Olive Leaf Extract (OLE) as a Novel Antioxidant That Ameliorates the Inflammatory Response in Cystic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Olive Leaf Extract (OLE) Production
2.3. Cytotoxicity MTT Assay
2.4. ROS Measurment
2.5. RNA Extraction and Quantification (qRT-PCR)
2.6. CFTR Channel Function
2.7. Statistical Analysis
3. Results
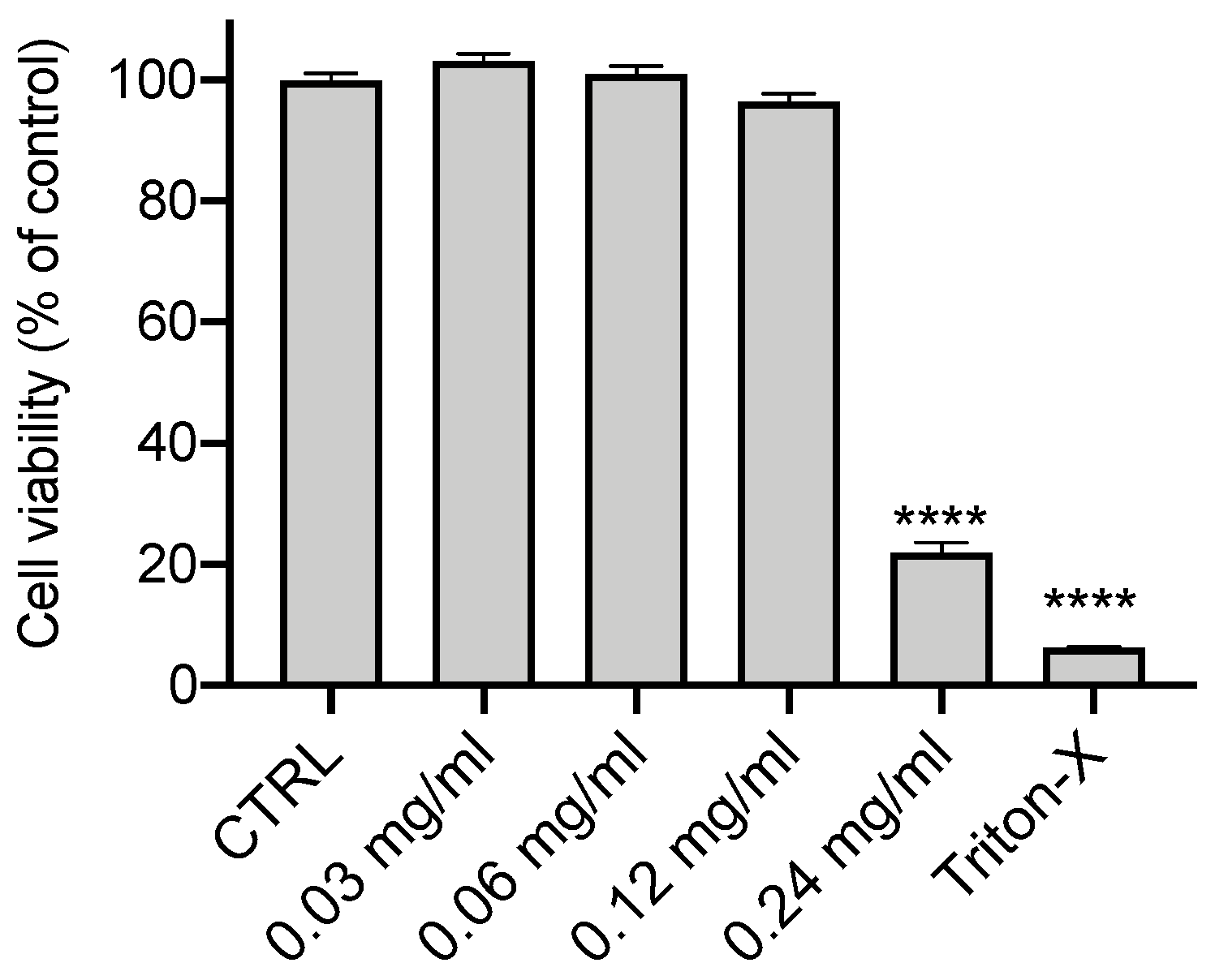
3.1. Effect of Olive Leaf Extract on the Cell Viability of CFBE Cells
3.2. OLE Reduces Oxidative Stress (ROS) in CFBE Cells
3.3. Anti-Inflammatory Activity of OLE
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Sullivan, B.P.; Freedman, S.D. Cystic fibrosis. Lancet 2009, 373, 1891–1904. [Google Scholar] [CrossRef]
- Cuevas-Ocana, S.; Laselva, O.; Avolio, J.; Nenna, R. The era of CFTR modulators: Improvements made and remaining challenges. Breathe 2020, 16, 200016. [Google Scholar] [CrossRef] [PubMed]
- Lund-Palau, H.; Turnbull, A.R.; Bush, A.; Bardin, E.; Cameron, L.; Soren, O.; Wierre-Gore, N.; Alton, E.W.; Bundy, J.G.; Connett, G.; et al. Pseudomonas aeruginosa infection in cystic fibrosis: Pathophysiological mechanisms and therapeutic approaches. Expert Rev. Respir. Med. 2016, 10, 685–697. [Google Scholar] [CrossRef]
- Chmiel, J.F.; Berger, M.; Konstan, M.W. The role of inflammation in the pathophysiology of CF lung disease. Clin. Rev. Allergy Immunol. 2002, 23, 5–27. [Google Scholar] [CrossRef]
- Chmiel, J.F.; Konstan, M.W. Inflammation and anti-inflammatory therapies for cystic fibrosis. Clin. Chest Med. 2007, 28, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.A.L.; Laselva, O.; Lopes-Pacheco, M. Advances in Preclinical In Vitro Models for the Translation of Precision Medicine for Cystic Fibrosis. J. Pers. Med. 2022, 12, 1321. [Google Scholar] [CrossRef]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.; Burton, B.; Stack, J.H.; Straley, K.S.; Decker, C.J.; Miller, M.; McCartney, J.; Olson, E.R.; et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. USA 2011, 108, 18843–18848. [Google Scholar] [CrossRef]
- Keating, D.; Marigowda, G.; Burr, L.; Daines, C.; Mall, M.A.; McKone, E.F.; Ramsey, B.W.; Rowe, S.M.; Sass, L.A.; Tullis, E.; et al. VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 2018, 379, 1612–1620. [Google Scholar] [CrossRef] [PubMed]
- Ntimbane, T.; Comte, B.; Mailhot, G.; Berthiaume, Y.; Poitout, V.; Prentki, M.; Rabasa-Lhoret, R.; Levy, E. Cystic fibrosis-related diabetes: From CFTR dysfunction to oxidative stress. Clin. Biochem. Rev. 2009, 30, 153–177. [Google Scholar]
- van der Vliet, A.; Eiserich, J.P.; Marelich, G.P.; Halliwell, B.; Cross, C.E. Oxidative stress in cystic fibrosis: Does it occur and does it matter? Adv. Pharmacol. 1997, 38, 491–513. [Google Scholar] [CrossRef]
