ML216 Prevents DNA Damage-Induced Senescence by Modulating DBC1–BLM Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Antibodies
2.2. Transfection and Infection
2.3. Immunoprecipitation (IP)
2.4. RT-PCR and RNA Sequencing
2.5. HR Repair Assays Design and Procedure
2.6. Cell Viability Assay
2.7. Apoptosis Assay
2.8. DNA Damage-Induced Senescence
2.9. Immunofluorescence Staining
2.10. Mouse Model and Drug Treatment
2.11. Muscle Endurance Capacity Analysis
2.12. Lung Function Analysis
2.13. Y-Maze Test
2.14. β-Galactosidase Staining for Cells and Lung Tissues
2.15. Hydroxyproline Assay
2.16. Histology
2.17. Immunohistochemistry
2.18. ELISA Assay
2.19. Statistical Analysis
3. Results
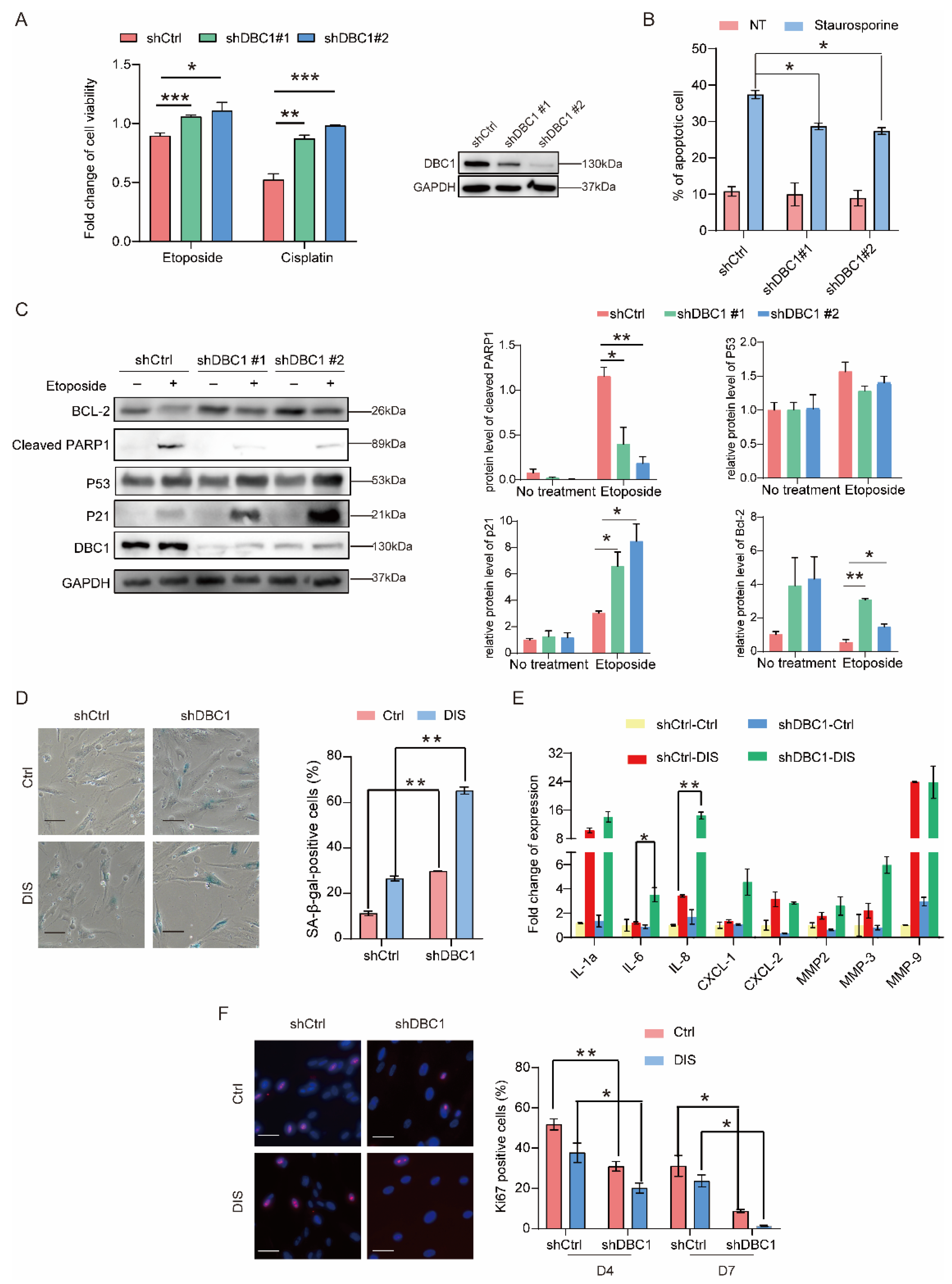
3.1. DBC1 Deficiency Increases Senescence Levels and Reduces Apoptosis in Response to DNA Damage
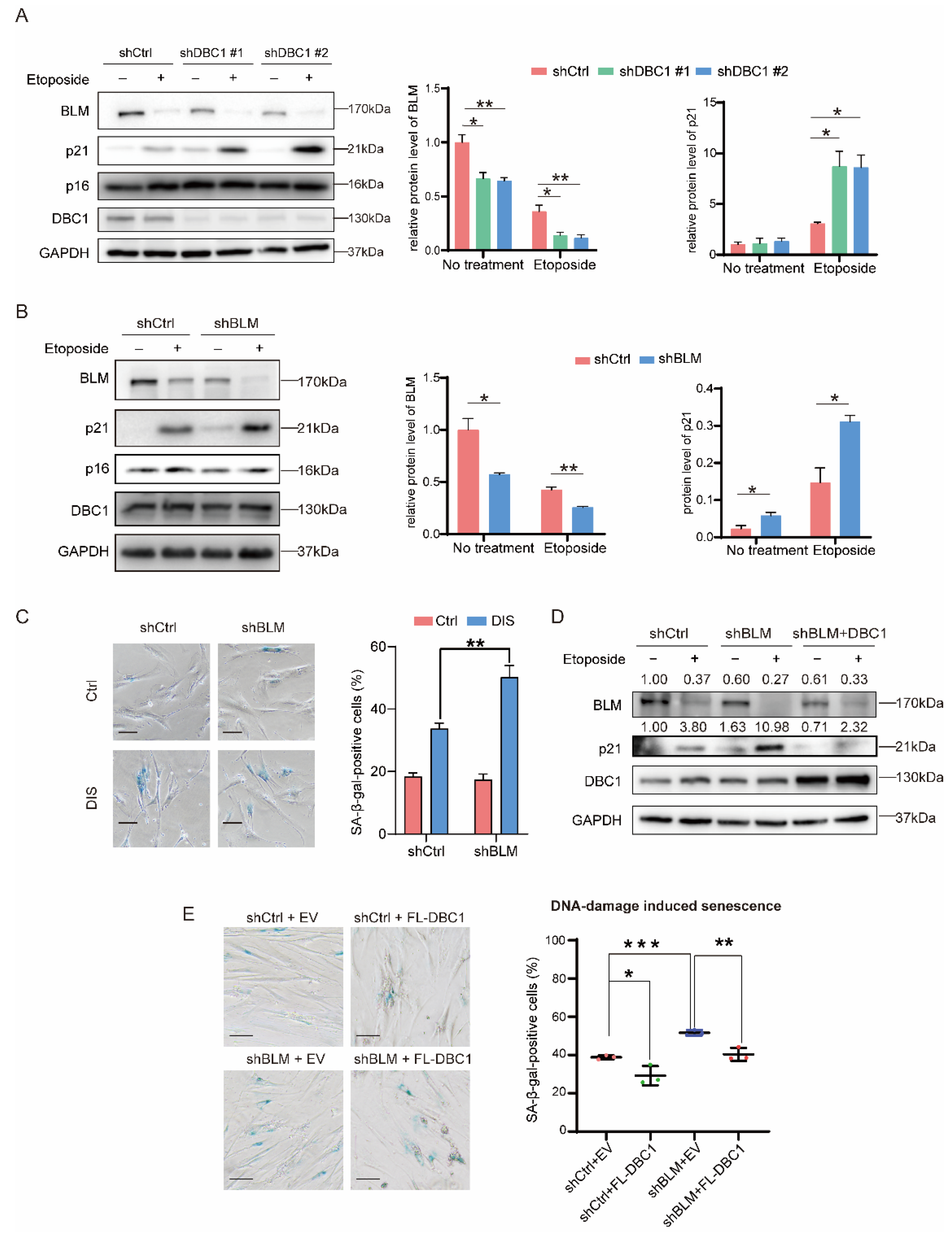
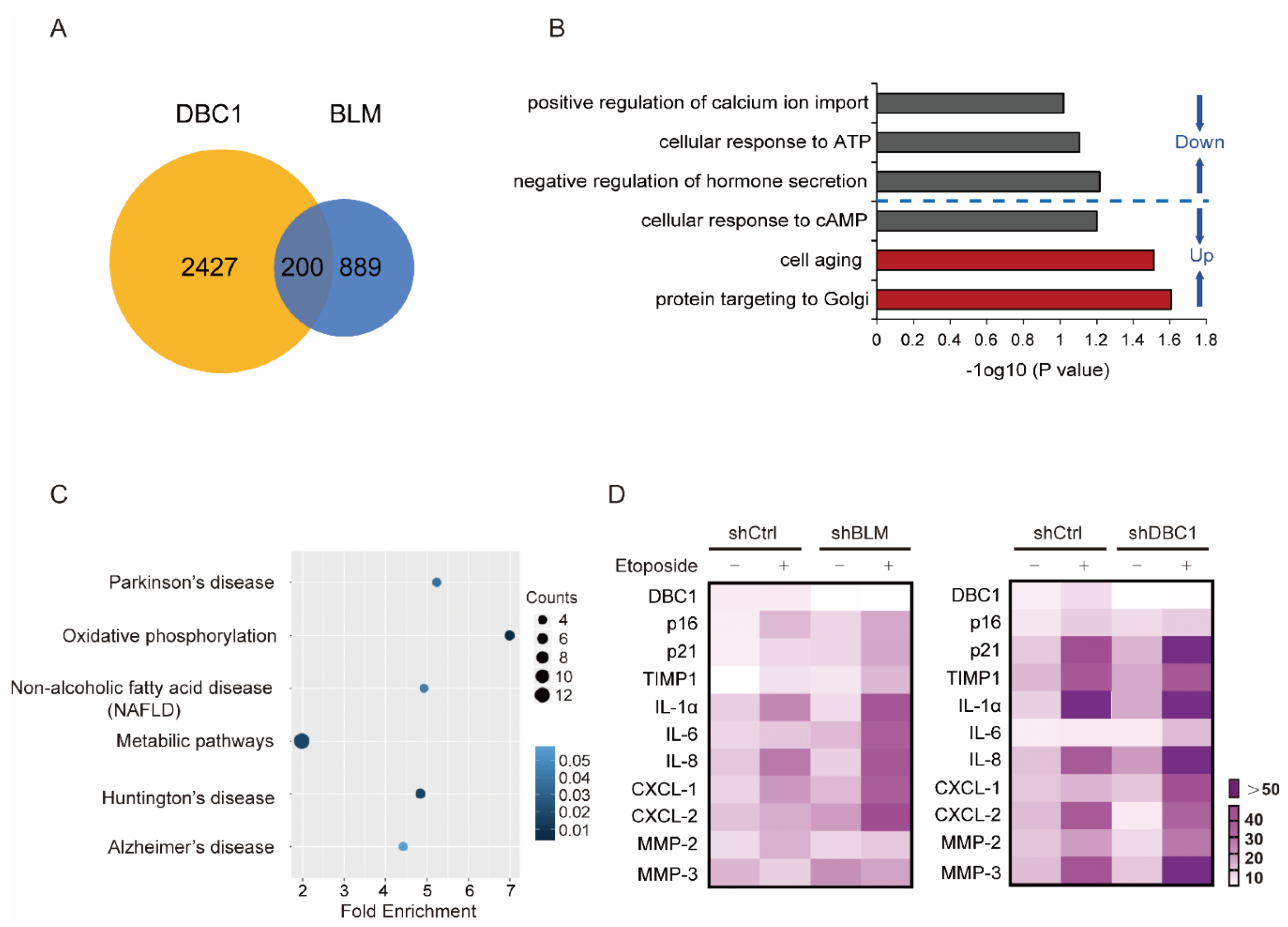
3.2. DBC1 Regulates DNA Damage-Induced Senescence by Maintaining BLM Abundance
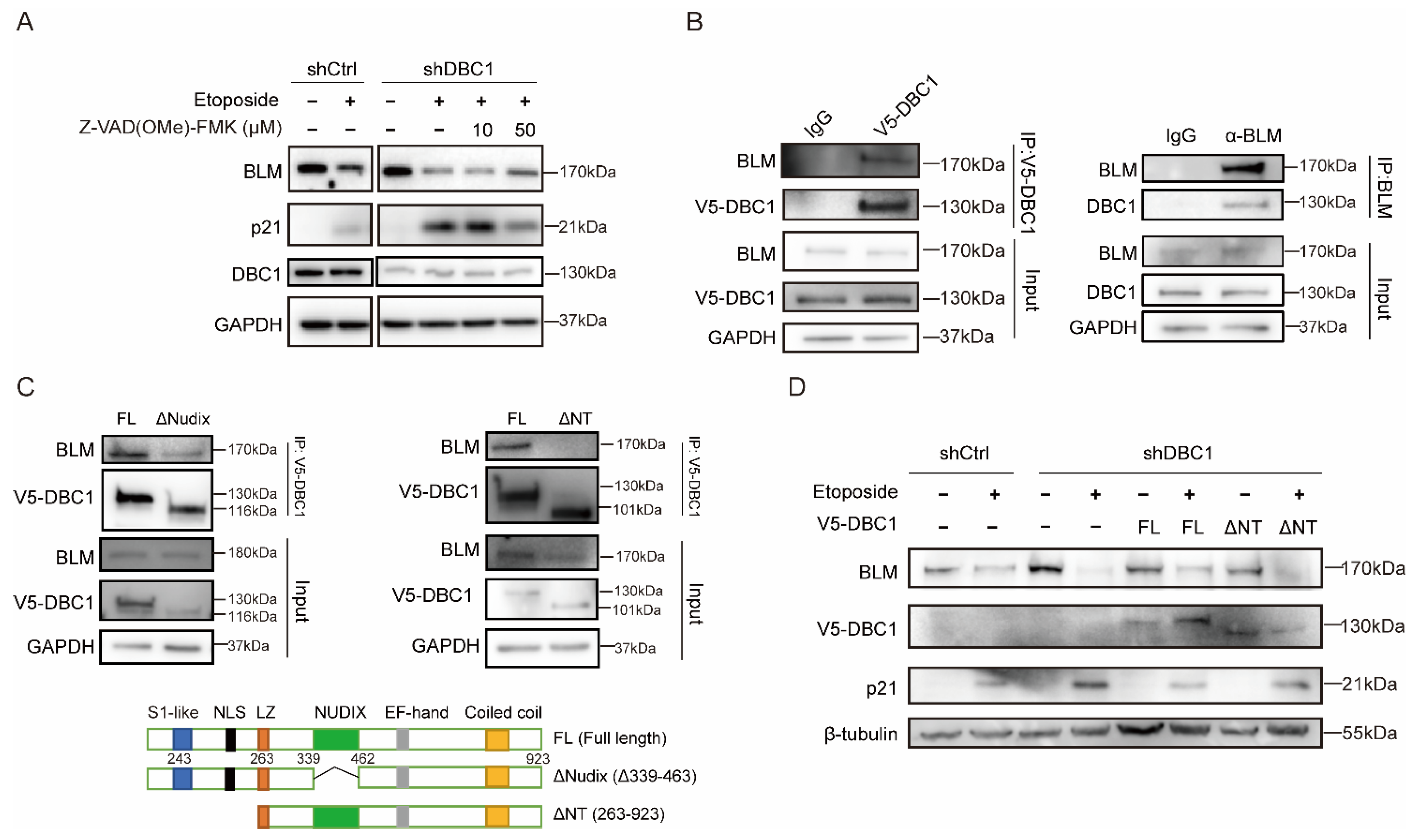
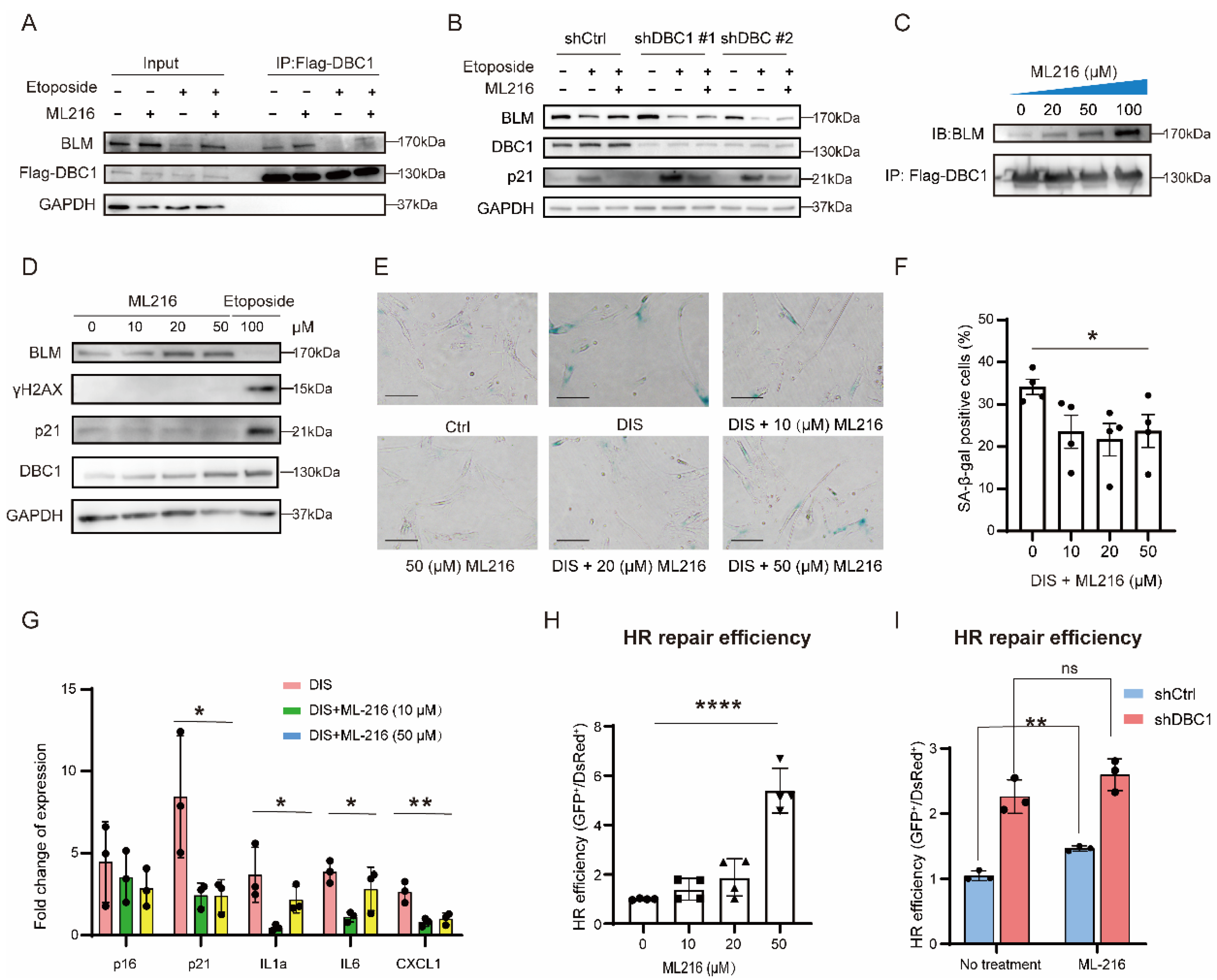
3.3. DBC1 Binds to BLM to Prevent its Degradation
3.4. ML216 Protects BLM from Degradation by Promoting the DBC1–BLM Interaction and Suppresses DNA Damage-Induced Senescence
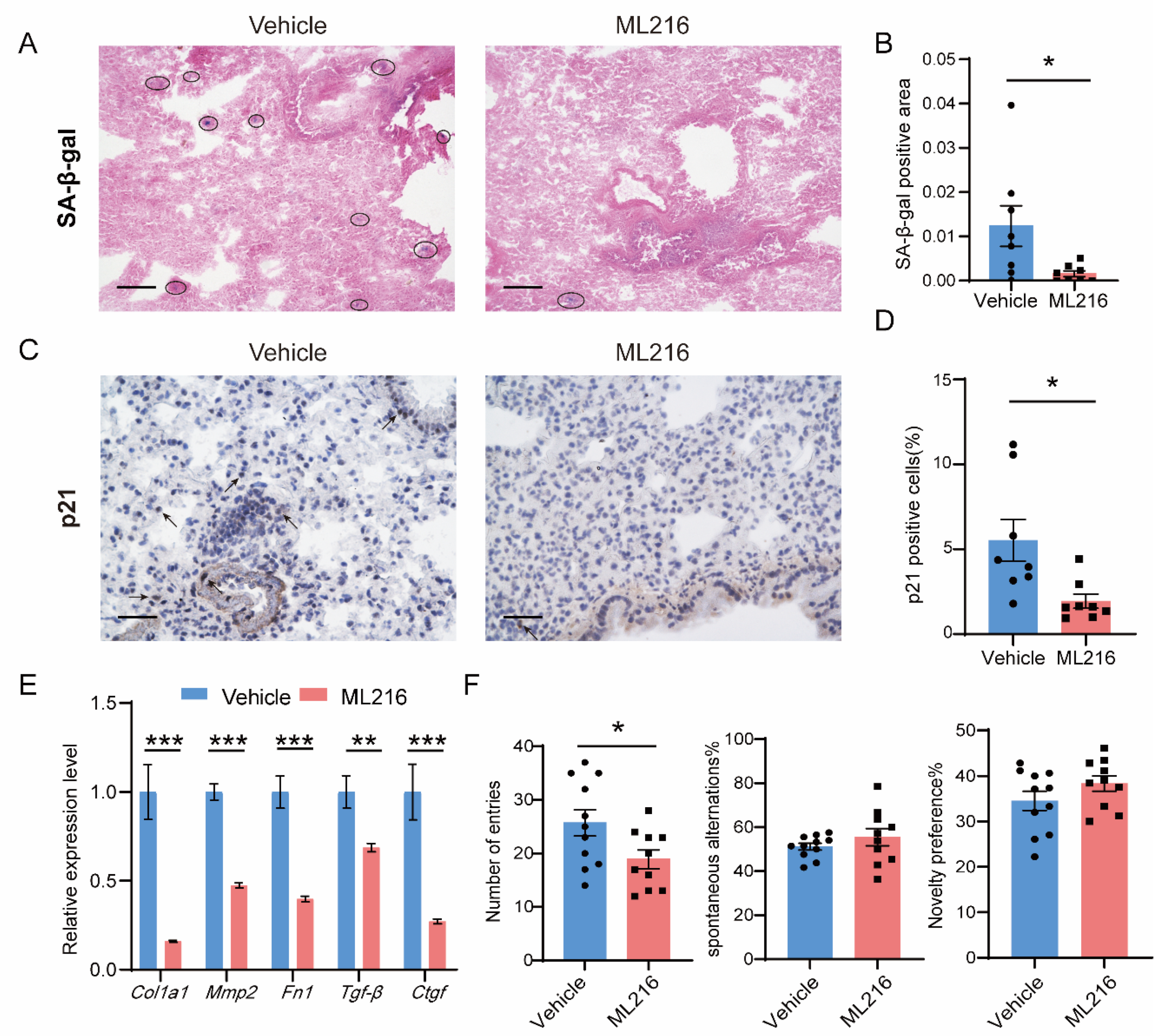
3.5. ML216 Alleviates Senescence In Vivo and Improves the Health in Naturally Aged Mice
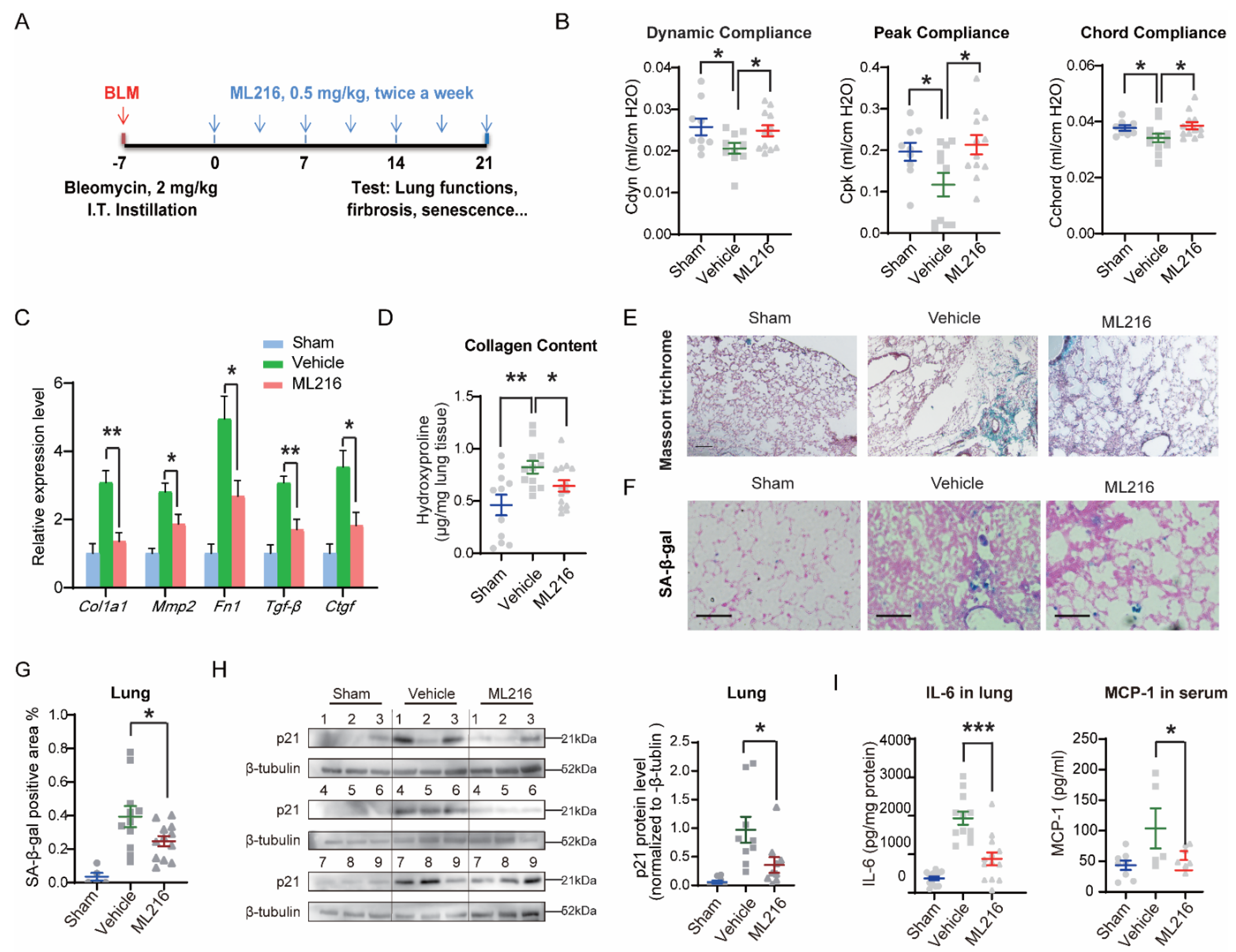
3.6. ML216 Reduces Senescence-Associated Pathological Changes in IPF Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jurk, D.; Maddick, M.; Nelson, G.; Martin-Ruiz, C.; von Zglinicki, T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 2009, 8, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; van Deursen, J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef]
