Leveraging Plasma Membrane Repair Therapeutics for Treating Neurodegenerative Diseases
Abstract
:1. Introduction
2. Neurodegenerative Diseases with Membrane Damage Implications
2.1. Pathogenic Proteins and the Plasma Membrane
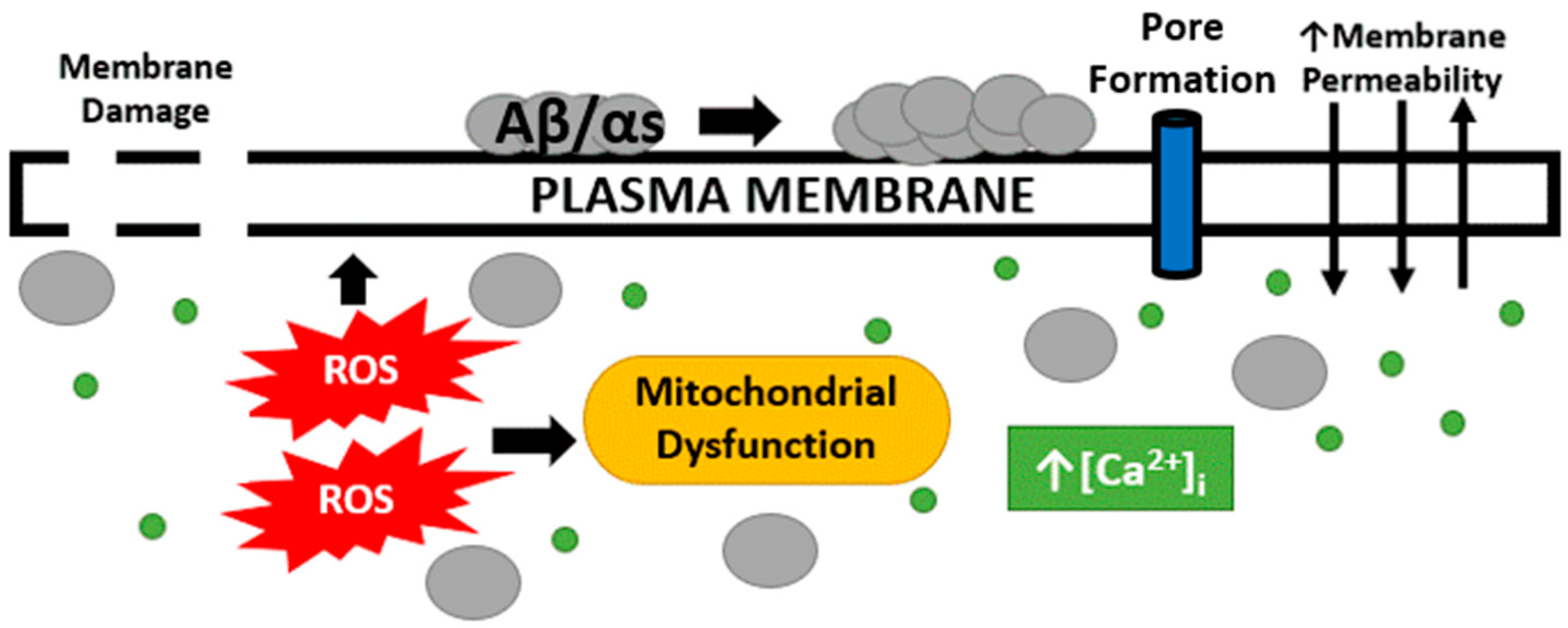
2.2. Changes in Membrane Permeability, Oxidative Stress and Mitochondrial Dysfunction
2.3. Membrane Damage and Pore Formation by Proteins Associated with Neurodegeneration
2.4. Membrane Repair Proteins Involved in Neurodegeneration
3. Enhancing Cell Membrane Repair as a Therapeutic Approach
| Molecule | Target Tissue(s) | Effect on Membrane Repair | References |
|---|---|---|---|
| Recombinant TRIM72/MG53 | Heart, skeletal muscle, kidney, liver, peripheral nervous system | Increases membrane repair capacity; accumulates at the injury site | Weisleder et al. (2012) [16], Gushchina et al. (2017) [17], Paleo et al. (2020) [44] |
| Recombinant Annexin A6 | Skeletal muscle | Enhances membrane repair, protects against skeletal muscle damage | Demonbreun et al. (2019) [125] |
| Recombinant Annexin A5 | Myotubes | Rescues membrane repair from annexin A5 knockdown | Carmeille et al. (2016) [30] |
| Recombinant Annexin A1 | Heart | Reduced ischemia-reperfusion damage | D’Amico et al. (2000) [132] |
| Recombinant Annexin A2 | Brain | Enhances blood brain barrier integrity | Cheng et al. (2021) [133] |
| P188 | Skeletal muscle, cardiac muscle, lung, brain | Increases membrane resealing | Moloughney and Weisleder (2012) [128], Kwiatkowski et al. (2020) [131], Spurney et al. (2011) [130], Tang et al. (2021) [134], Gu et al. (2013) [135] |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McNeil, P.; Vogel, S.; Miyake, K.; Terasaki, M. Patching plasma membrane disruptions with cytoplasmic membrane. J. Cell Sci. 2000, 113, 1891–1902. [Google Scholar] [CrossRef]
- McNeil, P.L.; Baker, M.M. Cell surface events during resealing visualized by scanning-electron microscopy. Cell Tissue Res. 2001, 304, 141–146. [Google Scholar] [CrossRef]
- McNeil, P.L.; Khakee, R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am. J. Pathol. 1992, 140, 1097–1109. [Google Scholar]
- Howard, A.C.; McNeil, A.K.; McNeil, P.L. Promotion of plasma membrane repair by vitamin E. Nat. Commun. 2011, 2, 597. [Google Scholar] [CrossRef] [Green Version]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Bansal, D.; Miyake, K.; Vogel, S.S.; Groh, S.; Chen, C.-C.; Williamson, R.; McNeil, P.L.; Campbell, K.P. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 2003, 423, 168–172. [Google Scholar] [CrossRef]
- Cai, C.; Weisleder, N.; Ko, J.-K.; Komazaki, S.; Sunada, Y.; Nishi, M.; Takeshima, H.; Ma, J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J. Biol. Chem. 2009, 284, 15894–15902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duann, P.; Li, H.; Lin, P.; Tan, T.; Wang, Z.; Chen, K.; Zhou, X.; Gumpper, K.; Zhu, H.; Ludwig, T.; et al. MG53-mediated cell membrane repair protects against acute kidney injury. Sci. Transl. Med. 2015, 7, 279ra36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Hu, Y.-H.; Han, Y.; Wang, Y.-B.; Zhang, Y.; Zhang, X.-Q.; He, D.-F.; Ren, H.-M.; Liu, Y.-K.; Wang, H.-Y.; et al. MG53 protects against contrast-induced acute kidney injury by reducing cell membrane damage and apoptosis. Acta Pharmacol. Sin. 2020, 41, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata-Endo, H.; Kato, J.; Tonouchi, A.; Chung, Y.W.; Sun, J.; Stevens, L.A.; Zhu, J.; Aponte, A.M.; Springer, D.A.; San, H.; et al. Role of a TRIM72 ADP-ribosylation cycle in myocardial injury and membrane repair. J. Clin. Investig. 2018, 3, e97898. [Google Scholar] [CrossRef] [Green Version]
- Houang, E.M.; Bartos, J.; Hackel, B.J.; Lodge, T.P.; Yannopoulos, D.; Bates, F.S.; Metzger, J.M. Cardiac Muscle Membrane Stabilization in Myocardial Reperfusion Injury. JACC Basic Transl. Sci. 2019, 4, 275–287. [Google Scholar] [CrossRef]
- Han, R.; Bansal, D.; Miyake, K.; Muniz, V.P.; Weiss, R.M.; McNeil, P.L.; Campbell, K.P. Dysferlin-mediated membrane repair protects the heart from stress-induced left ventricular injury. J. Clin. Investig. 2007, 117, 1805–1813. [Google Scholar] [CrossRef]
- Cong, X.; Nagre, N.; Herrera, J.; Pearson, A.C.; Pepper, I.; Morehouse, R.; Ji, H.-L.; Jiang, D.; Hubmayr, R.D.; Zhao, X. TRIM72 promotes alveolar epithelial cell membrane repair and ameliorates lung fibrosis. Respir. Res. 2020, 21, 132. [Google Scholar] [CrossRef]
