Effects of Scaffolds on Urine- and Urothelial Carcinoma Tissue-Derived Organoids from Bladder Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
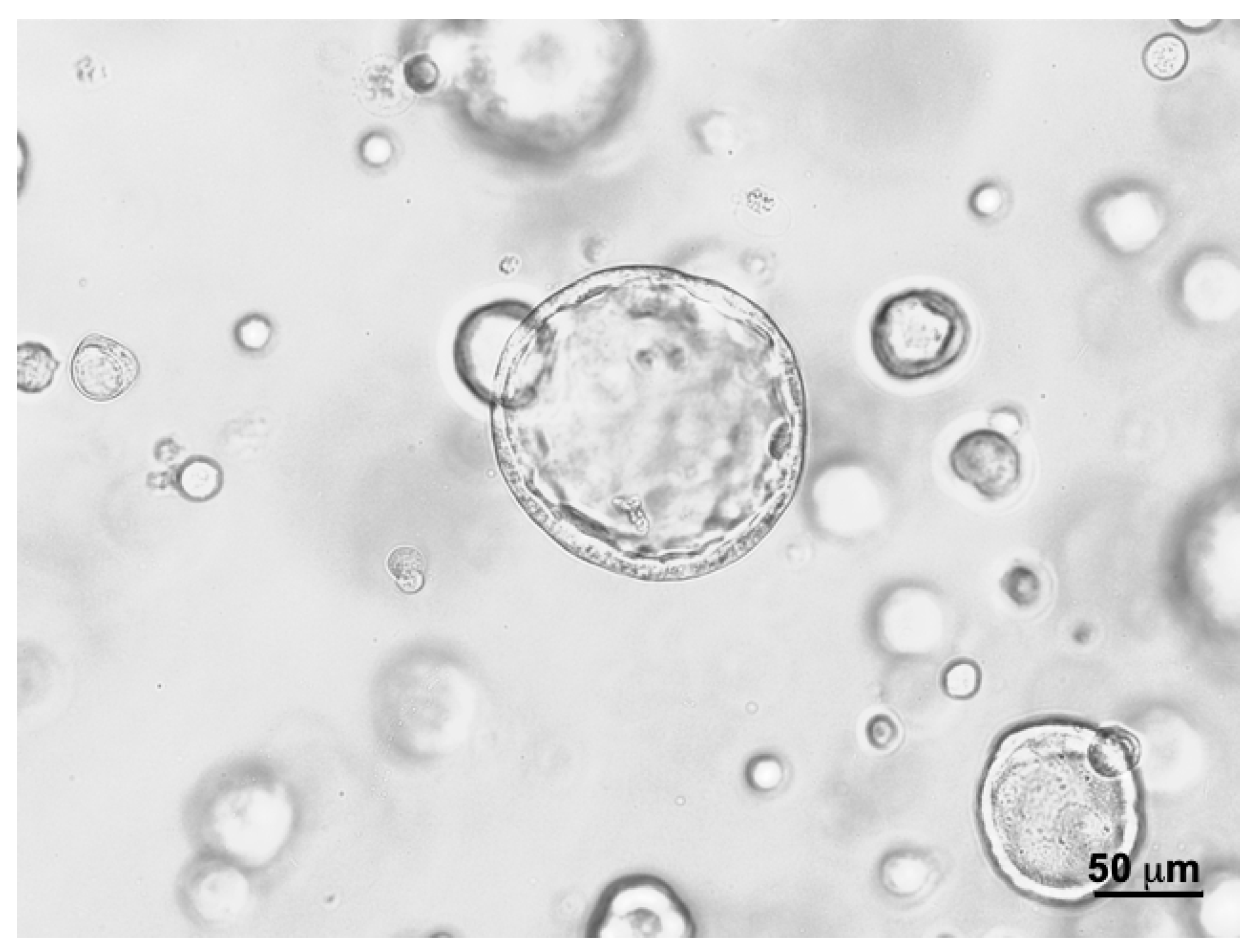
2.1. Culture of Bladder Cancer Cell Line, RT112, in 3D Organoids
2.2. Isolation of Cells from Urothelial Carcinoma Tissue from Bladder Cancer Patients
2.3. Isolation of Cells from Rinsing Urine of Bladder Cancer Patients
