Adenovirus-Based Gene Therapy for Bone Regeneration: A Comparative Analysis of In Vivo and Ex Vivo BMP2 Gene Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Matrices
2.2. Cell Cultures
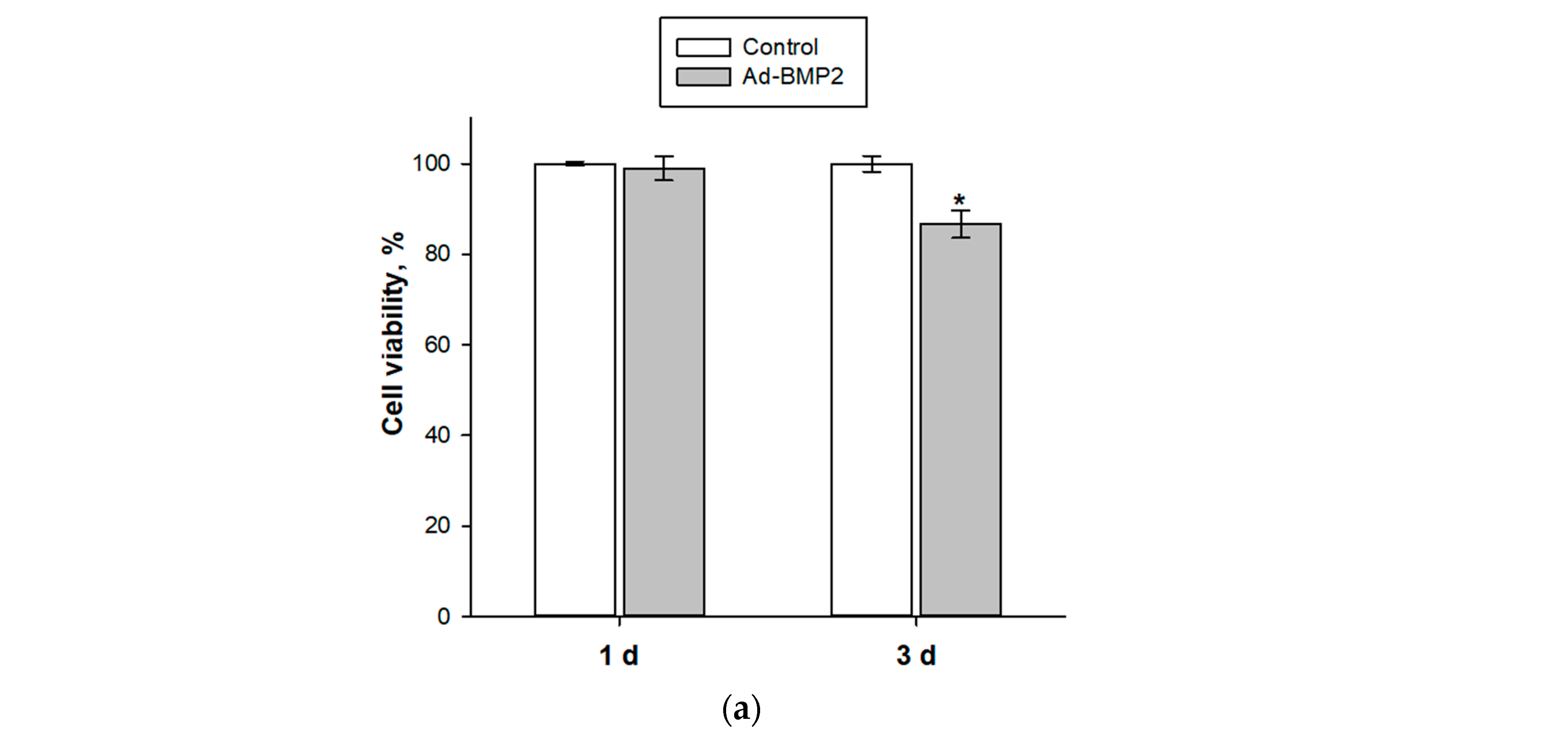
2.3. MTT Test
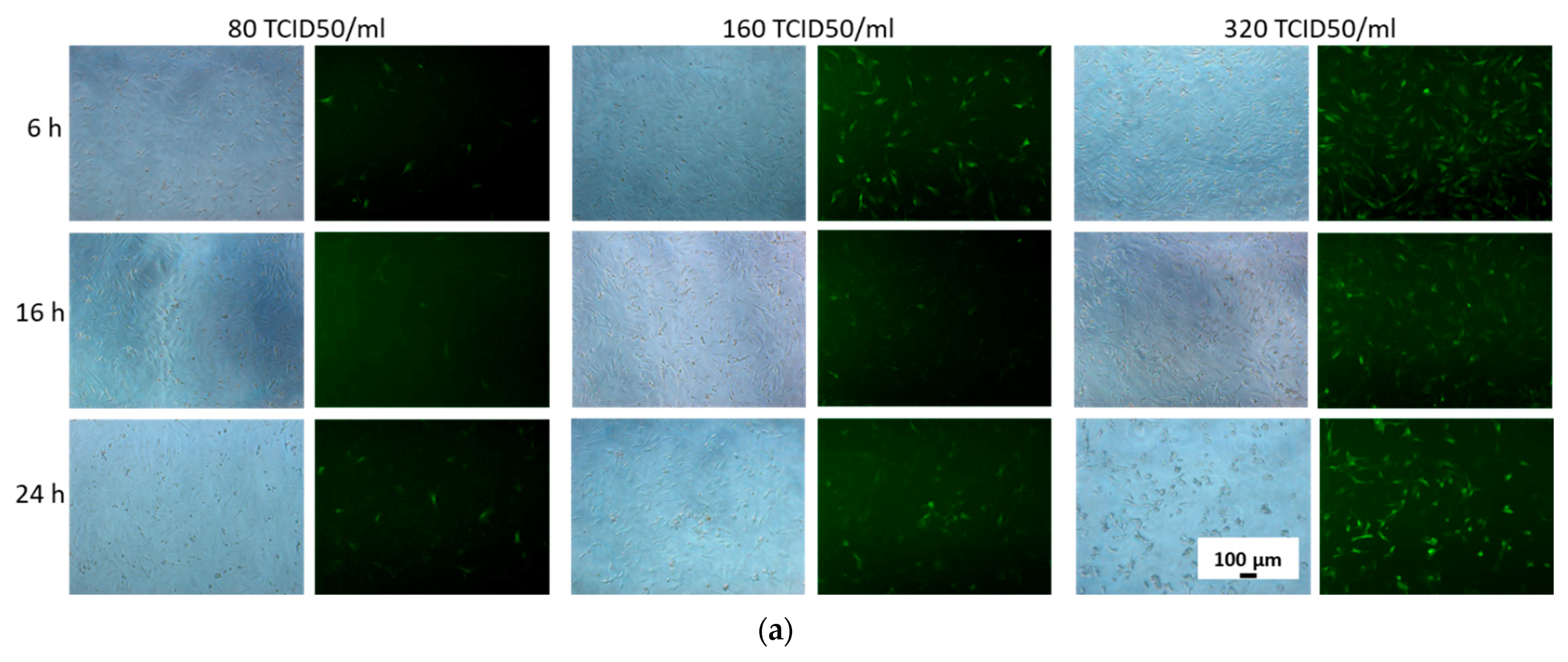
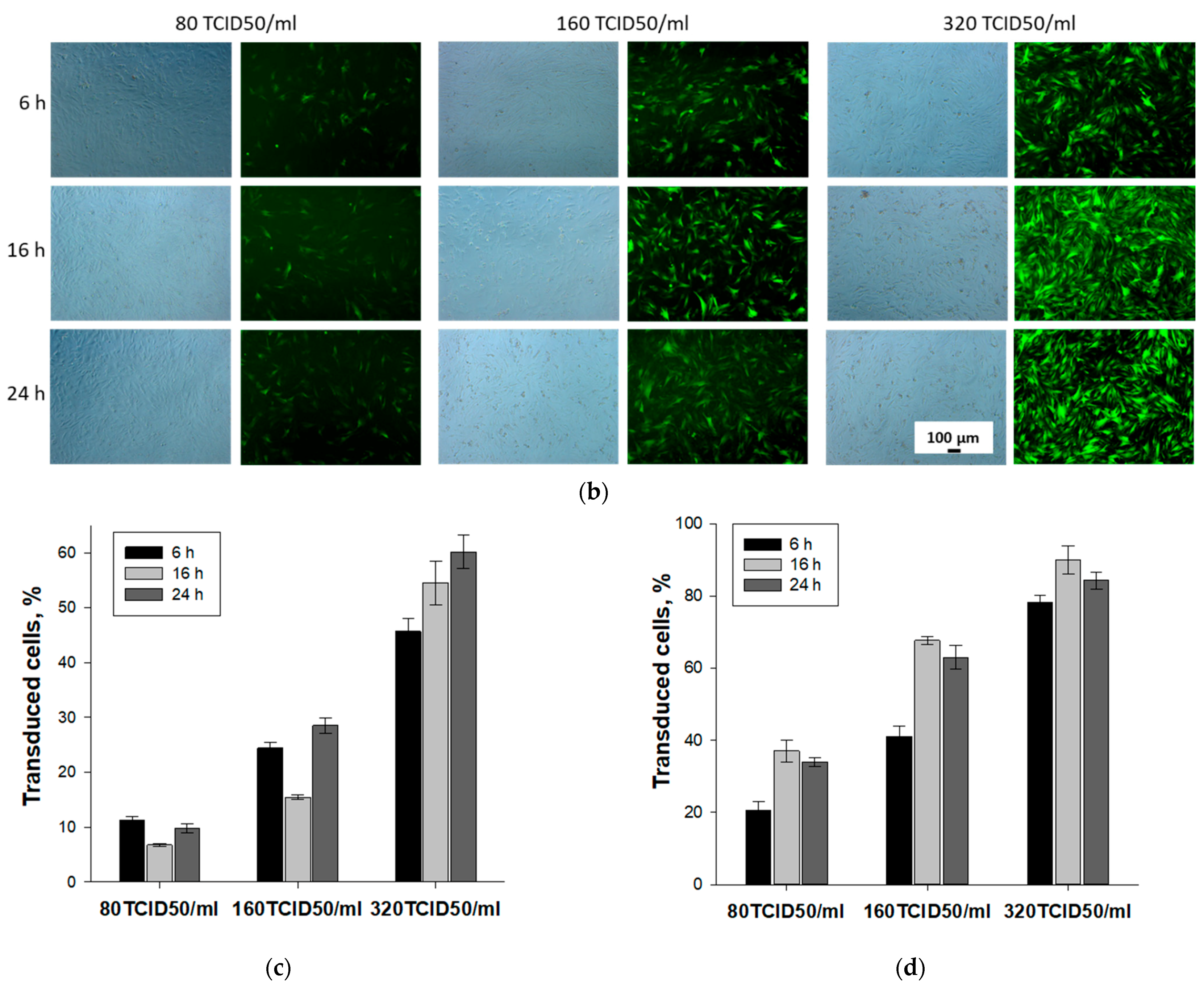
2.4. Transduction Efficiency
2.5. Osteogenic Differentiation of MSCs
2.6. Matrices’ Cytocompatibility In Vitro
2.7. In Vivo Studies
2.8. Micro-CT
2.9. Histological Assay
2.10. Statistical Analysis
3. Results
3.1. Selection of Conditions for Efficient Adenoviral Transduction of MSCs
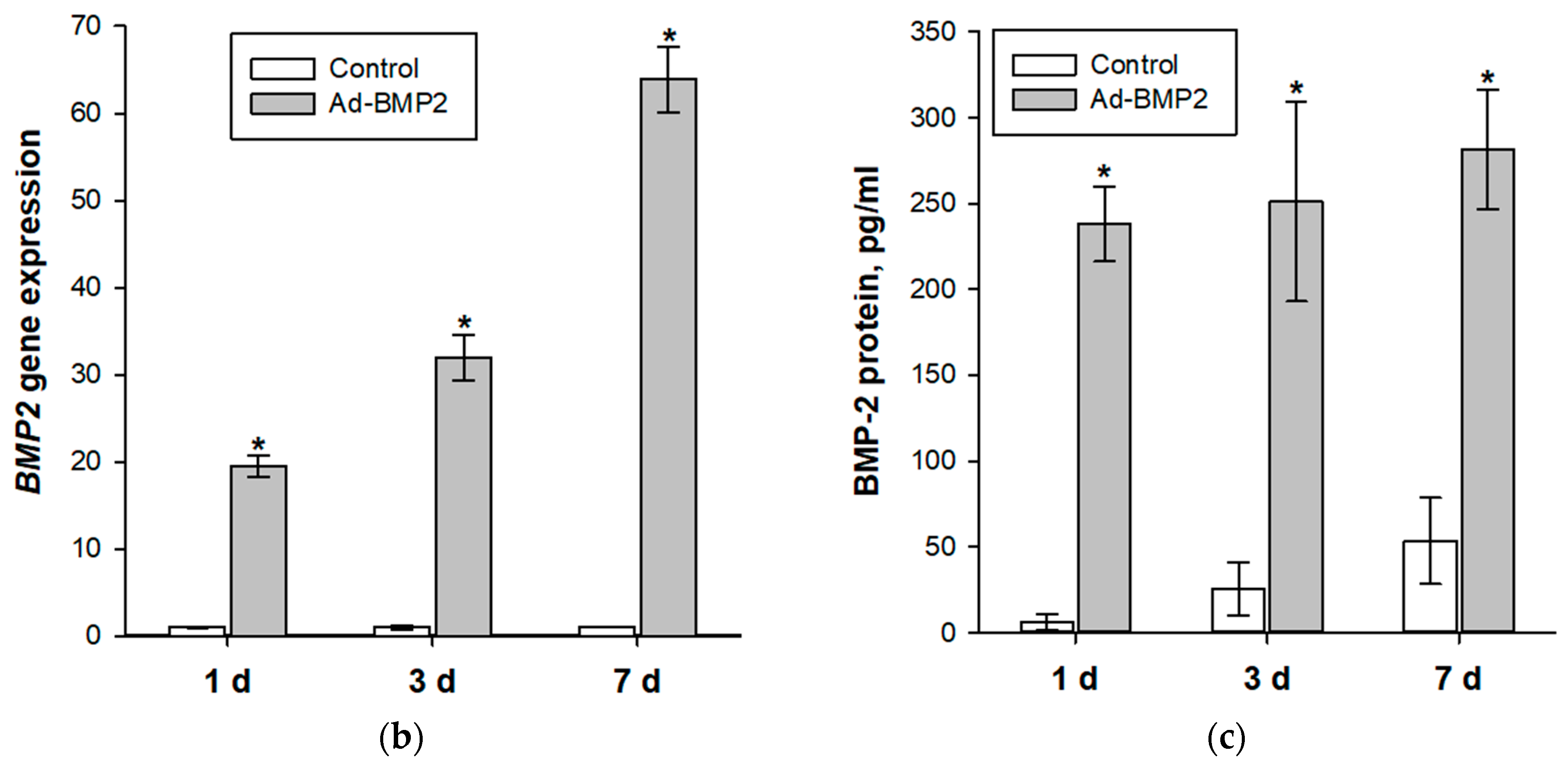
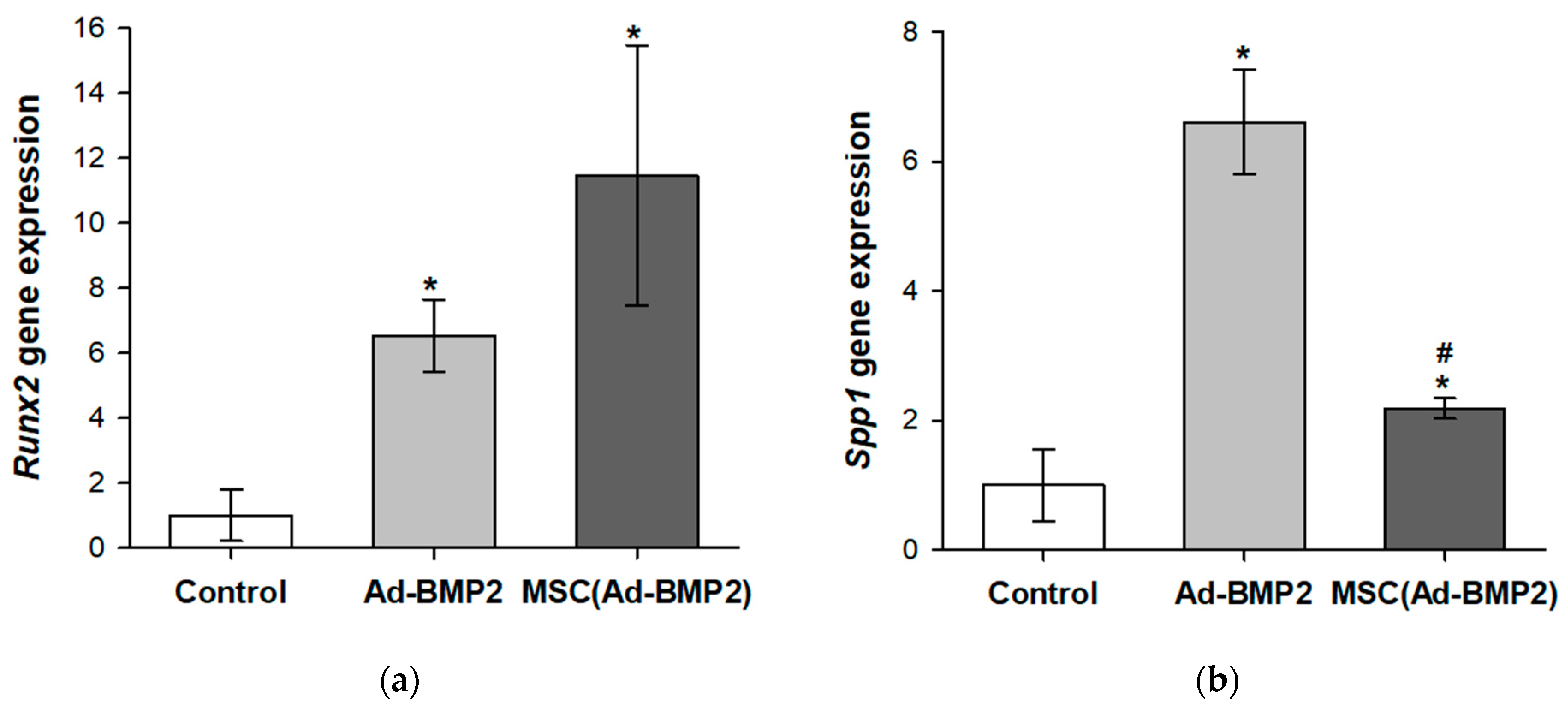
3.2. Evaluation of Osteogenic Differentiation of MSCs upon Transduction by Ad-BMP2 and upon Co-Cultivation with MSC(Ad-BMP2)
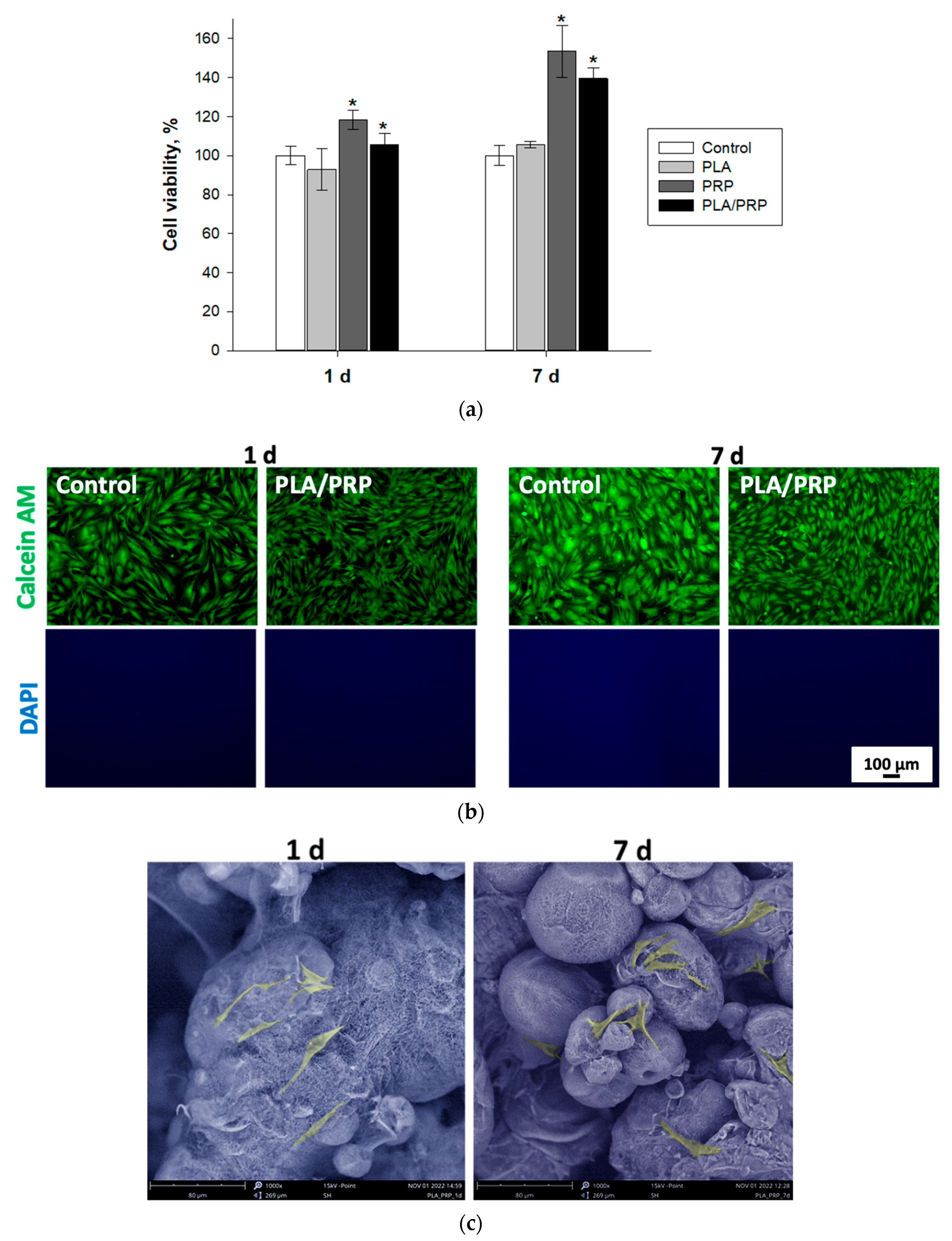
3.3. Assessment of Biocompatibility of Matrices In Vitro
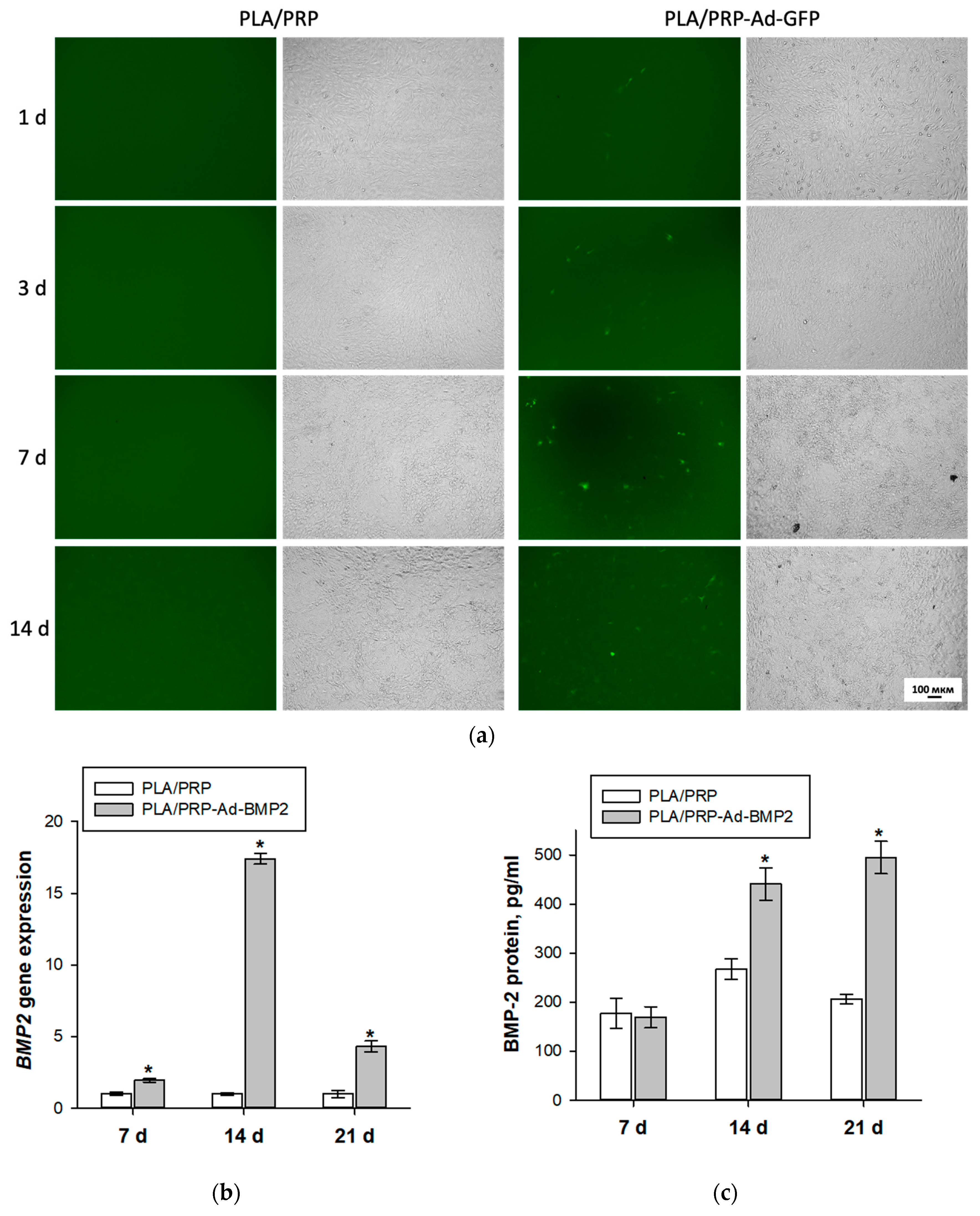
3.4. Transducing Ability of Adenovirus Constructs Impregnated into PLA/PRP Matrices
3.5. Osteoinductive Properties of PLA/PRP-Ad-BMP2 and PLA/PRP-MSC(Ad-BMP2) Matrices In Vitro
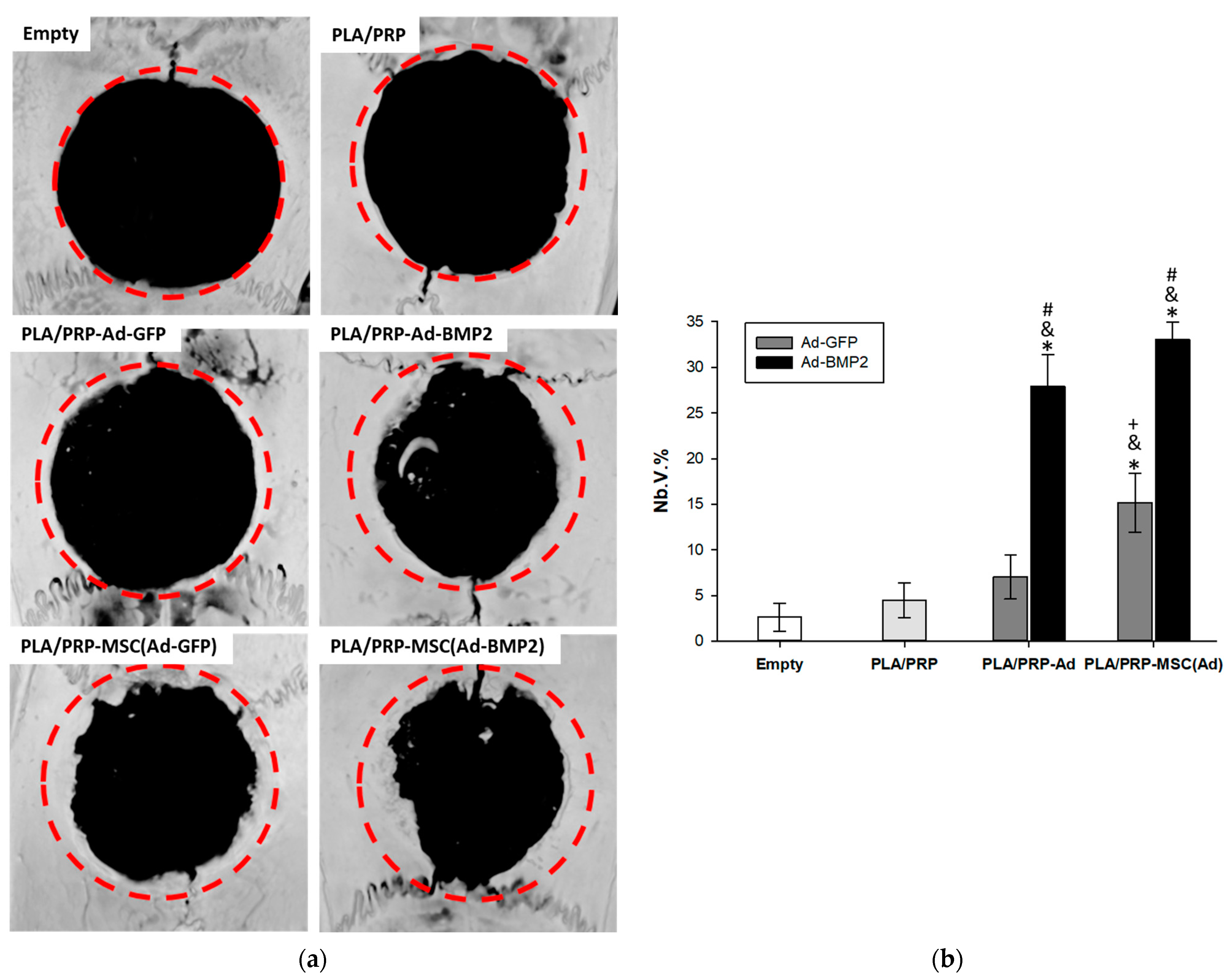
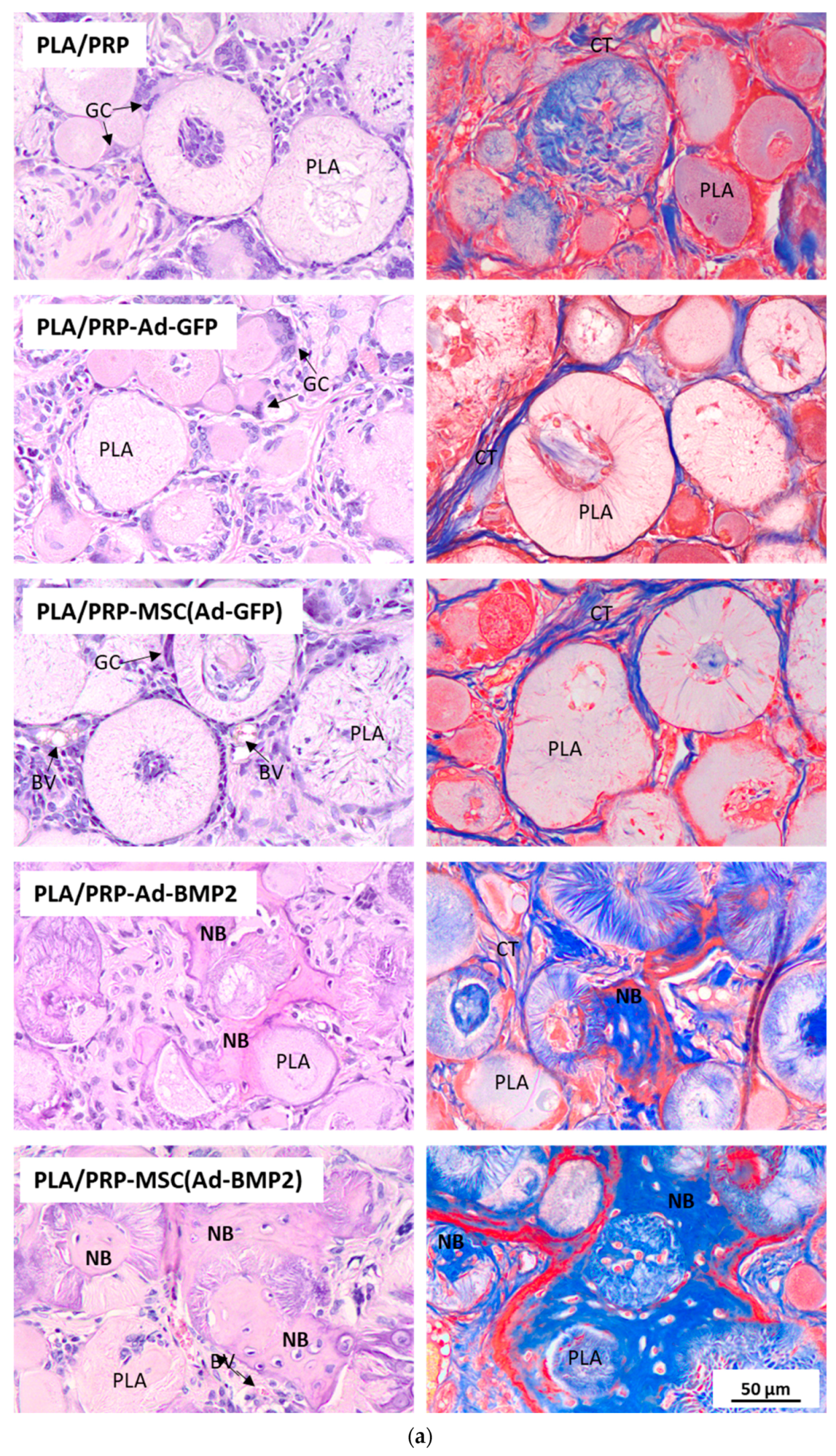
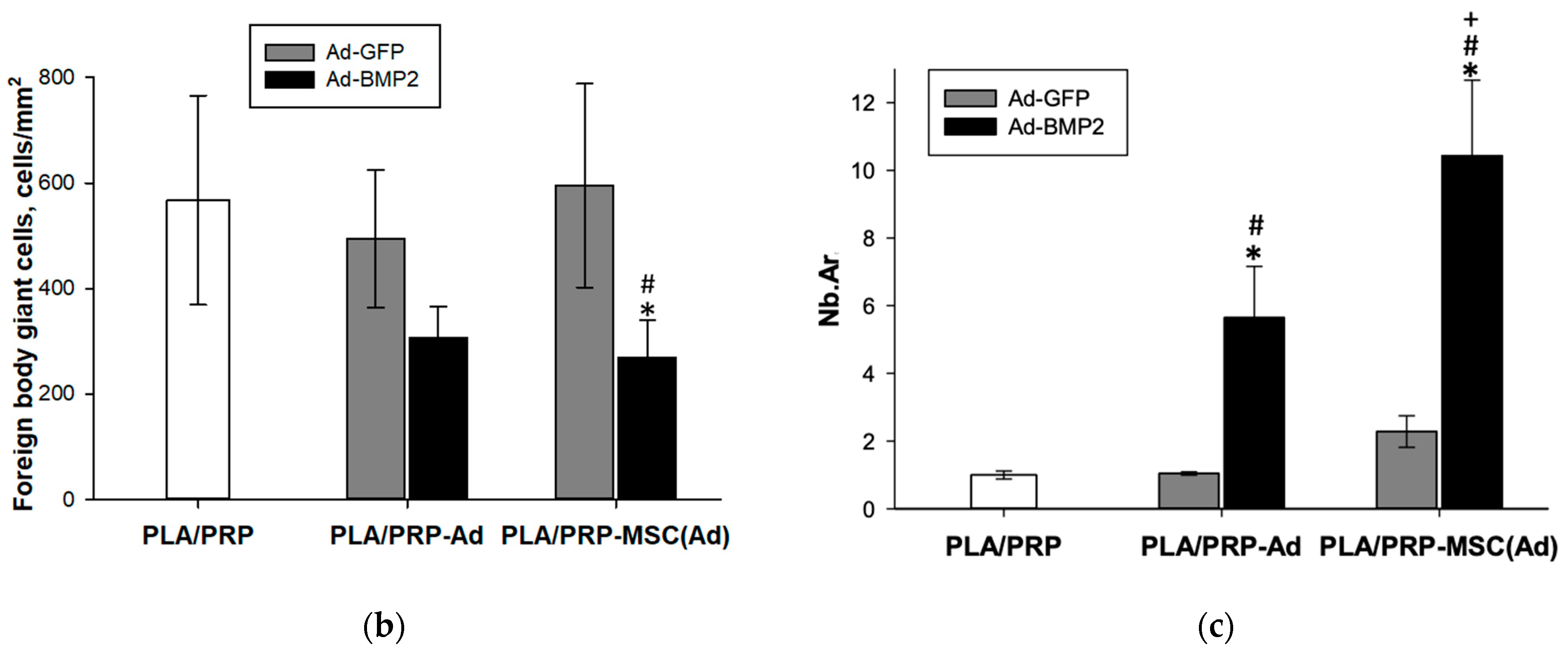
