Enhanced Physiological and Biochemical Performance of Mung Bean and Maize under Saline and Heavy Metal Stress through Application of Endophytic Fungal Strain SL3 and Exogenous IAA
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Screening of SL3 Fungal Strain
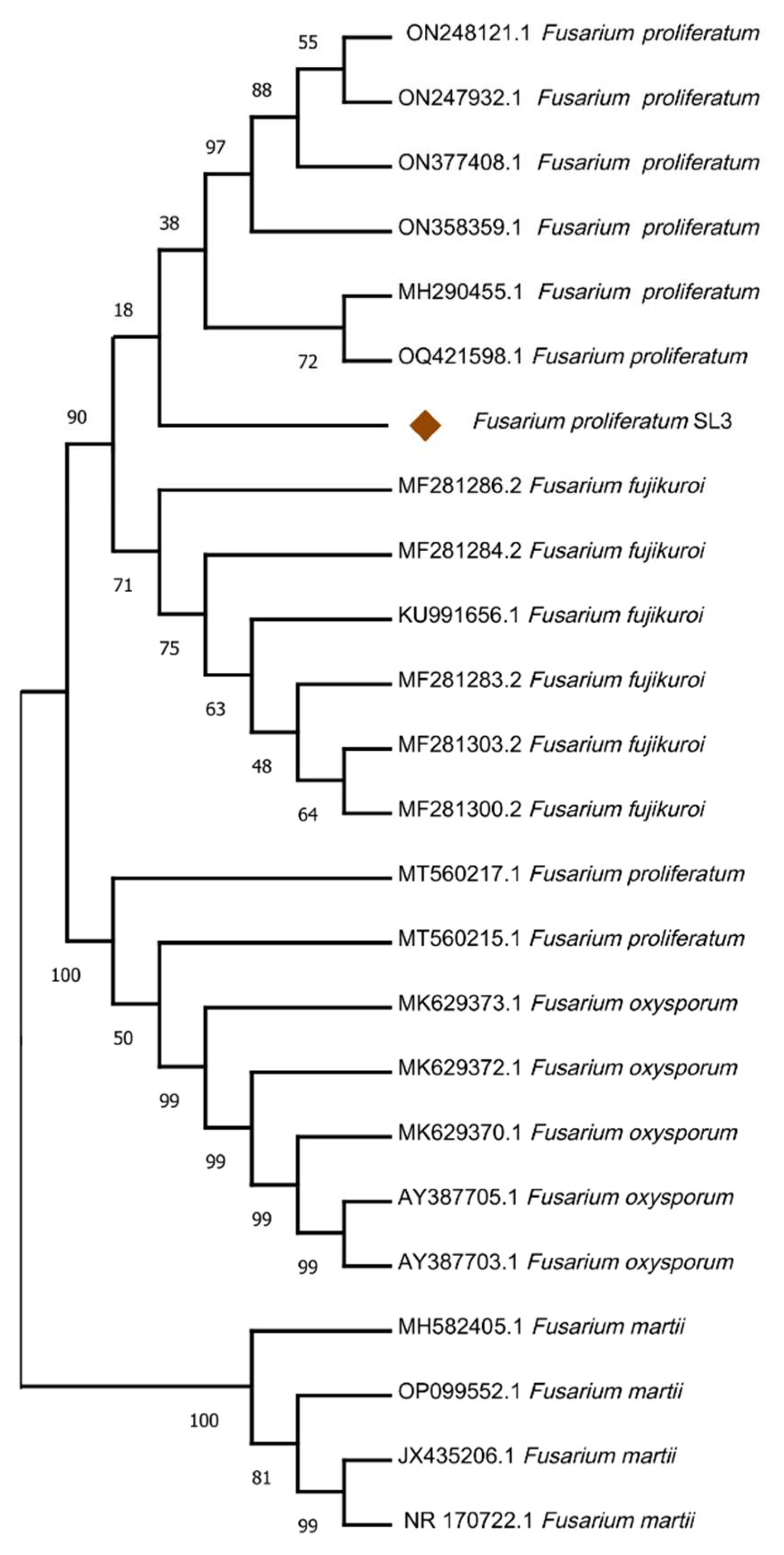
2.2. Identification and Phylogenetic Analysis of SL3
2.3. Determination of IAA by GC/MS
2.4. Determination of Siderophore Production
2.5. Extraction and Quantification of Gibberellins (GAs) in Culture Broth
2.6. Seeds Surface Sterilization and Germination
2.7. Experimental Design
2.8. Assessment of Growth Attributes
2.9. Assessment of Photosynthetic Pigments
2.10. Assessment of Total Protein Contents
2.11. Assessment of Catalase Activity
2.12. Assessment of Total Phenolic Content
2.13. Assessment of Polyphenol Oxidase
2.14. Assessment of Flavanol Contents
2.15. Statistical Analysis
3. Results
3.1. Assessment of SL3 Tolerance to NaCl and Pb
3.2. Quantitation of IAA in the Fungal Culture
3.3. Siderophore Production by SL3
3.4. Determination of Gibberellins in SL3 Culture Filtrate
3.5. Fungal Strain Identification and Phylogenetic Analysis
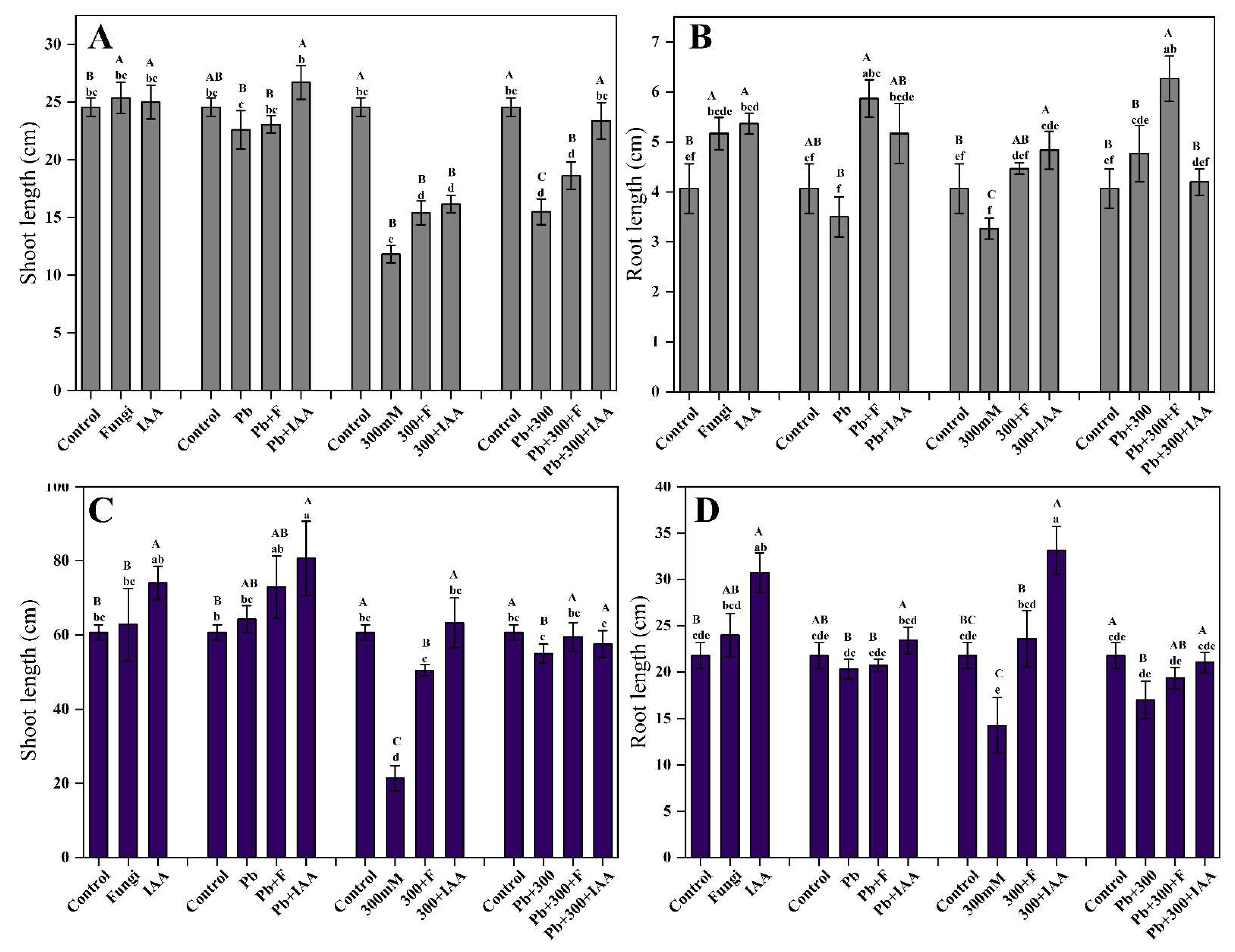
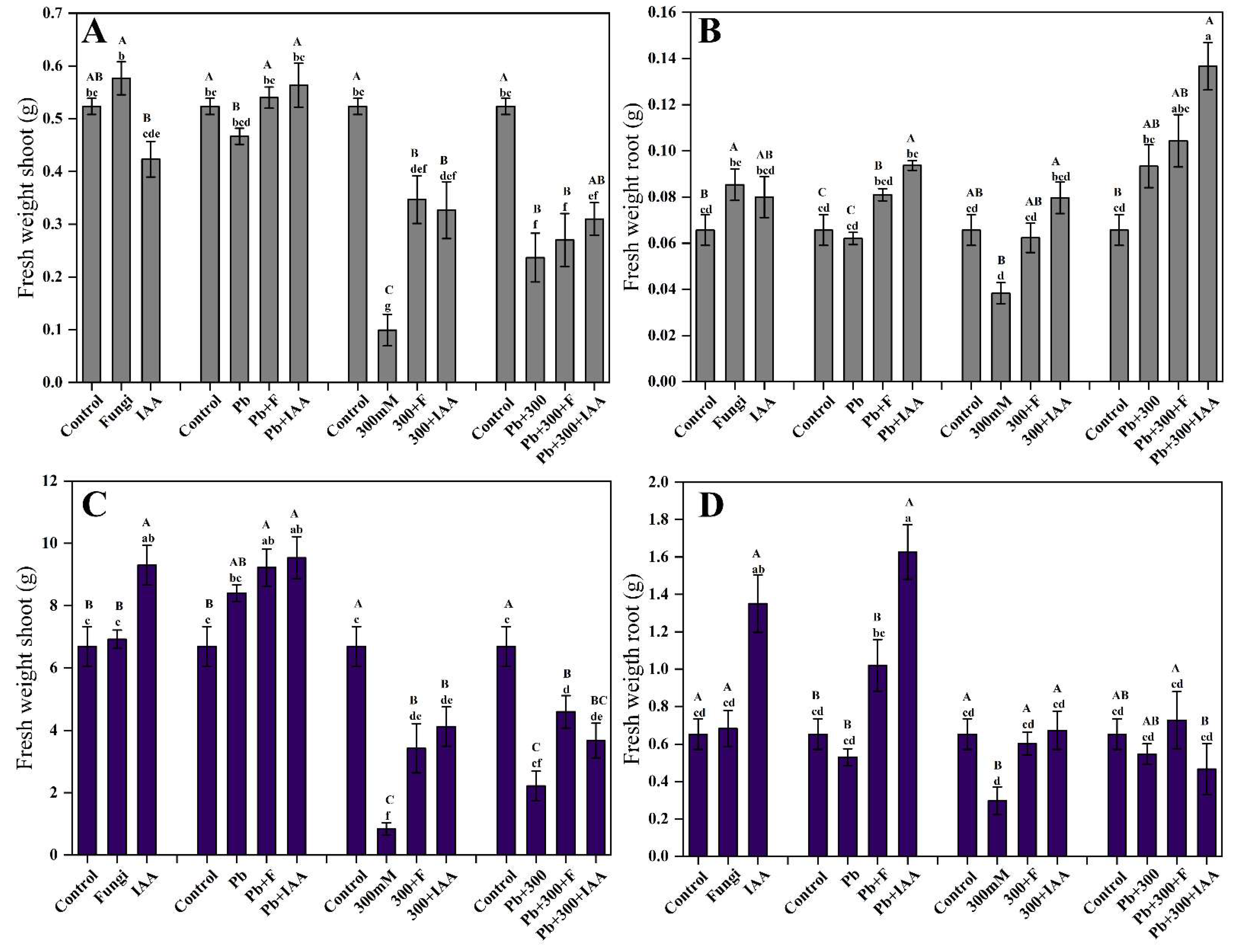
3.6. Effect of SL3 Inoculation and IAA Treatment on Growth Attributes under NaCl and Pb Stress
3.7. Effects of SL3 and IAA Treatment on Photosynthetic Pigments of Mung Bean and Maize under NaCl and Pb Stress
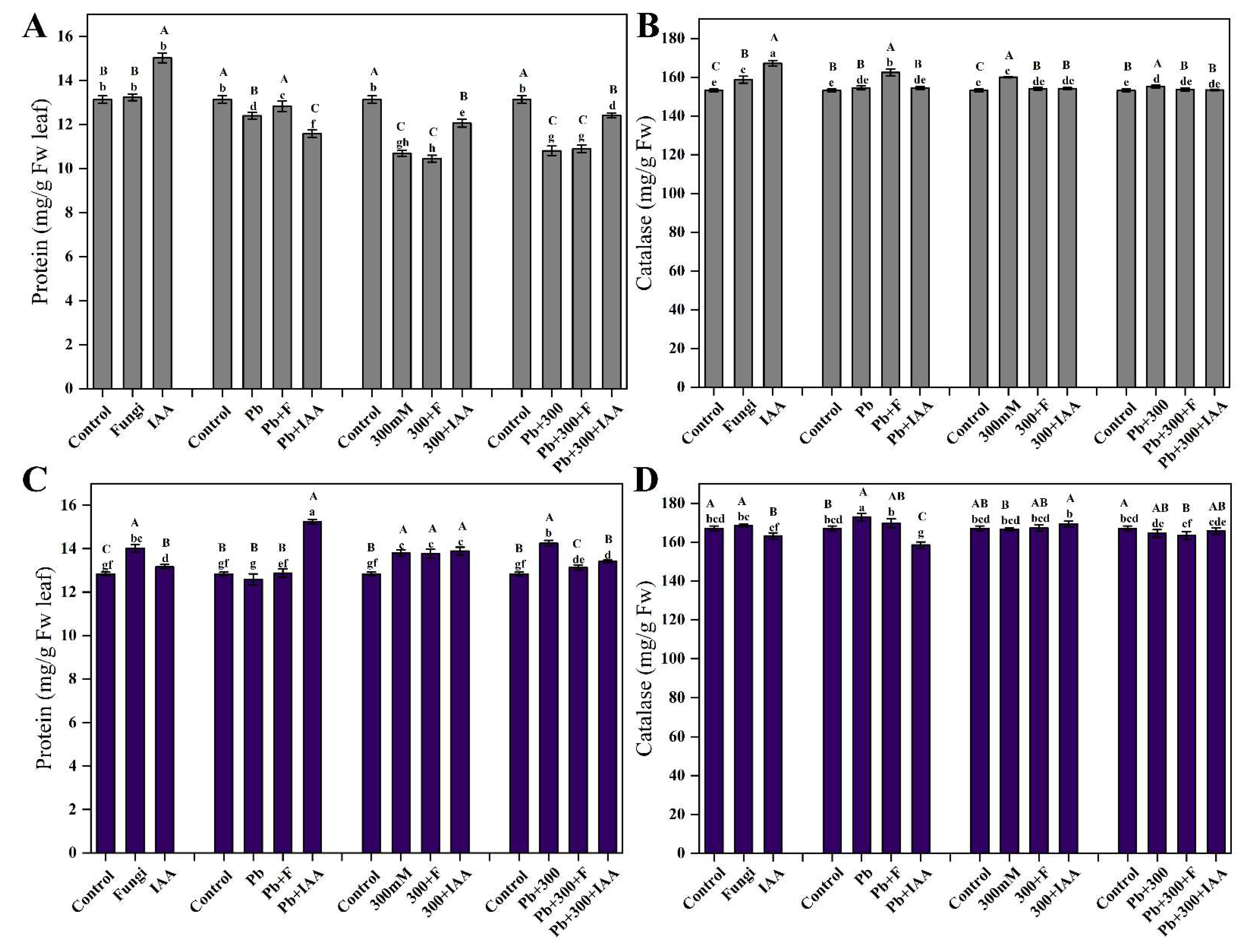
3.8. Effect of SL3 and IAA Treatments on Total Protein Contents of Mung Bean and Maize under NaCl and Pb Stress
3.9. Effect of SL3 and IAA Treatments on Catalase Activity of Mung Bean and Maize under NaCl and Pb Stress
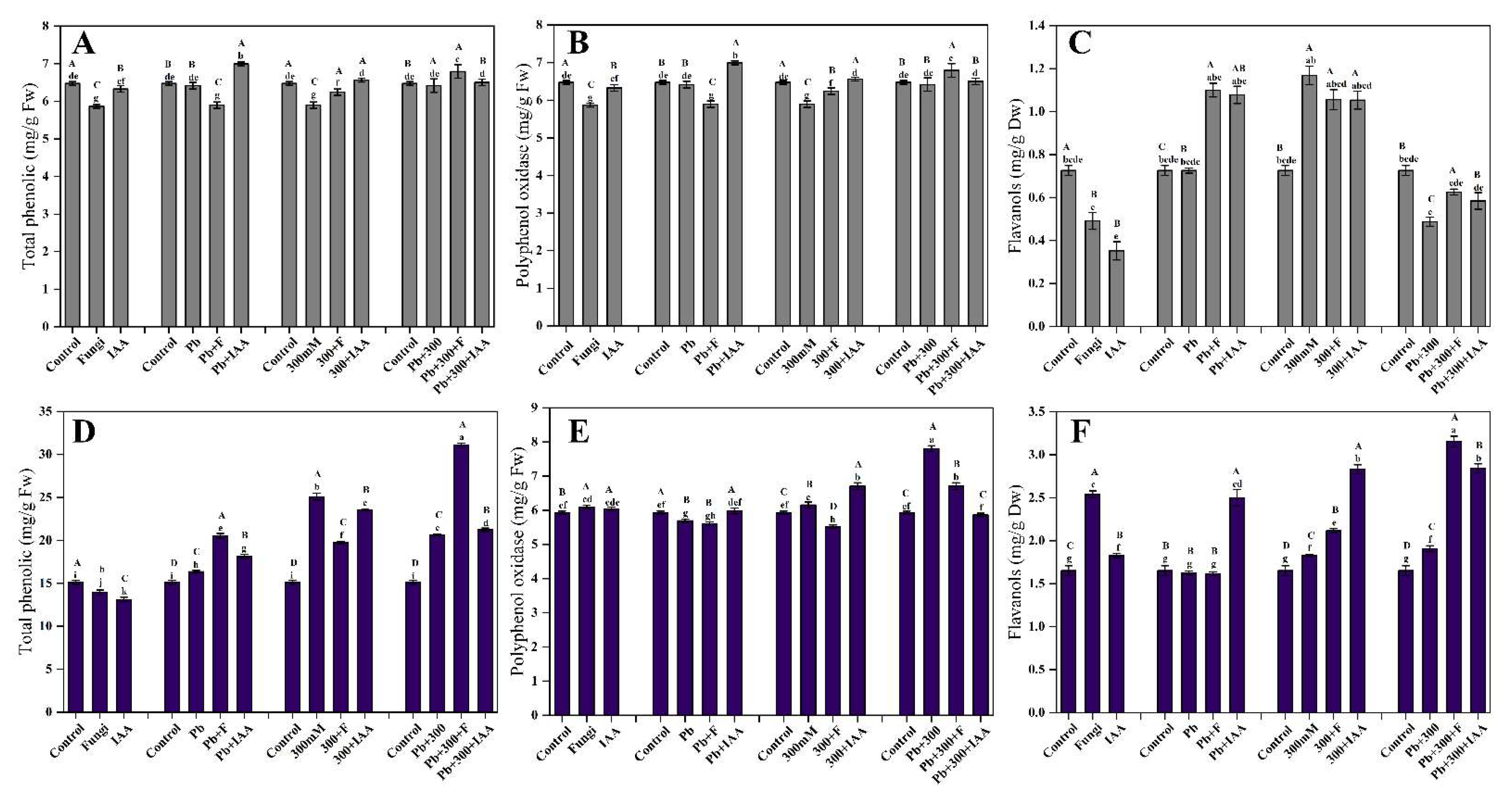
3.10. Effect of SL3 and IAA Treatments on total Phenolic Contents of Mung Bean and Maize under NaCl and Pb Stress
3.11. Effect of SL3 and IAA Treatments on Polyphenol Oxidase Content of Mung Bean and Maize under NaCl and Pb Stress
