Abstract
Background: The large-scale utilization of immunoglobulins in patients with inborn errors of immunity (IEIs) since 1952 prompted the discovery of their key role at high doses as immunomodulatory and anti-inflammatory therapy, in the treatment of IEI-related immune dysregulation disorders, according to labelled and off-label indications. Recent years have been dominated by a progressive imbalance between the gradual but constant increase in the use of immunoglobulins and their availability, exacerbated by the SARS-CoV-2 pandemic. Objectives: To provide pragmatic indications for a need-based application of high-dose immunoglobulins in the pediatric context. Sources: A literature search was performed using PubMed, from inception until 1st August 2023, including the following keywords: anti-inflammatory; children; high dose gammaglobulin; high dose immunoglobulin; immune dysregulation; immunomodulation; immunomodulatory; inflammation; intravenous gammaglobulin; intravenous immunoglobulin; off-label; pediatric; subcutaneous gammaglobulin; subcutaneous immunoglobulin. All article types were considered. Implications: In the light of the current imbalance between gammaglobulins’ demand and availability, this review advocates the urgency of a more conscious utilization of this medical product, giving indications about benefits, risks, cost-effectiveness, and administration routes of high-dose immunoglobulins in children with hematologic, neurologic, and inflammatory immune dysregulation disorders, prompting further research towards a responsible employment of gammaglobulins and improving the therapeutical decisional process.
1. Introduction
Immunoglobulin is a plasma-derived medicinal product (PDMPs) sourced from a large pool of healthy blood donors, guaranteeing a broad-spectrum specificity against pathogens, which was employed for the first time in the context of inborn errors of immunity (IEI) as replacement therapy (RT) in 1952 [1,2,3]. The large-scale utilization of immunoglobulins in IEI patients favored the discovery of their key role at high doses (HDs) as immunomodulant and anti-inflammatory therapy in IEI-related immune-dysregulation disorders, such as immune-mediated hematological conditions and rheumatic diseases. This fostered the progressive understanding of the wide-spread biological mechanisms responsible for their multiple therapeutic effects, giving a further boost to HD intravenous immunoglobulin (HD-IVIG) implementation in non-IEI-related immune–mediated neurological and inflammatory disorders. It is true that at the current state of the art, for some of these entities, such as Kawasaki disease (KD), multifocal motor neuropathy (MMN), and Guillain–Barré syndrome (GBS), HD-IVIG represents even the first-choice treatment [3,4].
Immunoglobulins are currently employed according to an approved indication by both the European Medicine Agency (EMA) and the Food and Drug Administration (FDA) [3,4,5,6] in the following pediatric disorders: KD in the acute phase [4,7], primary immune thrombocytopenia (ITP) [3,4,8,9], chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) [4,10] and MMN [4,11]. GBS deserves a separate discussion, as HD-IVIG utilization for its treatment is approved by the EMA but not by the FDA, despite being strongly recommended by evidenced-based medicine in severe forms presenting with an inability to walk independently [12,13,14].
In other pathological conditions, such as rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), systemic lupus erythematosus (SLE) and lupus nephritis (LN), catastrophic antiphospholipid syndrome (CAPS), hemophagocytic lymphohistiocytosis (HLH) and multisystem inflammatory syndrome in children (MIS-C), immunoglobulin employment is not sustained by EMA or FDA indications, playing generally a minor but non-negligible role [4].
The multiple mechanisms of action of IVIG rely on its particular two-component structure, in which the Fab variable and the Fcγ constant regions exert distinct functions [15,16] (Figure 1).
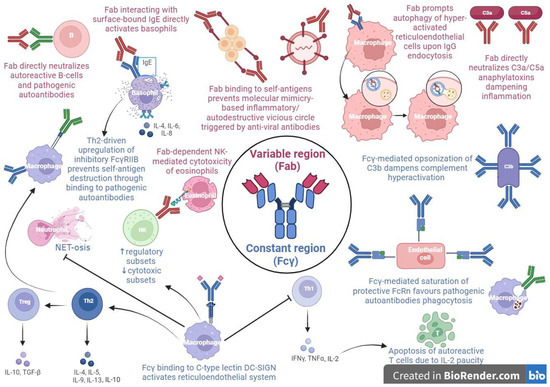
Figure 1.
The different mechanisms of action of high dose intravenous immunoglobulins (HD-IVIG) as anti-inflammatory and immunomodulatory agents.
In the context of pediatric hematological conditions, in particular ITP, the prominent role probably belongs to immunoglobulin’s Fcγ fragment, which seems to be implied in the positive modulation of the inhibitory Fcγ receptor IIB (FcγRIIB) expression on the phagocytic cells of the reticuloendothelial system. In fact, FcγRIIB binding to IgG autoantibodies prevents them from forming immune complexes with platelets, which would be destined to peripheral destruction by phagocytosis [17,18,19,20].
A further therapeutic immunomodulant action of IVIG is attributable to its saturating action on neonatal Fc receptors (FcRn), involved in the prevention of the clearance of IgG, including pathogenic aAbs, and thus in the prolongation of its half-life, maintaining high levels of disease-causing antibodies, as occurs in ITP [21].
In pediatric rheumatological disorders, such as arthritis, nephritis and myopathy, the mechanism of action is attributable to the Fab region. Specifically, the Fab fragment activates basophils upon interaction with surface-bound IgE, independently from the sialylated Fc/C-type lectin/IL-33 pathway proposed in mice; this signaling results in an enhanced secretion of interleukin (IL-4, IL-6, and IL-8) with a subsequent T helper 2 (Th2)-driven production of IL-4, resulting in the upregulation of the abovementioned FcγRIIB and thus in the rescue of aAbs targeted-cells [22,23,24].
Another Fab region-dependent mechanism in inflammatory myopathies involves IVIG-induced autophagy of hyper-activated reticuloendothelial system cells, upon their IgG endocytosis [25].
Further immunomodulant IVIG functions, in particular in SLE, could involve the dampening of complement hyperactivation by Fcγ fraction opsonization of C3b and Fab region-mediated neutralization of C3a/C5a anaphylatoxins, the accelerated clearance of immune complexes and cellular debris by FcRn saturation, the direct neutralization of pathogenic aAbs and autoreactive B-cells by the IVIG anti-idiotypic Fab fraction ligation with ANCA, dsDNA, BAFF, and APRIL, and the downregulation of the pro-inflammatory interferon cascade through a T helper 1 (Th1)–Th2 imbalance skewed towards a Th2 activation, with a subsequent negative regulation of autoreactive T cells due to decremental IL-2 levels [26,27,28,29,30,31].
Two additional immunomodulatory IVIG functions in rheumatologic diseases, especially in ANCA-associated vasculitis, consists, respectively, of the inhibition of a neutrophil extracellular trap formation in a lactoferrin-dependent manner, upon a FcγR-mediated neutrophil activation [32], and of a Fc segment-independent ROS-mediated cytotoxic effect on eosinophils via a largely caspase-independent pathway involving anti-sialic acid-binding Ig-like lectin-8 (Siglec-8) autoantibodies binding their surface receptor on granulocytes [33].
Eventually, in the setting of hyperinflammatory diseases, such as KD, IVIG impairs NK directly and impair antibody-dependent cell-mediated cytotoxicity activity in a Fc-dependent fashion, enhancing the functions of the NK regulatory cell subset [34]; these effects have been shown also in ITP [35] and CIDP [36,37].
Preliminary studies in the field of neuroinflammation address immunoglobulin’s immunomodulation role in the Fcγ region interaction with neural dendritic cells’ DC-SING, resulting in the modulation of the Th1–Th2–T helper 17 (Th17) differentiation, with an expansion of the regulatory T-cell compartment and strong Th2-driven FcγRIIB expression, at the expense of a Th17 subset’s inhibition.
Other IVIG anti-inflammatory mechanisms in neurological disorders could be due to the impairment of both innate and adaptive immune responses through a reduced Toll-like receptor expression and an ineffective T-cell/B-cell costimulation, respectively [38,39,40,41,42].
In the context of pediatric inflammatory disorders, such as KD and MIS-C, both the Fcγ and Fab fractions exert immunomodulant functions. The Fab region-mediated IVIG binding to self-antigens could dampen the pro-inflammatory vicious circle triggered by the molecular mimicry between viruses and the host tissues responsible for the development of post-viral KD and MIS-C. This prevents cross-linking among self-antigens and pathogenic autoantibodies, as occurs in dermatomyositis with the IVIG Fab fraction, protecting BP180 from degradation fostered by aAbs. Moreover, the FcRn saturation by the Fcγ region could accelerate the clearance of pathogenic aAbs implied in KD and MIS-C [15,43].
IVIG use, mostly off-label, is often limited to multi-refractory conditions as second- or third-line therapy; so, they should be employed as soon as possible in case of multi-refractoriness.
In the off-label context, indications about the optimal dosage, duration, and frequency of immunomodulatory IVIG are usually contingent to expert opinions, varying among different centers; they are also on the basis of the equipe expertise, of the patients’ preferences and comorbidity, and of the environmental affordability. In general, a HD-IVIG protocol ranges from 1000 to 3000 mg/kg of body weight, divided into 400 mg/day for generally 2–5 days [44,45].
In the light of the current imbalance between the gradual but constant increase in the use of immunoglobulins and their availability, we claim the urgency of a thoughtful and responsible utilization of immunoglobulin, according to the latest indications reported by national/international guidelines and reliable reviews.
The largest immunoglobulin consumption is mainly due, in a decrescent order, to acquired (secondary) immunodeficiencies (SID) followed by CIDP and IEI [3,46,47]. Considering the production gap existing between USA and Europe, due in part to different donors’ retention strategies, including non-profit and remunerated donations, the immunoglobulin business, dominated by Big Pharma’s proposal of new therapeutic frontiers and unsupported by high-quality evidence, has become more and more consolidated over the years [4,48,49].
In this background of PDMP’s paucity, our manuscript aims to provide a review of the most recent insights into the major indications of HD-IVIG application in the pediatric context, in order to sensitize healthcare professionals about the responsible employment of this important medical product in a perspective of cost-effectiveness.
2. Materials and Methods
2.1. Search Strategy
A literature search was performed using PubMed, from inception until 1st August 2023, on HD immunoglobulin use in children, including the following keywords: anti-inflammatory; children; high dose gammaglobulin; high dose immunoglobulin; immune dysregulation; immunomodulation; immunomodulatory; inflammation; intravenous gammaglobulin; intravenous immunoglobulin; off-label; pediatric; subcutaneous gammaglobulin; subcutaneous immunoglobulin. Additional publications were identified and included manually. All types of articles including systematic reviews, case series, and case reports were reviewed.
2.2. Eligibility Criteria
Articles were considered for inclusion based on the following criteria:
- they had to be meta-analysis, systematic reviews, reviews, clinical trials, retrospective and/or prospective observational studies, comparative studies, case series, or reports focusing on the immunomodulatory treatment of hematologic, neurologic, and inflammatory immune dysregulation disorders;
- the full text had to be available.
On the other hand, articles were excluded if they met any of the following criteria:
- they were editorials, or conference abstracts;
- the articles were written in a language other than English;
- full-text versions of the articles could not be obtained.
2.3. Study Selection
The titles and abstracts of the included publications underwent scrutiny by at least two authors. Any conflicts or disagreements that arose during this process were resolved through discussion with at least a third author. For articles deemed likely to meet the eligibility criteria, a comprehensive full-text review was conducted by at least two authors, and in cases of uncertainty, consensus was reached through discussions involving other co-authors, prior to decisions about inclusion or exclusion. In instances where full texts were not readily available through online databases, library requests were made to obtain the necessary documents.
2.4. Results
The initial search strategy identified 3279 publications. Following title and abstract review, the full texts of 336 articles were reviewed, resulting in 76 studies fulfilling the inclusion criteria.
Research articles analyzed for the writing of the review are reported in Table 1.

Table 1.
Research articles involving children assessing the anti-inflammatory and immunomodulatory effect of high-dose immunoglobulins (HD-IVIG).
3. Results
3.1. Immunoglobulins and Immune-Mediated Neurological Disorders
Although HD-IVIG is widely used in pediatric neurological disorders, the literature data on its benefits in these conditions are limited [125]. Available clinical trials are almost exclusively on adults and evidence for children mostly relies on with low sample sizes and restricted end-point measures (Gadian, 2017) [50].
3.1.1. Acute and Chronic Inflammatory Neuropathies
Inflammatory neuropathies are part of a various group of autoimmune diseases affecting the peripheral nervous system in terms of sensory and/or motor complaints, causing significant disability [126,127]. They can have an acute (i.e., GBS in its different variants) or chronic (i.e., CIDP) course, with both a protracted onset and relapsing–remitting or chronic–progressive impairment [128]. MMN is a different entity, which is extremely rare in children, causing non-symmetric arm weakness with no sensorial impairment [128,129]. HD-IVIG use is approved by both FDA and EMA in CIDP (Eftimov, 2013) and MMN (Keddie, 2022) [4,11]. HD-IVIG is the first choice also in GBS, despite only being approved by EMA, based on evidence of faster recovery and reduced morbidity [130,131,132,133,134]. The latest Cochrane meta-analysis about GBS (including both adults and children) found HD-IVIG to be equivalent to plasma exchange (PLEX) in end-point measures, such as time to unassisted walking and ventilation discontinuation (Hughes, 2014) [13]. The common dosage regimen is 2 g/kg dose in one, two or five days. Some studies reported superiority of the 2 day versus 5 day regimen with shorter recovery time [133], others found no difference but a higher relapse rate in the 2 day regimen [132]; a more recent one found the 5 day regimen to lead to faster recovery and a shorter hospitalization time, with no difference in long-term outcome [135].
3.1.2. Myasthenia Gravis (MG)
MG affects the neuromuscular junction, in most cases through specific autoantibodies such those against the acetylcholine (Ach) receptors [54,55]. This results in fatigable ptosis, ophthalmoplegia, dysarthria, dysphagia, and extremity weakness. Ocular symptoms can occur in isolation (ocular MG) or together with systemic symptoms (generalized MG).
Treatment is mainly derived from adult guidelines, given the analogous pathobiological mechanisms and the absence of pediatric clinical trials [136]. Pyridostigmine is usually the first line of therapy, although additive immunosuppressants (corticosteroids, steroid-sparing agents, and anti-B cell agents), and/or thymectomy are often required [136]. HD-IVIG and/or PLEX can induce a prompt short-lasting response in patients with myasthenic crises or severe motor impairment but can also be useful in maintaining remission through monthly cycles, in cases of refractoriness or intolerance to other treatment options [136,137]. In his systematic review, Gadian identified 67 pediatric patients in three studies testing IVIG efficacy in generalized and ocular MG. Efficacy was found in 67% of patients receiving IVIG with or without corticosteroids, 91% with PLEX and IVIG, and 100% with PLEX alone (Gadian, 2017) [50].
3.1.3. Autoimmune Encephalitis (AE)
AE is a heterogeneous group of hyper-inflammatory disorders presenting with an acute or subacute onset of neuropsychiatric symptoms (mental status alteration, seizures, or neurological focal deficits), resulting from an excessive response of the central nervous system (CNS) intrinsic immunity [138,139,140]. Autoantibodies targeting CNS cellular receptors, ion channels, or surface proteins have been increasingly recognized [138]. N-methyl D-aspartate receptor antibody encephalitis (NMDARE) is most frequent in children, although a prevalence of anti-myelin oligodendrocyte glycoprotein (MOG) antibodies emerged in some studies [141,142]. The AE first choice treatment consists of immunomodulant steroids with HD-IVIG or PLEX, followed by second-line immunosuppressants and anti-B lymphocytes agents [143]. Although widely used in clinical practice, all supporting data regarding the safety and efficacy of IVIG in AE come from retrospective case series [130]. The International Consensus Recommendations for the Treatment of Pediatric NMDARE recommend HD-IVIG (or alternatively PLEX) as an additional first line treatment after corticosteroids in severe cases (Nosadini, 2021). First-choice immunotherapy, such as oral corticosteroids or monthly HD-IVIG/intravenous corticosteroids, can be continued for up to 3–12 months, according to severity-based criteria. Refractoriness to first-line therapies is an indication to switch to second-line treatments, to be started 2 weeks after the initiation of first-choice drugs, preferring rituximab and switching to cyclophosphamide or tocilizumab after a further 1–3 months in case of refractoriness. Maintenance therapy beyond 6 months with mycophenolate mofetil or rituximab redosing should be considered in case of severe, relapsing, prolonged and second-line therapy-requiring disease [144].
3.1.4. Autoimmune Demyelinating Disorders of CNS
Acute disseminated encephalomyelitis (ADEM) is a CNS disorder consisting in an immune-mediated (often post-infectious) demyelinization process, resulting in polyfocal neurological deficits associated with encephalopathy and typical reversible white matter lesions at MRI [145]. It mostly occurs in children under 9 years old [146,147]. Anti-MOG antibodies are detected in at least 50% of monophasic and almost all multiphasic forms [145,148]. Observational studies and expert opinions remain to date the only available evidence about the treatment [149,150]. HD intravenous corticosteroids represent the first-choice treatment; HD-IVIG is recommended in the acute phase of monophasic ADEM when corticosteroid therapy fails or is contraindicated (Massa, 2021) [149]. Gadian et al. found HD-IVIG to be associated with complete recovery in 23 out of 26 patients treated (with or without steroids) [50].
The characteristic demyelination pattern of neuromyelitis optica spectrum disorder (NMOSD) includes the II nerve, area postrema, spinal cord, and, rarely, brainstem and diencephalon, and is frequently associated with antibodies against aquaporin-4 (AQP4-NMOSD) [151,152]. A few series (mainly adults’ ones) suggest a potential role of HD-IVIG in AQP4-NMOSD [153,154]. Nevertheless, the increasing and effective use of novel therapies overshadows their use [50].
HD-IVIG is also suggested in multiple sclerosis for acute relapses in the case of incomplete response to HD intravenous corticosteroid, but no systematic study is available (Jancic, 2016) [155,156].