2.4. Expansion of Cells in Organoid Cultures
2.5. Freezing and Thawing of Organoids
2.6. Immunofluorescence of Spheroids and Organoids and Fluorescence Microscopy
2.7. Statistics
3. Results
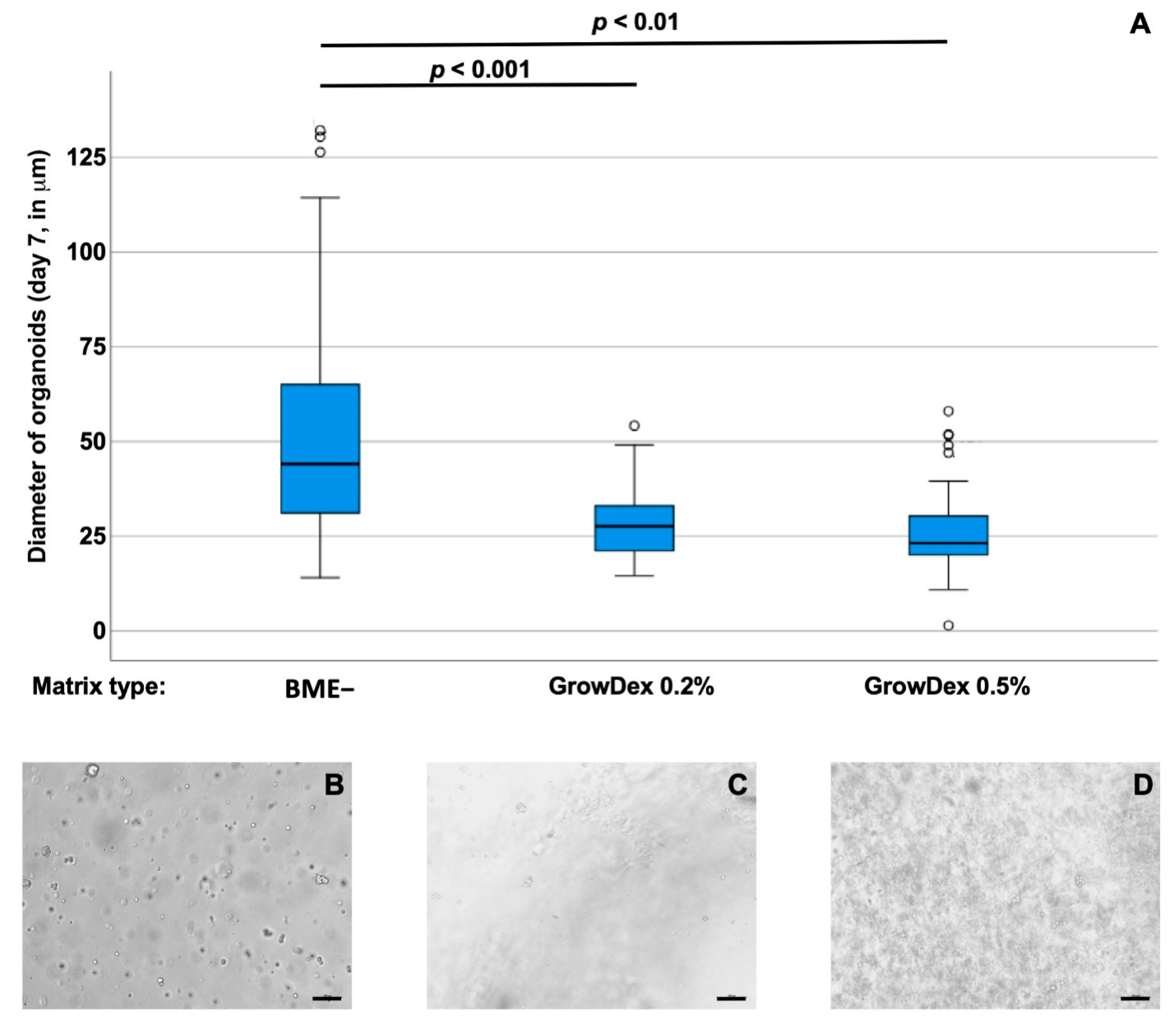
3.1. Optimizing Scaffold Concentrations for Bladder Cancer Cell Cultures
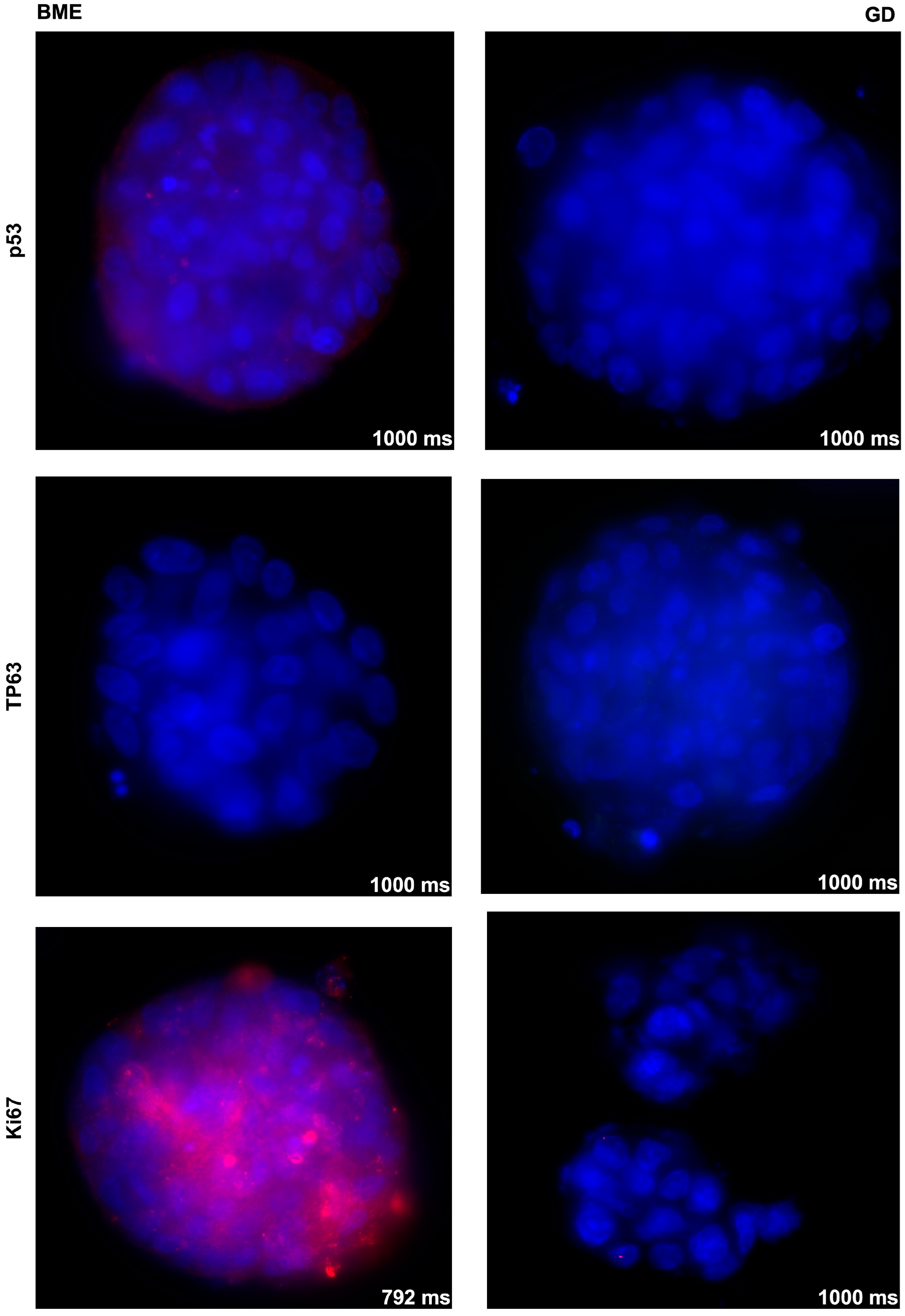
3.2. Effects of Basement Membrane Extract- Versus Cellulose-Derived Scaffolds on Urothelial Carcinoma-Derived Organoid BCO#140
3.3. Effects of Basement Membrane Extract- Versus Cellulose-Derived Scaffolds on Urine-Derived Organoid UCO#33
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Registry, D.C. 2018. Available online: https://iknl.nl/en/about-iknl (accessed on 20 May 2023).