3.12. Effects of SL3 and IAA Treatment on Flavanols Content of Mung Bean and Maize under NaCl and Pb Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Khan, S.; Zhang, Y.; Zhou, J.; Akhoundian, M.; Jan, S.A. CRISPR-Cas technology based genome editing for modification of salinity stress tolerance responses in rice (Oryza sativa L.). Mol. Biol. Rep. 2021, 48, 3605–3615. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Zhang, Y.; Akbar, F.; Khan, J. Abiotic Stress Tolerance in Cereals Through Genome Editing. In Omics Approach to Manage Abiotic Stress in Cereals; Roychoudhury, A., Aftab, T., Acharya, K., Eds.; Springer Nature: Singapore, 2022; pp. 295–319. [Google Scholar] [CrossRef]
- Afzal, M.; Hindawi, S.E.S.; Alghamdi, S.S.; Migdadi, H.H.; Khan, M.A.; Hasnain, M.U.; Arslan, M.; Habib ur Rahman, M.; Sohaib, M. Potential Breeding Strategies for Improving Salt Tolerance in Crop Plants. J. Plant Growth Regul. 2023, 42, 3365–3387. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef] [PubMed]
- Lubna; Asaf, S.; Hamayun, M.; Khan, A.L.; Waqas, M.; Khan, M.A.; Jan, R.; Lee, I.-J.; Hussain, A. Salt tolerance of Glycine max L. induced by endophytic fungus Aspergillus flavus CSH1, via regulating its endogenous hormones and antioxidative system. Plant Physiol. Biochem. 2018, 128, 13–23. [Google Scholar] [CrossRef]
- Jhuma, T.A.; Rafeya, J.; Sultana, S.; Rahman, M.T.; Karim, M.M. Isolation of Endophytic Salt-Tolerant Plant Growth-Promoting Rhizobacteria from Oryza sativa and Evaluation of Their Plant Growth-Promoting Traits under Salinity Stress Condition. Front. Sustain. Food Syst. 2021, 5, 687531. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Kang, S.-M.; Kim, Y.-H.; Lee, I.-J. Endophytic Fungi Produce Gibberellins and Indoleacetic Acid and Promotes Host-Plant Growth during Stress. Molecules 2012, 17, 10754–10773. [Google Scholar] [CrossRef]
- Kruasuwan, W.; Lohmaneeratana, K.; Munnoch, J.T.; Vongsangnak, W.; Jantrasuriyarat, C.; Hoskisson, P.A.; Thamchaipenet, A. Transcriptome Landscapes of Salt-Susceptible Rice Cultivar IR29 Associated with a Plant Growth Promoting Endophytic Streptomyces. Rice 2023, 16, 6. [Google Scholar] [CrossRef]
- Khan, A.L.; Halo, B.A.; Elyassi, A.; Ali, S.; Al-Hosni, K.; Hussain, J.; Al-Harrasi, A.; Lee, I.-J. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electron. J. Biotechnol. 2016, 21, 58–64. [Google Scholar] [CrossRef]
- Dubey, A.; Malla, M.A.; Kumar, A.; Dayanandan, S.; Khan, M.L. Plants endophytes: Unveiling hidden agenda for bioprospecting toward sustainable agriculture. Crit. Rev. Biotechnol. 2020, 40, 1210–1231. [Google Scholar] [CrossRef]