- Montgomery, S.T.; Mall, M.A.; Kicic, A.; Stick, S.M.; Arest, C.F. Hypoxia and sterile inflammation in cystic fibrosis airways: Mechanisms and potential therapies. Eur. Respir. J. 2017, 49, 1600903. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.P.; Konstan, M.W.; Chmiel, J.F. Anti-inflammatory therapies for cystic fibrosis-related lung disease. Clin. Rev. Allergy Immunol. 2008, 35, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Doring, G.; Heltshe, S.L.; Lands, L.C.; Hilliard, K.A.; Koker, P.; Bhattacharya, S.; Staab, A.; Hamilton, A.; Investigators; et al. A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J. Cyst. Fibros. 2014, 13, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Schluchter, M.D.; Xue, W.; Davis, P.B. Clinical use of Ibuprofen is associated with slower FEV1 decline in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007, 176, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef]
- Karygianni, L.; Cecere, M.; Skaltsounis, A.L.; Argyropoulou, A.; Hellwig, E.; Aligiannis, N.; Wittmer, A.; Al-Ahmad, A. High-level antimicrobial efficacy of representative Mediterranean natural plant extracts against oral microorganisms. BioMed Res. Int. 2014, 2014, 839019. [Google Scholar] [CrossRef]
- de la Puerta, R.; Martinez Dominguez, M.E.; Ruiz-Gutierrez, V.; Flavill, J.A.; Hoult, J.R. Effects of virgin olive oil phenolics on scavenging of reactive nitrogen species and upon nitrergic neurotransmission. Life Sci. 2001, 69, 1213–1222. [Google Scholar] [CrossRef]
- de la Puerta, R.; Ruiz Gutierrez, V.; Hoult, J.R. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999, 57, 445–449. [Google Scholar] [CrossRef]
- Joshi, G.; Johnson, J.A. The Nrf2-ARE pathway: A valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Pat. CNS Drug Discov. 2012, 7, 218–229. [Google Scholar] [CrossRef]
- Abbattiscianni, A.C.; Favia, M.; Mancini, M.T.; Cardone, R.A.; Guerra, L.; Monterisi, S.; Castellani, S.; Laselva, O.; Di Sole, F.; Conese, M.; et al. Correctors of mutant CFTR enhance subcortical cAMP-PKA signaling through modulating ezrin phosphorylation and cytoskeleton organization. J. Cell Sci. 2016, 129, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Laselva, O.; Criscione, M.L.; Allegretta, C.; Di Gioia, S.; Liso, A.; Conese, M. Insulin-Like Growth Factor Binding Protein (IGFBP-6) as a Novel Regulator of Inflammatory Response in Cystic Fibrosis Airway Cells. Front. Mol. Biosci. 2022, 9, 905468. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Totaro, M.P.; Caponio, F.; Pasqualone, A.; Summo, C. Olive Leaf Extract (OLE) Addition as Tool to Reduce Nitrate and Nitrite in Ripened Sausages. Foods 2022, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Crescenzi, M.A.; Piacente, S.; Altamura, G.; Caponio, F.; Montoro, P. Metabolomics Approach to Characterize Green Olive Leaf Extracts Classified Based on Variety and Season. Plants 2022, 11, 3321. [Google Scholar] [CrossRef]
- Laselva, O.; Allegretta, C.; Di Gioia, S.; Avolio, C.; Conese, M. Anti-Inflammatory and Anti-Oxidant Effect of Dimethyl Fumarate in Cystic Fibrosis Bronchial Epithelial Cells. Cells 2021, 10, 2132. [Google Scholar] [CrossRef]
