Riboflavin and Its Derivates as Potential Photosensitizers in the Photodynamic Treatment of Skin Cancers
Abstract
1. Introduction
2. Blue Light in PDT Therapy for Skin Cancer and Diseases
3. Photoreactivity of Flavin Derivatives: Photochemical Aspects
4. Photosensitization with Flavin Sensitizers: Biophysical Approach
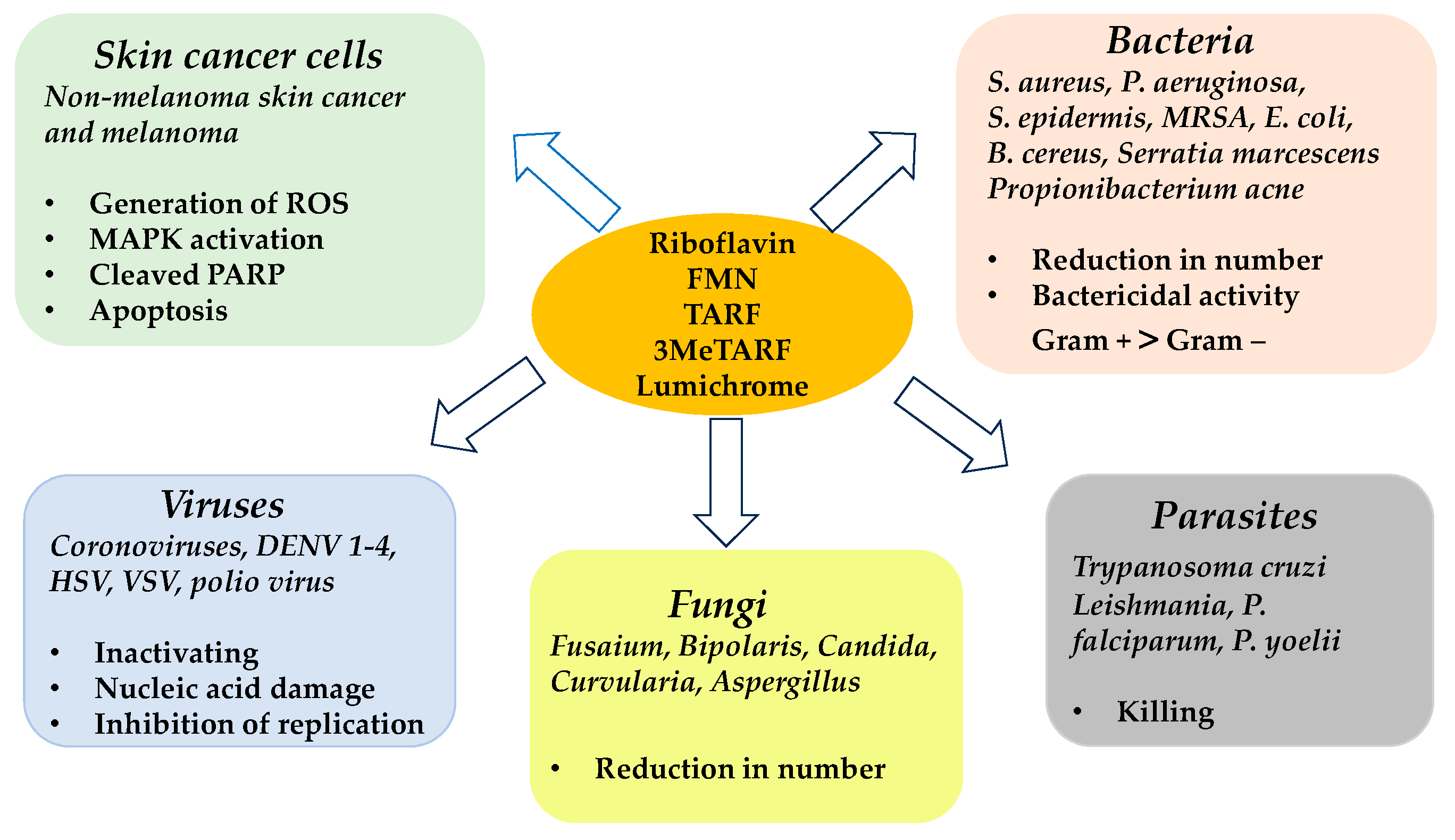
5. Riboflavin and Its Derivates in PDT: Biological Issues
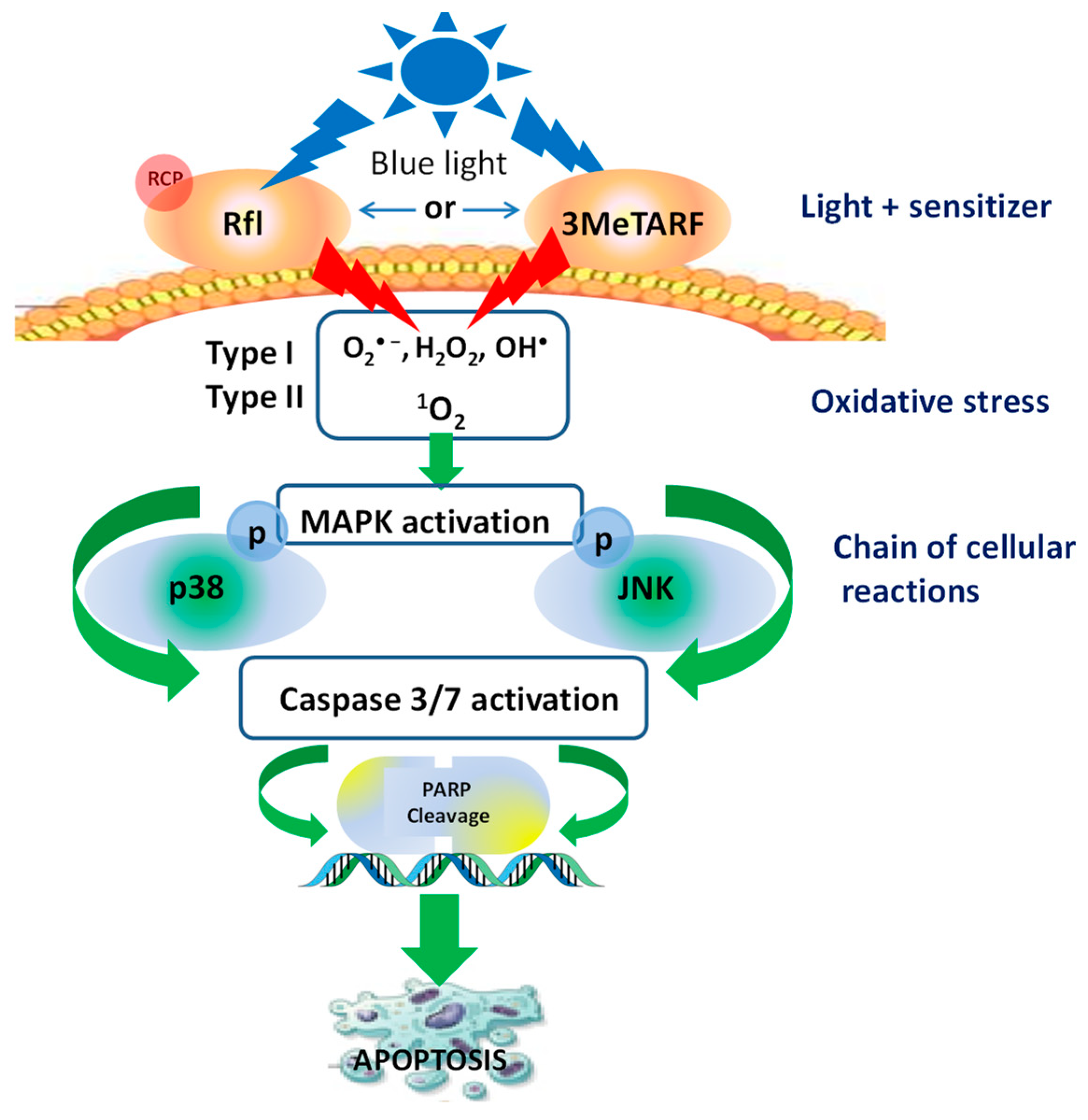
6. Apoptosis as a Mechanism of 3MeTARF-Induced Melanoma and Non-Melanoma Skin Cancer Cell Death
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agostinis, P.; Breyssens, H.; Buytaert, E.; Hendrickx, N. Regulatory pathways in photodynamic therapy induced apoptosis. Photochem. Photobiol. Sci. 2004, 3, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photo-chemistry and cellular localization. Photodiagn. Photodyn. 2004, 1, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Fotodynamiczna Metoda Rozpoznawania i Leczenia Nowotworów; Scientific editoship Alfreda Graczyk; Publishing House Bellona: Warsaw, Poland, 1999.
- Songca, S.P.; Adjei, Y. Applications of antimicrobial photodynamic therapy against bacterial biofilms. Int. J. Mol. Sci. 2022, 23, 3209. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.J.; Jemec, G.B.; Arendrup, M.C.; Saunte, D.M.L. Photodynamic therapy treatment of superficial fungal infections: A systematic review. Photodiagnosis Photodyn. Ther. 2020, 31, 101774. [Google Scholar] [CrossRef]
- Piksa, M.; Lian, C.; Samuel, I.C.; Pawlik, K.J.; Samuel, I.D.W.; Matczyszyn, K. The role of the light source in antimicrobial photodynamic therapy. Chem. Soc. Rev. 2023, 52, 1697–1722. [Google Scholar] [CrossRef]
- Gilaberte, Y.; Rezusta, A.; Juarranz, A.; Hamblin, M.R. Editorial: Antimicrobial photodynamic therapy: A new paradigm in the fight against infections. Front. Med. 2021, 8, 788888. [Google Scholar] [CrossRef]
- Juzeniene, A.; Peng, Q.; Moan, J. Milestones in the development of photodynamic therapy and fluorescence diagnosis. Photochem. Photobiol. Sci. 2007, 6, 1234–1245. [Google Scholar] [CrossRef]
- Mroz, P.; Tegos, G.P.; Gali, H.; Wharton, T.; Sarna, T.; Hamblin, M.R. Photodynamic therapy with fullerenes. Photochem. Photobiol. Sci. 2007, 6, 1139–1149. [Google Scholar] [CrossRef]
- Vatansever, F.; de Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered, around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef]
- Bastos, E.L.; Quina, F.H.; Baptista, M.S. Endogenous photosensitizers in human skin. Chem. Rev. 2023, 123, 9720–9785. [Google Scholar] [CrossRef]
- Iqbal, J.; Husain, A.; Gupta, A. Photooxidation of acyclovir with thermally generated triplet excited ketones. A comparison with Type I and II photosensitizers. Chem. Pharm. Bull. 2006, 54, 519–521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baptista, M.S.; Cadet, J.; Greer, A.; Thomas, A.H. Photosensitization reactions of biomolecules: Definition, targets and mechanisms. Photochem. Photobiol. 2021, 97, 1456–1483. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II photosensitized oxidation reactions: Guidelines and mechanistic pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Min, D.B.; Boff, J.M. Chemistry and reaction of singlet oxygen in foods. Compr. Rev. Food Sci. Food Saf. 2002, 1, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Mang, T.S. Lasers and light sources for PDT: Past, present and future. Photodiagnosis Photodyn. Ther. 2004, 1, 43–48. [Google Scholar] [CrossRef]