- Ur Rahman, S.; Li, Y.; Hussain, S.; Hussain, B.; Khan, W.-u.-D.; Riaz, L.; Nadeem Ashraf, M.; Athar Khaliq, M.; Du, Z.; Cheng, H. Role of phytohormones in heavy metal tolerance in plants: A review. Ecol. Indic. 2023, 146, 109844. [Google Scholar] [CrossRef]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone Priming: Regulator for Heavy Metal Stress in Plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef]
- Bianco, C.; Defez, R. Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J. Exp. Bot. 2009, 60, 3097–3107. [Google Scholar] [CrossRef]
- Kahraman, A.; Adali, M.; Onder, M.; Koc, N.; Kaya, C. Mung bean [Vigna radiata (L.) Wilczek] as human food. Int. J. Agric. Econ. Dev. 2014, 2, 9. [Google Scholar]
- HanumanthaRao, B.; Nair, R.M.; Nayyar, H. Salinity and high temperature tolerance in mungbean [Vigna radiata (L.) Wilczek] from a physiological perspective. Front. Plant Sci. 2016, 7, 957. [Google Scholar] [CrossRef]
- Dahiya, P.; Linnemann, A.; Van Boekel, M.; Khetarpaul, N.; Grewal, R.; Nout, M. Mung bean: Technological and nutritional potential. Crit. Rev. Food Sci. Nutr. 2015, 55, 670–688. [Google Scholar] [CrossRef]
- Chaudhary, D.P.; Kumar, S.; Yadav, O.P. Nutritive Value of Maize: Improvements, Applications and Constraints. In Maize: Nutrition Dynamics and Novel Uses; Chaudhary, D.P., Kumar, S., Langyan, S., Eds.; Springer: New Delhi, India, 2014; pp. 3–17. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A.; McCulley, L.; Roh, R.; Lopez-Ridaura, S.; Palacios-Rojas, N.; Gunaratna, N.S. Maize agro-food systems to ensure food and nutrition security in reference to the Sustainable Development Goals. Glob. Food Secur. 2020, 25, 100327. [Google Scholar] [CrossRef]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef]
- Lacava, P.T.; Bogas, A.C.; Cruz, F.D.P.N. Plant Growth Promotion and Biocontrol by Endophytic and Rhizospheric Microorganisms from the Tropics: A Review and Perspectives. Front. Sustain. Food Syst. 2022, 6, 796113. [Google Scholar] [CrossRef]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of Indole-3-acetic Acid (IAA) Production in Klebsiellaby LC-MS/MS and the Salkowski Method. Bio-Protocol 2019, 9, e3230. [Google Scholar] [CrossRef]
