Unveiling the Neural Environment in Cancer: Exploring the Role of Neural Circuit Players and Potential Therapeutic Strategies
Abstract
1. Introduction
2. Neurogenesis and Neoneurogenesis
2.1. Neurogenesis of Nerve Stem Cells (NSCs)
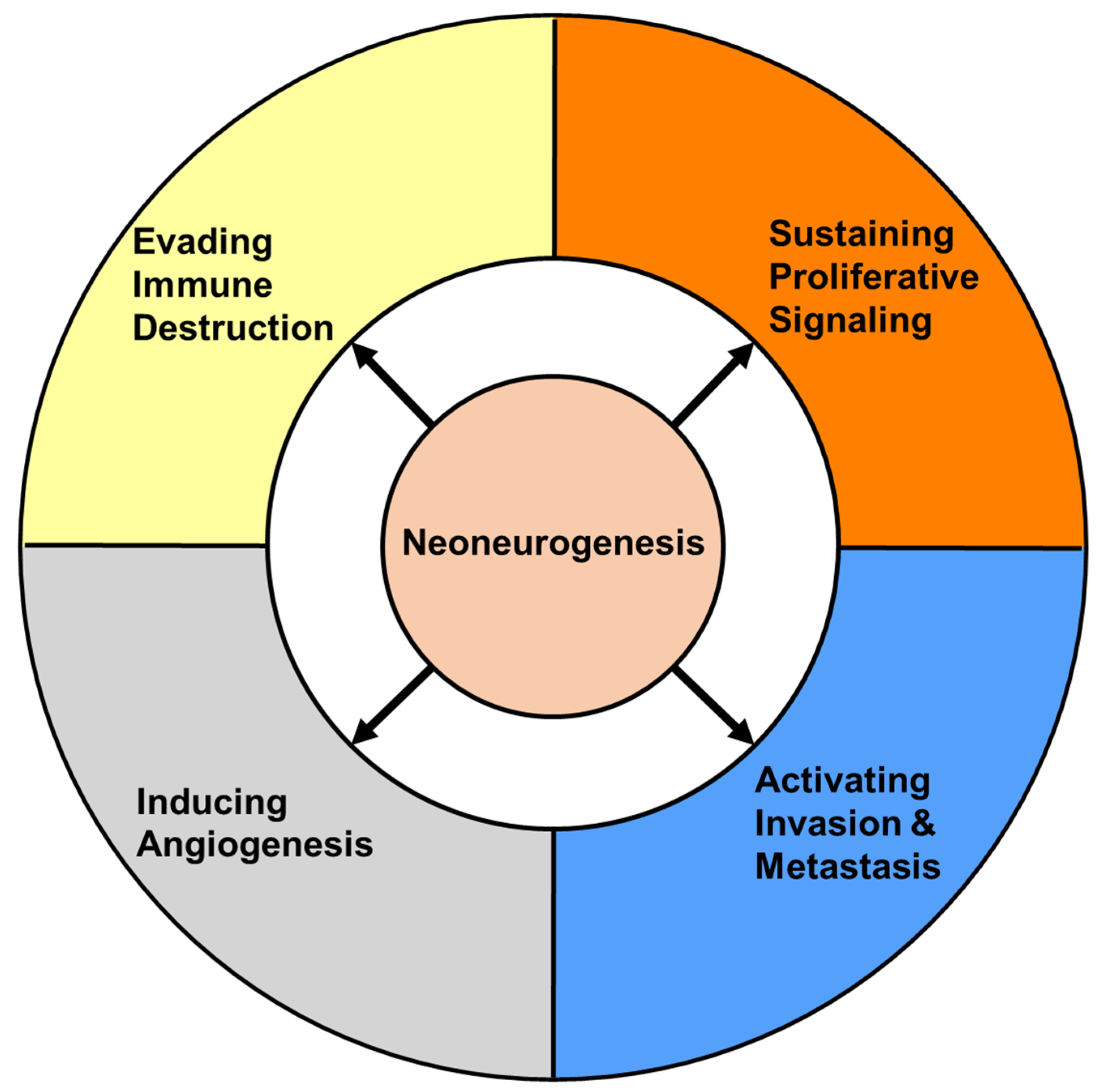
2.2. Prognostic Importance of Neoneurogenesis in Cancer
3. The Relationship between Neurogenesis and Cancer
3.1. The Effects of Cancer on Nerves
3.2. The Effects of Nerves on Cancer
3.2.1. Paracrine Signaling
3.2.2. Chemical Synapse
3.3. The Effects of Nerves on the Tumor Microenvironment
3.3.1. The Effects of Nerves on Angiogenesis
3.3.2. The Effects of Nerves on the Immune System
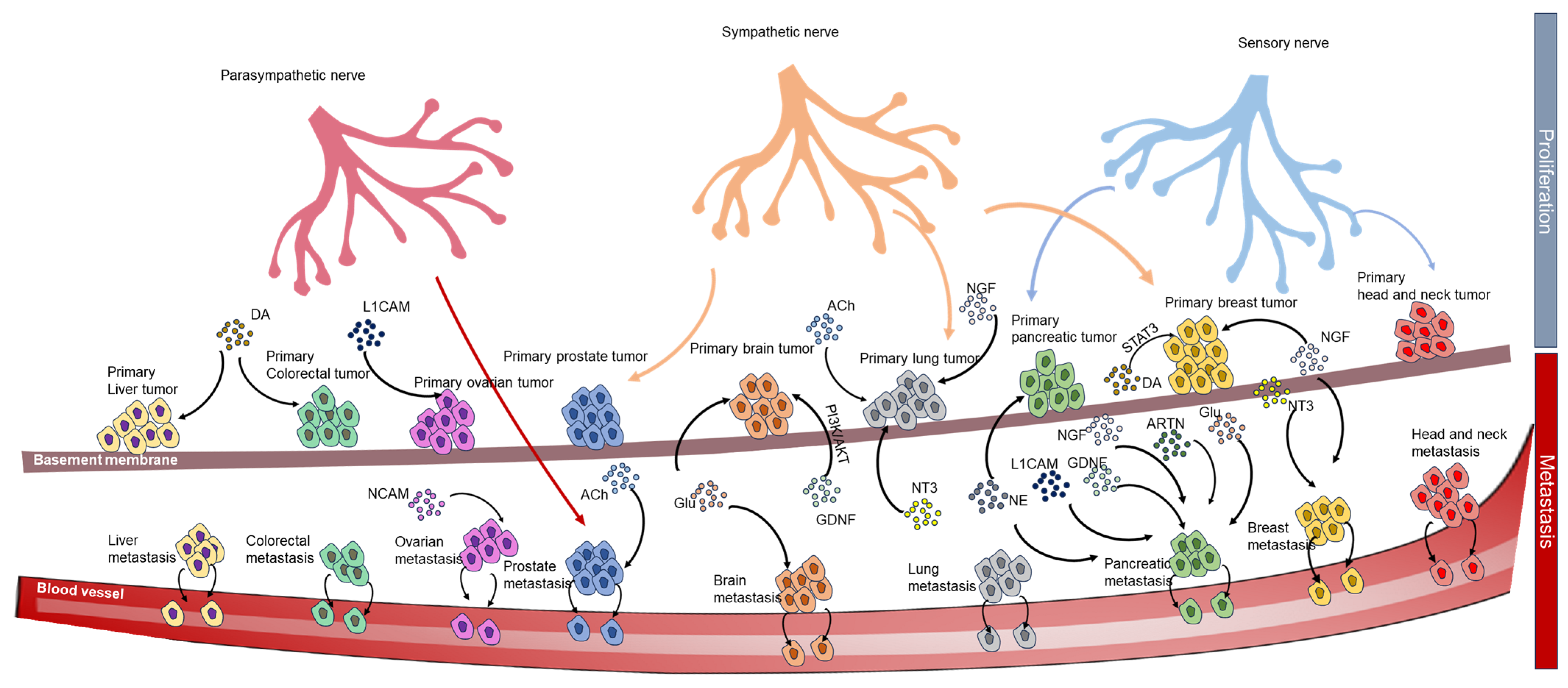
4. The Neural Circuit of Cancer
4.1. Receptors in the Neural Circuit of Cancers
4.1.1. Neurotrophic Receptor Tyrosine Kinase (Trks)
4.1.2. Nerve Growth Factor Receptor (NGFR)
4.1.3. Glial Cell Line-Derived Family Receptor Alpha (GFRα) Family
4.1.4. L1 Cell Adhesion Molecule (L1CAM)
4.1.5. Neural Cell Adhesion Molecule (NCAM)
4.1.6. Acetylcholine Receptors (AChRs)
4.1.7. AMPA, NMDA and Metabotropic Glutamate Receptors
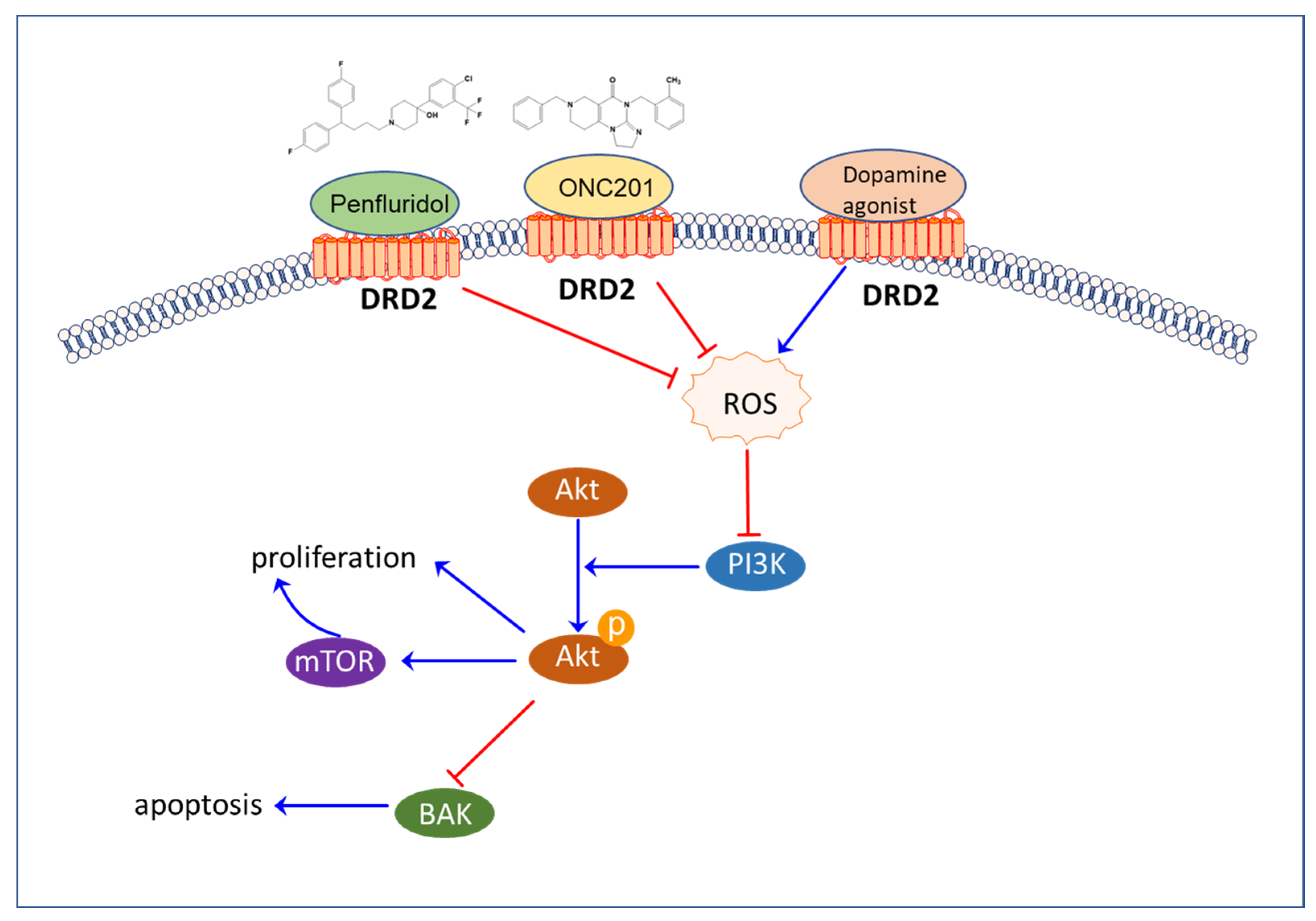
4.1.8. Dopamine Receptors
4.2. Ligands in the Neural Circuits of Cancers
4.2.1. Brain-Derived Neurotrophic Factor (BDNF), Neurotrophin-3 (NT-3), and Nerve Growth Factor (NGF)
4.2.2. Glial Cell-Derived Neurotrophic Factor (GDNF), Neurturin (NRTN), and Artemin (ARTN)
4.2.3. Glutamate
4.2.4. Acetylcholine
4.2.5. Dopamine
4.3. Reactive Oxygen Species (ROS) in the Neural Circuits of Cancers
4.3.1. The Role of ROS in Neurogenesis
4.3.2. The Role of ROS in Cancer
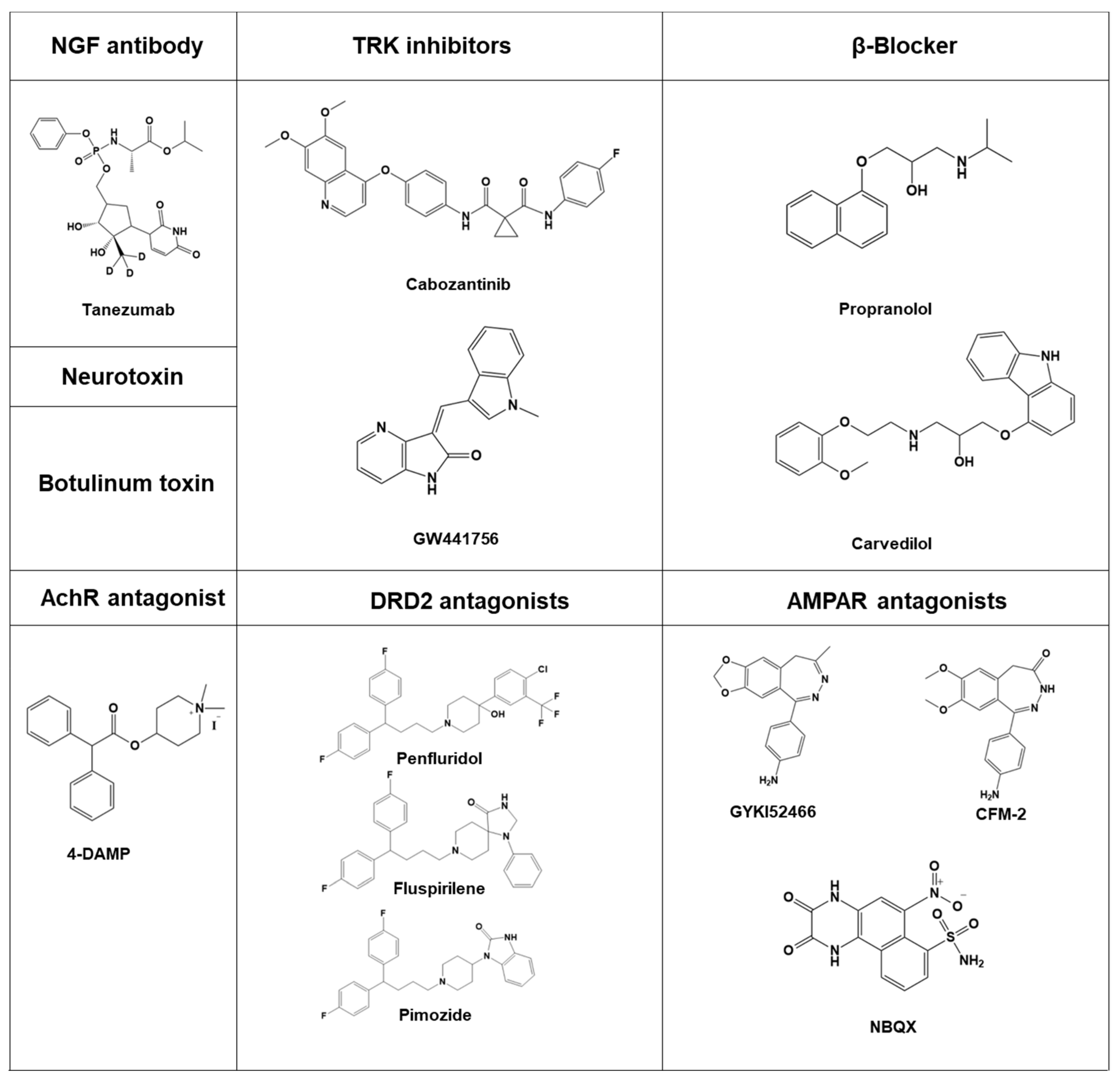
5. Modulators of the Neural Circuit as Anticancer Agents
6. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Lolas, G.; Bianchi, A.; Syrigos, K.N. Tumour-induced neoneurogenesis and perineural tumour growth: A mathematical approach. Sci. Rep. 2016, 6, 20684. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Wang, H.C.; Yuan, Z.; Huang, J.; Zheng, Q. Norepinephrine stimulates pancreatic cancer cell proliferation, migration and invasion via β-adrenergic receptor-dependent activation of P38/MAPK pathway. Hepatogastroenterology 2012, 59, 889–893. [Google Scholar] [CrossRef]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef]
- Vishwakarma, S.K.; Bardia, A.; Tiwari, S.K.; Paspala, S.A.; Khan, A.A. Current concept in neural regeneration research: NSCs isolation, characterization and transplantation in various neurodegenerative diseases and stroke: A review. J. Adv. Res. 2014, 5, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Overall, R.W.; Kempermann, G. The Small World of Adult Hippocampal Neurogenesis. Front. Neurosci. 2018, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-Ubieta, L.; Bonni, A. Transcriptional regulation of neuronal polarity and morphogenesis in the mammalian brain. Neuron 2011, 72, 22–40. [Google Scholar] [CrossRef]
- Kristiansen, M.; Ham, J. Programmed cell death during neuronal development: The sympathetic neuron model. Cell Death Differ. 2014, 21, 1025–1035. [Google Scholar] [CrossRef]
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef]
- Gage, F.H. Adult neurogenesis in mammals. Science 2019, 364, 827–828. [Google Scholar] [CrossRef]
- Niklison-Chirou, M.V.; Agostini, M.; Amelio, I.; Melino, G. Regulation of Adult Neurogenesis in Mammalian Brain. Int. J. Mol. Sci. 2020, 21, 4869. [Google Scholar] [CrossRef]
- Zahalka, A.H.; Frenette, P.S. Nerves in cancer. Nat. Rev. Cancer 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Ding, Y.; Lee, M.; Gao, Y.; Bu, P.; Coarfa, C.; Miles, B.; Sreekumar, A.; Creighton, C.J.; Ayala, G. Neuropeptide Y nerve paracrine regulation of prostate cancer oncogenesis and therapy resistance. Prostate 2021, 81, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Dubeykovskaya, Z.; Si, Y.; Chen, X.; Worthley, D.L.; Renz, B.W.; Urbanska, A.M.; Hayakawa, Y.; Xu, T.; Westphalen, C.B.; Dubeykovskiy, A.; et al. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat. Commun. 2016, 7, 10517. [Google Scholar] [CrossRef]
- Yoneda, T.; Hiasa, M.; Okui, T. Crosstalk Between Sensory Nerves and Cancer in Bone. Curr. Osteoporos. Rep. 2018, 16, 648–656. [Google Scholar] [CrossRef]