3.1.5. Myelin Oligodendrocyte Glycoprotein (MOG) Associated Disorder (MOGAD)
MOGAD includes different types of demyelinating (such as ADEM, optic neuritis, transverse myelitis, and NMOSD-like phenotype with simultaneous optic neuritis and transverse myelitis [75]), and non-demyelinating (such as meningoencephalitis, encephalitis of the cortex with seizures and of the brainstem, and CNS disorders mimicking vasculitis) manifestations [157,158]. The timely initiation and proper duration of immunotherapy is important, as prompt treatment and corticosteroid therapy longer than weeks have been associated with lower risk of subsequent relapse in a recent study [159]. A pediatric systematic review revealed HD-IVIG is often used after corticosteroids in the acute phase of MOGAD (67%) although there is no randomized controlled trial [55]. Moreover, retrospective data on HD-IVIG use as long-term prophylactic therapy showed that 70% of pediatric patients on monthly HD-IVIG (9–16 months on treatment) were relapse-free [152]. Rituximab, mycophenolate mofetil, or azathioprine can be an alternative as maintenance therapy when used alone, or combined with HD-IVIG (Bruijstens, 2020) [160].
3.1.6. Opsoclonus-Myoclonus-Ataxia Syndrome (OMAS)
OMAS in children usually develops by the first 2 years of life with an acute onset consisting in irritability, ataxia, tremor, myoclonus, drooling, and, later, opsoclonus, defined as chaotic, rapid, multidirectional movements of the eyes in the absence of saccadic intervals. It seems to be mediated by the immune humoral response [141,142], and an underlying tumor (mostly neuroblastoma) is present in about 50% of cases [142]. The prompt Initiation of immunotherapy is indicated because this disorder rarely remits spontaneously, and frequently shows a chronic-relapsing course, with a risk of irreversible neurologic sequelae. A stepwise use of adrenocorticotropic hormone (ACTH) or steroids, PLEX, HD-IVIG, and cyclophosphamide or rituximab is adopted [141]. HD-IVIG, in conjunction with other immunomodulators, is recommended to ameliorate outcomes and reduce further flares in OMAS [50,145]. The adjunction of HD-IVIG in neuroblastoma-associated OMAS treated with cyclophosphamide and corticosteroids showed a better response rate to treatment (81% vs. 41%) in a recent clinical trial [57]. The suggested posology is 2 g/kg distributed across 2–5 days, followed by monthly 1–2 g/kg for up to 12 months (Rossor, 2022) [141].
3.1.7. Other Neurological Disorders with Limited or Inconclusive Evidence
In Rasmussen’s encephalitis [161,162], HD-IVIG use has shown no or time-limited anti-seizure efficacy but seems to have some efficacy in slowing the disease’s progression and reducing the level of disability when used in the early stages of the disease [125,139,163,164].
Immunomodulatory drugs have been increasingly used in febrile infection-related epilepsy syndrome (FIRES), because of proof of cytokine-mediated etiology and minimal response to anti-seizure medications [53,54,165,166,167]. Nevertheless, there is, to date, poor evidence about HD-IVIG effectiveness, limited to a very small percentage of cases [54,168].
In Sydenham’s chorea [92], immunomodulatory therapy did not seem to change the short-term outcome compared with symptomatic therapy alone (i.e., antiepileptics and antipsychotics); nevertheless, individuals without immunomodulatory therapy seemed to have a higher relapse rate [59]. One study demonstrated short-term benefits of HD-IVIG, but data on long-term neurological and psychiatric outcomes were not available [60,61].
In pediatric acute-onset neuropsychiatric syndrome (PANS), HD-IVIG are often considered the preferred treatment [169,170]; nevertheless, a recent meta-analysis revealed a very low grade of evidence of beneficial effects of antibacterial, anti-inflammatory, or immuno-modulating treatments in these patients. Moreover, a moderate grade of evidence of adverse effects was found, particularly related to HD-IVIG (Johnson, 2021) [58].
3.2. Immunoglobulins in Hematological Conditions
3.2.1. Immune Thrombocytopenia (ITP)
ITP is a rare immune-mediated acquired bleeding disorder affecting the megakaryocyte lineage of children and adults (the incidence in pediatric population is 3–5 per 100,000/year). ITP is classified as primary when a trigger cannot be identified, or as secondary when it occurs in the context of other pathological conditions (e.g., infections, immunodeficiencies, lymphoproliferative and rheumatological disorders) [171].
In most cases, pediatric patients with newly diagnosed ITP do not have significant bleeding symptoms or other risk factors and may not need any therapy [172].
When a treatment for ITP is required, it is based on the administration of steroids (intravenous HD methylprednisolone or HD dexamethasone), HD-IVIG, and, least commonly, anti-Rh-D immunoglobulin [173]. Second-line treatment options include the monoclonal CD20-antibody rituximab, thrombopoietin-receptor agonists (TPO-Ras) and splenectomy [174].
Numerous studies have shown the effectiveness of HD-IVIG in raising platelet counts in children with ITP, administered alone [69,71,73,74] or in combination with steroids [72,175]. HD-IVIG provides a faster response than steroids, generally increasing the platelet count within 24 h of administration; this is particularly important if bleeding is present in the early stages of the disease. Furthermore, HD-IVIG has been shown to be effective in reducing the development of chronic ITP [69].
In a recent study Mikhail et al. demonstrated that the effectiveness of HD-IVIG in rising platelet count is affected by the initial count, but not by demographic features (i.e., age and sex) [68].
Higashide et al. tried to identify predictive factors for the response to HD-IVIG in newly diagnosed ITP patients, showing that age ≥23 months is the only significant unfavorable factor for long-term response, for which corticosteroid therapy in adjunct with HD-IVIG should be considered as an initial treatment [70].
In the most recent guideline for ITP, the American Society of Hematology recommends administering HD-IVIG in a single dose of 0.8 to 1.0 g/kg, with the possibility of a second dose in unresponsive patients (Provan, 2019) [172].
3.2.2. Autoimmune Hemolytic Anemia (AIHA)
AIHA is a heterogeneous disease characterized by the presence of autoantibodies directed against the patient’s red blood cells. It is very rare in a pediatric setting, with an estimated incidence of 0.8 per 100,000/year [176]. Based on the optimal temperature at which autoantibodies bind erythrocytes and cause hemolysis, AIHA is traditionally classified as warm (IgG-mediated), cold (IgM-mediated) and mixed type.
Warm-AIHA is the most common form in the pediatric population and rarely resolves without treatment [177].
Because of the small number of randomized controlled studies, the treatment of warm-AIHA, especially in children, is still not based on solid evidence [178]. Corticosteroids still represent the first line of treatment, inducing a partial remission in 60–70% of the patients [179]. When steroids alone do not provide sufficient response, second-line treatments, such as splenectomy and immunosuppressive drugs, namely azathioprine, cyclophosphamide, and mycophenolate mofetil, should be considered.
The role of HD-IVIG in the management of AIHA, alone or in combination with prednisone, is controversial and probably linked to its evidence-based effectiveness in ITP and the relatively low-incident adverse effects [65,178,180].
A mixed prospective-retrospective study by Flores et al. demonstrated responsiveness to HD-IVIG in 39% of the 73 patients involved, with an even higher response rate in the pediatric population examined (6 of the 11 children) [64]. Volgaridou et al., in a recent 2021 review, suggest the employment of HD-IVIG (dose: 1 g/kg/day for 2 days) as an additional first-line treatment in children with an inadequate initial response to steroids [177].
3.2.3. Autoimmune Neutropenia (AIN)
AIN is a disease characterized by the presence of circulating autoantibodies targeting neutrophil-specific antigens. Primary autoimmune neutropenia typically affects pediatric patients and generally is self-limited. The neutropenia is classified as a neutrophil count <1.5 × 109/L, but despite the low number of neutrophils, these patients present a mild infectious risk.
In cases where neutropenia is associated with infections, the first line of therapy is represented by the granulocyte colony-stimulating factor (G-CSF) [181]. HD-IVIG is not routinely used in the treatment of AIN in children, but cases of increased neutrophil counts in patients being treated with HD-IVIG are described in the literature [66].
3.2.4. Acquired Hemophilia (AHA)
AHA is a rare coagulopathy caused by the presence of autoantibodies directed against the coagulation factor VIII (FVIII), resulting in an increased risk of bleeding, which can be severe. Generally, AHA occurs in elderly patients with comorbidities, but it has been described in children and postpartum women [182].
Therapeutic options include corticosteroids, cyclophosphamide, cyclosporine, and rituximab.
Despite the presence of several case series describing clinical improvement after immunoglobulin therapy [62,63], HD-IVIG displays a minor role in the management of AHA (Kruse-Jarres, 2017) [183,184].
3.2.5. Neonatal Alloimmune Thrombocytopenia (NAIT)
NAIT is a rare disorder, with an estimated incidence of 1 in 1000 births, caused by the formation of maternal alloantibodies which target fetal platelet antigens inherited from the father. NAIT can result in fetal and neonatal intracranial hemorrhage [185].
Due to the high recurrence risk, in cases of subsequent pregnancies a close surveillance is necessary and, when needed, prophylactic interventions must be taken; the antenatal management of NAIT consists of weekly HD-IVIG infusions during affected pregnancies (Lieberman, 2019) [186].
The first-line treatment of NAIT newborns with <30,000/μL platelets consists of multiple antigen-negative platelet transfusions; in the case of a persistently low platelet count despite transfusions, HD-IVIG can be administered [75].
HD-IVIG is effective in approximately 65% of cases of NAIT [187], although HD-IVIG’s effectiveness seems to be limited when it is applied after antenatal maternal treatment with HD-IVIG [76].
3.2.6. Post-Transfusion Purpura (PTP)
PTP is a severe and rare transfusion reaction, caused by the development of deep thrombocytopenia within 5–10 days of a blood transfusion. In almost all cases, PTP is characterized by the development of antibodies targeting the human platelet antigen 1 on the donor platelets [188].
Possible treatment options include HD-IVIG, systemic corticosteroids, plasmapheresis, and/or rituximab, variously combined. The success of HD-IVIG therapy in slowing platelet destruction in adult patients is stated in many case reports and suggests a possible use in pediatric patients [77,78,79,189].
3.2.7. Thrombotic Thrombocytopenic Purpura (TTP)
TTP is a rare thrombotic microangiopathy, caused by the deficiency of a specific protease targeting the von Willebrand factor, called ADAMTS13. About 10% of all TTP cases occurs in childhood [190].
Microangiopathic hemolytic anemia, consumption thrombocytopenia, and organ injury configure the typical clinical scenario of TTP [191].
The standard treatment of the acute phase of child-onset TTP is based on therapeutical plasma therapy and steroids; in patients unresponsive to first-line therapy, immunomodulatory therapy with rituximab may be considered [190].
The employment of HD-IVIG for the management of TTP, based on few case reports and small-size group series on adult subjects, remains controversial and is currently indicated only in those cases refractory to plasma therapy and in chronic-relapsing TTP (Ding, 2018) [80,81,192].
3.3. Immunoglobulins and Inflammatory Diseases
3.3.1. Juvenile Idiopathic Arthritis (JIA)
JIA represents the most frequent rheumatic disease of childhood and gathers heterogeneous conditions. HD-IVIG use for JIA has remained controversial and interest in its efficacy has progressively decreased, largely due to the discovery of new biological treatments in the last 20 years [193]. A trial involving 25 patients suffering from resistant polyarticular JIA demonstrated that the HD-IVIG-treated group experienced a short beneficial effect on clinical symptoms, compared to the placebo [112]. For theoligo- and polyarticular subsets, current guidelines recommend the use of steroidal anti-inflammatory drugs (NSAIDs), corticosteroid intra-articular injection, and disease-modifying anti-rheumatic drugs (DMARDs), especially methotrexate, followed by biological DMARDs in the case of resistant forms (Onel, 2022) [194]. More studies were conducted on systemic JIA (sJIA), a subgroup with visceral manifestations and different treatments [195]. Despite the fact that mild forms could respond to NSAIDs, glucocorticoids and biological therapy with monoclonal antibodies directed against IL-1 and IL-6 are recommended [194]. In the past, off-label HD-IVIG therapy had been attempted. Although in open-label studies an initial improvement in both clinical and laboratory index suggested a potential role in the management of sJIA [113,115,116], Silverman et al. failed to demonstrate statistically significant results between HD-IVIG and the placebo [114]. Therefore, its role in sJIA remained controversial, and the advent of biological therapies limited its application in clinical settings [194,196].
3.3.2. Juvenile Dermatomyositis (JDM)
JDM represents a rare vasculopathy disease, although it is one of the most common inflammatory muscular diseases that affects children. Alongside muscular and cutaneous involvement, clinical features may include systemic symptoms and pulmonary and gastrointestinal manifestations [197]. HD corticosteroids with progressive weaning and the start of immunosuppressive drugs, like methotrexate, represent the mainstay of pharmacologic treatment [198]. In the past, various case series reported that HD-IVIG could potentially be used for JDM, especially in severe, refractory, and steroid-resistant forms [92,94,95,98], as an effective add-on treatment, with improvement in muscle strength, skin manifestations, and steroid-dosage reduction [93,96,97]. In one of the largest investigated cohorts, Lam et al. showed that the HD-IVIG-treated group had comparable or reduced disease than the control patients, and this lasted 4 years after diagnosis, especially for the steroid-resistant patients [91]. More recent clinical trials on adult patients with active dermatomyositis demonstrated better clinical outcomes in those treated with a 16-weeks HD-IVIG course, compared to the placebo group [90].
Current consensus treatment plans include HD-IVIG as a second-line therapy for resistant forms. In particular, current guidelines state that intravenous immunoglobulin may be a useful adjunct for resistant disease, particularly when skin features are prominent (strength of recommendation C) and, in the case of calcinosis cutis, at a monthly dose of 2 g/kg (Enders, 2017) [199,200].
3.3.3. Childhood-Onset Systemic Lupus Erythematosus (cSLE)
cSLE management includes hydroxychloroquine, corticosteroids, and immunosuppressive agents, tailored on disease manifestations and severity. In adult patients, the use of HD-IVIG showed successful results for many complications, like nephritis, hematological and neurological symptoms, vasculitis, and pleuropericarditis [201]. At a pediatric age, HD-IVIG is used as a first-line therapy for hematological abnormalities, especially for SLE-associated thrombocytopenia, although the uncombined use could result in a transient response (Rodriguez, 2017) [202]. A retrospective multicentered study on 215 pediatric patients confirmed a good clinical response to HD-IVIG as a single or combined treatment with other drugs [118]. Some small studies reported the efficacy of prenatal maternal immunoglobulin infusions to prevent cardiac neonatal lupus erythematosus, although the results remained inconclusive [123]. A few case reports on the efficacy of HD-IVIG on neuropsychiatric symptoms [203], like chorea and SLE-associated GBS, can be found in the literature [119,124,204]. A more recent case report showed the successful use of HD-IVIG combined with methylprednisolone and cyclophosphamide for cSLE-associated macrophage activation syndrome (MAS) and neuroimaging alterations [117]. However, given the paucity of well-designed trials, data are not enough to guide strong recommendations (Papachristos, 2020) [205].
3.3.4. Henoch-Schönlein Purpura (HSP)
HSP is a common self-resolving pediatric vasculitis, sometimes associated with gastrointestinal and renal complications for which treatment is debated (Oni, 2019) [206]. Corticosteroids represent the principal therapy in severe forms; HD-IVIG use presented successful results as a second-line option for resistant cases, showing the sustained resolution of bleeding [83,85,89] and of intestinal pneumatosis in one patient [86]. In one case, given the preclusion of steroids because of severe bleeding gastritis, HD-IVIG treatment alone showed a good efficacy on disease flare-up [88]. A recent case–control study, conducted on 64 patients under 18 years of age with gastrointestinal symptoms, refractoriness, or a dependence on glucocorticoids, highlighted a better response to HD-IVIG in younger children [82]. Therefore, pediatric randomized studies are needed to better define its use in this setting. Despite some isolated case reports in adult patients [207], no further trial is available on the role of IVIG in pediatric renal injury prevention and treatment [208]. Regarding purpuric rash, at the present there is no agreement on the treatment of isolated HSP skin symptoms, for which corticosteroids usually represent the first choice, especially in severe hemorrhagic bullous forms [84,209]. Nonetheless, a few cases reporting successful cutaneous healing after HD-IVIG as a second-line drug can be found [84]: in one patient, an improvement in purpuric rash was associated with the resolution of neurological symptoms caused by a brain hemorrhage [87]. However, given the heterogeneity of the published studies on the impact of HD-IVIG on extra-intestinal manifestations, further investigations are needed to evaluate their role.
3.3.5. Kawasaki Disease (KD)
Since the late 1980s, HD-IVIG has been effectively employed as the keystone treatment for KD, significantly modifying the natural course of the disease. Coronary artery aneurysms (CAAs) represent the main KD complication, potentially leading to sudden death, myocardial ischemia, and chronic cardiomyopathy. After the systematic use of HD-IVIG (2 g/kg) during the KD acute phase, in addition to aspirin, the rate of coronary artery aneurysms (CAAs) dropped from 25%, with a mortality rate of 1–2%, to a rate of 3–4% [210] and a mortality rate of 0.1% (McCrindle, 2017) [211]. The precise mechanism of action of HD-IVIG towards KD has not been completely exploited yet [210]. HD-IVIG appears to exert a “generalized” immune-suppressive effect on many levels by (1) neutralizing microbial toxins, (2) suppressing T- and B-lymphocyte activation and favoring their apoptosis in peripheral blood, (3) regulating the Treg/Th17 cell equilibrium, (4) reducing cytokine release, and (5) restoring effector molecules in dendritic cell subsets [212]. However, individual characteristics can vary among KD patients, identifying some children who require supplemental immune-modulatory adjunctive treatments: those patients unresponsive to the first HD-IVIG infusion, and those who are at high risk for developing CAA.
Since HD-IVIG resistance has been reported to correlate with high serum levels of TNF-α, IL-6, and IL-10 and with low levels of IL-5 and eosinophil counts [211], these molecular pathways have been targeted by using corticosteroids, anti-IL-1, anti-TNF-α, or cyclosporin, often with satisfactory outcomes [213].
Treatment with HD-IVIG should be undertaken before day 10 of fever, because it has been shown to decrease the incidence of coronary lesions. However, HD-IVIG can also be infused later, in the case of the persistence of fever and high inflammatory markers. The maximal dose is 2 g/kg, repeatable after 36 h if the fever persists. In the case of HD-IVIG resistance or forms at high risk for coronary involvement, steroids, or biologics, such as anti-IL-1 or anti-TNFα drugs, can be added as second step or directly, as adjunctive first-line therapy (Broderick, 2023) [99].
