Is Cutaneous T-Cell Lymphoma Caused by Ultraviolet Radiation? A Comparison of UV Mutational Signatures in Malignant Melanoma and Mycosis Fungoides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Data Analysis
3. Results
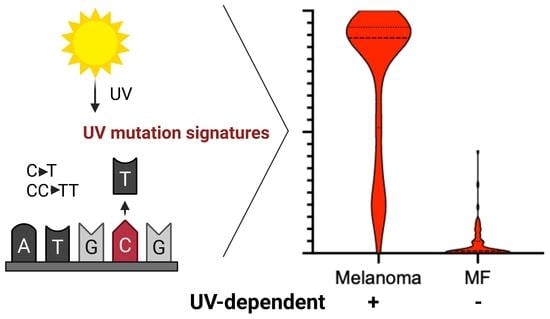
3.1. UV Mutational Signature Is Dominant in Malignant Melanoma
3.2. UV Mutations in Mycosis Fungoides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cerroni, L. Mycosis Fungoides-Clinical and Histopathologic Features, Differential Diagnosis, and Treatment. Semin. Cutan. Med. Surg. 2018, 37, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.J.; Clark, R.A.; Watanabe, R.; Kupper, T.S. Sezary Syndrome and Mycosis Fungoides Arise from Distinct T-Cell Subsets: A Biologic Rationale for Their Distinct Clinical Behaviors. Blood 2010, 116, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Hennessey, D.; O’Keefe, S.; Patterson, J.; Wang, W.; Wong, G.K.-S.; Gniadecki, R. Skin Colonization by Circulating Neoplastic Clones in Cutaneous T-Cell Lymphoma. Blood 2019, 134, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Hennessey, D.; Gniadecki, R. Clonotype Pattern in T-Cell Lymphomas Map the Cell of Origin to Immature Lymphoid Precursors. Blood Adv. 2022, 6, 2334–2345. [Google Scholar] [CrossRef]
- Ziegler, A.; Jonason, A.S.; Leffell, D.J.; Simon, J.A.; Sharma, H.W.; Kimmelman, J.; Remington, L.; Jacks, T.; Brash, D.E. Sunburn and p53 in the Onset of Skin Cancer. Nature 1994, 372, 773–776. [Google Scholar] [CrossRef]
- Brash, D.E. UV Signature Mutations. Photochem. Photobiol. 2015, 91, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Dousset, L.; Poizeau, F.; Robert, C.; Mansard, S.; Mortier, L.; Caumont, C.; Routier, É.; Dupuy, A.; Rouanet, J.; Battistella, M.; et al. Positive Association Between Location of Melanoma, Ultraviolet Signature, Tumor Mutational Burden, and Response to Anti-PD-1 Therapy. JCO Precis. Oncol. 2021, 5, 1821–1829. [Google Scholar] [CrossRef]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.-M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-Genome Landscapes of Major Melanoma Subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef]
- Adami, J.; Frisch, M.; Yuen, J.; Glimelius, B.; Melbye, M. Evidence of an Association between Non-Hodgkin’s Lymphoma and Skin Cancer. BMJ 1995, 310, 1491–1495. [Google Scholar] [CrossRef] [Green Version]
- Morales-Suárez-Varela, M.M.; Olsen, J.; Johansen, P.; Kaerlev, L.; Guénel, P.; Arveux, P.; Wingren, G.; Hardell, L.; Ahrens, W.; Stang, A.; et al. Occupational Sun Exposure and Mycosis Fungoides: A European Multicenter Case-Control Study. J. Occup. Environ. Med. 2006, 48, 390–393. [Google Scholar] [CrossRef]
- DeStefano, C.B.; Desale, S.; Fernandez, S.J.; Shenoy, A.G. The Impact of Environmental Ultraviolet Exposure on the Clinical Course of Mycosis Fungoides. J. Am. Acad. Dermatol. 2019, 81, 1074–1077. [Google Scholar] [CrossRef]
