The Triticeae CBF Gene Cluster—To Frost Resistance and Beyond
Abstract
1. Triticeae Crops and Abiotic Stress
1.1. Triticeae as Staple Food and Adaptable Crops
1.2. Cold and Drought Issues for Triticeae in the Climate Change Era
2. CBF Gene Cluster and Its Central Role in Response to Frost and Drought
2.1. C-Repeat Binding Factors and Cluster Organization in Triticeae
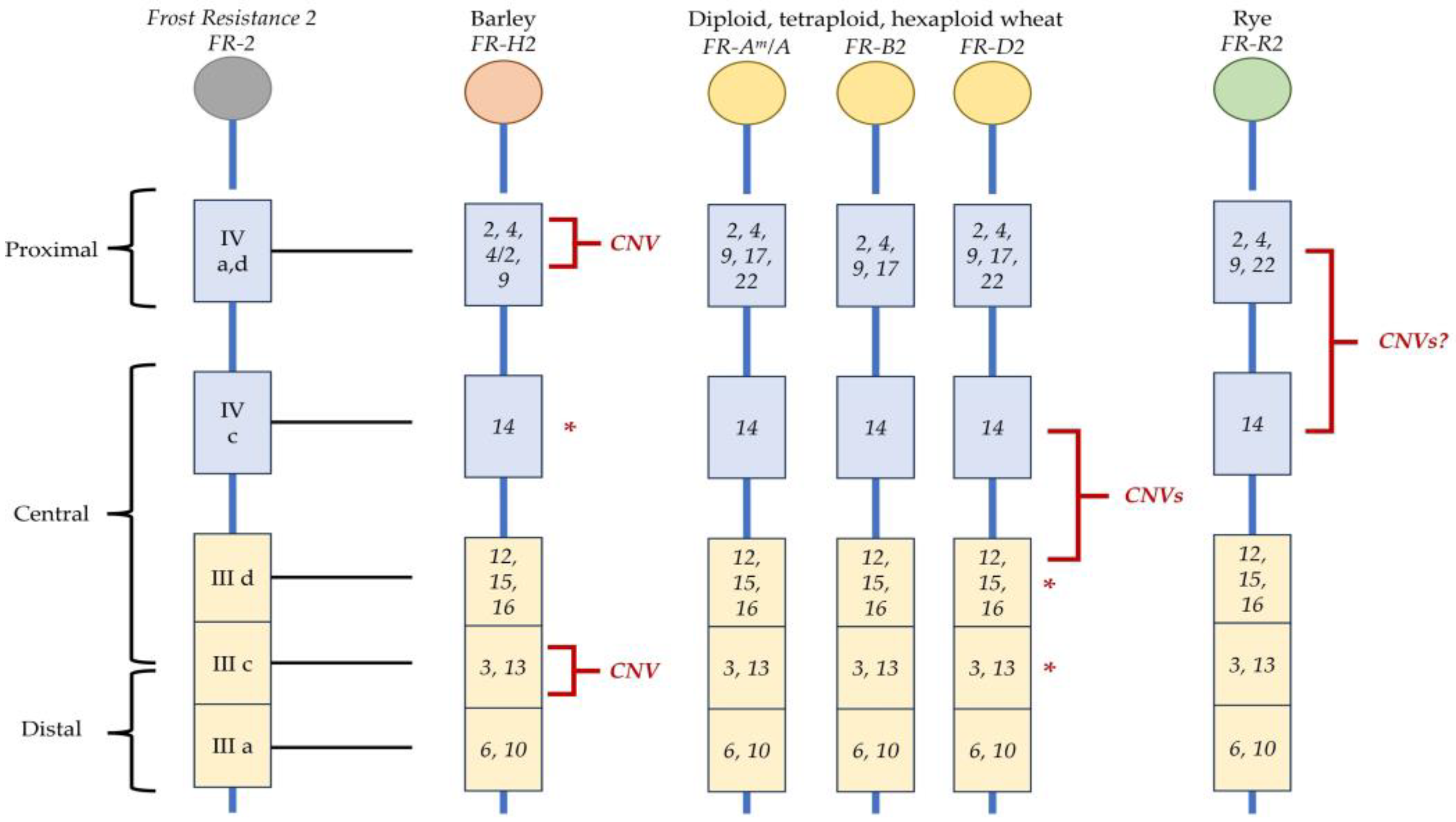
2.2. Role of the ICE-CBF-COR Pathway in Cold Acclimation

2.3. FR-2 in Barley—A Synergistic Action of CNV and HvCBF14?
2.4. FR-2 in Wheats—CBF Cluster Ploidy
2.5. FR-2 in Rye—Evidence of ICE1 Involvement in the Tolerance
2.6. New Frontiers for CBF Genes? CBF Genes in the Drought Stress Adaptative Response
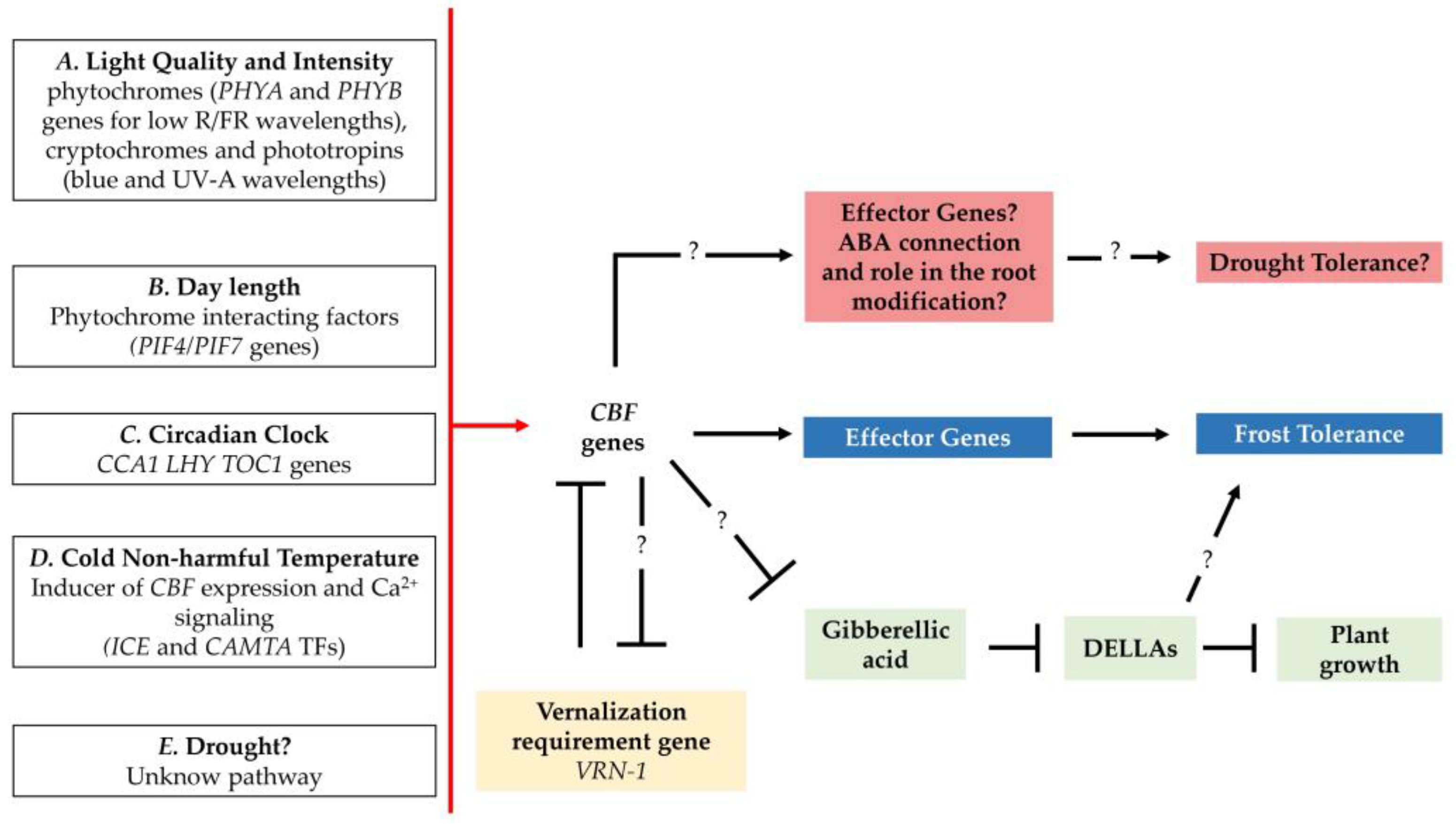
3. Prospects for Triticeae Improvement against Abiotic Stresses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pingali, P.L. Green Revolution: Impacts, Limits, and the Path Ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed]
- World Population Prospects 2022, Population Growth Rate File, Estimates Table United Nations Department of Economic and Social Affairs. 2022. Available online: https://population.un.org/wpp/ (accessed on 29 November 2022).
