Immunity against Non-Melanoma Skin Cancer and the Effect of Immunosuppressive Medication on Non-Melanoma Skin Cancer Risk in Solid Organ Transplant Recipients
Abstract
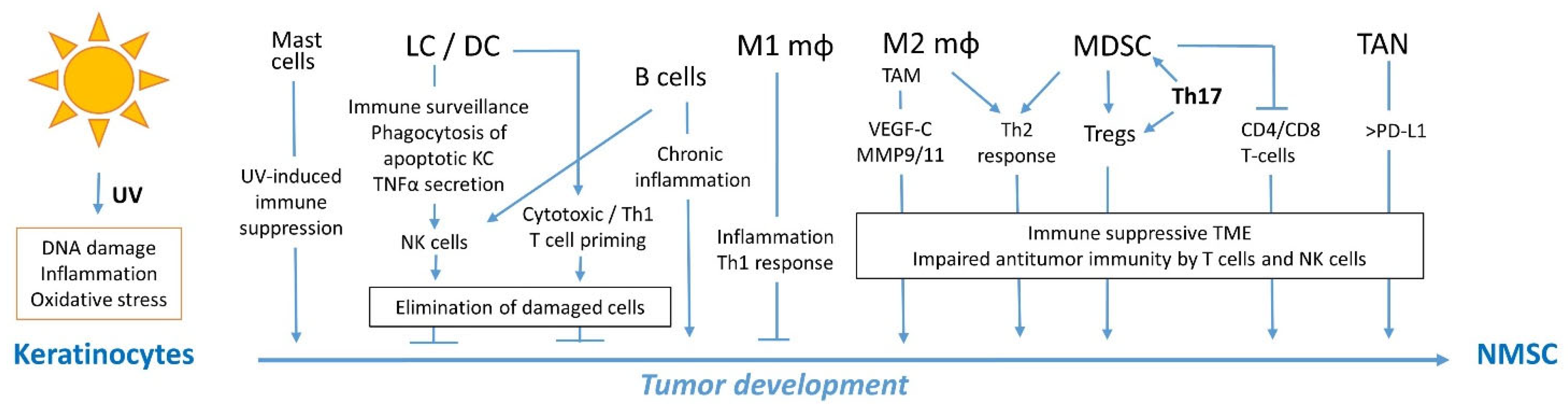
1. Introduction
2. Immunity against NMSC
2.1. Innate Immunity against NMSC
2.1.1. Dendritic Cells
2.1.2. Macrophages
2.1.3. Myeloid-Derived Suppressor Cells
2.1.4. Neutrophils
2.1.5. Mast Cells
2.1.6. Innate lymphoid Cells and Natural Killer Cells
2.1.7. NKT Cells and γδ T Cells
2.2. Adaptive Immunity against NMSC
2.2.1. T Cell Responses
2.2.2. B Cell Responses
2.3. Increased Risk of Non-Melanoma Skin Cancer (NMSC) in Solid Organ Transplant Recipients (SOTRs)
2.3.1. Calcineurin Inhibitors
2.3.2. Purine Analogues
2.3.3. mTOR Inhibitors
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cives, M.; Mannavola, F.; Lospalluti, L.; Sergi, M.C.; Cazzato, G.; Filoni, E.; Cavallo, F.; Giudice, G.; Stucci, L.S.; Porta, C.; et al. Non-Melanoma Skin Cancers: Biological and Clinical Features. Int. J. Mol. Sci. 2020, 21, 5394. [Google Scholar] [CrossRef] [PubMed]
- Coghill, A.E.; Johnson, L.G.; Berg, D.; Resler, A.J.; Leca, N.; Madeleine, M.M. Immunosuppressive Medications and Squamous Cell Skin Carcinoma: Nested Case-Control Study Within the Skin Cancer after Organ Transplant (SCOT) Cohort. Am. J. Transpl. 2016, 16, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shu, G.; Wang, S. The risk of non-melanoma skin cancer in HIV-infected patients: New data and meta-analysis. Int. J. STD AIDS 2016, 27, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, H.M.; Cherikh, W.S.; Cheng, Y.; Hanto, D.W.; Kahan, B.D. Maintenance Immunosuppression with Target-of-Rapamycin Inhibitors is Associated with a Reduced Incidence of De Novo Malignancies. Transplantation 2005, 80, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Yanofsky, V.R.; Mitsui, H.; Felsen, D.; Carucci, J.A. Understanding Dendritic Cells and Their Role in Cutaneous Carcinoma and Cancer Immunotherapy. Clin. Dev. Immunol. 2013, 2013, 624123. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, S.; Kocyigit, P.; Alp, A.; Erdem, C.; Gürgey, E. Treatment of Basal Cell Carcinoma Located in the Head and Neck Region with Intralesional Interferon α-2: Evaluation of Long-Term Follow-Up Results. Clin. Drug Investig. 2005, 25, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Yavel, R.M.; Gross, V.L.; Brody, N. Intralesional interferon α-2b in the treatment of basal cell carcinoma and squamous cell carcinoma: Revisited. Dermatol. Surg. 2004, 30, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Clifford, J.L.; Walch, E.; Yang, X.; Xu, X.; Alberts, D.S.; Clayman, G.L.; El-Naggar, A.K.; Lotan, R.; Lippman, S.M. Suppression of Type I Interferon Signaling Proteins Is an Early Event in Squamous Skin Carcinogenesis. Clin. Cancer Res. 2002, 8, 2067–2072. [Google Scholar]
- Kim, Y.; He, Y.Y. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M.; Fukunaga, A.; Washio, K.; Taguchi, K.; Oda, Y.; Ogura, K.; Nishigori, C. Anti-Inflammatory Role of Langerhans Cells and Apoptotic Keratinocytes in Ultraviolet-B-Induced Cutaneous Inflammation. J. Immunol. 2017, 199, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Ortner, D.; Tripp, C.H.; Komenda, K.; Dubrac, S.; Zelger, B.; Hermann, M.; Doppler, W.; Tymoszuk, P.Z.; Boon, L.; Clausen, B.E.; et al. Langerhans cells and NK cells cooperate in the inhibition of chemical skin carcinogenesis. Oncoimmunology 2017, 6, e1260215. [Google Scholar] [CrossRef] [PubMed]