- Newton, R. Solar Ultraviolet Radiation Is Not a Major Cause of Primary Cutaneous Non-Hodgkin’s Lymphoma. BMJ 1997, 314, 1483–1484. [Google Scholar] [CrossRef] [Green Version]
- McGregor, J.M.; Crook, T.; Fraser-Andrews, E.A.; Rozycka, M.; Crossland, S.; Brooks, L.; Whittaker, S.J. Spectrum of p53 Gene Mutations Suggests a Possible Role for Ultraviolet Radiation in the Pathogenesis of Advanced Cutaneous Lymphomas. J. Investig. Dermatol. 1999, 112, 317–321. [Google Scholar] [CrossRef]
- Jones, C.L.; Degasperi, A.; Grandi, V.; Amarante, T.D.; Genomics England Research Consortium; Mitchell, T.J.; Nik-Zainal, S.; Whittaker, S.J. Spectrum of Mutational Signatures in T-Cell Lymphoma Reveals a Key Role for UV Radiation in Cutaneous T-Cell Lymphoma. Sci. Rep. 2021, 11, 3962. [Google Scholar] [CrossRef]
- Wooler, G.; Melchior, L.; Ralfkiaer, E.; Rahbek Gjerdrum, L.M.; Gniadecki, R. Gene Status Affects Survival in Advanced Mycosis Fungoides. Front. Med. 2016, 3, 51. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [Green Version]
- ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium Pan-Cancer Analysis of Whole Genomes. Nature 2020, 578, 82–93. [CrossRef] [Green Version]
- Iyer, A.; Hennessey, D.; O’Keefe, S.; Patterson, J.; Wang, W.; Salopek, T.; Wong, G.K.-S.; Gniadecki, R. Clonotypic Heterogeneity in Cutaneous T-Cell Lymphoma (mycosis Fungoides) Revealed by Comprehensive Whole-Exome Sequencing. Blood Adv. 2019, 3, 1175–1184. [Google Scholar] [CrossRef] [Green Version]
- SignatureAnalyzer. Available online: https://github.com/broadinstitute/SignatureAnalyzer-GPU (accessed on 12 June 2023).
- Pfeifer, G.P.; You, Y.-H.; Besaratinia, A. Mutations Induced by Ultraviolet Light. Mutat. Res. 2005, 571, 19–31. [Google Scholar] [CrossRef]
- Iyer, A.; Hennessey, D.; O’Keefe, S.; Patterson, J.; Wang, W.; Wong, G.K.-S.; Gniadecki, R. Branched Evolution and Genomic Intratumor Heterogeneity in the Pathogenesis of Cutaneous T-Cell Lymphoma. Blood Adv. 2020, 4, 2489–2500. [Google Scholar] [CrossRef]
- Sivanand, A.; Hennessey, D.; Iyer, A.; O’Keefe, S.; Surmanowicz, P.; Vaid, G.; Xiao, Z.; Gniadecki, R. The Neoantigen Landscape of Mycosis Fungoides. Front. Immunol. 2020, 11, 561234. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.Z.X.; Hennessey, D.; Iyer, A.; O’Keefe, S.; Zhang, F.; Sivanand, A.; Gniadecki, R. Transcriptomic Changes during Stage Progression of Mycosis Fungoides. Br. J. Dermatol. 2021, 186, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Shi, X.; Lan, S.; Jin, H.; Wu, D. Effect of Melanoma Stem Cells on Melanoma Metastasis. Oncol. Lett. 2021, 22, 566. [Google Scholar] [CrossRef] [PubMed]
- Schatton, T.; Frank, M.H. Cancer Stem Cells and Human Malignant Melanoma. Pigment. Cell Melanoma Res. 2008, 21, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Schatton, T.; Murphy, G.F.; Frank, N.Y.; Yamaura, K.; Waaga-Gasser, A.M.; Gasser, M.; Zhan, Q.; Jordan, S.; Duncan, L.M.; Weishaupt, C.; et al. Identification of Cells Initiating Human Melanomas. Nature 2008, 451, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Peterson, S.C.; Eberl, M.; Vagnozzi, A.N.; Belkadi, A.; Veniaminova, N.A.; Verhaegen, M.E.; Bichakjian, C.K.; Ward, N.L.; Dlugosz, A.A.; Wong, S.Y. Basal Cell Carcinoma Preferentially Arises from Stem Cells within Hair Follicle and Mechanosensory Niches. Cell Stem Cell 2015, 16, 400–412. [Google Scholar] [CrossRef] [Green Version]