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic Strategies for Improving Crop Yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Evenson, R.E.; Gollin, D. Assessing the Impact of the Green Revolution, 1960 to 2000. Science 2003, 300, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P. The Genes of the Green Revolution. Trends Genet. 2003, 19, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Wik, M.; Pingali, P.; Broca, S. Global Agricultural Performance: Past Trends and Future Prospects; World Bank: Washington, DC, USA, 2008; p. 40. [Google Scholar]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a Cultivated Planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Pingali, P.L. The Green Revolution and Crop Biodiversity. In Biological Extinction; Dasgupta, P., Raven, P., McIvor, A., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 175–192. ISBN 978-1-108-66867-5. [Google Scholar]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the Intensification of Agriculture for Global Food Security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. Improving Photosynthetic Efficiency for Greater Yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef]
- Schröter, D.; Cramer, W.; Leemans, R.; Prentice, I.C.; Araújo, M.B.; Arnell, N.W.; Bondeau, A.; Bugmann, H.; Carter, T.R.; Gracia, C.A.; et al. Ecosystem Service Supply and Vulnerability to Global Change in Europe. Science 2005, 310, 1333–1337. [Google Scholar] [CrossRef]
- Kole, C.; Muthamilarasan, M.; Henry, R.; Edwards, D.; Sharma, R.; Abberton, M.; Batley, J.; Bentley, A.; Blakeney, M.; Bryant, J.; et al. Application of Genomics-Assisted Breeding for Generation of Climate Resilient Crops: Progress and Prospects. Front. Plant Sci. 2015, 6, 563. [Google Scholar] [CrossRef]
- Grassini, P.; Eskridge, K.M.; Cassman, K.G. Distinguishing between Yield Advances and Yield Plateaus in Historical Crop Production Trends. Nat. Commun. 2013, 4, 2918. [Google Scholar] [CrossRef]
- Khoury, C.K.; Brush, S.; Costich, D.E.; Curry, H.A.; De Haan, S.; Engels, J.M.M.; Guarino, L.; Hoban, S.; Mercer, K.L.; Miller, A.J.; et al. Crop Genetic Erosion: Understanding and Responding to Loss of Crop Diversity. New Phytol. 2022, 233, 84–118. [Google Scholar] [CrossRef] [PubMed]
- Haussmann, B.I.G.; Parzies, H.K.; Presterl, T.; Suciz, Z.; Miedaner, T. Plant Genetic Resources in Crop Improvement. Plant Genet. Resour. Charact. Util. 2004, 2, 3–21. [Google Scholar] [CrossRef]
- Corrado, G.; Rao, R. Special Issue: Plant Genetics and Biotechnology in Biodiversity. Diversity 2018, 10, 19. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, L.; Li, Z.; Jin, J.; Luo, J.; Gao, G. Identification and Analysis of Unitary Loss of Long-Established Protein-Coding Genes in Poaceae Shows Evidences for Biased Gene Loss and Putatively Functional Transcription of Relics. BMC Evol. Biol. 2015, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Zenda, T.; Liu, S.; Dong, A.; Duan, H. Advances in Cereal Crop Genomics for Resilience under Climate Change. Life 2021, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The Molecular Genetics of Crop Domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change and Land: IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems, 1st ed.; Cambridge University Press: Cambridge, UK, 2022; ISBN 978-1-00-915798-8. [Google Scholar]
- Kovak, E.; Blaustein-Rejto, D.; Qaim, M. Genetically Modified Crops Support Climate Change Mitigation. Trends Plant Sci. 2022, 27, 627–629. [Google Scholar] [CrossRef]
- Challinor, A.J.; Watson, J.; Lobell, D.B.; Howden, S.M.; Smith, D.R.; Chhetri, N. A Meta-Analysis of Crop Yield under Climate Change and Adaptation. Nat. Clim. Change 2014, 4, 287–291. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Quilligan, E.; Aggarwal, P.K.; Bansal, K.C.; Cavalieri, A.J.; Chapman, S.C.; Chapotin, S.M.; Datta, S.K.; Duveiller, E.; Gill, K.S.; et al. An Integrated Approach to Maintaining Cereal Productivity under Climate Change. Glob. Food Secur. 2016, 8, 9–18. [Google Scholar] [CrossRef]
- Muehlbauer, G.J.; Feuillet, C. (Eds.) Genetics and Genomics of the Triticeae; Springer US: New York, NY, USA, 2009; ISBN 978-0-387-77488-6. [Google Scholar]
- Wang, J.; Vanga, S.; Saxena, R.; Orsat, V.; Raghavan, V. Effect of Climate Change on the Yield of Cereal Crops: A Review. Climate 2018, 6, 41. [Google Scholar] [CrossRef]
- FAO; STAT. License: CC BY-NC-SA 3.0 IGO. Available online: https://www.fao.org/faostat/en/#home (accessed on 7 October 2022).
- Barkworth, M.E.; Von Bothmer, R. Scientific Names in the Triticeae. In Genetics and Genomics of the Triticeae; Muehlbauer, G.J., Feuillet, C., Eds.; Springer US: New York, NY, USA, 2009; pp. 3–30. ISBN 978-0-387-77488-6. [Google Scholar]
- Hyles, J.; Bloomfield, M.T.; Hunt, J.R.; Trethowan, R.M.; Trevaskis, B. Phenology and Related Traits for Wheat Adaptation. Heredity 2020, 125, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, M.; Himmelbach, A.; Börner, A.; Mascher, M. Genetic Diversity and Relationship between Domesticated Rye and Its Wild Relatives as Revealed through Genotyping-by-sequencing. Evol. Appl. 2019, 12, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.M.; Dennis, E.S.; Finnegan, E.J. The Low Temperature Response Pathways for Cold Acclimation and Vernalization Are Independent: Low Temperature Response Pathways. Plant Cell Environ. 2011, 34, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Trevaskis, B.; Hemming, M.N.; Peacock, W.J.; Dennis, E.S. HvVRN2 Responds to Daylength, Whereas HvVRN1 Is Regulated by Vernalization and Developmental Status. Plant Physiol. 2006, 140, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Molecular Basis of Plant Cold Acclimation: Insights Gained from Studying the CBF Cold Response Pathway: Figure 1. Plant Physiol. 2010, 154, 571–577. [Google Scholar] [CrossRef] [PubMed]
- von Bothmer, R.; Sato, K.; Komatsuda, T.; Yasuda, S.; Fischbeck, G. The Domestication of Cultivated Barley. In Developments in Plant Genetics and Breeding; Elsevier: Amsterdam, The Netherlands, 2003; Volume 7, pp. 9–27. ISBN 978-0-444-50585-9. [Google Scholar]
- von Zitzewitz, J.; Cuesta-Marcos, A.; Condon, F.; Castro, A.J.; Chao, S.; Corey, A.; Filichkin, T.; Fisk, S.P.; Gutierrez, L.; Haggard, K.; et al. The Genetics of Winterhardiness in Barley: Perspectives from Genome-Wide Association Mapping. Plant Genome 2011, 4, 76–91. [Google Scholar] [CrossRef]
- Stockinger, E.J. Winter Hardiness and the CBF Genes in the Triticeae. In Plant Cold Hardiness: From the Laboratory to the Field; Gusta, L.V., Wisniewski, M.E., Tanino, K.K., Eds.; CABI: Wallingford, UK, 2009; pp. 119–130. ISBN 978-1-84593-513-9. [Google Scholar]
- Muñoz-Amatriaín, M.; Hernandez, J.; Herb, D.; Baenziger, P.S.; Bochard, A.M.; Capettini, F.; Casas, A.; Cuesta-Marcos, A.; Einfeldt, C.; Fisk, S.; et al. Perspectives on Low Temperature Tolerance and Vernalization Sensitivity in Barley: Prospects for Facultative Growth Habit. Front. Plant Sci. 2020, 11, 585927. [Google Scholar] [CrossRef]
- Rosicka-Kaczmarek, J.; Makowski, B.; Nebesny, E.; Tkaczyk, M.; Komisarczyk, A.; Nita, Z. Composition and Thermodynamic Properties of Starches from Facultative Wheat Varieties. Food Hydrocoll. 2016, 54, 66–76. [Google Scholar] [CrossRef]
- Deng, P.; Wang, M.; Feng, K.; Cui, L.; Tong, W.; Song, W.; Nie, X. Genome-Wide Characterization of Microsatellites in Triticeae Species: Abundance, Distribution and Evolution. Sci. Rep. 2016, 6, 32224. [Google Scholar] [CrossRef]
- Feldman, M.; Levy, A.A. Origin and Evolution of Wheat and Related Triticeae Species. In Alien Introgression in Wheat; Molnár-Láng, M., Ceoloni, C., Doležel, J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 21–76. ISBN 978-3-319-23493-9. [Google Scholar]
- Kumlehn, J.; Zimmermann, G.; Berger, C.; Marthe, C.; Hensel, G. Triticeae Cereals. In Genetic Modification of Plants; Kempken, F., Jung, C., Eds.; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 2010; Volume 64, pp. 287–306. ISBN 978-3-642-02390-3. [Google Scholar]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Maccaferri, M.; Sanguineti, M.C.; Giuliani, S.; Tuberosa, R. Genomics of Tolerance to Abiotic Stress in the Triticeae. In Genetics and Genomics of the Triticeae; Muehlbauer, G.J., Feuillet, C., Eds.; Springer US: New York, NY, USA, 2009; pp. 481–558. ISBN 978-0-387-77488-6. [Google Scholar]
- Thomashow, M.F. PLANT COLD ACCLIMATION: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Yuanyuan, K.; Bruno, A.K.; Lele, Z.; Jincai, L. Cold Stress in Wheat: Plant Acclimation Responses and Management Strategies. Front. Plant Sci. 2021, 12, 676884. [Google Scholar] [CrossRef] [PubMed]
- Praba, M.L.; Cairns, J.E.; Babu, R.C.; Lafitte, H.R. Identification of Physiological Traits Underlying Cultivar Differences in Drought Tolerance in Rice and Wheat. J. Agron. Crop Sci. 2009, 195, 30–46. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; p. 36. ISBN 978-90-481-2666-8. [Google Scholar]
- Preston, J.C.; Fjellheim, S. Understanding Past, and Predicting Future, Niche Transitions Based on Grass Flowering Time Variation. Plant Physiol. 2020, 183, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, I.; Pu, X.; Naseer, M.A.; Bohoussou, Y.N.; Liu, Y.; Farooq, M.; Zhang, J.; Zhang, Y.; Wang, Z.; Sun, Z. Cold and Drought Stresses in Wheat: A Global Meta-Analysis of 21st Century. J. Plant Growth Regul. 2023, 42, 5379–5395. [Google Scholar] [CrossRef]
- Ozturk, T.; Ceber, Z.P.; Türkeş, M.; Kurnaz, M.L. Projections of Climate Change in the Mediterranean Basin by Using Downscaled Global Climate Model Outputs: Projections of climate change in mediterranean by using global models. Int. J. Climatol. 2015, 35, 4276–4292. [Google Scholar] [CrossRef]
- Cohen, J.; Agel, L.; Barlow, M.; Garfinkel, C.I. Linking Arctic Variability and Change with Extreme Winter Weather in the United States. Science 2021, 373, 1116–1121. [Google Scholar] [CrossRef]
- Huang, J.; Hitchcock, P.; Maycock, A.C.; McKenna, C.M.; Tian, W. Northern Hemisphere Cold Air Outbreaks Are More Likely to Be Severe during Weak Polar Vortex Conditions. Commun. Earth Environ. 2021, 2, 147. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Rizza, F.; Karsai, I.; Morcia, C.; Badeck, F.-W.; Terzi, V.; Pagani, D.; Kiss, T.; Stanca, A.M. Association between the Allele Compositions of Major Plant Developmental Genes and Frost Tolerance in Barley (Hordeum Vulgare L.) Germplasm of Different Origin. Mol. Breed. 2016, 36, 156. [Google Scholar] [CrossRef]
- Current Map|U.S. Drought Monitor. Available online: https://droughtmonitor.unl.edu/ (accessed on 6 March 2023).
- Drought Reports—European Drought Observatory—JRC European Commission. Available online: https://edo.jrc.ec.europa.eu/edov2/php/index.php?id=1051 (accessed on 6 March 2023).
- Graph|U.S. Climate Extremes Index (CEI) | National Centers for Environmental Information (NCEI). Available online: https://www.ncei.noaa.gov/access/monitoring/cei/graph/us/01-12/cei (accessed on 6 March 2023).
