The Multi-Faceted Nature of Renalase for Mitochondrial Dysfunction Improvement in Cardiac Disease
Abstract
:1. Introduction
2. Mitochondrial Pathophysiology within the Concept of Cardiac Disease
3. The Molecular Signature of Renalase
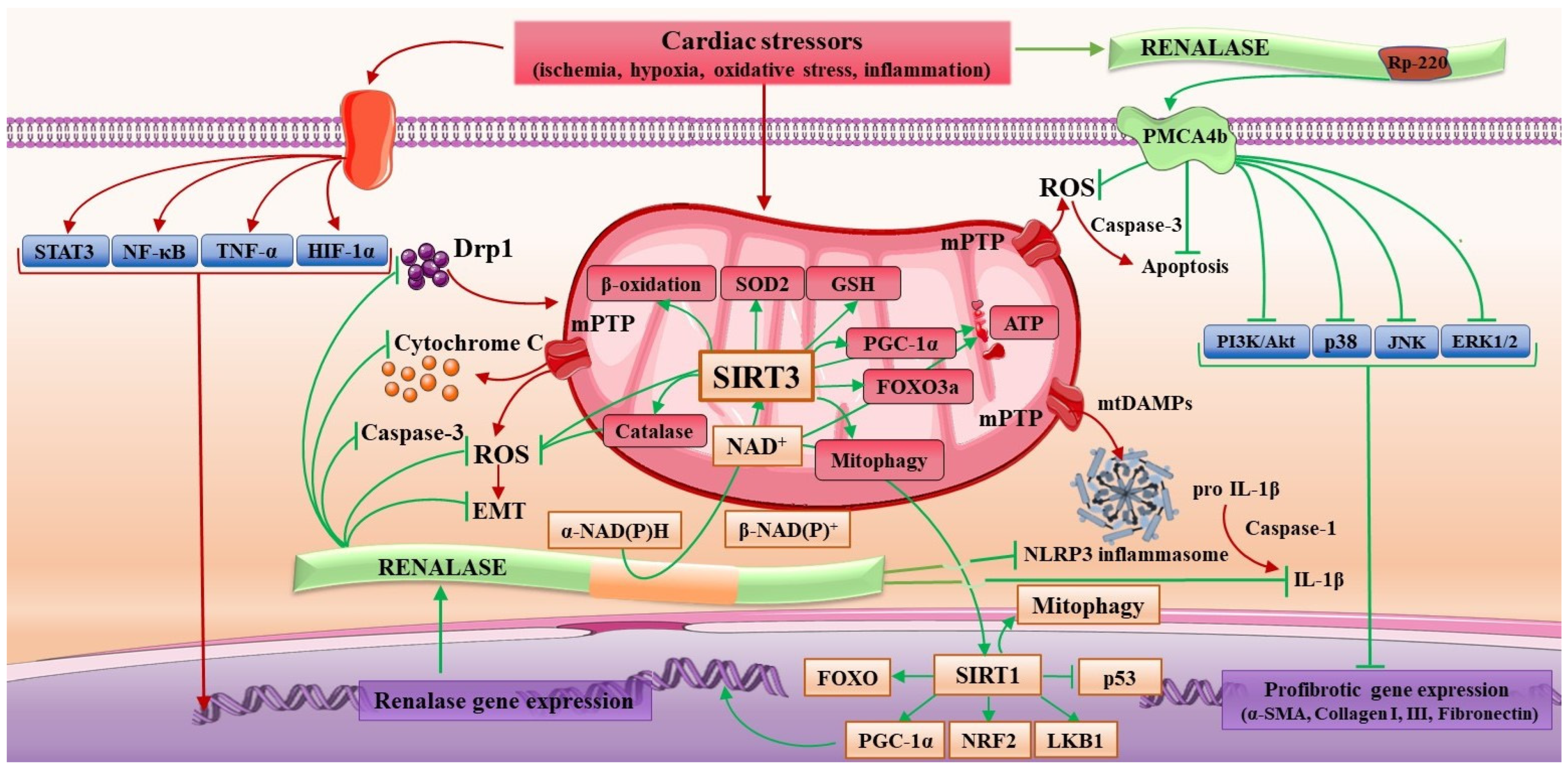
4. The Network of Renalase/NAD+/Sirtuins in Regard to Mitochondrial Dysfunction within Cardiac Disease
5. The Interplay of Renalase and ROS in Regard to Mitochondrial Dysfunction in Cardiac Disease
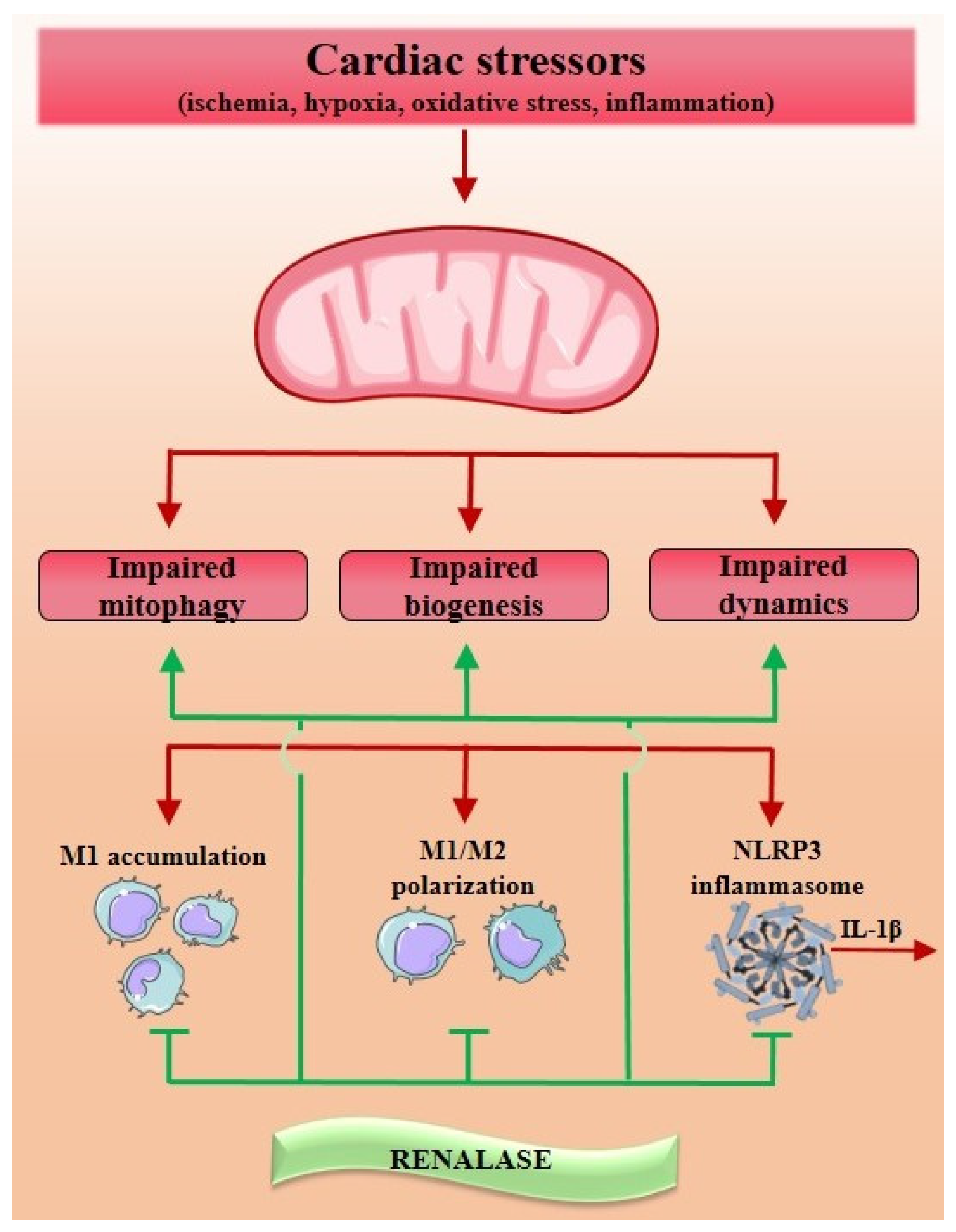
6. The Intertwist of Renalase and Inflammation in Regard to Mitochondrial Dysfunction in Cardiac Disease
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Alizadeh, G.; Gholipour, K.; Azami-Aghdash, S.; Dehnavieh, R.; JafarAbadi, M.A.; Azmin, M.; Khodayari-Zarnaq, R. Social, Economic, Technological, and Environmental Factors Affecting Cardiovascular Diseases: A Systematic Review and Thematic Analysis. Int. J. Prev. Med. 2022, 13, 78. [Google Scholar]
- Taylor, C.J.; Ordóñez-Mena, J.M.; Roalfe, A.K.; Lay-Flurrie, S.; Jones, N.R.; Marshall, T.; Hobbs, F.D.R. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: Population based cohort study. BMJ 2019, 364, 1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Vinas, A.; Corral-Partearroyo, C.; Gil-Girbau, M.; Penarrubia-Maria, M.T.; Gallardo-Gonzalez, C.; Olmos-Palenzuela, M.D.; Aznar-Lou, I.; Serrano-Blanco, A.; Rubio-Valera, M. Effectiveness and cost-effectiveness of an intervention to improve Initial Medication Adherence to treatments for cardiovascular diseases and diabetes in primary care: Study protocol for a pragmatic cluster randomised controlled trial and economic model (the IMA-cRCT study). BMC Prim. Care 2022, 23, 170. [Google Scholar]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef] [Green Version]
- Ciccarelli, G.; Conte, S.; Cimmino, G.; Maiorano, P.; Morrione, A.; Giordano, A. Mitochondrial Dysfunction: The Hidden Player in the Pathogenesis of Atherosclerosis? Int. J. Mol. Sci. 2023, 24, 1086. [Google Scholar] [CrossRef]
- Yang, M.; Linn, B.S.; Zhang, Y.; Ren, J. Mitophagy and mitochondrial integrity in cardiac ischemia-reperfusion injury. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 186, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz-Bahaghighat, H.; Darwesh, A.M.; Sosnowski, D.K.; Seubert, J.M. Mitochondrial Dysfunction and Inflammaging in Heart Failure: Novel Roles of CYP-Derived Epoxylipids. Cells 2020, 9, 1565. [Google Scholar] [CrossRef]
- Chang, X.; Liu, R.; Li, R.; Peng, Y.; Zhu, P.; Zhou, H. Molecular Mechanisms of Mitochondrial Quality Control in Ischemic Cardiomyopathy. Int. J. Biol. Sci. 2023, 19, 426–448. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Q.; Feng, X.; Liu, Y.; Zhou, Y. Mitochondrial Dysfunction in Cardiovascular Diseases: Potential Targets for Treatment. Front. Cell Dev. Biol. 2022, 10, 841523. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Dang, Q.Y.; Fan, X.R.; Han, C.T.; Xu, S.Z.; Li, P.Y. Assessment of mitochondrial dysfunction and implications in cardiovascular disorders. Life Sci. 2022, 306, 120834. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lv, J.; Pan, Z.; Wang, D.; Zhao, L.; Guo, X. Mitochondrial dysfunction in heart failure and its therapeutic implications. Front. Cardiovasc. Med. 2022, 9, 945142. [Google Scholar] [CrossRef]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Zeng, Z.L.; Yang, S.; Li, A.; Zu, X.; Liu, J. Mitochondrial Stress in Metabolic Inflammation: Modest Benefits and Full Losses. Oxid. Med. Cell. Longev. 2022, 2022, 8803404. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.L.; Chen, S.D.; Lin, K.J.; Liou, C.W.; Chuang, Y.C.; Wang, P.W.; Chuang, J.H.; Lin, T.K. Quality Matters? The Involvement of Mitochondrial Quality Control in Cardiovascular Disease. Front. Cell. Dev. Biol. 2021, 9, 636295. [Google Scholar] [CrossRef] [PubMed]
- Kiyuna, L.A.; Albuquerque, R.P.E.; Chen, C.H.; Mochly-Rosen, D.; Ferreira, J.C.B. Targeting mitochondrial dysfunction and oxidative stress in heart failure: Challenges and opportunities. Free Radic. Biol. Med. 2018, 129, 155–168. [Google Scholar] [CrossRef]
- Wang, Q.; Su, H.; Liu, J. Protective Effect of Natural Medicinal Plants on Cardiomyocyte Injury in Heart Failure: Targeting the Dysregulation of Mitochondrial Homeostasis and Mitophagy. Oxid. Med. Cell. Longev. 2022, 2022, 3617086. [Google Scholar] [CrossRef]
- Banoth, B.; Cassel, S.L. Mitochondria in innate immune signaling. Transl. Res. 2018, 202, 52–68. [Google Scholar] [CrossRef]
- Song, L.; Liang, J.; Wang, W.; Gao, J.; Chai, H.; Tan, Y.; Zheng, L.; Xue, M.; Shi, D. Global Trends in Research of Mitochondrial Biogenesis over past 20 Years: A Bibliometric Analysis. Oxid. Med. Cell. Longev. 2023, 2023, 7291284. [Google Scholar] [CrossRef]
- Anderson, E.J.; Lustig, M.E.; Boyle, K.E.; Woodlief, T.L.; Kane, D.A.; Lin, C.; Price, J.W., 3rd; Kang, L.; Rabinovitch, P.S.; Szeto, H.H.; et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009, 3, 573–581. [Google Scholar] [CrossRef]
- Raimundo, N. Mitochondrial pathology: Stress signals from the energy factory. Trends Mol. Med. 2014, 20, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Shemiakova, T.; Ivanova, E.; Wu, W.K.; Kirichenko, T.V.; Starodubova, A.V.; Orekhov, A.N. Atherosclerosis as Mitochondriopathy: Repositioning the Disease to Help Finding New Therapies. Front. Cardiovasc. Med. 2021, 8, 660473. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, D.D.L.; Garcia, A.A.; Lee, L.; Mochly-Rosen, D.; Ferreira, J.C.B. Mitochondrial Fusion, Fission, and Mitophagy in Cardiac Diseases: Challenges and Therapeutic Opportunities. Antioxid. Redox Signal. 2022, 36, 844–886. [Google Scholar] [CrossRef] [PubMed]