- Lubna; Asaf, S.; Hamayun, M.; Gul, H.; Lee, I.-J.; Hussain, A. Aspergillus niger CSR3 regulates plant endogenous hormones and secondary metabolites by producing gibberellins and indoleacetic acid. J. Plant Interact. 2018, 13, 100–111. [Google Scholar] [CrossRef]
- Ullah, I.; Khan, A.R.; Park, G.-S.; Lim, J.-H.; Waqas, M.; Lee, I.-J.; Shin, J.-H. Analysis of phytohormones and phosphate solubilization in Photorhabdus spp. Food Sci. Biotechnol. 2013, 22, 25–31. [Google Scholar] [CrossRef]
- Vellore, J.M. Iron Acquisition in Rhodococcus Erythrolpolis: The Isolation of Mutant (s) that Do Not Produce a Siderophore; East Tennessee State University: Johnson City, TN, USA, 2001. [Google Scholar]
- Wildermuth, E.; Stark, H.; Friedrich, G.; Ebenhöch, F.L.; Kühborth, B.; Silver, J.; Rituper, R. Iron compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Novopol’tseva, V.M.; Labzina, L.; Atianina, T.F. The preparation of iron (III) chloride solution from salt with an unknown water content. Lab. Delo 1991, 9, 10–11. [Google Scholar]
- Lee, I.-J.; Foster, K.R.; Morgan, P.W. Photoperiod control of gibberellin levels and flowering in sorghum. Plant Physiol. 1998, 116, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Khalmuratova, I.; Choi, D.-H.; Woo, J.-R.; Jeong, M.-J.; Oh, Y.; Kim, Y.-G.; Lee, I.-J.; Choo, Y.-S.; Kim, J.-G. Diversity and plant growth-promoting effects of fungal endophytes isolated from salt-tolerant plants. J. Microbiol. Biotechnol. 2020, 30, 1680–1687. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Arulsekar, S.; Parfitt, D.E. Isozyme Analysis Procedures for Stone Fruits, Almond, Grape, Walnut, Pistachio, and Fig. HortScience 1986, 21, 928–933. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Illera, A.; Sanz, M.; Beltrán, S.; Melgosa, R.; Solaesa, A.; Ruiz, M. Evaluation of HPCD batch treatments on enzyme inactivation kinetics and selected quality characteristics of cloudy juice from Golden delicious apples. J. Food Eng. 2018, 221, 141–150. [Google Scholar] [CrossRef]
- Park, Y.-S.; Jung, S.-T.; Kang, S.-G.; Heo, B.G.; Arancibia-Avila, P.; Toledo, F.; Drzewiecki, J.; Namiesnik, J.; Gorinstein, S. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008, 107, 640–648. [Google Scholar] [CrossRef]