- Francis, A.J.; Vavrus, J.S.; Cohen, J. Amplified Arctic Warming and Mid-Latitude Weather: New Perspectives on Emerging Connections. WIREs Clim. Change 2017, 8, e474. [Google Scholar] [CrossRef]
- Tao, F.; Zhang, Z.; Xiao, D.; Zhang, S.; Rötter, R.P.; Shi, W.; Liu, Y.; Wang, M.; Liu, F.; Zhang, H. Responses of Wheat Growth and Yield to Climate Change in Different Climate Zones of China, 1981–2009. Agric. For. Meteorol. 2014, 189–190, 91–104. [Google Scholar] [CrossRef]
- Willick, I.R.; Tanino, K.K.; Gusta, L.V. The Impact of Global Climate Change on the Freezing Tolerance of Winter Cereals in Western Canada. J. Agro. Crop Sci. 2021, 207, 88–99. [Google Scholar] [CrossRef]
- Chen, F.; He, J.; Jin, G.; Chen, Z.-H.; Dai, F. Identification of Novel microRNAs for Cold Deacclimation in Barley. Plant Growth Regul. 2020, 92, 389–400. [Google Scholar] [CrossRef]
- Barlow, K.M.; Christy, B.P.; O’Leary, G.J.; Riffkin, P.A.; Nuttall, J.G. Simulating the Impact of Extreme Heat and Frost Events on Wheat Crop Production: A Review. Field Crops Res. 2015, 171, 109–119. [Google Scholar] [CrossRef]
- Penfield, S.; Warner, S.; Wilkinson, L. Molecular Responses to Chilling in a Warming Climate and Their Impacts on Plant Reproductive Development and Yield. J. Exp. Bot. 2021, 72, 7374–7383. [Google Scholar] [CrossRef]
- Trnka, M.; Rötter, R.P.; Ruiz-Ramos, M.; Kersebaum, K.C.; Olesen, J.E.; Žalud, Z.; Semenov, M.A. Adverse Weather Conditions for European Wheat Production Will Become More Frequent with Climate Change. Nat. Clim. Change 2014, 4, 637–643. [Google Scholar] [CrossRef]
- Frederiks, T.M.; Christopher, J.T.; Sutherland, M.W.; Borrell, A.K. Post-Head-Emergence Frost in Wheat and Barley: Defining the Problem, Assessing the Damage, and Identifying Resistance. EXBOTJ 2015, 66, 3487–3498. [Google Scholar] [CrossRef]
- Faranda, D.; Pascale, S.; Bulut, B. Persistent Anticyclonic Conditions and Climate Change Exacerbated the Exceptional 2022 European-Mediterranean Drought. Environ. Res. Lett. 2023, 18, 034030. [Google Scholar] [CrossRef]
- Perkins-Kirkpatrick, S.E.; Lewis, S.C. Increasing Trends in Regional Heatwaves. Nat. Commun. 2020, 11, 3357. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising Temperatures Reduce Global Wheat Production. Nat. Clim. Change 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Neupane, D.; Adhikari, P.; Bhattarai, D.; Rana, B.; Ahmed, Z.; Sharma, U.; Adhikari, D. Does Climate Change Affect the Yield of the Top Three Cereals and Food Security in the World? Earth 2022, 3, 45–71. [Google Scholar] [CrossRef]
- Gammans, M.; Mérel, P.; Ortiz-Bobea, A. Negative Impacts of Climate Change on Cereal Yields: Statistical Evidence from France. Environ. Res. Lett. 2017, 12, 054007. [Google Scholar] [CrossRef]
- Cammarano, D.; Ceccarelli, S.; Grando, S.; Romagosa, I.; Benbelkacem, A.; Akar, T.; Al-Yassin, A.; Pecchioni, N.; Francia, E.; Ronga, D. The Impact of Climate Change on Barley Yield in the Mediterranean Basin. Eur. J. Agron. 2019, 106, 1–11. [Google Scholar] [CrossRef]
- Dhillon, T.; Pearce, S.P.; Stockinger, E.J.; Distelfeld, A.; Li, C.; Knox, A.K.; Vashegyi, I.; Vágújfalvi, A.; Galiba, G.; Dubcovsky, J. Regulation of Freezing Tolerance and Flowering in Temperate Cereals: The VRN-1 Connection. Plant Physiol. 2010, 153, 1846–1858. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, F. Regulation by Vrn-1/Fr-1 Chromosomal Intervals of CBF-Mediated Cor/Lea Gene Expression and Freezing Tolerance in Common Wheat. J. Exp. Bot. 2005, 56, 887–895. [Google Scholar] [CrossRef]
- Szùcs, P.; Skinner, J.S.; Karsai, I.; Cuesta-Marcos, A.; Haggard, K.G.; Corey, A.E.; Chen, T.H.H.; Hayes, M.P. Validation of the VRN-H2/VRN-H1 Epistatic Model in Barley Reveals That Intron Length Variation in VRN-H1 May Account for a Continuum of Vernalization Sensitivity. Mol. Genet. Genom. 2007, 277, 249–261. [Google Scholar] [CrossRef]
- Francia, E.; Rizza, F.; Cattivelli, L.; Stanca, A.M.; Galiba, G.; Tóth, B.; Hayes, P.M.; Skinner, J.S.; Pecchioni, N. Two Loci on Chromosome 5H Determine Low-Temperature Tolerance in a ‘Nure’ (Winter) × ‘Tremois’ (Spring) Barley Map. Theor. Appl. Genet. 2004, 108, 670–680. [Google Scholar] [CrossRef]
- Vágújfalvi, A.; Aprile, A.; Miller, A.; Dubcovsky, J.; Delugu, G.; Galiba, G.; Cattivelli, L. The Expression of Several Cbf Genes at the Fr-A2 Locus Is Linked to Frost Resistance in Wheat. Mol. Genet. Genom. 2005, 274, 506–514. [Google Scholar] [CrossRef]
- Dong, C.; Ma, Y.; Zheng, D.; Wisniewski, M.; Cheng, Z.-M. Meta-Analysis of the Effect of Overexpression of Dehydration-Responsive Element Binding Family Genes on Temperature Stress Tolerance and Related Responses. Front. Plant Sci. 2018, 9, 713. [Google Scholar] [CrossRef]
- Yang, Y.; Al-Baidhani, H.H.J.; Harris, J.; Riboni, M.; Li, Y.; Mazonka, I.; Bazanova, N.; Chirkova, L.; Sarfraz Hussain, S.; Hrmova, M.; et al. DREB/CBF Expression in Wheat and Barley Using the Stress-inducible Promoters of HD-Zip I Genes: Impact on Plant Development, Stress Tolerance and Yield. Plant Biotechnol. J. 2020, 18, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ren, Y.; Tang, Z.; Shi, W.; Zhou, M. Characterization and Expression Profiling of the ICE-CBF-COR Genes in Wheat. PeerJ 2019, 7, e8190. [Google Scholar] [CrossRef] [PubMed]
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic Stress in Crop Production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of Abiotic Stress on Plants: A Systems Biology Perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef]
- Ranty, B.; Aldon, D.; Cotelle, V.; Galaud, J.-P.; Thuleau, P.; Mazars, C. Calcium Sensors as Key Hubs in Plant Responses to Biotic and Abiotic Stresses. Front. Plant Sci. 2016, 7, 327. [Google Scholar] [CrossRef]
- Jan, A.U.; Hadi, F.; Ahmad, A.; Rahman, K. Role of CBF/DREB Gene Expression in Abiotic Stress Tolerance. A Review. Int. J. Hortic. Agric. 2017. [Google Scholar]
- Rodríguez-Vázquez, R.; Carrieri, V. A Proteomic Approach to Abiotic and Biotic Stress in Barley: A Review. Plant Mol. Biol. Rep. 2023. [Google Scholar] [CrossRef]
- Mastrangelo, A.M.; Mare, C.; Mazzucotelli, E.; Francia, E.; Arru, L.; Di Fonzo, N.; Pecchioni, N.; Cattivelli, L. Genetic Bases of Resistance to Abiotic Stresses in Durum Wheat (Triticum Turgidum Ssp. Durum). In Durum Wheat Breeding: Current Approaches and Future Stategies; Food Products Press, An Imprint of The Haworth Press, Inc.: Binghamton, NY, USA, 2005; Volume 1, pp. 255–289. [Google Scholar]
- Park, S.; Lee, C.; Doherty, C.J.; Gilmour, S.J.; Kim, Y.; Thomashow, M.F. Regulation of the Arabidopsis CBF Regulon by a Complex Low-temperature Regulatory Network. Plant J. 2015, 82, 193–207. [Google Scholar] [CrossRef]
- Akhtar, M.; Jaiswal, A.; Taj, G.; Jaiswal, J.P.; Qureshi, M.I.; Singh, N.K. DREB1/CBF Transcription Factors: Their Structure, Function and Role in Abiotic Stress Tolerance in Plants. J. Genet. 2012, 91, 385–395. [Google Scholar] [CrossRef]
- Heidarvand, L.; Maali Amiri, R. What Happens in Plant Molecular Responses to Cold Stress? Acta Physiol. Plant 2010, 32, 419–431. [Google Scholar] [CrossRef]
- Choi, D.-W.; Zhu, B.; Close, T.J. The Barley (Hordeum Vulgare L.) Dehydrin Multigene Family: Sequences, Allele Types, Chromosome Assignments, and Expression Characteristics of 11 Dhn Genes of Cv Dicktoo. Theor. Appl. Genet. 1999, 98, 1234–1247. [Google Scholar] [CrossRef]
- Ahmad, M.; Alabd, A.; Gao, Y.; Yu, W.; Jamil, W.; Wang, X.; Wei, J.; Ni, J.; Teng, Y.; Bai, S. Three Stress-Responsive NAC Transcription Factors, Pp-SNACs, Differentially and Synergistically Regulate Abiotic Stress in Pear. Sci. Hortic. 2022, 305, 111393. [Google Scholar] [CrossRef]
- Ahres, M.; Gierczik, K.; Boldizsár, Á.; Vítámvás, P.; Galiba, G. Temperature and Light-Quality-Dependent Regulation of Freezing Tolerance in Barley. Plants 2020, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ding, Y.; Yang, S. Molecular Regulation of CBF Signaling in Cold Acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef]
- Skinner, J.S.; von Zitzewitz, J.; Szűcs, P.; Marquez-Cedillo, L.; Filichkin, T.; Amundsen, K.; Stockinger, E.J.; Thomashow, M.F.; Chen, T.H.H.; Hayes, P.M. Structural, Functional, and Phylogenetic Characterization of a Large CBF Gene Family in Barley. Plant Mol. Biol. 2005, 59, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, E.J.; Gilmour, S.J.; Thomashow, M.F. Arabidopsis Thaliana CBF1 Encodes an AP2 Domain-Containing Transcriptional Activator That Binds to the C-Repeat/DRE, a Cis-Acting DNA Regulatory Element That Stimulates Transcription in Response to Low Temperature and Water Deficit. Proc. Natl. Acad. Sci. USA 1997, 94, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.; Bargues, M.; Terol, J.; Pérez-Alonso, M.; Salinas, J. The Arabidopsis CBF Gene Family Is Composed of Three Genes Encoding AP2 Domain-Containing Proteins Whose Expression Is Regulated by Low Temperature but Not by Abscisic Acid or Dehydration1. Plant Physiol. 1999, 119, 463–470. [Google Scholar] [CrossRef]