- Nagre, N.; Cong, X.; Ji, H.-L.; Schreiber, J.M.; Fu, H.; Pepper, I.; Warren, S.; Sill, J.M.; Hubmayr, R.D.; Zhao, X. Inhaled TRIM72 Protein Protects Ventilation Injury to the Lung through Injury-guided Cell Repair. Am. J. Respir. Cell Mol. Biol. 2018, 59, 635–647. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, B.; Zhu, H.; Li, H.; Han, Y.; Chen, K.; Wang, Z.; Zeng, J.; Liu, Y.; Wang, X.; et al. MG53 permeates through blood-brain barrier to protect ischemic brain injury. Oncotarget 2016, 7, 22474–22485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisleder, N.; Takizawa, N.; Lin, P.; Wang, X.; Cao, C.; Zhang, Y.; Tan, T.; Ferrante, C.; Zhu, H.; Chen, P.-J.; et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci. Transl. Med. 2012, 4, 139ra85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gushchina, L.V.; Bhattacharya, S.; McElhanon, K.E.; Choi, J.H.; Manring, H.; Beck, E.X.; Alloush, J.; Weisleder, N. Treatment with Recombinant Human MG53 Protein Increases Membrane Integrity in a Mouse Model of Limb Girdle Muscular Dystrophy 2B. Mol. Ther. 2017, 25, 2360–2371. [Google Scholar] [CrossRef] [Green Version]
- Yao, W.; Li, H.; Han, X.; Chen, C.; Zhang, Y.; Tai, W.L.; Xia, Z.; Hei, Z. MG53 anchored by dysferlin to cell membrane reduces hepatocyte apoptosis which induced by ischaemia/reperfusion injury in vivo and in vitro. J. Cell. Mol. Med. 2017, 21, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Segura, E.; Rookhuizen, D.C.; Baudon, B.; Heurtebise-Chrétien, S.; Burgdorf, N.; Maurin, M.; Kapp, E.A.; Simpson, R.J.; Kozik, P.; et al. Endocytic membrane repair by ESCRT-III controls antigen export to the cytosol during antigen cross-presentation. Cell Rep. 2022, 40, 111205. [Google Scholar] [CrossRef]
- Shukla, S.; Larsen, K.P.; Ou, C.; Rose, K.; Hurley, J.H. Hurley, In vitro reconstitution of calcium-dependent recruitment of the human ESCRT machinery in lysosomal membrane repair. Proc. Natl. Acad. Sci. USA 2022, 119, e2205590119. [Google Scholar] [CrossRef]
- Jimenez, A.J.; Maiuri, P.; Lafaurie-Janvore, J.; Divoux, S.; Piel, M.; Perez, F. ESCRT Machinery Is Required for Plasma Membrane Repair. Science 2014, 343, 1247136. [Google Scholar] [CrossRef]
- Los, F.C.; Kao, C.-Y.; Smitham, J.; McDonald, K.L.; Ha, C.; Peixoto, C.A.; Aroian, R.V. RAB-5- and RAB-11-Dependent Vesicle-Trafficking Pathways Are Required for Plasma Membrane Repair after Attack by Bacterial Pore-Forming Toxin. Cell Host Microbe 2011, 9, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, M.; Keyel, M.; Shi, G.; Bhattacharjee, P.; Roth, R.; Heuser, J.E.; Keyel, P.A. Intrinsic repair protects cells from pore-forming toxins by microvesicle shedding. Cell Death Differ. 2017, 24, 798–808. [Google Scholar] [CrossRef] [Green Version]
- Corrotte, M.; Fernandes, M.C.; Tam, C.; Andrews, N.W. Toxin Pores Endocytosed During Plasma Membrane Repair Traffic into the Lumen of <scp>MVB</scp> s for Degradation. Traffic 2011, 13, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Idone, V.; Tam, C.; Goss, J.W.; Toomre, D.; Pypaert, M.; Andrews, N.W. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 2008, 180, 905–914. [Google Scholar] [CrossRef] [Green Version]
- Keyel, P.A.; Loultcheva, L.; Roth, R.; Salter, R.D.; Watkins, S.C.; Yokoyama, W.M.; Heuser, J.E. Streptolysin O clearance through sequestration into blebs that bud passively from the plasma membrane. J. Cell Sci. 2011, 124, 2414–2423. [Google Scholar] [CrossRef] [Green Version]
- Blazek, A.D.; Paleo, B.J.; Weisleder, N. Plasma Membrane Repair: A Central Process for Maintaining Cellular Homeostasis. Physiology 2015, 30, 438–448. [Google Scholar] [CrossRef] [Green Version]
- McNeil, P. Membrane repair redux: Redox of MG53. Nat. Cell Biol. 2009, 11, 7–9. [Google Scholar] [CrossRef]
- Cai, C.; Masumiya, H.; Weisleder, N.; Matsuda, N.; Nishi, M.; Hwang, M.; Ko, J.-K.; Lin, P.; Thornton, A.; Zhao, X.; et al. MG53 nucleates assembly of cell membrane repair machinery. Nat. Cell Biol. 2008, 11, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmeille, R.; Bouvet, F.; Tan, S.; Croissant, C.; Gounou, C.; Mamchaoui, K.; Mouly, V.; Brisson, A.R.; Bouter, A. Membrane repair of human skeletal muscle cells requires Annexin-A5. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2267–2279. [Google Scholar] [CrossRef]
- Croissant, C.; Gounou, C.; Bouvet, F.; Tan, S.; Bouter, A. Annexin-A6 in Membrane Repair of Human Skeletal Muscle Cell: A Role in the Cap Subdomain. Cells 2020, 9, 1742. [Google Scholar] [CrossRef]