- Shin, S.H.; Lim, Y.; Lee, S.E.; Yang, N.W.; Rhee, J.H. CAS agar diffusion assay for the measurement of siderophores in biological fluids. J. Microbiol. Methods 2001, 44, 89–95. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, R.; Rai, S.; Bano, A.; Pathak, N.; Fujita, M.; Kumar, M.; Hasanuzzaman, M. Mechanistic insights of plant growth promoting bacteria mediated drought and salt stress tolerance in plants for sustainable agriculture. Int. J. Mol. Sci. 2022, 23, 3741. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Kratovalieva, S.; Cvetanowska, L. Influence of different lead concentrations to some morpho-physiological parameters at tomato (Lycopersicon esculentum Mill.) in experimental conditions. Maced Agric. Rev. 2001, 48, 35–41. [Google Scholar]
- Angelova, V.R.; Babrikov, T.D.; Ivanov, K.I. Bioaccumulation and distribution of lead, zinc, and cadmium in crops of Solanaceae family. Commun. Soil Sci. Plant Anal. 2009, 40, 2248–2263. [Google Scholar] [CrossRef]
- Khan, I.; Asaf, S.; Jan, R.; Bilal, S.; Lubna; Khan, A.L.; Kim, K.-M.; Al-Harrasi, A. Genome-wide annotation and expression analysis of WRKY and bHLH transcriptional factor families reveal their involvement under cadmium stress in tomato (Solanum lycopersicum L.). Front. Plant Sci. 2023, 14, 1100895. [Google Scholar] [CrossRef]
- Finnegan, P.M.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-Metal-Induced Reactive Oxygen Species: Phytotoxicity and Physicochemical Changes in Plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer International Publishing: Cham, Switzerland, 2014; Volume 232, pp. 1–44. [Google Scholar] [CrossRef]
- Ashraf, M.; Akram, N.A. Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnol. Adv. 2009, 27, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Verma, J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Aizaz, M.; Ahmad, W.; Asaf, S.; Khan, I.; Saad Jan, S.; Salim Alamri, S.; Bilal, S.; Jan, R.; Kim, K.-M.; Al-Harrasi, A. Characterization of the Seed Biopriming, Plant Growth-Promoting and Salinity-Ameliorating Potential of Halophilic Fungi Isolated from Hypersaline Habitats. Int. J. Mol. Sci. 2023, 24, 4904. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Li, Z.; Wang, L.; Dai, T.; Kang, Q.; Niu, D. Exogenous of Indole-3-Acetic Acid Application Alleviates Copper Toxicity in Spinach Seedlings by Enhancing Antioxidant Systems and Nitrogen Metabolism. Toxics 2019, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Ma, C.; Yuan, S.; Xie, B.; Li, Q.; Wang, Q.; Shao, M. IAA Plays an Important Role in Alkaline Stress Tolerance by Modulating Root Development and ROS Detoxifying Systems in Rice Plants. Int. J. Mol. Sci. 2022, 23, 4817. [Google Scholar] [CrossRef]