- Jaglo-Ottosen, K.R.; Gilmour, S.J.; Zarka, D.G.; Schabenberger, O.; Thomashow, M.F. Arabidopsis CBF1 Overexpression Induces COR Genes and Enhances Freezing Tolerance. Science 1998, 280, 104–106. [Google Scholar] [CrossRef]
- Tondelli, A.; Francia, E.; Barabaschi, D.; Pasquariello, M.; Pecchioni, N. Inside the CBF Locus in Poaceae. Plant Sci. 2011, 180, 39–45. [Google Scholar] [CrossRef]
- Welling, A.; Palva, E.T. Molecular Control of Cold Acclimation in Trees. Physiol Plant 2006, 127, 167–181. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Ye, M.; Lu, H.; Wang, D.; Chen, Q. Evolutionary History of the C-Repeat Binding Factor/Dehydration-Responsive Element-Binding 1 (CBF/DREB1) Protein Family in 43 Plant Species and Characterization of CBF/DREB1 Proteins in Solanum Tuberosum. BMC Evol. Biol. 2020, 20, 142. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ban, Q.; Hao, J.; Zhu, X.; Cheng, Y.; Mao, J.; Lin, M.; Xia, E.; Li, Y. Genome-Wide Characterization of the C-Repeat Binding Factor (CBF) Gene Family Involved in the Response to Abiotic Stresses in Tea Plant (Camellia Sinensis). Front. Plant Sci. 2020, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Cai, H.; Fu, H.; An, Z.; Fang, J.; Hu, Y.; Guo, D.; Huang, H. Functional Characterization of Hevea Brasiliensis CRT/DRE Binding Factor 1 Gene Revealed Regulation Potential in the CBF Pathway of Tropical Perennial Tree. PLoS ONE 2015, 10, e0137634. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Huang, T.; Lu, C.; Dang, P.; Zhang, M.; Guan, X.; Wen, P.; Wang, T.-C.; Chen, Y.; Siddique, K.H.M. Benefits and Limitations of Straw Mulching and Incorporation on Maize Yield, Water Use Efficiency, and Nitrogen Use Efficiency. Agric. Water Manag. 2021, 256, 107128. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB Genes in Rice, Oryza Sativa L., Encode Transcription Activators That Function in Drought-, High-Salt- and Cold-Responsive Gene Expression: DREB Transcription Activators in Rice. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-Wide Analysis of the ERF Gene Family in Arabidopsis and Rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef]
- Navarro, M.; Marque, G.; Ayax, C.; Keller, G.; Borges, J.P.; Marque, C.; Teulières, C. Complementary Regulation of Four Eucalyptus CBF Genes under Various Cold Conditions. J. Exp. Bot. 2009, 60, 2713–2724. [Google Scholar] [CrossRef]
- Galiba, G.; Vágújfalvi, A.; Li, C.; Soltész, A.; Dubcovsky, J. Regulatory Genes Involved in the Determination of Frost Tolerance in Temperate Cereals. Plant Sci. 2009, 176, 12–19. [Google Scholar] [CrossRef]
- Campoli, C.; Matus-Cádiz, M.A.; Pozniak, C.J.; Cattivelli, L.; Fowler, D.B. Comparative Expression of Cbf Genes in the Triticeae under Different Acclimation Induction Temperatures. Mol. Genet. Genom. 2009, 282, 141–152. [Google Scholar] [CrossRef]
- Badawi, M.; Danyluk, J.; Boucho, B.; Houde, M.; Sarhan, F. The CBF Gene Family in Hexaploid Wheat and Its Relationship to the Phylogenetic Complexity of Cereal CBFs. Mol. Genet. Genom. 2007, 277, 533–554. [Google Scholar] [CrossRef]
- Francia, E.; Barabaschi, D.; Tondelli, A.; Laidò, G.; Rizza, F.; Stanca, A.M.; Busconi, M.; Fogher, C.; Stockinger, E.J.; Pecchioni, N. Fine Mapping of a HvCBF Gene Cluster at the Frost Resistance Locus Fr-H2 in Barley. Theor. Appl. Genet. 2007, 115, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.M.; Blake, T.; Chen, T.H.H.; Tragoonrung, S.; Chen, F.; Pan, A.; Liu, B. Quantitative Trait Loci on Barley ( Hordeum Vulgare L.) Chromosome 7 Associated with Components of Winterhardiness. Genome 1993, 36, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Vágújfalvi, A.; Galiba, G.; Cattivelli, L.; Dubcovsky, J. The Cold-Regulated Transcriptional Activator Cbf3 Is Linked to the Frost-Tolerance Locus Fr-A2 on Wheat Chromosome 5A. Mol. Gen. Genom. 2003, 269, 60–67. [Google Scholar] [CrossRef]
- Båga, M.; Bahrani, H.; Larsen, J.; Hackauf, B.; Graf, R.J.; Laroche, A.; Chibbar, R.N. Association Mapping of Autumn-Seeded Rye (Secale Cereale L.) Reveals Genetic Linkages between Genes Controlling Winter Hardiness and Plant Development. Sci. Rep. 2022, 12, 5793. [Google Scholar] [CrossRef]
- Galiba, G.; Quarrie, S.A.; Sutka, J.; Morgounov, A.; Snape, J.W. RFLP Mapping of the Vernalization (Vrnl) and Frost Resistance (Frl) Genes on Chromosome 5A of Wheat. Theor. Appl. Genet. 1995, 90, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Amasino, R. Vernalization, Competence, and the Epigenetic Memory of Winter. Plant Cell 2004, 16, 2553–2559. [Google Scholar] [CrossRef] [PubMed]
- Rabanus-Wallace, M.T.; Hackauf, B.; Mascher, M.; Lux, T.; Wicker, T.; Gundlach, H.; Baez, M.; Houben, A.; Mayer, K.F.X.; Guo, L.; et al. Chromosome-Scale Genome Assembly Provides Insights into Rye Biology, Evolution and Agronomic Potential. Nat. Genet. 2021, 53, 564–573. [Google Scholar] [CrossRef]
- The International Wheat Genome Sequencing Consortium (IWGSC); Appels, R.; Eversole, K.; Stein, N.; Feuillet, C.; Keller, B.; Rogers, J.; Pozniak, C.J.; Choulet, F.; Distelfeld, A.; et al. Shifting the Limits in Wheat Research and Breeding Using a Fully Annotated Reference Genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef]
- Pasquariello, M.; Barabaschi, D.; Himmelbach, A.; Steuernagel, B.; Ariyadasa, R.; Stein, N.; Gandolfi, F.; Tenedini, E.; Bernardis, I.; Tagliafico, E.; et al. The Barley Frost Resistance-H2 Locus. Funct. Integr. Genom. 2014, 14, 85–100. [Google Scholar] [CrossRef]
- Mareri, L.; Milc, J.; Laviano, L.; Buti, M.; Vautrin, S.; Cauet, S.; Mascagni, F.; Natali, L.; Cavallini, A.; Bergès, H.; et al. Influence of CNV on Transcript Levels of HvCBF Genes at Fr-H2 Locus Revealed by Resequencing in Resistant Barley Cv. ‘Nure’ and Expression Analysis. Plant Sci. 2020, 290, 110305. [Google Scholar] [CrossRef]
- Miller, A.K.; Galiba, G.; Dubcovsky, J. A Cluster of 11 CBF Transcription Factors Is Located at the Frost Tolerance Locus Fr-A m 2 in Triticum Monococcum. Mol. Genet. Genom. 2006, 275, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Knox, A.K.; Li, C.; Vágújfalvi, A.; Galiba, G.; Stockinger, E.J.; Dubcovsky, J. Identification of Candidate CBF Genes for the Frost Tolerance Locus Fr-A m 2 in Triticum Monococcum. Plant Mol. Biol. 2008, 67, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, S.; Che, H.; Djillali, Z.; Dumont, E.; Nankeu, J.; Danyluk, J. Wheat CBF Gene Family: Identification of Polymorphisms in the CBF Coding Sequence. Genome 2012, 55, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Knox, A.K.; Dhillon, T.; Cheng, H.; Tondelli, A.; Pecchioni, N.; Stockinger, E.J. CBF Gene Copy Number Variation at Frost Resistance-2 Is Associated with Levels of Freezing Tolerance in Temperate-Climate Cereals. Theor. Appl. Genet. 2010, 121, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Francia, E.; Morcia, C.; Pasquariello, M.; Mazzamurro, V.; Milc, J.A.; Rizza, F.; Terzi, V.; Pecchioni, N. Copy Number Variation at the HvCBF4–HvCBF2 Genomic Segment Is a Major Component of Frost Resistance in Barley. Plant Mol. Biol. 2016, 92, 161–175. [Google Scholar] [CrossRef]
- Sieber, A.-N.; Longin, C.F.H.; Leiser, W.L.; Würschum, T. Copy Number Variation of CBF-A14 at the Fr-A2 Locus Determines Frost Tolerance in Winter Durum Wheat. Theor. Appl. Genet. 2016, 129, 1087–1097. [Google Scholar] [CrossRef]
- Zhu, J.; Pearce, S.; Burke, A.; See, D.R.; Skinner, D.Z.; Dubcovsky, J.; Garland-Campbell, K. Copy Number and Haplotype Variation at the VRN-A1 and Central FR-A2 Loci Are Associated with Frost Tolerance in Hexaploid Wheat. Theor. Appl. Genet. 2014, 127, 1183–1197. [Google Scholar] [CrossRef]
- Hüner, N.P.A.; Dahal, K.; Bode, R.; Kurepin, L.V.; Ivanov, A.G. Photosynthetic Acclimation, Vernalization, Crop Productivity and ‘the Grand Design of Photosynthesis’. J. Plant Physiol. 2016, 203, 29–43. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T. Wheat and Barley Dehydrins under Cold, Drought, and Salinity €“ What Can LEA-II Proteins Tell Us about Plant Stress Response? Front. Plant Sci. 2014, 5, 343. [Google Scholar] [CrossRef]
- Hüner, N.; Öquist, G.; Hurry, V.M.; Krol, M.; Falk, S.; Griffith, M. Photosynthesis, Photoinhibition and Low Temperature Acclimation in Cold Tolerant Plants. Photosynth. Res. 1993, 37, 19–39. [Google Scholar] [CrossRef]
- Fiust, A.; Rapacz, M. Downregulation of Three Novel Candidate Genes Is Important for Freezing Tolerance of Field and Laboratory Cold Acclimated Barley. J. Plant Physiol. 2020, 244, 153049. [Google Scholar] [CrossRef] [PubMed]