- Richters, A.; Aben, K.K.H.; Kiemeney, L. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.G.K.; Jubber, I.; Black, P.C.; Esperto, F.; Figueroa, J.D.; Kamat, A.M.; Catto, J.W. Epidemiology of Bladder Cancer: A Systematic Review and Contemporary Update of Risk Factors in 2018. Eur. Urol. 2018, 74, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Amado, F.; Bernardo, C.; Costa, C.; Freitas, R.; Oliveira, P.; Palmeira, C.; Pinto-Leite, R. What we have learned from urinary bladder cancer models. J. Cancer Metastasis Treat. 2016, 2, 51–58. [Google Scholar] [CrossRef]
- John, B.A.; Said, N. Insights from animal models of bladder cancer: Recent advances, challenges, and opportunities. Oncotarget 2017, 8, 57766–57781. [Google Scholar] [CrossRef]
- Zhang, N.; Li, D.; Shao, J.; Wang, X. Animal models for bladder cancer: The model establishment and evaluation (Review). Oncol. Lett. 2015, 9, 1515–1519. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar]
- Page, R.; Baneux, P.; Vail, D.; Duda, L.; Olson, P.; Anestidou, L.; Dybdal, N.; Golab, G.; Shelton, W.; Salgaller, M.; et al. Conduct, Oversight, and Ethical Considerations of Clinical Trials in Companion Animals with Cancer: Report of a Workshop on Best Practice Recommendations. J. Vet. Intern. Med. 2016, 30, 527–535. [Google Scholar] [CrossRef]
- Nelson, C.M.; Bissell, M.J. Of extracellular matrix, scaffolds, and signaling: Tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2006, 22, 287–309. [Google Scholar] [CrossRef]
- Corro, C.; Novellasdemunt, L.; Li, V.S.W. A brief history of organoids. Am. J. Physiol. Cell Physiol. 2020, 319, C151–C165. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef]
- Bartfeld, S.; Clevers, H. Stem cell-derived organoids and their application for medical research and patient treatment. J. Mol. Med. 2017, 95, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Ebner-Peking, P.; Krisch, L.; Wolf, M.; Hochmann, S.; Hoog, A.; Vári, B.; Muigg, K.; Poupardin, R.; Scharler, C.; Schmidhuber, S.; et al. Self-assembly of differentiated progenitor cells facilitates spheroid human skin organoid formation and planar skin regeneration. Theranostics 2021, 11, 8430–8447. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Hu, W.; Matulay, J.T.; Silva, M.V.; Owczarek, T.B.; Kim, K.; Chua, C.W.; Barlow, L.M.J.; Kandoth, C.; Williams, A.B.; et al. Tumor Evolution and Drug Response in Patient-Derived Organoid Models of Bladder Cancer. Cell 2018, 173, 515–528.e17. [Google Scholar] [CrossRef] [PubMed]
- Mullenders, J.; de Jongh, E.; Brousali, A.; Roosen, M.; Blom, J.P.A.; Begthel, H.; Korving, J.; Jonges, T.; Kranenburg, O.; Meijer, R.; et al. Mouse and human urothelial cancer organoids: A tool for bladder cancer research. Proc. Natl. Acad. Sci. USA 2019, 116, 4567–4574. [Google Scholar] [CrossRef]
- Kim, E.; Choi, S.; Kang, B.; Kong, J.; Kim, Y.; Yoon, W.H.; Lee, H.-R.; Kim, S.; Kim, H.-M.; Lee, H.; et al. Creation of bladder assembloids mimicking tissue regeneration and cancer. Nature 2020, 588, 664–669. [Google Scholar] [CrossRef]
- Geng, R.; Harland, N.; Montes-Mojarro, I.A.; Fend, F.; Aicher, W.K.; Stenzl, A.; Amend, B. CD24: A Marker for an Extended Expansion Potential of Urothelial Cancer Cell Organoids In Vitro? Int. J. Mol. Sci. 2022, 23, 5453. [Google Scholar] [CrossRef]