- Ranjbarvaziri, S.; Kooiker, K.B.; Ellenberger, M.; Fajardo, G.; Zhao, M.; Roest, A.S.V.; Woldeyes, R.A.; Koyano, T.T.; Fong, R.; Ma, N.; et al. Altered Cardiac Energetics and Mitochondrial Dysfunction in Hypertrophic Cardiomyopathy. Circulation 2021, 144, 1714–1731. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, G.; Wang, P.; Velazquez, H.; Yao, X.; Li, Y.; Wu, Y.; Peixoto, A.; Crowley, S.; Desir, G.V. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J. Clin. Investig. 2005, 115, 1275–1280. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Desir, G.V. Renalase, a new renal hormone: Its role in health and disease. Curr. Opin. Nephrol. Hypertens. 2007, 16, 373–378. [Google Scholar] [CrossRef]
- Desir, G.V.; Wang, L.; Peixoto, A.J. Human renalase: A review of its biology, function, and implications for hypertension. J. Am. Soc. Hypertens. 2012, 6, 417–426. [Google Scholar] [CrossRef]
- Li, H.; Guo, J.; Liu, H.; Niu, Y.; Wang, L.; Huang, K.; Wang, J. Renalase as a Novel Biomarker for Evaluating the Severity of Hepatic Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2016, 2016, 3178562. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Gu, J.; Guo, J.; Chen, K.; Li, H.; Wang, J. Renalase Attenuates Mouse Fatty Liver Ischemia/Reperfusion Injury through Mitigating Oxidative Stress and Mitochondrial Damage via Activating SIRT1. Oxid. Med. Cell. Longev. 2019, 2019, 7534285. [Google Scholar] [CrossRef] [Green Version]
- Tokinoya, K.; Sekine, N.; Aoki, K.; Ono, S.; Kuji, T.; Sugasawa, T.; Yoshida, Y.; Takekoshi, K. Effects of renalase deficiency on liver fibrosis markers in a nonalcoholic steatohepatitis mouse model. Mol. Med. Rep. 2021, 23, 210. [Google Scholar] [CrossRef]
- Aoki, K.; Yanazawa, K.; Tokinoya, K.; Sugasawa, T.; Suzuki, T.; Yoshida, Y.; Nakano, T.; Omi, N.; Kawakami, Y.; Takekoshi, K. Renalase is localized to the small intestine crypt and expressed upon the activation of NF-kappaB p65 in mice model of fasting-induced oxidative stress. Life Sci. 2021, 267, 118904. [Google Scholar] [CrossRef]
- Kolodecik, T.R.; Reed, A.M.; Date, K.; Shugrue, C.A.; Patel, V.; Chung, S.L.; Desir, G.V.; Gorelick, F.S. The serum protein renalase reduces injury in experimental pancreatitis. J. Biol. Chem. 2017, 292, 21047–21059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Wang, M.; Guo, X.; Hu, J.; Chen, T.M.; Finn, S.M.B.; Lacy, J.; Kunstman, J.W.; Cha, C.H.; Bellin, M.D.; et al. Renalase is a novel tissue and serological biomarker in pancreatic ductal adenocarcinoma. PLoS ONE 2021, 16, e0250539. [Google Scholar] [CrossRef] [PubMed]
- Fedchenko, V.; Globa, A.; Buneeva, O.; Medvedev, A. Renalase mRNA levels in the brain, heart, and kidneys of spontaneously hypertensive rats with moderate and high hypertension. Med. Sci. Monit. Basic Res. 2013, 19, 267–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokinoya, K.; Yoshida, Y.; Sugasawa, T.; Takekoshi, K. Moderate-intensity exercise increases renalase levels in the blood and skeletal muscle of rats. FEBS Open Bio 2020, 10, 1005–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokinoya, K.; Shiromoto, J.; Sugasawa, T.; Yoshida, Y.; Aoki, K.; Nakagawa, Y.; Ohmori, H.; Takekoshi, K. Influence of acute exercise on renalase and its regulatory mechanism. Life Sci. 2018, 210, 235–242. [Google Scholar] [CrossRef]
- Luo, M.; Cao, S.; Lv, D.; He, L.; He, Z.; Li, L.; Li, Y.; Luo, S.; Chang, Q. Aerobic Exercise Training Improves Renal Injury in Spontaneously Hypertensive Rats by Increasing Renalase Expression in Medulla. Front. Cardiovasc. Med. 2022, 9, 922705. [Google Scholar] [CrossRef]
- Tokinoya, K.; Ono, S.; Aoki, K.; Yanazawa, K.; Shishikura, Y.; Sugasawa, T.; Takekoshi, K. Gene expression level of renalase in the skeletal muscles is increased with high-intensity exercise training in mice on a high-fat diet. Physiol. Int. 2021, 108, 274–284. [Google Scholar] [CrossRef]
- Potts, L.; Phillips, C.; Hwang, M.; Fulcher, S.; Choi, H. Rescue of human corneal epithelial cells after alkaline insult using renalase derived peptide, RP-220. Int. J. Ophthalmol. 2019, 12, 1667–1673. [Google Scholar] [CrossRef]
- Wang, M.; Silva, T.; Toothaker, J.M.; McCourt, B.T.; Shugrue, C.; Desir, G.; Gorelick, F.; Konnikova, L. Renalase and its receptor, PMCA4b, are expressed in the placenta throughout the human gestation. Sci. Rep. 2022, 12, 4953. [Google Scholar] [CrossRef]
- Guo, X.; Hollander, L.; MacPherson, D.; Wang, L.; Velazquez, H.; Chang, J.; Safirstein, R.; Cha, C.; Gorelick, F.; Desir, G.V. Inhibition of renalase expression and signaling has antitumor activity in pancreatic cancer. Sci. Rep. 2016, 6, 22996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollander, L.; Guo, X.; Velazquez, H.; Chang, J.; Safirstein, R.; Kluger, H.; Cha, C.; Desir, G.V. Renalase Expression by Melanoma and Tumor-Associated Macrophages Promotes Tumor Growth through a STAT3-Mediated Mechanism. Cancer Res. 2016, 76, 3884–3894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Jessel, S.; Qu, R.; Kluger, Y.; Chen, T.M.; Hollander, L.; Safirstein, R.; Nelson, B.; Cha, C.; Bosenberg, M.; et al. Inhibition of renalase drives tumour rejection by promoting T cell activation. Eur. J. Cancer 2022, 165, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Pointer, T.C.; Gorelick, F.S.; Desir, V.G. Renalase: A Multi-Functional Signaling Molecule with Roles in Gastrointestinal Disease. Cells 2021, 10, 2006. [Google Scholar] [CrossRef]
- Fedchenko, V.I.; Kaloshin, A.A.; Mezhevikina, L.M.; Buneeva, O.A.; Medvedev, A.E. Construction of the coding sequence of the transcription variant 2 of the human Renalase gene and its expression in the prokaryotic system. Int. J. Mol. Sci. 2013, 14, 12764–12779. [Google Scholar] [CrossRef] [Green Version]