- Grachtchouk, M.; Pero, J.; Yang, S.H.; Ermilov, A.N.; Michael, L.E.; Wang, A.; Wilbert, D.; Patel, R.M.; Ferris, J.; Diener, J.; et al. Basal Cell Carcinomas in Mice Arise from Hair Follicle Stem Cells and Multiple Epithelial Progenitor Populations. J. Clin. Investig. 2011, 121, 1768–1781. [Google Scholar] [CrossRef] [Green Version]
- Jian, Z.; Strait, A.; Jimeno, A.; Wang, X.-J. Cancer Stem Cells in Squamous Cell Carcinoma. J. Investig. Dermatol. 2017, 137, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, X.; Parmentier, L.; King, B.; Bezrukov, F.; Kaya, G.; Zoete, V.; Seplyarskiy, V.B.; Sharpe, H.J.; McKee, T.; Letourneau, A.; et al. Genomic Analysis Identifies New Drivers and Progression Pathways in Skin Basal Cell Carcinoma. Nat. Genet. 2016, 48, 398–406. [Google Scholar] [CrossRef]
- Martincorena, I.; Roshan, A.; Gerstung, M.; Ellis, P.; Van Loo, P.; McLaren, S.; Wedge, D.C.; Fullam, A.; Alexandrov, L.B.; Tubio, J.M.; et al. Tumor Evolution. High Burden and Pervasive Positive Selection of Somatic Mutations in Normal Human Skin. Science 2015, 348, 880–886. [Google Scholar] [CrossRef] [Green Version]
- Royer-Bertrand, B.; Torsello, M.; Rimoldi, D.; El Zaoui, I.; Cisarova, K.; Pescini-Gobert, R.; Raynaud, F.; Zografos, L.; Schalenbourg, A.; Speiser, D.; et al. Comprehensive Genetic Landscape of Uveal Melanoma by Whole-Genome Sequencing. Am. J. Hum. Genet. 2016, 99, 1190–1198. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.; Van der Weyden, L.; Schott, C.R.; Foote, A.; Constantino-Casas, F.; Smith, S.; Dobson, J.M.; Murchison, E.P.; Wu, H.; Yeh, I.; et al. Cross-Species Genomic Landscape Comparison of Human Mucosal Melanoma with Canine Oral and Equine Melanoma. Nat. Commun. 2019, 10, 353. [Google Scholar] [CrossRef]
- Yang, C.; Sanchez-Vega, F.; Chang, J.C.; Chatila, W.K.; Shoushtari, A.N.; Ladanyi, M.; Travis, W.D.; Busam, K.J.; Rekhtman, N. Lung-Only Melanoma: UV Mutational Signature Supports Origin from Occult Cutaneous Primaries and Argues against the Concept of Primary Pulmonary Melanoma. Mod. Pathol. 2020, 33, 2244–2255. [Google Scholar] [CrossRef]
- Clark, R.A. Resident Memory T Cells in Human Health and Disease. Sci. Transl. Med. 2015, 7, 269rv1. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.A.; Watanabe, R.; Teague, J.E.; Schlapbach, C.; Tawa, M.C.; Adams, N.; Dorosario, A.A.; Chaney, K.S.; Cutler, C.S.; Leboeuf, N.R.; et al. Skin Effector Memory T Cells Do Not Recirculate and Provide Immune Protection in Alemtuzumab-Treated CTCL Patients. Sci. Transl. Med. 2012, 4, 117ra7. [Google Scholar] [CrossRef] [Green Version]
- Wulf, H.C.; Sandby-Møller, J.; Kobayasi, T.; Gniadecki, R. Skin Aging and Natural Photoprotection. Micron 2004, 35, 185–191. [Google Scholar] [CrossRef]