- Laselva, O.; Moraes, T.J.; He, G.; Bartlett, C.; Szarics, I.; Ouyang, H.; Gunawardena, T.N.A.; Strug, L.; Bear, C.E.; Gonska, T. The CFTR Mutation c.3453G > C (D1152H) Confers an Anion Selectivity Defect in Primary Airway Tissue that Can Be Rescued by Ivacaftor. J. Pers. Med. 2020, 10, 40. [Google Scholar] [CrossRef]
- Laselva, O.; Erwood, S.; Du, K.; Ivakine, Z.; Bear, C.E. Activity of lumacaftor is not conserved in zebrafish Cftr bearing the major cystic fibrosis-causing mutation. FASEB Bioadv. 2019, 1, 661–670. [Google Scholar] [CrossRef]
- Laselva, O.; McCormack, J.; Bartlett, C.; Ip, W.; Gunawardena, T.N.A.; Ouyang, H.; Eckford, P.D.W.; Gonska, T.; Moraes, T.J.; Bear, C.E. Preclinical Studies of a Rare CF-Causing Mutation in the Second Nucleotide Binding Domain (c.3700A > G) Show Robust Functional Rescue in Primary Nasal Cultures by Novel CFTR Modulators. J. Pers. Med. 2020, 10, 209. [Google Scholar] [CrossRef]
- Cao, H.; Ouyang, H.; Laselva, O.; Bartlett, C.; Zhou, Z.P.; Duan, C.; Gunawardena, T.; Avolio, J.; Bear, C.E.; Gonska, T.; et al. A helper-dependent adenoviral vector rescues CFTR to wild-type functional levels in cystic fibrosis epithelial cells harbouring class I mutations. Eur. Respir. J. 2020, 56, 2000205. [Google Scholar] [CrossRef]
- Laselva, O.; Qureshi, Z.; Zeng, Z.W.; Petrotchenko, E.V.; Ramjeesingh, M.; Hamilton, C.M.; Huan, L.J.; Borchers, C.H.; Pomes, R.; Young, R.; et al. Identification of binding sites for ivacaftor on the cystic fibrosis transmembrane conductance regulator. iScience 2021, 24, 102542. [Google Scholar] [CrossRef]
- Visioli, F.; Bellomo, G.; Galli, C. Free radical-scavenging properties of olive oil polyphenols. Biochem. Biophys. Res. Commun. 1998, 247, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Guidotti, V.; Bucciantini, M.; Parri, M.; Nediani, C.; Cerbai, E.; Stefani, M.; Berti, A. Oleuropein aglycon prevents cytotoxic amyloid aggregation of human amylin. J. Nutr. Biochem. 2010, 21, 726–735. [Google Scholar] [CrossRef]
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef]
- Murotomi, K.; Umeno, A.; Yasunaga, M.; Shichiri, M.; Ishida, N.; Koike, T.; Matsuo, T.; Abe, H.; Yoshida, Y.; Nakajima, Y. Oleuropein-Rich Diet Attenuates Hyperglycemia and Impaired Glucose Tolerance in Type 2 Diabetes Model Mouse. J. Agric. Food Chem. 2015, 63, 6715–6722. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Corona, G.; Yaqoob, P.; Spencer, J.P.; Rowland, I. Secoiridoids delivered as olive leaf extract induce acute improvements in human vascular function and reduction of an inflammatory cytokine: A randomised, double-blind, placebo-controlled, cross-over trial. Br. J. Nutr. 2015, 114, 75–83. [Google Scholar] [CrossRef]
- Rigacci, S.; Miceli, C.; Nediani, C.; Berti, A.; Cascella, R.; Pantano, D.; Nardiello, P.; Luccarini, I.; Casamenti, F.; Stefani, M. Oleuropein aglycone induces autophagy via the AMPK/mTOR signalling pathway: A mechanistic insight. Oncotarget 2015, 6, 35344–35357. [Google Scholar] [CrossRef]