- Bussian, T.J.; Aziz, A.; Meyer, C.F.; Swenson, B.L.; van Deursen, J.M.; Baker, D.J. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 2018, 562, 578–582. [Google Scholar] [CrossRef]
- Jeon, O.H.; Kim, C.; Laberge, R.M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.M.; Demaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W.; et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016, 22, 78–83. [Google Scholar] [CrossRef]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef]
- Tilstra, J.S.; Robinson, A.R.; Wang, J.; Gregg, S.Q.; Clauson, C.L.; Reay, D.P.; Nasto, L.A.; St Croix, C.M.; Usas, A.; Vo, N.; et al. NF-kappaB inhibition delays DNA damage-induced senescence and aging in mice. J. Clin. Investig. 2012, 122, 2601–2612. [Google Scholar] [CrossRef]
- Xu, M.; Tchkonia, T.; Ding, H.; Ogrodnik, M.; Lubbers, E.R.; Pirtskhalava, T.; White, T.A.; Johnson, K.O.; Stout, M.B.; Mezera, V.; et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. USA 2015, 112, E6301–E6310. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef]
- von Kobbe, C. Targeting senescent cells: Approaches, opportunities, challenges. Aging (Albany NY) 2019, 11, 12844–12861. [Google Scholar] [CrossRef]
- de Keizer, P.L. The Fountain of Youth by Targeting Senescent Cells? Trends Mol. Med. 2017, 23, 6–17. [Google Scholar] [CrossRef]
- Munoz-Espin, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb Perspect. Med. 2015, 5, a025130. [Google Scholar] [CrossRef]