3.6. Osteoinductive Properties of PLA/PRP-Ad-BMP2 and PLA/PRP-MSC(Ad-BMP2) Matrices In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hosseinkhani, H.; Domb, A.J.; Sharifzadeh, G.; Nahum, V. Gene Therapy for Regenerative Medicine. Pharmaceutics 2023, 15, 856. [Google Scholar] [CrossRef] [PubMed]
- Laird, N.Z.; Acri, T.M.; Tingle, K.; Salem, A.K. Gene- and RNAi-activated scaffolds for bone tissue engineering: Current progress and future directions. Adv. Drug Deliv. Rev. 2021, 174, 613–627. [Google Scholar] [CrossRef] [PubMed]
- De la Vega, R.E.; Panos, J.; Van Griensven, M.; Evans, C.H.; Balmayor, E.R. Gene Therapy for Bone Healing: Lessons Learned and New Approaches. Transl. Res. 2021, 236, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Le, Y.; Zhang, Z.; Nian, X.; Liu, B.; Yang, X. Viral Vector-Based Gene Therapy. Int. J. Mol. Sci. 2023, 24, 7736. [Google Scholar] [CrossRef]
- Sakurai, F.; Tachibana, M.; Mizuguchi, H. Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet. 2022, 42, 100432. [Google Scholar] [CrossRef]
- Crystal, R.G. The First Effective In Vivo Gene Delivery Vector. Hum. Gene Ther. 2014, 11, 3–11. [Google Scholar] [CrossRef]
- Le Gars, M.; Hendriks, J.; Sadoff, J.; Ryser, M.; Struyf, F.; Douoguih, M.; Schuitemaker, H. Immunogenicity and efficacy of Ad26.COV2.S: An adenoviral vector–based COVID-19 vaccine. Immunol. Rev. 2022, 310, 47–60. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, K.H.; Kim, S.; Lee, Y.M.; Seol, Y.J. BMP-2 gene delivery-based bone regeneration in dentistry. Pharmaceutics 2019, 11, 393. [Google Scholar] [CrossRef]
- Carreira, A.C.O.; Zambuzzi, W.F.; Rossi, M.C.; Filho, R.A.; Sogayar, M.C.; Granjeiro, J.M. Bone Morphogenetic Proteins: Promising Molecules for Bone Healing, Bioengineering, and Regenerative Medicine. Vitam. Horm. 2015, 99, 293–322. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef] [PubMed]
- Nedorubova, I.A.; Bukharova, T.B.; Mokrousova, V.O.; Khvorostina, M.A.; Vasilyev, A.V.; Nedorubov, A.A.; Grigoriev, T.E.; Zagoskin, Y.D.; Chvalun, S.N.; Kutsev, S.I.; et al. Comparative Efficiency of Gene-Activated Matrices Based on Chitosan Hydrogel and PRP Impregnated with BMP2 Polyplexes for Bone Regeneration. Int. J. Mol. Sci. 2022, 23, 14720. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, A.V.; Kuznetsova, V.S.; Bukharova, T.B.; Osidak, E.O.; Grigoriev, T.E.; Zagoskin, Y.D.; Nedorubova, I.A.; Domogatsky, S.P.; Babichenko, I.I.; Zorina, O.A.; et al. Osteoinductive Moldable and Curable Bone Substitutes Based. Polymers 2021, 13, 3974. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, A.V.; Kuznetsova, V.S.; Bukharova, T.B.; Grigoriev, T.E.; Zagoskin, Y.D.; Nedorubova, I.A.; Babichenko, I.I.; Chvalun, S.N.; Goldstein, D.V.; Kulakov, A.A. Influence of the degree of deacetylation of chitosan and bmp-2 concentration on biocompatibility and osteogenic properties of bmp-2/pla granule-loaded chitosan/β-glycerophosphate hydrogels. Molecules 2021, 26, 261. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.M.; Oreffo, R.O.C. Osteogenesis and angiogenesis: The potential for engineering bone. Eur. Cells Mater. 2008, 15, 100–114. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. The simpler, the better: Tissue vascularization using the body’s own resources. Trends Biotechnol. 2022, 40, 281–290. [Google Scholar] [CrossRef]
- Etulain, J. Platelets in wound healing and regenerative medicine. Platelets 2018, 29, 556–568. [Google Scholar] [CrossRef]
- Desai, A.P.; Sahoo, N.; Pal, A.K.; Chowdhury, S.K.R. Efficacy of Platelet-Rich Plasma in Enhancing the Osteogenic Potential of Bone Graft in Oral and Maxillofacial Region. J. Maxillofac. Oral Surg. 2020, 20, 282–295. [Google Scholar] [CrossRef]
- Vasilyev, A.V.; Bukharova, T.B.; Kuznetsova, V.S.; Zagoskin, Y.D.; Minaeva, S.A.; Grigoriev, T.E.; Antonov, E.N.; Osidak, E.O.; Galitsyna, E.V.; Babichenko, I.I.; et al. Comparison of Impregnated Bone Morphogenetic Protein-2 Release Kinetics from Biopolymer Scaffolds. Inorg. Mater. Appl. Res. 2019, 10, 1093–1100. [Google Scholar] [CrossRef]