- Fedchenko, V.; Kopylov, A.T.; Buneeva, O.A.; Kaloshin, A.A.; Victor, G.; Zgoda, V.G.; Medvedev, A.M. Proteomic profiling data of HEK293 proteins bound to human recombinant renalases-1 and -2. Data Brief 2018, 21, 1477–1482. [Google Scholar] [CrossRef]
- Fedchenko, V.; Kopylov, A.; Kozlova, N.; Buneeva, O.; Kaloshin, A.; Zgoda, V.; Medvedev, A. Renalase Secreted by Human Kidney HEK293T Cells Lacks its N-Terminal Peptide: Implications for Putative Mechanisms of Renalase Action. Kidney Blood Press. Res. 2016, 41, 593–603. [Google Scholar] [CrossRef]
- Wang, L.; Velazquez, H.; Chang, J.; Safirstein, R.; Desir, G.V. Identification of a receptor for extracellular renalase. PLoS ONE 2015, 10, e0122932. [Google Scholar] [CrossRef]
- Du, M.; Huang, K.; Huang, D.; Yang, L.; Gao, L.; Wang, X.; Huang, D.; Li, X.; Wang, C.; Zhang, F.; et al. Renalase is a novel target gene of hypoxia-inducible factor-1 in protection against cardiac ischaemia–reperfusion injury. Cardiovasc. Res. 2014, 105, 182–191. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, G.; Xing, T.; Lu, Z.; Li, J.; Peng, C.; Liu, G.; Wang, N. Renalase contributes to the renal protection of delayed ischaemic preconditioning via the regulation of hypoxia-inducible factor-1alpha. J. Cell. Mol. Med. 2015, 19, 1400–1409. [Google Scholar] [CrossRef]
- Sonawane, P.J.; Gupta, V.; Sasi, B.K.; Kalyani, A.; Natarajan, B.; Khan, A.A.; Sahu, B.S.; Mahapatra, N.R. Transcriptional Regulation of the Novel Monoamine Oxidase Renalase: Crucial Roles of Transcription Factors Sp1, STAT3, and ZBP89. Biochemistry 2014, 53, 6878–6892. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cai, H.; Zhao, Q.; Xing, T.; Li, J.; Wang, N. Epinephrine evokes renalase secretion via alpha-adrenoceptor/NF-kappaB pathways in renal proximal tubular epithelial cells. Kidney Blood Press. Res. 2014, 39, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Beaupre, B.A.; Carmichael, B.R.; Hoag, M.R.; Shah, D.D.; Moran, G.R. Renalase is an alpha-NAD(P)H oxidase/anomerase. J. Am. Chem. Soc. 2013, 135, 13980–13987. [Google Scholar] [CrossRef] [PubMed]
- Beaupre, B.A.; Hoag, M.R.; Carmichael, B.R.; Moran, G.R. Kinetics and equilibria of the reductive and oxidative half-reactions of human renalase with alpha-NADPH. Biochemistry 2013, 52, 8929–8937. [Google Scholar] [CrossRef]
- Beaupre, B.A.; Hoag, M.R.; Roman, J.; Försterling, F.H.; Moran, G.R. Metabolic Function for Human Renalase: Oxidation of Isomeric Forms of β-NAD(P)H that Are Inhibitory to Primary Metabolism. Biochemistry 2015, 54, 795–806. [Google Scholar] [CrossRef]
- Sun, W.; Liu, C.; Chen, Q.; Liu, N.; Yan, Y.; Liu, B. SIRT3: A New Regulator of Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 7293861. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Huang, Y.; Xu, C.; An, P.; Luo, Y.; Jiao, L.; Luo, J.; Li, Y. Mitochondrial Dysfunction and Therapeutic Perspectives in Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 16053. [Google Scholar] [CrossRef]
- Miao, R.; Wang, L.; Chen, Z.; Ge, S.; Li, L.; Zhang, K.; Chen, Y.; Guo, W.; Duan, X.; Zhu, M.; et al. Advances in the study of nicotinamide adenine dinucleotide phosphate oxidase in myocardial remodeling. Front. Cardiovasc. Med. 2022, 9, 1000578. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Velazquez, H.; Wang, P.; Li, G.; Liu, D.; Sampaio-Maia, B.; Quelhas-Santos, J.; Russell, K.; Russell, R.; et al. Renalase deficiency aggravates ischemic myocardial damage. Kidney Int. 2011, 79, 853–860. [Google Scholar] [CrossRef] [Green Version]
- Morrison, C.S.; Paskaleva, E.E.; Rios, M.A.; Beusse, T.R.; Blair, E.M.; Lin, L.Q.; Hu, J.R.; Gorby, A.H.; Dodds, D.R.; Armiger, W.B.; et al. Improved soluble expression and use of recombinant human renalase. PLoS ONE 2020, 15, e0242109. [Google Scholar] [CrossRef]
- Wang, L.; Velazquez, H.; Moeckel, G.; Chang, J.; Ham, A.; Lee, H.T.; Safirstein, R.; Desir, G.V. Renalase prevents AKI independent of amine oxidase activity. J. Am. Soc. Nephrol. 2014, 25, 1226–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Safirstein, R.; Velazquez, H.; Guo, X.-J.; Hollander, L.; Chang, J.; Chen, T.-M.; Mu, J.-J.; Desir, G.V. Extracellular renalase protects cells and organs by outside-in signalling. J. Cell. Mol. Med. 2017, 21, 1260–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wu, W.; Liu, W.; Zhou, M. Roles and mechanisms of renalase in cardiovascular disease: A promising therapeutic target. Biomed Pharmacother. 2020, 131, 110712. [Google Scholar] [CrossRef]
- Farzaneh-Far, R.; Desir, G.V.; Na, B.; Schiller, N.B.; Whooley, M.A. A functional polymorphism in renalase (Glu37Asp) is associated with cardiac hypertrophy, dysfunction, and ischemia: Data from the heart and soul study. PLoS ONE 2010, 5, e13496. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, C.; Hu, G.L.; Chu, C.; Zhang, X.Y.; Du, M.F.; Zou, T.; Zhou, Q.; Liao, Y.Y.; Ma, Q.; et al. Associations of Renalase With Blood Pressure and Hypertension in Chinese Adults. Front. Cardiovasc. Med. 2022, 9, 800427. [Google Scholar] [CrossRef]
- Safdar, B.; Guo, X.; Johnson, C.; D’Onofrio, G.; Dziura, J.; Sinusas, A.J.; Testani, J.; Rao, V.; Desir, G. Elevated renalase levels in patients with acute coronary microvascular dysfunction-A possible biomarker for ischemia. Int. J. Cardiol. 2019, 279, 155–161. [Google Scholar] [CrossRef]
- Orlowska-Baranowska, E.; Gadomska, V.B.L.; Gora, J.; Baranowski, R.; Pedzich-Placha, E.; Zakrzewski, D.; Dlugosz, A.; Kossowska, H.; Zebrowska, A.; Zakoscielna, E.; et al. Functional polymorphism of the renalase gene is associated with cardiac hypertrophy in female patients with aortic stenosis. PLoS ONE 2017, 12, e0186729. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Mahapatra, N.R. Renalase: A novel regulator of cardiometabolic and renal diseases. Hypertens. Res. 2022, 45, 1582–1598. [Google Scholar] [CrossRef]