- Hüner, N.P.A.; Bode, R.; Dahal, K.; Busch, F.A.; Possmayer, M.; Szyszka, B.; Rosso, D.; Ensminger, I.; Krol, M.; Ivanov, A.G.; et al. Shedding Some Light on Cold Acclimation, Cold Adaptation, and Phenotypic Plasticity. Botany 2013, 91, 127–136. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, Y.; Yang, S. Cold Signal Transduction and Its Interplay with Phytohormones During Cold Acclimation. Plant Cell Physiol. 2015, 56, 7–15. [Google Scholar] [CrossRef]
- Kashyap, P.; Deswal, R. Phytohormones Regulating the Master Regulators of CBF Dependent Cold Stress Signaling Pathway. In Genetic Enhancement of Crops for Tolerance to Abiotic Stress: Mechanisms and Approaches, Vol. I; Rajpal, V.R., Sehgal, D., Kumar, A., Raina, S.N., Eds.; Sustainable Development and Biodiversity; Springer International Publishing: Cham, Switzerland, 2019; Volume 20, pp. 249–264. ISBN 978-3-319-91955-3. [Google Scholar]
- Galiba, G.; Tóth, B. Cold Stress. In Encyclopedia of Applied Plant Sciences; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–7. ISBN 978-0-12-394808-3. [Google Scholar]
- Maibam, P.; Nawkar, G.; Park, J.; Sahi, V.; Lee, S.; Kang, C. The Influence of Light Quality, Circadian Rhythm, and Photoperiod on the CBF-Mediated Freezing Tolerance. Int. J. Mol. Sci. 2013, 14, 11527–11543. [Google Scholar] [CrossRef] [PubMed]
- Kurepin, L.; Dahal, K.; Savitch, L.; Singh, J.; Bode, R.; Ivanov, A.; Hurry, V.; Hüner, N. Role of CBFs as Integrators of Chloroplast Redox, Phytochrome and Plant Hormone Signaling during Cold Acclimation. Int. J. Mol. Sci. 2013, 14, 12729–12763. [Google Scholar] [CrossRef]
- Vaultier, M.-N.; Cantrel, C.; Vergnolle, C.; Justin, A.-M.; Demandre, C.; Benhassaine-Kesri, G.; Çiçek, D.; Zachowski, A.; Ruelland, E. Desaturase Mutants Reveal That Membrane Rigidification Acts as a Cold Perception Mechanism Upstream of the Diacylglycerol Kinase Pathway in Arabidopsis Cells. FEBS Lett. 2006, 580, 4218–4223. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.-P. The DNA-Binding Activity of an AP2 Transcriptional Activator HvCBF2 Involved in Regulation of Low-Temperature Responsive Genes in Barley Is Modulated by Temperature. Plant J. 2003, 33, 373–383. [Google Scholar] [CrossRef]
- Jin, Y.; Zhai, S.; Wang, W.; Ding, X.; Guo, Z.; Bai, L.; Wang, S. Identification of Genes from the ICE–CBF–COR Pathway under Cold Stress in Aegilops–Triticum Composite Group and the Evolution Analysis with Those from Triticeae. Physiol. Mol. Biol. Plants 2018, 24, 211–229. [Google Scholar] [CrossRef]
- Fowler, D.B. Cold Acclimation Threshold Induction Temperatures in Cereals. Crop Sci. 2008, 48, 1147. [Google Scholar] [CrossRef]
- Rizza, F.; Pagani, D.; Gut, M.; Prášil, I.T.; Lago, C.; Tondelli, A.; Orrù, L.; Mazzucotelli, E.; Francia, E.; Badeck, F.-W.; et al. Diversity in the Response to Low Temperature in Representative Barley Genotypes Cultivated in Europe. Crop Sci. 2011, 51, 2759–2779. [Google Scholar] [CrossRef]
- Crosatti, C.; Marè, C.; Mazzucotelli, E.; Belloni, S.; Barilli, S.; Bassi, R.; Dubcovskyi, J.; Galiba, G.; Stanca, A.M.; Cattivelli, L. Genetic Analysis of the Expression of the Cold-Regulated Gene Cor14b: A Way toward the Identification of Components of the Cold Response Signal Transduction in Triticeae. Can. J. Bot. 2003, 81, 1162–1167. [Google Scholar] [CrossRef]
- Cha, J.-K.; O’Connor, K.; Alahmad, S.; Lee, J.-H.; Dinglasan, E.; Park, H.; Lee, S.-M.; Hirsz, D.; Kwon, S.-W.; Kwon, Y.; et al. Speed Vernalization to Accelerate Generation Advance in Winter Cereal Crops. Mol. Plant 2022, 15, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chong, K. Remembering Winter through Vernalisation. Nat. Plants 2018, 4, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Pecchioni, N.; Kosová, K.; Vítámvás, P.; Prášil, I.T.; Milc, J.A.; Francia, E.; Gulyás, Z.; Kocsy, G.; Galiba, G. Genomics of Low-Temperature Tolerance for an Increased Sustainability of Wheat and Barley Production. In Genomics of Plant Genetic Resources; Tuberosa, R., Graner, A., Frison, E., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 149–183. ISBN 978-94-007-7574-9. [Google Scholar]
- Miura, K.; Furumoto, T. Cold Signaling and Cold Response in Plants. Int. J. Mol. Sci. 2013, 14, 5312–5337. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Yang, Q.; Zeng, J.; Jin, G.; Chen, Z.-H.; Zhang, G.; Dai, F. Identification of Mild Freezing Shock Response Pathways in Barley Based on Transcriptome Profiling. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Badawi, M.; Reddy, Y.V.; Agharbaoui, Z.; Tominaga, Y.; Danyluk, J.; Sarhan, F.; Houde, M. Structure and Functional Analysis of Wheat ICE (Inducer of CBF Expression) Genes. Plant Cell Physiol. 2008, 49, 1237–1249. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Ohta, M.; Kanrar, S.; Lee, B.; Hong, X.; Agarwal, M.; Zhu, J.-K. ICE1: A Regulator of Cold-Induced Transcriptome and Freezing Tolerance in Arabidopsis. Genes Dev. 2003, 17, 1043–1054. [Google Scholar] [CrossRef]
- Gierczik, K.; Novák, A.; Ahres, M.; Székely, A.; Soltész, A.; Boldizsár, Á.; Gulyás, Z.; Kalapos, B.; Monostori, I.; Kozma-Bognár, L.; et al. Circadian and Light Regulated Expression of CBFs and Their Upstream Signalling Genes in Barley. Int. J. Mol. Sci. 2017, 18, 1828. [Google Scholar] [CrossRef]
- Dhillon, T.; Morohashi, K.; Stockinger, E.J. CBF2A–CBF4B Genomic Region Copy Numbers alongside the Circadian Clock Play Key Regulatory Mechanisms Driving Expression of FR-H2 CBFs. Plant Mol. Biol. 2017, 94, 333–347. [Google Scholar] [CrossRef]
- Lee, C.-M.; Thomashow, M.F. Photoperiodic Regulation of the C-Repeat Binding Factor (CBF) Cold Acclimation Pathway and Freezing Tolerance in Arabidopsis Thaliana. Proc. Natl. Acad. Sci. USA 2012, 109, 15054–15059. [Google Scholar] [CrossRef]
- Liu, T.L.; Newton, L.; Liu, M.-J.; Shiu, S.-H.; Farré, E.M. A G-Box-Like Motif Is Necessary for Transcriptional Regulation by Circadian Pseudo-Response Regulators in Arabidopsis. Plant Physiol. 2016, 170, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Ahres, M.; Pálmai, T.; Kovács, T.; Kovács, L.; Lacek, J.; Vankova, R.; Galiba, G.; Borbély, P. The Effect of White Light Spectrum Modifications by Excess of Blue Light on the Frost Tolerance, Lipid- and Hormone Composition of Barley in the Early Pre-Hardening Phase. Plants 2022, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Ahres, M.; Pálmai, T.; Gierczik, K.; Dobrev, P.; Vanková, R.; Galiba, G. The Impact of Far-Red Light Supplementation on Hormonal Responses to Cold Acclimation in Barley. Biomolecules 2021, 11, 450. [Google Scholar] [CrossRef]
- Monostori, I.; Heilmann, M.; Kocsy, G.; Rakszegi, M.; Ahres, M.; Altenbach, S.B.; Szalai, G.; Pál, M.; Toldi, D.; Simon-Sarkadi, L.; et al. LED Lighting—Modification of Growth, Metabolism, Yield and Flour Composition in Wheat by Spectral Quality and Intensity. Front. Plant Sci. 2018, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Novák, A.; Boldizsár, Á.; Gierczik, K.; Vágújfalvi, A.; Ádám, É.; Kozma-Bognár, L.; Galiba, G. Light and Temperature Signalling at the Level of CBF14 Gene Expression in Wheat and Barley. Plant Mol. Biol. Rep. 2017, 35, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Kovács, T.; Ahres, M.; Pálmai, T.; Kovács, L.; Uemura, M.; Crosatti, C.; Galiba, G. Decreased R:FR Ratio in Incident White Light Affects the Composition of Barley Leaf Lipidome and Freezing Tolerance in a Temperature-Dependent Manner. Int. J. Mol. Sci. 2020, 21, 7557. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, G.; Zhuang, M.; Yin, J.; Wang, X. Molecular Cloning and Functional Characterization of TaIRI9 Gene in Wheat (Triticum Aestivum L.). Gene 2021, 791, 145694. [Google Scholar] [CrossRef]
- Distelfeld, A.; Li, C.; Dubcovsky, J. Regulation of Flowering in Temperate Cereals. Curr. Opin. Plant Biol. 2009, 12, 178–184. [Google Scholar] [CrossRef]
- Trevaskis, B.; Hemming, M.N.; Dennis, E.S.; Peacock, W.J. The Molecular Basis of Vernalization-Induced Flowering in Cereals. Trends Plant Sci. 2007, 12, 352–357. [Google Scholar] [CrossRef]
- Karsai, I.; Szűcs, P.; Mészáros, K.; Filichkina, T.; Hayes, P.M.; Skinner, J.S.; Láng, L.; Bedő, Z. The Vrn-H2 Locus Is a Major Determinant of Flowering Time in a Facultative × Winter Growth Habit Barley (Hordeum Vulgare L.) Mapping Population. Theor. Appl. Genet. 2005, 110, 1458–1466. [Google Scholar] [CrossRef]
- Fernández-Calleja, M.; Casas, A.M.; Igartua, E. Major Flowering Time Genes of Barley: Allelic Diversity, Effects, and Comparison with Wheat. Theor. Appl. Genet. 2021, 134, 1867–1897. [Google Scholar] [CrossRef] [PubMed]