- Petr, M.A.; Tulika, T.; Carmona-Marin, L.M.; Scheibye-Knudsen, M. Protecting the Aging Genome. Trends Cell Biol. 2020, 30, 117–132. [Google Scholar] [CrossRef]
- Kim, J.E.; Chen, J.; Lou, Z. DBC1 is a negative regulator of SIRT1. Nature 2008, 451, 583–586. [Google Scholar] [CrossRef]
- Hiraike, H.; Wada-Hiraike, O.; Nakagawa, S.; Koyama, S.; Miyamoto, Y.; Sone, K.; Tanikawa, M.; Tsuruga, T.; Nagasaka, K.; Matsumoto, Y.; et al. Identification of DBC1 as a transcriptional repressor for BRCA1. Br. J. Cancer 2010, 102, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bonkowski, M.S.; Moniot, S.; Zhang, D.; Hubbard, B.P.; Ling, A.J.; Rajman, L.A.; Qin, B.; Lou, Z.; Gorbunova, V.; et al. A conserved NAD(+) binding pocket that regulates protein-protein interactions during aging. Science 2017, 355, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Escande, C.; Nin, V.; Pirtskhalava, T.; Chini, C.C.; Thereza Barbosa, M.; Mathison, A.; Urrutia, R.; Tchkonia, T.; Kirkland, J.L.; Chini, E.N. Deleted in Breast Cancer 1 regulates cellular senescence during obesity. Aging Cell 2014, 13, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Colman, L.; Contreras, P.; Chini, C.C.; Carlomagno, A.; Leyva, A.; Bresque, M.; Marmisolle, I.; Quijano, C.; Duran, R.; et al. A novel form of Deleted in breast cancer 1 (DBC1) lacking the N-terminal domain does not bind SIRT1 and is dynamically regulated in vivo. Sci. Rep. 2019, 9, 14381. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, K.A.; Gangloff, S.; Rothstein, R. The RecQ DNA helicases in DNA repair. Annu. Rev. Genet. 2010, 44, 393–417. [Google Scholar] [CrossRef] [PubMed]
- Cunniff, C.; Bassetti, J.A.; Ellis, N.A. Bloom’s Syndrome: Clinical Spectrum, Molecular Pathogenesis, and Cancer Predisposition. Mol. Syndromol. 2017, 8, 4–23. [Google Scholar] [CrossRef]