- McVary, K.T.; McKenna, K.E.; Lee, C. Prostate innervation. Prostate Suppl. 1998, 8, 2–13. [Google Scholar] [CrossRef]
- Huang, D.; Su, S.; Cui, X.; Shen, X.; Zeng, Y.; Wu, W.; Chen, J.; Chen, F.; He, C.; Liu, J.; et al. Nerve fibers in breast cancer tissues indicate aggressive tumor progression. Medicine 2014, 93, e172. [Google Scholar] [CrossRef]
- Kappos, E.A.; Engels, P.E.; Tremp, M.; Sieber, P.K.; von Felten, S.; Madduri, S.; Meyer Zu Schwabedissen, M.; Fischmann, A.; Schaefer, D.J.; Kalbermatten, D.F. Denervation leads to volume regression in breast cancer. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 833–839. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Shao, J.X.; Wang, B.; Yao, Y.N.; Pan, Z.J.; Shen, Q.; Zhou, J.Y. Autonomic nervous infiltration positively correlates with pathological risk grading and poor prognosis in patients with lung adenocarcinoma. Thorac. Cancer 2016, 7, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Albo, D.; Akay, C.L.; Marshall, C.L.; Wilks, J.A.; Verstovsek, G.; Liu, H.; Agarwal, N.; Berger, D.H.; Ayala, G.E. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer 2011, 117, 4834–4845. [Google Scholar] [CrossRef]
- Schwartz, E.S.; Christianson, J.A.; Chen, X.; La, J.H.; Davis, B.M.; Albers, K.M.; Gebhart, G.F. Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology 2011, 140, 1283–1291.e2. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.S.; La, J.H.; Scheff, N.N.; Davis, B.M.; Albers, K.M.; Gebhart, G.F. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J. Neurosci. 2013, 33, 5603–5611. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Tang, L.; Xie, Y.; Xianyu, Y.; Zhang, L.; Wang, P.; Hamada, Y.; Jiang, K.; Zheng, W.; Jiang, X. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat. Commun. 2017, 8, 15130. [Google Scholar] [CrossRef] [PubMed]
- Stopczynski, R.E.; Normolle, D.P.; Hartman, D.J.; Ying, H.; DeBerry, J.J.; Bielefeldt, K.; Rhim, A.D.; DePinho, R.A.; Albers, K.M.; Davis, B.M. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014, 74, 1718–1727. [Google Scholar] [CrossRef]
- Peterson, S.C.; Eberl, M.; Vagnozzi, A.N.; Belkadi, A.; Veniaminova, N.A.; Verhaegen, M.E.; Bichakjian, C.K.; Ward, N.L.; Dlugosz, A.A.; Wong, S.Y. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 2015, 16, 400–412. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef]
- Polli-Lopes, A.C.; Zucoloto, S.; de Queirós Cunha, F.; da Silva Figueiredo, L.A.; Garcia, S.B. Myenteric denervation reduces the incidence of gastric tumors in rats. Cancer Lett. 2003, 190, 45–50. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Sakitani, K.; Konishi, M.; Asfaha, S.; Niikura, R.; Tomita, H.; Renz, B.W.; Tailor, Y.; Macchini, M.; Middelhoff, M.; et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 2017, 31, 21–34. [Google Scholar] [CrossRef]
- Yang, E.V.; Eubank, T.D. The impact of adrenergic signaling in skin cancer progression: Possible repurposing of β-blockers for treatment of skin cancer. Cancer Biomark. 2013, 13, 155–160. [Google Scholar] [CrossRef]
- Phadke, S.; Clamon, G. Beta blockade as adjunctive breast cancer therapy: A review. Crit. Rev. Oncol. Hematol. 2019, 138, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Lin, J.H.; Arcuino, G.; Gao, Q.; Yang, J.; Nedergaard, M. Glutamate release promotes growth of malignant gliomas. Nat. Med. 2001, 7, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Michael, I.P.; Zhang, P.; Saghafinia, S.; Knott, G.; Jiao, W.; McCabe, B.D.; Galván, J.A.; Robinson, H.P.C.; Zlobec, I.; et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 2019, 573, 526–531. [Google Scholar] [CrossRef]
- Friedman, J.R.; Richbart, S.D.; Merritt, J.C.; Brown, K.C.; Nolan, N.A.; Akers, A.T.; Lau, J.K.; Robateau, Z.R.; Miles, S.L.; Dasgupta, P. Acetylcholine signaling system in progression of lung cancers. Pharmacol. Ther. 2019, 194, 222–254. [Google Scholar] [CrossRef] [PubMed]
- Romon, R.; Adriaenssens, E.; Lagadec, C.; Germain, E.; Hondermarck, H.; Le Bourhis, X. Nerve growth factor promotes breast cancer angiogenesis by activating multiple pathways. Mol. Cancer 2010, 9, 157. [Google Scholar] [CrossRef]
- Chakroborty, D.; Sarkar, C.; Mitra, R.B.; Banerjee, S.; Dasgupta, P.S.; Basu, S. Depleted dopamine in gastric cancer tissues: Dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin. Cancer Res. 2004, 10, 4349–4356. [Google Scholar] [CrossRef]