- Brancaleon, L.; Moseley, H. Laser and non-laser light sources for photodynamic therapy. Lasers Med. Sci. 2002, 17, 173–186. [Google Scholar] [CrossRef]
- Moan, J. The Photochemical yield of singlet oxygen from porphyrins in different states of aggregation. Photochem. Photobiol. 1984, 39, 445–449. [Google Scholar] [CrossRef]
- Mang, T.S.; Dougherty, T.J.; Potter, W.R.; Boyle, D.G.; Somer, S.; Moan, J. Photobleaching of porphyrins used in photodynamic therapy and implications for therapy. Photochem. Photobiol. 1987, 45, 501–506. [Google Scholar] [CrossRef]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.H.; Childs, C.J.; Sibata, C.H. Photosensitizers in clinical PDT. Photodiagnosis Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M. Antifungal photodynamic therapy. Microbiol. Res. 2008, 163, 1–12. [Google Scholar] [CrossRef]
- Nakamura, S.; Mukai, T.; Senoh, M. Candela-class high-brightness InGaN/AlGaN double-heterostructure blue-light-emitting diodes. Appl. Phys. Lett. 1994, 64, 1687–1689. [Google Scholar] [CrossRef]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist. Updat. 2012, 15, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Visible light-induced killing of bacteria as a function of wavelength: Im-plication for wound healing. Laser Surg. Med. 2010, 42, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl. Environ. Microbiol. 2009, 75, 1932–1937. [Google Scholar] [CrossRef]
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J. Investig. Dermatol. 2010, 130, 259–269. [Google Scholar] [CrossRef]
- Barolet, D. Light-emitting diodes (LEDs) in dermatology. Semin. Cutan. Med. Surg. 2008, 27, 227–238. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Jagdeo, J.; Austin, E.; Mamalis, A.; Wong, C.; Ho, D.; Siegel, D.M. Light-emitting diodes in dermatology: A systematic review of randomized controlled trials MS. Lasers Surg. Med. 2018, 50, 613–628. [Google Scholar] [CrossRef]
- Heelis, P.F. The photophysical and photochemical properties of flavins (isoalloxazines). Chem. Soc. Rev. 1982, 11, 15–39. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, S.; Sheraz, M.A.; Vaid, F.H.; Ansari, I.A. Effect of divalent anions on photodegradation kinetics and pathways of riboflavin in aqueous solution. Int. J. Pharm. 2010, 390, 174–182. [Google Scholar] [CrossRef]
- Insińska-Rak, M.; Golczak, A.; Sikorski, M. Photochemistry of riboflavin derivatives in methanolic solutions. J. Phys. Chem. A 2012, 116, 1199–1207. [Google Scholar] [CrossRef]
- Insińska-Rak, M.; Prukała, D.; Golczak, A.; Fornal, E.; Sikorski, M. Riboflavin degradation products; combined photochemical and mass spectrometry approach. J. Photochem. Photobiol. A Chem. 2020, 403, 112837. [Google Scholar] [CrossRef]
- Wolnicka-Glubisz, A.; Pawlak, A.; Insinska-Rak, M.; Zadlo, A. Analysis of photoreactivity and phototoxicity of riboflavin’s analogue 3MeTARF. J. Photochem. Photobiol. B Biol. 2020, 205, 111820. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Ahmed, S.; Anwar, Z.; Sheraz, M.A.; Sikorski, M. Photostability and photostabilization of drugs and drug products. Int. J. Photoenergy 2016, 2016, 1–19. [Google Scholar] [CrossRef]
- Ribes, J.; Cossard, P.; Al Yaman, K.; Bestel, I.; Badarau, E. Investigating the photosensitization activities of flavins irradiated by blue LEDs. RSC Adv. 2023, 13, 2355–2364. [Google Scholar] [CrossRef]