- Nguyen, G.H.; Dexheimer, T.S.; Rosenthal, A.S.; Chu, W.K.; Singh, D.K.; Mosedale, G.; Bachrati, C.Z.; Schultz, L.; Sakurai, M.; Savitsky, P.; et al. A small molecule inhibitor of the BLM helicase modulates chromosome stability in human cells. Chem. Biol. 2013, 20, 55–62. [Google Scholar] [CrossRef]
- Magni, M.; Buscemi, G.; Zannini, L. Cell cycle and apoptosis regulator 2 at the interface between DNA damage response and cell physiology. Mutat. Res. 2018, 776, 1–9. [Google Scholar] [CrossRef]
- Sharpless, N.E.; Sherr, C.J. Forging a signature of in vivo senescence. Nat. Rev. Cancer 2015, 15, 397–408. [Google Scholar] [CrossRef]
- Freire, R.; d’Adda Di Fagagna, F.; Wu, L.; Pedrazzi, G.; Stagljar, I.; Hickson, I.D.; Jackson, S.P. Cleavage of the Bloom’s syndrome gene product during apoptosis by caspase-3 results in an impaired interaction with topoisomerase IIIalpha. Nucleic Acids Res. 2001, 29, 3172–3180. [Google Scholar] [CrossRef]
- Bischof, O.; Kim, S.H.; Irving, J.; Beresten, S.; Ellis, N.A.; Campisi, J. Regulation and localization of the Bloom syndrome protein in response to DNA damage. J. Cell Biol. 2001, 153, 367–380. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; Martin, O.A.; Bonner, W.M. p21: A Two-Faced Genome Guardian. Trends Mol. Med. 2017, 23, 310–319. [Google Scholar] [CrossRef]
- Qin, B.; Minter-Dykhouse, K.; Yu, J.; Zhang, J.; Liu, T.; Zhang, H.; Lee, S.; Kim, J.; Wang, L.; Lou, Z. DBC1 functions as a tumor suppressor by regulating p53 stability. Cell Rep. 2015, 10, 1324–1334. [Google Scholar] [CrossRef]