- Stojanovic, D.; Mitic, V.; Stojanovic, M.; Petrovic, D.; Ignjatovic, A.; Milojkovic, M.; Dunjic, O.; Milenkovic, J.; Bojanic, V.; Ilic, M.D. The Discriminatory Ability of Renalase and Biomarkers of Cardiac Remodeling for the Prediction of Ischemia in Chronic Heart Failure Patients With the Regard to the Ejection Fraction. Front. Cardiovasc. Med. 2021, 8, 691513. [Google Scholar] [CrossRef]
- Stojanovic, D.; Mitic, V.; Petrovic, D.; Stojanovic, M.; Ignjatovic, A.; Stefanovic, N.; Cvetkovic, T.; Bojanic, V.; Kocic, G.; Ilic, M.D. Association of Plasma Renalase and Left Ventricle Mass Index in Heart Failure Patients Stratified to the Category of the Ejection Fraction: A Pilot Study. Dis. Markers 2019, 2019, 7265160. [Google Scholar] [CrossRef] [Green Version]
- Martynowicz, H.; Wieckiewicz, M.; Poreba, R.; Wojakowska, A.; Smardz, J.; Januszewska, L.; Markiewicz-Gorka, I.; Mazur, G.; Pawlas, K.; Gac, P. The Relationship between Sleep Bruxism Intensity and Renalase Concentration-An Enzyme Involved in Hypertension Development. J. Clin. Med. 2019, 9, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, I.T.; Sheu, W.H. Serum Renalase Levels Are Predicted by Brain-Derived Neurotrophic Factor and Associated with Cardiovascular Events and Mortality after Percutaneous Coronary Intervention. J. Clin. Med. 2018, 7, 437. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Sheu, W.H.; Lee, W.J.; Wang, J.; Fu, C.P.; Liang, K.W.; Lee, I.T. Synergistic effect of renalase and chronic kidney disease on endothelin-1 in patients with coronary artery disease—A cross-sectional study. Sci. Rep. 2018, 8, 7378. [Google Scholar] [CrossRef] [PubMed]
- Czubilińska-Łada, J.; Badeński, A.; Świętochowska, E.; Nowak-Borzęcka, L.; Sadownik, B.; Behrendt, J.; Szczepańska, M. The influence of cord blood renalase and advanced oxidation protein products (AOPPs) on perinatal and anthropometric parameters of newborns of mothers with gestational hypertension. Adv. Clin. Exp. Med. 2022, 31, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bai, Y.; Feng, Y.; Zhang, Q.; Diao, Z.; Liu, W. Renalase Prevents Renal Fibrosis by Inhibiting Endoplasmic Reticulum Stress and Down-Regulating GSK-3β/Snail Signaling. Int. J. Med. Sci. 2023, 20, 669–681. [Google Scholar] [CrossRef]
- Wisniewska, M.; Serwin, N.; Dziedziejko, V.; Marchelek-Mysliwiec, M.; Dołegowska, B.; Domanski, L.; Ciechanowski, K.; Safranow, K.; Pawlik, A. Renalase in Haemodialysis Patients with Chronic Kidney Disease. J. Clin. Med. 2021, 10, 680. [Google Scholar] [CrossRef]
- Dziedzic, M.; Powrózek, T.; Orłowska, E.; Koch, W.; Kukula-Koch, W.; Gaweł, K.; Bednarek-Skublewska, A.; Małecka-Massalska, T.; Milanowski, J.; Petkowicz, B.; et al. Relationship between microRNA-146a expression and plasma renalase levels in hemodialyzed patients. PLoS ONE 2017, 12, e0179218. [Google Scholar] [CrossRef] [Green Version]
- Stojanovic, D.; Cvetkovic, T.; Stojanovic, M.; Stefanovic, N.; Velickovic-Radovanovic, R.; Zivkovic, N. Renalase Assessment With Regard to Kidney Function, Lipid Disturbances, and Endothelial Dysfunction Parameters in Stable Renal Transplant Recipients. Prog. Transplant. 2017, 27, 125–130. [Google Scholar] [CrossRef]
- Stojanovic, D.; Stojanovic, M.; Milenkovic, J.; Velickov, A.; Ignjatovic, A.; Milojkovic, M. Renalase Challenges the Oxidative Stress and Fibroproliferative Response in COVID-19. Oxid. Med. Cell. Longev. 2022, 2022, 4032704. [Google Scholar] [CrossRef]
- Safdar, B.; Wang, M.; Guo, X.; Cha., C.; Chun, H.J.; Deng, Y.; Dziura, J.; El-Khoury, J.M.; Gorelick, F.; Ko, A.I.; et al. Association of renalase with clinical outcomes in hospitalized patients with COVID-19. PLoS ONE 2022, 17, e0264178. [Google Scholar] [CrossRef]
- Carrico, C.; Meyer, J.G.; He, W.; Gibson, B.W.; Verdin, E. The mitochondrial acylome emerges: Proteomics, regulation by sirtuins, and metabolic and disease implications. Cell Metab. 2018, 27, 497–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horton, J.L.; Martin, O.J.; Lai, L.; Riley, N.M.; Richards, A.L.; Vega, R.V.; Leone, T.L.; Pagliarini, D.J.; Muoio, D.M.; Bedi, K.C.; et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight. 2016, 2, e84897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Ji, R.; Liao, X.; Castillero, E.; Kennel, P.J.; Brunjes, D.L.; Franz, M.; Möbius-Winkler, S.; Drosatos, K.; George, I.; et al. MicroRNA-195 regulates metabolism in failing myocardium via alterations in sirtuin 3 expression and mitochondrial protein acetylation. Circulation 2018, 137, 2052–2067. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar]
- Lee, C.F.; Chavez, J.D.; Garcia-Menendez, L.; Choi, Y.; Roe, N.D.; Chiao, Y.A.; Edgar, J.S.; Goo, Y.A.; Goodlett, D.R.; Bruce, J.E.; et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation 2016, 134, 883–894. [Google Scholar] [CrossRef] [Green Version]
- Hershberger, K.A.; Martin, A.S.; Hirschey, M.D. Role of NAD+ and mitochondrial sirtuins in cardiac and renal diseases. Nat. Rev. Nephrol. 2017, 13, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Winnik, S.; Auwerx, J.; Sinclair, D.A.; Matter, C.M. Protective effects of sirtuins in cardiovascular diseases: From bench to bedside. Eur. Heart J. 2015, 36, 3404–3412. [Google Scholar] [CrossRef] [Green Version]
- Cercillieux, A.; Ciarlo, E.; Canto, C. Balancing NAD+ deficits with nicotinamide riboside: Therapeutic possibilities and limitations. Cell. Mol. Life Sci. 2022, 79, 463. [Google Scholar] [CrossRef]
- Lin, Q.; Zuo, W.; Liu, Y.; Wu, K.; Liu, Q. NAD+ and cardiovascular diseases. Clin. Chim. Acta 2021, 515, 104–110. [Google Scholar] [CrossRef]
- Abdellatif, M.; Sedej, S.; Kroemer, G. NAD+ Metabolism in Cardiac Health, Aging, and Disease. Circulation 2021, 144, 1795–1817. [Google Scholar] [CrossRef]