3.3.6. Multisystem Inflammatory Syndrome in Children (MIS-C)
Since the COVID-19 pandemic, MIS-C has emerged as a severe complication of SARS-CoV-2 infection among children. The clinical and laboratorist analogies between MIS-C and KD led physicians to use HD-IVIG for the treatment of MIS-C since its early appearance, during the acute phase [214,215,216]. HD-IVIG is most frequently associated with glucocorticoids and biologic agents in most severe cases. The association treatment showed satisfactory outcomes in improving cardiac function, CAAs, and the mortality rate [217,218,219,220]. However, no substantial differences were found in terms of inotropic or ventilatory support, or in reduction in disease severity when comparing three different therapeutic regimens: HD-IVIG alone versus steroids alone versus HD-IVIG + steroids [218]. On the other hand, when comparing HD-IVIG + glucosteroid and HD-IVIG, patients’ Intensive Care Unit (ICU) stay was shorter and the need for additional intensification immunomodulatory treatment was lower in the first treatment group [110], as well as the need for inotropic support and the risk for new or persistent cardiovascular dysfunction [109].
Despite there is not an international consensus on MIS-C management, the American College of Rheumatology has confirmed HD-IVIG as its first choice treatment in MIS-C, in association with methylprednisolone (Henderson, 2022) [221].
HD-IVIG acts by multiple mechanisms, one of which seems to induce the death of neutrophils and consequently the clearance of granulocyte-mediated inflammation through PI3K- and NADPH-oxidase-dependent pathways rather than on apoptosis, caspase-1-dependent pyroptosis, necroptosis, or ferroptosis [222,223]. Notably, the reduction in neutrophils counts; IL-1-beta expressing neutrophils were documented in patients receiving HD-IVIG as the unique immunomodulatory treatment. Several markers of inflammation decrease after HD-IVIG [224], which might also block out endothelial and dendritic cell, monocyte and T-lymphocyte functions [225], that are exaggerated in MIS-C.
One complication and continuum of severity of both KD and MIS-C is represented by MAS, a potentially life-threatening variant of hemophagocytic lymphohistiocytosis (HLH) presenting in the context of rheumatological (autoimmune or inflammatory) disorders [226], caused by apoptosis-resistant cytotoxic T-cells and histiocytes, resulting in a massive cytokines release, multi-organ injury, and hemophagocytosis (Ravelli, 2016). Despite the laboratorist features of MIS-C show analogies with MAS, the trend of some markers rise differently [227,228,229], supporting the need for a new definition of MAS in MIS-C. Conflicting data have been reported about HD-IVIG use in MAS, thus its use is still debated: the rationale is supported by its anti-inflammatory action through the inhibition of complement activation, its blocking of antibody Fc fragments and macrophage Fc receptors, and its neutralization of cytokines. Thus, HD-IVIG can be added to steroids or anakinra, but it does not represent the first line of treatment.
HD-IVIG resistance is particularly expressed in clinical patterns complicated by KD Shock Syndrome or MAS, further reflecting a difference in the immunological molecular pathways involved.
In MIS-C myocardial injury can be present in up to 80% of cases [230] and is most likely due to the aberrant systemic inflammation rather than the classic viral myocarditis [231], where the myocyte damage is either directly virus-induced and innate and adaptive immunity-mediated [220]. In addition to these mechanisms, HD-IVIG seems to interfere with the immunological response in myocarditis by disrupting the complement cascade, inhibiting leukocyte adhesion and metalloproteinase release [232] and augmenting anti-inflammatory cytokines with a subsequent reduced production of nitric oxide, which is partly responsible for negative inotropic effects [233]. Despite a Cochrane review which concluded that the evidence of HD-IVIG use in presumed viral myocarditis remains uncertain (Robinson, 2020), if an ongoing infection, or both a post-infectious or non-infectious inflammatory disorder is involved in the pathogenic mechanism, HD-IVIG can be effective, due to its anti-inflammatory, antiviral and immunomodulatory effects [234]. Notably, both the European Society of Cardiology (Caforio, 2013) [235] and the American Heart Association (McCrindle, 2017) [220] encourage HD-IVIG employment due to its immunomodulatory action and the leak of major side-effects, for both viral and autoimmune forms.
3.4. Monitoring, Prevention, and Treatment of Adverse Effects
In order to guarantee a better surveillance of major adverse events of IVIG and to prevent minor ones, it is recommended to observe a slow infusion rate, to select the minimum practicable concentration, and to rely on premedication with steroids, antihistamines, and/or NSAIDs, taking into account their potential pro-hemolytic and nephrotoxic effects [236] (Table 2).

Table 2.
IVIG-related major adverse events and the relative monitoring, preventive, and therapeutic strategies in a pediatric context.
B-cell depleting treatments targeting CD19, CD20, B-cell activating factors (BAFFs), and B-cell maturation antigens (BCMAs) play a key role in the context of antibody-mediated autoimmune disorders; in particular, chimeric anti-CD20 rituximab can be employed in rheumatoid arthritis, systemic lupus erythematosus (SLE), and granulomatosis in polyangiitis, humanized anti-CD20 ocrelizumab, and ofatumumab in multiple sclerosis, veltuzumab in immune thrombocytopenia, humanized anti-CD19 inebilizumab in neuromyelitis optica spectrum disorders, and anti-BAFF belimumab in SLE. However, acquired hypogammaglobulinemia is a short- and long-term side effect associated with these monoclonal antibodies [239,248,249,250]. The prevalence of long-lasting hypogammaglobulinemia is conditioned by the type of autoimmune disorder, pre-existent hypogammaglobulinemia, therapy duration, and concomitant adjunctive immunosuppressants [251]. The ever-expanding clinical application of these treatments, along with CD19-targeted chimeric antigen receptor T cell (CAR T) employment in hematological malignancies [252], led to an increase in patients with SID showing susceptibility to encapsulated bacteria and recommended as candidates for prophylactic immunoglobulin replacement therapy (IgRT), at a dose of 400 up to 600 mg/kg every 3–4 weeks, with recommended trough IgG levels ranging from 750 to 850 mg/dL [239,253].
A further relevant issue about gammaglobulins’ employment regards their presence in the commercial preparations of several autoantibodies, whose potential pathogenicity in the recipients, despite being improbable, is currently unknown [254,255,256,257,258].
The recent SARS-CoV-2 pandemic, prompting studies on MIS-C and KD, strengthened the evidence about the fact that temporary anti-Ro52, anti-Ro60, anti-La, and anti-Gastric ATPase IgG antibodies in these patients are attributable to IVIG administration; though, these antibodies show a relative rapid decay under the seronegative cut-off over 2–4 months [259].
A further 2020 study assessed the presence in IVIG preparations of anti-neuronal IgG autoantibodies against glutamic acid decarboxylase (GAD) and aquaporin-4 (AQP4), in titers similar to those of patients with type 1 diabetes and NMOSD, respectively; however, these IVIG-related autoantibodies seem to target only linear epitopes, being part of the natural humoral immune repertoire and, thus, not playing a definite pathogenic role interacting with structural epitopes, as demonstrated by animal passive transfer models [260]. Another detail endorsing the harmlessness of IVIG-related antibodies, in this case of anti-myelin associated glycoprotein ones, is the fact that pathogenic antibodies in demyelinating neuropathies belong prevalently to the IgM isotype [260].
The detection of autoantibodies in patients receiving gammaglobulins can favor, more or less properly, inpatient and outpatient rheumatology and endocrinology consultations. In this context, it is worth considering that low-titer antibodies, such as ANA, anti-Ro, and anti-thyroid antibodies, on the one hand, can also be detected in otherwise healthy people with no clinically manifested disorders, independently from immunoglobulin administration; on the other hand their positivity could be falsified by a high epitope density-driven cross-reactivity at the linear epitope-recognizing enzyme-linked immunosorbent assay dependent on gammaglobulins therapy [261]. So, a standardized approach to attribute pathogenic significance to autoantibodies in immunoglobulin recipients is currently lacking; nonetheless, it is important to consider past laboratory positivity prior to IVIG/SCIG therapy, to employ confirmative structural epitope-recognizing cell-based assays, and to repeat the tests after a time sufficient for the natural decay of these antibodies, being guided by the clinic scenario rather than laboratory features [261].
4. Discussion
The review exclusively focused on pediatric indication and use of immunoglobulins to treat inflammatory and autoimmune conditions.
The first reported use of HD-IVIG was described in February 1981 by Imbach et al. on six children, two with idiopathic aplastic anemia and four with refractory ITP, showing ITP resolution within 5–10 days of HD-IVIG therapy, successfully followed with a single infusion every 1–3 weeks [262]. In June 1981, Imbach et al. reported a further seven and six pediatric probands effectively treated with HD-IVIG, without adverse effects, affected by chronic/intermittent and acute ITP, respectively [263]. This prompted the opening of new frontiers in the field of HD-IVIG employment as immunomodulatory and anti-inflammatory therapy, to the extent that Furusho et al. in December 1983 and Fateh-Moghadam et al. in April 1984 published the first case series about the successful use of HD-IVIG in children with KD [107] and young adults with MG [264], respectively.
The very first step towards the discovery of the immunomodulant properties of HD-IVIG was made in 1977 by Péchadre et al., showing a remarkable clinical and electroencephalographic improvement in eight of ten children suffering from severe refractory epilepsy, when treated with large doses of placental gammaglobulins [265].
Since then, several improvements in terms of immunoglobulin production, tolerability, and product availability have led to the large use of HD-IVIG in various pediatric diseases. Although the labelled indications of HD-IVIG are limited, it has been shown to be clinically beneficial in many diseases, displaying a high safety profile which contributed to its large usage, not only in adults but also in pediatric contexts [3,4]. However, especially for HD-IVIG, a deep knowledge of current clinical labelled indications, risks, and benefits should be critically considered.
According to the latest England and Northern Ireland National Immunoglobulin Database Data Report 2020/2021, there has been a general reduction in the yearly number of patients on immunoglobulin therapy for immunologic, neurologic, and infectious conditions (Appendix A) from 2018–2019 to 2020–2021, with a subsequent diminishment in the yearly recorded immunoglobulins volume issued since 2018–2019 [46]. This is in contrast with an ever-expanding demand for immunoglobulins therapy, with a 6–8% annual growth; in particular, SID [3] is currently the leading medical condition for immunoglobulin administration, followed by CIDP and IEIs, according to the latest report of the Australian National Blood Authority, dating back to December 2022 [47].
These considerations fit with the first comprehensive summary of IgG use in Poland over a 5-year period, published in 2023, according to which the neurological and IEI labelled drug programs accounted, respectively, for 34.1% and 21.4% of total IgG consumption, while the remnant 44.5% was employed outside the above-mentioned drug programs, including off-label indications in which HD-IVIG/-SCIG effectiveness is weak, doubtful, or unclear [266].
Similar findings have been reported in the United Kingdom, where SID appears to be driving an increased demand for IgRT [3]. It is worth noting that recommendations for the treatment of SID are not comprehensively defined or strictly specified and vary globally [267,268,269].
Recent updates from the EMA have revised the indications for IgRT in SID. According to the updated guidance, IgRT is recommended for all patients experiencing severe or recurrent infections, those with ineffective responses to antimicrobial treatments, and individuals presenting with either proven specific antibody failure or serum IgG levels below 4 g/L. Proven specific antibody failure is specifically defined as the inability to achieve at least a twofold increase in IgG antibody titer, in response to pneumococcal polysaccharide and polypeptide antigen vaccines [267].
It is worth noting that the supporting evidence from large controlled clinical trials to substantiate these EMA indications for SID treatment is currently lacking. So, there is an urgent need for further research, aligning with the call for the harmonization of existing clinical practices, as there are discrepancies in guidelines across European countries with respect to the initiation, dosing, and discontinuation of IgRT in SID [270]. To address these disparities and provide guidance in areas where there is uncertainty or variation, expert opinions have been offered and referenced in a 2023 European expert question–answer-based review on IgRT employment in SID (Cinetto, 2023) [271].
Despite the intravenous route being, to date, the only administration process with FDA approval for the use of HD in inflammatory and autoimmune conditions, there is an ever- and ever-increasing SCIG utilization in clinical practice, especially for immunoglobulins enriched with human recombinant hyaluronidase, allowing higher infusion volumes and a quicker administration compared to the other products [272].
It is a worthy reminder that, except for KD [4,7], ITP [3,4,8,9], CIDP [4,10], MMN [4,11] and GBS [12,13,14], HD-IVIG is currently off-label for all the other immune-dysregulation disorders, such as RA, JIA, SLE, LN, CAPS, HLH, and MIS-C, playing generally a minor but non-negligible role [4].
Furthermore, in the above-mentioned pediatric disorders, HD-IVIG utilization is preferred over corticosteroids and monoclonal antibodies, not only for its evidence-based medicine effectiveness but also for its minor systemic adverse effects and greater availability and feasibility [4].
The COVID-19 era accelerated the process that had already begun in the 1990s in the United Kingdom and in the 2000s in the United States, when rapid infusion of SCIG was first introduced, gradually replacing the intravenous route by reason of its clinical advantages [273,274,275,276,277,278]. Indeed, SCIG guarantees steady plasmatic immunoglobulin G levels, elevated tolerability, and less systemic adverse effects than IVIG, a more flexible self-made administration with a subsequent better adherence to therapy, a higher quality of life, and a reduction in hospital access, which in turn leads to a decrease in infectious exposures, which is potentially dangerous, especially for patients with IEI and SID on IgRT [272]. Furthermore, the shift from IVIG to SCIG is boosted by the subcutaneous route-related economic advantages and cost reductions demonstrated by several research studies, in the face of major and minor studies assessing the non-inferiority and even the superiority of SCIG in respect to IVIG [272,279,280,281,282].
The ongoing shift from IVIG to SCIG is witnessed also by the aforementioned comprehensive summary, published in 2023, of IgG use in Poland over a 5-year period, according to which, in 2020, 78% of Polish children with IEI received SCIG [266].
From an economic standpoint, there is growing evidence to support the potential benefits of lifelong SCIG treatment. For instance, a cost minimization model, assessing the expenses of IVIG versus SCIG from the perspective of the Spanish National Healthcare System, assumed that all IVIG infusions were hospital-administered, while 95% of SCIG infusions were carried out at home [283]. These economic models, applied also in other European and American healthcare systems, suggest that SCIG could be a cost-effective alternative to IVIG, not only in Spain but also in Switzerland [284,285], Canada [286,287] and Pennsylvania [288], both for neurological and IEI labelled drug programs.
These considerations endorse the fact that, during an acute event, although IVIG availability could be more immediate, it is important to consider the use of SCIG, especially in view of the relatively low immunoglobulin volumes needed. This is, first of all, because the subcutaneous route is technically more feasible, particularly in long-term treatment (e.g., myasthenia gravis), which is frequently complicated by a depletion of the venous heritage; secondly, this is due to the fact that the amount of immunoglobulins needed in children, being weight dependent, is lower, resulting in cost and product savings.
On the other hand, the ever-increasing strictness of criteria to be considered a suitable donor [4,289], along with the SARS-CoV-2 pandemic, has led to a decrease in blood and plasma donation [278], resulting in difficulty finding the immunoglobulins in general, with a poor availability of the subcutaneous product in particular. Indeed, in the pandemic era, immunoglobulin use was reserved mainly for patients affected by predominantly antibody deficiencies, undergoing IgRT as a first priority.
Autoimmune and inflammatory conditions in the pediatric population may underlie a predisposing factor related to an unbalanced immune system; the severe and acute onset of symptoms often justify an off-label employment of HD-IVIG. Indeed, in selected cases, it should be important to check the immunological status before immunoglobulin is used, considering inflammatory/autoimmune disorders as a potential red flag for an IEI [290,291].
Considering that HD-IVIG requires four to five times the dose employed in patients on IgRT, further studies to validate the standardized protocols, while reconsidering the benefits, risks, cost-effectiveness, and different administration routes of immunoglobulins, for children with immune-dysregulation disorders, are needed to guide clinicians towards a thoughtful and responsible employment of this important medical product and to improve the therapeutical decisional process. The field is waiting for the development and validation of alternative and/or complementary immunomodulatory therapies reducing the demand for HD immunoglobulins [3], such as FcRn inhibitors (nipocalimab, rozanolixizumab, batoclimab, and efgartigimod), which are presently in clinical trials for CIDP and MG treatment [292,293]; an anti-CD19 antibody provoking B-cell exhaustion (inebilizumab) and an anti-IL-6 receptor antibody affecting the B- and T-cell maturing process (satralizumab), which are, to date, under investigation for use in NMOSD [294,295]; the proteasome inhibitor bortezomib, anecdotally used for pediatric refractory ITP [296]; and novel complement inhibitors (zilucoplan and ravulizumab), at present in clinical trials for subjects with MG [297].
In response to a rising IVIG/SCIG demand, the strategies to apply in order to ensure consistency and coherence in IVIG/SCIG utilization practices could consist of:
- Formulating international guidelines on which a spectrum of other global, national, regional, and local initiatives should be aligned, encompassing consensus documents, audit programs, and monitoring frameworks.
- Implementing robust data collection mechanisms for IVIG/SCIG usage, IVIG/SCIG-requiring disease prevalence and diagnoses (e.g., IEI registries), as well as dosage and clinical outcome data, thereby facilitating an evidence-based evaluation of the efficacy of IVIG-SCIG prescriptions.
- Exploring viable alternatives to IVIG/SCIG therapy to mitigate the growing demand and address supply constraints.
- Ensuring access to proficient immunologists who possess expertise in the judicious prescription of IVIG/SCIG, while also fostering collaboration among less experienced clinicians and their more seasoned counterparts to facilitate knowledge sharing and skill development.
- Refraining from utilizing IVIG/SCIG in cases where its efficacy is tenuous, uncertain, or not well-established, unless the situation involves a life-threatening condition that may potentially benefit from rescue HD-IVIG. Whenever possible and if suitable, it is preferable to explore alternative, safe, and cost-effective therapeutic options. This approach is aimed at preserving the availability of IVIG/SCIG for patients with IEI primarily suffering from antibody deficiencies, for whom IgRT serves as a crucial and life-saving treatment.
Author Contributions
Conceptualization: F.C. and D.Z.; methodology: F.C. and D.Z.; writing—original draft preparation: M.M., A.C., E.B., L.L., A.F., E.F. (Emanuele Filice) and M.F.; table: M.M., A.C. and E.B.; figure: M.M.; writing—review and editing: F.C., M.M., D.Z., A.M., E.F. (Elena Facchini), M.E.C., D.M.C., M.L. and A.P.; supervision: F.C., D.Z., D.M.C., M.L. and A.P. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
APC was funded by Bando F. CARISBO U-GOV: 2021_CARISBORMT_PESSION_A.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available in the figure and tables of this article.