- Lu, H.; Fang, E.F.; Sykora, P.; Kulikowicz, T.; Zhang, Y.; Becker, K.G.; Croteau, D.L.; Bohr, V.A. Senescence induced by RECQL4 dysfunction contributes to Rothmund-Thomson syndrome features in mice. Cell Death Dis. 2014, 5, e1226. [Google Scholar] [CrossRef]
- Lu, H.; Davis, A.J. Human RecQ Helicases in DNA Double-Strand Break Repair. Front. Cell Dev. Biol. 2021, 9, 640755. [Google Scholar] [CrossRef]
- Yosef, R.; Pilpel, N.; Papismadov, N.; Gal, H.; Ovadya, Y.; Vadai, E.; Miller, S.; Porat, Z.; Ben-Dor, S.; Krizhanovsky, V. p21 maintains senescent cell viability under persistent DNA damage response by restraining JNK and caspase signaling. EMBO J. 2017, 36, 2280–2295. [Google Scholar] [CrossRef]
- Murtha, L.A.; Morten, M.; Schuliga, M.J.; Mabotuwana, N.S.; Hardy, S.A.; Waters, D.W.; Burgess, J.K.; Ngo, D.T.; Sverdlov, A.L.; Knight, D.A.; et al. The Role of Pathological Aging in Cardiac and Pulmonary Fibrosis. Aging Dis. 2019, 10, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Beaudet, G.; Bouet, V.; Jozet-Alves, C.; Schumann-Bard, P.; Dauphin, F.; Paizanis, E.; Boulouard, M.; Freret, T. Spatial memory deficit across aging: Current insights of the role of 5-HT7 receptors. Front. Behav. Neurosci. 2014, 8, 448. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhou, H.; Zhu, Y.; Sun, Q.; Ji, Y.; Xue, A.; Wang, Y.; Chen, W.; Yu, X.; Wang, L.; et al. Elimination of senescent cells by beta-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 2020, 30, 574–589. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Geng, Y.; Li, L.; Li, X.; Yan, X.; Fang, Y.; Li, X.; Dong, S.; Liu, X.; Li, X.; et al. Blocking follistatin-like 1 attenuates bleomycin-induced pulmonary fibrosis in mice. J. Exp. Med. 2015, 212, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Plantier, L.; Cazes, A.; Dinh-Xuan, A.T.; Bancal, C.; Marchand-Adam, S.; Crestani, B. Physiology of the lung in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2018, 27, 147. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.J.; Chen, J.H.; Rocha, N.; Semple, R.K. Human pluripotent stem cell-based models suggest preadipocyte senescence as a possible cause of metabolic complications of Werner and Bloom Syndromes. Sci. Rep. 2020, 10, 7490. [Google Scholar] [CrossRef] [PubMed]
- Szekely, A.M.; Bleichert, F.; Numann, A.; Van Komen, S.; Manasanch, E.; Ben Nasr, A.; Canaan, A.; Weissman, S.M. Werner protein protects nonproliferating cells from oxidative DNA damage. Mol. Cell. Biol. 2005, 25, 10492–10506. [Google Scholar] [CrossRef] [PubMed]
- Colman, L.; Caggiani, M.; Leyva, A.; Bresque, M.; Liechocki, S.; Maya-Monteiro, C.M.; Mazal, D.; Batthyany, C.; Calliari, A.; Contreras, P.; et al. The protein Deleted in Breast Cancer-1 (DBC1) regulates vascular response and formation of aortic dissection during Angiotensin II infusion. Sci. Rep. 2020, 10, 6772. [Google Scholar] [CrossRef]
- Xu, J.; Liu, P.; Meng, X.; Bai, J.; Fu, S.; Guan, R.; Sun, W. Association between sister chromatid exchange and double minute chromosomes in human tumor cells. Mol. Cytogenet. 2015, 8, 91. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Chen, J.; Gong, Z. TopBP1 stabilizes BLM protein to suppress sister chromatid exchange. Mol. Cell 2015, 57, 955–956. [Google Scholar] [CrossRef][Green Version]