- Furio, L.; Berthier-Vergnes, O.; Ducarre, B.; Schmitt, D.; Peguet-Navarro, J. UVA radiation impairs phenotypic and functional maturation of human dermal dendritic cells. J. Investig. Dermatol. 2005, 125, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Bluth, M.J.; Zaba, L.C.; Moussai, D.; Suarez-Farinas, M.; Kaporis, H.; Fan, L.; Pierson, K.C.; White, T.R.; Pitts-Kiefer, A.; Fuentes-Duculan, J.; et al. Myeloid dendritic cells from human cutaneous squamous cell carcinoma are poor stimulators of T-cell proliferation. J. Investig. Dermatol. 2009, 129, 2451–2462. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, S.R.; Tampa, M.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Caruntu, A.; Lupu, M.; Matei, C.; Constantin, C.; Neagu, M. Tumour Microenvironment in Skin Carcinogenesis. Adv. Exp. Med. Biol. 2020, 1226, 123–142. [Google Scholar] [CrossRef]
- Jensen, T.O.; Schmidt, H.; Møller, H.J.; Høyer, M.; Maniecki, M.B.; Sjoegren, P.; Christensen, I.J.; Steiniche, T. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J. Clin. Oncol. 2009, 27, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, S.; Yasuda, K.; Suzuki, K.; Tahara, K.; Higashi, H.; Era, S. Macrophage infiltration and its prognostic implications in breast cancer: The relationship with VEGF expression and microvessel density. Oncol. Rep. 2005, 14, 425–431. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, J.S.; Fuentes-Duculan, J.; Suárez-Fariñas, M.; Pierson, K.C.; Pitts-Kiefer, A.; Fan, L.; Belkin, D.A.; Wang, C.Q.; Bhuvanendran, S.; Johnson-Huang, L.M.; et al. Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated. J. Investig. Dermatol. 2011, 131, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Moussai, D.; Mitsui, H.; Pettersen, J.S.; Pierson, K.C.; Shah, K.R.; Suárez-Fariñas, M.; Cardinale, I.R.; Bluth, M.J.; Krueger, J.G.; Carucci, J.A. The human cutaneous squamous cell carcinoma microenvironment is characterized by increased lymphatic density and enhanced expression of macrophage-derived VEGF-C. J. Investig. Dermatol. 2011, 131, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Dollinger, E.; Bergman, D.; Zhou, P.; Atwood, S.X.; Nie, Q. Divergent Resistance Mechanisms to Immunotherapy Explain Responses in Different Skin Cancers. Cancers 2020, 12, 2946. [Google Scholar] [CrossRef]
- Saeidi, V.; Doudican, N.; Carucci, J.A. Understanding the squamous cell carcinoma immune microenvironment. Front. Immunol. 2023, 14, 1084873. [Google Scholar] [CrossRef]
- Katoh, M. Genomic testing, tumor microenvironment and targeted therapy of Hedgehog-related human cancers. Clin. Sci. 2019, 133, 953–970. [Google Scholar] [CrossRef] [PubMed]
- Weide, B.; Martens, A.; Zelba, H.; Stutz, C.; Derhovanessian, E.; Di Giacomo, A.M.; Maio, M.; Sucker, A.; Schilling, B.; Schadendorf, D.; et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: Comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin. Cancer Res. 2014, 20, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Sarhan, D.; Steven, A.; Seliger, B.; Kiessling, R.; Lundqvist, A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin. Cancer Res. 2014, 20, 4096–4106. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.Y.; Ma, G.; Weber, K.J.; Ozao-Choy, J.; Wang, G.; Yin, B.; Divino, C.M.; Chen, S.H. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010, 70, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Shan, F.; Qu, N.; Huang, H.; Handley, M.; Griffin, N.; Zhang, S.; Cao, X. Regulatory role of methionine enkephalin in myeloid-derived suppressor cells and macrophages in human cutaneous squamous cell carcinoma. Int. Immunopharmacol. 2021, 99, 107996. [Google Scholar] [CrossRef]