- Boye, T.L.; Nylandsted, J. Annexins in plasma membrane repair. Biol. Chem. 2016, 397, 961–969. [Google Scholar] [CrossRef]
- Lennon, N.J.; Kho, A.; Bacskai, B.J.; Perlmutter, S.L.; Hyman, B.T.; Brown, R.H. Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J. Biol. Chem. 2003, 278, 50466–50473. [Google Scholar] [CrossRef] [Green Version]
- Boye, T.L.; Maeda, K.; Pezeshkian, W.; Sønder, S.L.; Haeger, S.C.; Gerke, V.; Simonsen, A.C.; Nylandsted, J. Annexin A4 and A6 induce membrane curvature and constriction during cell membrane repair. Nat. Commun. 2017, 8, 1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDade, J.R.; Michele, D.E. Membrane damage-induced vesicle-vesicle fusion of dysferlin-containing vesicles in muscle cells requires microtubules and kinesin. Hum. Mol. Genet. 2013, 23, 1677–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defour, A.; Van der Meulen, J.H.; Bhat, R.; Bigot, A.; Bashir, R.; Nagaraju, K.; Jaiswal, J.K. Dysferlin regulates cell membrane repair by facilitating injury-triggered acid sphingomyelinase secretion. Cell Death Dis. 2014, 5, e1306. [Google Scholar] [CrossRef] [Green Version]
- Codding, S.; Marty, N.; Abdullah, N.; Johnson, C.P. Dysferlin binds SNAREs (soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptors) and stimulates membrane fusion in a calcium-sensitive manner. J. Biol. Chem. 2016, 291, 14575–14584. [Google Scholar] [CrossRef] [Green Version]
- Vogel, K.; Cabaniols, J.-P.; Roche, P. Targeting of SNAP-25 to Membranes Is Mediated by Its Association with the Target SNARE Syntaxin. J. Biol. Chem. 2000, 275, 2959–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreetama, S.C.; Takano, T.; Nedergaard, M.; Simon, S.M.; Jaiswal, J.K. Injured astrocytes are repaired by Synaptotagmin XI-regulated lysosome exocytosis. Cell Death Differ. 2015, 23, 596–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, A.P.K.; Gerke, V. The resealing factor S100A11 interacts with annexins and extended synaptotagmin-1 in the course of plasma membrane wound repair. Front. Cell Dev. Biol. 2022, 10, 968164. [Google Scholar] [CrossRef]
- Clarke, M.S.F.; Caldwell, R.W.; Chiao, H.; Miyake, K.; McNeil, P.L. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circ. Res. 1995, 76, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kellett, T.; Wang, S.; Nishi, M.; Nagre, N.; Zhou, B.; Flodby, P.; Shilo, K.; Ghadiali, S.N.; Takeshima, H.; et al. TRIM72 is required for effective repair of alveolar epithelial cell wounding. Am. J. Physiol. Cell. Mol. Physiol. 2014, 307, L449–L459. [Google Scholar] [CrossRef] [Green Version]
- McNeil, P.L.; Ito, S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology 1989, 96, 1238–1248. [Google Scholar] [CrossRef]
- Paleo, B.J.; Madalena, K.M.; Mital, R.; McElhanon, K.E.; Kwiatkowski, T.A.; Rose, A.L.; Lerch, J.K.; Weisleder, N. Enhancing membrane repair increases regeneration in a sciatic injury model. PLoS ONE 2020, 15, e0231194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, S.T.; McNeil, P.L. Membrane Repair: Mechanisms and Pathophysiology. Physiol. Rev. 2015, 95, 1205–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNeil, P.L.; Ito, S. Molecular traffic through plasma membrane disruptions of cells in vivo. J. Cell Sci. 1990, 96, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; Caler, E.V.; Andrews, N.W. Plasma Membrane Repair Is Mediated by Ca2+-Regulated Exocytosis of Lysosomes. Cell 2001, 106, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Ammendolia, D.A.; Bement, W.M.; Brumell, J.H. Plasma membrane integrity: Implications for health and disease. BMC Biol. 2021, 19, 71. [Google Scholar] [CrossRef]
- Cong, X.; Hubmayr, R.D.; Li, C.; Zhao, X. Plasma membrane wounding and repair in pulmonary diseases. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 312, L371–L391. [Google Scholar] [CrossRef] [Green Version]
- Rigoni, M.; Negro, S. Signals Orchestrating Peripheral Nerve Repair. Cells 2020, 9, 1768. [Google Scholar] [CrossRef]
- Javaid, S.F.; Giebel, C.; AB Khan, M.; Hashim, M.J. Epidemiology of Alzheimer’s disease and other dementias: Rising global burden and forecasted trends. F1000Research 2021, 10, 425. [Google Scholar] [CrossRef]
- Maserejian, L.; Vinikoor-Imler; Dilley, A. Estimation of the 2020 Global Population of Parkinson’s Disease (PD). Mov. Disord. 2022, 35. [Google Scholar]
- Alzheimer’s Association. 2022 Alzheimer’s Disease Facts and Figures; Alzheimer’s Association: Chicago, IL, USA, 2022. [Google Scholar]