Acknowledgments
Figure 1 was created with Biorender.com, accessed on 14 August 2023.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
Immunoglobulins and Infectious Conditions
As concerns the role of IVIG for treatment in the hyperimmune phase of septic infection, IVIG was used as an adjuvant treatment in sepsis and septic shock due to their bactericidal (the neutralization and opsonization of IgG and IgM antibodies, stimulation of phagocytosis, and neutralization of bacterial toxins) and inflammatory-modulating actions (the suppression of proinflammatory cytokine release from endotoxin- and superantigen-activated blood cells) [298].
In the pediatric population IVIG has historically been used, above all, for neonatal sepsis [299,300,301]; nonetheless, a randomized multicentric study involving 3493 infants in 2011 demonstrated that the use of IVIG has no effects on the outcome of newborns with suspected or confirmed neonatal sepsis [302], mirroring the results of a 2013 Cochrane by Alejandria et al. investigating almost exclusively adults and newborns [303].
Considering the infectious context in a more general way, on the one hand, it is important to recall the potential paradoxical detrimental effect of IVIG in infections sustained via Zika, Ebola, and SARS-CoV-2; this is driven by the so-called antibody-dependent enhancement mechanism in which low-titer, non-neutralizing antibodies bind to the viral particles, prompting their entry into the reticuloendothelial system via the Fc receptor and triggering an hyper-inflammatory response [304,305,306]. On the other hand, it is worth mentioning the promising role of IVIG in the management of organ transplantation-related pediatric infections.
As for pediatric kidney transplant patients, Mosca et al., in 2023, sustained the employment of IVIG in the case of a highly replicative BK virus infection, which was unresponsive to a reduction in the immunosuppressive regimen, relying on a monthly dose of 0.5 g/kg for 4 months for a viral load under 4 log10 copies/mL, escalating up to 1 g/kg per month in children with BK virus-associated nephropathy or viremia persistently over 4 log10 copies/mL, and up to 2 g/kg per month in case of donor-specific HLA antibodies or biopsy-confirmed graft rejection [307].
References
- Bruton, O.C. Agammaglobulinemia. Pediatrics 1952, 9, 722–728. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Clinical Investigation of Human Immunoglobulin for Intravenous Administration (IVIg). EMA/CHMP/BPWP/94033/2007 Rev. 4. Draft Committee for Medicinal Products for Human (CHMP). 13 October 2020. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-human-normal-immunoglobulin-intravenous-administration-ivig-rev-4_en.pdf (accessed on 1 August 2023).
- Prevot, J.; Jolles, S. Global immunoglobulin supply: Steaming towards the iceberg? Curr. Opin. Allergy Clin. Immunol. 2020, 20, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.; De Angelis, V.; Vuk, T.; Garraud, O.; Lozano, M.; Politis, D. Review of indications for immunoglobulin (IG) use: Narrowing the gap between supply and demand. Transfus. Clin. Biol. 2021, 28, 96–122. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Model List of Essential Medicines for Children, 8th List, 2021; World Health Organization: Geneva, Switzerland, 2021.
- World Health Organization Model List of Essential Medicines, 22nd List, 2021; World Health Organization: Geneva, Switzerland, 2021.
- Marchesi, A.; De Jacobis, I.T.; Rigante, D.; Rimini, A.; Malorni, W.; Corsello, G.; Bossi, G.; Buonuomo, S.; Cardinale, F.; Cortis, E.; et al. Kawasaki disease: Guidelines of the Italian Society of Pediatrics, part I—Definition, epidemiology, etiopathogenesis, clinical expression and management of the acute phase. Ital. J. Pediatr. 2018, 44, 102. [Google Scholar] [CrossRef] [PubMed]
- Parodi, E.; Russo, G.; Farruggia, P.; Notarangelo, L.D.; Giraudo, M.T.; Nardi, M.; Giona, F.; Giordano, P.; Ramenghi, U.; Barone, A.; et al. Management strategies for newly diagnosed immune thrombocytopenia in Italian AIEOP Centres: Do we overtreat? Data from a multicentre, prospective cohort study. Blood Transfus. 2020, 18, 396–405. [Google Scholar] [CrossRef] [PubMed]
- DeSouza, S.; Angelini, D. Updated guidelines for immune thrombocytopenic purpura: Expanded management options. Clevel. Clin. J. Med. 2021, 88, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Eftimov, F.; Winer, J.B.; Vermeulen, M.; de Haan, R.; van Schaik, I.N. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst. Rev. 2013, 12, CD001797. [Google Scholar] [CrossRef] [PubMed]
- Keddie, S.; Eftimov, F.; van den Berg, L.H.; Brassington, R.; de Haan, R.J.; van Schaik, I.N. Immunoglobulin for multifocal motor neuropathy. Cochrane Database Syst. Rev. 2022, 11, CD004429. [Google Scholar] [CrossRef]
- Doets, A.Y.; Hughes, R.A.; Brassington, R.; Hadden, R.D.; Pritchard, J. Pharmacological treatment other than corticosteroids, intravenous immunoglobulin and plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst. Rev. 2020, 11, CD008630. [Google Scholar] [CrossRef]
- Hughes, R.A.; Swan, A.V.; van Doorn, P.A. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst. Rev. 2014, 2019, CD002063. [Google Scholar] [CrossRef]
- Verboon, C.; Harbo, T.; Cornblath, D.R.; Hughes, R.A.C.; van Doorn, P.A.; Lunn, M.P.; Gorson, K.C.; Barroso, F.; Kuwabara, S.; Galassi, G.; et al. Intravenous immunoglobulin treatment for mild Guillain-Barré syndrome: An international observational study. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.H.O.; Enk, A.H. High-dose intravenous immunoglobulin in skin autoimmune disease. Front Immunol. 2019, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Bayry, J.; Ahmed, E.A.; Toscano-Rivero, D.; Vonniessen, N.; Genest, G.; Cohen, C.G.; Dembele, M.; Kaveri, S.V.; Mazer, B.D. Intravenous Immunoglobulin: Mechanism of Action in Autoimmune and Inflammatory Conditions. J. Allergy Clin. Immunol. Pract. 2023, 11, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Clynes, R. Immune complexes as therapy for autoimmunity. J. Clin. Investig. 2005, 115, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Siragam, V.; Brinc, D.; Crow, A.R.; Song, S.; Freedman, J.; Lazarus, A.H. Can antibodies with specificity for soluble antigens mimic the therapeutic effects of intravenous IgG in the treatment of autoimmune disease? J. Clin. Investig. 2005, 115, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, A.; Towers, T.L.; Ravetch, J.V. Anti-inflammatory Activity of IVIG Mediated Through the Inhibitory Fc Receptor. Science 2001, 291, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Debré, M.; Griscelli, C.; Bonnet, M.-C.; Carosella, E.; Philippe, N.; Reinert, P.; Vilmer, E.; Kaplan, C.; Fridman, W.; Teillaud, J.-L. Infusion of Fcγ fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet 1993, 342, 945–949. [Google Scholar] [CrossRef]
- Crow, A.R.; Suppa, S.J.; Chen, X.; Mott, P.J.; Lazarus, A.H. The neonatal Fc receptor (FcRn) is not required for IVIg or anti-CD44 monoclonal antibody–mediated amelioration of murine immune thrombocytopenia. Blood 2011, 118, 6403–6406. [Google Scholar] [CrossRef]
- Anthony, R.M.; Kobayashi, T.; Wermeling, F.; Ravetch, J.V. Intravenous gammaglobulin suppresses inflammation through a novel TH2 pathway. Nature 2011, 475, 110–113. [Google Scholar] [CrossRef]
- Anthony, R.M.; Wermeling, F.; Karlsson, M.C.I.; Ravetch, J.V. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc. Natl. Acad. Sci. USA 2008, 105, 19571–19578. [Google Scholar] [CrossRef]
- Galeotti, C.; Stephen-Victor, E.; Karnam, A.; Das, M.; Gilardin, L.; Maddur, M.S.; Wymann, S.; Vonarburg, C.; Chevailler, A.; Dimitrov, J.D.; et al. Intravenous immunoglobulin induces IL-4 in human basophils by signaling through surface-bound IgE. J. Allergy Clin. Immunol. 2019, 144, 524–535.e8. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Karnam, A.; Stephen-Victor, E.; Gilardin, L.; Bhatt, B.; Sharma, V.K.; Rambabu, N.; Patil, V.; Lecerf, M.; Käsermann, F.; et al. Intravenous immunoglobulin mediates anti-inflammatory effects in peripheral blood mononuclear cells by inducing autophagy. Cell Death Dis. 2020, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Martínez, T.; Garcia-Robledo, J.E.; Plata, I.; Urbano, M.-A.; Posso-Osorio, I.; Rios-Serna, L.J.; Barrera, M.C.; Tobón, G.J. Mechanisms of action and historical facts on the use of intravenous immunoglobulins in systemic lupus erythematosus. Autoimmun. Rev. 2019, 18, 279–286. [Google Scholar] [CrossRef]
- Shoenfeld, Y. Efficacy of IVIG affinity-purified anti-double-stranded DNA anti-idiotypic antibodies in the treatment of an experimental murine model of systemic lupus erythematosus. Int. Immunol. 2002, 14, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Dietrich, G.; Kazatchkine, M.D. Antiidiotypic suppression of autoantibodies with normal polyspecific immunoglobulins. Res. Immunol. 1989, 140, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Basta, M.; Van Goor, F.; Luccioli, S.; Billings, E.M.; Vortmeyer, A.O.; Baranyi, L.; Szebeni, J.; Alving, C.R.; Carroll, M.C.; Berkower, I.; et al. F(ab)′2-mediated neutralization of C3a and C5a anaphylatoxins: A novel effector function of immunoglobulins. Nat. Med. 2003, 9, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Spycher, M.; Matozan, K.; Minnig, K.; Zehnder, R.; Miescher, S.; Hoefferer, L.; Rieben, R. In vitro comparison of the complement-scavenging capacity of different intravenous immunoglobulin preparations. Vox Sang. 2009, 97, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Udi, N.; Yehuda, S. Intravenous immunoglobulin—Indications and mechanisms in cardiovascular diseases. Autoimmun. Rev. 2008, 7, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Uozumi, R.; Iguchi, R.; Masuda, S.; Nishibata, Y.; Nakazawa, D.; Tomaru, U.; Ishizu, A. Pharmaceutical immunoglobulins reduce neutrophil extracellular trap formation and ameliorate the development of MPO-ANCA-associated vasculitis. Mod. Rheumatol. 2020, 30, 544–550. [Google Scholar] [CrossRef]
- von Gunten, S.; Vogel, M.; Schaub, A.; Stadler, B.M.; Miescher, S.; Crocker, P.R.; Simon, H.-U. Intravenous immunoglobulin preparations contain anti–Siglec-8 autoantibodies. J. Allergy Clin. Immunol. 2007, 119, 1005–1011. [Google Scholar] [CrossRef]
- McAlpine, S.M.; Roberts, S.E.; Heath, J.J.; Käsermann, F.; Issekutz, A.C.; Issekutz, T.B.; Derfalvi, B. High Dose Intravenous IgG Therapy Modulates Multiple NK Cell and T Cell Functions in Patients with Immune Dysregulation. Front. Immunol. 2021, 12, 660506. [Google Scholar] [CrossRef]
- Ebbo, M.; Audonnet, S.; Grados, A.; Benarous, L.; Mahevas, M.; Godeau, B.; Viallard, J.; Piperoglou, C.; Cognet, C.; Farnarier, C.; et al. NK cell compartment in the peripheral blood and spleen in adult patients with primary immune thrombocytopenia. Clin. Immunol. 2017, 177, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Mausberg, A.K.; Heininger, M.K.; Zu Horste, G.M.; Cordes, S.; Fleischer, M.; Szepanowski, F.; Kleinschnitz, C.; Hartung, H.-P.; Kieseier, B.C.; Stettner, M. NK cell markers predict the efficacy of IV immunoglobulins in CIDP. Neurol.-Neuroimmunol. Neuroinflamm. 2020, 7, e884. [Google Scholar] [CrossRef] [PubMed]
- Bohn, A.B.; Nederby, L.; Harbo, T.; Skovbo, A.; Vorup-Jensen, T.; Krog, J.; Jakobsen, J.; Hokland, M.E. The effect of IgG levels on the number of natural killer cells and their Fc receptors in chronic inflammatory demyelinating polyradiculoneuropathy. Eur. J. Neurol. 2011, 18, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Maddur, M.S.; Sharma, M.; Hegde, P.; Lacroix-Desmazes, S.; Kaveri, S.V.; Bayry, J. Inhibitory Effect of IVIG on IL-17 Production by Th17 Cells is Independent of Anti-IL-17 Antibodies in the Immunoglobulin Preparations. J. Clin. Immunol. 2013, 33, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Maddur, M.S.; Vani, J.; Hegde, P.; Lacroix-Desmazes, S.; Kaveri, S.V.; Bayry, J. Inhibition of differentiation, amplification, and function of human TH17 cells by intravenous immunoglobulin. J. Allergy Clin. Immunol. 2011, 127, 823–830.e7. [Google Scholar] [CrossRef]
- Séïté, J.-F.; Goutsmedt, C.; Youinou, P.; Pers, J.-O.; Hillion, S. Intravenous immunoglobulin induces a functional silencing program similar to anergy in human B cells. J. Allergy Clin. Immunol. 2014, 133, 181–188.e9. [Google Scholar] [CrossRef]
- Séité, J.F.; Guerrier, T.; Cornec, D.; Jamin, C.; Youinou, P.; Hillion, S. TLR9 responses of B cells are repressed by intravenous immunoglobulin through the recruitment of phosphatase. J. Autoimmun. 2011, 37, 190–197. [Google Scholar] [CrossRef]
- Trinath, J.; Hegde, P.; Sharma, M.; Maddur, M.S.; Rabin, M.; Vallat, J.-M.; Magy, L.; Balaji, K.N.; Kaveri, S.V.; Bayry, J. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase-2–dependent prostaglandin E2 in human dendritic cells. Blood 2013, 122, 1419–1427. [Google Scholar] [CrossRef]
- Kamaguchi, M.; Iwata, H.; Mori, Y.; Toyonaga, E.; Ujiie, H.; Kitagawa, Y.; Shimizu, H. Anti-idiotypic Antibodies against BP-IgG Prevent Type XVII Collagen Depletion. Front. Immunol. 2017, 8, 1669. [Google Scholar] [CrossRef]
- Patwa, H.S. Dosing and individualized treatment—Patient-centric treatment: Changing practice guidelines. Clin. Exp. Immunol. 2014, 178, 36–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zandman-Goddard, G.; Krauthammer, A.; Levy, Y.; Langevitz, P.; Shoenfeld, Y. Long-Term Therapy with Intravenous Immunoglobulin is Beneficial in Patients with Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2012, 42, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Mdsas, M.F. Immunoglobulin Database Data Update 2020/21 September 2022 Compiled by Mark Foster MDSAS. Available online: https://igd.mdsas.com/wp-content/uploads/immunoglobulindatabasedataupdate202021.pdf (accessed on 1 August 2023).
- Ig Usage Data and Statistics—July 2022 | National Blood Authority. Available online: https://www.blood.gov.au/ig-usage-data-and-statistics (accessed on 1 August 2023).
- George, R. Nine Pints: A Journey through the Money, Medicine and Mysteries of Blood; Metropolitan Books: New York, NY, USA, 2018. [Google Scholar]
- Marketing Research Bureau. Plasma Economics: How Demand for Plasma Proteins Affects Plasma Fractionation Volumes. Available online: https://marketingresearchbureau.com/plasma-industry/plasma-economics-concept-of-plasma-market-driver/ (accessed on 1 August 2023).