- Lauritzen, K.H.; Olsen, M.B.; Ahmed, M.S.; Yang, K.; Rinholm, J.E.; Bergersen, L.H.; Esbensen, Q.Y.; Sverkeli, L.J.; Ziegler, M.; Attramadal, H.; et al. Instability in NAD+ metabolism leads to impaired cardiac mitochondrial function and communication. Elife 2021, 10, e59828. [Google Scholar] [CrossRef] [PubMed]
- Ministrini, S.; Puspitasari, Y.M.; Beer, G.; Liberale, L.; Montecucco, F.; Camici, G.G. Sirtuin 1 in Endothelial Dysfunction and Cardiovascular Aging. Front. Physiol. 2021, 12, 733696. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M.L. SIRT1 and SIRT6 Signaling Pathways in Cardiovascular Disease Protection. Antioxid. Redox Signal. 2018, 28, 711–732. [Google Scholar] [CrossRef] [PubMed]
- Koentges, C.; Bode, C.; Bugger, H. SIRT3 in Cardiac Physiology and Disease. Front. Cardiovasc. Med. 2016, 13, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, M.; Zhao, Q.; Sun, X.; Qian, H.; Lyu, S.; Chen, R.; Xia, H.; Yuan, W. Sirtuin 3: Emerging therapeutic target for cardiovascular diseases. Free. Radic. Biol. Med. 2022, 180, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, S.; Zhang, B.; Liu, J. SIRT3 as a potential therapeutic target for heart failure. Pharmacol. Res. 2021, 165, 105432. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.R.; Liu, B. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics 2020, 10, 8315–8342. [Google Scholar] [CrossRef]
- Herranz, D.; Serrano, M. SIRT1: Recent lessons from mouse models. Nat. Rev. Cancer 2010, 10, 819–823. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef]
- Borzsei, D.; Sebestyen, J.; Szabo, R.; Lesi, Z.N.; Palszabo, A.; Palszabo, P.; Szasz, A.; Priksz, D.; Juhasz, B.; Veszelka, M.; et al. Resveratrol as a Promising Polyphenol in Age-Associated Cardiac Alterations. Oxid. Med. Cell. Longev. 2022, 2022, 7911222. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [Green Version]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiese, K. Targeting the core of neurodegeneration: FoxO, mTOR, and SIRT1. Neural Regen. Res. 2021, 16, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.X.; van Tits, L.J.; Lohmann, C.; Arsiwala, T.; Winnik, S.; Tailleux, A.; Stein, S.; Gomes, A.P.; Suri, V.; Ellis, J.L.; et al. The Sirt1 activator SRT3025 provides atheroprotection in Apoe-/- mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. Eur. Heart J. 2015, 36, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becatti, M.; Taddei, N.; Cecchi, C.; Nassi, N.; Nassi, P.A.; Fiorillo, C. SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell. Mol. Life Sci. 2012, 69, 2245–2260. [Google Scholar] [CrossRef]
- Yamamoto, T.; Byun, J.; Zhai, P.; Ikeda, Y.; Oka, S.; Sadoshima, J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS ONE 2014, 9, e98972. [Google Scholar] [CrossRef] [Green Version]
- Fasano, C.; Disciglio, V.; Bertora, S.; Signorile, M.L.; Simone, C. FOXO3a from the Nucleus to the Mitochondria: A Round Trip in Cellular Stress Response. Cells 2019, 8, 1110. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Gao, B.; Li, N.; Wang, J.; Qiu, C.; Zhang, G.; Liu, M.; Zhang, R.; Li, C.; Ji, G.; et al. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1973–1983. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Pandey, A.; Xiao, L.; Arslanbaeva, L.; Sidorova, T.; Lopez, M.G.; Billings, F.T., 4th; Verdin, E.; Auwerx, J.; Harrison, D.G.; et al. Mitochondrial Deacetylase Sirt3 Reduces Vascular Dysfunction and Hypertension While Sirt3 Depletion in Essential Hypertension Is Linked to Vascular Inflammation and Oxidative Stress. Circ. Res. 2020, 126, 439–452. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Li, Q.; Yuan, Y.; Zhang, C.; Wu, L.; Liu, X.; Cao, W.; Guo, H.; Duan, S.; Xu, X.; et al. Renalase attenuates mitochondrial fission in cisplatin-induced acute kidney injury via modulating sirtuin-3. Life Sci. 2019, 222, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Wang, X.; Wang, Y.; Zhang, Q.; Liu, W. Renalase contributes to protection against renal fibrosis via inhibiting oxidative stress in rats. Int. Urol. Nephrol. 2018, 50, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhao, Q.; Li, J.; Xing, T.; Wang, F.; Wang, N. Renalase protects against contrast-induced nephropathy in Sprague-Dawley rats. PLoS ONE 2015, 10, e0116583. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Lu, Z.; Wang, F.; Jiang, Z.; Lu, L.; Miao, N.; Wang, N. Renalase attenuates hypertension, renal injury and cardiac remodelling in rats with subtotal nephrectomy. J. Cell. Mol. Med. 2016, 20, 1106–1117. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Yin, J.; Lu, Z.; Zhang, G.; Li, J.; Xing, T.; Zhuang, S.; Wang, N. Limb ischemic preconditioning protects against contrast-induced nephropathy via renalase. EBioMedicine 2016, 9, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Liu, X.; Zhao, T.; Liang, R.; Wu, R.; Zhang, F.; Kong, Y.; Liu, L.; Xing, T.; Wang, N.; et al. A protective role of renalase in diabetic nephropathy. Clin. Sci. 2020, 134, 75–85. [Google Scholar] [CrossRef]
- Guo, X.; Xu, L.; Velazquez, H.; Chen, T.M.; Williams, R.M.; Heller, D.A.; Burtness, B.; Safirstein, R.; Desir, G.V. Kidney-Targeted Renalase Agonist Prevents Cisplatin-Induced Chronic Kidney Disease by Inhibiting Regulated Necrosis and Inflammation. J. Am. Soc. Nephrol. 2022, 33, 342–356. [Google Scholar] [CrossRef]