- Brown, J.P.; Wei, W.; Sedivy, J.M. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science 1997, 277, 831–834. [Google Scholar] [CrossRef]
- Aoshiba, K.; Tsuji, T.; Nagai, A. Bleomycin induces cellular senescence in alveolar epithelial cells. Eur. Respir. J. 2003, 22, 436–443. [Google Scholar] [CrossRef]
- Chu, W.K.; Hickson, I.D. RecQ helicases: Multifunctional genome caretakers. Nat. Rev. Cancer 2009, 9, 644–654. [Google Scholar] [CrossRef]
- Ouyang, K.J.; Woo, L.L.; Zhu, J.; Huo, D.; Matunis, M.J.; Ellis, N.A. SUMO modification regulates BLM and RAD51 interaction at damaged replication forks. PLoS Biol. 2009, 7, e1000252. [Google Scholar] [CrossRef]
- Mao, F.J.; Sidorova, J.M.; Lauper, J.M.; Emond, M.J.; Monnat, R.J. The human WRN and BLM RecQ helicases differentially regulate cell proliferation and survival after chemotherapeutic DNA damage. Cancer Res. 2010, 70, 6548–6555. [Google Scholar] [CrossRef]
- Beamish, H.; Kedar, P.; Kaneko, H.; Chen, P.; Fukao, T.; Peng, C.; Beresten, S.; Gueven, N.; Purdie, D.; Lees-Miller, S.; et al. Functional link between BLM defective in Bloom’s syndrome and the ataxia-telangiectasia-mutated protein, ATM. J. Biol. Chem. 2002, 277, 30515–30523. [Google Scholar] [CrossRef]
- Bugreev, D.V.; Yu, X.; Egelman, E.H.; Mazin, A.V. Novel pro- and anti-recombination activities of the Bloom’s syndrome helicase. Genes Dev. 2007, 21, 3085–3094. [Google Scholar] [CrossRef]
- Srivastava, V.; Modi, P.; Tripathi, V.; Mudgal, R.; De, S.; Sengupta, S. BLM helicase stimulates the ATPase and chromatin-remodeling activities of RAD54. J. Cell Sci. 2009, 122, 3093–3103. [Google Scholar] [CrossRef][Green Version]