- Moore, W.M.; Baylor, J. The photochemistry of riboflavin IV. The photobleaching of some nitrogen-9 substituted isoalloxazines and flavins. J. Am. Chem. Soc. 1969, 91, 7170–7179. [Google Scholar] [CrossRef]
- Jorns, M.S.; Schollnhammer, G.; Hemmerich, P. Intramolecular addition of the riboflavin side chain. Anion-catalyzed neutral photochemistry. JBIC J. Biol. Inorg. Chem. 1975, 57, 35–48. [Google Scholar] [CrossRef]
- Larson, R.A.; Stackhouse, P.L.; Crowley, T.O. Riboflavin tetraacetate: A potentially useful photosensitizing agent for the treatment of contaminated waters. Environ. Sci. Technol. 1992, 26, 1792–1798. [Google Scholar] [CrossRef]
- Remucal, C.K.; McNeill, K. Photosensitized amino acid degradation in the presence of riboflavin and its derivatives. Environ. Sci. Technol. 2011, 45, 5230–5237. [Google Scholar] [CrossRef]
- Banekovich, C.; Matuszczak, B. 2′,3′,4′,5′-Tetraacetyl-N(3)-carboxymethylriboflavin derivatives: Synthesis and fluorescence studies. Tetrahedron Lett. 2005, 46, 5053–5056. [Google Scholar] [CrossRef]
- Sikorska, E.; Khmelinskii, I.; Komasa, A.; Koput, J.; Ferreira, L.F.; Herance, J.R.; Bourdelande, J.L.; Williams, S.L.; Worrall, D.R.; Insińska-Rak, M.; et al. Spectroscopy and photophysics of flavin related compounds: Riboflavin and iso-(6,7)-riboflavin. Chem. Phys. 2005, 314, 239–247. [Google Scholar] [CrossRef]
- Silva, A.V.; López-Sánchez, A.; Junqueira, H.C.; Rivas, L.; Baptista, M.S.; Orellana, G. Riboflavin derivatives for enhanced photodynamic activity against Leishmania parasites. Tetrahedron 2015, 71, 457–462. [Google Scholar] [CrossRef]
- Insińska-Rak, M.; Sikorska, E.; Bourdelande, J.L.; Khmelinskii, I.V.; Prukała, W.; Dobek, K.; Karolczak, J.; Machado, I.F.; Fer-reira, L.F.V.; Dulewicz, E. New photochemically stable riboflavin analogue-3-methyl-riboflavin tetraacetate. J. Photochem. Photobiol. A 2007, 186, 14–23. [Google Scholar] [CrossRef]
- Lafaye, C.; Aumonier, S.; Torra, J.; Signor, L.; von Stetten, D.; Noirclerc-Savoye, M.; Shu, X.; Ruiz González, R.; Gotthard, G.; Royant, A.; et al. Ribofavin binding proteins for singlet oxygen production. Photochem. Photobiol. Sci. 2022, 21, 1545–1555. [Google Scholar] [CrossRef]
- Mogensen, D.J.; Etzerodt, M.; Ogilby, P.R. Photoinduced bleaching in an efficient singlet oxygen photosensitizing protein: Identifying a culprit in the flavin-binding LOV-based protein SOPP3. J. Photochem. Photobiol. A Chem. 2022, 429, 113894. [Google Scholar] [CrossRef]
- Westberg, M.; Brenghoj, M.; Etzerodt, M.; Ogilby, P.R. No photon wasted: An efficient and selective singlet oxygen photosen-sitizing protein. J. Phys. Chem. B 2017, 121, 9366–9371. [Google Scholar] [CrossRef]
- Insińska-Rak, M.; Sikorska, E.; Bourdelande, J.L.; Khmelinskii, I.V.; Prukała, W.; Dobek, K.; Karolczak, J.; Machado, I.F.; Ferreira, L.F.; Komasa, A.; et al. Spectroscopy and photophysics of flavin-related compounds: 5-deaza-riboflavin. J. Mol. Struct. 2005, 783, 184–190. [Google Scholar] [CrossRef]
- Sikorska, E.; Khmelinskii, I.V.; Prukała, W.; Williams, S.L.; Patel, M.; Worrall, D.R.; Bourdelande, J.L.; Koput, J.; Sikorski, M. Spectroscopy and photophysics of lumiflavins and lumichromes. J. Phys. Chem. A 2004, 108, 1501–1508. [Google Scholar] [CrossRef]
- Zirak, P.; Penzkofer, A.; Mathes, T.; Hegemann, P. Absorption and emission spectroscopic characterization of BLUF protein Slr1694 from Synechocystis sp. PCC6803 with roseoflavin cofactor. J. Photochem. Photobiol. B Biol. 2009, 97, 61–70. [Google Scholar] [CrossRef]