- Seddon, A.; Hock, B.; Miller, A.; Frei, L.; Pearson, J.; McKenzie, J.; Simcock, J.; Currie, M. Cutaneous squamous cell carcinomas with markers of increased metastatic risk are associated with elevated numbers of neutrophils and/or granulocytic myeloid derived suppressor cells. J. Dermatol. Sci. 2016, 83, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. J. Immunol. Res. 2015, 2015, 983698. [Google Scholar] [CrossRef] [PubMed]
- Khou, S.; Popa, A.; Luci, C.; Bihl, F.; Meghraoui-Kheddar, A.; Bourdely, P.; Salavagione, E.; Cosson, E.; Rubod, A.; Cazareth, J.; et al. Tumor-Associated Neutrophils Dampen Adaptive Immunity and Promote Cutaneous Squamous Cell Carcinoma Development. Cancers 2020, 12, 1860. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Grimbaldeston, M.A.; Swift, G.J.; Jaksic, A.; Noonan, F.P.; Finlay-Jones, J.J. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J. Exp. Med. 1998, 187, 2045–2053. [Google Scholar] [CrossRef]
- Grimbaldeston, M.A.; Skov, L.; Baadsgaard, O.; Skov, B.G.; Marshman, G.; Finlay-Jones, J.J.; Hart, P.H. Communications: High dermal mast cell prevalence is a predisposing factor for basal cell carcinoma in humans. J. Investig. Dermatol. 2000, 115, 317–320. [Google Scholar] [CrossRef]
- Wu, S.Y.; Fu, T.; Jiang, Y.Z.; Shao, Z.M. Natural killer cells in cancer biology and therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef]
- Wagner, M.; Koyasu, S. Cancer Immunoediting by Innate Lymphoid Cells. Trends Immunol. 2019, 40, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Luci, C.; Bihl, F.; Bourdely, P.; Khou, S.; Popa, A.; Meghraoui-Kheddar, A.; Vermeulen, O.; Elaldi, R.; Poissonnet, G.; Sudaka, A.; et al. Cutaneous Squamous Cell Carcinoma Development Is Associated with a Temporal Infiltration of ILC1 and NK Cells with Immune Dysfunctions. J. Investig. Dermatol. 2021, 141, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Amôr, N.G.; de Oliveira, C.E.; Gasparoto, T.H.; Vilas Boas, V.G.; Perri, G.; Kaneno, R.; Lara, V.S.; Garlet, G.P.; da Silva, J.S.; Martins, G.A.; et al. ST2/IL-33 signaling promotes malignant development of experimental squamous cell carcinoma by decreasing NK cells cytotoxicity and modulating the intratumoral cell infiltrate. Oncotarget 2018, 9, 30894–30904. [Google Scholar] [CrossRef]
- Hope, C.M.; Troelnikov, A.; Hanf, W.; Jesudason, S.; Coates, P.T.; Heeger, P.S.; Carroll, R.P. Peripheral natural killer cell and allo-stimulated T-cell function in kidney transplant recipients associate with cancer risk and immunosuppression-related complications. Kidney Int. 2015, 88, 1374–1382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Devillier, R.; Chrétien, A.S.; Pagliardini, T.; Salem, N.; Blaise, D.; Olive, D. Mechanisms of NK cell dysfunction in the tumor microenvironment and current clinical approaches to harness NK cell potential for immunotherapy. J. Leukoc. Biol. 2021, 109, 1071–1088. [Google Scholar] [CrossRef]
- McKee, S.J.; Mattarollo, S.R.; Leggatt, G.R. Immunosuppressive roles of natural killer T (NKT) cells in the skin. J. Leukoc. Biol. 2014, 96, 49–54. [Google Scholar] [CrossRef]
- Girardi, M.; Oppenheim, D.E.; Steele, C.R.; Lewis, J.M.; Glusac, E.; Filler, R.; Hobby, P.; Sutton, B.; Tigelaar, R.E.; Hayday, A.C. Regulation of cutaneous malignancy by γδ T cells. Science 2001, 294, 605–609. [Google Scholar] [CrossRef]
- Pardo, J.; Aguilo, J.I.; Anel, A.; Martin, P.; Joeckel, L.; Borner, C.; Wallich, R.; Müllbacher, A.; Froelich, C.J.; Simon, M.M. The biology of cytotoxic cell granule exocytosis pathway: Granzymes have evolved to induce cell death and inflammation. Microbes Infect. 2009, 11, 452–459. [Google Scholar] [CrossRef]