- Shamshoum, H.; Vlavcheski, F.; Tsiani, E. Anticancer effects of oleuropein. Biofactors 2017, 43, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; la Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef]
- Ranieri, M.; Di Mise, A.; Centrone, M.; D’Agostino, M.; Tingskov, S.J.; Venneri, M.; Pellegrino, T.; Difonzo, G.; Caponio, F.; Norregaard, R.; et al. Olive Leaf Extract (OLE) impaired vasopressin-induced aquaporin-2 trafficking through the activation of the calcium-sensing receptor. Sci. Rep. 2021, 11, 4537. [Google Scholar] [CrossRef]
- Giacometti, J.; Grubic-Kezele, T. Olive Leaf Polyphenols Attenuate the Clinical Course of Experimental Autoimmune Encephalomyelitis and Provide Neuroprotection by Reducing Oxidative Stress, Regulating Microglia and SIRT1, and Preserving Myelin Integrity. Oxidative Med. Cell. Longev. 2020, 2020, 6125638. [Google Scholar] [CrossRef]
- Sarbishegi, M.; Charkhat Gorgich, E.A.; Khajavi, O.; Komeili, G.; Salimi, S. The neuroprotective effects of hydro-alcoholic extract of olive (Olea europaea L.) leaf on rotenone-induced Parkinson’s disease in rat. Metab. Brain Dis. 2018, 33, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, F.; Chamkha, M.; Sayadi, S. Protective effect of olive leaves phenolic compounds against neurodegenerative disorders: Promising alternative for Alzheimer and Parkinson diseases modulation. Food Chem. Toxicol. 2022, 159, 112752. [Google Scholar] [CrossRef]
- Saibandith, B.; Spencer, J.P.E.; Rowland, I.R.; Commane, D.M. Olive Polyphenols and the Metabolic Syndrome. Molecules 2017, 22, 1082. [Google Scholar] [CrossRef]
- Burja, B.; Kuret, T.; Janko, T.; Topalovic, D.; Zivkovic, L.; Mrak-Poljsak, K.; Spremo-Potparevic, B.; Zigon, P.; Distler, O.; Cucnik, S.; et al. Olive Leaf Extract Attenuates Inflammatory Activation and DNA Damage in Human Arterial Endothelial Cells. Front. Cardiovasc. Med. 2019, 6, 56. [Google Scholar] [CrossRef]
- Vezza, T.; Algieri, F.; Rodriguez-Nogales, A.; Garrido-Mesa, J.; Utrilla, M.P.; Talhaoui, N.; Gomez-Caravaca, A.M.; Segura-Carretero, A.; Rodriguez-Cabezas, M.E.; Monteleone, G.; et al. Immunomodulatory properties of Olea europaea leaf extract in intestinal inflammation. Mol. Nutr. Food Res. 2017, 61, 1601066. [Google Scholar] [CrossRef]
- Fito, M.; de la Torre, R.; Farre-Albaladejo, M.; Khymenetz, O.; Marrugat, J.; Covas, M.I. Bioavailability and antioxidant effects of olive oil phenolic compounds in humans: A review. Ann. Ist Super Sanita 2007, 43, 375–381. [Google Scholar] [PubMed]
- Giner, E.; Recio, M.C.; Rios, J.L.; Giner, R.M. Oleuropein protects against dextran sodium sulfate-induced chronic colitis in mice. J. Nat. Prod. 2013, 76, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.J.; Choi, H.S.; Yoon, K.Y.; Lee, O.H.; Kim, K.J.; Lee, B.Y. Oleuropein suppresses LPS-induced inflammatory responses in RAW 264.7 cell and zebrafish. J. Agric. Food Chem. 2015, 63, 2098–2105. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef]