- Lee, H.T.; Kim, J.Y.; Kim, M.; Wang, P.; Tang, L.; Baroni, S.; D’Agati, V.D.; Desir, G.V. Renalase protects against ischemic AKI. J. Am. Soc. Nephrol. 2013, 24, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, L.; Deng, D.; Zhang, Q.; Liu, W. Renalase Protects against Renal Fibrosis by Inhibiting the Activation of the ERK Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 855. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Quan, C.; Yang, Y.; Liang, Z.; Jiang, W.; Li, X. Renalase improves pressure overload-induced heart failure in rats by regulating extracellular signal-regulated protein kinase 1/2 signaling. Hypertens. Res. 2021, 44, 481–488. [Google Scholar] [CrossRef]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.; Zhu, Z.; Wang, Y.; Wen, Z.; Jiang, Z.; Zhang, L.; Pang, Y.; Lu, J. Research progress on the relationship between mitochondrial function and heart failure: A bibliometric study from 2002 to 2021. Front. Mol. Biosci. 2022, 9, 1036364. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, E.; Kass, D.A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 2007, 49, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, Z.; Zhang, W.; Liu, X. Mitochondrial dysfunction and mitochondrial therapies in heart failure. Pharmacol. Res. 2022, 175, 106038. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Gao, J.; Ding, S.L.; Wang, K.; Jiao, J.Q.; Wang, Y.; Sun, T.; Zhou, L.Y.; Long, B.; Zhang, X.J.; et al. Oxidative Modification of miR-184 Enables It to Target Bcl-xL and Bcl-w. Mol. Cell. 2015, 59, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Pinti, M.V.; Hathaway, Q.A.; Hollander, J.M. Role of microRNA in metabolic shift during heart failure. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.Y.; Wang, W.; Chen, J.; Ocorr, K.; Bodmer, R. ROS regulate cardiac function via a distinct paracrine mechanism. Cell Rep. 2014, 10, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Lovisa, S. Epithelial-to-Mesenchymal Transition in Fibrosis: Concepts and Targeting Strategies. Front. Pharmacol. 2021, 12, 737570. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, S.; Cao, Y.; Kong, G.; Jiang, F.; Li, Y.; Wang, Q.; Tang, M.; Zhang, Q.; Wang, Q.; et al. Gasotransmitters: Potential Therapeutic Molecules of Fibrotic Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 3206982. [Google Scholar] [CrossRef]
- Stojanovic, D.; Mitic, V.; Stojanovic, M.; Milenkovic, J.; Ignjatovic, A.; Milojkovic, M. The Scientific Rationale for the Introduction of Renalase in the Concept of Cardiac Fibrosis. Front. Cardiovasc. Med. 2022, 9, 845878. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, X.; Liu, D.; Cui, C.; Wang, X. Endothelial-to-Mesenchymal Transition: Role in Cardiac Fibrosis. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, F.; Kaneko, E.; Sugimoto, T.; Ishijima, T.; Wakamatsu, M.; Yuasa, A.; Sampei, R.; Mori, K.; Nose, K.; Shibanuma, M. A mitochondrial thioredoxin-sensitive mechanism regulates TGF-β-mediated gene expression associated with epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2014, 443, 821–827. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, L.; Wen, J.; Zhang, F.; Gu, S.; Wang, F.; Yin, J.; Wang, N. Cardiac-specific renalase overexpression alleviates CKD-induced pathological cardiac remodeling in mice. Front. Cardiovasc. Med. 2022, 9, 1061146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef]
- Teuber, J.P.; Essandoh, K.; Hummel, S.L.; Madamanchi, N.R.; Brody, M.J. NADPH Oxidases in Diastolic Dysfunction and Heart Failure with Preserved Ejection Fraction. Antioxidants 2022, 11, 1822. [Google Scholar] [CrossRef]
- Parajuli, N.; Patel, V.B.; Wang, W.; Basu, R.; Oudit, G.Y. Loss of NOX2 (gp91phox) prevents oxidative stress and progression to advanced heart failure. Clin. Sci. 2014, 127, 331–340. [Google Scholar] [CrossRef]
- Czarkowska-Paczek, B.; Zendzian-Piotrowska, M.; Gala, K.; Sobol, M.; Paczek, L. Exercise differentially regulates renalase expression in skeletal muscle and kidney. Tohoku J. Exp. Med. 2013, 231, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Pahlavani, H.A. Exercise-induced signaling pathways to counteracting cardiac apoptotic processes. Front. Cell. Dev. Biol. 2022, 10, 950927. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Yang, A.-L.; Lin, Y.-M.; Wu, F.-N.; Lin, J.A.; Chan, Y.-S.; Tsai, F.-J.; Tsai, C.-H.; Kuo, C.-H.; Lee, S.-D. Anti-apoptotic and pro-survival effects of exercise training on hypertensive hearts. J. Appl. Physiol. 2012, 112, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Yang, A.; Lin, Y.; Wu, F.; Lin, J.A.; Chan, Y.; Tsai, F.; Tsai, C.; Kuo, C.; Lee, S. Anti-apoptotic and pro-survival effect of exercise training on early aged hypertensive rat cerebral cortex. Aging 2021, 112, 883–891. [Google Scholar]
- Schefold, J.C.; Filippatos, G.; Hasenfuss, G.; Anker, S.D.; von Haehling, S. Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat. Rev. Nephrol. 2016, 12, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, A.; Fontanella, A.M.; Burke, G.W.; Merscher, S.; Fornoni, A. Mitochondrial Contribution to Inflammation in Diabetic Kidney Disease. Cells 2022, 11, 3635. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Sánchez-Lozada, L.G.; Osorio-Alonso, H.; Tapia, E.; Scholze, A. New Pathogenic Concepts and Therapeutic Approaches to Oxidative Stress in Chronic Kidney Disease. Oxid. Med. Cell. Longev. 2016, 2016, 6043601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Aparicio-Trejo, O.E.; Pedraza-Chaverri, J. Mitochondrial Redox Signaling and Oxidative Stress in Kidney Diseases. Biomolecules 2021, 11, 1144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef]
- Tang, C.; Cai, J.; Yin, X.M.; Weinberg, J.M.; Venkatachalam, M.A.; Dong, Z. Mitochondrial quality control in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 299–318. [Google Scholar] [CrossRef]
- Jin, L.; Yu, B.; Armando, I.; Han, F. Mitochondrial DNA-Mediated Inflammation in Acute Kidney Injury and Chronic Kidney Disease. Oxid. Med. Cell. Longev. 2021, 2021, 9985603. [Google Scholar] [CrossRef]