- Chacon, J.N.; McLearie, J.; Sinclair, R.S. Singlet oxygen yields and radical contributions in the dye- sensitized photo-oxidation in methanol of esters of poly- unsaturated fatty-acids (oleic, linoleic, linolenic and archidonic). Photochem. Photobiol. 1988, 47, 647–656. [Google Scholar] [CrossRef]
- Moore, W.M.; McDaniels, J.C.; Hen, J.A. The photochemistry of riboflavin—VI. The photophysical properties of isoalloxazines. Photochem. Photobiol. 1977, 25, 505–512. [Google Scholar] [CrossRef]
- Holzer, W.; Penzkofer, A.; Hegemann, P. Absorption and emission spectroscopic characterisation of the LOV2-His domain of phot from Chlamydomonas reinhardtii. Chem. Phys. 2005, 308, 79–91. [Google Scholar] [CrossRef]
- Bertolotti, S.G.; Previtali, C.M.; Rufs, A.M.; Encinas, M.V. Riboflavin triethanolamine as photoinitiator system of vinyl polymerization. A mechanistic study by laser flash photolysis. Macromolecules 1999, 32, 2920–2924. [Google Scholar] [CrossRef]
- Sikorski, M.; Sikorska, E.; Koziolowa, A.; Moreno, R.G.; Bourdelande, J.; Steer, R.; Wilkinson, F. Photophysical properties of lumichromes in water. J. Photochem. Photobiol. B Biol. 2001, 60, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The health benefits of a forgotten natural vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Singh, P.K.; Srivastava, A.; Singh, P.P. Synthetic applications of flavin photocatalysis: A review. RSC Adv. 2021, 11, 14251–14259. [Google Scholar] [CrossRef]
- Lindén, A.A.; Krüger, L.; Bäckvall, J.-E. Highly selective sulfoxidation of allylic and vinylic sulfides by hydrogen peroxide using a flavin as catalyst. J. Org. Chem. 2003, 68, 5890–5896. [Google Scholar] [CrossRef]
- Cui, H.; Hwang, H.-M.; Cook, S.; Zeng, K. Effect of photosensitizer riboflavin on the fate of 2,4,6-trinitrotoluene in a freshwater environment. Chemosphere 2001, 44, 621–625. [Google Scholar] [CrossRef]
- Lu, C.Y.; Han, Z.H.; Liu, G.S.; Cai, X.C.; Chen, Y.L.; Yao, S. Photophysical and photochemical processes of riboflavin (vitamin B2) by means of the transient absorption spectra in aqueous solution. Sci. China Ser. B Chem. 2001, 44, 39–48. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Lin, W.-Z.; Wang, W.-F.; Han, Z.-H.; Zheng, Z.-D.; Yao, S.-D. Kinetic observation of rapid electron transfer between pyrimidine electron adducts and sensitizers of riboflavin, flavin adenine dinucleotide (FAD) and chloranil: A pulse radiolysis study. Radiat. Phys. Chem. 2000, 59, 61–66. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Wang, W.-F.; Lin, W.-Z.; Han, Z.-H.; Yao, S.-D.; Lin, N.-Y. Generation and photosensitization properties of the oxidized radical of riboflavin: A laser flash photolysis study. J. Photochem. Photobiol. B Biol. 1999, 52, 111–116. [Google Scholar] [CrossRef]
- Cardoso, D.R.; Libardi, S.H.; Skibsted, L.H. Riboflavin as a photosensitizer. Effects on human health and food quality. Food Funct. 2012, 3, 487–502. [Google Scholar] [CrossRef] [PubMed]
- King, J.M.; Min, D.B. Riboflavin-photosensitized singlet oxygen oxidation product of vitamin D2. J. Am. Oil Chem. Soc. 2002, 79, 983–987. [Google Scholar] [CrossRef]
- King, J.M.; Min, D.B. Riboflavin photosensitized singlet oxygen oxidation of vitamin D. J. Food Sci. 1998, 63, 31–34. [Google Scholar] [CrossRef]