- Marras, C.; Beck, J.C.; Bower, J.H.; Roberts, E.; Ritz, B.; Ross, G.W.; Abbott, R.D.; Savica, R.; Van Den Eeden, S.K.; Willis, A.W.; et al. Prevalence of Parkinson’s disease across North America. NPJ Parkinson’s Dis. 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crews, L.; Masliah, E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum. Mol. Genet. 2010, 19, R12–R20. [Google Scholar] [CrossRef] [Green Version]
- Harvey, R.J.; Skelton-Robinson, M.; Rossor, M.N. The prevalence and causes of dementia in people under the age of 65 years. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1206–1209. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [Green Version]
- Mullan, M.; Crawford, F.; Axelman, K.; Houlden, H.; Lilius, L.; Winblad, B.; Lannfelt, L. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N–terminus of β–amyloid. Nat. Genet. 1992, 1, 345–347. [Google Scholar] [CrossRef]
- Achouri-Rassas, A.; Ben Ali, N.; Fray, S.; Fredj, S.H.; Kechaou, M.; Zakraoui, N.O.; Cherif, A.; Chabbi, S.; Anane, N.; Messaoud, T.; et al. Novel presenilin 1 mutation (p.I83T) in Tunisian family with early-onset Alzheimer’s disease. Neurobiol. Aging 2015, 36, 2904.e9–2904.e11. [Google Scholar] [CrossRef]
- Lanoiselée, H.-M.; Nicolas, G.; Wallon, D.; Rovelet-Lecrux, A.; Lacour, M.; Rousseau, S.; Richard, A.-C.; Pasquier, F.; Rollin-Sillaire, A.; Martinaud, O.; et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med. 2017, 14, e1002270. [Google Scholar] [CrossRef] [Green Version]
- Eryilmaz, I.E.; Bakar, M.; Egeli, U.; Cecener, G.; Yurdacan, B.; Colak, D.K.; Tunca, B. Evaluation of the Clinical Features Accompanied by the Gene Mutations. Alzheimer Dis. Assoc. Disord. 2021, 35, 214–222. [Google Scholar] [CrossRef]
- Sleegers, K.; Roks, G.; Theuns, J.; Aulchenko, Y.S.; Rademakers, R.; Cruts, M.; van Gool, W.A.; Van Broeckhoven, C.; Heutink, P.; Oostra, B.A.; et al. Familial clustering and genetic risk for dementia in a genetically isolated Dutch population. Brain 2004, 127, 1641–1649. [Google Scholar] [CrossRef]
- Bulgart, H.R.; Neczypor, E.W.; Wold, L.E.; Mackos, A.R. Microbial involvement in Alzheimer disease development and progression. Mol. Neurodegener. 2020, 15, 42. [Google Scholar] [CrossRef]
- Bramblett, G.T.; Goedert, M.; Jakes, R.; Merrick, S.E.; Trojanowski, J.Q.; Lee, V.M. Abnormal tau phosphorylation at Ser396 in alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron 1993, 10, 1089–1099. [Google Scholar] [CrossRef]
- Strang, K.H.; Golde, T.E.; Giasson, B.I. MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Lab. Investig. 2019, 99, 912–928. [Google Scholar] [CrossRef]
- Siddiqui, I.J.; Pervaiz, N.; Abbasi, A.A. The Parkinson Disease gene SNCA: Evolutionary and structural insights with pathological implication. Sci. Rep. 2016, 6, 24475. [Google Scholar] [CrossRef]
- Fang, Y.-Q.; Mao, F.; Zhu, M.-J.; Li, X.-H. Compound heterozygous mutations in PARK2 causing early-onset Parkinson disease. Medicine 2019, 98, e14228. [Google Scholar] [CrossRef] [PubMed]
- Siuda, J.; Jasinska-Myga, B.; Boczarska-Jedynak, M.; Opala, G.; Fiesel, F.C.; Moussaud-Lamodière, E.L.; Scarffe, L.A.; Dawson, V.L.; Ross, O.A.; Springer, W.; et al. Early-onset Parkinson’s disease due to PINK1 p.Q456X mutation—Clinical and functional study. Park. Relat. Disord. 2014, 20, 1274–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, C.; Markello, T.; Zein, W.M.; Bishop, R.; Groden, C.; Gahl, W.; Toro, C. PARK7-Related Early Onset Parkinson Disease in the Setting of Complete Uniparental Isodisomy of Chromosome 1. Neurol. Genet. 2021, 7, e606. [Google Scholar] [CrossRef]
- Clark, L.N.; Wang, Y.; Karlins, E.; Saito, L.; Mejia-Santana, H.; Harris, J.; Louis, E.D.; Cote, L.J.; Andrews, H.; Fahn, S.; et al. Frequency of LRRK2 mutations in early- and late-onset Parkinson disease. Neurology 2006, 67, 1786–1791. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flagmeier, P.; Meisl, G.; Vendruscolo, M.; Knowles, T.P.J.; Dobson, C.M.; Buell, A.K.; Galvagnion, C. Mutations associated with familial Parkinson’s disease alter the initiation and amplification steps of α-synuclein aggregation. Proc. Natl. Acad. Sci. USA 2016, 113, 10328–10333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arispe, N.; Diaz, J.C.; Simakova, O. Abeta ion channels. Prospects for treating Alzheimer’s disease with Amyloid Beta channel blockers. Biochim. Biophys. Acta Biomembr. 2007, 1768, 1952–1965. [Google Scholar] [CrossRef] [Green Version]