- Zahalka, A.H.; Arnal-Estapé, A.; Maryanovich, M.; Nakahara, F.; Cruz, C.D.; Finley, L.W.S.; Frenette, P.S. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 2017, 358, 321–326. [Google Scholar] [CrossRef]
- Kamiya, A.; Hayama, Y.; Kato, S.; Shimomura, A.; Shimomura, T.; Irie, K.; Kaneko, R.; Yanagawa, Y.; Kobayashi, K.; Ochiya, T. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 2019, 22, 1289–1305. [Google Scholar] [CrossRef]
- Partecke, L.I.; Speerforck, S.; Käding, A.; Seubert, F.; Kühn, S.; Lorenz, E.; Schwandke, S.; Sendler, M.; Keßler, W.; Trung, D.N.; et al. Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology 2016, 16, 423–433. [Google Scholar] [CrossRef]
- Makwana, M.; Raivich, G. Molecular mechanisms in successful peripheral regeneration. FEBS J. 2005, 272, 2628–2638. [Google Scholar] [CrossRef]
- Lu, R.; Fan, C.; Shangguan, W.; Liu, Y.; Li, Y.; Shang, Y.; Yin, D.; Zhang, S.; Huang, Q.; Li, X.; et al. Neurons generated from carcinoma stem cells support cancer progression. Signal Transduct. Target. Ther. 2017, 2, 16036. [Google Scholar] [CrossRef]
- Idiaquez, J.; Benarroch, E.; Nogues, M. Evaluation and Management of Autonomic Disorders: A Case-Based Practical Guide; Springer: Cham, Swizerland, 2018; pp. 3–18. [Google Scholar]
- McVary, K.T.; Razzaq, A.; Lee, C.; Venegas, M.F.; Rademaker, A.; McKenna, K.E. Growth of the Rat Prostate Gland is Facilitated by the Autonomic Nervous System. Biol. Reprod. 1994, 51, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Philippe Mauffrey, N.T.; Barroca, V.; Bemelmans, A.-P.; Firlej, V.; Allory, Y.; Roméo, P.-H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678. [Google Scholar] [CrossRef]
- Moran Amit, H.T.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; Knutsen, E.; et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 2020, 578, 449–454. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lin, C.P.; Ho, J.J.; He, X.; Okada, N.; Bu, P.; Zhong, Y.; Kim, S.Y.; Bennett, M.J.; Chen, C.; et al. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat. Cell Biol. 2011, 13, 1353–1360. [Google Scholar] [CrossRef]
- Agostini, M.; Tucci, P.; Killick, R.; Candi, E.; Sayan, B.S.; Rivetti di val Cervo, P.; Nicotera, P.; McKeon, F.; Knight, R.A.; Mak, T.W.; et al. Neuronal differentiation by TAp73 is mediated by microRNA-34a regulation of synaptic protein targets. Proc. Natl. Acad. Sci. USA 2011, 108, 21093–21098. [Google Scholar] [CrossRef]
- Jauhari, A.; Singh, T.; Singh, P.; Parmar, D.; Yadav, S. Regulation of miR-34 Family in Neuronal Development. Mol. Neurobiol. 2018, 55, 936–945. [Google Scholar] [CrossRef]
- Handly, L.N.; Pilko, A.; Wollman, R. Paracrine communication maximizes cellular response fidelity in wound signaling. Elife 2015, 4, e09652. [Google Scholar] [CrossRef] [PubMed]
- Saloman, J.L.; Albers, K.M.; Rhim, A.D.; Davis, B.M. Can Stopping Nerves, Stop Cancer? Trends Neurosci. 2016, 39, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, Q.; Lu, Z.; Wang, L.; Ding, L.; Xia, L.; Zhang, H.; Wang, M.; Chen, Y.; Li, G. Role of the nervous system in cancers: A review. Cell Death Discov. 2021, 7, 76. [Google Scholar] [CrossRef]
- Silverman, D.A.; Martinez, V.K.; Dougherty, P.M.; Myers, J.N.; Calin, G.A.; Amit, M. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer Res. 2021, 81, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Spini, A.; Roberto, G.; Gini, R.; Bartolini, C.; Bazzani, L.; Donnini, S.; Crispino, S.; Ziche, M. Evidence of β-blockers drug repurposing for the treatment of triple negative breast cancer: A systematic review. Neoplasma 2019, 66, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Grytli, H.H.; Fagerland, M.W.; Fosså, S.D.; Taskén, K.A. Association between use of β-blockers and prostate cancer-specific survival: A cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur. Urol. 2014, 65, 635–641. [Google Scholar] [CrossRef]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, L.C.; LaMantia, A.-S.; McNamara, J.O.; Williams, S.M. (Eds.) Chemical Synapses. In Neuroscience, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK11009/ (accessed on 13 June 2023).