- Grininger, M.; Staudt, H.; Johansson, P.; Wachtveitl, J.; Oesterhelt, D. Dodecin is the key player in flavin homeostasis of archaea. Pediatrics 2009, 284, 13068–13076. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, M.; Khmelinskii, I.; Sikorska, E. Spectral Properties of flavins. In Flavin-Based Catalysis; Cibulka, R., Fraaije, M., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2021; pp. 67–96. [Google Scholar]
- Climent, T.; Gonzalez-Luque, R.; Merchan, M.; Serrano-Andres, L. Theoretical insight into the spectroscopy and photochem-istry of isoalloxazine, the flavin core ring. J. Phys. Chem. A 2006, 110, 13584–13590. [Google Scholar] [CrossRef]
- Prukała, D.; Gierszewski, M.; Pędziński, T.; Sikorski, M. Influence of pH on spectral and photophysical properties of 9-methyl-5-deazaalloxazine and 10-ethyl-5-deaza-isoalloxazine. J. Photochem. Photobiol. A Chem. 2014, 275, 12–20. [Google Scholar] [CrossRef]
- Senda, T.; Senda, M.; Kimura, S.; Ishida, T. Redox control of protein conformation in flavoproteins. Antioxid. Redox Signal. 2009, 11, 1741–1766. [Google Scholar] [CrossRef]
- Kao, Y.-T.; Saxena, C.; He, T.-F.; Guo, L.; Wang, L.; Sancar, A.; Zhong, D. Ultrafast dynamics of flavins in five redox states. J. Am. Chem. Soc. 2008, 130, 13132–13139. [Google Scholar] [CrossRef]
- McBride, R.A.; Barnard, D.T.; Jacoby-Morris, K.; Harun-Or-Rashid, M.; Stanley, J. Reduced flavin in aqueous solution is non-fluorescent. Biochemistry 2023, 62, 759–769. [Google Scholar] [CrossRef]
- Visser, A.J.W.G.; Ghisla, S.; Massey, V.; Muller, F.; Veeger, C. Fluorescence properties of reduced flavins and flavoproteins. JBIC J. Biol. Inorg. Chem. 1979, 101, 13–21. [Google Scholar] [CrossRef]
- Zhuang, B.; Aleksandrov, A.; Seo, D.; Vos, M.H. Excited-state properties of fully reduced flavins in ferredoxin–NADP+ oxi-doreductase. J. Phys. Chem. Lett. 2023, 14, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Chang, K.-C.; Chen, L.-Y.; Hu, A. Low-dose blue light irradiation enhances the antimicrobial activities of cur-cumin against Propionibacterium acnes. J. Photochem. Photobiol. B 2018, 189, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wolnicka-Glubisz, A.; Wisniewska-Becker, A. Dual action of curcumin as an anti- and pro-oxidant from a biophysical perspective. Antioxidants 2023, 12, 1725. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, E.; Weng, Q.; Zhou, L.; Li, Q. Optimization of hydrogel containing toluidine blue O for photodynamic therapy in treating acne. Lasers Med. Sci. 2019, 34, 1535–1545. [Google Scholar] [CrossRef]
- Wangsuwan, S.; Meephansan, J. Comparative study of photodynamic therapy with riboflavin-tryptophan Gel and 13% 5-Aminolevulinic acid in the treatment of mild to moderate acne vulgaris. Clin. Cosmet. Investig. Dermatol. 2019, 12, 805–814. [Google Scholar] [CrossRef]
- Corbin, F. Pathogen inactivation of blood components: Current status and introduction of an approach using riboflavin as a photosensitizer. Int. J. Hematol. 2002, 76, 253–257. [Google Scholar] [CrossRef]
- Reddy, H.L.; Dayan, A.D.; Cavagnaro, J.; Gad, S.; Li, J.; Goodrich, R.P. Toxicity testing of a novel riboflavin-based technology for pathogen reduction and white blood cell inactivation. Transfus. Med. Rev. 2008, 22, 133–153. [Google Scholar] [CrossRef]
- Balint, B.; Jovicic-Gojkov, D.; Todorovic-Balint, M.; Subota, V.; Pavlovic, M.; Goodrich, R. Plasma constituent integrity in pre-storage vs. post-storage riboflavin and UV-light treatment—A comparative study. Transf. Apher. Sci. 2013, 49, 434–443. [Google Scholar] [CrossRef]