- Wei, Y.; Amend, B.; Todenhöfer, T.; Lipke, N.; Aicher, W.K.; Fend, F.; Stenzl, A.; Harland, N. Urinary Tract Tumor Organoids Reveal Eminent Differences in Drug Sensitivities When Compared to 2-Dimensional Culture Systems. Int. J. Mol. Sci. 2022, 23, 6305. [Google Scholar] [CrossRef]
- Li, Z.; Xu, H.; Gong, Y.; Chen, W.; Zhan, Y.; Yu, L.; Sun, Y.; Li, A.; He, S.; Guan, B.; et al. Patient-Derived Upper Tract Urothelial Carcinoma Organoids as a Platform for Drug Screening. Adv. Sci. 2022, 9, e2103999. [Google Scholar] [CrossRef]
- Medle, B.; Sjödahl, G.; Eriksson, P.; Liedberg, F.; Höglund, M.; Bernardo, C. Patient-Derived Bladder Cancer Organoid Models in Tumor Biology and Drug Testing: A Systematic Review. Cancers 2022, 14, 2062. [Google Scholar] [CrossRef]
- Yu, L.; Li, Z.; Mei, H.; Li, W.; Chen, D.; Liu, L.; Zhang, Z.; Sun, Y.; Song, F.; Chen, W.; et al. Patient-derived organoids of bladder cancer recapitulate antigen expression profiles and serve as a personal evaluation model for CAR-T cells in vitro. Clin. Transl. Immunol. 2021, 10, e1248. [Google Scholar] [CrossRef]
- Zhang, S.; Wan, Z.; Kamm, R.D. Vascularized organoids on a chip: Strategies for engineering organoids with functional vasculature. Lab Chip 2021, 21, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Duzagac, F.; Saorin, G.; Memeo, L.; Canzonieri, V.; Rizzolio, F. Microfluidic Organoids-on-a-Chip: Quantum Leap in Cancer Research. Cancers 2021, 13, 737. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Benton, G.; Kleinman, H.K.; George, J.; Arnaoutova, I. Multiple uses of basement membrane-like matrix BME/Matrigel) in vitro and in vivo with cancer cells. Int. J. Cancer 2011, 128, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kaur, I.; Rawal, P.; Tripathi, D.M.; Vasudevan, A. Non-matrigel scaffolds for organoid cultures. Cancer Lett. 2021, 504, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.J.; Franks, L.M.; Carbonell, A.W. Markers of neoplastic transformation in epithelial cell lines derived from human carcinomas. J. Natl. Cancer Inst. 1977, 58, 1743–1751. [Google Scholar] [CrossRef]
- VanDussen, K.L.; Sonnek, N.M.; Stappenbeck, T.S. L-WRN conditioned medium for gastrointestinal epithelial stem cell culture shows replicable batch-to-batch activity levels across multiple research teams. Stem. Cell Res. 2019, 37, 101430. [Google Scholar] [CrossRef]
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a Type of Three-Dimensional Cell Cultures—Examples of Methods of Preparation and the Most Important Application. Int. J. Mol. Sci. 2020, 21, 6225. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Yu, H. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Leitinger, B.; Hohenester, E. Mammalian collagen receptors. Matrix Biol. 2007, 26, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, Y.; Hozumi, K.; Katagiri, F.; Nomizu, M.; Kleinman, H.K.; Koblinski, J.E. Laminin-111-derived peptides and cancer. Cell Adhes. Migr. 2013, 7, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, A.P.; Metcalfe, A.D.; Romer, L.H.; Streuli, C.H. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J. Cell Biol. 2000, 149, 431–446. [Google Scholar] [CrossRef]
- Munger, J.S.; Sheppard, D. Cross talk among TGF-β signaling pathways, integrins, and the extracellular matrix. Cold. Spring Harb. Perspect. Biol. 2011, 3, a005017. [Google Scholar] [CrossRef] [PubMed]