- Andrieux, P.; Chevillard, C.; Cunha-Neto, E.; Nunes, J.P.S. Mitochondria as a Cellular Hub in Infection and Inflammation. Int. J. Mol. Sci. 2021, 22, 11338. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, M.; Li, Q.; Xu, X.; Ou, W.; Zhang, Y.; Xiao, H.; Yu, H.; Zheng, Y.; Liang, Y.; et al. Targeting Mitochondria-Inflammation Circuit by β-Hydroxybutyrate Mitigates HFpEF. Circ. Res. 2021, 128, 232–245. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Hikoso, S.; Yamaguchi, O.; Taneike, M.; Takeda, T.; Tamai, T.; Oyabu, J.; Murakawa, T.; Nakayama, H.; Nishida, K.; et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 2012, 485, 251–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, H.; Otsu, K. Mitochondrial DNA as an inflammatory mediator in cardiovascular diseases. Biochem. J. 2018, 475, 839–852. [Google Scholar] [CrossRef]
- Bouhamida, E.; Morciano, G.; Perrone, M.; Kahsay, A.E.; Della Sala, M.; Wieckowski, M.R.; Fiorica, F.; Pinton, P.; Giorgi, C.; Patergnani, S. The Interplay of Hypoxia Signaling on Mitochondrial Dysfunction and Inflammation in Cardiovascular Diseases and Cancer: From Molecular Mechanisms to Therapeutic Approaches. Biology 2022, 11, 300. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Zhou, J.; Li, T. Mitochondrial DNA Is a Vital Driving Force in Ischemia-Reperfusion Injury in Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 6235747. [Google Scholar] [CrossRef] [PubMed]
- Patergnani, S.; Bouhamida, E.; Leo, S.; Pinton, P.; Rimessi, A. Mitochondrial Oxidative Stress and “Mito-Inflammation”: Actors in the Diseases. Biomedicines 2021, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Nakahira, K.; Hisata, S.; Choi, A.M. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal. 2015, 23, 1329–1350. [Google Scholar] [CrossRef] [Green Version]
- Lafuse, W.P.; Wozniak, D.J.; Rajaram, M.V.S. Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair. Cells 2020, 10, 51. [Google Scholar] [CrossRef]
- Marín-Aguilar, F.; Lechuga-Vieco, A.V.; Alcocer-Gómez, E.; Castejón-Vega, B.; Lucas, J.; Garrido, C.; Peralta-Garcia, A.; Pérez-Pulido, A.J.; Varela-López, A.; Quiles, J.L.; et al. NLRP3 inflammasome suppression improves longevity and prevents cardiac aging in male mice. Aging Cell 2020, 19, e13050. [Google Scholar] [CrossRef] [Green Version]
- Bracey, N.A.; Beck, P.L.; Muruve, D.A.; Hirota, S.A.; Guo, J.; Jabagi, H.; Wright, J.R., Jr.; Macdonald, J.A.; Lees-Miller, J.P.; Roach, D.; et al. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1β. Exp. Physiol. 2013, 98, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Pinar, A.A.; Scott, T.E.; Huuskes, B.M.; Cáceres, F.E.T.; Kemp-Harper, B.K.; Samuel, C.S. Targeting the NLRP3 inflammasome to treat cardiovascular fibrosis. Pharmacol. Ther. 2020, 209, 107511. [Google Scholar] [CrossRef] [PubMed]
- Butts, B.; Gary, R.A.; Dunbar, S.B.; Butler, J. The Importance of NLRP3 Inflammasome in Heart Failure. J. Card. Fail. 2015, 21, 586–593. [Google Scholar] [CrossRef] [Green Version]
- Yap, J.; Cabrera-Fuentes, H.A.; Irei, J.; Hausenloy, D.J.; Boisvert, W.A. Role of Macrophages in Cardioprotection. Int. J. Mol. Sci. 2019, 20, 2474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toba, H.; Cannon, P.L.; Yabluchanskiy, A.; Iyer, R.P.; D’Armiento, J.; Lindsey, M.L. Transgenic overexpression of macrophage matrix metalloproteinase-9 exacerbates age-related cardiac hypertrophy, vessel rarefaction, inflammation, and fibrosis. Am. J. Physiol. Heart. Circ. Physiol. 2017, 312, 375–383. [Google Scholar] [CrossRef] [Green Version]
- van Beek, A.A.; Van den Bossche, J.; Mastroberardino, P.G.; de Winther, M.P.J.; Leenen, P.J.M. Metabolic Alterations in Aging Macrophages: Ingredients for Inflammaging? Trends Immunol. 2019, 40, 113–127. [Google Scholar] [CrossRef]
- Javadov, S.; Jang, S.; Agostini, B. Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: Therapeutic perspectives. Pharmacol. Ther. 2014, 144, 202–225. [Google Scholar] [CrossRef] [Green Version]
- Mailloux, R.J. Mitochondrial Antioxidants and the Maintenance of Cellular Hydrogen Peroxide Levels. Oxid. Med. Cell. Longev. 2018, 2018, 7857251. [Google Scholar] [CrossRef]
- Roy, N.; Chakraborty, S.; Chowdhury, B.P.; Banerjee, S.; Halder, K.; Majumder, S.; Majumdar, S.; Sen, P.C. Regulation of PKC mediated signaling by calcium during visceral leishmaniasis. PLoS ONE 2014, 9, e110843. [Google Scholar] [CrossRef] [Green Version]
| Research Model | Mainstay Findings | Therapeutic Gain | Ref |
|---|---|---|---|
| Acute low- and high-intensity treadmill exercise | Renalase expression is upregulated upon NF-κB or HIF-1α, depending on the type of the muscle fibers and the exercise intensity (acute low- and high-intensity training) and represents a response to exercise-induced oxidative stress. | Antioxidative skeletal muscles protection | [36] |
| Aerobic training in spontaneously hypertensive rats | Increased renalase in the renal medulla ameliorates the degree of oxidative stress (decreased NOX2 and NOX4 expression) and apoptosis (decreased Bax and cleaved-caspase-3 expression; increased Bcl-2 expression) upon aerobic exercise and improves hypertensive kidney injury. | Amelioration of hypertensive kidney injury | [37] |
| Ischemic myocardial damage | Renalase deficiency in knockout mice results in increased plasma catecholamine levels, hypertension, mild ventricular hypertrophy, and an increased degree of myocardial necrosis, while recombinant renalase supplementation significantly ameliorates cardiac injury. These cardio-protective traits are likely obtained through the ability of renalase to oxidize NADH, thereby efficiently recovering cellular NAD+ content and the NAD+/NADH ratio. | Mitigation of cardiac injury in renalase-deficient conditions | [59] |