- Ramadoss, D.; Lakkineni, V.K.; Bose, P.; Ali, S.; Annapurna, K. Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. SpringerPlus 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Kumar, V.; Raghuvanshi, N.; Pandey, A.K.; Kumar, A.; Thoday-Kennedy, E.; Kant, S. Role of halotolerant plant growth-promoting rhizobacteria in mitigating salinity stress: Recent advances and possibilities. Agriculture 2023, 13, 168. [Google Scholar] [CrossRef]
- Saleem, S.; Iqbal, A.; Ahmed, F.; Ahmad, M. Phytobeneficial and salt stress mitigating efficacy of IAA producing salt tolerant strains in Gossypium hirsutum. Saudi J. Biol. Sci. 2021, 28, 5317–5324. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Hashem, A. Pseudomonas induces salinity tolerance in cotton (Gossypium hirsutum) and resistance to Fusarium root rot through the modulation of indole-3-acetic acid. Saudi J. Biol. Sci. 2015, 22, 773–779. [Google Scholar] [CrossRef]
- Shi, Y.; Lou, K.; Li, C. Growth and photosynthetic efficiency promotion of sugar beet (Beta vulgaris L.) by endophytic bacteria. Photosynth. Res. 2010, 105, 5–13. [Google Scholar] [CrossRef]
- Li, X.; Bu, N.; Li, Y.; Ma, L.; Xin, S.; Zhang, L. Growth, photosynthesis and antioxidant responses of endophyte infected and non-infected rice under lead stress conditions. J. Hazard. Mater. 2012, 213–214, 55–61. [Google Scholar] [CrossRef]
- Ali, B.; Wang, X.; Saleem, M.H.; Azeem, M.A.; Afridi, M.S.; Nadeem, M.; Ghazal, M.; Batool, T.; Qayyum, A.; Alatawi, A.; et al. Bacillus mycoides PM35 Reinforces Photosynthetic Efficiency, Antioxidant Defense, Expression of Stress-Responsive Genes, and Ameliorates the Effects of Salinity Stress in Maize. Life 2022, 12, 219. [Google Scholar] [CrossRef]
- Maslennikova, D.; Koryakov, I.; Yuldashev, R.; Avtushenko, I.; Yakupova, A.; Lastochkina, O. Endophytic Plant Growth-Promoting Bacterium Bacillus subtilis Reduces the Toxic Effect of Cadmium on Wheat Plants. Microorganisms 2023, 11, 1653. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Jan, F.G.; Rahman, I.U.; Iqbal, A.; Hamayun, M. IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS ONE 2018, 13, e0208150. [Google Scholar] [CrossRef]
