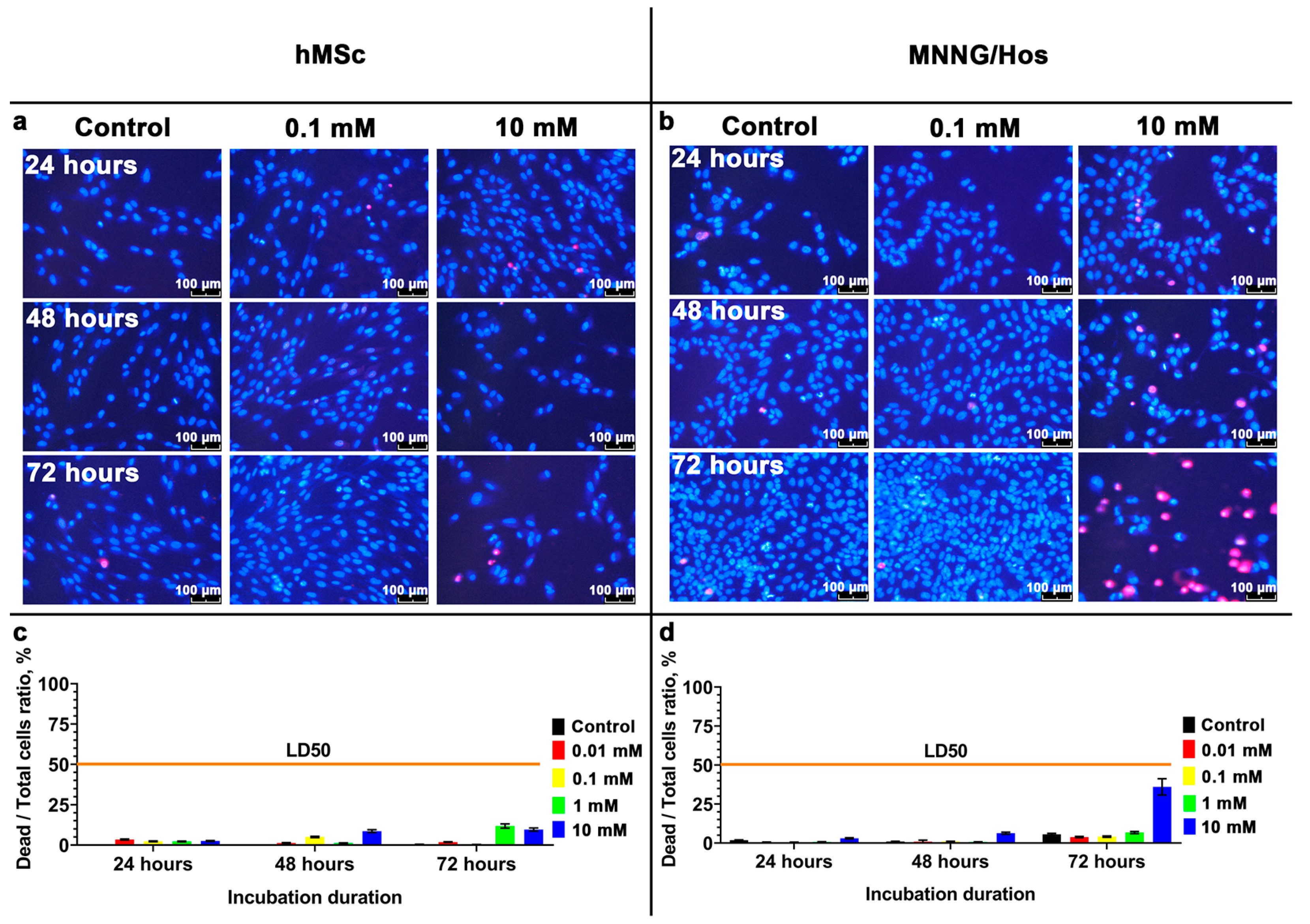
2.4. Live/Dead Assay
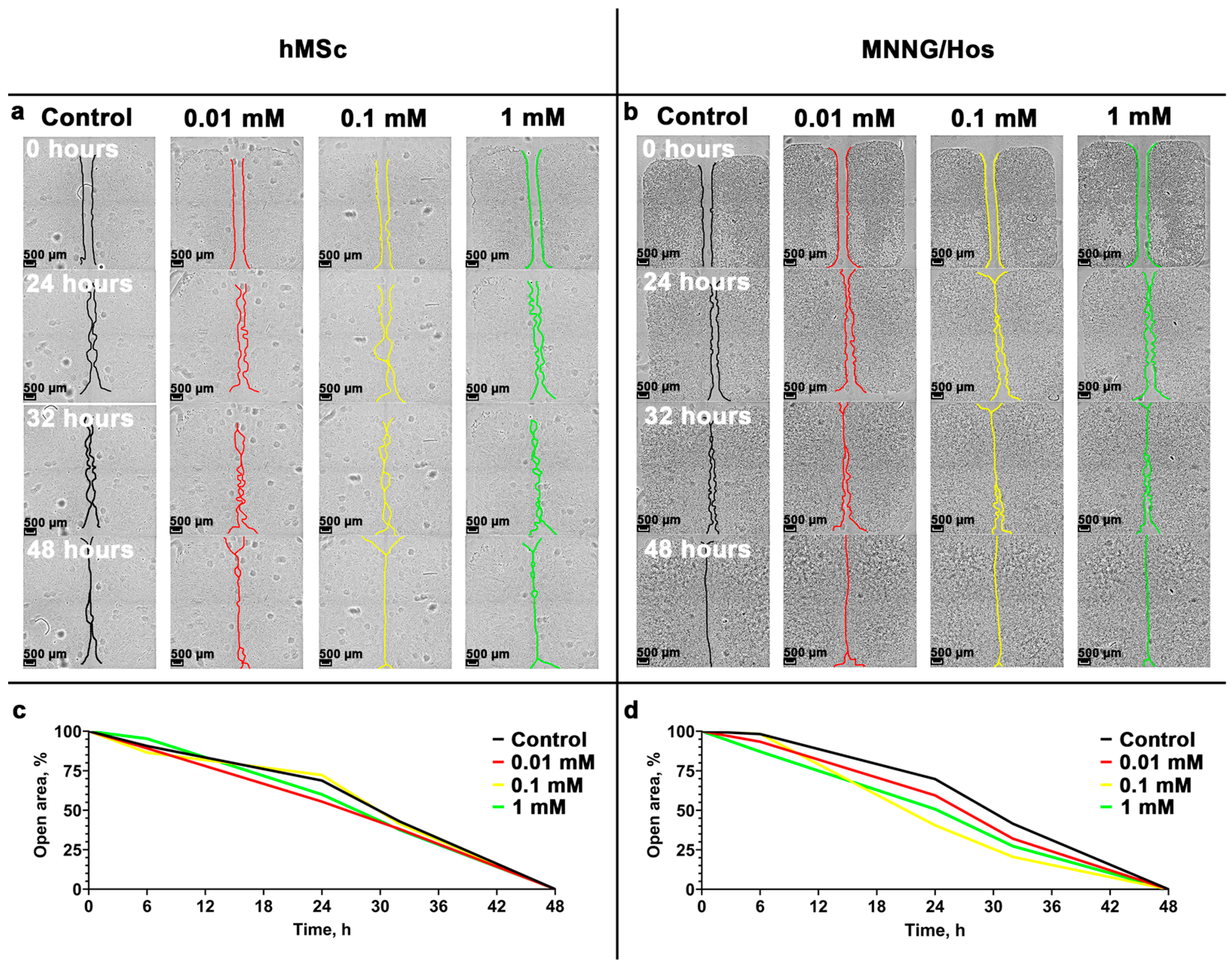
2.5. Wound-Healing Assay
2.6. Real-Time Polymerase Chain Reaction (RT-PCR)
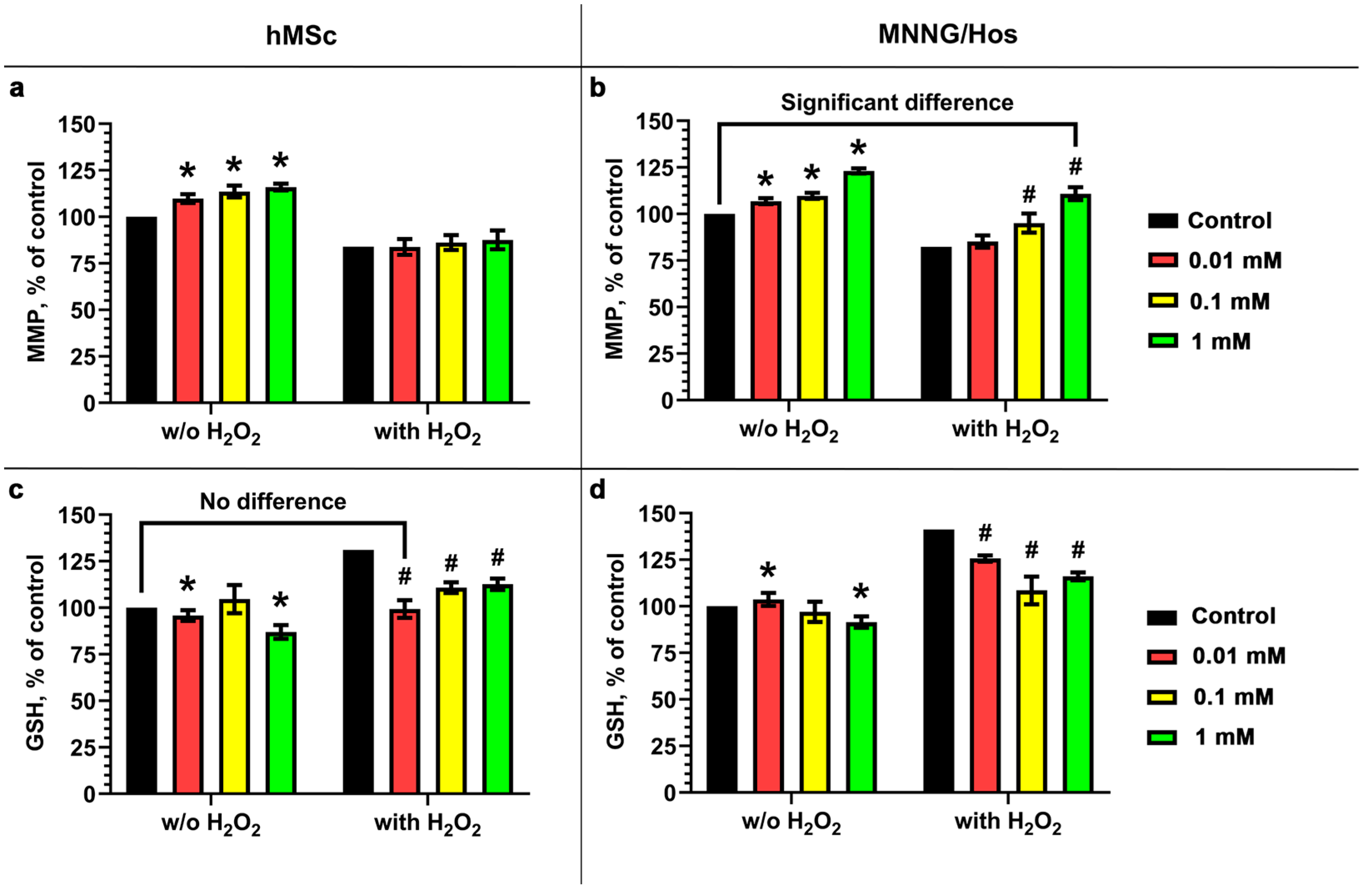
2.7. Antioxidant Activity Analysis of Calcein-Modified CeO2 NPs
2.8. Statistical Analysis
3. Results and Discussion
3.1. The Analysis of Calcein-Modified CeO2 NPs
3.2. Cytotoxicity Analysis of the Calcein-Modified CeO2 NPs
3.3. Cell Migration Analysis
3.4. Antioxidant Activity of the Calcein-Modified CeO2 NPs
3.5. Functional Activity of the Calcein-Modified CeO2 NPs
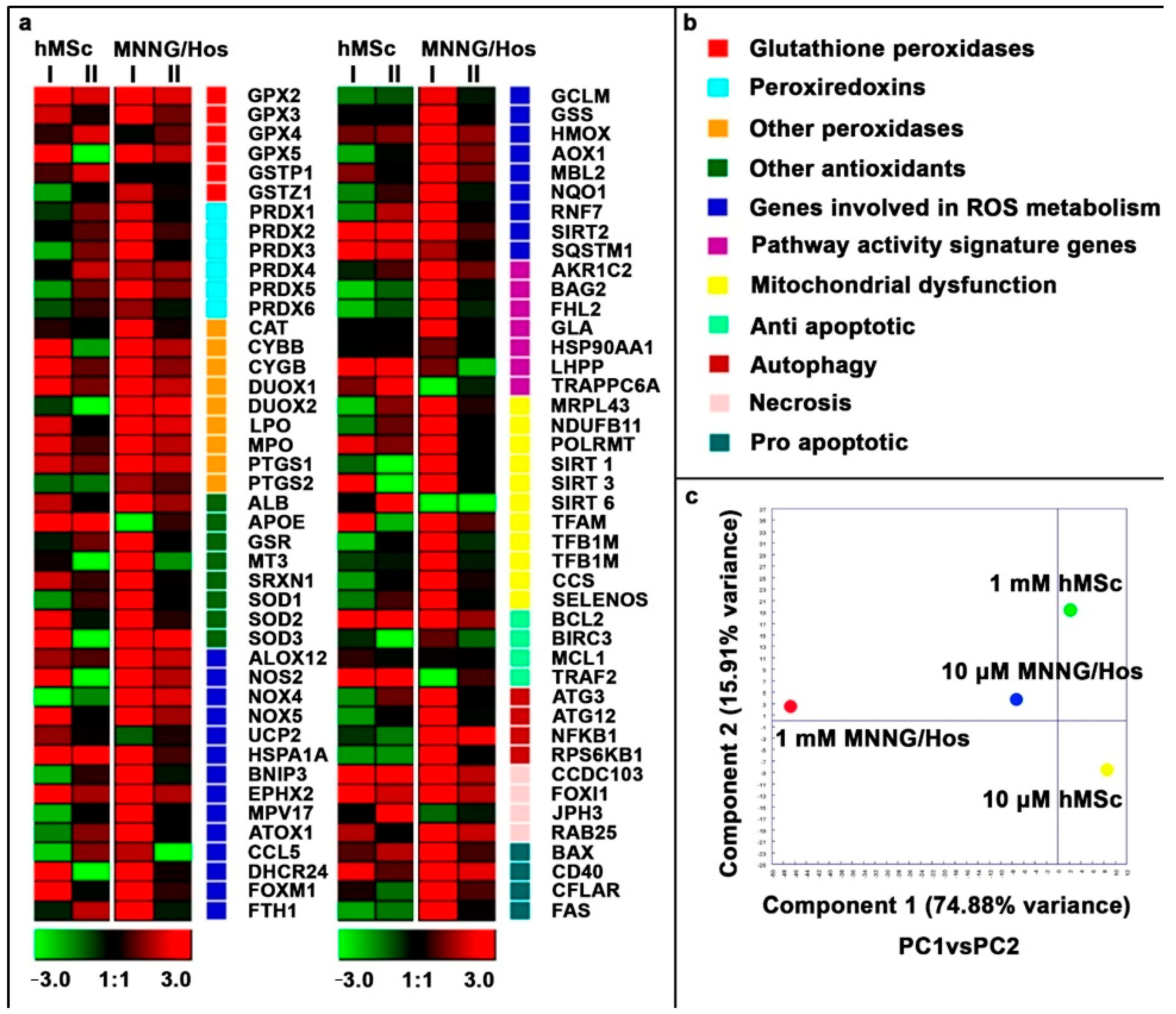
3.6. Gene Expression Analysis
3.6.1. Glutathione Peroxidase Gene Family Analysis
3.6.2. Peroxiredoxin Gene Family Analysis
3.6.3. Peroxidase Gene Family Analysis
3.6.4. Antioxidant Gene Family Analysis
3.6.5. Analysis of Genes Involved in ROS Metabolism
3.6.6. Pathway Signature Gene Analysis
3.6.7. Mitochondrial Dysfunction Gene Analysis
3.6.8. Anti-Apoptotic Gene Analysis
3.6.9. Autophagy Gene Analysis
3.6.10. Necrosis Gene Analysis
3.6.11. Pro-Apoptotic Gene Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Y.; Tian, Z.; Zhai, W. Insights on catalytic mechanism of CeO2 as multiple nanozymes. Nano Res. 2022, 15, 10328–10342. [Google Scholar] [CrossRef] [PubMed]
- Dan, M.; Wu, P.; Grulke, E.A.; Graham, U.M.; Unrine, J.M.; Yokel, R.A. Ceria-engineered nanomaterial distribution in, and clearance from, blood: Size matters. Nanomedicine 2012, 7, 95–110. [Google Scholar] [CrossRef]