- Tharmalingam, S.; Hampson, D.R. The Calcium-Sensing Receptor and Integrins in Cellular Differentiation and Migration. Front. Physiol. 2016, 7, 190. [Google Scholar] [CrossRef] [PubMed]
- Warstat, K.; Meckbach, D.; Weis-Klemm, M.; Hack, A.; Klein, G.; de Zwart, P.; Aicher, W.K. TGF-beta enhances the integrin alpha2beta1-mediated attachment of mesenchymal stem cells to type I collagen. Stem. Cells Dev. 2010, 19, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.M.; Truong, T.N.; Schwartz, M.A. Integrins regulate the apoptotic response to DNA damage through modulation of p53. Proc. Natl. Acad. Sci. USA 2002, 99, 3627–3632. [Google Scholar] [CrossRef]
- Lauer-Fields, J.L.; Malkar, N.B.; Richet, G.; Drauz, K.; Fields, G.B. Melanoma cell CD44 interaction with the alpha 1(IV)1263-1277 region from basement membrane collagen is modulated by ligand glycosylation. J Biol Chem. 2003, 278, 14321–14330. [Google Scholar] [CrossRef]
- Xu, H.; Niu, M.; Yuan, X.; Wu, K.; Liu, A. CD44 as a tumor biomarker and therapeutic target. Exp. Hematol. Oncol. 2020, 9, 36. [Google Scholar] [CrossRef]
- Szatanek, R.; Baj-Krzyworzeka, M. CD44 and Tumor-Derived Extracellular Vesicles (TEVs). Possible Gateway to Cancer Metastasis. Int. J. Mol. Sci. 2021, 22, 1463. [Google Scholar] [CrossRef]
- Wu, C.T.; Lin, W.Y.; Chang, Y.H.; Chen, W.C.; Chen, M.F. Impact of CD44 expression on radiation response for bladder cancer. J. Cancer. 2017, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Vogel, W.; Brakebusch, C.; Fässler, R.; Alves, F.; Ruggiero, F.; Pawson, T. Discoidin domain receptor 1 is activated independently of beta(1) integrin. J. Biol. Chem. 2000, 275, 5779–5784. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Rui, W.; He, W.; Shao, Y.; Sun, F.; Zhou, W.; Wu, Y.; Zhu, Y. Discoidin domain receptor 1 activity drives an aggressive phenotype in bladder cancer. Am. J. Transl. Res. 2017, 9, 2500–2507. [Google Scholar] [PubMed]
- Rao, N.C.; Barsky, S.H.; Terranova, V.P.; Liotta, L.A. Isolation of a tumor cell laminin receptor. Biochem. Biophys. Res. Commun. 1983, 111, 804–808. [Google Scholar] [CrossRef]
- Terranova, V.P.; Rao, C.N.; Kalebic, T.; Margulies, I.M.; Liotta, L.A. Laminin receptor on human breast carcinoma cells. Proc. Natl. Acad. Sci. USA 1983, 80, 444–448. [Google Scholar] [CrossRef]
- Umbaugh, C.S.; Diaz-Quiñones, A.; Neto, M.F.; Shearer, J.J.; Figueiredo, M.L. A dock derived compound against laminin receptor (37 LR) exhibits anti-cancer properties in a prostate cancer cell line model. Oncotarget 2018, 9, 5958–5978. [Google Scholar] [CrossRef]
- Qing, J.; Du, X.; Chen, Y.; Chan, P.; Li, H.; Wu, P.; Marsters, S.; Stawicki, S.; Tien, J.; Totpal, K.; et al. Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. J. Clin. Investig. 2009, 119, 1216–1229. [Google Scholar] [CrossRef] [PubMed]
- Kacew, A.; Sweis, R.F. FGFR3 Alterations in the Era of Immunotherapy for Urothelial Bladder Cancer. Front. Immunol. 2020, 11, 575258. [Google Scholar] [CrossRef]
- Aicher, W.K.; Korn, M.; Reitnauer, L.; Maurer, F.B.; Hennenlotter, J.; Black, P.C.; Todenhofer, T.; Bedke, J.; Stenzl, A. Expression patterns of the immune checkpoint ligand CD276 in urothelial carcinoma. BMC Urol. 2021, 21, 60. [Google Scholar] [CrossRef]
- Picarda, E.; Ohaegbulam, K.C.; Zang, X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin. Cancer Res. 2016, 22, 3425–3431. [Google Scholar] [CrossRef]