- Nasti, T.H.; Iqbal, O.; Tamimi, I.A.; Geise, J.T.; Katiyar, S.K.; Yusuf, N. Differential roles of T-cell subsets in regulation of ultraviolet radiation induced cutaneous photocarcinogenesis. Photochem. Photobiol. 2011, 87, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Veitch, M.; Kelly, G.A.; Tuong, Z.K.; Cruz, J.G.; Frazer, I.H.; Wells, J.W. IFN-γ Critically Enables the Intratumoural Infiltration of CXCR3+ CD8+ T Cells to Drive Squamous Cell Carcinoma Regression. Cancers 2021, 13, 2131. [Google Scholar] [CrossRef] [PubMed]
- Linedale, R.; Schmidt, C.; King, B.T.; Ganko, A.G.; Simpson, F.; Panizza, B.J.; Leggatt, G.R. Elevated frequencies of CD8 T cells expressing PD-1, CTLA-4 and Tim-3 within tumour from perineural squamous cell carcinoma patients. PLoS ONE 2017, 12, e0175755. [Google Scholar] [CrossRef]
- Sen, D.R.; Kaminski, J.; Barnitz, R.A.; Kurachi, M.; Gerdemann, U.; Yates, K.B.; Tsao, H.W.; Godec, J.; LaFleur, M.W.; Brown, F.D.; et al. The epigenetic landscape of T cell exhaustion. Science 2016, 354, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Beksaç, B.; İlter, N.; Erdem, Ö.; Çakmak, P.; Çenetoğlu, S.; Yapar, D. Sparsity of dendritic cells and cytotoxic T cells in tumor microenvironment may lead to recurrence in basal cell carcinoma. Int. J. Dermatol. 2020, 59, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.L.; Sharman, A.R.; Allen, R.O.; Ye, T.; Lee, J.H.; Low, T.H.; Ch’ng, S.; Palme, C.E.; Ashford, B.; Ranson, M.; et al. High-Dimensional and Spatial Analysis Reveals Immune Landscape-Dependent Progression in Cutaneous Squamous Cell Carcinoma. Clin. Cancer Res. 2022, 28, 4677–4688. [Google Scholar] [CrossRef]
- Kosmidis, M.; Dziunycz, P.; Suarez-Farinas, M.; Muhleisen, B.; Scharer, L.; Lauchli, S.; Hafner, J.; French, L.E.; Schmidt-Weber, C.; Carucci, J.A.; et al. Immunosuppression affects CD4+ mRNA expression and induces Th2 dominance in the microenvironment of cutaneous squamous cell carcinoma in organ transplant recipients. J. Immunother. 2010, 33, 538–546. [Google Scholar] [CrossRef]
- Wu, L.; Chen, X.; Zhao, J.; Martin, B.; Zepp, J.A.; Ko, J.S.; Gu, C.; Cai, G.; Ouyang, W.; Sen, G.; et al. A novel IL-17 signaling pathway controlling keratinocyte proliferation and tumorigenesis via the TRAF4-ERK5 axis. J. Exp. Med. 2015, 212, 1571–1587. [Google Scholar] [CrossRef]
- Li, H.; Prasad, R.; Katiyar, S.K.; Yusuf, N.; Elmets, C.A.; Xu, H. Interleukin-17 mediated inflammatory responses are required for ultraviolet radiation-induced immune suppression. Photochem. Photobiol. 2015, 91, 235–241. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Targeting Treg cells in cancer immunotherapy. Eur. J. Immunol. 2019, 49, 1140–1146. [Google Scholar] [CrossRef]
- Loser, K.; Apelt, J.; Voskort, M.; Mohaupt, M.; Balkow, S.; Schwarz, T.; Grabbe, S.; Beissert, S. IL-10 controls ultraviolet-induced carcinogenesis in mice. J. Immunol. 2007, 179, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Azzimonti, B.; Zavattaro, E.; Provasi, M.; Vidali, M.; Conca, A.; Catalano, E.; Rimondini, L.; Colombo, E.; Valente, G. Intense Foxp3+ CD25+ regulatory T-cell infiltration is associated with high-grade cutaneous squamous cell carcinoma and counterbalanced by CD8+/Foxp3+ CD25+ ratio. Br. J. Dermatol. 2015, 172, 64–73. [Google Scholar] [CrossRef]
- Langowski, J.L.; Zhang, X.; Wu, L.; Mattson, J.D.; Chen, T.; Smith, K.; Basham, B.; McClanahan, T.; Kastelein, R.A.; Oft, M. IL-23 promotes tumour incidence and growth. Nature 2006, 442, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Yuen, G.J.; Demissie, E.; Pillai, S. B lymphocytes and cancer: A love-hate relationship. Trends Cancer 2016, 2, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Sautes-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Byers, C.; Gill, M.; Kurtansky, N.R.; Alessi-Fox, C.; Harman, M.; Cordova, M.; Gonzalez, S.; Guitera, P.; Rotemberg, V.; Marghoob, A.; et al. Tertiary lymphoid structures accompanied by fibrillary matrix morphology impact anti-tumor immunity in basal cell carcinomas. Front. Med. 2022, 9, 981074. [Google Scholar] [CrossRef]