- Gniadecka, M.; Wulf, H.C.; Mortensen, N.N.; Poulsen, T. Photoprotection in Vitiligo and Normal Skin. A Quantitative Assessment of the Role of Stratum Corneum, Viable Epidermis and Pigmentation. Acta Derm. Venereol. 1996, 76, 429–432. [Google Scholar]
- Lindberg, M.; Boström, M.; Elliott, K.; Larsson, E. Intragenomic Variability and Extended Sequence Patterns in the Mutational Signature of Ultraviolet Light. Proc. Natl. Acad. Sci. USA 2019, 116, 20411–20417. [Google Scholar] [CrossRef] [Green Version]




| All | ESP 1 | LSP 1 | TMR 1 | |
|---|---|---|---|---|
| Patients (number) | 35 | |||
| Female (%) | 25.70% | |||
| White (%) | 96% | |||
| Age (mean [range]) | 68 (47–90) | |||
| Samples (n) | 61 | 27 | 14 | 20 |
| IA | 6 | 6 | NA | NA |
| IB | 21 | 21 | NA | NA |
| IIB | 28 | NA | 11 | 17 |
| III | 2 | NA | 1 | 1 |
| IVA | 4 | NA | 2 | 2 |
| Primary Tumor | Metastases | N/A | Total | |||
|---|---|---|---|---|---|---|
| Regional 1 | Lymph Node | Distant | ||||
| Patients (number) | 63 | 61 | 170 | 43 | 3 | 340 |
| Female (%) | 36.5 | 37.7 | 37.1 | 39.5 | 33.3 | 37.4 |
| White (%) | 93.7 | 98.4 | 98.8 | 95.3 | 100.0 | 97.4 |
| Age (mean [range]) | 63 (24–90) | 57 (19–81) | 54 (15–87) | 58 (25–85) | 74 (68–83) | 61 (15–90) |
| Stage 1 (%) | 0.0 | 16.4 | 27.1 | 16.3 | 0.0 | 18.5 |
| Stage 2 (%) | 54.0 | 23.0 | 14.7 | 27.9 | 33.3 | 25.3 |
| Stage 3 (%) | 36.5 | 39.3 | 43.5 | 25.6 | 66.7 | 39.4 |
| Stage 4 (%) | 4.8 | 6.6 | 2.9 | 14.0 | 0.0 | 5.3 |
| Stage N/A (%) | 4.8 | 14.8 | 11.8 | 16.3 | 0.0 | 11.5 |
| Head and neck (%) | 9.5 | 3.3 | 6.5 | 11.6 | 33.3 | 7.4 |
| Trunk (%) | 42.9 | 37.7 | 35.3 | 27.9 | 33.3 | 36.2 |
| Extremities (%) | 41.3 | 42.6 | 42.4 | 44.2 | 0.0 | 42.1 |
| N/A and other (%) | 6.3 | 16.4 | 15.9 | 16.3 | 33.3 | 14.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gniadecki, R.; O’Keefe, S.; Hennessey, D.; Iyer, A. Is Cutaneous T-Cell Lymphoma Caused by Ultraviolet Radiation? A Comparison of UV Mutational Signatures in Malignant Melanoma and Mycosis Fungoides. Cells 2023, 12, 1616. https://doi.org/10.3390/cells12121616
Gniadecki R, O’Keefe S, Hennessey D, Iyer A. Is Cutaneous T-Cell Lymphoma Caused by Ultraviolet Radiation? A Comparison of UV Mutational Signatures in Malignant Melanoma and Mycosis Fungoides. Cells. 2023; 12(12):1616. https://doi.org/10.3390/cells12121616
Chicago/Turabian StyleGniadecki, Robert, Sandra O’Keefe, Dylan Hennessey, and Aishwarya Iyer. 2023. "Is Cutaneous T-Cell Lymphoma Caused by Ultraviolet Radiation? A Comparison of UV Mutational Signatures in Malignant Melanoma and Mycosis Fungoides" Cells 12, no. 12: 1616. https://doi.org/10.3390/cells12121616
APA StyleGniadecki, R., O’Keefe, S., Hennessey, D., & Iyer, A. (2023). Is Cutaneous T-Cell Lymphoma Caused by Ultraviolet Radiation? A Comparison of UV Mutational Signatures in Malignant Melanoma and Mycosis Fungoides. Cells, 12(12), 1616. https://doi.org/10.3390/cells12121616