- Kiss, B.; Berg, N.S.v.D.; Ertsey, R.; McKenna, K.; Mach, K.E.; Zhang, C.A.; Volkmer, J.-P.; Weissman, I.L.; Rosenthal, E.L.; Liao, J.C. CD47-Targeted Near-Infrared Photoimmunotherapy for Human Bladder Cancer. Clin. Cancer Res. 2019, 25, 3561–3571. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Guo, X.; Mo, N.; Zhang, J.; Li, C. Frontiers in Bladder Cancer Genomic Research. Front. Oncol. 2021, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- BahBahrami, S.; Kazemi, B.; Zali, H.; Black, P.C.; Basiri, A.; Bandehpour, M.; Hedayati, M.; Sahebkar, A. Discovering Therapeutic Protein Targets for Bladder Cancer Using Proteomic Data Analysis. Curr. Mol. Pharmacol. 2020, 13, 150–172. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.-L.; et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014, 25, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Bretz, N.P.; Salnikov, A.V.; Perne, C.; Keller, S.; Wang, X.; Mierke, C.T.; Fogel, M.; Erbe-Hofmann, N.; Schlange, T.; Moldenhauer, G.; et al. CD24 controls Src/STAT3 activity in human tumors. Cell Mol. Life Sci. 2012, 69, 3863–3879. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, S.; Shen, H.; Xu, K.; Chen, J.; Li, H.; Xu, Y.; Xu, A.; Chen, B.; Kaku, H.; et al. Clinical significance of CD24 as a predictor of bladder cancer recurrence. Oncol. Lett. 2013, 6, 96–100. [Google Scholar] [CrossRef]
- Panagiotou, E.; Syrigos, N.K.; Charpidou, A.; Kotteas, E.; Vathiotis, I.A. CD24: A Novel Target for Cancer Immunotherapy. J. Pers. Med. 2022, 12, 1235. [Google Scholar] [CrossRef]




| BCO#140 | UCO#33 | |
|---|---|---|
| Sex | male | male |
| Age | 62 | 85 |
| Pathology | ||
| pT | 1 | a |
| pL | 1 | n.a. |
| pV | 1 | n.a. |
| Grading | high grade | low grade |
| CIS | yes | no |
| Urine cytology | positive | positive |
| Target | Dilution | Clone | Company |
|---|---|---|---|
| AE1/AE3 antigens | 1:200 | AE1/Ae3 | Merck |
| CD24 | 1:100 | PR19925 | Abcam |
| CD44 | 1:200 | MEM-263 | Abcam |
| CK7 | 1:300 | EPRY1619Y | Abcam |
| CK20 | 1:100 | XQ1 | Merck |
| GATA-3 | 1:100 | serum | Abcam |
| Ki67 | 1:100 | ARG57562 | Biomol-Argio |
| p53 | 1:100 | PAb 240 | Invitrogen |
| TP63 | 1:100 | 10H7L17 | Invitrogen |
| gt-a-ms IgG 1 | 1:1000 | serum | Jackson Immuno Res. |
| gt-a-rb IgG 2 | 1:1000 | serum | Jackson Immuno Res. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walz, S.; Pollehne, P.; Vollmer, P.; Aicher, W.K.; Stenzl, A.; Harland, N.; Amend, B. Effects of Scaffolds on Urine- and Urothelial Carcinoma Tissue-Derived Organoids from Bladder Cancer Patients. Cells 2023, 12, 2108. https://doi.org/10.3390/cells12162108
Walz S, Pollehne P, Vollmer P, Aicher WK, Stenzl A, Harland N, Amend B. Effects of Scaffolds on Urine- and Urothelial Carcinoma Tissue-Derived Organoids from Bladder Cancer Patients. Cells. 2023; 12(16):2108. https://doi.org/10.3390/cells12162108
Chicago/Turabian StyleWalz, Simon, Paul Pollehne, Philipp Vollmer, Wilhelm K. Aicher, Arnulf Stenzl, Niklas Harland, and Bastian Amend. 2023. "Effects of Scaffolds on Urine- and Urothelial Carcinoma Tissue-Derived Organoids from Bladder Cancer Patients" Cells 12, no. 16: 2108. https://doi.org/10.3390/cells12162108
APA StyleWalz, S., Pollehne, P., Vollmer, P., Aicher, W. K., Stenzl, A., Harland, N., & Amend, B. (2023). Effects of Scaffolds on Urine- and Urothelial Carcinoma Tissue-Derived Organoids from Bladder Cancer Patients. Cells, 12(16), 2108. https://doi.org/10.3390/cells12162108