- Thai, A.A.; Young, R.J.; Bressel, M.; Angel, C.; McDowell, L.; Tiong, A.; Bucknell, N.W.; Fellowes, A.; Xu, H.; Trigos, A.; et al. Comprehensive profiling identifies tumour and immune-microenvironmental differences in clinical subsets of cutaneous squamous cell carcinoma. Br. J. Dermatol. 2023. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Korets, L.V.; Coussens, L.M. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 2005, 7, 411–423. [Google Scholar] [CrossRef]
- Schioppa, T.; Moore, R.; Thompson, R.G.; Rosser, E.C.; Kulbe, H.; Nedospasov, S.; Mauri, C.; Coussens, L.M.; Balkwill, F.R. B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 10662–10667. [Google Scholar] [CrossRef] [PubMed]
- Kok, L.F.; Ferguson, A.L.; Marshall, J.E.; Tse, B.C.Y.; Halliday, G.M.; Byrne, S.N. B Cell-Targeted Immunotherapy Limits Tumor Growth, Enhances Survival, and Prevents Lymph Node Metastasis of UV-Induced Keratinocyte Cancers in Mice. J. Investig. Dermatol. 2020, 140, 1459–1463. [Google Scholar] [CrossRef]
- Harwood, C.A.; Mesher, D.; McGregor, J.M.; Mitchell, L.; Leedham-Green, M.; Raftery, M.; Cerio, R.; Leigh, I.M.; Sasieni, P.; Proby, C.M. A surveillance model for skin cancer in organ transplant recipients: A 22-year prospective study in an ethnically diverse population. Am. J. Transpl. 2013, 13, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.; Schmook, T.; Sachse, M.M.; Sterry, W.; Stockfleth, E. Comparative epidemiology and pathogenic factors for nonmelanoma skin cancer in organ transplant patients. Dermatol. Surg. 2004, 30, 622–627. [Google Scholar] [CrossRef]
- Plasmeijer, E.I.; Sachse, M.M.; Gebhardt, C.; Geusau, A.; Bouwes Bavinck, J.N. Cutaneous squamous cell carcinoma (cSCC) and immunosurveillance—The impact of immunosuppression on frequency of cSCC. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. 8), 33–37. [Google Scholar] [CrossRef]
- Berg, D.; Otley, C.C. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J. Am. Acad. Dermatol. 2002, 47, 1–17; quiz 18–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Yang, I.H.; Lee, Y.H.; Im, S.A.; Song, S.; Li, H.; Han, K.; Kim, K.; Eo, S.K.; Lee, C.K. Cyclosporin A and tacrolimus, but not rapamycin, inhibit MHC-restricted antigen presentation pathways in dendritic cells. Blood 2005, 105, 3951–3955. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Koyasu, S. Mechanisms of action of cyclosporine. Immunopharmacology 2000, 47, 119–125. [Google Scholar] [CrossRef]
- Wang, X.J.; Liefer, K.M.; Tsai, S.; O’Malley, B.W.; Roop, D.R. Development of gene-switch transgenic mice that inducibly express transforming growth factor beta1 in the epidermis. Proc. Natl. Acad. Sci. USA 1999, 96, 8483–8488. [Google Scholar] [CrossRef]
- Weeks, B.H.; He, W.; Olson, K.L.; Wang, X.J. Inducible expression of transforming growth factor beta1 in papillomas causes rapid metastasis. Cancer Res. 2001, 61, 7435–7443. [Google Scholar]
- Wu, F.; Weigel, K.J.; Zhou, H.; Wang, X.J. Paradoxical roles of TGF-β signaling in suppressing and promoting squamous cell carcinoma. Acta Biochim. Biophys. Sin. 2018, 50, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.L.; Reh, D.; Li, A.G.; Woods, J.; Corless, C.L.; Kulesz-Martin, M.; Wang, X.J. Overexpression of transforming growth factor beta1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Res. 2004, 64, 4405–4410. [Google Scholar] [CrossRef]