- Gadian, J.; Kirk, E.; Holliday, K.; Lim, M.; Absoud, M. Systematic review of immunoglobulin use in paediatric neurological and neurodevelopmental disorders. Dev. Med. Child Neurol. 2017, 59, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Vitaliti, G.; Tabatabaie, O.; Matin, N.; Ledda, C.; Pavone, P.; Lubrano, R.; Serra, A.; Di Mauro, P.; Cocuzza, S.; Falsaperla, R. The usefulness of immunotherapy in pediatric neurodegenerative disorders: A systematic review of literature data. Hum. Vaccines Immunother. 2015, 11, 2749–2763. [Google Scholar] [CrossRef] [PubMed]
- Racosta, J.M.; Sposato, L.A.; Kimpinski, K. Subcutaneous versus intravenous immunoglobulin for chronic autoimmune neuropathies: A meta-analysis. Muscle Nerve 2017, 55, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, N.; Hirsch, L.J.; Sculier, C.; Loddenkemper, T.; van Baalen, A.; Lancrenon, J.; Emmery, M.; Specchio, N.; Farias-Moeller, R.; Wong, N.; et al. New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES): State of the art and perspectives. Epilepsia 2018, 59, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Kramer, U.; Chi, C.-S.; Lin, K.-L.; Specchio, N.; Sahin, M.; Olson, H.; Nabbout, R.; Kluger, G.; Lin, J.-J.; van Baalen, A. Febrile infection-related epilepsy syndrome (FIRES): Pathogenesis, treatment, and outcome. Epilepsia 2011, 52, 1956–1965. [Google Scholar] [CrossRef]
- Klein da Costa, B.; Banwell, B.L.; Sato, D.K. Treatment of MOG-IgG associated disease in paediatric patients: A systematic review. Mult. Scler. Relat. Disord. 2021, 56, 103216. [Google Scholar] [CrossRef]
- Nosadini, M.; Eyre, M.; Molteni, E.; Thomas, T.; Irani, S.R.; Dalmau, J.; Dale, R.C.; Lim, M.; Anlar, B.; Armangue, T.; et al. Use and Safety of Immunotherapeutic Management of N-Methyl-d-Aspartate Receptor Antibody Encephalitis: A Meta-analysis. JAMA Neurol. 2021, 78, 1333–1344. [Google Scholar] [CrossRef]
- de Alarcon, P.A.; Matthay, K.K.; London, W.B.; Naranjo, A.; Tenney, S.C.; Panzer, J.A.; Hogarty, M.D.; Park, J.R.; Maris, J.M.; Cohn, S.L. Intravenous immunoglobulin with prednisone and risk-adapted chemotherapy for children with opsoclonus myoclonus ataxia syndrome associated with neuroblastoma (ANBL00P3): A randomised, open-label, phase 3 trial. Lancet Child Adolesc. Health 2018, 2, 25. [Google Scholar] [CrossRef]
- Johnson, M.; Ehlers, S.; Fernell, E.; Hajjari, P.; Wartenberg, C.; Wallerstedt, S.M. Anti-inflammatory, antibacterial and immunomodulatory treatment in children with symptoms corresponding to the research condition PANS (Pediatric Acuteonset Neuropsychiatric Syndrome): A systematic review. PLoS ONE 2021, 16, e0253844. [Google Scholar] [CrossRef] [PubMed]
- Orsini, A.; Foiadelli, T.; Magistrali, M.; Carli, N.; Bagnasco, I.; Dassi, P.; Verrotti, A.; Marcotulli, D.; Canavese, C.; Nicita, F.; et al. A nationwide study on Sydenham’s chorea: Clinical features, treatment and prognostic factors: A multicenter cohort study on Sydenham’s chorea. Eur. J. Paediatr. Neurol. 2022, 36, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.S.; Nosadini, M.; Grattan-Smith, P.; Dale, R.C. Intravenous immunoglobulin in acute Sydenham’s chorea: A systematic review Selected studies and critical appraisal. J. Paediatr. Child Health 2015, 51, 12. [Google Scholar] [CrossRef]
- Orsini, A.; Foiadelli, T.; Sica, A.; Santangelo, A.; Carli, N.; Bonuccelli, A.; Consolini, R.; D’elios, S.; Loddo, N.; Verrotti, A.; et al. Psychopathological Impact in Patients with History of Rheumatic Fever with or without Sydenham’s Chorea: A Multicenter Prospective Study. Int. J. Environ. Res. Public Health 2022, 19, 10586. [Google Scholar] [CrossRef] [PubMed]
- Gianella-Borradori, A.; Hirt, A.; Lüthy, A.; Wagner, H.P.; Imbach, P. Haemophilia due to factor VIII inhibitors in a patient suffering from an autoimmune disease: Treatment with intravenous immunoglobulin: A case report. Blut 1984, 48, 403–407. [Google Scholar] [CrossRef]
- Sultan, Y.; Kazatchkine, M.D.; Maisonneuve, P.; Nydegger, U.E. Anti-idiotypic suppression of autoantibodies to factor VIII (antihaemophilic factor) by high-dose intravenous gammaglobulin. Lancet 1984, 2, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; Cunningham-Rundles, C.; Newland, A.C.; Bussel, J.B. Efficacy of intravenous immunoglobulin in the treatment of autoimmune hemolytic anemia: Results in 73 patients. Am. J. Hematol. 1993, 44, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Mathai, S.S.; Bawa, K.S.; Gupta, G.; Mehrishi, R.N. Auto Immune Hemolytic Anaemia in Infancy: Case Report. Med. J. Armed Forces India 1999, 55, 61–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hilgartner, M.W.; Bussel, J. Use of intravenous gamma globulin for the treatment of autoimmune neutropenia of childhood and autoimmune hemolytic anemia. Am. J. Med. 1987, 83, 25–29. [Google Scholar] [CrossRef]
- Bussel, J.; Lalezari, P.; Hilgartner, M.; Partin, J.; Fikrig, S.; O’malley, J.; Barandun, S. Reversal of neutropenia with intravenous gammaglobulin in autoimmune neutropenia of infancy. Blood 1983, 62, 398–400. [Google Scholar] [CrossRef]
- Mikhail, D.B.; Zhu, J.; Pradhan, S.M.; Freiberg, A.S. Rate of Rise of Platelet Count After IVIG for Pediatric Immune Thrombocytopenia. J. Pediatr. Hematol. 2022, 44, e672–e676. [Google Scholar] [CrossRef] [PubMed]
- Heitink-Pollé, K.M.J.; Uiterwaal, C.S.P.M.; Porcelijn, L.; Tamminga, R.Y.J.; Smiers, F.J.; van Woerden, N.L.; Wesseling, J.; Vidarsson, G.; Laarhoven, A.G.; de Haas, M.; et al. Intravenous immunoglobulin vs observation in childhood immune thrombocytopenia: A randomized controlled trial. Blood 2018, 132, 883–891. [Google Scholar] [CrossRef]
- Higashide, Y.; Hori, T.; Yoto, Y.; Kabutoya, H.; Honjo, S.; Sakai, Y.; Nojima, M.; Yoda, M.; Yamamoto, M.; Tsutsumi, H. Predictive factors of response to IVIG in pediatric immune thrombocytopenic purpura. Pediatr. Int. 2018, 60, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.Y.; Hsieh, K.S.; Chiou, Y.H.; Chang, Y.H.; Ger, L.P. A comparative study of initial use of intravenous immunoglobulin and prednisolone treatments in childhood idiopathic thrombocytopenic purpur. Acta Paediatr. Taiwanica 2006, 47, 226–231. [Google Scholar]
- Gereige, R.S.; Barrios, N.J. Treatment of childhood acute immune thrombocytopenic purpura with high-dose methylprednisolone, intravenous immunoglobulin, or the combination of both. P. R. Health Sci. J. 2000, 19, 15–18. [Google Scholar] [PubMed]
- Fujisawa, K.; Iyori, H.; Ohkawa, H.; Konishi, S.; Bessho, F.; Shirahata, A.; Miyazaki, S.; Akatsuka, J. A prospective, randomized trial of conventional, dose-accelerated corticosteroids and intravenous immunoglobulin in children with newly diagnosed idiopathic thrombocytopenic purpura. Int. J. Hematol. 2000, 72, 376–383. [Google Scholar]
- Rosthøj, S.; Nielsen, S.M.; Pedersen, F.K. Randomized comparison of intravenous immunoglobulin and methylprednisolone pulse therapy in children with newly diagnosed idiopathic thrombocytic purpura. The Danish ITP Study Group. Ugeskr. Laeger 1998, 160, 1640–1644. [Google Scholar]
- Winkelhorst, D.; Oepkes, D.; Lopriore, E. Fetal and neonatal alloimmune thrombocytopenia: Evidence based antenatal and postnatal management strategies. Expert Rev. Hematol. 2017, 10, 729–737. [Google Scholar] [CrossRef]
- Mueller-Eckhardt, C.; Kiefel, V.; Grubert, A. High-dose IgG treatment for neonatal alloimmune thrombocytopenia. Blut 1989, 59, 145–146. [Google Scholar] [CrossRef]
- Ziman, A.; Klapper, E.; Pepkowitz, S.; Smith, R.; Garratty, G.; Goldfinger, D. A second case of post-transfusion purpura caused by HPA-5a antibodies: Successful treatment with intravenous immunoglobulin. Vox Sang. 2002, 83, 165–166. [Google Scholar] [CrossRef]
- Kroll, H.; Kiefel, V.; Mueller-Eckhardt, C. Post-transfusion purpura: Clinical and immunologic studies in 38 patients. Infusionsther. Transfusionsmed. 1993, 20, 198–204. [Google Scholar] [PubMed]
- Mueller-Eckhardt, C.; Kiefel, V. High-dose IgG for post-transfusion purpura-revisited. Blut 1988, 57, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Baer, M.R.; Hess, J.R.; Zimrin, A.B. Intravenous immunoglobulin as salvage therapy for refractory thrombotic thrombocytopenic purpura. Am. J. Hematol. 2018, 93, E77–E79. [Google Scholar] [CrossRef] [PubMed]
- Centurioni, R.; Bobbio-Pallavicini, E.; Porta, C.; Rodeghiero, F.; Gugliotta, L.; Billio, A.; Tacconi, F.; Ascari, E. Treatment of thrombotic thrombocytopenic purpura with high-dose immunoglobulins. Results in 17 patients. Italian Cooperative Group for TTP. Haematologica 1995, 80, 325–331. [Google Scholar] [PubMed]
- Zhang, X.; Che, R.; Xu, H.; Ding, G.; Zhao, F.; Huang, S.; Zhang, A. Hemoperfusion and intravenous immunoglobulins for refractory gastrointestinal involvement in pediatric Henoch-Schönlein purpura: A single-center retrospective cohort study. BMC Pediatr. 2022, 22, 692. [Google Scholar] [CrossRef] [PubMed]
- Morotti, F.; Bracciolini, G.; Caorsi, R.; Cattaneo, L.; Gattorno, M.; Ravelli, A.; Felici, E. Intravenous immunoglobulin for corticosteroid-resistant intestinal Henoch-Schönlein purpura: Worth a controlled trial against corticosteroids? Rheumatology 2021, 60, 3868–3871. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.; Mauro, S.; Rega, R.; Martemucci, L.; Sottile, R. Successful treatment of hemorrhagic bullous Henoch-Schonlein purpura with intravenous immunoglobulins. Pediatr. Dermatol. 2019, 36, e34–e36. [Google Scholar] [CrossRef] [PubMed]
- Cherqaoui, B.; Chausset, A.; Stephan, J.L.; Merlin, E. Intravenous immunoglobulins for severe gastrointestinal involvement in pediatric Henoch-Schönlein purpura: A French retrospective study. Arch. Pediatr. 2016, 23, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Gibson, D.P. Pneumatosis intestinalis associated with Henoch-Schönlein purpura. Pediatrics 2014, 134, e880–e883. [Google Scholar] [CrossRef]
- de Maddi, F.; Dinardo, R.; Buonocore, M.C.; Dinardo, M.; Bartolomei, B.; Rigante, D. Intravenous immunoglobulin in Henoch-Schönlein purpura complicated by cerebral hemorrhage. Rheumatol. Int. 2013, 33, 2451–2453. [Google Scholar] [CrossRef]
- Fagbemi, A.A.O.; Torrente, F.; Hilson, A.J.W.; Thomson, M.A.; Heuschkel, R.B.; Murch, S.H. Massive gastrointestinal haemorrhage in isolated intestinal Henoch-Schonlein purpura with response to intravenous immunoglobulin infusion. Eur. J. Pediatr. 2007, 166, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Lamireau, T.; Rebouissoux, L.; Hehunstre, J.P. Intravenous immunoglobulin therapy for severe digestive manifestations of Henoch-Schönlein purpura. Acta Paediatr. 2001, 90, 1081–1082. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Charles-Schoeman, C.; Schessl, J.; Bata-Csörgő, Z.; Dimachkie, M.M.; Griger, Z.; Moiseev, S.; Oddis, C.; Schiopu, E.; Vencovský, J.; et al. Trial of Intravenous Immune Globulin in Dermatomyositis. N. Engl. J. Med. 2022, 387, 1264–1278. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.G.; Manlhiot, C.; Pullenayegum, E.M.; Feldman, B.M. Efficacy of intravenous Ig therapy in juvenile dermatomyositis. Ann. Rheum. Dis. 2011, 70, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.M.; Bingham, C.A.; Kahn, P.J.; Eichenfield, A.H.; Imundo, L.F. Favorable Outcome of Juvenile Dermatomyositis Treated without Systemic Corticosteroids. J. Pediatr. 2010, 156, 302–307. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Mayouf, S.M.; Laxer, R.M.; Schneider, R.; Silverman, E.D.; Feldman, B.M. Intravenous immunoglobulin therapy for juvenile dermatomyositis: Efficacy and safety. J. Rheumatol. 2000, 27, 2498–2503. [Google Scholar]
- Sansome, A.; Dubowitz, V. Intravenous immunoglobulin in juvenile dermatomyositis—Four year review of nine cases. Arch. Dis. Child. 1995, 72, 25. [Google Scholar] [CrossRef][Green Version]
- Collet, K.; Dalac, S.; Maerens, B.; Courtois, J.; Izac, M.; Lambert, D. Juvenile dermatomyositis: Treatment with intravenous gammaglobulin. Br. J. Dermatol. 1994, 130, 231–234. [Google Scholar] [CrossRef]
- Dalakas, M.C.; Illa, I.; Dambrosia, J.M.; Soueidan, S.A.; Stein, D.P.; Otero, C.; Dinsmore, S.T.; McCrosky, S. A Controlled Trial of High-Dose Intravenous Immune Globulin Infusions as Treatment for Dermatomyositis. N. Engl. J. Med. 1993, 329, 1993–2000. [Google Scholar] [CrossRef]
- Lang, B.A.; Laxer, R.M.; Murphy, G.; Silverman, E.D.; Roifman, C.M. Treatment of dermatomyositis with intravenous gammaglobulin. Am. J. Med. 1991, 91, 169–172. [Google Scholar] [CrossRef]
- Roifman, C.M.; Schaffer, F.M.; Wachsmuth, S.E.; Murphy, G.; Gelfand, E.W. Reversal of Chronic Polymyositis Following Intravenous Immune Serum Globulin Therapy. JAMA 1987, 258, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Broderick, C.; Kobayashi, S.; Suto, M.; Ito, S.; Kobayashi, T. Intravenous immunoglobulin for the treatment of Kawasaki disease. Cochrane Database Syst. Rev. 2023, 2023, CD014884. [Google Scholar] [CrossRef]
- Lei, W.-T.; Chang, L.-S.; Zeng, B.-Y.; Tu, Y.-K.; Uehara, R.; Matsuoka, Y.J.; Su, K.-P.; Lee, P.-C.; Cavalcante, J.L.; Stubbs, B.; et al. Pharmacologic interventions for Kawasaki disease in children: A network meta-analysis of 56 randomized controlled trials. eBioMedicine 2022, 78, 103946. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jiang, J.; Shi, X.; Qin, J.; Dong, J.; Xu, L.; Huang, C.; Liu, Y.; Zheng, Y.; Hou, M.; et al. Nomogram to predict risk of resistance to intravenous immunoglobulin in children hospitalized with Kawasaki disease in Eastern China. Ann. Med. 2022, 54, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Suzuki, H.; Onouchi, Y.; Ebata, R.; Terai, M.; Fuse, S.; Okajima, Y.; Kurotobi, S.; Hirai, K.; Soga, T.; et al. Efficacy of primary treatment with immunoglobulin plus ciclosporin for prevention of coronary artery abnormalities in patients with Kawasaki disease predicted to be at increased risk of non-response to intravenous immunoglobulin (KAICA): A randomised controlled, open-label, blinded-endpoints, phase 3 trial. Lancet 2019, 393, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Dionne, A.; Le, C.-K.; Poupart, S.; Autmizguine, J.; Meloche-Dumas, L.; Turgeon, J.; Fournier, A.; Dahdah, N. Profile of resistance to IVIG treatment in patients with Kawasaki disease and concomitant infection. PLoS ONE 2018, 13, e0206001. [Google Scholar] [CrossRef] [PubMed]
- Akca, U.K.; Aydin, E.A.; Aykan, H.H.; Serin, O.; Sag, E.; Demir, S.; Atalay, E.; Kasap, M.; Batu, E.D.; Karagoz, T.; et al. Comparison of IVIG resistance predictive models in Kawasaki disease. Pediatr. Res. 2022, 91, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Sun, L.C.; Wu, E.T.; Wang, J.K.; Lue, H.C.; Wu, M.H. Acute and late coronary outcomes in 1073 patients with Kawasaki disease with and without intravenous γ-immunoglobulin therapy. Arch. Dis. Child. 2015, 100, 542–547. [Google Scholar] [CrossRef]
- Newburger, J.W.; Takahashi, M.; Burns, J.C.; Beiser, A.S.; Chung, K.J.; Duffy, C.E.; Glode, M.P.; Mason, W.H.; Reddy, V.; Sanders, S.P.; et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N. Engl. J. Med. 1986, 315, 341–347. [Google Scholar] [CrossRef]
- Furusho, K.; Sato, K.; Soeda, T.; Matsumoto, H.; Okabe, T.; Hirota, T.; Kawada, S. High-dose intravenous gammaglobulin for kawasaki disease. Lancet 1983, 322, 1359. [Google Scholar] [CrossRef]
- Mahmoud, S.; El-Kalliny, M.; Kotby, A.; El-Ganzoury, M.; Fouda, E.; Ibrahim, H. Treatment of MIS-C in Children and Adolescents. Curr. Pediatr. Rep. 2022, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Son, M.B.F.; Murray, N.; Friedman, K.; Young, C.C.; Newhams, M.M.; Feldstein, L.R.; Loftis, L.L.; Tarquinio, K.M.; Singh, A.R.; Heidemann, S.M.; et al. Multisystem Inflammatory Syndrome in Children—Initial Therapy and Outcomes. N. Engl. J. Med. 2021, 385, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Ouldali, N.; Toubiana, J.; Antona, D.; Javouhey, E.; Madhi, F.; Lorrot, M.; Léger, P.-L.; Galeotti, C.; Claude, C.; Wiedemann, A.; et al. Association of Intravenous Immunoglobulins Plus Methylprednisolone vs Immunoglobulins Alone with Course of Fever in Multisystem Inflammatory Syndrome in Children. JAMA 2021, 325, 855. [Google Scholar] [CrossRef] [PubMed]