- Boss, A.; Kao, C.H.; Murray, P.M.; Marlow, G.; Barnett, M.P.; Ferguson, L.R. Human Intervention Study to Assess the Effects of Supplementation with Olive Leaf Extract on Peripheral Blood Mononuclear Cell Gene Expression. Int. J. Mol. Sci. 2016, 17, 2019. [Google Scholar] [CrossRef]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- Schroeder, T.H.; Lee, M.M.; Yacono, P.W.; Cannon, C.L.; Gerceker, A.A.; Golan, D.E.; Pier, G.B. CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF-kappa B translocation. Proc. Natl. Acad. Sci. USA 2002, 99, 6907–6912. [Google Scholar] [CrossRef]
- Rocca, J.; Manin, S.; Hulin, A.; Aissat, A.; Verbecq-Morlot, W.; Pruliere-Escabasse, V.; Wohlhuter-Haddad, A.; Epaud, R.; Fanen, P.; Tarze, A. New use for an old drug: COX-independent anti-inflammatory effects of sulindac in models of cystic fibrosis. Br. J. Pharmacol. 2016, 173, 1728–1741. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Ma, H.; Qin, S.; Zhao, S. Osteoarthritis is Prevented in Rats by Verbascoside via Nuclear Factor kappa B (NF-kappaB) Pathway Downregulation. Med. Sci. Monit. 2020, 26, e921276. [Google Scholar] [CrossRef]
- Difonzo, G.; Pasqualone, A.; Silletti, R.; Cosmai, L.; Summo, C.; Paradiso, V.M.; Caponio, F. Use of olive leaf extract to reduce lipid oxidation of baked snacks. Food Res. Int. 2018, 108, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Pine, R.; Lozano-Sanchez, J.; Herrero, M.; Ibanez, E.; Segura-Carretero, A.; Fernandez-Gutierrez, A. HPLC-ESI-QTOF-MS as a powerful analytical tool for characterising phenolic compounds in olive-leaf extracts. Phytochem. Anal. 2013, 24, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.H.; Lee, B.Y.; Lee, J.; Lee, H.B.; Son, J.Y.; Park, C.S.; Shetty, K.; Kim, Y.C. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour. Technol. 2009, 100, 6107–6113. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Maisto, M.; Tenore, G.C.; Ianaro, A. Olive Leaf Extract, from Olea europaea L., Reduces Palmitate-Induced Inflammation via Regulation of Murine Macrophages Polarization. Nutrients 2020, 12, 3663. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Germoush, M.O.; Al-Anazi, K.M.; Mahmoud, A.H.; Farah, M.A.; Allam, A.A. Commiphora molmol protects against methotrexate-induced nephrotoxicity by up-regulating Nrf2/ARE/HO-1 signaling. Biomed. Pharmacother. 2018, 106, 499–509. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Germoush, M.O.; Alotaibi, M.F.; Hussein, O.E. Possible involvement of Nrf2 and PPARgamma up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed. Pharmacother. 2017, 86, 297–306. [Google Scholar] [CrossRef]
- Stanton, B.A.; Coutermarsh, B.; Barnaby, R.; Hogan, D. Pseudomonas aeruginosa Reduces VX-809 Stimulated F508del-CFTR Chloride Secretion by Airway Epithelial Cells. PLoS ONE 2015, 10, e0127742. [Google Scholar] [CrossRef] [PubMed]