- Jabeen, S.; Thirumalai, V. The interplay between electrical and chemical synaptogenesis. J. Neurophysiol. 2018, 120, 1914–1922. [Google Scholar] [CrossRef]
- Choquet, D.; Triller, A. The dynamic synapse. Neuron 2013, 80, 691–703. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Rzeski, W.; Turski, L.; Ikonomidou, C. Glutamate antagonists limit tumor growth. Proc. Natl. Acad. Sci. USA 2001, 98, 6372–6377. [Google Scholar] [CrossRef]
- Gambrill, A.C.; Barria, A. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc. Natl. Acad. Sci. USA 2011, 108, 5855–5860. [Google Scholar] [CrossRef]
- Li, L.; Hanahan, D. Hijacking the neuronal NMDAR signaling circuit to promote tumor growth and invasion. Cell 2013, 153, 86–100. [Google Scholar] [CrossRef]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Role of the Nervous System in Tumor Angiogenesis. Cancer Microenviron. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Eichmann, A.; Brunet, I. Arterial innervation in development and disease. Sci. Transl. Med. 2014, 6, 252ps9. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Li, S.; Xu, H.X.; Wu, C.T.; Wang, W.Q.; Jin, W.; Gao, H.L.; Li, H.; Zhang, S.R.; Xu, J.Z.; Qi, Z.H.; et al. Angiogenesis in pancreatic cancer: Current research status and clinical implications. Angiogenesis 2019, 22, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, M.C.; Sarkar, C. Morphology of angiogenesis in human cancer: A conceptual overview, histoprognostic perspective and significance of neoangiogenesis. Histopathology 2005, 46, 481–489. [Google Scholar] [CrossRef]
- Wrona, D. Neural-immune interactions: An integrative view of the bidirectional relationship between the brain and immune systems. J. Neuroimmunol. 2006, 172, 38–58. [Google Scholar] [CrossRef]
- Procaccini, C.; Pucino, V.; De Rosa, V.; Marone, G.; Matarese, G. Neuro-endocrine networks controlling immune system in health and disease. Front. Immunol. 2014, 5, 143. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdés-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S.; et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011, 334, 98–101. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef]
- Cox, M.A.; Duncan, G.S.; Lin, G.H.Y.; Steinberg, B.E.; Yu, L.X.; Brenner, D.; Buckler, L.N.; Elia, A.J.; Wakeham, A.C.; Nieman, B.; et al. Choline acetyltransferase-expressing T cells are required to control chronic viral infection. Science 2019, 363, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Salmon, H.; Remark, R.; Gnjatic, S.; Merad, M. Host tissue determinants of tumour immunity. Nat. Rev. Cancer 2019, 19, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Partecke, L.I.; Günther, C.; Hagemann, S.; Jacobi, C.; Merkel, M.; Sendler, M.; van Rooijen, N.; Käding, A.; Nguyen Trung, D.; Lorenz, E.; et al. Induction of M2-macrophages by tumour cells and tumour growth promotion by M2-macrophages: A quid pro quo in pancreatic cancer. Pancreatology 2013, 13, 508–516. [Google Scholar] [CrossRef]
- Sanchez, L.R.; Borriello, L.; Entenberg, D.; Condeelis, J.S.; Oktay, M.H.; Karagiannis, G.S. The emerging roles of macrophages in cancer metastasis and response to chemotherapy. J. Leukoc. Biol. 2019, 106, 259–274. [Google Scholar] [CrossRef]
- Partecke, L.I.; Käding, A.; Trung, D.N.; Diedrich, S.; Sendler, M.; Weiss, F.; Kühn, J.-P.; Mayerle, J.; Beyer, K.; Von Bernstorff, W.; et al. Subdiaphragmatic vagotomy promotes tumor growth and reduces survival via TNFα in a murine pancreatic cancer model. Oncotarget 2017, 8, 22501–22512. [Google Scholar] [CrossRef]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.; Morizono, K.; Karanikolas, B.D.; Wu, L.; et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef]
- Guldner, I.H.; Wang, Q.; Yang, L.; Golomb, S.M.; Zhao, Z.; Lopez, J.A.; Brunory, A.; Howe, E.N.; Zhang, Y.; Palakurthi, B.; et al. CNS-Native Myeloid Cells Drive Immune Suppression in the Brain Metastatic Niche through Cxcl10. Cell 2020, 183, 1234–1248.e25. [Google Scholar] [CrossRef]
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef]
- You, H.; Baluszek, S.; Kaminska, B. Immune Microenvironment of Brain Metastases-Are Microglia and Other Brain Macrophages Little Helpers? Front. Immunol. 2019, 10, 1941. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Beutel, G.; Rhein, M.; Meyer, J.; Koenecke, C.; Neumann, T.; Yang, M.; Krauter, J.; von Neuhoff, N.; Heuser, M.; et al. High-affinity neurotrophin receptors and ligands promote leukemogenesis. Blood 2009, 113, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.A. Selectivity in neurotrophin signaling: Theme and variations. Annu. Rev. Neurosci. 2003, 26, 299–330. [Google Scholar] [CrossRef]
- Stoleru, B.; Popescu, A.M.; Tache, D.E.; Neamtu, O.M.; Emami, G.; Tataranu, L.G.; Buteica, A.S.; Dricu, A.; Purcaru, S.O. Tropomyosin-receptor-kinases signaling in the nervous system. Maedica 2013, 8, 43–48. [Google Scholar]
- Miknyoczki, S.J.; Wan, W.; Chang, H.; Dobrzanski, P.; Ruggeri, B.A.; Dionne, C.A.; Buchkovich, K. The neurotrophin-trk receptor axes are critical for the growth and progression of human prostatic carcinoma and pancreatic ductal adenocarcinoma xenografts in nude mice. Clin. Cancer Res. 2002, 8, 1924–1931. [Google Scholar]
- Passiglia, F.; Caparica, R.; Giovannetti, E.; Giallombardo, M.; Listi, A.; Diana, P.; Cirrincione, G.; Caglevic, C.; Raez, L.E.; Russo, A.; et al. The potential of neurotrophic tyrosine kinase (NTRK) inhibitors for treating lung cancer. Expert Opin. Investig. Drugs 2016, 25, 385–392. [Google Scholar] [CrossRef]
- Miknyoczki, S.J.; Lang, D.; Huang, L.; Klein-Szanto, A.J.; Dionne, C.A.; Ruggeri, B.A. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: Expression patterns and effects on in vitro invasive behavior. Int. J. Cancer 1999, 81, 417–427. [Google Scholar] [CrossRef]
- Pundavela, J.; Roselli, S.; Faulkner, S.; Attia, J.; Scott, R.J.; Thorne, R.F.; Forbes, J.F.; Bradshaw, R.A.; Walker, M.M.; Jobling, P.; et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol. Oncol. 2015, 9, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Canossa, M.; Twiss, J.L.; Verity, A.N.; Shooter, E.M. p75(NGFR) and TrkA receptors collaborate to rapidly activate a p75(NGFR)-associated protein kinase. EMBO J. 1996, 15, 3369–3376. [Google Scholar] [CrossRef]
- Bruno, F.; Abondio, P.; Montesanto, A.; Luiselli, D.; Bruni, A.C.; Maletta, R. The Nerve Growth Factor Receptor (NGFR/p75(NTR)): A Major Player in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 3200. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Villadsen, R.; Sørlie, T.; Fogh, L.; Grønlund, S.Z.; Fridriksdottir, A.J.; Kuhn, I.; Rank, F.; Wielenga, V.T.; Solvang, H.; et al. Tumor initiating but differentiated luminal-like breast cancer cells are highly invasive in the absence of basal-like activity. Proc. Natl. Acad. Sci. USA 2012, 109, 6124–6129. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Huang, S.; Wang, J.; Zhang, Y.; Xiong, Y.; Zeng, S.X.; Lu, H. Inactivating p53 is essential for nerve growth factor receptor to promote melanoma-initiating cell-stemmed tumorigenesis. Cell Death Dis. 2020, 11, 550. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, K.; Yuan, M.; Luo, K.Q. Nerve growth factor receptor increases the tumor growth and metastatic potential of triple-negative breast cancer cells. Oncogene 2021, 40, 2165–2181. [Google Scholar] [CrossRef]
- Li, Q.; Cao, Z.; Zhao, S. The Emerging Portrait of Glial Cell Line-derived Neurotrophic Factor Family Receptor Alpha (GFRα) in Cancers. Int. J. Med. Sci. 2022, 19, 659–668. [Google Scholar] [CrossRef]
- Arighi, E.; Borrello, M.G.; Sariola, H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005, 16, 441–467. [Google Scholar] [CrossRef]
- Boulay, A.; Breuleux, M.; Stephan, C.; Fux, C.; Brisken, C.; Fiche, M.; Wartmann, M.; Stumm, M.; Lane, H.A.; Hynes, N.E. The Ret receptor tyrosine kinase pathway functionally interacts with the ERalpha pathway in breast cancer. Cancer Res. 2008, 68, 3743–3751. [Google Scholar] [CrossRef]
- Kim, M.; Jung, J.Y.; Choi, S.; Lee, H.; Morales, L.D.; Koh, J.T.; Kim, S.H.; Choi, Y.D.; Choi, C.; Slaga, T.J.; et al. GFRA1 promotes cisplatin-induced chemoresistance in osteosarcoma by inducing autophagy. Autophagy 2017, 13, 149–168. [Google Scholar] [CrossRef]