- Ohata, K.; Espinoza, J.L.; Lu, X.; Kondo, Y.; Nakao, S. Mycophenolic acid inhibits natural killer cell proliferation and cytotoxic function: A possible disadvantage of including mycophenolate mofetil in the graft-versus-host disease prophylaxis regimen. Biol. Blood Marrow Transpl. 2011, 17, 205–213. [Google Scholar] [CrossRef]
- Zhang, S.; Fujita, H.; Mitsui, H.; Yanofsky, V.R.; Fuentes-Duculan, J.; Pettersen, J.S.; Suárez-Fariñas, M.; Gonzalez, J.; Wang, C.Q.; Krueger, J.G.; et al. Increased Tc22 and Treg/CD8 ratio contribute to aggressive growth of transplant associated squamous cell carcinoma. PLoS ONE 2013, 8, e62154. [Google Scholar] [CrossRef] [PubMed]
- Abikhair, M.; Mitsui, H.; Yanofsky, V.; Roudiani, N.; Ovits, C.; Bryan, T.; Oberyszyn, T.M.; Tober, K.L.; Gonzalez, J.; Krueger, J.G.; et al. Cyclosporine A immunosuppression drives catastrophic squamous cell carcinoma through IL-22. JCI Insight 2016, 1, e86434. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Nguyen, B.C.; Dziunycz, P.; Chang, S.; Brooks, Y.; Lefort, K.; Hofbauer, G.F.; Dotto, G.P. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature 2010, 465, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Dziunycz, P.J.; Lefort, K.; Wu, X.; Freiberger, S.N.; Neu, J.; Djerbi, N.; Iotzowa-Weiss, G.; French, L.E.; Dotto, G.P.; Hofbauer, G.F.L. The oncogene ATF3 is potentiated by cyclosporine A and ultraviolet light A. J. Investig. Dermatol. 2014, 134, 1998–2004. [Google Scholar] [CrossRef]
- Tiefenthaler, M.; Hofer, S.; Ebner, S.; Ivarsson, L.; Neyer, S.; Herold, M.; Mayer, G.; Fritsch, P.; Heufler, C. In vitro treatment of dendritic cells with tacrolimus: Impaired T-cell activation and IP-10 expression. Nephrol. Dial. Transpl. 2004, 19, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Maltzman, J.S.; Koretzky, G.A. Azathioprine: Old drug, new actions. J. Clin. Investig. 2003, 111, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Tiede, I.; Fritz, G.; Strand, S.; Poppe, D.; Dvorsky, R.; Strand, D.; Lehr, H.A.; Wirtz, S.; Becker, C.; Atreya, R.; et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J. Clin. Investig. 2003, 111, 1133–1145. [Google Scholar] [CrossRef]
- O’Donovan, P.; Perrett, C.M.; Zhang, X.; Montaner, B.; Xu, Y.Z.; Harwood, C.A.; McGregor, J.M.; Walker, S.L.; Hanaoka, F.; Karran, P. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science 2005, 309, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.C. Mechanisms of action of mycophenolate mofetil. Lupus 2005, 14 (Suppl. 1), s2–s8. [Google Scholar] [CrossRef] [PubMed]
- Haidinger, M.; Poglitsch, M.; Geyeregger, R.; Kasturi, S.; Zeyda, M.; Zlabinger, G.J.; Pulendran, B.; Hörl, W.H.; Säemann, M.D.; Weichhart, T. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J. Immunol. 2010, 185, 3919–3931. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Veitch, M.; Bridge, J.A.; Overgaard, N.H.; Cruz, J.L.; Linedale, R.; Franklin, M.E.; Saunders, N.A.; Simpson, F.; Frazer, I.H.; et al. Clinically-Relevant Rapamycin Treatment Regimens Enhance CD8+ Effector Memory T Cell Function In The Skin and Allow their Infiltration into Cutaneous Squamous Cell Carcinoma. Oncoimmunology 2018, 7, e1479627. [Google Scholar] [CrossRef] [PubMed]
- Easton, J.B.; Houghton, P.J. mTOR and cancer therapy. Oncogene 2006, 25, 6436–6446. [Google Scholar] [CrossRef] [PubMed]
- DeTemple, V.; Satzger, I.; Walter, A.; Schaper, K.; Gutzmer, R. Effects of mammalian target of rapamycin inhibitors on cytokine production and differentiation in keratinocytes. Exp. Dermatol. 2016, 25, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, J.; Furstenberger, G.; Magin, T.M. Loss of keratin 10 leads to mitogen-activated protein kinase (MAPK) activation, increased keratinocyte turnover, and decreased tumor formation in mice. J. Investig. Dermatol. 2004, 123, 973–981. [Google Scholar] [CrossRef]