- Faure, S.; Higgins, J.; Turner, A.; Laurie, D.A. The FLOWERING LOCUS T-Like Gene Family in Barley (Hordeum Vulgare). Genetics 2007, 176, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Fu, D.; Li, C.; Blechl, A.; Tranquilli, G.; Bonafede, M.; Sanchez, A.; Valarik, M.; Yasuda, S.; Dubcovsky, J. The Wheat and Barley Vernalization Gene VRN3 Is an Orthologue of FT. Proc. Natl. Acad. Sci. USA 2006, 103, 19581–19586. [Google Scholar] [CrossRef]
- Monteagudo, A.; Igartua, E.; Contreras-Moreira, B.; Gracia, M.P.; Ramos, J.; Karsai, I.; Casas, A.M. Fine-Tuning of the Flowering Time Control in Winter Barley: The Importance of HvOS2 and HvVRN2 in Non-Inductive Conditions. BMC Plant Biol. 2019, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Hemming, M.N.; Fieg, S.; James Peacock, W.; Dennis, E.S.; Trevaskis, B. Regions Associated with Repression of the Barley (Hordeum Vulgare) VERNALIZATION1 Gene Are Not Required for Cold Induction. Mol. Genet. Genom. 2009, 282, 107–117. [Google Scholar] [CrossRef]
- Cuesta-Marcos, A.; Szűcs, P.; Close, T.J.; Filichkin, T.; Muehlbauer, G.J.; Smith, K.P.; Hayes, P.M. Genome-Wide SNPs and Re-Sequencing of Growth Habit and Inflorescence Genes in Barley: Implications for Association Mapping in Germplasm Arrays Varying in Size and Structure. BMC Genom. 2010, 11, 707. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Marcos, A.; Muñoz-Amatriaín, M.; Filichkin, T.; Karsai, I.; Trevaskis, B.; Yasuda, S.; Hayes, P.; Sato, K. The Relationships between Development and Low Temperature Tolerance in Barley Near Isogenic Lines Differing for Flowering Behavior. Plant Cell Physiol. 2015, 56, 2312–2324. [Google Scholar] [CrossRef]
- Maeda, A.E.; Nakamichi, N. Plant Clock Modifications for Adapting Flowering Time to Local Environments. Plant Physiol. 2022, 190, 952–967. [Google Scholar] [CrossRef]
- Shcherban, A.B.; Strygina, K.V.; Salina, E.A. VRN-1 Gene- Associated Prerequisites of Spring Growth Habit in Wild Tetraploid Wheat T. Dicoccoides and the Diploid A Genome Species. BMC Plant Biol. 2015, 15, 94. [Google Scholar] [CrossRef]
- Tóth, B.; Galiba, G.; Fehér, E.; Sutka, J.; Snape, J.W. Mapping Genes Affecting Flowering Time and Frost Resistance on Chromosome 5B of Wheat. Theor. Appl. Genet. 2003, 107, 509–514. [Google Scholar] [CrossRef]
- Todorovska, E.G.; Kolev, S.; Christov, N.K.; Balint, A.; Kocsy, G.; Vágújfalvi, A.; Galiba, G. The Expression of CBF Genes at Fr-2 Locus Is Associated with the Level of Frost Tolerance in Bulgarian Winter Wheat Cultivars. Biotechnol. Biotechnol. Equip. 2014, 28, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, E.J.; Skinner, J.S.; Gardner, K.G.; Francia, E.; Pecchioni, N. Expression Levels of Barley Cbf Genes at the Frost Resistance—H2 Locus Are Dependent upon Alleles at Fr-H1 and Fr-H2: Expression Levels of Barley Cbf Genes. Plant J. 2007, 51, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Casao, M.C.; Wang, P.; Sato, K.; Hayes, P.M.; Finnegan, E.J.; Trevaskis, B. Direct Links between the Vernalization Response and Other Key Traits of Cereal Crops. Nat. Commun. 2015, 6, 5882. [Google Scholar] [CrossRef] [PubMed]
- Jayakodi, M.; Padmarasu, S.; Haberer, G.; Bonthala, V.S.; Gundlach, H.; Monat, C.; Lux, T.; Kamal, N.; Lang, D.; Himmelbach, A.; et al. The Barley Pan-Genome Reveals the Hidden Legacy of Mutation Breeding. Nature 2020, 588, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Galiba, G.; Stockinger, E.J.; Francia, E.; Milc, J.A.; Kocsy, G.; Pecchioni, N. Freezing Tolerance in the Triticeae. In Translational Genomics for Crop Breeding; Wiley Blackwell: Hoboken, NJ, USA, 2013; Volume 2. [Google Scholar]
- Visioni, A.; Tondelli, A.; Francia, E.; Pswarayi, A.; Malosetti, M.; Russell, J.; Thomas, W.; Waugh, R.; Pecchioni, N.; Romagosa, I.; et al. Genome-Wide Association Mapping of Frost Tolerance in Barley (Hordeum Vulgare L.). BMC Genom. 2013, 14, 424. [Google Scholar] [CrossRef] [PubMed]
- Stein, N.; Muehlbauer, G.J. (Eds.) The Barley Genome; Compendium of Plant Genomes; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-92527-1. [Google Scholar]
- Fricano, A.; Rizza, F.; Faccioli, P.; Pagani, D.; Pavan, P.; Stella, A.; Rossini, L.; Piffanelli, P.; Cattivelli, L. Genetic Variants of HvCbf14 Are Statistically Associated with Frost Tolerance in a European Germplasm Collection of Hordeum Vulgare. Theor. Appl. Genet. 2009, 119, 1335–1348. [Google Scholar] [CrossRef][Green Version]
- Guerra, D.; Morcia, C.; Badeck, F.; Rizza, F.; Delbono, S.; Francia, E.; Milc, J.A.; Monostori, I.; Galiba, G.; Cattivelli, L.; et al. Extensive Allele Mining Discovers Novel Genetic Diversity in the Loci Controlling Frost Tolerance in Barley. Theor. Appl. Genet. 2022, 135, 553–569. [Google Scholar] [CrossRef]
- Novák, A.; Boldizsár, Á.; Ádám, É.; Kozma-Bognár, L.; Majláth, I.; Båga, M.; Tóth, B.; Chibbar, R.; Galiba, G. Light-Quality and Temperature-Dependent CBF14 Gene Expression Modulates Freezing Tolerance in Cereals. EXBOTJ 2016, 67, 1285–1295. [Google Scholar] [CrossRef]
- Francia, E.; Pecchioni, N.; Policriti, A.; Scalabrin, S. CNV and Structural Variation in Plants: Prospects of NGS Approaches. In Advances in the Understanding of Biological Sciences Using Next Generation Sequencing (NGS) Approaches; Sablok, G., Kumar, S., Ueno, S., Kuo, J., Varotto, C., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 211–232. ISBN 978-3-319-17156-2. [Google Scholar]
- Cook, D.E.; Lee, T.G.; Guo, X.; Melito, S.; Wang, K.; Bayless, A.M.; Wang, J.; Hughes, T.J.; Willis, D.K.; Clemente, T.E.; et al. Copy Number Variation of Multiple Genes at Rhg1 Mediates Nematode Resistance in Soybean. Science 2012, 338, 1206–1209. [Google Scholar] [CrossRef]
- Maron, L.G.; Guimarães, C.T.; Kirst, M.; Albert, P.S.; Birchler, J.A.; Bradbury, P.J.; Buckler, E.S.; Coluccio, A.E.; Danilova, T.V.; Kudrna, D.; et al. Aluminum Tolerance in Maize Is Associated with Higher MATE1 Gene Copy Number. Proc. Natl. Acad. Sci. USA 2013, 110, 5241–5246. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, S.; Du, X.; Mateo, R.G.; Guo, W.; Li, A.; Wang, Z.; Wu, S.; Chen, J.; Liu, J.; et al. Genomic Analysis of Medicago Ruthenica Provides Insights into Its Tolerance to Abiotic Stress and Demographic History. Mol. Ecol. Resour. 2021, 21, 1641–1657. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadian, A.; Patel, D.A.; Edwards, D.; Batley, J. Copy Number Variation and Disease Resistance in Plants. Theor. Appl. Genet. 2017, 130, 2479–2490. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T.; Baumann, U.; Hayes, J.; Collins, N.C.; Shi, B.-J.; Schnurbusch, T.; Hay, A.; Mayo, G.; Pallotta, M.; Tester, M.; et al. Boron-Toxicity Tolerance in Barley Arising from Efflux Transporter Amplification. Science 2007, 318, 1446–1449. [Google Scholar] [CrossRef] [PubMed]
- Jeknić, Z.; Pillman, K.A.; Dhillon, T.; Skinner, J.S.; Veisz, O.; Cuesta-Marcos, A.; Hayes, P.M.; Jacobs, A.K.; Chen, T.H.H.; Stockinger, E.J. Hv-CBF2A Overexpression in Barley Accelerates COR Gene Transcript Accumulation and Acquisition of Freezing Tolerance during Cold Acclimation. Plant Mol. Biol. 2014, 84, 67–82. [Google Scholar] [CrossRef]
- Kopeć, P.; Rapacz, M.; Arora, R. Post-Translational Activation of CBF for Inducing Freezing Tolerance. Trends Plant Sci. 2022, 27, 415–417. [Google Scholar] [CrossRef]
- Vashegyi, I.; Marozsán-Tóth, Z.; Galiba, G.; Dobrev, P.I.; Vankova, R.; Tóth, B. Cold Response of Dedifferentiated Barley Cells at the Gene Expression, Hormone Composition, and Freezing Tolerance Levels: Studies on Callus Cultures. Mol. Biotechnol. 2013, 54, 337–349. [Google Scholar] [CrossRef]
- Ogihara, Y.; Takumi, S.; Handa, H. (Eds.) Advances in Wheat Genetics: From Genome to Field; Springer: Tokyo, Japan, 2015; ISBN 978-4-431-55674-9. [Google Scholar]
- Maccaferri, M.; Harris, N.S.; Twardziok, S.O.; Pasam, R.K.; Gundlach, H.; Spannagl, M.; Ormanbekova, D.; Lux, T.; Prade, V.M.; Milner, S.G.; et al. Durum Wheat Genome Highlights Past Domestication Signatures and Future Improvement Targets. Nat. Genet. 2019, 51, 885–895. [Google Scholar] [CrossRef]
- Smith, D.B.; Flavell, R.B. Characterisation of the Wheat Genome by Renaturation Kinetics. Chromosoma 1975, 50. [Google Scholar] [CrossRef]
- Pearce, S.; Zhu, J.; Boldizsár, Á.; Vágújfalvi, A.; Burke, A.; Garland-Campbell, K.; Galiba, G.; Dubcovsky, J. Large Deletions in the CBF Gene Cluster at the Fr-B2 Locus Are Associated with Reduced Frost Tolerance in Wheat. Theor. Appl. Genet. 2013, 126, 2683–2697. [Google Scholar] [CrossRef]
- Bolouri, P.; Haliloğlu, K.; Mohammadi, S.A.; Türkoğlu, A.; İlhan, E.; Niedbała, G.; Szulc, P.; Niazian, M. Identification of Novel QTLs Associated with Frost Tolerance in Winter Wheat (Triticum Aestivum L.). Plants 2023, 12, 1641. [Google Scholar] [CrossRef]