- Belhadjer, Z.; Auriau, J.; Méot, M.; Oualha, M.; Renolleau, S.; Houyel, L.; Bonnet, D. Addition of Corticosteroids to Immunoglobulins Is Associated with Recovery of Cardiac Function in Multi-Inflammatory Syndrome in Children. Circulation 2020, 142, 2282–2284. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.H.; Lovell, D.J.; Silverman, E.D.; Sundel, R.P.; Tague, B.L.; Ruperto, N. Intravenous immunoglobulin in the treatment of polyarticular juvenile rheumatoid arthritis: A phase I/II study. Pediatric Rheumatology Collaborative Study Group. J. Rheumatol. 1996, 23, 919–924. [Google Scholar] [PubMed]
- Uziel, Y.; Laxer, R.M.; Schneider, R.; Silverman, E.D. Intravenous immunoglobulin therapy in systemic onset juvenile rheumatoid arthritis: A followup study. J. Rheumatol. 1996, 23, 910–918. [Google Scholar] [PubMed]
- Silverman, E.D.; Cawkwell, G.D.; Lovell, D.J.; Laxer, R.M.; Lehman, T.J.; Passo, M.H.; Zemel, L.S.; Giannini, E.H. Intravenous immunoglobulin in the treatment of systemic juvenile rheumatoid arthritis: A randomized placebo controlled trial. Pediatric Rheumatology Collaborative Study Group. J. Rheumatol. 1994, 21, 2353–2358. [Google Scholar] [PubMed]
- Silverman, E.D.; Laxer, R.M.; Greenwald, M.; Gelfand, E.; Shore, A.; Stein, L.D.; Roifman, C.M. Intravenous gamma globulin therapy in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 1990, 33, 1015–1022. [Google Scholar] [CrossRef]
- Prieur, A.M.; Adleff, A.; Debre, M.; Boulate, P.; Griscelli, C. High dose immunoglobulin therapy in severe juvenile chronic arthritis: Long-term follow-up in 16 patients. Clin. Exp. Rheumatol. 1990, 8, 603–608. [Google Scholar]
- Shi, N.; Wang, X.; Zou, L.; Yang, X.; Ma, Q.; Lu, M. Case Report: Macrophage Activation Syndrome and Widespread Neuroimaging Abnormality in Childhood-Onset Systemic Lupus Erythematosus. Front. Pediatr. 2021, 9, 767115. [Google Scholar] [CrossRef]
- Akca, Ü.K.; Batu, E.D.; Kısaarslan, A.P.; Poyrazoğlu, H.; Ayaz, N.A.; Sözeri, B.; Sağ, E.; Atalay, E.; Demir, S.; Karadağ, Ş.G.; et al. Hematological involvement in pediatric systemic lupus erythematosus: A multi-center study. Lupus 2021, 30, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Baglan, E.; Ozdel, S.; Yazıcı, M.U.; Azapağası, E.; Çelik, H.; Yüksel, D.; Uçan, B.; Karakaya, D.; Bulbul, M. A novel therapeutic approach using the Zipper method to treat chorea in a pediatric-onset systemic lupus erythematosus patient. Lupus 2021, 30, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Lube, G.E.; Ferriani, M.P.L.; Campos, L.M.A.; Terreri, M.T.; Bonfá, E.; Magalhães, C.S.; Aikawa, N.E.; Piotto, D.P.; Peracchi, O.A.B.; dos Santos, M.C.; et al. Evans Syndrome at Childhood-Onset Systemic Lupus Erythematosus Diagnosis: A Large Multicenter Study. Pediatr. Blood Cancer 2016, 63, 1238–1243. [Google Scholar] [CrossRef]
- Brogna, C.; Manna, R.; Contaldo, I.; Romeo, D.M.; Stefanini, M.C.; Chiaretti, A.; Mercuri, E.; Mariotti, P. Intravenous immunoglobulin for Pediatric Neuropsychiatric Lupus Triggered by Epstein-Barr Virus Cerebral Infection. Isr. Med. Assoc. J. 2016, 18, 763–766. [Google Scholar] [PubMed]
- Friedman, D.M.; Llanos, C.; Izmirly, P.M.; Brock, B.; Byron, J.; Copel, J.; Cummiskey, K.; Dooley, M.A.; Foley, J.; Graves, C.; et al. Evaluation of fetuses in a study of intravenous immunoglobulin as preventive therapy for congenital heart block: Results of a multicenter, prospective, open-label clinical trial. Arthritis Rheum. 2010, 62, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Pisoni, C.N.; Brucato, A.; Ruffatti, A.; Espinosa, G.; Cervera, R.; Belmonte-Serrano, M.; Sánchez-Román, J.; García-Hernández, F.G.; Tincani, A.; Bertero, M.T.; et al. Failure of intravenous immunoglobulin to prevent congenital heart block: Findings of a multicenter, prospective, observational study. Arthritis Rheum. 2010, 62, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Nakajima, M.; Nishio, K.; Sogami, J.; Tsubakimoto, A.; Yoshioka, A.; Shirai, T. Guillain-Barre syndrome in a child with systemic lupus erythematosus and anti-Ro/SSA and anti-La/SSB autoantibodies. Br. J. Dermatol. 2000, 143, 1050–1054. [Google Scholar] [CrossRef]
- Nosadini, M.; Mohammad, S.S.; Suppiej, A.; Sartori, S.; Dale, R.C. Intravenous immunoglobulin in paediatric neurology: Safety, adherence to guidelines, and long-term outcome. Dev. Med. Child Neurol. 2016, 58, 1180–1192. [Google Scholar] [CrossRef]
- Schafflick, D.; Kieseier, B.C.; Wiendl, H.; Meyer zu Horste, G. Novel pathomechanisms in inflammatory neuropathies. J. Neuroinflamm. 2017, 14, 232. [Google Scholar] [CrossRef]
- Querol, L.; Lleixà, C. Novel Immunological and Therapeutic Insights in Guillain-Barré Syndrome and CIDP. Neurotherapeutics 2021, 18, 2222–2235. [Google Scholar] [CrossRef]
- Korinthenberg, R.; Trollmann, R.; Felderhoff-Müser, U.; Bernert, G.; Hackenberg, A.; Hufnagel, M.; Pohl, M.; Hahn, G.; Mentzel, H.; Sommer, C.; et al. Diagnosis and treatment of Guillain-Barré Syndrome in childhood and adolescence: An evidence- and consensus-based guideline. Eur. J. Paediatr. Neurol. 2020, 25, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Ishii, R.; Kusunoki, S.; Chiyonobu, T. Childhood-onset multifocal motor neuropathy with IgM antibodies to GM2 and GalNac-GD1a. Brain Dev. 2020, 42, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C. Update on Intravenous Immunoglobulin in Neurology: Modulating Neuro-autoimmunity, Evolving Factors on Efficacy and Dosing and Challenges on Stopping Chronic IVIg Therapy. Neurotherapeutics 2021, 18, 2397–2418. [Google Scholar] [CrossRef]
- Lünemann, J.D.; Quast, I.; Dalakas, M.C. Efficacy of Intravenous Immunoglobulin in Neurological Diseases. Neurotherapeutics 2015, 13, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Korinthenberg, R.; Schessl, J.; Kirschner, J.; Mönting, J.S. Intravenously administered immunoglobulin in the treatment of childhood Guillain-Barré syndrome: A randomized trial. Pediatrics 2005, 116, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Kanra, G.; Ozon, A.; Vajsar, J.; Castagna, L.; Secmeer, G.; Topaloglu, H. Intravenous immunoglobulin treatment in children with Guillain-Barré syndrome. Eur. J. Paediatr. Neurol. 1997, 1, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R. The role of IVIg in autoimmune neuropathies: The latest evidence. J. Neurol. 2008, 255, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Ram, D.; Aziz, M. G99(P) IVIG for Guillain-Barré syndrome: Which regimen should I choose? Arch. Dis. Child. 2016, 101 (Suppl. 1), A57. [Google Scholar] [CrossRef]
- Ramdas, S.; Della Marina, A.; Ryan, M.M.; McWilliam, K.; Klein, A.; Jacquier, D.; Alabaf, S.; Childs, A.-M.; Parasuraman, D.; Beeson, D.; et al. Rituximab in juvenile myasthenia gravis-an international cohort study and literature review. Eur. J. Paediatr. Neurol. 2022, 40, 5–10. [Google Scholar] [CrossRef]
- Liew, W.K.M.; Kang, P.B. Update on juvenile myasthenia gravis. Curr. Opin. Pediatr. 2013, 25, 694–700. [Google Scholar] [CrossRef]
- Dalmau, J.; Graus, F. Antibody-Mediated Encephalitis. N. Engl. J. Med. 2018, 378, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Armangue, T.; Petit-Pedrol, M.; Dalmau, J. Autoimmune Encephalitis in Children. J. Child Neurol. 2012, 27, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Cellucci, T.; Van Mater, H.; Graus, F.; Muscal, E.; Gallentine, W.; Klein-Gitelman, M.S.; Benseler, S.M.; Frankovich, J.; Gorman, M.P.; Van Haren, K.; et al. Clinical approach to the diagnosis of autoimmune encephalitis in the pediatric patient. Neurol.-Neuroimmunol. Neuroinflamm. 2020, 7, e663. [Google Scholar] [CrossRef] [PubMed]
- Rossor, T.; Yeh, E.A.; Khakoo, Y.; Angelini, P.; Hemingway, C.; Irani, S.R.; Schleiermacher, G.; Santosh, P.; Lotze, T.; Dale, R.C.; et al. Diagnosis and Management of Opsoclonus-Myoclonus-Ataxia Syndrome in Children: An International Perspective. Neurol.-Neuroimmunol. Neuroinflamm. 2022, 9, e1153. [Google Scholar] [CrossRef] [PubMed]
- Armangué, T.; Sabater, L.; Torres-Vega, E.; Martínez-Hernández, E.; Ariño, H.; Petit-Pedrol, M.; Planagumà, J.; Bataller, L.; Dalmau, J.; Graus, F. Clinical and immunological features of opsoclonus-myoclonus syndrome in the era of neuronal cell surface antibodies. JAMA Neurol. 2016, 73, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Nosadini, M.; Mohammad, S.S.; Ramanathan, S.; Brilot, F.; Dale, R.C. Immune therapy in autoimmune encephalitis: A systematic review. Expert Rev. Neurother. 2015, 15, 1391–1419. [Google Scholar] [CrossRef] [PubMed]
- Nosadini, M.; Thomas, T.; Eyre, M.; Anlar, B.; Armangue, T.; Benseler, S.M.; Cellucci, T.; Deiva, K.; Gallentine, W.; Gombolay, G.; et al. International Consensus Recommendations for the Treatment of Pediatric NMDAR Antibody Encephalitis. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8, e1052. [Google Scholar] [CrossRef] [PubMed]
- Rossor, T.; Benetou, C.; Wright, S.; Duignan, S.; Lascelles, K.; Robinson, R.; Das, K.; Ciccarelli, O.; Wassmer, E.; Hemingway, C.; et al. Early predictors of epilepsy and subsequent relapse in children with acute disseminated encephalomyelitis. Mult. Scler. J. 2020, 26, 333–342. [Google Scholar] [CrossRef]
- Santoro, J.D.; Chitnis, T. Diagnostic Considerations in Acute Disseminated Encephalomyelitis and the Interface with MOG Antibody. Neuropediatrics 2019, 50, 273–279. [Google Scholar] [CrossRef]
- Krupp, L.B.; Tardieu, M.; Amato, M.P.; Banwell, B.; Chitnis, T.; Dale, R.C.; Ghezzi, A.; Hintzen, R.; Kornberg, A.; Pohl, D.; et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: Revisions to the 2007 definitions. Mult. Scler. J. 2013, 19, 1261–1267. [Google Scholar] [CrossRef]
- Baumann, M.; Bartels, F.; Finke, C.; Adamsbaum, C.; Hacohen, Y.; Rostásy, K.; E.U. Paediatric Mog Consortium; Bruijstens, A.L.; Wendel, E.-M.; Lechner, C.; et al. E.U. paediatric MOG consortium consensus: Part 2—Neuroimaging features of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur. J. Paediatr. Neurol. 2020, 29, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.; Fracchiolla, A.; Neglia, C.; Argentiero, A.; Esposito, S. Update on Acute Disseminated Encephalomyelitis in Children and Adolescents. Children 2021, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Pohl, D.; Alper, G.; Van Haren, K.; Kornberg, A.J.; Lucchinetti, C.F.; Tenembaum, S.; Belman, A.L. Acute disseminated encephalomyelitis. Neurology 2016, 87 (Suppl. 2), S38–S45. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; De Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Banwell, B.; Fadda, G.; Armangue, T.; Hacohen, Y.; Chitnis, T. Paediatric Multiple Sclerosis and Antibody-Associated Demyelination: Clinical, Imaging, and Biological Considerations for Diagnosis and Care. Lancet Neurol. 2021, 20, 136–149. [Google Scholar]
- Magraner, M.J.; Coret, F.; Casanova, B. The effect of intravenous immunoglobulin on neuromyelitis optica. Neurologia 2013, 28, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Elsone, L.; Panicker, J.; Mutch, K.; Boggild, M.; Appleton, R.; Jacob, A. Role of intravenous immunoglobulin in the treatment of acute relapses of neuromyelitis optica: Experience in 10 patients. Mult. Scler. J. 2013, 20, 501–504. [Google Scholar] [CrossRef]
- Pena, J.A.; Lotze, T.E. Pediatric Multiple Sclerosis: Current Concepts and Consensus Definitions. Autoimmune Dis. 2013, 2013, 673947. [Google Scholar] [CrossRef]
- Jancic, J.; Nikolic, B.; Ivancevic, N.; Djuric, V.; Zaletel, I.; Stevanovic, D.; Peric, S.; Anker, J.N.V.D.; Samardzic, J. Multiple Sclerosis in Pediatrics: Current Concepts and Treatment Options. Neurol. Ther. 2016, 5, 131. [Google Scholar] [CrossRef]
- Armangue, T.; Olivé-Cirera, G.; Martínez-Hernandez, E.; Sepulveda, M.; Ruiz-Garcia, R.; Muñoz-Batista, M.; Ariño, H.; González-Álvarez, V.; Felipe-Rucián, A.; Martínez-González, M.J.; et al. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies: A multicentre observational study. Lancet Neurol. 2020, 19, 234–246. [Google Scholar] [CrossRef]
- Bruijstens, A.L.; Lechner, C.; Flet-Berliac, L.; Deiva, K.; Neuteboom, R.F.; Hemingway, C.; Wassmer, E.; Wendel, E.M.; Breu, M.; de Chalus, A.; et al. paediatric MOG consortium consensus: Part 1—Classification of clinical phenotypes of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur. J. Paediatr. Neurol. 2020, 29, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Nosadini, M.; Eyre, M.; Giacomini, T.; Valeriani, M.; Della Corte, M.; Praticò, A.D.; Annovazzi, P.; Cordani, R.; Cordelli, D.M.; Crichiutti, G.; et al. Early Immunotherapy and Longer Corticosteroid Treatment Are Associated with Lower Risk of Relapsing Disease Course in Pediatric MOGAD. Neurol.-Neuroimmunol. Neuroinflamm. 2023, 10, e200065. [Google Scholar] [CrossRef] [PubMed]
- Bruijstens, A.L.; Wendel, E.-M.; Lechner, C.; Bartels, F.; Finke, C.; Breu, M.; Flet-Berliac, L.; de Chalus, A.; Adamsbaum, C.; Capobianco, M.; et al. E.U. paediatric MOG consortium consensus: Part 5—Treatment of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur. J. Paediatr. Neurol. 2020, 29, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Varadkar, S.; Bien, C.G.; Kruse, C.A.; Jensen, F.E.; Bauer, J.; Pardo, C.A.; Vincent, A.; Mathern, G.W.; Cross, J.H. Rasmussen’s encephalitis: Clinical features, pathobiology, and treatment advances. Lancet Neurol. 2014, 13, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Bien, C.G.; Tiemeier, H.; Sassen, R.; Kuczaty, S.; Urbach, H.; von Lehe, M.; Becker, A.J.; Bast, T.; Herkenrath, P.; Karenfort, M.; et al. Rasmussen encephalitis: Incidence and course under randomized therapy with tacrolimus or intravenous immunoglobulins. Epilepsia 2013, 54, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Ramesha, K.; Rajesh, B.; Ashalatha, R.; Kesavadas, C.; Abraham, M.; Radhakrishnan, V.; Sarma, P.; Radhakrishnan, K. Rasmussen’s encephalitis: Experience from a developing country based on a group of medically and surgically treated patients. Seizure 2009, 18, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Granata, T.; Fusco, L.; Gobbi, G.; Freri, E.; Ragona, F.; Broggi, G.; Mantegazza, R.; Giordano, L.; Villani, F.; Capovilla, G.; et al. Experience with immunomodulatory treatments in Rasmussen’s encephalitis. Neurology 2003, 61, 1807–1810. [Google Scholar] [CrossRef] [PubMed]
- Specchio, N.; Pietrafusa, N. New-onset refractory status epilepticus and febrile infection-related epilepsy syndrome. Dev. Med. Child Neurol. 2020, 62, 897–905. [Google Scholar] [CrossRef]
- Hirsch, L.J.; Gaspard, N.; van Baalen, A.; Nabbout, R.; Demeret, S.; Loddenkemper, T.; Navarro, V.; Specchio, N.; Lagae, L.; Rossetti, A.O.; et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia 2018, 59, 739–744. [Google Scholar] [CrossRef]
- Gofshteyn, J.S.; Wilfong, A.; Devinsky, O.; Bluvstein, J.; Charuta, J.; Ciliberto, M.A.; Laux, L.; Marsh, E.D. Cannabidiol as a Potential Treatment for Febrile Infection-Related Epilepsy Syndrome (FIRES) in the Acute and Chronic Phases. J. Child Neurol. 2017, 32, 35–40. [Google Scholar] [CrossRef]
- Specchio, N.; Fusco, L.; Claps, D.; Vigevano, F. Epileptic encephalopathy in children possibly related to immune-mediated pathogenesis. Brain Dev. 2010, 32, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Frankovich, J.; Swedo, S.; Murphy, T.; Dale, R.C.; Agalliu, D.; Williams, K.; Daines, M.; Hornig, M.; Chugani, H.; Sanger, T.; et al. Clinical Management of Pediatric Acute-Onset Neuropsychiatric Syndrome: Part II—Use of Immunomodulatory Therapies. J. Child Adolesc. Psychopharmacol. 2017, 27, 574–593. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Frankovich, J.; Cunningham, M.W.; Latimer, M.E.; Murphy, T.K.; Thienemann, M.; Williams, K.; Walter, J.; Swedo, S.E. Clinical Evaluation of Youth with Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS): Recommendations from the 2013 PANS Consensus Conference. J. Child Adolesc. Psychopharmacol. 2015, 25, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kühne, T. Diagnosis and management of immune thrombocytopenia in childhood. Hamostaseologie 2017, 37, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Provan, D.; Arnold, D.M.; Bussel, J.B.; Chong, B.H.; Cooper, N.; Gernsheimer, T.; Ghanima, W.; Godeau, B.; González-López, T.J.; Grainger, J.; et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019, 3, 3780–3817. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, A.; Economou, M.; Tragiannidis, A.; Karatza, E.; Tsatra, I.; Gombakis, N.; Athanassiadou-Piperopoulou, F.; Athanasiou-Metaxa, M. Standard-dose intravenous anti-D immunoglobulin versus intravenous immunoglobulin in the treatment of newly diagnosed childhood primary immune thrombocytopenia. J. Pediatr. Hematol. Oncol. 2011, 33, 265–269. [Google Scholar] [CrossRef]