- Bukharova, T.B.; Arutyunyan, I.V.; Shustrov, S.A.; Alekseeva, I.S.; Fedyunina, I.A.; Logovskaya, L.V.; Volkov, A.V.; Rzhaninova, A.A.; Grigor’yan, A.S.; Kulakov, A.A.; et al. Tissue engineering construct on the basis of multipotent stromal adipose tissue cells and osteomatrix for regeneration of the bone tissue. Bull. Exp. Biol. Med. 2011, 152, 153–158. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, X.; Fernandes, G.; Dziak, R.; Ionita, C.N.; Li, C.; Wang, C.; Yang, S. The combination of nano-calcium sulfate/platelet rich plasma gel scaffold with BMP2 gene-modified mesenchymal stem cells promotes bone regeneration in rat critical-sized calvarial defects. Stem Cell Res. Ther. 2017, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Wang, C.; Yuan, X.; Liu, Z.; Dziak, R.; Yang, S. Combination of Controlled Release Platelet-Rich Plasma Alginate Beads and Bone Morphogenetic Protein-2 Genetically Modified Mesenchymal Stem Cells for Bone Regeneration. J. Periodontol. 2016, 87, 470–480. [Google Scholar] [CrossRef]
- Cicciù, M.; Scott, A.; Cicciù, D.; Tandon, R.; Maiorana, C. Recombinant human bone morphogenetic protein-2 promote and stabilize hard and soft tissue healing for large mandibular new bone reconstruction defects. J. Craniofac. Surg. 2014, 25, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, Q.Y.; Song, Y.T.; Huang, K.; Jiang, Y.L.; Chen, J.; Wang, R.; Zou, C.Y.; Li, Q.J.; Qin, B.Q.; et al. Accelerated bone defect regeneration through sequential activation of the M1 and M2 phenotypes of macrophages by a composite BMP-2@SIS hydrogel: An immunomodulatory perspective. Compos. Part B Eng. 2022, 243, 110149. [Google Scholar] [CrossRef]
- Park, S.; Heo, H.A.; Lee, K.B.; Kim, H.G.; Pyo, S.W. Improved Bone Regeneration with Multiporous PLGA Scaffold and BMP-2-Transduced Human Adipose-Derived Stem Cells by Cell-Permeable Peptide. Implant Dent. 2017, 26, 4–11. [Google Scholar] [CrossRef]
- Müller, C.W.; Hildebrandt, K.; Gerich, T.; Krettek, C.; van Griensven, M.; Rosado Balmayor, E. BMP-2-transduced human bone marrow stem cells enhance neo-bone formation in a rat critical-sized femur defect. J. Tissue Eng. Regen. Med. 2017, 11, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.S.; Barry, M.A.; Mikos, A.G.; Jansen, J.A. In Vivo Evaluation of Gene Therapy Vectors in Ex Vivo-Derived Marrow Stromal Cells for Bone Regeneration in a Rat Critical-Size Calvarial Defect Model. Hum. Gene Ther. 2003, 14, 1689–1701. [Google Scholar] [CrossRef]