- Li, L.; Han, C.; Yang, J.; Tian, Z.; Jiang, R.; Yang, F.; Jiao, K.; Qi, M.; Liu, L.; Zhang, B.; et al. Comprehensive Transcriptome Analysis of Responses during Cold Stress in Wheat (Triticum Aestivum L.). Genes 2023, 14, 844. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.B.; Dvorak, J.; Gusta, L.v. Comparative Cold Hardiness of Several Triticum Species and Secale Cereale L.1. Crop Sci. 1977, 17, 941–943. [Google Scholar] [CrossRef]
- Limin, A.E.; Fowler, D.B. Cold Hardiness of Some Relatives of Hexaploid Wheat. Can. J. Bot. 1981, 59, 572–573. [Google Scholar] [CrossRef]
- Båga, M.; Chodaparambil, S.V.; Limin, A.E.; Pecar, M.; Fowler, D.B.; Chibbar, R.N. Identification of Quantitative Trait Loci and Associated Candidate Genes for Low-Temperature Tolerance in Cold-Hardy Winter Wheat. Funct. Integr. Genom. 2006, 7, 53–68. [Google Scholar] [CrossRef]
- Saripalli, G.; Adhikari, L.; Amos, C.; Kibriya, A.; Ahmed, H.I.; Heuberger, M.; Raupp, J.; Athiyannan, N.; Wicker, T.; Abrouk, M.; et al. Integration of Genetic and Genomics Resources in Einkorn Wheat Enables Precision Mapping of Important Traits. Commun. Biol. 2023, 6, 835. [Google Scholar] [CrossRef]
- Dhillon, T.; Stockinger, E.J. Cbf14 Copy Number Variation in the A, B, and D Genomes of Diploid and Polyploid Wheat. Theor. Appl. Genet. 2013, 126, 2777–2789. [Google Scholar] [CrossRef]
- Würschum, T.; Longin, H.F.C.; Hahn, V.; Tucker, M.R.; Leiser, W.L. Copy Number Variations of CBF Genes at the Fr-A2 Locus Are Essential Components of Winter Hardiness in Wheat. Plant J. 2017, 89, 764–773. [Google Scholar] [CrossRef]
- Babben, S.; Schliephake, E.; Janitza, P.; Berner, T.; Keilwagen, J.; Koch, M.; Arana-Ceballos, F.A.; Templer, S.E.; Chesnokov, Y.; Pshenichnikova, T.; et al. Association Genetics Studies on Frost Tolerance in Wheat (Triticum Aestivum L.) Reveal New Highly Conserved Amino Acid Substitutions in CBF-A3, CBF-A15, VRN3 and PPD1 Genes. BMC Genom. 2018, 19, 409. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; Zhang, H.; Jiao, B.; Wang, H.; Zhou, S. Transcriptome Analysis during Vernalization in Wheat (Triticum Aestivum L.). BMC Genom. Data 2023, 24, 43. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Y.; Liu, Z.; Zou, J.; Li, Q. Computational Genomics Insights into Cold Acclimation in Wheat. Front. Genet. 2022, 13, 1015673. [Google Scholar] [CrossRef]
- Singh, K.; Singh, S.P.; Yadav, M.K. Physio-Biochemical Assessment and CBF Genes Expression Analysis in Wheat under Dehydration Condition. Biologia 2022, 77, 1851–1860. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, M.; Wang, J.; Yang, N.; Wang, K.; Xi, J.; Wu, C.; Xi, T.; Zheng, J.; Zhang, J. Isoform Sequencing Provides Insight Into Freezing Response of Common Wheat (Triticum Aestivum L.). Front. Genet. 2020, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.J.; Seo, Y.W. Identification of Novel C-Repeat Binding Factor (CBF) Genes in Rye (Secale Cereale L.) and Expression Studies. Gene 2019, 684, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Alptekin, B.; Langridge, P.; Budak, H. Abiotic Stress miRNomes in the Triticeae. Funct. Integr. Genom. 2017, 17, 145–170. [Google Scholar] [CrossRef]
- Mago, R.; Miah, H.; Lawrence, G.J.; Wellings, C.R.; Spielmeyer, W.; Bariana, H.S.; McIntosh, R.A.; Pryor, A.J.; Ellis, J.G. High-Resolution Mapping and Mutation Analysis Separate the Rust Resistance Genes Sr31, Lr26 and Yr9 on the Short Arm of Rye Chromosome 1. Theor. Appl. Genet. 2005, 112, 41–50. [Google Scholar] [CrossRef]
- Bartoš, J.; Paux, E.; Kofler, R.; Havránková, M.; Kopecký, D.; Suchánková, P.; Šafář, J.; Šimková, H.; Town, C.D.; Lelley, T.; et al. A First Survey of the Rye (Secale Cereale) Genome Composition through BAC End Sequencing of the Short Arm of Chromosome 1R. BMC Plant Biol 2008, 8, 95. [Google Scholar] [CrossRef]
- Flavell, R.B.; Bennett, M.D.; Smith, J.B.; Smith, D.B. Genome Size and the Proportion of Repeated Nucleotide Sequence DNA in Plants. Biochem. Genet. 1974, 12, 257–269. [Google Scholar] [CrossRef]
- Martis, M.M.; Zhou, R.; Haseneyer, G.; Schmutzer, T.; Vrána, J.; Kubaláková, M.; König, S.; Kugler, K.G.; Scholz, U.; Hackauf, B.; et al. Reticulate Evolution of the Rye Genome. Plant Cell 2013, 25, 3685–3698. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Yang, J.; He, H.; Jin, H.; Li, X.; Ren, T.; Ren, Z.; Li, F.; Han, X.; et al. A High-Quality Genome Assembly Highlights Rye Genomic Characteristics and Agronomically Important Genes. Nat. Genet. 2021, 53, 574–584. [Google Scholar] [CrossRef]
- Li, Y.; Böck, A.; Haseneyer, G.; Korzun, V.; Wilde, P.; Schön, C.-C.; Ankerst, D.P.; Bauer, E. Association Analysis of Frost Tolerance in Rye Using Candidate Genes and Phenotypic Data from Controlled, Semi-Controlled, and Field Phenotyping Platforms. BMC Plant Biol. 2011, 11, 146. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zhang, J.; Zhao, H.; Tan, S.; Xu, W.; Pan, J.; Yang, F.; Pi, E. ERF Subfamily Transcription Factors and Their Function in Plant Responses to Abiotic Stresses. Front. Plant Sci. 2022, 13, 1042084. [Google Scholar] [CrossRef] [PubMed]
- Haake, V.; Cook, D.; Riechmann, J.; Pineda, O.; Thomashow, M.F.; Zhang, J.Z. Transcription Factor CBF4 Is a Regulator of Drought Adaptation in Arabidopsis. Plant Physiol. 2002, 130, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Hrmova, M.; Hussain, S.S. Plant Transcription Factors Involved in Drought and Associated Stresses. Int. J. Mol. Sci. 2021, 22, 5662. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N. Abscisic Acid and Abiotic Stress Signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Molecular Responses to Drought Stress in Plants. Biol. Plant. 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Uno, Y.; Furihata, T.; Abe, H.; Yoshida, R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis Basic Leucine Zipper Transcription Factors Involved in an Abscisic Acid-Dependent Signal Transduction Pathway under Drought and High-Salinity Conditions. Proc. Natl. Acad. Sci. USA 2000, 97, 11632–11637. [Google Scholar] [CrossRef]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought Stress in Plants: Causes, Consequences, and Tolerance. In Drought Stress Tolerance in Plants, Vol 1; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–16. ISBN 978-3-319-28897-0. [Google Scholar]
- Xu, F.; Liu, Z.; Xie, H.; Zhu, J.; Zhang, J.; Kraus, J.; Blaschnig, T.; Nehls, R.; Wang, H. Increased Drought Tolerance through the Suppression of ESKMO1 Gene and Overexpression of CBF-Related Genes in Arabidopsis. PLoS ONE 2014, 9, e106509. [Google Scholar] [CrossRef][Green Version]
- Javadi, S.M.; Shobbar, Z.-S.; Ebrahimi, A.; Shahbazi, M. New Insights on Key Genes Involved in Drought Stress Response of Barley: Gene Networks Reconstruction, Hub, and Promoter Analysis. J. Genet. Eng. Biotechnol. 2021, 19, 2. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought Stress Tolerance in Wheat and Barley: Advances in Physiology, Breeding and Genetics Research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Hammer, G.L.; Christopher, J.T.; deVoil, P. Genotypic Variation in Seedling Root Architectural Traits and Implications for Drought Adaptation in Wheat (Triticum Aestivum L.). Plant Soil 2008, 303, 115–129. [Google Scholar] [CrossRef]
- Canè, M.A.; Maccaferri, M.; Nazemi, G.; Salvi, S.; Francia, R.; Colalongo, C.; Tuberosa, R. Association Mapping for Root Architectural Traits in Durum Wheat Seedlings as Related to Agronomic Performance. Mol. Breed. 2014, 34, 1629–1645. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.Q.; Shen, C.; Wu, L.H.; Tang, K.X.; Lin, J. CBF-Dependent Signaling Pathway: A Key Responder to Low Temperature Stress in Plants. Crit. Rev. Biotechnol. 2011, 31, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wang, Y.; Long, L.; Luo, H.; Shen, Q.; Broughton, S.; Wu, D.; Shu, X.; Dai, F.; Li, C.; et al. A Trypsin Family Protein Gene Controls Tillering and Leaf Shape in Barley. Plant Physiol. 2019, 181, 701–713. [Google Scholar] [CrossRef]
- Hussien, A.; Tavakol, E.; Horner, D.S.; Muñoz-Amatriaín, M.; Muehlbauer, G.J.; Rossini, L. Genetics of Tillering in Rice and Barley. Plant Genome 2014, 7. [Google Scholar] [CrossRef]
- Riaz, A.; Alqudah, A.M.; Kanwal, F.; Pillen, K.; Ye, L.; Dai, F.; Zhang, G. Advances in Studies on Physiological and Molecular Regulation of Barley Tillering. J. Integr. Agric. 2022, 22, 1–13. [Google Scholar] [CrossRef]
- Shang, Q.; Wang, Y.; Tang, H.; Sui, N.; Zhang, X.; Wang, F. Genetic, Hormonal, and Environmental Control of Tillering in Wheat. Crop J. 2021, 9, 986–991. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z.; Li, H.; Wang, M.; Onac, E.; Zhou, J.; Xia, X.; Shi, K.; Yu, J.; Zhou, Y. Phytochrome A and B Function Antagonistically to Regulate Cold Tolerance via Abscisic Acid-Dependent Jasmonate Signaling. Plant Physiol. 2016, 170, 459–471. [Google Scholar] [CrossRef]