- Trinh, N.T.; Bilodeau, C.; Maille, E.; Ruffin, M.; Quintal, M.C.; Desrosiers, M.Y.; Rousseau, S.; Brochiero, E. Deleterious impact of Pseudomonas aeruginosa on cystic fibrosis transmembrane conductance regulator function and rescue in airway epithelial cells. Eur. Respir. J. 2015, 45, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Laselva, O.; Stone, T.A.; Bear, C.E.; Deber, C.M. Anti-Infectives Restore ORKAMBI((R)) Rescue of F508del-CFTR Function in Human Bronchial Epithelial Cells Infected with Clinical Strains of P. aeruginosa. Biomolecules 2020, 10, 334. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef]
- Cantin, A.M.; Bilodeau, G.; Ouellet, C.; Liao, J.; Hanrahan, J.W. Oxidant stress suppresses CFTR expression. Am. J. Physiol. Cell Physiol. 2006, 290, C262–C270. [Google Scholar] [CrossRef]
- Le Gars, M.; Descamps, D.; Roussel, D.; Saussereau, E.; Guillot, L.; Ruffin, M.; Tabary, O.; Hong, S.S.; Boulanger, P.; Paulais, M.; et al. Neutrophil elastase degrades cystic fibrosis transmembrane conductance regulator via calpains and disables channel function in vitro and in vivo. Am. J. Respir. Crit. Care Med. 2013, 187, 170–179. [Google Scholar] [CrossRef] [PubMed]





| Gene | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) |
|---|---|---|
| IL-1β | TTACAGTGGCAATGAGGATGAC | TGTAGTGGTGGTCGGAGATTC |
| IL-6 | CGGTACATCCTCGACGGC | CTTGTTACATGTCTCCTTTCTCAGG |
| IL-8 | GACCACACTGCGCCAACA | GCTCTCTTCCATCAGAAAGTTACATAATTT |
| TNFα | GGACCTCTCTCTAATCAGCCCTC | TCGAGAAGATGATCTGACTGCC |
| Nrf2 | CACATCCAGTCAGAAACCAGTGG | GGAATGTCTGCGCCAAAAGCT |
| NF-kB p50 | CTGGTGATCGTGGAACAGCC | CAGAGCCTGCTGTCTTGTCC |
| NF-kB p65 | ATGCGCTTCCGCTACAAGTG | ACAATGGCCACTTGTCGGTG |
| HO-1 | ATGGAGCGTCCGCAACCCGACAG | TCACATGGCATAAAGCCCTACAG |
| NQO1 | GAAGAGCACTGATCGTACTGGC | GGGTCCTTCAGTTTACCTGTGAT |
| GAPDH | CAAGAGCACAAGAGGAAGAGAG | CTACATGGCAACTGTGAGGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegretta, C.; Difonzo, G.; Caponio, F.; Tamma, G.; Laselva, O. Olive Leaf Extract (OLE) as a Novel Antioxidant That Ameliorates the Inflammatory Response in Cystic Fibrosis. Cells 2023, 12, 1764. https://doi.org/10.3390/cells12131764
Allegretta C, Difonzo G, Caponio F, Tamma G, Laselva O. Olive Leaf Extract (OLE) as a Novel Antioxidant That Ameliorates the Inflammatory Response in Cystic Fibrosis. Cells. 2023; 12(13):1764. https://doi.org/10.3390/cells12131764
Chicago/Turabian StyleAllegretta, Caterina, Graziana Difonzo, Francesco Caponio, Grazia Tamma, and Onofrio Laselva. 2023. "Olive Leaf Extract (OLE) as a Novel Antioxidant That Ameliorates the Inflammatory Response in Cystic Fibrosis" Cells 12, no. 13: 1764. https://doi.org/10.3390/cells12131764
APA StyleAllegretta, C., Difonzo, G., Caponio, F., Tamma, G., & Laselva, O. (2023). Olive Leaf Extract (OLE) as a Novel Antioxidant That Ameliorates the Inflammatory Response in Cystic Fibrosis. Cells, 12(13), 1764. https://doi.org/10.3390/cells12131764