- Bennett, W.M.; Norman, D.J. Action and toxicity of cyclosporine. Annu. Rev. Med. 1986, 37, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Witte, E.; Wallace, E.; Döcke, W.D.; Kunz, S.; Asadullah, K.; Volk, H.D.; Sterry, W.; Sabat, R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasis. Eur. J. Immunol. 2006, 36, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Carucci, J.A. Cutaneous oncology in organ transplant recipients: Meeting the challenge of squamous cell carcinoma. J. Investig. Dermatol. 2004, 123, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.W.; Myhre, G.M.; Tschumper, R.; Sreekumar, R.; Jelinek, D.; McKean, D.J.; Lipsky, J.J.; Sandborn, W.J.; Egan, L.J. Selective inhibition of inflammatory gene expression in activated T lymphocytes: A mechanism of immune suppression by thiopurines. J. Pharmacol. Exp. Ther. 2005, 312, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Euvrard, S.; Morelon, E.; Rostaing, L.; Goffin, E.; Brocard, A.; Tromme, I.; Broeders, N.; del Marmol, V.; Chatelet, V.; Dompmartin, A.; et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N. Engl. J. Med. 2012, 367, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Turner, A.P.; Shaffer, V.O.; Gangappa, S.; Keller, S.A.; Bachmann, M.F.; Larsen, C.P.; Ahmed, R. mTOR regulates memory CD8 T-cell differentiation. Nature 2009, 460, 108–112. [Google Scholar] [CrossRef]
- Salgo, R.; Gossmann, J.; Schöfer, H.; Kachel, H.G.; Kuck, J.; Geiger, H.; Kaufmann, R.; Scheuermann, E.H. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: Reduced rate of (pre-)malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am. J. Transpl. 2010, 10, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Woltman, A.M.; de Fijter, J.W.; Kamerling, S.W.; van Der Kooij, S.W.; Paul, L.C.; Daha, M.R.; van Kooten, C. Rapamycin induces apoptosis in monocyte- and CD34-derived dendritic cells but not in monocytes and macrophages. Blood 2001, 98, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Dean, P.G.; Lund, W.J.; Larson, T.S.; Prieto, M.; Nyberg, S.L.; Ishitani, M.B.; Kremers, W.K.; Stegall, M.D. Wound-healing complications after kidney transplantation: A prospective, randomized comparison of sirolimus and tacrolimus. Transplantation 2004, 77, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Zaza, G.; Tomei, P.; Ria, P.; Granata, S.; Boschiero, L.; Lupo, A. Systemic and nonrenal adverse effects occurring in renal transplant patients treated with mTOR inhibitors. Clin. Dev. Immunol. 2013, 2013, 403280. [Google Scholar] [CrossRef]
| Drugs | DCs | Myeloid Cells | NK Cells | Lymphocytes | Non-Immunologic Effects on Tumorigenesis |
|---|---|---|---|---|---|
| Calcineurin inhibitors | |||||
| CsA | Impairs MHC-I antigen processing and presentation [65] | Induction of TGFβ1 production, which suppresses early tumor formation but also stimulates malignant transformation and metastasis of established tumors [66,67,68,69] Induction of granulocytic inflammation and proinflammatory cytokines IL-1β and TNFα [70] | Inhibition of NK cell proliferation; increases the proportion of CD16−CD56brightNK cells, which are not cytotoxic but express IL-10 and IL-13 [71] | Dephosphorylation of NFAT family members NFAT1, NFAT2 and NFAT4, resulting in the inhibition of IL-2 and IL-4 production and the decreased activation and proliferation of T cells [66] Increased Treg/CD8+ T cell ratios; lower IFNy-producing CD4+ T cells numbers [72] Induction of Th22 response [72,73] | Downregulation of NFAT in keratinocytes decreases the expression of tumor suppressor gene P53 [74], the carcinogenic effect of which is potentiated by UV exposure [75] |