- Iehisa, J.C.M.; Takumi, S. Variation in Abscisic Acid Responsiveness of Aegilops Tauschii and Hexaploid Wheat Synthetics Due to the D-Genome Diversity. Genes Genet. Syst. 2012, 87, 9–18. [Google Scholar] [CrossRef]
- Achard, P.; Renou, J.-P.; Berthomé, R.; Harberd, N.P.; Genschik, P. Plant DELLAs Restrain Growth and Promote Survival of Adversity by Reducing the Levels of Reactive Oxygen Species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef]
- Achard, P.; Gong, F.; Cheminant, S.; Alioua, M.; Hedden, P.; Genschik, P. The Cold-Inducible CBF1 Factor–Dependent Signaling Pathway Modulates the Accumulation of the Growth-Repressing DELLA Proteins via Its Effect on Gibberellin Metabolism. Plant Cell 2008, 20, 2117–2129. [Google Scholar] [CrossRef] [PubMed]
- Ergon, Å. Optimal Regulation of the Balance between Productivity and Overwintering of Perennial Grasses in a Warmer Climate. Agronomy 2017, 7, 19. [Google Scholar] [CrossRef]
- Sinha, D.; Maurya, A.K.; Abdi, G.; Majeed, M.; Agarwal, R.; Mukherjee, R.; Ganguly, S.; Aziz, R.; Bhatia, M.; Majgaonkar, A.; et al. Integrated Genomic Selection for Accelerating Breeding Programs of Climate-Smart Cereals. Genes 2023, 14, 1484. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Sandhu, K.S.; Kamal, R.; Kaur, K.; Singh, J.; Röder, M.S.; Muqaddasi, Q.H. Omics for the Improvement of Abiotic, Biotic, and Agronomic Traits in Major Cereal Crops: Applications, Challenges, and Prospects. Plants 2021, 10, 1989. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed Breeding Is a Powerful Tool to Accelerate Crop Research and Breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Forster, B.P.; Ellis, R.P.; Thomas, W.T.B.; Newton, A.C.; Tuberosa, R.; This, D.; El-Enein, R.A.; Bahri, M.H.; Salem, M.B. The Development and Application of Molecular Markers for Abiotic Stress Tolerance in Barley. J. Exp. Bot. 2000, 51, 19–27. [Google Scholar] [CrossRef]
- Leng, P.; Lübberstedt, T.; Xu, M. Genomics-Assisted Breeding—A Revolutionary Strategy for Crop Improvement. J. Integr. Agric. 2017, 16, 2674–2685. [Google Scholar] [CrossRef]
- Bhatta, M.; Sandro, P.; Smith, M.R.; Delaney, O.; Voss-Fels, K.P.; Gutierrez, L.; Hickey, L.T. Need for Speed: Manipulating Plant Growth to Accelerate Breeding Cycles. Curr. Opin. Plant Biol. 2021, 60, 101986. [Google Scholar] [CrossRef]
- Kumar, M.; Prusty, M.R.; Pandey, M.K.; Singh, P.K.; Bohra, A.; Guo, B.; Varshney, R.K. Application of CRISPR/Cas9-Mediated Gene Editing for Abiotic Stress Management in Crop Plants. Front. Plant Sci. 2023, 14, 1157678. [Google Scholar] [CrossRef]
- Bevan, M.W.; Uauy, C.; Wulff, B.B.H.; Zhou, J.; Krasileva, K.; Clark, M.D. Genomic Innovation for Crop Improvement. Nature 2017, 543, 346–354. [Google Scholar] [CrossRef]
- Lorenz, A.J.; Hamblin, M.T.; Jannink, J.-L. Performance of Single Nucleotide Polymorphisms versus Haplotypes for Genome-Wide Association Analysis in Barley. PloS ONE 2010, 5, e14079. [Google Scholar] [CrossRef] [PubMed]
- Voss-Fels, K.P.; Robinson, H.; Mudge, S.R.; Richard, C.; Newman, S.; Wittkop, B.; Stahl, A.; Friedt, W.; Frisch, M.; Gabur, I.; et al. VERNALIZATION1 Modulates Root System Architecture in Wheat and Barley. Mol. Plant 2018, 11, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Crossa, J.; Pérez-Rodríguez, P.; Cuevas, J.; Montesinos-López, O.; Jarquín, D.; de los Campos, G.; Burgueño, J.; González-Camacho, J.M.; Pérez-Elizalde, S.; Beyene, Y.; et al. Genomic Selection in Plant Breeding: Methods, Models, and Perspectives. Trends Plant Sci. 2017, 22, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Lozada, D.N.; Mason, R.E.; Sukumaran, S.; Dreisigacker, S. Validation of Grain Yield QTLs from Soft Winter Wheat Using a CIMMYT Spring Wheat Panel. Crop Sci. 2018, 58, 1964–1971. [Google Scholar] [CrossRef]
- Sun, C.; Hu, H.; Cheng, Y.; Yang, X.; Qiao, Q.; Wang, C.; Zhang, L.; Chen, D.; Zhao, S.; Dong, Z.; et al. Genomics-assisted Breeding: The Next-generation Wheat Breeding Era. Plant Breed. 2023, 142, 259–268. [Google Scholar] [CrossRef]
- Stockinger, E.J. The Breeding of Winter-Hardy Malting Barley. Plants 2021, 10, 1415. [Google Scholar] [CrossRef]
- Soleimani, V.D.; Baum, B.R.; Johnson, D.A. Genetic Diversity among Barley Cultivars Assessed by Sequence-Specific Amplification Polymorphism. Theor. Appl. Genet. 2005, 110, 1290–1300. [Google Scholar] [CrossRef]
- Fisk, S.P.; Cuesta-Marcos, A.; Cistué, L.; Russell, J.; Smith, K.P.; Baenziger, S.; Bedo, Z.; Corey, A.; Filichkin, T.; Karsai, I.; et al. FR-H3: A New QTL to Assist in the Development of Fall-Sown Barley with Superior Low Temperature Tolerance. Theor. Appl. Genet. 2013, 126, 335–347. [Google Scholar] [CrossRef]
- Matthies, I.E.; Malosetti, M.; Röder, M.S.; Van Eeuwijk, F. Genome-Wide Association Mapping for Kernel and Malting Quality Traits Using Historical European Barley Records. PloS ONE 2014, 9, e110046. [Google Scholar] [CrossRef][Green Version]
- Iglesias, A.; Quiroga, S.; Moneo, M.; Garrote, L. From Climate Change Impacts to the Development of Adaptation Strategies: Challenges for Agriculture in Europe. Clim. Change 2012, 112, 143–168. [Google Scholar] [CrossRef]
- Eagles, H.A.; Hyles, J.; Wilson, J.; Cane, K.; Forrest, K.L.; Hayden, M.J.; Ramm, K.; Trevaskis, B. A Linked SNP Marker to Genotype Fr-B2 in Wheat. Crop Pasture Sci. 2018, 69, 859. [Google Scholar] [CrossRef]
- Miedaner, T.; Korzun, V.; Bauer, E. Chapter 15—Genomics-Based Hybrid Rye Breeding. In Applications of Genetic and Genomic Research in Cereals; Miedaner, T., Korzun, V., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2019; pp. 329–348. ISBN 978-0-08-102163-7. [Google Scholar]
- Wilde, P.; Miedaner, T. Hybrid Rye Breeding. In The Rye Genome; Rabanus-Wallace, M.T., Stein, N., Eds.; Compendium of Plant Genomes; Springer International Publishing: Cham, Switzerland, 2021; pp. 13–41. ISBN 978-3-030-83383-1. [Google Scholar]
- Erath, W.; Bauer, E.; Fowler, D.B.; Gordillo, A.; Korzun, V.; Ponomareva, M.; Schmidt, M.; Schmiedchen, B.; Wilde, P.; Schön, C.-C. Exploring New Alleles for Frost Tolerance in Winter Rye. Theor. Appl. Genet. 2017, 130, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- Driedonks, N.; Rieu, I.; Vriezen, W.H. Breeding for Plant Heat Tolerance at Vegetative and Reproductive Stages. Plant Reprod. 2016, 29, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Bikard, D.; Cox, D.; Zhang, F.; Marraffini, L.A. RNA-Guided Editing of Bacterial Genomes Using CRISPR-Cas Systems. Nat. Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture. Annu. Rev. Plant Biol. 2019, 70, 667–697. [Google Scholar] [CrossRef]
- Jain, M. Function Genomics of Abiotic Stress Tolerance in Plants: A CRISPR Approach. Front. Plant Sci. 2015, 6, 375. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.-J.; Zhang, F.-J.; Zhang, G.-Z.; Jiang, X.-Y.; Yu, H.-M.; Hou, B.-K. The Arabidopsis UDP-Glycosyltransferases UGT79B2 and UGT79B3, Contribute to Cold, Salt and Drought Stress Tolerance via Modulating Anthocyanin Accumulation. Plant J. 2017, 89, 85–103. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Li, R.; Zhao, R.; Yang, M.; Sheng, J.; Shen, L. Reduced Drought Tolerance by CRISPR/Cas9-Mediated SlMAPK3 Mutagenesis in Tomato Plants. J. Agric. Food Chem. 2017, 65, 8674–8682. [Google Scholar] [CrossRef]
- Lv, K.; Li, J.; Zhao, K.; Chen, S.; Nie, J.; Zhang, W.; Liu, G.; Wei, H. Overexpression of an AP2/ERF Family Gene, BpERF13, in Birch Enhances Cold Tolerance through Upregulating CBF Genes and Mitigating Reactive Oxygen Species. Plant Sci. 2020, 292, 110375. [Google Scholar] [CrossRef]
- Kim, D.; Alptekin, B.; Budak, H. CRISPR/Cas9 Genome Editing in Wheat. Funct. Integr. Genom. 2018, 18, 31–41. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Z.; Xie, S.; Si, T.; Li, Y.; Zhu, J.-K. Mutational Evidence for the Critical Role of CBF Transcription Factors in Cold Acclimation in Arabidopsis. Plant Physiol. 2016, 171, 2744–2759. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caccialupi, G.; Milc, J.; Caradonia, F.; Nasar, M.F.; Francia, E. The Triticeae CBF Gene Cluster—To Frost Resistance and Beyond. Cells 2023, 12, 2606. https://doi.org/10.3390/cells12222606
Caccialupi G, Milc J, Caradonia F, Nasar MF, Francia E. The Triticeae CBF Gene Cluster—To Frost Resistance and Beyond. Cells. 2023; 12(22):2606. https://doi.org/10.3390/cells12222606
Chicago/Turabian StyleCaccialupi, Giovanni, Justyna Milc, Federica Caradonia, Muhammad Fazail Nasar, and Enrico Francia. 2023. "The Triticeae CBF Gene Cluster—To Frost Resistance and Beyond" Cells 12, no. 22: 2606. https://doi.org/10.3390/cells12222606
APA StyleCaccialupi, G., Milc, J., Caradonia, F., Nasar, M. F., & Francia, E. (2023). The Triticeae CBF Gene Cluster—To Frost Resistance and Beyond. Cells, 12(22), 2606. https://doi.org/10.3390/cells12222606