- Yokel, R.A.; Tseng, M.T.; Dan, M.; Unrine, J.M.; Graham, U.M.; Wu, P.; Grulke, E.A. Biodistribution and biopersistence of ceria engineered nanomaterials: Size dependence. Nanomedicine 2013, 9, 398–407. [Google Scholar] [CrossRef]
- Popov, A.L.; Popova, N.R.; Selezneva, I.I.; Akkizov, A.Y.; Ivanov, V.K. Cerium oxide nanoparticles stimulate proliferation of primary mouse embryonic fibroblasts in vitro. Mater. Sci. Eng. C 2016, 68, 406–413. [Google Scholar] [CrossRef]
- Popov, A.L.; Ermakov, A.M.; Savintseva, I.V.; Selezneva, I.I.; Poltavtseva, R.A.; Zaraisky, E.I.; Poltavtsev, A.M.; Stepanov, A.A.; Ivanov, V.K.; Sukhikh, G.T. Citrate-stabilized nanoparticles of CeO2 stimulate proliferation of human mesenchymal stem cells in vitro. Nanomech. Sci. Technol. Int. J. 2016, 7, 235–246. [Google Scholar] [CrossRef]
- Popov, A.L.; Ermakov, A.M.; Savintseva, I.V.; Selezneva, I.I.; Poltavtseva, R.A.; Zaraisky, E.I.; Poltavtsev, A.M.; Stepanova, I.E.; Ivanov, V.K.; Sukhikh, G.T. Biosafety and effect of nanoparticles of CeO2 on metabolic and proliferative activity of human mesenchemal stem cells in vitro. Int. J. Nanomech. Sci. Technol. 2016, 7, 165–175. [Google Scholar] [CrossRef]
- Gofman, I.V.; Nikolaeva, A.L.; Khripunov, A.K.; Ivan’kova, E.M.; Shabunin, A.S.; Yakimansky, A.V.; Romanov, D.P.; Popov, A.L.; Ermakov, A.M.; Solomevich, S.O.; et al. Bacterial Cellulose-Based Nanocomposites Containing Ceria and Their Use in the Process of Stem Cell Proliferation. Polymers 2021, 13, 1999. [Google Scholar] [CrossRef]