- Kim, M.; Kim, D.J. GFRA1: A Novel Molecular Target for the Prevention of Osteosarcoma Chemoresistance. Int. J. Mol. Sci. 2018, 19, 1078. [Google Scholar] [CrossRef]
- Huber, R.M.; Lucas, J.M.; Gomez-Sarosi, L.A.; Coleman, I.; Zhao, S.; Coleman, R.; Nelson, P.S. DNA damage induces GDNF secretion in the tumor microenvironment with paracrine effects promoting prostate cancer treatment resistance. Oncotarget 2015, 6, 2134–2147. [Google Scholar] [CrossRef]
- Li, Z.; Xie, J.; Fei, Y.; Gao, P.; Xie, Q.; Gao, W.; Xu, Z. GDNF family receptor alpha 2 promotes neuroblastoma cell proliferation by interacting with PTEN. Biochem. Biophys. Res. Commun. 2019, 510, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Eftang, L.L.; Klajic, J.; Kristensen, V.N.; Tost, J.; Esbensen, Q.Y.; Blom, G.P.; Bukholm, I.R.; Bukholm, G. GFRA3 promoter methylation may be associated with decreased postoperative survival in gastric cancer. BMC Cancer 2016, 16, 225. [Google Scholar] [CrossRef] [PubMed]
- Cavel, O.; Shomron, O.; Shabtay, A.; Vital, J.; Trejo-Leider, L.; Weizman, N.; Krelin, Y.; Fong, Y.; Wong, R.J.; Amit, M.; et al. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 2012, 72, 5733–5743. [Google Scholar] [CrossRef]
- Gil, Z.; Cavel, O.; Kelly, K.; Brader, P.; Rein, A.; Gao, S.P.; Carlson, D.L.; Shah, J.P.; Fong, Y.; Wong, R.J. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J. Natl. Cancer Inst. 2010, 102, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Altevogt, P.; Doberstein, K.; Fogel, M. L1CAM in human cancer. Int. J. Cancer 2016, 138, 1565–1576. [Google Scholar] [CrossRef]
- Terraneo, N.; Jacob, F.; Peitzsch, C.; Dubrovska, A.; Krudewig, C.; Huang, Y.L.; Heinzelmann-Schwarz, V.; Schibli, R.; Béhé, M.; Grünberg, J. L1 Cell Adhesion Molecule Confers Radioresistance to Ovarian Cancer and Defines a New Cancer Stem Cell Population. Cancers 2020, 12, 217. [Google Scholar] [CrossRef]
- Kasper, C.; Rasmussen, H.; Kastrup, J.S.; Ikemizu, S.; Jones, E.Y.; Berezin, V.; Bock, E.; Larsen, I.K. Structural basis of cell-cell adhesion by NCAM. Nat. Struct. Biol. 2000, 7, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.A.; Small, S.J.; Akeson, R. At least 27 alternatively spliced forms of the neural cell adhesion molecule mRNA are expressed during rat heart development. Mol. Cell. Biol. 1991, 11, 1654–1661. [Google Scholar] [CrossRef]
- Michalides, R.; Kwa, B.; Springall, D.; van Zandwijk, N.; Koopman, J.; Hilkens, J.; Mooi, W. NCAM and lung cancer. Int. J. Cancer Suppl. 1994, 8, 34–37. [Google Scholar] [CrossRef]
- Zecchini, S.; Bombardelli, L.; Decio, A.; Bianchi, M.; Mazzarol, G.; Sanguineti, F.; Aletti, G.; Maddaluno, L.; Berezin, V.; Bock, E.; et al. The adhesion molecule NCAM promotes ovarian cancer progression via FGFR signalling. EMBO Mol. Med. 2011, 3, 480–494. [Google Scholar] [CrossRef]
- Strang, C.E.; Long, Y.; Gavrikov, K.E.; Amthor, F.R.; Keyser, K.T. Nicotinic and muscarinic acetylcholine receptors shape ganglion cell response properties. J. Neurophysiol. 2015, 113, 203–217. [Google Scholar] [CrossRef]
- Kruse, A.C.; Kobilka, B.K.; Gautam, D.; Sexton, P.M.; Christopoulos, A.; Wess, J. Muscarinic acetylcholine receptors: Novel opportunities for drug development. Nat. Rev. Drug Discov. 2014, 13, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Dani, J.A. Neuronal Nicotinic Acetylcholine Receptor Structure and Function and Response to Nicotine. Int. Rev. Neurobiol. 2015, 124, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cheuk, I.W.Y.; Shin, V.Y.; Kwong, A. Acetylcholine receptors: Key players in cancer development. Surg. Oncol. 2019, 31, 46–53. [Google Scholar] [CrossRef]
- Saracino, L.; Zorzetto, M.; Inghilleri, S.; Pozzi, E.; Stella, G.M. Non-neuronal cholinergic system in airways and lung cancer susceptibility. Transl. Lung Cancer Res. 2013, 2, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Vogelzang, A.; Miyajima, M.; Sugiura, Y.; Wu, Y.; Chamoto, K.; Nakano, R.; Hatae, R.; Menzies, R.J.; Sonomura, K.; et al. B cell-derived GABA elicits IL-10(+) macrophages to limit anti-tumour immunity. Nature 2021, 599, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Samimi, R.; Xie, G.; Shant, J.; Drachenberg, C.; Wade, M.; Davis, R.J.; Nomikos, G.; Raufman, J.P. Acetylcholine release by human colon cancer cells mediates autocrine stimulation of cell proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G591–G597. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Sekhon, H.S.; Jia, Y.; Keller, J.A.; Blusztajn, J.K.; Mark, G.P.; Spindel, E.R. Acetylcholine is synthesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res. 2003, 63, 214–221. [Google Scholar]
- Nie, M.; Chen, N.; Pang, H.; Jiang, T.; Jiang, W.; Tian, P.; Yao, L.; Chen, Y.; DeBerardinis, R.J.; Li, W.; et al. Targeting acetylcholine signaling modulates persistent drug tolerance in EGFR-mutant lung cancer and impedes tumor relapse. J. Clin. Investig. 2022, 132, e160152. [Google Scholar] [CrossRef]
- Song, I.; Huganir, R.L. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002, 25, 578–588. [Google Scholar] [CrossRef]
- Greger, I.H.; Watson, J.F.; Cull-Candy, S.G. Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron 2017, 94, 713–730. [Google Scholar] [CrossRef] [PubMed]
- De Groot, J.; Sontheimer, H. Glutamate and the biology of gliomas. Glia 2011, 59, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer, H. A role for glutamate in growth and invasion of primary brain tumors. J. Neurochem. 2008, 105, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Herner, A.; Sauliunaite, D.; Michalski, C.W.; Erkan, M.; De Oliveira, T.; Abiatari, I.; Kong, B.; Esposito, I.; Friess, H.; Kleeff, J. Glutamate increases pancreatic cancer cell invasion and migration via AMPA receptor activation and Kras-MAPK signaling. Int. J. Cancer 2011, 129, 2349–2359. [Google Scholar] [CrossRef]
- Furukawa, H.; Singh, S.K.; Mancusso, R.; Gouaux, E. Subunit arrangement and function in NMDA receptors. Nature 2005, 438, 185–192. [Google Scholar] [CrossRef]
- Salussolia, C.L.; Prodromou, M.L.; Borker, P.; Wollmuth, L.P. Arrangement of subunits in functional NMDA receptors. J. Neurosci. 2011, 31, 11295–11304. [Google Scholar] [CrossRef]
- Abdul, M.; Hoosein, N. N-methyl-D-aspartate receptor in human prostate cancer. J. Membr. Biol. 2005, 205, 125–128. [Google Scholar] [CrossRef]
- North, W.G.; Gao, G.; Jensen, A.; Memoli, V.A.; Du, J. NMDA receptors are expressed by small-cell lung cancer and are potential targets for effective treatment. Clin. Pharmacol. 2010, 2, 31–40. [Google Scholar] [CrossRef]
- North, W.G.; Gao, G.; Memoli, V.A.; Pang, R.H.; Lynch, L. Breast cancer expresses functional NMDA receptors. Breast Cancer Res. Treat. 2010, 122, 307–314. [Google Scholar] [CrossRef]
- North, W.G.; Liu, F.; Lin, L.Z.; Tian, R.; Akerman, B. NMDA receptors are important regulators of pancreatic cancer and are potential targets for treatment. Clin. Pharmacol. 2017, 9, 79–86. [Google Scholar] [CrossRef]
- Stepulak, A.; Sifringer, M.; Rzeski, W.; Endesfelder, S.; Gratopp, A.; Pohl, E.E.; Bittigau, P.; Felderhoff-Mueser, U.; Kaindl, A.M.; Bührer, C.; et al. NMDA antagonist inhibits the extracellular signal-regulated kinase pathway and suppresses cancer growth. Proc. Natl. Acad. Sci. USA 2005, 102, 15605–15610. [Google Scholar] [CrossRef]
- Deutsch, S.I.; Tang, A.H.; Burket, J.A.; Benson, A.D. NMDA receptors on the surface of cancer cells: Target for chemotherapy? Biomed. Pharmacother. 2014, 68, 493–496. [Google Scholar] [CrossRef]
- Bonsi, P.; Cuomo, D.; De Persis, C.; Centonze, D.; Bernardi, G.; Calabresi, P.; Pisani, A. Modulatory action of metabotropic glutamate receptor (mGluR) 5 on mGluR1 function in striatal cholinergic interneurons. Neuropharmacology 2005, 49, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.J.; Wall, B.A.; Wangari-Talbot, J.; Chen, S. Metabotropic glutamate receptors in cancer. Neuropharmacology 2017, 115, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Matera, C.; Calvé, P.; Casadó-Anguera, V.; Sortino, R.; Gomila, A.M.J.; Moreno, E.; Gener, T.; Delgado-Sallent, C.; Nebot, P.; Costazza, D.; et al. Reversible Photocontrol of Dopaminergic Transmission in Wild-Type Animals. Int. J. Mol. Sci. 2022, 23, 10114. [Google Scholar] [CrossRef] [PubMed]