| Cisplatin- and hydrogen peroxide -induced acute kidney injury | Renalase deficiency leads to a significant renal macrophage infiltration, acute tubular necrosis, and apoptosis (increased caspase-3 activation and decreased Bcl-2 expression), while renalase treatment reduces tissue phenotype via PI3K/Akt and MAPKs activation and JNK downregulation. | AKI protection in patients receiving cisplatin therapy | [61] |
| Cisplatin-induced acute kidney injury | Renalase administration upon acute injury regulates ROS generation and oxidative stress levels, increases phosphorylation of Drp1 at serine 637, decreases Drp1 translocation to mitochondria, and enhances SIRT3 expression, thereby ameliorating mitochondrial morphology and dynamics in a SIRT3-dependent manner. | Reno-protection in patients receiving cisplatin therapy | [111] |
| Unilateral ureteral obstruction | Renalase administration suppresses the levels of MDA and ROS, reinforces SOD, and impedes oxidative stress-mediated EMT by effectively decreasing α-SMA expression, fibronectin, and collagens, while recovering the expression of E-cadherin and renal interstitial fibrosis. | Anti-fibrotic effects and mitigation of chronic kidney disease | [112] |
| Contrast-induced nephropathy | Renalase pretreatment suppresses the inflammatory response by decreasing TNF-α and MCP-1 levels as well as macrophage tissue accumulation; ameliorates the level of histological injury; decreases MDA; improves SOD levels; and abolishes apoptosis. | Reno-protection in patients receiving contrast | [113] |
| Subtotal nephrectomy | The systemic delivery of renalase reduces renal infiltration of total macrophages (CD68), specifically M1-like (CD86) and M2-like macrophages (CD163), and silences M1/M2 polarization, pro-inflammatory cytokines (TNF-α, MCP-1, and IL-6), and NADPH oxidase component expression. Renalase administration rescues overall cardio–renal structure and function by preventing hypertrophy and fibrosis via a decrease in the expression of collagen I and III, TIMP-1, and TGF-β1 and an increase in the expression of MMP-1 through the inhibition of ERK1/2 signalization and pro-fibrotic gene expression. | Cardiovascular and renal protection in patients with CKD | [114] |
| Contrast-induced nephropathy | Limb ischemic preconditioning-induced reno-protection depends on renalase upregulation via the TNF-α/NF-κB signaling and results in significantly reduced macrophage accumulation, improved renal function, tubular damage, and oxidative stress mitigation. | Reno-protection in patients receiving contrast | [115] |
| Diabetic nephropathy | Renalase downregulation leads to significant renal inflammation, mesangial hypertrophy, kidney injury, hypertension, and albuminuria, whereas renalase administration mitigates profibrotic gene expression and p21 expression via the impediment of the ERK1/2 pathway. | Mitigation of diabetic nephropathy progression | [116] |
| Cisplatin-induced chronic kidney disease | The therapy with kidney-targeted renalase agonist (RP81-MNP) weakens the pro-inflammatory state of chronic kidney disease by hindering the renal accumulation of neutrophils, CD4 T cells, dendritic cells, M1 macrophages (CD68), and myofibroblasts. In inflamed macrophages (M1), RP81-MNP downregulates chemokines (Cxcl2, Ccl12, Ccr12, Ccl7), proinflammatory cytokines (IL-1β, TNF-α), complement/coagulation factors (C1qa, C1qb, C1qc), antigen process/presenting molecules (H2-Aa, K2-k1, Cd74), genes involved in TLR signaling (Cd14, Spp1), and oxidative stress gene Gadd45. Moreover, renalase agonist administration results in decreased proinflammatory cytokine plasma concentration (IFN-Υ, IL-1β, IL-6, and TNF-α), reduced number of apoptotic cells, inhibited renal necrosis, and preserved epithelial components of the nephron and the vasculature. | Reno-protection in patients receiving cisplatin therapy | [117] |
| Ischemic acute kidney injury | Renalase treatment, before the ischemic injury, lessens neutrophil and macrophage infiltration and modulates renal tubular necrosis and apoptosis, while the depletion of renalase increases the expression of the kidney’s proinflammatory genes (TNF-α, MCP-1, and MIP-2). | Biomarker, prevention, and therapy for ischemic acute kidney injury | [118] |
| Unilateralureteral obstruction | Renalase ameliorates renal interstitial fibrosis, evidenced by the maintenance of E-cadherin expression and α-SMA as well as fibronectin and collagen-I downregulation, by inhibiting the activation of the ERK1/2 signaling pathway. | Antifibrotic effects for slowing the progression of CKD | [119] |
| Transverse aortic constriction-induced heart failure | Renalase alleviates pressure overload-induced heart failure through p38 and ERK1/2 signaling | Biomarker of cardiac hypertrophy and therapy for heart failure | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanovic, D.; Stojanovic, M.; Milenkovic, J.; Velickov, A.; Ignjatovic, A.; Milojkovic, M. The Multi-Faceted Nature of Renalase for Mitochondrial Dysfunction Improvement in Cardiac Disease. Cells 2023, 12, 1607. https://doi.org/10.3390/cells12121607
Stojanovic D, Stojanovic M, Milenkovic J, Velickov A, Ignjatovic A, Milojkovic M. The Multi-Faceted Nature of Renalase for Mitochondrial Dysfunction Improvement in Cardiac Disease. Cells. 2023; 12(12):1607. https://doi.org/10.3390/cells12121607
Chicago/Turabian StyleStojanovic, Dijana, Miodrag Stojanovic, Jelena Milenkovic, Aleksandra Velickov, Aleksandra Ignjatovic, and Maja Milojkovic. 2023. "The Multi-Faceted Nature of Renalase for Mitochondrial Dysfunction Improvement in Cardiac Disease" Cells 12, no. 12: 1607. https://doi.org/10.3390/cells12121607
APA StyleStojanovic, D., Stojanovic, M., Milenkovic, J., Velickov, A., Ignjatovic, A., & Milojkovic, M. (2023). The Multi-Faceted Nature of Renalase for Mitochondrial Dysfunction Improvement in Cardiac Disease. Cells, 12(12), 1607. https://doi.org/10.3390/cells12121607