- Popov, A.L.; Zaichkina, S.I.; Popova, N.R.; Rozanova, O.M.; Romanchenko, S.P.; Ivanova, O.S.; Smirnov, A.A.; Mironova, E.V.; Selezneva, I.I.; Ivanov, V.K. Radioprotective effects of ultra-small citrate-stabilized cerium oxide nanoparticles. RSC Adv. 2016, 6, 106141–106149. [Google Scholar] [CrossRef]
- Genchi, G.G.; Degl’Innocenti, A.; Martinelli, C.; Battaglini, M.; De Pasquale, D.; Prato, M.; Marras, S.; Pugliese, G.; Drago, F.; Mariani, A.; et al. Cerium Oxide Nanoparticle Administration to Skeletal Muscle Cells under Different Gravity and Radiation Conditions. ACS Appl. Mater. Interfaces 2021, 13, 40200–40213. [Google Scholar] [CrossRef]
- Popova, N.R.; Ermakov, A.M.; Popov, A.L.; Selezneva, I.I.; Akkizov, A.Y.; Ivanova, O.S.; Ivanov, V.K. Cerium Oxide Nanoparticles Protect Primary Embryonic Mouse Fibroblasts from Oxidative Stress Induced by Low-Temperature Argon Plasma Treatment. Nano Hybrids Compos. 2017, 13, 294–300. [Google Scholar] [CrossRef]
- Ermakov, A.M.; Kamenskikh, K.A.; Popov, A.L.; Ermakova, O.N.; Afanasyeva, V.A.; Ivanov, V.K. Additive effects of green LED light and cerium oxide nanoparticles on the planarian’s regeneration. Nanosyst. Phys. Chem. Math. 2021, 12, 175–181. [Google Scholar] [CrossRef]
- Filippova, K.O.; Ermakov, A.M.; Popov, A.L.; Ermakova, O.N.; Blagodatsky, A.S.; Chukavin, N.N.; Shcherbakov, A.B.; Baranchikov, A.E.; Ivanov, V.K. Mitogen-like Cerium-Based Nanoparticles Protect Schmidtea mediterranea against Severe Doses of X-rays. Int. J. Mol. Sci. 2023, 24, 1241. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Alpaslan, E.; Geilich, B.M.; Yazici, H.; Webster, T.J. pH-Controlled Cerium Oxide Nanoparticle Inhibition of Both Gram-Positive and Gram-Negative Bacteria Growth. Sci. Rep. 2017, 7, 45859. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Dong, Y.; Jia, T.; Liu, S.; Liu, J.; Yang, D.; He, F.; Gai, S.; Yang, P.; Lin, J. GSH-Depleted Nanozymes with Hyperthermia-Enhanced Dual Enzyme-Mimic Activities for Tumor Nanocatalytic Therapy. Adv. Mater. 2020, 32, e2002439. [Google Scholar] [CrossRef] [PubMed]
- Vinothkumar, G.; Lalitha, A.I.; Babu, K.S. Cerium Phosphate–Cerium Oxide Heterogeneous Composite Nanozymes with Enhanced Peroxidase-Like Biomimetic Activity for Glucose and Hydrogen Peroxide Sensing. Inorg. Chem. 2019, 58, 349–358. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.; Zeng, Y.P.; Hao, Y.H.; Huang, J.W.; Yang, Z.Y.; Li, R. Nanoceria-Mediated Drug Delivery for Targeted Photodynamic Therapy on Drug-Resistant Breast Cancer. ACS Appl. Mater. Interfaces 2016, 8, 31510–31523. [Google Scholar] [CrossRef]
- Naha, P.C.; Hsu, J.C.; Kim, J.; Shah, S.; Bouché, M.; Si-Mohamed, S.; Rosario-Berrios, D.N.; Douek, P.; Hajfathalian, M.; Yasini, P.; et al. Dextran-Coated Cerium Oxide Nanoparticles: A Computed Tomography Contrast Agent for Imaging the Gastrointestinal Tract and Inflammatory Bowel Disease. ACS Nano 2020, 14, 10187–10197. [Google Scholar] [CrossRef]