- Rondou, P.; Haegeman, G.; Van Craenenbroeck, K. The dopamine D4 receptor: Biochemical and signalling properties. Cell. Mol. Life Sci. 2010, 67, 1971–1986. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef]
- Martel, J.C.; Gatti McArthur, S. Dopamine Receptor Subtypes, Physiology and Pharmacology: New Ligands and Concepts in Schizophrenia. Front. Pharmacol. 2020, 11, 1003. [Google Scholar] [CrossRef]
- Rangel-Barajas, C.; Coronel, I.; Florán, B. Dopamine Receptors and Neurodegeneration. Aging Dis. 2015, 6, 349–368. [Google Scholar] [CrossRef]
- Mark, M.D.; Herlitze, S. G-protein mediated gating of inward-rectifier K+ channels. Eur. J. Biochem. 2000, 267, 5830–5836. [Google Scholar] [CrossRef]
- Brami-Cherrier, K.; Valjent, E.; Garcia, M.; Pagès, C.; Hipskind, R.A.; Caboche, J. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: A new route to cAMP response element-binding protein phosphorylation. J. Neurosci. 2002, 22, 8911–8921. [Google Scholar] [CrossRef] [PubMed]
- Mannoury la Cour, C.; Salles, M.J.; Pasteau, V.; Millan, M.J. Signaling pathways leading to phosphorylation of Akt and GSK-3β by activation of cloned human and rat cerebral D₂and D₃ receptors. Mol. Pharmacol. 2011, 79, 91–105. [Google Scholar] [CrossRef]
- Yin, J.; Chen, K.M.; Clark, M.J.; Hijazi, M.; Kumari, P.; Bai, X.C.; Sunahara, R.K.; Barth, P.; Rosenbaum, D.M. Structure of a D2 dopamine receptor-G-protein complex in a lipid membrane. Nature 2020, 584, 125–129. [Google Scholar] [CrossRef]
- Weissenrieder, J.S.; Neighbors, J.D.; Mailman, R.B.; Hohl, R.J. Cancer and the Dopamine D(2) Receptor: A Pharmacological Perspective. J. Pharmacol. Exp. Ther. 2019, 370, 111–126. [Google Scholar] [CrossRef]
- Kline, C.L.B.; Ralff, M.D.; Lulla, A.R.; Wagner, J.M.; Abbosh, P.H.; Dicker, D.T.; Allen, J.E.; El-Deiry, W.S. Role of Dopamine Receptors in the Anticancer Activity of ONC201. Neoplasia 2018, 20, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Tegowski, M.; Fan, C.; Baldwin, A.S. Thioridazine inhibits self-renewal in breast cancer cells via DRD2-dependent STAT3 inhibition, but induces a G(1) arrest independent of DRD2. J. Biol. Chem. 2018, 293, 15977–15990. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Bracci-Laudiero, L.; Bonini, S.; Manni, L. The expanding role of nerve growth factor: From neurotrophic activity to immunologic diseases. Allergy 1997, 52, 883–894. [Google Scholar] [CrossRef]
- Lee, S.; Hwang, C.; Marini, S.; Tower, R.J.; Qin, Q.; Negri, S.; Pagani, C.A.; Sun, Y.; Stepien, D.M.; Sorkin, M.; et al. NGF-TrkA signaling dictates neural ingrowth and aberrant osteochondral differentiation after soft tissue trauma. Nat. Commun. 2021, 12, 4939. [Google Scholar] [CrossRef]
- Dollé, L.; Adriaenssens, E.; El Yazidi-Belkoura, I.; Le Bourhis, X.; Nurcombe, V.; Hondermarck, H. Nerve growth factor receptors and signaling in breast cancer. Curr. Cancer Drug Targets 2004, 4, 463–470. [Google Scholar] [CrossRef]
- Descamps, S.; Toillon, R.A.; Adriaenssens, E.; Pawlowski, V.; Cool, S.M.; Nurcombe, V.; Le Bourhis, X.; Boilly, B.; Peyrat, J.P.; Hondermarck, H. Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. J. Biol. Chem. 2001, 276, 17864–17870. [Google Scholar] [CrossRef]
- Arrighi, N.; Bodei, S.; Zani, D.; Simeone, C.; Cunico, S.C.; Missale, C.; Spano, P.; Sigala, S. Nerve growth factor signaling in prostate health and disease. Growth Factors 2010, 28, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Sortino, M.A.; Condorelli, F.; Vancheri, C.; Chiarenza, A.; Bernardini, R.; Consoli, U.; Canonico, P.L. Mitogenic effect of nerve growth factor (NGF) in LNCaP prostate adenocarcinoma cells: Role of the high- and low-affinity NGF receptors. Mol. Endocrinol. 2000, 14, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Molloy, N.H.; Read, D.E.; Gorman, A.M. Nerve Growth Factor in Cancer Cell Death and Survival. Cancers 2011, 3, 510–530. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.B.; Standop, J.; Ulrich, A.; Wittel, U.; Friess, H.; Andrén-Sandberg, A.; Pour, P.M. Expression of nerve growth factors in pancreatic neural tissue and pancreatic cancer. J. Histochem. Cytochem. 2001, 49, 1205–1210. [Google Scholar] [CrossRef]
- Renz, B.W.; Takahashi, R.; Tanaka, T.; Macchini, M.; Hayakawa, Y.; Dantes, Z.; Maurer, H.C.; Chen, X.; Jiang, Z.; Westphalen, C.B.; et al. β2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018, 33, 75–90.e77. [Google Scholar] [CrossRef]
- Lam, C.T.; Yang, Z.F.; Lau, C.K.; Tam, K.H.; Fan, S.T.; Poon, R.T. Brain-derived neurotrophic factor promotes tumorigenesis via induction of neovascularization: Implication in hepatocellular carcinoma. Clin. Cancer Res. 2011, 17, 3123–3133. [Google Scholar] [CrossRef]
- Vanhecke, E.; Adriaenssens, E.; Verbeke, S.; Meignan, S.; Germain, E.; Berteaux, N.; Nurcombe, V.; Le Bourhis, X.; Hondermarck, H. Brain-derived neurotrophic factor and neurotrophin-4/5 are expressed in breast cancer and can be targeted to inhibit tumor cell survival. Clin. Cancer Res. 2011, 17, 1741–1752. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Tseng, L.M.; Hsu, C.Y.; Yang, M.H.; Chiu, J.H.; Shyr, Y.M. Brain-derived neurotrophic factor (BDNF) -TrKB signaling modulates cancer-endothelial cells interaction and affects the outcomes of triple negative breast cancer. PLoS ONE 2017, 12, e0178173. [Google Scholar] [CrossRef]
- Maisonpierre, P.C.; Belluscio, L.; Friedman, B.; Alderson, R.F.; Wiegand, S.J.; Furth, M.E.; Lindsay, R.M.; Yancopoulos, G.D. NT-3, BDNF, and NGF in the developing rat nervous system: Parallel as well as reciprocal patterns of expression. Neuron 1990, 5, 501–509. [Google Scholar] [CrossRef]
- Bové, M.; Monto, F.; Guillem-Llobat, P.; Ivorra, M.D.; Noguera, M.A.; Zambrano, A.; Sirerol-Piquer, M.S.; Requena, A.C.; García-Alonso, M.; Tejerina, T.; et al. NT3/TrkC Pathway Modulates the Expression of UCP-1 and Adipocyte Size in Human and Rodent Adipose Tissue. Front. Endocrinol. 2021, 12, 630097. [Google Scholar] [CrossRef]
- Butte, M.J.; Hwang, P.K.; Mobley, W.C.; Fletterick, R.J. Crystal structure of neurotrophin-3 homodimer shows distinct regions are used to bind its receptors. Biochemistry 1998, 37, 16846–16852. [Google Scholar] [CrossRef] [PubMed]
- Louie, E.; Chen, X.F.; Coomes, A.; Ji, K.; Tsirka, S.; Chen, E.I. Neurotrophin-3 modulates breast cancer cells and the microenvironment to promote the growth of breast cancer brain metastasis. Oncogene 2013, 32, 4064–4077. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Kim, J.S.; Ma, Y.E.; Cho, H.W.; Ju, H.Y.; Lee, S.H.; Lee, J.W.; Yoo, K.H.; Sung, K.W.; Koo, H.H. Differential Clinical Significance of Neurotrophin-3 Expression according to MYCN Amplification and TrkC Expression in Neuroblastoma. J. Korean Med. Sci. 2019, 34, e254. [Google Scholar] [CrossRef]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef]
- Heuckeroth, R.O.; Lampe, P.A.; Johnson, E.M.; Milbrandt, J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev. Biol. 1998, 200, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Ketterer, K.; Rao, S.; Friess, H.; Weiss, J.; Büchler, M.W.; Korc, M. Reverse transcription-PCR analysis of laser-captured cells points to potential paracrine and autocrine actions of neurotrophins in pancreatic cancer. Clin. Cancer Res. 2003, 9, 5127–5136. [Google Scholar] [PubMed]
- Funahashi, H.; Okada, Y.; Sawai, H.; Takahashi, H.; Matsuo, Y.; Takeyama, H.; Manabe, T. The role of glial cell line-derived neurotrophic factor (GDNF) and integrins for invasion and metastasis in human pancreatic cancer cells. J. Surg. Oncol. 2005, 91, 77–83. [Google Scholar] [CrossRef]
- Na’ara, S.; Amit, M.; Gil, Z. L1CAM induces perineural invasion of pancreas cancer cells by upregulation of metalloproteinase expression. Oncogene 2019, 38, 596–608. [Google Scholar] [CrossRef]
- Heuckeroth, R.O.; Enomoto, H.; Grider, J.R.; Golden, J.P.; Hanke, J.A.; Jackman, A.; Molliver, D.C.; Bardgett, M.E.; Snider, W.D.; Johnson, E.M., Jr.; et al. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron 1999, 22, 253–263. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas. TCGA Pan-Cancer Atlas. Accession Number: TCGA-2018-03-19. 2018. Available online: https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga (accessed on 13 June 2023).