- Neunert, C.; Terrell, D.R.; Arnold, D.M.; Buchanan, G.; Cines, D.B.; Cooper, N.; Cuker, A.; Despotovic, J.M.; George, J.N.; Grace, R.F.; et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019, 3, 3829–3866. [Google Scholar] [CrossRef]
- Carcao, M.; Silva, M.; David, M.; Klaassen, R.J.; Steele, M.; Price, V.; Wakefield, C.; Kim, L.; Stephens, D.; Blanchette, V.S. IVMP+IVIG raises platelet counts faster than IVIG alone: Results of a randomized, blinded trial in childhood ITP. Blood Adv. 2020, 4, 1492–1500. [Google Scholar] [CrossRef]
- Aladjidi, N.; Jutand, M.; Beaubois, C.; Fernandes, H.; Jeanpetit, J.; Coureau, G.; Gilleron, V.; Kostrzewa, A.; Lauroua, P.; Jeanne, M.; et al. Reliable assessment of the incidence of childhood autoimmune hemolytic anemia. Pediatr. Blood Cancer 2017, 64, e26683. [Google Scholar] [CrossRef]
- Voulgaridou, A.; Kalfa, T.A. Autoimmune hemolytic anemia in the pediatric setting. J. Clin. Med. 2021, 10, 216. [Google Scholar] [CrossRef]
- Zanella, A.; Barcellini, W. Treatment of autoimmune hemolytic anemias. Haematologica 2014, 99, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Zeerleder, S. Autoimmune haemolytic anaemia—A practical guide to cope with a diagnostic and therapeutic challenge. Neth. J. Med. 2011, 69, 177–184. [Google Scholar] [PubMed]
- Fan, J.; He, H.; Zhao, W.; Wang, Y.; Lu, J.; Li, J.; Li, J.; Xiao, P.; Lu, Y.; Chai, Y.; et al. Clinical Features and Treatment Outcomes of Childhood Autoimmune Hemolytic Anemia: A Retrospective Analysis of 68 Cases. J. Pediatr. Hematol. 2016, 38, e50–e55. [Google Scholar] [CrossRef] [PubMed]
- Afzal, W.; Owlia, M.B.; Hasni, S.; Newman, K.A. Autoimmune Neutropenia Updates: Etiology, Pathology, and Treatment. South Med. J. 2017, 110, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Charlebois, J.; Rivard, G.É.; St-Louis, J. Management of acquired hemophilia A: Review of current evidence. Transfus. Apher. Sci. 2018, 57, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Kruse-Jarres, R.; Kempton, C.L.; Baudo, F.; Collins, P.W.; Knoebl, P.; Leissinger, C.A.; Tiede, A.; Kessler, C.M. Acquired hemophilia A: Updated review of evidence and treatment guidance. Am. J. Hematol. 2017, 92, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.; Baudo, F.; Knoebl, P.; Lévesque, H.; Nemes, L.; Pellegrini, F.; Marco, P.; Tengborn, L.; Huth-Kühne, A. Immunosuppression for acquired hemophilia A: Results from the European Acquired Haemophilia Registry (EACH2). Blood 2012, 120, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B.; Vander Haar, E.L.; Berkowitz, R.L. New developments in fetal and neonatal alloimmune thrombocytopenia. Am. J. Obstet. Gynecol. 2021, 225, 120–127. [Google Scholar] [CrossRef]
- Lieberman, L.; Greinacher, A.; Murphy, M.F.; Bussel, J.; Bakchoul, T.; Corke, S.; Kjaer, M.; Kjeldsen-Kragh, J.; Bertrand, G.; Oepkes, D.; et al. Fetal and neonatal alloimmune thrombocytopenia: Recommendations for evidence-based practice, an international approach. Br. J. Haematol. 2019, 185, 549–562. [Google Scholar] [CrossRef]
- Ouwehand, W.H.; Smith, G.; Ranasinghe, E. Management of severe alloimmune thrombocytopenia in the newborn. Arch. Dis. Child.-Fetal Neonatal Ed. 2000, 82, 173F–175F. [Google Scholar] [CrossRef]
- de Kruijff, E.; van Gammeren, A.J.; Porcelijn, L.; van Esser, J.W.J. Post-transfusion purpura in a woman with acute myeloid leukemia. Neth. J. Med. 2019, 77, 81–83. [Google Scholar] [PubMed]
- Hamblin, T.J.; Abidi, S.M.N.; Nee, P.A.; Copplestone, A.; Mufti, G.J.; Oscier, D.G. Successful Treatment of Post-Transfusion Purpura with High Dose Immunoglobulins after Lack of Response to Plasma Exchange. Vox Sang. 1985, 49, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Joly, B.S.; Coppo, P.; Veyradier, A. Pediatric thrombotic thrombocytopenic purpura. Eur. J. Haematol. 2018, 101, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Joly, B.S.; Coppo, P.; Veyradier, A. An update on pathogenesis and diagnosis of thrombotic thrombocytopenic purpura. Expert Rev. Hematol. 2019, 12, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.C.; Arnold, D.M.; Leber, B.F.; Clare, R.; Molnar, G.J.; Kelton, J.G. Intravenous immunoglobulin as an adjunct to plasma exchange for the treatment of chronic thrombotic thrombocytopenic purpura. Vox Sang. 2007, 93, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Lovell, D.J.; Ruperto, N.; Giannini, E.H.; Martini, A. Advances from clinical trials in juvenile idiopathic arthritis. Nat. Rev. Rheumatol. 2013, 9, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Onel, K.B.; Horton, D.B.; Lovell, D.J.; Shenoi, S.; Cuello, C.A.; Angeles-Han, S.T.; Becker, M.L.; Cron, R.Q.; Feldman, B.M.; Ferguson, P.J.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Oligoarthritis, Temporomandibular Joint Arthritis, and Systemic Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2022, 74, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.Y.; Schneider, R. Systemic Juvenile Idiopathic Arthritis. Pediatr. Clin. N. Am. 2018, 65, 691–709. [Google Scholar] [CrossRef]
- Bayry, J.; Negi, V.S.; Kaveri, S.V. Intravenous immunoglobulin therapy in rheumatic diseases. Nat. Rev. Rheumatol. 2011, 7, 349–359. [Google Scholar] [CrossRef]
- Feldman, B.M.; Rider, L.G.; Reed, A.M.; Pachman, L.M. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet 2008, 371, 2201–2212. [Google Scholar] [CrossRef]
- Kobayashi, I.; Akioka, S.; Kobayashi, N.; Iwata, N.; Takezaki, S.; Nakaseko, H.; Sato, S.; Nishida, Y.; Nozawa, T.; Yamasaki, Y.; et al. Clinical practice guidance for juvenile dermatomyositis (JDM) 2018-Update. Mod. Rheumatol. 2020, 30, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Enders, F.B.; Bader-Meunier, B.; Baildam, E.; Constantin, T.; Dolezalova, P.; Feldman, B.M.; Lahdenne, P.; Magnusson, B.; Nistala, K.; Ozen, S.; et al. Consensus-based recommendations for the management of juvenile dermatomyositis. Ann. Rheum. Dis. 2017, 76, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Waldman, R.; DeWane, M.E.; Lu, J. Dermatomyositis: Diagnosis and treatment. J. Am. Acad. Dermatol. 2020, 82, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Zandman-Goddard, G.; Levy, Y.; Shoenfeld, Y. Intravenous Immunoglobulin Therapy and Systemic Lupus Erythematosus. Clin. Rev. Allergy Immunol. 2005, 29, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.M.; Wagner-Weiner, L. Intravenous Immunoglobulin in Pediatric Rheumatology: When to Use It and What Is the Evidence. Pediatr. Ann. 2017, 46, e19–e24. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.P.S.T.; Nascimento, B.R.; Calderaro, D.C.; Ferreira, G.A.; Correa, H. Neuropsychiatric Syndromes in Childhood-Onset Systemic Lupus Erythematosus: A Systematic Review and Meta-analysis. J. Clin. Rheumatol. 2021, 27, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Brogna, C.; Mariotti, P.; Manna, R. Conventional and intravenous immunoglobulin therapy in paediatric antiphospholipid antibodies-related chorea. Lupus 2014, 23, 1449–1451. [Google Scholar] [CrossRef]
- Papachristos, D.A.; Oon, S.; Hanly, J.G.; Nikpour, M. Management of inflammatory neurologic and psychiatric manifestations of systemic lupus erythematosus: A systematic review. Semin. Arthritis Rheum. 2021, 51, 49–71. [Google Scholar] [CrossRef]
- Oni, L.; Sampath, S. Childhood IgA vasculitis (Henoch Schonlein Purpura)-advances and knowledge gaps. Front. Pediatr. 2019, 7, 257. [Google Scholar] [CrossRef]
- Rostoker, G.; Desvaux-Belghiti, D.; Pilatte, Y.; Petit-Phar, M.; Philippon, C.; Deforges, L.; Terzidis, H.; Intrator, L.; Andre, C.; Adnot, S.; et al. High-dose immunoglobulin therapy for severe IgA nephropathy and Henoch- Schonlein purpura. Ann. Intern. Med. 1994, 120, 476–484. [Google Scholar] [CrossRef]
- Hahn, D.; Hodson, E.M.; Craig, J.C. Interventions for preventing and treating kidney disease in IgA vasculitis. Cochrane Database Syst. Rev. 2023, 2023, CD005128. [Google Scholar] [CrossRef]
- Nothhaft, M.; Klepper, J.; Kneitz, H.; Meyer, T.; Hamm, H.; Morbach, H. Hemorrhagic Bullous Henoch-Schönlein Purpura: Case Report and Review of the Literature. Front. Pediatr. 2019, 6, 413. [Google Scholar] [CrossRef] [PubMed]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Yang, H.W.; Lin, T.Y.; Yang, K.D. Perspective of Immunopathogenesis and Immunotherapies for Kawasaki Disease. Front. Pediatr. 2021, 9, 697632. [Google Scholar] [CrossRef]
- Wang, N.; Chen, Z.; Zhang, F.; Zhang, Q.; Sun, L.; Lv, H.; Wang, B.; Shen, J.; Zhou, X.; Chen, F.; et al. Intravenous Immunoglobulin Therapy Restores the Quantity and Phenotype of Circulating Dendritic Cells and CD4+ T Cells in Children with Acute Kawasaki Disease. Front. Immunol. 2022, 13, 802690. [Google Scholar] [CrossRef] [PubMed]
- Tremoulet, A.H. Adjunctive therapies in Kawasaki disease. Int. J. Rheum Dis. 2018, 21, 76–79. [Google Scholar] [CrossRef]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef] [PubMed]
- Toubiana, J.; Poirault, C.; Corsia, A.; Bajolle, F.; Fourgeaud, J.; Angoulvant, F.; Debray, A.; Basmaci, R.; Salvador, E.; Biscardi, S.; et al. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: Prospective observational study. BMJ 2020, 369, m2094. [Google Scholar] [CrossRef]
- Verdoni, L.; Mazza, A.; Gervasoni, A.; Martelli, L.; Ruggeri, M.; Ciuffreda, M.; Bonanomi, E.; D’Antiga, L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet 2020, 395, 1771–1778. [Google Scholar] [CrossRef]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Ferris, A.; Kernan, K.F.; Schulert, G.S.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 2. Arthritis Rheumatol. 2021, 73, e13–e29. [Google Scholar] [CrossRef]
- McArdle, A.J.; Vito, O.; Patel, H.; Seaby, E.G.; Shah, P.; Wilson, C.; Broderick, C.; Nijman, R.; Tremoulet, A.H.; Munblit, D.; et al. Treatment of Multisystem Inflammatory Syndrome in Children. N. Engl. J. Med. 2021, 385, 11–22. [Google Scholar] [CrossRef]
- Rauniyar, R.; Mishra, A.; Kharel, S.; Giri, S.; Rauniyar, R.; Yadav, S.; Chaudhary, G. IVIG plus Glucocorticoids versus IVIG Alone in Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with COVID-19: A Systematic Review and Meta-Analysis. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 9458653. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.M.; Lal, A.K.; Chen, S.; Čiháková, D.; Cooper, L.T.; Deshpande, S.; Godown, J.; Grosse-Wortmann, L.; Robinson, J.D.; Towbin, J.A.; et al. Diagnosis and Management of Myocarditis in Children. Circulation 2021, 144, E123–E135. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Kernan, K.F.; Schulert, G.S.; Seo, P.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS–CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 3. Arthritis Rheumatol. 2022, 74, E1–E20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Shamie, I.; Lee, J.C.; Nowell, C.J.; Peng, W.; Angulo, S.; Le, L.N.; Liu, Y.; Miao, H.; Xiong, H.; et al. Immune response to intravenous immunoglobulin in patients with Kawasaki disease and MIS-C. J. Clin. Investig. 2021, 131, e147076. [Google Scholar] [CrossRef] [PubMed]
- Ganigara, M.; Sharma, C.; Bayry, J. Unraveling the mechanisms of IVIG immunotherapy in MIS-C. Cell Rep. Med. 2021, 2, 100431. [Google Scholar] [CrossRef] [PubMed]
- Syrimi, E.; Fennell, E.; Richter, A.; Vrljicak, P.; Stark, R.; Ott, S.; Murray, P.G.; Al-Abadi, E.; Chikermane, A.; Dawson, P.; et al. The immune landscape of SARS-CoV-2-associated Multisystem Inflammatory Syndrome in Children (MIS-C) from acute disease to recovery. iScience 2021, 24, 103215. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, C.; Kaveri, S.V.; Bayry, J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int. Immunol. 2017, 29, 491–498. [Google Scholar] [CrossRef]
- Ravelli, A.; Minoia, F.; Davì, S.; Horne, A.; Bovis, F.; Pistorio, A.; Aricò, M.; Avcin, T.; Behrens, E.M.; De Benedetti, F.; et al. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol. 2016, 68, 566–576. [Google Scholar] [CrossRef]
- Lee, P.Y.; Day-Lewis, M.; Henderson, L.A.; Friedman, K.G.; Lo, J.; Roberts, J.E.; Lo, M.S.; Platt, C.D.; Chou, J.; Hoyt, K.J.; et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J. Clin. Investig. 2020, 130, 5942–5950. [Google Scholar] [CrossRef]
- Rodriguez-Smith, J.J.; Verweyen, E.L.; Clay, G.M.; Esteban, Y.M.; de Loizaga, S.R.; Baker, E.J.; Do, T.; Dhakal, S.; Lang, S.M.; Grom, A.A.; et al. Inflammatory biomarkers in COVID-19-associated multisystem inflammatory syndrome in children, Kawasaki disease, and macrophage activation syndrome: A cohort study. Lancet Rheumatol. 2021, 3, e574–e584. [Google Scholar] [CrossRef] [PubMed]
- Yener, G.O.; Kısaarslan, A.P.; Ulu, K.; Atalay, E.; Haşlak, F.; Özdel, S.; Yücel, B.B.; Yıldırım, D.G.; Çakmak, F.; Öztürk, K.; et al. Differences and similarities of multisystem inflammatory syndrome in children, Kawasaki disease and macrophage activating syndrome due to systemic juvenile idiopathic arthritis: A comparative study. Rheumatol. Int. 2022, 42, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Valverde, I.; Singh, Y.; Sanchez-De-Toledo, J.; Theocharis, P.; Chikermane, A.; Di Filippo, S.; Kuciñska, B.; Mannarino, S.; Tamariz-Martel, A.; Gutierrez-Larraya, F.; et al. Acute Cardiovascular Manifestations in 286 Children with Multisystem Inflammatory Syndrome Associated With COVID-19 Infection in Europe. Circulation 2021, 143, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Kelleman, M.; West, Z.; Peter, A.; Dove, M.; Butto, A.; Oster, M.E. Comparison of Multisystem Inflammatory Syndrome in Children-Related Myocarditis, Classic Viral Myocarditis, and COVID-19 Vaccine-Related Myocarditis in Children. J. Am. Heart Assoc. 2022, 11, e024393. [Google Scholar] [CrossRef]
- Maisch, B. Cardio-Immunology of Myocarditis: Focus on Immune Mechanisms and Treatment Options. Front. Cardiovasc. Med. 2019, 6, 48. [Google Scholar] [CrossRef]
- Huang, X.; Sun, Y.; Su, G.; Li, Y.; Shuai, X. Intravenous Immunoglobulin Therapy for Acute Myocarditis in Children and Adults. Int. Heart J. 2019, 60, 359–365. [Google Scholar] [CrossRef]
- Robinson, J.; Hartling, L.; Vandermeer, B.; Sebastianski, M.; Klassen, T.P. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst. Rev. 2020, 2020, CD004370. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, X.; Wang, X.; Xiao, Z. Adverse Effects of Immunoglobulin Therapy. Front. Immunol. 2018, 9, 1299. [Google Scholar] [CrossRef]
- Dantal, J. Intravenous Immunoglobulins: In-Depth Review of Excipients and Acute Kidney Injury Risk. Am. J. Nephrol. 2013, 38, 275–284. [Google Scholar] [CrossRef]
- Jiang, M.; Kimber, J.S.; Gupta, A.; Kovoor, J.; Stretton, B.; Ravindran, J.; Hissaria, P.; Smith, W.B.; Bacchi, S. Adverse Reactions Associated with Intravenous Immunoglobulin Administration in the Treatment of Neurological Disorders: A Systematic Review. Int. Arch. Allergy Immunol. 2023, 184, 513–528. [Google Scholar] [CrossRef]
- Freeman, C.M.; Squire, J.D.; Joshi, A.Y. Immunoglobulin treatment for B-cell immunodeficiencies. J. Immunol. Methods 2022, 509, 113336. [Google Scholar] [CrossRef] [PubMed]
- Sandler, S.G.; Eder, A.F.; Goldman, M.; Winters, J.L. The entity of immunoglobulin A-related anaphylactic transfusion reactions is not evidence based. Transfusion 2015, 55, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Tacquard, C.; Boudjedir, K.; Carlier, M.; Muller, J.Y.; Gomis, P.; Mertes, P.M. Hypersensitivity transfusion reactions due to IgA deficiency are rare according to French hemovigilance data. J. Allergy Clin. Immunol. 2017, 140, 884–885. [Google Scholar] [CrossRef] [PubMed]
- Yanagihashi, M.; Okamoto, R.; Morioka, H.; Sawada, M.; Matsumoto, S.; Ikeda, T.; Kano, O. Coronary spastic angina after the administration of intravenous immunoglobulin in myasthenia gravis: A case report. BMC Neurol. 2020, 20, 319. [Google Scholar] [CrossRef] [PubMed]
- Binitha, M.; Nandakumar, G.; Thomas, D. Suspected cardiac toxicity to intravenous immunoglobulin used for treatment of scleromyxedema. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 248. [Google Scholar] [CrossRef] [PubMed]