- Bougioukli, S.; Chateau, M.; Morales, H.; Vakhshori, V.; Sugiyama, O.; Oakes, D.; Longjohn, D.; Cannon, P.; Lieberman, J.R. Limited potential of AAV-mediated gene therapy in transducing human mesenchymal stem cells for bone repair applications. Gene Ther. 2021, 28, 729–739. [Google Scholar] [CrossRef]
- Yao, S.; Rong, W.; Yuan, Y. Optimization of adeno-associated virus (AAV) gene delivery into human bone marrow stem cells (hBMSCs). Stem Cell Investig. 2023, 10, 3. [Google Scholar] [CrossRef]
- Fausther-Bovendo, H.; Kobinger, G.P. Pre-existing immunity against Ad vectors: Humoral, cellular, and innate response, what’s important? Hum. Vaccines Immunother. 2014, 10, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Bai, B.; Yan, G.; Zhang, S.; Liu, Q.; Chen, Y.; Tan, X.; Zeng, Y. Construction of doxycycline-mediated BMP-2 transgene combining with APA microcapsules for bone repair. Artif. Cells Nanomed. Biotechnol. 2016, 44, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Gabal, Y.; Ramsey, J.D. Surface modification of adenovirus vector to improve immunogenicity and tropism. Methods Mol. Biol. 2021, 2183, 357–366. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukharova, T.B.; Nedorubova, I.A.; Mokrousova, V.O.; Meglei, A.Y.; Basina, V.P.; Nedorubov, A.A.; Vasilyev, A.V.; Grigoriev, T.E.; Zagoskin, Y.D.; Chvalun, S.N.; et al. Adenovirus-Based Gene Therapy for Bone Regeneration: A Comparative Analysis of In Vivo and Ex Vivo BMP2 Gene Delivery. Cells 2023, 12, 1762. https://doi.org/10.3390/cells12131762