- Baloh, R.H.; Tansey, M.G.; Lampe, P.A.; Fahrner, T.J.; Enomoto, H.; Simburger, K.S.; Leitner, M.L.; Araki, T.; Johnson, E.M., Jr.; Milbrandt, J. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron 1998, 21, 1291–1302. [Google Scholar] [CrossRef]
- Airaksinen, M.S.; Saarma, M. The GDNF family: Signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002, 3, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Na’ara, S.; Gil, Z. Mechanisms of cancer dissemination along nerves. Nat. Rev. Cancer 2016, 16, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Demir, I.E.; D’Haese, J.G.; Tieftrunk, E.; Kujundzic, K.; Schorn, S.; Xing, B.; Kehl, T.; Friess, H.; Ceyhan, G.O. The neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis 2014, 35, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Bo, H.; Wang, Y.; Zhang, J.; Zhu, M. Neurotrophic Factor Artemin Promotes Invasiveness and Neurotrophic Function of Pancreatic Adenocarcinoma In Vivo and In Vitro. Pancreas 2015, 44, 134–143. [Google Scholar] [CrossRef][Green Version]
- Ceyhan, G.O.; Giese, N.A.; Erkan, M.; Kerscher, A.G.; Wente, M.N.; Giese, T.; Büchler, M.W.; Friess, H. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann. Surg. 2006, 244, 274–281. [Google Scholar] [CrossRef]
- Pal, M.M. Glutamate: The Master Neurotransmitter and Its Implications in Chronic Stress and Mood Disorders. Front. Hum. Neurosci. 2021, 15, 722323. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef]
- Vicini, S. The role of GABA and glutamate on adult neurogenesis. J. Physiol. 2008, 586, 3737–3738. [Google Scholar] [CrossRef]
- Fazzari, J.; Lin, H.; Murphy, C.; Ungard, R.; Singh, G. Inhibitors of glutamate release from breast cancer cells; new targets for cancer-induced bone-pain. Sci. Rep. 2015, 5, 8380. [Google Scholar] [CrossRef]
- Seidlitz, E.P.; Sharma, M.K.; Saikali, Z.; Ghert, M.; Singh, G. Cancer cell lines release glutamate into the extracellular environment. Clin. Exp. Metastasis 2009, 26, 781–787. [Google Scholar] [CrossRef]
- Grant, C.E.; Flis, A.L.; Ryan, B.M. Understanding the role of dopamine in cancer: Past, present and future. Carcinogenesis 2022, 43, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Pan, J.; Chen, Y.; Xing, W.; Li, Q.; Wang, D.; Zhou, X.; Xie, J.; Miao, C.; Yuan, Y.; et al. Increased dopamine and its receptor dopamine receptor D1 promote tumor growth in human hepatocellular carcinoma. Cancer Commun. 2020, 40, 694–710. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.; Kim, C.Y.; Lee, J.M.; Ryu, B.; Kim, J.; Shin, C.; Park, J.H. Pimozide Inhibits the Human Prostate Cancer Cells Through the Generation of Reactive Oxygen Species. Front. Pharmacol. 2019, 10, 1517. [Google Scholar] [CrossRef]
- Shi, X.N.; Li, H.; Yao, H.; Liu, X.; Li, L.; Leung, K.S.; Kung, H.F.; Lu, D.; Wong, M.H.; Lin, M.C. In Silico Identification and In Vitro and In Vivo Validation of Anti-Psychotic Drug Fluspirilene as a Potential CDK2 Inhibitor and a Candidate Anti-Cancer Drug. PLoS ONE 2015, 10, e0132072. [Google Scholar] [CrossRef] [PubMed]
- Tuan, N.M.; Lee, C.H. Penfluridol as a Candidate of Drug Repurposing for Anticancer Agent. Molecules 2019, 24, 3659. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; Murali, M.G.; Shailender, G.; Malla, R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Pérez Estrada, C.; Covacu, R.; Sankavaram, S.R.; Svensson, M.; Brundin, L. Oxidative stress increases neurogenesis and oligodendrogenesis in adult neural progenitor cells. Stem Cells Dev. 2014, 23, 2311–2327. [Google Scholar] [CrossRef]
- Le Belle, J.E.; Orozco, N.M.; Paucar, A.A.; Saxe, J.P.; Mottahedeh, J.; Pyle, A.D.; Wu, H.; Kornblum, H.I. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 2011, 8, 59–71. [Google Scholar] [CrossRef]
- Yuan, T.F.; Gu, S.; Shan, C.; Marchado, S.; Arias-Carrión, O. Oxidative Stress and Adult Neurogenesis. Stem Cell Rev. Rep. 2015, 11, 706–709. [Google Scholar] [CrossRef]
- Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 2005, 10, 1881–1896. [Google Scholar] [CrossRef]
- Burdick, A.D.; Davis, J.W., 2nd; Liu, K.J.; Hudson, L.G.; Shi, H.; Monske, M.L.; Burchiel, S.W. Benzo(a)pyrene quinones increase cell proliferation, generate reactive oxygen species, and transactivate the epidermal growth factor receptor in breast epithelial cells. Cancer Res. 2003, 63, 7825–7833. [Google Scholar]
- Park, S.A.; Na, H.K.; Kim, E.H.; Cha, Y.N.; Surh, Y.J. 4-hydroxyestradiol induces anchorage-independent growth of human mammary epithelial cells via activation of IkappaB kinase: Potential role of reactive oxygen species. Cancer Res. 2009, 69, 2416–2424. [Google Scholar] [CrossRef] [PubMed]
- Janssen-Heininger, Y.M.; Poynter, M.E.; Baeuerle, P.A. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic. Biol. Med. 2000, 28, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, J.G.; Tafani, M.; Farber, J.L. Tumor necrosis factor induces phosphorylation and translocation of BAD through a phosphatidylinositide-3-OH kinase-dependent pathway. J. Biol. Chem. 1999, 274, 19411–19416. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Deng, X. Nicotine inactivation of the proapoptotic function of Bax through phosphorylation. J. Biol. Chem. 2005, 280, 10781–10789. [Google Scholar] [CrossRef]
- Limaye, V.; Li, X.; Hahn, C.; Xia, P.; Berndt, M.C.; Vadas, M.A.; Gamble, J.R. Sphingosine kinase-1 enhances endothelial cell survival through a PECAM-1-dependent activation of PI-3K/Akt and regulation of Bcl-2 family members. Blood 2005, 105, 3169–3177. [Google Scholar] [CrossRef]
- Liu, L.Z.; Hu, X.W.; Xia, C.; He, J.; Zhou, Q.; Shi, X.; Fang, J.; Jiang, B.H. Reactive oxygen species regulate epidermal growth factor-induced vascular endothelial growth factor and hypoxia-inducible factor-1alpha expression through activation of AKT and P70S6K1 in human ovarian cancer cells. Free Radic. Biol. Med. 2006, 41, 1521–1533. [Google Scholar] [CrossRef]
- Mochizuki, T.; Furuta, S.; Mitsushita, J.; Shang, W.H.; Ito, M.; Yokoo, Y.; Yamaura, M.; Ishizone, S.; Nakayama, J.; Konagai, A.; et al. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene 2006, 25, 3699–3707. [Google Scholar] [CrossRef]
- Jimenez-Andrade, J.M.; Ghilardi, J.R.; Castañeda-Corral, G.; Kuskowski, M.A.; Mantyh, P.W. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 2011, 152, 2564–2574. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.M.; Scholz, M.C.; Bono, J.S.D.; Vogelzang, N.J.; Souza, P.L.D.; Marx, G.M.; Vaishampayan, U.N.; George, S.; Schwarz, J.K.; Antonarakis, E.S.; et al. Final analysis of COMET-2: Cabozantinib (Cabo) versus mitoxantrone/prednisone (MP) in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) with moderate to severe pain who were previously treated with docetaxel (D) and abiraterone (A) and/or enzalutamide (E). J. Clin. Oncol. 2015, 33, 141. [Google Scholar] [CrossRef]
- Bauer, T.; Cho, B.C.; Heist, R.; Bazhenova, L.; Werner, T.; Goel, S.; Kim, D.W.; Adkins, D.; Carvajal, R.D.; Alva, A.; et al. First-in-human phase 1/1b study to evaluate sitravatinib in patients with advanced solid tumors. Investig. New Drugs 2022, 40, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Assayag, J.; Pollak, M.N.; Azoulay, L. Post-diagnostic use of beta-blockers and the risk of death in patients with prostate cancer. Eur. J. Cancer 2014, 50, 2838–2845. [Google Scholar] [CrossRef]