- Hornsey, V.S.; Drummond, O.; Morrison, A.; McMillan, L.; MacGregor, I.R.; Prowse, C.V. Pathogen reduction of fresh plasma using riboflavin and ultraviolet light: Effects on plasma coagulation proteins. Transfusion 2009, 49, 2167–2172. [Google Scholar] [CrossRef]
- Smith, J.; Rock, G. Protein quality in Mirasol pathogen reduction technology–treated, apheresis-derived fresh-frozen plasma. Transfusion 2010, 50, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Farah, N.; Chin, V.K.; Chong, P.P.; Lim, W.F.; Lim, C.W.; Basir, R.; Chang, S.K.; Lee, T.Y. Riboflavin as a promising antimicrobial agent? A multi-perspective review. Curr. Res. Microb. Sci. 2022, 3, 100111. [Google Scholar] [CrossRef] [PubMed]
- Buninowska, I.; Wroński, N.; Insińska-Rak, M.; Sikorski, M.; Wolnicka-Glubisz, A. Tetraacetyl riboflavin derivative mediates caspase 3/7 activation via MAPK in A431 cells upon blue light influence. Photochem. Photobiol. 2023. [Google Scholar] [CrossRef]
- Akasov, R.A.; Sholina, N.V.; Khochenkov, D.A.; Alova, A.; Gorelkin, P.V.; Erofeev, A.S.; Generalova, A.N.; Khaydukov, E.V. Photodynamic therapy of melanoma by blue-light photoactivation of flavin mononucleotide. Sci. Rep. 2019, 9, 9679. [Google Scholar] [CrossRef]
- Bergh, V.J.V.; Bruzell, E.; Hegge, A.B.; Tønnesen, H.H. Influence of formulation on photoinactivation of bacteria by lumichrome. Die Pharm. 2015, 70. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, L.; He, C.; Tai, S.; Zhu, L.; Ma, C.; Yang, T.; Cheng, F.; Sun, X.; Cui, R.; et al. An experimental study on riboflavin photosensitization treatment for inactivation of circulating HCT116 tumor cells. J. Photochem. Photobiol. B 2019, 196, 111496. [Google Scholar] [CrossRef]
- Salman, M.; Naseem, I. Riboflavin as adjuvant with cisplatin: Study in mouse skin cancer model. Front. Biosci. 2015, 7, 242–254. [Google Scholar] [CrossRef]
- Yang, M.-Y.; Chang, C.-J.; Chen, L.-Y. Blue light induced reactive oxygen species from flavin mononucleotide and flavin ad-enine dinucleotide on lethality of HeLa cells. J. Photochem. Photobiol. B 2017, 173, 325–332. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Cives, M.; Mannavola, F.; Lospalluti, L.; Sergi, M.C.; Cazzato, G.; Filoni, E.; Cavallo, F.; Giudice, G.; Stucci, L.S.; Porta, C.; et al. Non-melanoma skin cancers: Biological and clinical features. Int. J. Mol. Sci. 2020, 21, 5394. [Google Scholar] [CrossRef]
- Juarez, A.V.; Sosa, L.d.V.; De Paul, A.L.; Costa, A.P.; Farina, M.; Leal, R.B.; Torres, A.I.; Pons, P. Riboflavin acetate induces apoptosis in squamous carcinoma cells after photodynamic therapy. J. Photochem. Photobiol. B Biol. 2015, 153, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, B. CRC Handbook of Food Additives; Borenstein, B., Ed.; The Chemical Rubber Co.: Boca Raton, FL, USA; Washington, DC, USA, 1972; pp. 85–111. [Google Scholar]
- Bartmann, L.; Schumacher, D.; von Stillfried, S.; Sternkopf, M.; Alampour-Rajabi, S.; van Zandvoort, M.A.M.J.; Kiessling, F.; Wu, Z. Evaluation of riboflavin transporters as targets for drug delivery and theranostics. Front. Pharmacol. 2019, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Peechakara, B.V.; Gupta, M. Vitamin B2 (Riboflavin). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023, bookshelf ID: NBK525977. National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK525977/ (accessed on 28 August 2023).