- Benadiba, J.; Robitaille, N.; Lambert, G.; Itaj, N.K.; Pastore, Y. Intravenous immunoglobulin-associated thrombosis: Is it such a rare event? Report of a pediatric case and of the Quebec Hemovigilance System. Transfusion 2015, 55, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, J.; Al Amri, A.; Ghatasheh, G. Transfusion-Related Acute Lung Injury After Immunoglobulin Infusion for Kawasaki Disease: A Case Report and Literature Review. Glob. Pediatr. Health 2017, 4, 2333794X1774654. [Google Scholar] [CrossRef]
- Baudel, J.L.; Vigneron, C.; Pras-Landre, V.; Joffre, J.; Marjot, F.; Ait-Oufella, H.; Bigé, N.; Maury, E.; Guidet, B.; Fain, O.; et al. Transfusion-related acute lung injury (TRALI) after intravenous immunoglobulins: French multicentre study and literature review. Clin. Rheumatol. 2020, 39, 541–546. [Google Scholar] [CrossRef]
- Yu, Y.; Lian, Z. Update on transfusion-related acute lung injury: An overview of its pathogenesis and management. Front. Immunol. 2023, 14, 1175387. [Google Scholar] [CrossRef]
- Cappell, K.M.; Sherry, R.M.; Yang, J.C.; Goff, S.L.; Vanasse, D.A.; McIntyre, L.; Rosenberg, S.A.; Kochenderfer, J.N. Long-Term Follow-Up of Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy. J. Clin. Oncol. 2020, 38, 3805–3815. [Google Scholar] [CrossRef] [PubMed]
- Makatsori, M.; Kiani-Alikhan, S.; Manson, A.L.; Verma, N.; Leandro, M.; Gurugama, N.P.; Longhurst, H.J.; Grigoriadou, S.; Buckland, M.; Kanfer, E.; et al. Hypogammaglobulinaemia after rituximab treatment—Incidence and outcomes. QJM 2014, 107, 821–828. [Google Scholar] [CrossRef]
- Stohl, W.; Schwarting, A.; Okada, M.; Scheinberg, M.; Doria, A.; Hammer, A.E.; Kleoudis, C.; Groark, J.; Bass, D.; Fox, N.L.; et al. Efficacy and Safety of Subcutaneous Belimumab in Systemic Lupus Erythematosus: A Fifty-Two–Week Randomized, Double-Blind, Placebo-Controlled Study. Arthritis Rheumatol. 2017, 69, 1016–1027. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Vital, E.M.; McElvenny, D.M.; Hensor, E.M.A.; Das, S.; Dass, S.; Rawstron, A.C.; Buch, M.H.; Emery, P.; Savic, S. Predicting Severe Infection and Effects of Hypogammaglobulinemia During Therapy with Rituximab in Rheumatic and Musculoskeletal Diseases. Arthritis Rheumatol. 2019, 71, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Fresa, A.; Bellesi, S.; Metafuni, E.; Maiolo, E.; Pansini, I.; Frioni, F.; Autore, F.; Limongiello, M.A.; Innocenti, I.; et al. Hematopoiesis and immune reconstitution after CD19 directed chimeric antigen receptor T-cells (CAR-T): A comprehensive review on incidence, risk factors and current management. Eur. J. Haematol. 2023, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Lee, M.; Lortan, J.; Lopez-Granados, E.; Misbah, S.; Chapel, H. Infection outcomes in patients with common variable immunodeficiency disorders: Relationship to immunoglobulin therapy over 22 years. J. Allergy Clin. Immunol. 2010, 125, 1354–1360.e4. [Google Scholar] [CrossRef]
- Khan, S.; Davies, L.; Cowley, D.; Wild, G.; Sewell, W.A.C. Anti-acetylcholine receptor antibody reactivity of IgG in commercial immunoglobulin preparations. Clin. Neurol. Neurosurg. 2010, 112, 835–836. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Sims, S.; Raine, D.; Sewell, W.A.C. Both autoantibodies and pathogen-specific antibodies are present in immunoglobulin preparations and reflect characteristics of the donor population. J. Am. Acad. Dermatol. 2008, 59, 1089–1090. [Google Scholar] [CrossRef]
- Smith, T.D.; Cunningham-Rundles, C. Detection of anti–glutamic acid decarboxylase antibodies in immunoglobulin products. J. Allergy Clin. Immunol. Pract. 2018, 6, 260–261. [Google Scholar] [CrossRef]
- Grüter, T.; Ott, A.; Meyer, W.; Jarius, S.; Kinner, M.; Motte, J.; Pitarokoili, K.; Gold, R.; Komorowski, L.; Ayzenberg, I. Effects of IVIg treatment on autoantibody testing in neurological patients: Marked reduction in sensitivity but reliable specificity. J. Neurol. 2020, 267, 715–720. [Google Scholar] [CrossRef]
- van der Molen, R.G.; Hamann, D.; Jacobs, J.F.; van der Meer, A.; de Jong, J.; Kramer, C.; Strengers, P.F.; van der Meer, J.W. Anti-SSA antibodies are present in immunoglobulin preparations. Transfusion 2015, 55, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Burbelo, P.D.; Castagnoli, R.; Shimizu, C.; Delmonte, O.M.; Dobbs, K.; Discepolo, V.; Vecchio, A.L.; Guarino, A.; Licciardi, F.; Ramenghi, U.; et al. Autoantibodies Against Proteins Previously Associated with Autoimmunity in Adult and Pediatric Patients With COVID-19 and Children With MIS-C. Front. Immunol. 2022, 13, 841126. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadou, M.M.; Alexopoulos, H.; Akrivou, S.; Gola, E.; Dalakas, M.C. Anti-Neuronal Antibodies Within the IVIg Preparations: Importance in Clinical Practice. Neurotherapeutics 2020, 17, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Fnu, Z.; Uddin, A.; Navetta-Modrov, B.; Patnaik, A.; Kaell, A. Inpatient Rheumatology Consultation Prompted by Positive Autoantibodies in Patients Receiving Intravenous Immunoglobulin Therapy: A Case Series and Literature Review. Cureus 2023, 15, e37008. [Google Scholar] [CrossRef] [PubMed]
- Imbach, P.; Barandun, S.; Baumgartner, C.; Hirt, A.; Hofer, F.; Wagner, H.P. High-dose intravenous gammaglobulin therapy of refractory, in particular idiopathic thrombocytopenia in childhood. Helv. Paediatr. Acta 1981, 36, 81–86. [Google Scholar] [PubMed]
- Imbach, P.; D’Apuzzo, V.; Hirt, A.; Rossi, E.; Vest, M.; Barandun, S.; Baumgartner, C.; Morell, A.; Schöni, M.; Wagner, H. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet 1981, 317, 1228–1231. [Google Scholar] [CrossRef] [PubMed]
- Fateh-Moghadam, A.; Wick, M.; Besinger, U.; Geursen, R.G. High-dose intravenous gammaglobulin for myasthenia gravis. Lancet 1984, 323, 848–849. [Google Scholar] [CrossRef]
- Péchadre, J.; Sauvezie, B.; Osier, C.; Gibert, J. Traitement des encéphalopathies épileptiques de l’enfant par les gamma-globulines Résultats préliminaires. Rev. D’electroencéphalograph. Neurophysiol. Clin. 1977, 7, 443–447. [Google Scholar] [CrossRef]
- Więsik-Szewczyk, E.; Ziętkiewicz, M.; Radziwilska-Muc, A.; Jahnz-Różyk, K. Increased Access to Immunoglobulin Replacement Therapy for Patients with Primary Immunodeficiency in Poland Based on Clinical Usage Data of Immunoglobulin G over a 5-Year Period. J. Clin. Med. 2023, 12, 2431. [Google Scholar] [CrossRef]
- EMA. Guideline on the Clinical Investigation of Human Normal Immunoglobulin for Intravenous Administration (IVIg); EMA: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Criteria for Clinical Use of Immunoglobulin in Australia. Available online: https://www.criteria.blood.gov.au/checkeligibility (accessed on 1 August 2023).
- AIFA. Documento di Indirizzo Sull’uso Delle Immunoglobuline Umane in Condizioni di Carenza; AIFA: Rome, Italy, 2022. [Google Scholar]
- Na, I.; Buckland, M.; Agostini, C.; Edgar, J.D.M.; Friman, V.; Michallet, M.; Sánchez-Ramón, S.; Scheibenbogen, C.; Quinti, I. Current clinical practice and challenges in the management of secondary immunodeficiency in hematological malignancies. Eur. J. Haematol. 2019, 102, 447–456. [Google Scholar] [CrossRef]
- Cinetto, F.; Francisco, I.E.; Fenchel, K.; Scarpa, R.; Montefusco, V.; Pluta, A.; Wolf, H.M. Use of immunoglobulin replacement therapy in patients with secondary antibody deficiency in daily practice: A European expert Q&A-based review. Expert Rev. Hematol. 2023, 16, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kobayashi, R.H.; Litzman, J.; Cherwin, L.; Hoeller, S.; Kreuwel, H. Subcutaneous immunoglobulin 16.5% for the treatment of pediatric patients with primary antibody immunodeficiency. Expert Rev. Clin. Immunol. 2023, 19, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Gardulf, A.; Bjorvell, H.; Andersen, V.; Bjorkander, J.; Ericson, D.; Froland, S.S.; Gustafson, R.; Hammarstrom, L.; Nystrom, T.; Soeberg, B.; et al. Lifelong treatment with gammaglobulin for primary antibody deficiencies: The patients’ experiences of subcutaneous self-infusions and home therapy. J. Adv. Nurs. 1995, 21, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Hammarstrom, L.; Gardulf, A.; Edvard Smith, C.I.; Gardulf, A. Home treatment of hypogammaglobulinaemia with subcutaneous gammaglobulin by rapid infusion. Lancet 1991, 338, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Gardulf, A.; Björvell, H.; Gustafson, R.; Hammarström, L.; Smith, C.I. Safety of rapid subcutaneous gammaglobulin infusions in patients with primary antibody deficiency. Immunodeficiency 1993, 4, 81–84. [Google Scholar] [PubMed]
- Gaspar, J.; Gerritsen, B.; Jones, A. Immunoglobulin replacement treatment by rapid subcutaneous infusion. Arch. Dis. Child. 1998, 79, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Chouksey, A.; Duff, K.; Wasserbauer, N.; Berger, M. Subcutaneous Immunoglobulin-G Replacement Therapy with Preparations Currently Available in the United States for Intravenous or Intramuscular Use: Reasons and Regimens. Allergy Asthma Clin. Immunol. 2005, 1, 120. [Google Scholar] [CrossRef] [PubMed]
- Gammon, R.; Katz, L.M.; Strauss, D.; Rowe, K.; Menitove, J.; Benjamin, R.J.; Goel, R.; Borge, D.; Reichenberg, S.; Smith, R. Beyond COVID-19 and lessons learned in the United States. Transfus. Med. 2023, 33, 6–15. [Google Scholar] [CrossRef]
- Chapel, H.M.; Spickett, G.P.; Ericson, D.; Engl, W.; Eibl, M.M.; Bjorkander, J. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J. Clin. Immunol. 2000, 20, 94–100. [Google Scholar] [CrossRef]
- Gul, Y.; Kapakli, H.; Guner, S.N.; Alan, H.B.; Hazar, E.; Keles, S.; Reisli, I. Long-Term Experience of Subcutaneous Immunoglobulin Therapy in Pediatric Primary Immunodeficient Patients with Low and Normal Body Weight. J. Clin. Immunol. 2022, 42, 64–71. [Google Scholar] [CrossRef]
- Hagan, J.B.; Fasano, M.B.; Spector, S.; Wasserman, R.L.; Melamed, I.; Rojavin, M.A.; Zenker, O.; Orange, J.S. Efficacy and Safety of a New 20% Immunoglobulin Preparation for Subcutaneous Administration, IgPro20, in Patients with Primary Immunodeficiency. J. Clin. Immunol. 2010, 30, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Ochs, H.D.; Gupta, S.; Kiessling, P.; Nicolay, U.; Berger, M. Safety and Efficacy of Self-Administered Subcutaneous Immunoglobulin in Patients with Primary Immunodeficiency Diseases. J. Clin. Immunol. 2006, 26, 265–273. [Google Scholar] [CrossRef]
- Alsina, L.; Montoro, J.B.; Moral, P.M.; Neth, O.; Pica, M.O.; Sánchez-Ramón, S.; Presa, M.; Oyagüez, I.; Casado, M.; González-Granado, L.I. Cost-minimization analysis of immunoglobulin treatment of primary immunodeficiency diseases in Spain. Eur. J. Health Econ. 2022, 23, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Perraudin, C.; Bourdin, A.; Vicino, A.; Kuntzer, T.; Bugnon, O.; Berger, J. Home-based subcutaneous immunoglobulin for chronic inflammatory demyelinating polyneuropathy patients: A Swiss cost-minimization analysis. PLoS ONE 2020, 15, e0242630. [Google Scholar] [CrossRef] [PubMed]
- Perraudin, C.; Bourdin, A.; Spertini, F.; Berger, J.; Bugnon, O. Switching Patients to Home-Based Subcutaneous Immunoglobulin: An Economic Evaluation of an Interprofessional Drug Therapy Management Program. J. Clin. Immunol. 2016, 36, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, B.; Martins, K.J.B.; Tran, D.T.; Blain, H.; Richer, L.; Klarenbach, S.W. Economic impact of self-administered subcutaneous versus clinic-administered intravenous immunoglobulin G therapy in Alberta, Canada: A population-based cohort study. Allergy Asthma Clin. Immunol. 2022, 18, 99. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.W.; Song, C.; Isaranuwatchai, W.; Betschel, S. Home-based subcutaneous immunoglobulin therapy vs hospital-based intravenous immunoglobulin therapy: A prospective economic analysis. Ann. Allergy Asthma Immunol. 2018, 120, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Romberg, N. High cost of immunoglobulin replacement therapy. Ann. Allergy Asthma Immunol. 2022, 129, 645. [Google Scholar] [CrossRef]
- Garraud, O. Injectable immunoglobulins, immunodeficiency and off-label clinical trials, source plasma and ethical concerns and debates. Transfus. Apher. Sci. 2019, 58, 529–530. [Google Scholar] [CrossRef]
- Costagliola, G.; Peroni, D.G.; Consolini, R. Beyond Infections: New Warning Signs for Inborn Errors of Immunity in Children. Front. Pediatr. 2022, 10, 855445. [Google Scholar] [CrossRef]
- Lindahl, H.; Bryceson, Y.T. Neuroinflammation Associated with Inborn Errors of Immunity. Front. Immunol. 2022, 12, 827815. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhu, L.-N.; Hou, H.-M.; Wang, S.; Zhang, S.; Wang, G.-G.; Guo, Z.-Y. FcRn inhibitors: A novel option for the treatment of myasthenia gravis. Neural Regen. Res. 2022, 18, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Briani, C.; Visentin, A. Therapeutic Monoclonal Antibody Therapies in Chronic Autoimmune Demyelinating Neuropathies. Neurotherapeutics 2022, 19, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.A. Satralizumab: First Approval. Drugs 2020, 80, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Inebilizumab: First Approval. Drugs 2020, 80, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Gottardi, F.; Moratti, M.; Belotti, T.; Ferrari, S.; Selva, P.; Bassi, M.; Zama, D.; Pession, A. Refractory immune thrombocytopenia successfully treated with bortezomib in a child with 22q11.2 deletion syndrome, complicated by Evans syndrome and hypogammaglobulinemia. Platelets 2022, 33, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Dodig, D.; Genge, A.; Selchen, D.; Freedman, M.S. Complement Inhibition in Myasthenia Gravis and Neuromyelitis Optica Spectrum Disorder. Can. J. Neurol. Sci. J. Can. Des Sci. Neurol. 2023, 50, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Werdan, K. Pathophysiology of Septic Shock and Multiple Organ Dysfunction Syndrome and Various Therapeutic Approaches with Special Emphasis on Immunoglobulins. Ther. Apher. Dial. 2001, 5, 115–122. [Google Scholar] [CrossRef]
- Friedman, C.; Wender, D.; Temple, D.; Rawson, J. Intravenous Gamma Globulin as Adjunct Therapy for Severe Group B Streptococcal Disease in the Newborn. Am. J. Perinatol. 1990, 7, 1–4. [Google Scholar] [CrossRef]
- Kaul, R.; McGeer, A.; Norrby-Teglund, A.; Kotb, M.; Schwartz, B.; O’Rourke, K.; Talbot, J.; Low, D.E. Intravenous Immunoglobulin Therapy for Streptococcal Toxic Shock Syndrome—A Comparative Observational Study. Clin. Infect. Dis. 1999, 28, 800–807. [Google Scholar] [CrossRef]
- Jenson, H.B.; Pollock, B.H. The role of intravenous immunoglobulin for the prevention and treatment of neonatal sepsis. Semin. Perinatol. 1998, 22, 50–63. [Google Scholar] [CrossRef] [PubMed]
- INIS Collaborative Group. Treatment of Neonatal Sepsis with Intravenous Immune Globulin. N. Engl. J. Med. 2011, 365, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Alejandria, M.M.; Lansang, M.A.D.; Dans, L.F.; Mantaring, J.B., III. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst. Rev. 2013, 2018, CD001090. [Google Scholar] [CrossRef] [PubMed]
- Perricone, C.; Triggianese, P.; Bursi, R.; Cafaro, G.; Bartoloni, E.; Chimenti, M.S.; Gerli, R.; Perricone, R. Intravenous Immunoglobulins at the Crossroad of Autoimmunity and Viral Infections. Microorganisms 2021, 9, 121. [Google Scholar] [CrossRef]
- Pinto, A.K.; Hassert, M.; Han, X.; Barker, D.; Carnelley, T.; Branche, E.; Steffen, T.L.; Stone, E.T.; Geerling, E.; Viramontes, K.M.; et al. The Ability of Zika virus Intravenous Immunoglobulin to Protect from or Enhance Zika Virus Disease. Front. Immunol. 2021, 12, 717425. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, P.; D’alanno, G.; Biserni, G.B.; Moratti, M.; Conti, F.; Fabi, M.; Lanari, M. Exploring Factors Influencing Changes in Incidence and Severity of Multisystem Inflammatory Syndrome in Children. Pathogens 2023, 12, 997. [Google Scholar] [CrossRef]
- Mosca, M.; Bacchetta, J.; Chamouard, V.; Rascle, P.; Dubois, V.; Paul, S.; Mekki, Y.; Picard, C.; Bertholet-Thomas, A.; Ranchin, B.; et al. IVIg therapy in the management of BK virus infections in pediatric kidney transplant patients. Arch. Pédiatr. 2023, 30, 165–171. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).