- García, A.; Cámara, J.A.; Boullosa, A.M.; Gustà, M.F.; Mondragón, L.; Schwartz, S., Jr.; Casals, E.; Abasolo, I.; Bastús, N.G.; Puntes, V. Nanoceria as Safe Contrast Agents for X-ray CT Imaging. Nanomaterials 2023, 13, 2208. [Google Scholar] [CrossRef]
- Popov, A.L.; Savintseva, I.V.; Kozlova, T.O.; Ivanova, O.S.; Zhukov, I.V.; Baranchikov, A.E.; Yurkovskaya, A.V.; Savelov, A.A.; Ermakov, A.M.; Popova, N.R.; et al. Heavily Gd-doped non-toxic cerium oxide nanoparticles for MRI labeling of stem cells. Molecules 2023, 28, 1165. [Google Scholar] [CrossRef]
- Popov, A.; Abakumov, M.; Savintseva, I.; Ermakov, A.; Popova, N.; Ivanova, O.; Kolmanovich, D.; Baranchikov, A.; Ivanov, V. Biocompatible dextran-coated gadolinium-doped cerium oxide nanoparticles as MRI contrast agents with high T1 relaxivity and selective cytotoxicity to cancer cells. J. Mater. Chem. B 2021, 9, 6586–6599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhai, X.; Ma, T.; Huang, Y.; Yan, C.; Du, Y. Multifunctional cerium doped carbon dots nanoplatform and its applications for wound healing. Chem. Eng. J. 2021, 423, 130301. [Google Scholar] [CrossRef]
- Khabirova, S.; Aleshin, G.; Plakhova, T.; Zubenko, A.; Shchukina, A.; Fedorova, O.; Averin, A.; Belova, E.; Bazarkina, E.; Kvashnina, K.; et al. CeO2-Azacrown Conjugate as a Nanoplatform for Combined Radiopharmaceuticals. Nanomaterials 2022, 12, 4484. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.M.; Konduru, N.V.; Jimenez, R.J.; Pyrgiotakis, G.; Demokritou, P.; Wohlleben, W.; Brain, J.D. Bioavailability, distribution and clearance of tracheally instilled, gavaged or injected cerium dioxide nanoparticles and ionic cerium. Environ. Sci. Nano 2014, 1, 561–573. [Google Scholar] [CrossRef]