- Johnson, F.B.; Lombard, D.B.; Neff, N.F.; Mastrangelo, M.A.; Dewolf, W.; Ellis, N.A.; Marciniak, R.A.; Yin, Y.; Jaenisch, R.; Guarente, L. Association of the Bloom syndrome protein with topoisomerase IIIalpha in somatic and meiotic cells. Cancer Res. 2000, 60, 1162–1167. [Google Scholar]
- Langland, G.; Kordich, J.; Creaney, J.; Goss, K.H.; Lillard-Wetherell, K.; Bebenek, K.; Kunkel, T.A.; Groden, J. The Bloom’s syndrome protein (BLM) interacts with MLH1 but is not required for DNA mismatch repair. J. Biol. Chem. 2001, 276, 30031–30035. [Google Scholar] [CrossRef]
- Mohaghegh, P.; Karow, J.K.; Brosh, R.M., Jr.; Bohr, V.A.; Hickson, I.D. The Bloom’s and Werner’s syndrome proteins are DNA structure-specific helicases. Nucleic Acids Res. 2001, 29, 2843–2849. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, F.; Han, X.; Zhang, X.; Wang, S.; Liang, N.; Tan, Q.; Sha, W.; Li, J. ML216 Prevents DNA Damage-Induced Senescence by Modulating DBC1–BLM Interaction. Cells 2023, 12, 145. https://doi.org/10.3390/cells12010145
Cui F, Han X, Zhang X, Wang S, Liang N, Tan Q, Sha W, Li J. ML216 Prevents DNA Damage-Induced Senescence by Modulating DBC1–BLM Interaction. Cells. 2023; 12(1):145. https://doi.org/10.3390/cells12010145
Chicago/Turabian StyleCui, Feng, Xueying Han, Xiaoqian Zhang, Siqi Wang, Na Liang, Qing Tan, Wuga Sha, and Jun Li. 2023. "ML216 Prevents DNA Damage-Induced Senescence by Modulating DBC1–BLM Interaction" Cells 12, no. 1: 145. https://doi.org/10.3390/cells12010145
APA StyleCui, F., Han, X., Zhang, X., Wang, S., Liang, N., Tan, Q., Sha, W., & Li, J. (2023). ML216 Prevents DNA Damage-Induced Senescence by Modulating DBC1–BLM Interaction. Cells, 12(1), 145. https://doi.org/10.3390/cells12010145