- Kaya, I.; Jennische, E.; Dunevall, J.; Lange, S.; Ewing, A.G.; Malmberg, P.; Baykal, A.T.; Fletcher, J.S. Spatial Lipidomics Reveals Region and Long Chain Base Specific Accumulations of Monosialogangliosides in Amyloid Plaques in Familial Alzheimer’s Disease Mice (5xFAD) Brain. ACS Chem. Neurosci. 2019, 11, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, E.; Cascella, R.; Becatti, M.; Marrazza, G.; Dobson, C.M.; Chiti, F.; Stefani, M.; Cecchi, C. Binding affinity of amyloid oligomers to cellular membranes is a generic indicator of cellular dysfunction in protein misfolding diseases. Sci. Rep. 2016, 6, 32721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciacca, M.F.; Kotler, S.A.; Brender, J.R.; Chen, J.; Lee, D.-K.; Ramamoorthy, A. Two-Step Mechanism of Membrane Disruption by Aβ through Membrane Fragmentation and Pore Formation. Biophys. J. 2012, 103, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Julien, C.; Tomberlin, C.; Roberts, C.M.; Akram, A.; Silverman, M.A.; Link, C.D. In vivo induction of membrane damage by beta-amyloid peptide oligomers. Acta Neuropathol. Commun. 2018, 6, 131. [Google Scholar] [CrossRef] [Green Version]
- Brandt, R.; Léger, J.; Lee, G. Interaction of tau with the neural plasma membrane mediated by tau’s amino-terminal projection domain. J. Cell Biol. 1995, 131, 1327–1340. [Google Scholar] [CrossRef]
- Gauthier-Kemper, A.; Alonso, M.S.; Sündermann, F.; Niewidok, B.; Fernandez, M.-P.; Bakota, L.; Heinisch, J.J.; Brandt, R. Annexins A2 and A6 interact with the extreme N terminus of tau and thereby contribute to tau’s axonal localization. J. Biol. Chem. 2018, 293, 8065–8076. [Google Scholar] [CrossRef] [Green Version]
- Koerdt, S.N.; Gerke, V. Annexin A2 is involved in Ca 2+ -dependent plasma membrane repair in primary human endothelial cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 1046–1053. [Google Scholar] [CrossRef]
- Jones, E.M.; Dubey, M.; Camp, P.J.; Vernon, B.C.; Biernat, J.; Mandelkow, E.; Majewski, J.; Chi, E.Y. Interaction of Tau Protein with Model Lipid Membranes Induces Tau Structural Compaction and Membrane Disruption. Biochemistry 2012, 51, 2539–2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, W.S.; Jonas, A.; Clayton, D.F.; George, J.M. Stabilization of α-Synuclein Secondary Structure upon Binding to Synthetic Membranes. J. Biol. Chem. 1998, 273, 9443–9449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matharu, B.; Gibson, G.; Parsons, R.; Huckerby, T.N.; Moore, S.A.; Cooper, L.J.; Millichamp, R.; Allsop, D.; Austen, B. Galantamine inhibits β-amyloid aggregation and cytotoxicity. J. Neurol. Sci. 2009, 280, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Lin, H.; Lal, R. Fresh and nonfibrillar amyloid β protein(1–40) induces rapid cellular degeneration in aged human fibroblasts: Evidence for AβP-channel-mediated cellular toxicity. FASEB J. 2000, 14, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, Y.; Kozak, J.A.; Kayed, R.; Chanturiya, A.; Glabe, C.; Hall, J.E. Soluble Amyloid Oligomers Increase Bilayer Conductance by Altering Dielectric Structure. J. Gen. Physiol. 2006, 128, 637–647. [Google Scholar] [CrossRef] [Green Version]
- Flach, K.; Hilbrich, I.; Schiffmann, A.; Gärtner, U.; Krüger, M.; Leonhardt, M.; Waschipky, H.; Wick, L.; Arendt, T.; Holzer, M. Tau Oligomers Impair Artificial Membrane Integrity and Cellular Viability. J. Biol. Chem. 2012, 287, 43223. [Google Scholar] [CrossRef] [Green Version]
- Datta, D.; Leslie, S.N.; Wang, M.; Morozov, Y.M.; Yang, S.; Mentone, S.; Zeiss, C.; Duque, A.; Rakic, P.; Horvath, T.L.; et al. Age-related calcium dysregulation linked with tau pathology and impaired cognition in non-human primates. Alzheimer’s Dement. 2021, 17, 920–932. [Google Scholar] [CrossRef]
- van Rooijen, B.D.; Claessens, M.M.; Subramaniam, V. Lipid bilayer disruption by oligomeric α-synuclein depends on bilayer charge and accessibility of the hydrophobic core. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 1271–1278. [Google Scholar] [CrossRef] [Green Version]