- Muñoz, M.A.; Pacheco, A.; Becker, M.I.; Silva, E.; Ebensperger, R.; Garcia, A.M.; De Ioannes, A.E.; Edwards, A.M. Different cell death mechanisms are induced by a hydrophobic flavin in human tumor cells after visible light irradiation. J. Photochem. Photobiol. B Biol. 2011, 103, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Crocker, L.B.; Lee, J.H.; Mital, S.; Mills, G.C.; Schack, S.; Bistrović-Popov, A.; Franck, C.O.; Mela, I.; Kaminski, C.F.; Christie, G.; et al. Tuning riboflavin derivatives for photodynamic inactivation of pathogens. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arbiser, J.L.; Bonner, M.Y.; Gilbert, L.C. Targeting the duality of cancer. Npj Precis. Oncol. 2017, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Lossi, L. The concept of intrinsic versus extrinsic apoptosis. Biochem. J. 2022, 479, 357–384. [Google Scholar] [CrossRef]
- Hu, H.; Xu, F.; Yang, W.; Ren, J.; Ge, W.; Yang, P. Apoptosis as an underlying mechanism in lymphocytes induced by riboflavin and ultraviolet light. Transfus. Apher. Sci. 2020, 59, 102899. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Kirkin, V.; Joos, S.; Zörnig, M. The role of Bcl-2 family members in tumorigenesis. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2004, 1644, 229–249. [Google Scholar] [CrossRef]
- Ahmad, I.; Fasiliullah, Q.; Vaid, F.H.M. A study of simultaneous photolysis and photoaddition reactions of riboflavin in aqueous solution. J. Photochem. Photobiol. B 2004, 75, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Niemz, A.; Imbriglio, J.; Rotello, V.M. Model systems for flavoenzyme activity: One- and two-electron reduction of flavins in aprotic hydrophobic environments. J. Am. Chem. Soc. 1997, 119, 887–892. [Google Scholar] [CrossRef]
- Grajek, H.; Żurkowska, G.; Kuśba, J. Influence of diffusion on nonradiative energy transfer between FMN molecules in aqueous solutions. J. Photochem. Photobiol. B Biol. 2005, 80, 145–155. [Google Scholar] [CrossRef] [PubMed]
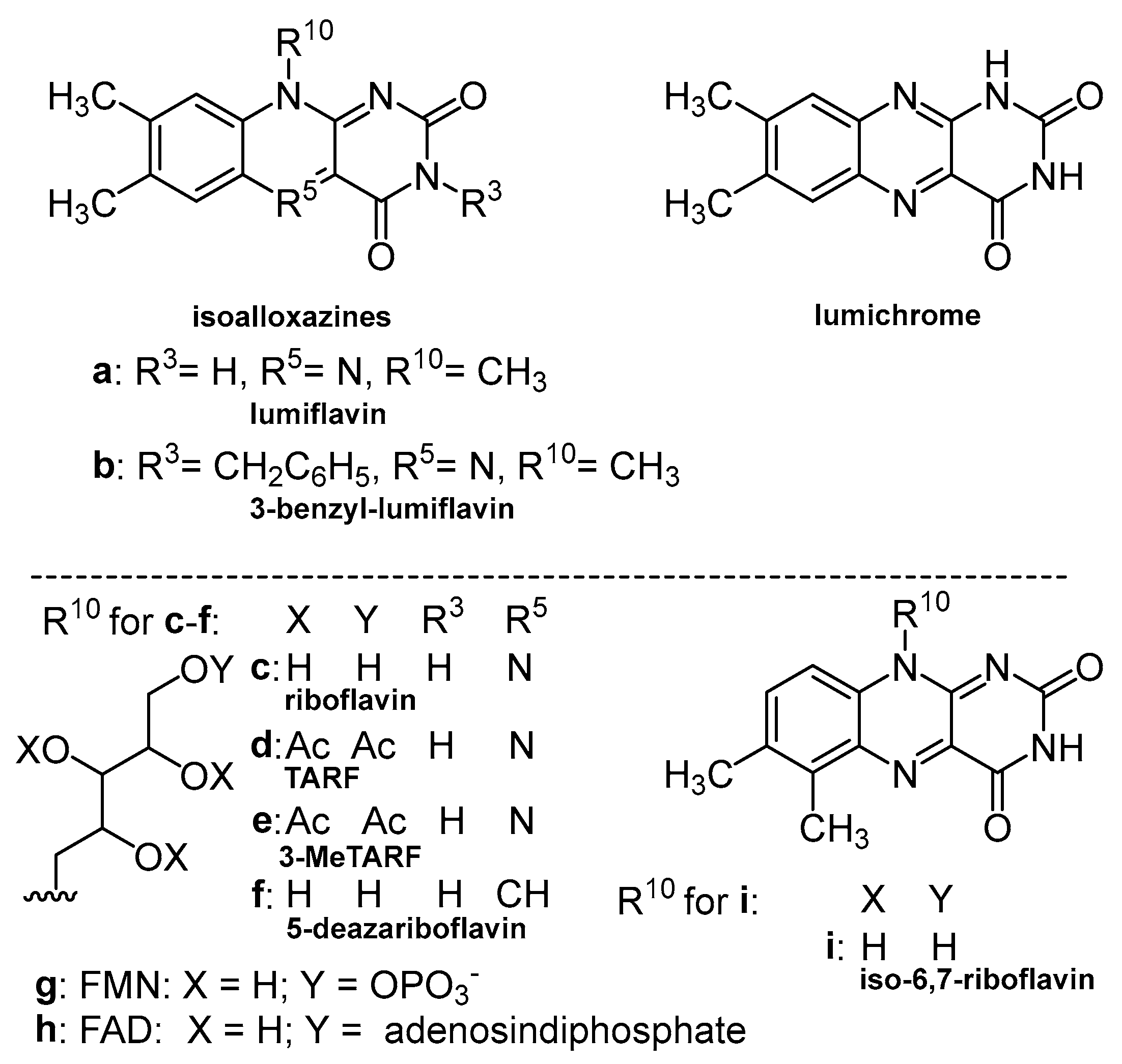
| Sensitizer | λA [nm] | λF [nm] | ΦF | τF [ns] | ΦISC | ΦΔ |
|---|---|---|---|---|---|---|