- Zholobak, N.M.; Shcherbakov, A.B.; Vitukova, E.O.; Yegorova, A.V.; Scripinets, Y.V.; Leonenko, I.I.; Baranchikov AYe Antonovich, V.P.; Ivanov, V.K. Direct monitoring of the interaction between ROS and cerium dioxide nanoparticles in living cells. RSC Adv. 2014, 4, 51703–51710. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, H.; Selke, M.; Wang, X. Selective cytotoxicity effect of cerium oxide nanoparticles under UV irradiation. J. Biomed. Nanotechnol. 2014, 10, 278–286. [Google Scholar] [CrossRef]
- Aplak, E.; von Montfort, C.; Haasler, L.; Stucki, D.; Steckel, B.; Reichert, A.S.; Stahl, W.; Brenneisen, P. CNP mediated selective toxicity onmelanom acellsis accompaniedbymitochond rialdysfunction. PLoS ONE 2020, 15, e0227926. [Google Scholar] [CrossRef]
- Zholobak, N.M.; Shcherbakov, A.B.; Ivanova, O.S.; Reukov, V.; Baranchikov, A.E.; Ivanov, V.K. Nanoceria-curcumin conjugate: Synthesis and selective cytotoxicity against cancer cells under oxidative stress conditions. J. Photochem. Photobiol. B 2020, 209, 111921. [Google Scholar] [CrossRef]
- Şekeroğlu, A.Z.; Şekeroğlu, V.; Aydın, B.; KYedier, S. Cerium oxide nanoparticles exert antitumor effects and enhance paclitaxel toxicity and activity against breast cancer cells. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 579–589. [Google Scholar] [CrossRef]