- Caruana, M.; Neuner, J.; Högen, T.; Schmidt, F.; Kamp, F.; Scerri, C.; Giese, A.; Vassallo, N. Polyphenolic compounds are novel protective agents against lipid membrane damage by α-synuclein aggregates in vitro. Biochim. Biophys. Acta (BBA) Biomembr. 2012, 1818, 2502–2510. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, K.; Matsuzaki-Kobayashi, M.; Hasegawa, T.; Kikuchi, A.; Sugeno, N.; Itoyama, Y.; Wang, Y.; Yao, P.J.; Bushlin, I.; Takeda, A. Plasma membrane ion permeability induced by mutant alpha-synuclein contributes to the degeneration of neural cells. J. Neurochem. 2006, 97, 1071–1077. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, M.; Li, H.; Wei, T.; Tang, C.; Zhou, Y.; Long, X. Effects of Low-level Lipid Peroxidation on the Permeability of Nitroaromatic Molecules across a Membrane: A Computational Study. ACS Omega 2020, 5, 4798–4806. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Canevari, L.; Duchen, M.R. β-Amyloid Peptides Induce Mitochondrial Dysfunction and Oxidative Stress in Astrocytes and Death of Neurons through Activation of NADPH Oxidase. J. Neurosci. 2004, 24, 565–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadowaki, H.; Nishitoh, H.; Urano, F.; Sadamitsu, C.; Matsuzawa, A.; Takeda, K.; Masutani, H.; Yodoi, J.; Urano, Y.; Nagano, T.; et al. Amyloid β induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death Differ. 2004, 12, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoji, M.; Iwakami, N.; Takeuchi, S.; Waragai, M.; Suzuki, M.; Kanazawa, I.; Lippa, C.F.; Ono, S.; Okazawa, H. JNK activation is associated with intracellular β-amyloid accumulation. Mol. Brain Res. 2000, 85, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Dias-Santagata, D.; Fulga, T.A.; Duttaroy, A.; Feany, M.B. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J. Clin. Investig. 2007, 117, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Raina, A.K.; Rottkamp, C.A.; Aliev, G.; Perry, G.; Boux, H.; Smith, M.A. Activation and redistribution of c-Jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer’s disease. J. Neurochem. 2001, 76, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Deas, E.; Cremades, N.; Angelova, P.R.; Ludtmann, M.H.; Yao, Z.; Chen, S.; Horrocks, M.H.; Banushi, B.; Little, D.; Devine, M.J.; et al. Alpha-Synuclein Oligomers Interact with Metal Ions to Induce Oxidative Stress and Neuronal Death in Parkinson’s Disease. Antioxid. Redox Signal. 2016, 24, 376–391. [Google Scholar] [CrossRef] [Green Version]
- Scudamore, O.; Ciossek, T. Increased Oxidative Stress Exacerbates α-Synuclein Aggregation In Vivo. J. Neuropathol. Exp. Neurol. 2018, 77, 443–453. [Google Scholar] [CrossRef]
- Kagan, B.L.; Hirakura, Y.; Azimov, R.; Azimova, R.; Lin, M.-C. The channel hypothesis of Alzheimer’s disease: Current status. Peptides 2002, 23, 1311–1315. [Google Scholar] [CrossRef]
- Arispe, N.; Pollard, H.B.; Rojas, E. Giant Multilevel Cation Channels Formed by Alzheimer Disease Amyloid Beta Protein [ABP-(1-40)] in Bilayer Membranes. Proc. Natl. Acad. Sci. USA 1993, 90, 10573–10577. [Google Scholar] [CrossRef] [Green Version]
- Arispe, N.; Pollard, H.B.; Rojas, E. Zn2+ interaction with Alzheimer amyloid beta protein calcium channels. Proc. Natl. Acad. Sci. USA 1996, 93, 1710–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirakura, Y.; Lin, M.-C.; Kagan, B.L. Alzheimer amyloid aβ1-42 channels: Effects of solvent, pH, and congo red. J. Neurosci. Res. 1999, 57, 458–466. [Google Scholar] [CrossRef]
- Wu, C.; Scott, J.; Shea, J.-E. Binding of Congo Red to Amyloid Protofibrils of the Alzheimer Aβ9–40 Peptide Probed by Molecular Dynamics Simulations. Biophys. J. 2012, 103, 550–557. [Google Scholar] [CrossRef] [Green Version]
- Quist, A.; Doudevski, I.; Lin, H.; Azimova, R.; Ng, D.; Frangione, B.; Kagan, B.; Ghiso, J.; Lal, R. Amyloid ion channels: A common structural link for protein-misfolding disease. Proc. Natl. Acad. Sci. USA 2005, 102, 10427–10432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-Y.; Cho, M.-K.; Kumar, A.; Maier, E.; Siebenhaar, C.; Becker, S.; Fernandez, C.O.; Lashuel, H.A.; Benz, R.; Lange, A.; et al. Structural Properties of Pore-Forming Oligomers of α-Synuclein. J. Am. Chem. Soc. 2009, 131, 17482–17489. [Google Scholar] [CrossRef] [PubMed]
- Volles, M.J.; Lansbury, J.P.T. Vesicle Permeabilization by Protofibrillar α-Synuclein Is Sensitive to Parkinson’s Disease-Linked Mutations and Occurs by a Pore-like Mechanism. Biochemistry 2002, 41, 4595–4602. [Google Scholar] [CrossRef]
- Tsigelny, I.F.; Sharikov, Y.; Wrasidlo, W.; Gonzalez, T.; Desplats, P.A.; Crews, L.; Spencer, B.; Masliah, E. Role of α-synuclein penetration into the membrane in the mechanisms of oligomer pore formation. FEBS J. 2012, 279, 1000–1013. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, F.; Levin, J.; Kamp, F.; Kretzschmar, H.; Giese, A.; Bötzel, K. Single-Channel Electrophysiology Reveals a Distinct and Uniform Pore Complex Formed by α-Synuclein Oligomers in Lipid Membranes. PLoS ONE 2012, 7, e42545. [Google Scholar] [CrossRef] [Green Version]