- Tufail, M.A.; Bejarano, A.; Shakoor, A.; Naeem, A.; Arif, M.S.; Dar, A.A.; Farooq, T.H.; Pertot, I.; Puopolo, G. Can Bacterial Endophytes Be Used as a Promising Bio-Inoculant for the Mitigation of Salinity Stress in Crop Plants?—A Global Meta-Analysis of the Last Decade (2011–2020). Microorganisms 2021, 9, 1861. [Google Scholar] [CrossRef]
- Almuhayawi, M.S.; Abdel-Mawgoud, M.; Al Jaouni, S.K.; Almuhayawi, S.M.; Alruhaili, M.H.; Selim, S.; AbdElgawad, H. Bacterial Endophytes as a Promising Approach to Enhance the Growth and Accumulation of Bioactive Metabolites of Three Species of Chenopodium Sprouts. Plants 2021, 10, 2745. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem.-Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxidative Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Auten, R.L.; Davis, J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Jaleel, C.A.; Azooz, M.; Nabi, G. Generation of ROS and non-enzymatic antioxidants during abiotic stress in plants. Bot. Res. Int. 2009, 2, 11–20. [Google Scholar]
- Sarker, U.; Oba, S. Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef]
- Hagaggi, N.S.A.; Mohamed, A.A.A. Enhancement of Zea mays (L.) growth performance using indole acetic acid producing endophyte Mixta theicola isolated from Solenostemma argel (Hayne). South Afr. J. Bot. 2020, 134, 64–71. [Google Scholar] [CrossRef]
- Abd El-Samad, H.M. The physiological response of wheat plants to exogenous application of gibberellic acid (GA3) or indole-3-acetic acid (IAA) with endogenous ethylene under salt stress conditions. Int. J. Plant Physiol. Biochem. 2013, 5, 58–64. [Google Scholar]
- Kaya, C.; Ashraf, M.; Dikilitas, M.; Tuna, A.L. Alleviation of salt stress-induced adverse effects on maize plants by exogenous application of indoleacetic acid (IAA) and inorganic nutrients-A field trial. Aust. J. Crop Sci. 2013, 7, 249–254. [Google Scholar]
- Hussain, A.; Ismail, A.; Qadir, M.; Hamayun, M.; Mehmood, A. Thermal stress alleviating potential of endophytic fungus rhizopus oryzae inoculated to sunflower (Helianthus annuus L.) and soybean (Glycine max L.). Pak. J. Bot. 2020, 52, 1857–1865. [Google Scholar]