- Weng, Q.; Sun, H.; Fang, C.; Xia, F.; Liao, H.; Lee, J.; Wang, J.; Xie, A.; Ren, J.; Guo, X.; et al. Catalytic activity tunable ceria nanoparticles prevent chemotherapy-induced acute kidney injury without interference with chemotherapeutics. Nat. Commun. 2021, 12, 1436. [Google Scholar] [CrossRef]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar] [PubMed]
- Paul, C.D.; Mistriotis, P.; Konstantopoulos, K. Cancer cell motility: Lessons from migration in confined spaces. Nat. Rev. Cancer 2017, 17, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999, 27, 922–935. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chukavin, N.N.; Ivanov, V.K.; Popov, A.L. Calcein-Modified CeO2 for Intracellular ROS Detection: Mechanisms of Action and Cytotoxicity Analysis In Vitro. Cells 2023, 12, 2416. https://doi.org/10.3390/cells12192416
Chukavin NN, Ivanov VK, Popov AL. Calcein-Modified CeO2 for Intracellular ROS Detection: Mechanisms of Action and Cytotoxicity Analysis In Vitro. Cells. 2023; 12(19):2416. https://doi.org/10.3390/cells12192416
Chicago/Turabian StyleChukavin, Nikita N., Vladimir K. Ivanov, and Anton L. Popov. 2023. "Calcein-Modified CeO2 for Intracellular ROS Detection: Mechanisms of Action and Cytotoxicity Analysis In Vitro" Cells 12, no. 19: 2416. https://doi.org/10.3390/cells12192416
APA StyleChukavin, N. N., Ivanov, V. K., & Popov, A. L. (2023). Calcein-Modified CeO2 for Intracellular ROS Detection: Mechanisms of Action and Cytotoxicity Analysis In Vitro. Cells, 12(19), 2416. https://doi.org/10.3390/cells12192416