- Kanekiyo, T.; Xu, H.; Bu, G. ApoE and Aβ in Alzheimer’s Disease: Accidental Encounters or Partners? Neuron 2014, 81, 740–754. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Kanekiyo, T.; Shinohara, M.; Zhang, Y.; LaDu, M.J.; Xu, H.; Bu, G. Differential Regulation of Amyloid-β Endocytic Trafficking and Lysosomal Degradation by Apolipoprotein E Isoforms. J. Biol. Chem. 2012, 287, 44593–44601. [Google Scholar] [CrossRef] [Green Version]
- Yajima, R.; Tokutake, T.; Koyama, A.; Kasuga, K.; Tezuka, T.; Nishizawa, M.; Ikeuchi, T. ApoE-isoform-dependent cellular uptake of amyloid-β is mediated by lipoprotein receptor LR11/SorLA. Biochem. Biophys. Res. Commun. 2015, 456, 482–488. [Google Scholar] [CrossRef]
- Nuriel, T.; Peng, K.Y.; Ashok, A.; Dillman, A.A.; Figueroa, H.Y.; Apuzzo, J.; Ambat, J.; Levy, E.; Cookson, M.R.; Mathews, P.M.; et al. The Endosomal–Lysosomal Pathway Is Dysregulated by APOE4 Expression in Vivo. Front. Neurosci. 2017, 11, 702. [Google Scholar] [CrossRef] [Green Version]
- Chua, X.Y.; Chong, J.R.; Cheng, A.L.; Lee, J.H.; Ballard, C.; Aarsland, D.; Francis, P.T.; Lai, M.K. Elevation of inactive cleaved annexin A1 in the neocortex is associated with amyloid, inflammatory and apoptotic markers in neurodegenerative dementias. Neurochem. Int. 2021, 152, 105251. [Google Scholar] [CrossRef]
- Chua, X.Y.; Chong, J.R.; Lee, J.; Attems, J.; Aarsland, D.; Francis, P.T.; Lai, M.K.P. Cleaved Annexin A1 is elevated in neurodegenerative dementia and is associated with pathological burden of amyloid and inflammatory cytokines. Alzheimer’s Dement. 2020, 16, e037636. [Google Scholar] [CrossRef]
- Li, C.; Ou, R.; Gu, X.; Hou, Y.; Chen, Y.; Wei, Q.; Zhang, L.; Lin, J.; Liu, K.; Huang, J.; et al. ANXA1 and the risk for early-onset Parkinson’s disease. Neurobiol. Aging 2022, 112, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Sohma, H.; Imai, S.-I.; Takei, N.; Honda, H.; Matsumoto, K.; Utsumi, K.; Matsuki, K.; Hashimoto, E.; Saito, T.; Kokai, Y. Evaluation of annexin A5 as a biomarker for Alzheimer’s disease and dementia with lewy bodies. Front. Aging Neurosci. 2013, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermes, I.; Steur, E.N.J.; Reutelingsperger, C.; Haanen, C. Decreased concentration of Annexin V in Parkinsonian cerebrospinal fluid: Speculation on the underlying cause. Mov. Disord. 1999, 14, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Q.; Davis-Turak, J.; Li, Y.; Karydas, A.M.; Hsu, S.C.; Sears, R.; Chatzopoulou, D.; Huang, A.Y.; Wojta, K.J.; et al. A Multiancestral Genome-Wide Exome Array Study of Alzheimer Disease, Frontotemporal Dementia, and Progressive Supranuclear Palsy. JAMA Neurol. 2015, 72, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Galvin, J.E.; Palamand, D.; Strider, J.; Milone, M.; Pestronk, A. The muscle protein dysferlin accumulates in the Alzheimer brain. Acta Neuropathol. 2006, 112, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Zhou, X.; Wang, Y.; Li, Z.; Wang, Y.; Shi, J.; Guan, F. MG53 protein rejuvenates hUC-MSCs and facilitates their therapeutic effects in AD mice by activating Nrf2 signaling pathway. Redox Biol. 2022, 53, 102325. [Google Scholar] [CrossRef]
- Ries, M.; Watts, H.; Mota, B.C.; Lopez, M.Y.; Donat, C.K.; Baxan, N.; Pickering, J.A.; Chau, T.W.; Semmler, A.; Gurung, B.; et al. Annexin A1 restores cerebrovascular integrity concomitant with reduced amyloid-β and tau pathology. Brain 2021, 144, 1526–1541. [Google Scholar] [CrossRef] [PubMed]
- Mina, E.W.; Lasagna-Reeves, C.; Glabe, C.G.; Kayed, R. Poloxamer 188 Copolymer Membrane Sealant Rescues Toxicity of Amyloid Oligomers In Vitro. J. Mol. Biol. 2009, 391, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R.; Di Meco, A.; Kemal, S.; Popovic, J.; Chandra, S.; Sadleir, K.R. Poloxamer-188 Exacerbates Brain Amyloidosis, Presynaptic Dystrophies, and Pathogenic Microglial Activation in 5XFAD Mice. Curr. Alzheimer Res. 2022, 19, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Demonbreun, A.; Fallon, K.; Oosterbaan, C.C.; Bogdanovic, E.; Warner, J.L.; Sell, J.J.; Page, P.G.; Quattrocelli, M.; Barefield, D.Y.; McNally, E.M. Recombinant annexin A6 promotes membrane repair and protects against muscle injury. J. Clin. Investig. 2019, 129, 4657–4670. [Google Scholar] [CrossRef] [Green Version]