| TAC | Inhibition of CXCL10 and IL-12 production by DC, impairing their T cell priming ability [76] Impairs MHC-I antigen processing and presentation [65] | - | Inhibition of NK cell proliferation; increases the proportion of CD16−CD56brightNK cells, which are not cytotoxic but express IL-10 and IL-13 [71] | Dephosphorylation of NFAT, resulting in the inhibition of IL-2 and IL-4 production, decreased the activation and proliferation of T cells [66] | - |
| Purine analogues | |||||
| AZA | - | - | Inhibition of DNA and RNA synthesis, resulting in the suppression of lymphocyte proliferation [71,77] Downregulation of Bcl-xL, resulting in increased apoptosis in CD4+ T cells Inhibition of pro-inflammatory gene expression [77,78] | Increases the photosensitivity of the skin. Promotes the accumulation of 6-thioguanine in the DNA, leading to increased oxidative stress and mutagenesis upon UV irradiation [79] | |
| MMF | - | - | Inhibition of NK cell proliferation; decreases the proportion of CD16−CD56brightNK cells, which are anti-inflammatory [71]. Downregulation of activating NK cell receptors and NK cell cytotoxicity [71] | Inhibition of DNA and RNA synthesis, resulting in the suppression of lymphocyte proliferation [71,77] Downregulation of VCAM-1 expression and the inhibition of recruitment and the migration of lymphocytes [77,80]. | - |
| mTOR inhibitors | |||||
| SIR | Decreased expression of costimulatory molecules and inflammatory cytokines by moDC Increased expression of NF-kB and other pro-inflammatory cytokines by mDC upon stimulation, and the downregulation of IL-10 and oncogene STAT3 [81] | Prevention of reduced immunosurveillance and constitutive development of cSCC via the downregulation of IL-10 and STAT3 and the upregulation of pro-inflammatory cytokines [81] | - | Binding to FKBP12, which inhibits mTOR signaling, leading to the inhibition of IL-2 signaling and T cell proliferation. Increased differentiation and enhanced CD8+ memory T cell function in the skin, against new antigenic challenges [82] | Anti-proliferative and anti-neoplastic activity [83] Upregulation of cytokine IL-6, resulting in the downregulation of CK10 [84] and less skin tumor formation [85] |
| EV | - | - | - | Binding to mTOR complex 1, leading to the inhibition of IL-2 signaling and T cell proliferation [4] | Anti-proliferative and anti-neoplastic activity [83] Upregulation of cytokine IL-6, resulting in the downregulation of CK10 [84] and less skin tumor formation [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakker, D.; Bakker, W.J.; Bekkenk, M.W.; Luiten, R.M. Immunity against Non-Melanoma Skin Cancer and the Effect of Immunosuppressive Medication on Non-Melanoma Skin Cancer Risk in Solid Organ Transplant Recipients. Cells 2023, 12, 2441. https://doi.org/10.3390/cells12202441
Bakker D, Bakker WJ, Bekkenk MW, Luiten RM. Immunity against Non-Melanoma Skin Cancer and the Effect of Immunosuppressive Medication on Non-Melanoma Skin Cancer Risk in Solid Organ Transplant Recipients. Cells. 2023; 12(20):2441. https://doi.org/10.3390/cells12202441
Chicago/Turabian StyleBakker, Dixie, Walbert J. Bakker, Marcel W. Bekkenk, and Rosalie M. Luiten. 2023. "Immunity against Non-Melanoma Skin Cancer and the Effect of Immunosuppressive Medication on Non-Melanoma Skin Cancer Risk in Solid Organ Transplant Recipients" Cells 12, no. 20: 2441. https://doi.org/10.3390/cells12202441
APA StyleBakker, D., Bakker, W. J., Bekkenk, M. W., & Luiten, R. M. (2023). Immunity against Non-Melanoma Skin Cancer and the Effect of Immunosuppressive Medication on Non-Melanoma Skin Cancer Risk in Solid Organ Transplant Recipients. Cells, 12(20), 2441. https://doi.org/10.3390/cells12202441