Bukharova TB, Nedorubova IA, Mokrousova VO, Meglei AY, Basina VP, Nedorubov AA, Vasilyev AV, Grigoriev TE, Zagoskin YD, Chvalun SN, et al. Adenovirus-Based Gene Therapy for Bone Regeneration: A Comparative Analysis of In Vivo and Ex Vivo BMP2 Gene Delivery. Cells. 2023; 12(13):1762. https://doi.org/10.3390/cells12131762
Chicago/Turabian StyleBukharova, Tatiana Borisovna, Irina Alekseevna Nedorubova, Viktoria Olegovna Mokrousova, Anastasiia Yurevna Meglei, Viktoriia Pavlovna Basina, Andrey Anatolevich Nedorubov, Andrey Vyacheslavovich Vasilyev, Timofei Evgenevich Grigoriev, Yuriy Dmitrievich Zagoskin, Sergei Nicolaevich Chvalun, and et al. 2023. "Adenovirus-Based Gene Therapy for Bone Regeneration: A Comparative Analysis of In Vivo and Ex Vivo BMP2 Gene Delivery" Cells 12, no. 13: 1762. https://doi.org/10.3390/cells12131762
APA StyleBukharova, T. B., Nedorubova, I. A., Mokrousova, V. O., Meglei, A. Y., Basina, V. P., Nedorubov, A. A., Vasilyev, A. V., Grigoriev, T. E., Zagoskin, Y. D., Chvalun, S. N., Kutsev, S. I., & Goldshtein, D. V. (2023). Adenovirus-Based Gene Therapy for Bone Regeneration: A Comparative Analysis of In Vivo and Ex Vivo BMP2 Gene Delivery. Cells, 12(13), 1762. https://doi.org/10.3390/cells12131762