- Fullenkamp, D.E.; Willis, A.B.; Curtin, J.L.; Amaral, A.P.; Harris, S.I.; Burridge, P.W.; Demonbreun, A.R.; McNally, E.M. Recombinant annexin A6 promotes membrane repair in a stem cell derived-cardiomyocyte model of dystrophic cardiomyopathy. bioRxiv 2022. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Wang, J.-Y.; Kausik, R.; Lee, K.Y.C.; Han, S. Nature of interactions between PEO-PPO-PEO triblock copolymers and lipid membranes: (II) role of hydration dynamics revealed by dynamic nuclear polarization. Biomacromolecules 2012, 13, 2624–2633. [Google Scholar] [CrossRef] [Green Version]
- Moloughney, J.G.; Weisleder, N. Poloxamer 188 (P188) as a Membrane Resealing Reagent in Biomedical Applications. Recent Pat. Biotechnol. 2012, 6, 200–211. [Google Scholar] [CrossRef]
- Ng, R.; Metzger, J.M.; Claflin, D.R.; Faulkner, J.A.; Sloboda, D.D.; Brooks, S.V.; Call, J.A.; Eckhoff, M.D.; Baltgalvis, K.A.; Warren, G.L.; et al. Poloxamer 188 reduces the contraction-induced force decline in lumbrical muscles from mdx mice. Am. J. Physiol. Physiol. 2008, 295, C146–C150. [Google Scholar] [CrossRef] [Green Version]
- Spurney, C.F.; Guerron, A.D.; Yu, Q.; Sali, A.; van der Meulen, J.H.; Hoffman, E.P.; Nagaraju, K. Membrane Sealant Poloxamer P188 Protects Against Isoproterenol Induced Cardiomyopathy in Dystrophin Deficient Mice. BMC Cardiovasc. Disord. 2011, 11, 20. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, T.A.; Rose, A.L.; Jung, R.; Capati, A.; Hallak, D.; Yan, R.; Weisleder, N. Multiple poloxamers increase plasma membrane repair capacity in muscle and nonmuscle cells. Am. J. Physiol. Physiol. 2020, 318, C253–C262. [Google Scholar] [CrossRef]
- D’Amico, M.; Di Filippo, C.; La, M.; Solito, E.; McLean, P.G.; JFlower, R.J.; Oliani, S.M.; Perretti, M. Lipocortin 1 reduces myocardial ischemia-reperfusion injury by affecting local leukocyte recruitment. FASEB J. 2000, 14, 1867–1869. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wang, X.; Jiang, Y.; Li, Y.; Liao, Z.; Li, W.; Yu, Z.; Whalen, M.J.; Lok, J.; Dumont, A.S.; et al. Recombinant Annexin A2 Administration Improves Outcomes After Traumatic Brain Injury in Mice. Front. Pharmacol. 2021, 12, 708469. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-E.; Liao, W.-I.; Pao, H.-P.; Hsu, C.-W.; Wu, S.-Y.; Huang, K.-L.; Chu, S.-J. Poloxamer 188 Attenuates Ischemia-Reperfusion-Induced Lung Injury by Maintaining Cell Membrane Integrity and Inhibiting Multiple Signaling Pathways. Front. Pharmacol. 2021, 12, 650573. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-H.; Ge, J.-B.; Li, M.; Xu, H.-D.; Wu, F.; Qin, Z.-H. Poloxamer 188 protects neurons against ischemia/reperfusion injury through preserving integrity of cell membranes and blood brain barrier. PLoS ONE 2013, 8, e61641. [Google Scholar] [CrossRef] [PubMed]

| Organ/Tissue | Type of Damage | Cell Type | References |
|---|---|---|---|
| Skeletal muscle | Aperiodic, eccentric contractions, saponin, chronic damage (e.g., muscular dystrophy) | Myocytes, myotubes | Cooper and McNeil (2015) [45], Carmeille et al. (2016) [30], Defour et al. (2014) [36] |
| Cardiac muscle | Ischemia/reperfusion injury | Cardiomyocytes | Houang et al. (2019) [11], Han et al. (2007) [12] |
| Skin | Aperiodic | Epidermal cells, fibroblasts, etc. | McNeil and Ito (1990) [46], Reddy et al. (2001) [47] |
| Gastrointestinal tract | Cyclic | Epithelial cells, smooth muscle cells | McNeil and Ito (1989) [43], Ammendolia et al. (2021) [48] |
| data | |||
| Respiratory | Stretch, overventilation, saponin | Epithelial cells, endothelial cells, smooth muscle cells | Ammendolia et al. (2021) [48], Cong et al. (2017) [49], Cong et al. (2020) [13] |
| Peripheral Nervous System | Crush injury, nerve transection | Sciatic nerve, Schwann cells | Paleo et al. (2020) [44], Rigonia and Negro (2020) [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulgart, H.R.; Goncalves, I.; Weisleder, N. Leveraging Plasma Membrane Repair Therapeutics for Treating Neurodegenerative Diseases. Cells 2023, 12, 1660. https://doi.org/10.3390/cells12121660
Bulgart HR, Goncalves I, Weisleder N. Leveraging Plasma Membrane Repair Therapeutics for Treating Neurodegenerative Diseases. Cells. 2023; 12(12):1660. https://doi.org/10.3390/cells12121660
Chicago/Turabian StyleBulgart, Hannah R., Isabella Goncalves, and Noah Weisleder. 2023. "Leveraging Plasma Membrane Repair Therapeutics for Treating Neurodegenerative Diseases" Cells 12, no. 12: 1660. https://doi.org/10.3390/cells12121660
APA StyleBulgart, H. R., Goncalves, I., & Weisleder, N. (2023). Leveraging Plasma Membrane Repair Therapeutics for Treating Neurodegenerative Diseases. Cells, 12(12), 1660. https://doi.org/10.3390/cells12121660