- Wang, J.M.; McKenna, K.E.; McVary, K.T.; Lee, C. Requirement of innervation for maintenance of structural and functional integrity in the rat prostate. Biol. Reprod. 1991, 44, 1171–1176. [Google Scholar] [CrossRef]
- Iida, M.; Miyazaki, I.; Tanaka, K.; Kabuto, H.; Iwata-Ichikawa, E.; Ogawa, N. Dopamine D2 receptor-mediated antioxidant and neuroprotective effects of ropinirole, a dopamine agonist. Brain Res. 1999, 838, 51–59. [Google Scholar] [CrossRef]
- Charvin, D.; Vanhoutte, P.; Pagès, C.; Borrelli, E.; Caboche, J. Unraveling a role for dopamine in Huntington’s disease: The dual role of reactive oxygen species and D2 receptor stimulation. Proc. Natl. Acad. Sci. USA 2005, 102, 12218–12223. [Google Scholar] [CrossRef]
- Sawada, H.; Ibi, M.; Kihara, T.; Urushitani, M.; Akaike, A.; Kimura, J.; Shimohama, S. Dopamine D2-type agonists protect mesencephalic neurons from glutamate neurotoxicity: Mechanisms of neuroprotective treatment against oxidative stress. Ann. Neurol. 1998, 44, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Bai, L.Y.; Tsai, M.H.; Chu, P.C.; Chiu, C.F.; Chen, M.Y.; Chiu, S.J.; Chiang, J.H.; Weng, J.R. Pharmacological exploitation of the phenothiazine antipsychotics to develop novel antitumor agents-A drug repurposing strategy. Sci. Rep. 2016, 6, 27540. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wen, Y.C.; Ku, C.C.; Yang, Y.C.; Chow, J.M.; Yang, S.F.; Lee, W.J.; Chien, M.H. Penfluridol triggers cytoprotective autophagy and cellular apoptosis through ROS induction and activation of the PP2A-modulated MAPK pathway in acute myeloid leukemia with different FLT3 statuses. J. Biomed. Sci. 2019, 26, 63. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, J.; Fang, Z.; Pierce, S.R.; West, L.; Staley, A.; Tucker, K.; Yin, Y.; Sun, W.; Kong, W.; et al. Anti-Tumor and Anti-Invasive Effects of ONC201 on Ovarian Cancer Cells and a Transgenic Mouse Model of Serous Ovarian Cancer. Front. Oncol. 2022, 12, 789450. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.R.; Fang, Z.; Yin, Y.; West, L.; Asher, M.; Hao, T.; Zhang, X.; Tucker, K.; Staley, A.; Fan, Y.; et al. Targeting dopamine receptor D2 as a novel therapeutic strategy in endometrial cancer. J. Exp. Clin. Cancer Res. 2021, 40, 61. [Google Scholar] [CrossRef] [PubMed]
- Dakir, E.-H.; Pickard, A.; Srivastava, K.; McCrudden, C.M.; Gross, S.R.; Lloyd, S.; Zhang, S.D.; Margariti, A.; Morgan, R.; Rudland, P.S.; et al. The anti-psychotic drug pimozide is a novel chemotherapeutic for breast cancer. Oncotarget 2018, 9, 34889–34910. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, P.; Zhou, X.Z.; Ji, Y.X.; Yuan, S.; Liu, S.P.; Zhang, Q.G. Pimozide inhibits the growth of breast cancer cells by alleviating the Warburg effect through the P53 signaling pathway. Biomed. Pharmacother. 2022, 150, 113063. [Google Scholar] [CrossRef] [PubMed]
- Spindel, E.R. Muscarinic receptor agonists and antagonists: Effects on cancer. In Muscarinic Receptors; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 208, pp. 451–468. [Google Scholar] [CrossRef]
- Yin, Q.Q.; Xu, L.H.; Zhang, M.; Xu, C. Muscarinic acetylcholine receptor M1 mediates prostate cancer cell migration and invasion through hedgehog signaling. Asian J. Androl. 2018, 20, 608–614. [Google Scholar] [CrossRef]
- Song, P.; Sekhon, H.S.; Lu, A.; Arredondo, J.; Sauer, D.; Gravett, C.; Mark, G.P.; Grando, S.A.; Spindel, E.R. M3 muscarinic receptor antagonists inhibit small cell lung carcinoma growth and mitogen-activated protein kinase phosphorylation induced by acetylcholine secretion. Cancer Res. 2007, 67, 3936–3944. [Google Scholar] [CrossRef]
- Jandaghi, P.; Najafabadi, H.S.; Bauer, A.S.; Papadakis, A.I.; Fassan, M.; Hall, A.; Monast, A.; von Knebel Doeberitz, M.; Neoptolemos, J.P.; Costello, E.; et al. Expression of DRD2 Is Increased in Human Pancreatic Ductal Adenocarcinoma and Inhibitors Slow Tumor Growth in Mice. Gastroenterology 2016, 151, 1218–1231. [Google Scholar] [CrossRef]


| Receptors | Ligand | Type of Innervation | Therapeutic Options | |
|---|---|---|---|---|
| Lung cancer | GFRα [97] | ARTN [97] | Sympathetic innervation [19] Cholinergic innervation [121] | NTRK inhibitor [89] |
| mAChRs [117] | ACh [120] | |||
| NMDARs [130] | NA | |||
| DRD2 [146] | NA | |||
| Trks [89] | NA | |||
| NCAM [111] | Small molecules [111] | |||
| Pancreatic cancer | GFRα [97] | ARTN [97,178], GDNF [97,105,168] NRTN [97] | Sensory innervation [23,24] | Beta blocker [2,39] NGF knockdown [23,88] Anti-NT3 [88] DRD2 blocker [221] |
| DRD2 [221] | NA | |||
| Trks [88,90] | BDNF, NT-3, NT-4/5 [88] | |||
| AMPARs [126], NMDARs [132] | Glutamate [126] | |||
| Prostate cancer | GFRα1 [97,102] | GDNF [97] | Sympathetic innervation [18] parasympathetic innervation [18] | Beta blocker [54,207] β2 and β3 receptor deletion[18] DRD2 blocker [186] |
| NMDAR [129,183] | Glutamate [183] | |||
| Trks [88] | NT-3, BDNF, NGF [88] | |||
| Breast cancer | DRD2 [146] | NA | Sympathetic innervation [38,79] Sensory innervation [91] | Surgical denervation [17] Beta blockers [31,53] |
| NMDA [131] | Glutamate [182,183] | |||
| Trks [89] | BDNF [159], NT-3 [164] | |||
| GFRα [97,99] | GDNF [97] | |||
| NGFR [94,96] | NGF [91] | |||
| L1CAM [107] | NA | |||
| AChRs [116] | NA | |||
| Gastric cancer | L1CAM [107] | Small molecules [107] | Cholinergic innervation [29] Vagal innervation [27] | Acetylcholine blocker (botulinum toxin A) [27] Surgical denervation [27] |
| GFRα [97,104] | NA | |||
| Trks [29,88] | NGF [29] | |||
| Head and neck cancer | Trks [89] | NA | Autonomic innervation derived from sensory nerves [45] | NA |
| Skin cancer | Trks [89] | NA | NA | Beta blocker [30] |
| L1CAM [107] | Small molecules [107] | |||
| Ovarian cancer | DRD2 [146] | NA | NA | NA |
| L1CAM [108] | NA | |||
| NCAM [112] | NA | |||
| Leukemia | DRD2 [146] | NA | NA | DRD2 blocker [213] |
| GFRα [97] | ARTN, NRTN [97] | |||
| Colorectal cancer | GFRα [97] | GDNF [97] | Autonomic innervation [20] | NA |
| NA | Ach [119] | |||
| NMDARs [129] | NA | |||
| Trks [89] | NA | |||
| Cervical cancer | DRD2 [116] | NA | NA | NA |
| Esophageal cancer | DRD2 [116] | NA | NA | NA |
| Liver cancer | L1CAM [107] | Small molecules [107] | NA | DRD1 blocker [185] DRD2 blocker [187] |
| DRD1 [185] | Dopamine [185] | |||
| Brain cancer | AMPARs [124,125] | Glutamate [124] | NA | NA |
| DRD2 [146] | Dopamine [146] | |||
| Trks [150,160,165] | NGF [150], NT-3 [165] | |||
| GFRα [97] | GDNF [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.M.; Ngoc, D.T.M.; Choi, J.-H.; Lee, C.-H. Unveiling the Neural Environment in Cancer: Exploring the Role of Neural Circuit Players and Potential Therapeutic Strategies. Cells 2023, 12, 1996. https://doi.org/10.3390/cells12151996
Nguyen TM, Ngoc DTM, Choi J-H, Lee C-H. Unveiling the Neural Environment in Cancer: Exploring the Role of Neural Circuit Players and Potential Therapeutic Strategies. Cells. 2023; 12(15):1996. https://doi.org/10.3390/cells12151996
Chicago/Turabian StyleNguyen, Tuan Minh, Dinh Thi Minh Ngoc, Jung-Hye Choi, and Chang-Hoon Lee. 2023. "Unveiling the Neural Environment in Cancer: Exploring the Role of Neural Circuit Players and Potential Therapeutic Strategies" Cells 12, no. 15: 1996. https://doi.org/10.3390/cells12151996
APA StyleNguyen, T. M., Ngoc, D. T. M., Choi, J.-H., & Lee, C.-H. (2023). Unveiling the Neural Environment in Cancer: Exploring the Role of Neural Circuit Players and Potential Therapeutic Strategies. Cells, 12(15), 1996. https://doi.org/10.3390/cells12151996