- El-Gendi, H.; Al-Askar, A.A.; Király, L.; Samy, M.A.; Moawad, H.; Abdelkhalek, A. Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Horticulturae 2022, 8, 301. [Google Scholar] [CrossRef]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 2015, 35, 62–74. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.N.; Tiwari, R.K.; Sahu, P.K.; Yadav, J.; Srivastava, A.K.; Kumar, S. Salinity Alleviation and Reduction in Oxidative Stress by Endophytic and Rhizospheric Microbes in Two Rice Cultivars. Plants 2023, 12, 976. [Google Scholar] [CrossRef]
- Poveda, J.; Baptista, P.; Sacristán, S.; Velasco, P. Editorial: Beneficial effects of fungal endophytes in major agricultural crops. Front. Plant Sci. 2022, 13, 1061112. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aizaz, M.; Khan, I.; Lubna; Asaf, S.; Bilal, S.; Jan, R.; Khan, A.L.; Kim, K.-M.; AL-Harrasi, A. Enhanced Physiological and Biochemical Performance of Mung Bean and Maize under Saline and Heavy Metal Stress through Application of Endophytic Fungal Strain SL3 and Exogenous IAA. Cells 2023, 12, 1960. https://doi.org/10.3390/cells12151960
Aizaz M, Khan I, Lubna, Asaf S, Bilal S, Jan R, Khan AL, Kim K-M, AL-Harrasi A. Enhanced Physiological and Biochemical Performance of Mung Bean and Maize under Saline and Heavy Metal Stress through Application of Endophytic Fungal Strain SL3 and Exogenous IAA. Cells. 2023; 12(15):1960. https://doi.org/10.3390/cells12151960
Chicago/Turabian StyleAizaz, Muhammad, Ibrahim Khan, Lubna, Sajjad Asaf, Saqib Bilal, Rahmatullah Jan, Abdul Latif Khan, Kyung-Min Kim, and Ahmed AL-Harrasi. 2023. "Enhanced Physiological and Biochemical Performance of Mung Bean and Maize under Saline and Heavy Metal Stress through Application of Endophytic Fungal Strain SL3 and Exogenous IAA" Cells 12, no. 15: 1960. https://doi.org/10.3390/cells12151960
APA StyleAizaz, M., Khan, I., Lubna, Asaf, S., Bilal, S., Jan, R., Khan, A. L., Kim, K.-M., & AL-Harrasi, A. (2023). Enhanced Physiological and Biochemical Performance of Mung Bean and Maize under Saline and Heavy Metal Stress through Application of Endophytic Fungal Strain SL3 and Exogenous IAA. Cells, 12(15), 1960. https://doi.org/10.3390/cells12151960