| RiboflavinMeOH [a] | 444 | 532 | 0.39 | 6.4/5.4 [k] | 0.6 [l] | 0.51 |
| Riboflavinwater [b] | 444 | 537 | 0.28 | 5.1 | 0.7 [m] | 0.48 [l] |
| TARFMeOH [c] | 446 | 525 | 0.46 | - | - | - |
| TARFwater [b] | 446 | - | - | - | 0.66 | |
| 3MeTARFMeOH [d] | 448 | 513 | 0.089/0.12 [c] | 5.4 | - | 0.61/0.46 [e] |
| 3MeTARFwater [d] | 451 | 520 | 0.11 | 4.4 | - | - |
| 3MeTARFPBS/DMSO [e] | 450 | - | - | - | - | 0.49 |
| FMNTRIS(d) [f] | - | - | - | - | - | 0.57 |
| FMNwater [g] | - | - | 0.23 [n] | 5.1 [n] | - | 0.59 |
| miniSOG [FMN]TRIS(d) [f] | - | - | 0.4 | 5.5 | 0.6 | 0.04 |
| miniSOG [Rfl]TRIS(d) [f] | - | - | - | - | - | 0.10 |
| SOPP3 [h] | - | - | - | - | - | 0.61D2O |
| Iso-6,7-riboflavinMeOH [a] | 447 | 552 | 0.20 | 4.2 | - | 0.70 |
| 5-deazariboflavinMeOH [i] | 400 | 455 | 0.11 | 3.98 | - | 0.33 |
| LumichromeMeOH [j] | 384 | 453 | 0.032 | 1.04 [o] | - | 0.85 |
| Lumichromewater [j] | 385 | 479 | 0.088 | 2.7 | 0.63 [b]/0.69 [p] | 0.36D2O [p] |
| [Ref.]: [a] [42]; [b] [40]; [c] [43]; [d] [44]; [e] [34]; [f] [45]; [g] [46]; [h] [47]; [i] [48]; [j] [49]; [k] [50]; [l] [51]; [m] [52]; [n] [53]; [o] [54]; [p] [55]. | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Insińska-Rak, M.; Sikorski, M.; Wolnicka-Glubisz, A. Riboflavin and Its Derivates as Potential Photosensitizers in the Photodynamic Treatment of Skin Cancers. Cells 2023, 12, 2304. https://doi.org/10.3390/cells12182304
Insińska-Rak M, Sikorski M, Wolnicka-Glubisz A. Riboflavin and Its Derivates as Potential Photosensitizers in the Photodynamic Treatment of Skin Cancers. Cells. 2023; 12(18):2304. https://doi.org/10.3390/cells12182304
Chicago/Turabian StyleInsińska-Rak, Małgorzata, Marek Sikorski, and Agnieszka Wolnicka-Glubisz. 2023. "Riboflavin and Its Derivates as Potential Photosensitizers in the Photodynamic Treatment of Skin Cancers" Cells 12, no. 18: 2304. https://doi.org/10.3390/cells12182304
APA StyleInsińska-Rak, M., Sikorski, M., & Wolnicka-Glubisz, A. (2023). Riboflavin and Its Derivates as Potential Photosensitizers in the Photodynamic Treatment of Skin Cancers. Cells, 12(18), 2304. https://doi.org/10.3390/cells12182304








