Trifloroside Induces Bioactive Effects on Differentiation, Adhesion, Migration, and Mineralization in Pre-Osteoblast MC3T3E-1 Cells
Abstract
:1. Introduction
2. Results
2.1. Isolation of TriFs from Gentianae Scabrae Radix and Its Effect on Cellular Toxicity
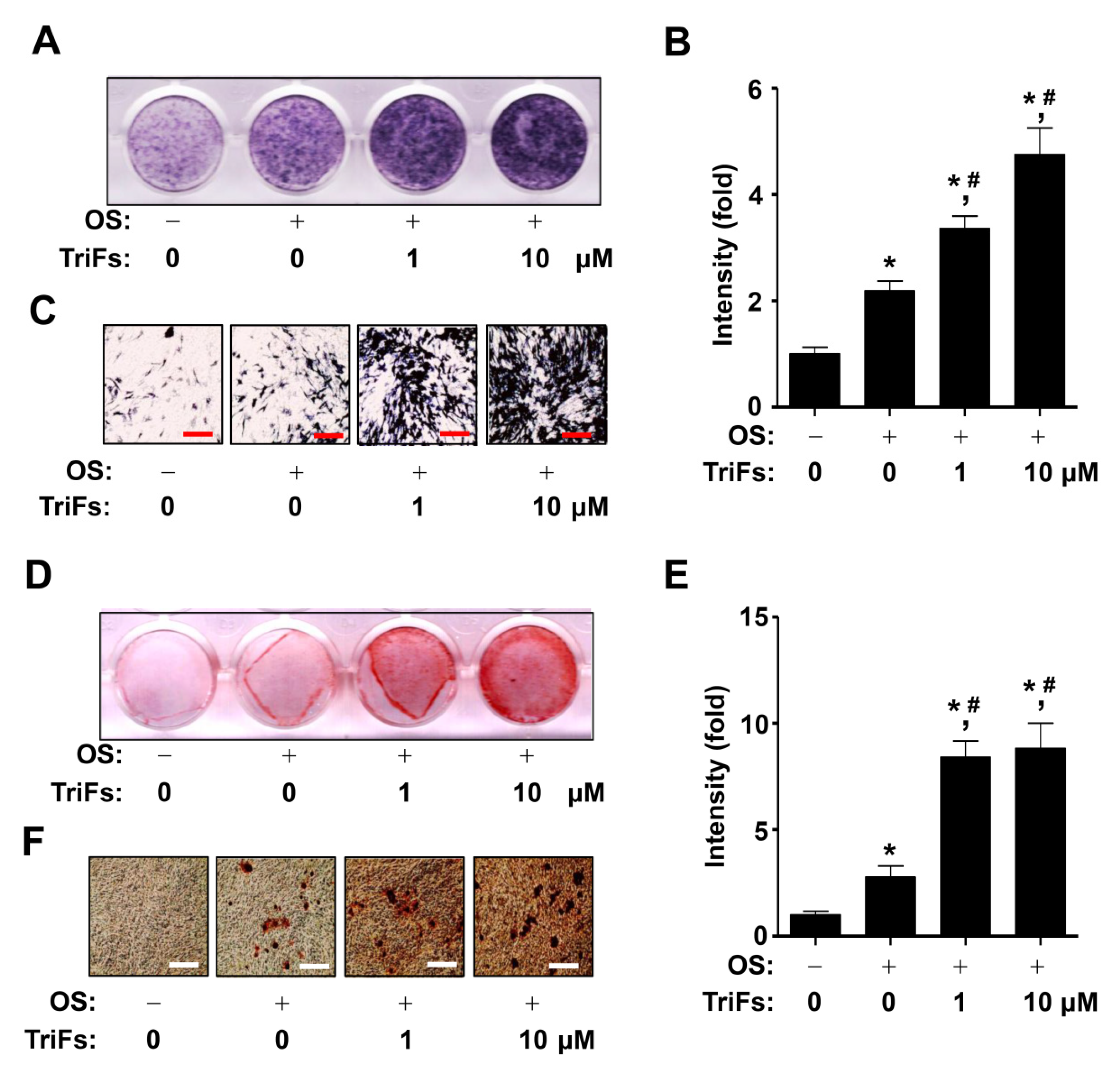
2.2. TriFs Accelerates Osteoblast Differentiation and Maturation
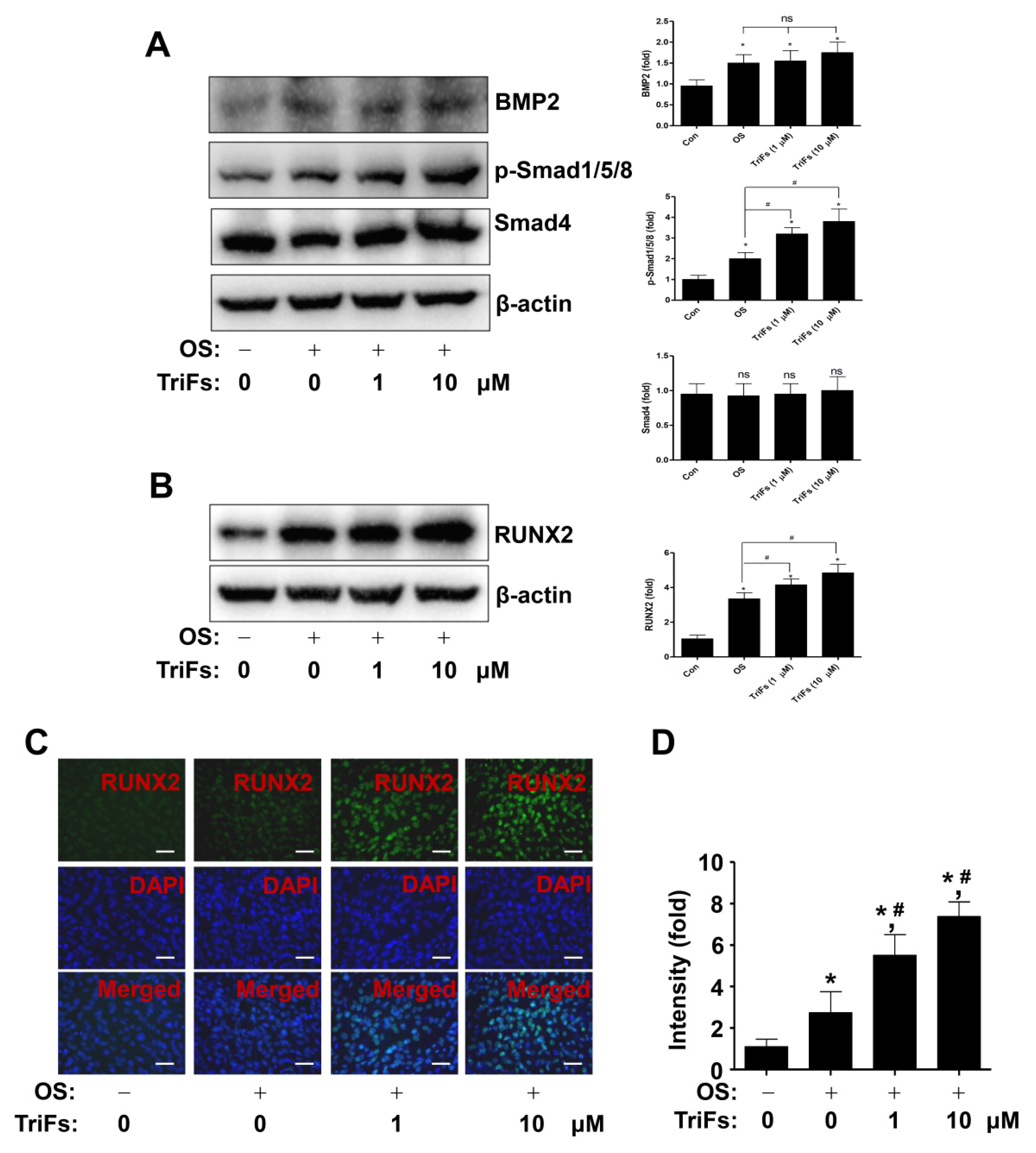
2.3. TriFs Regulates Multiple Signaling Pathways in Osteoblast Differentiation
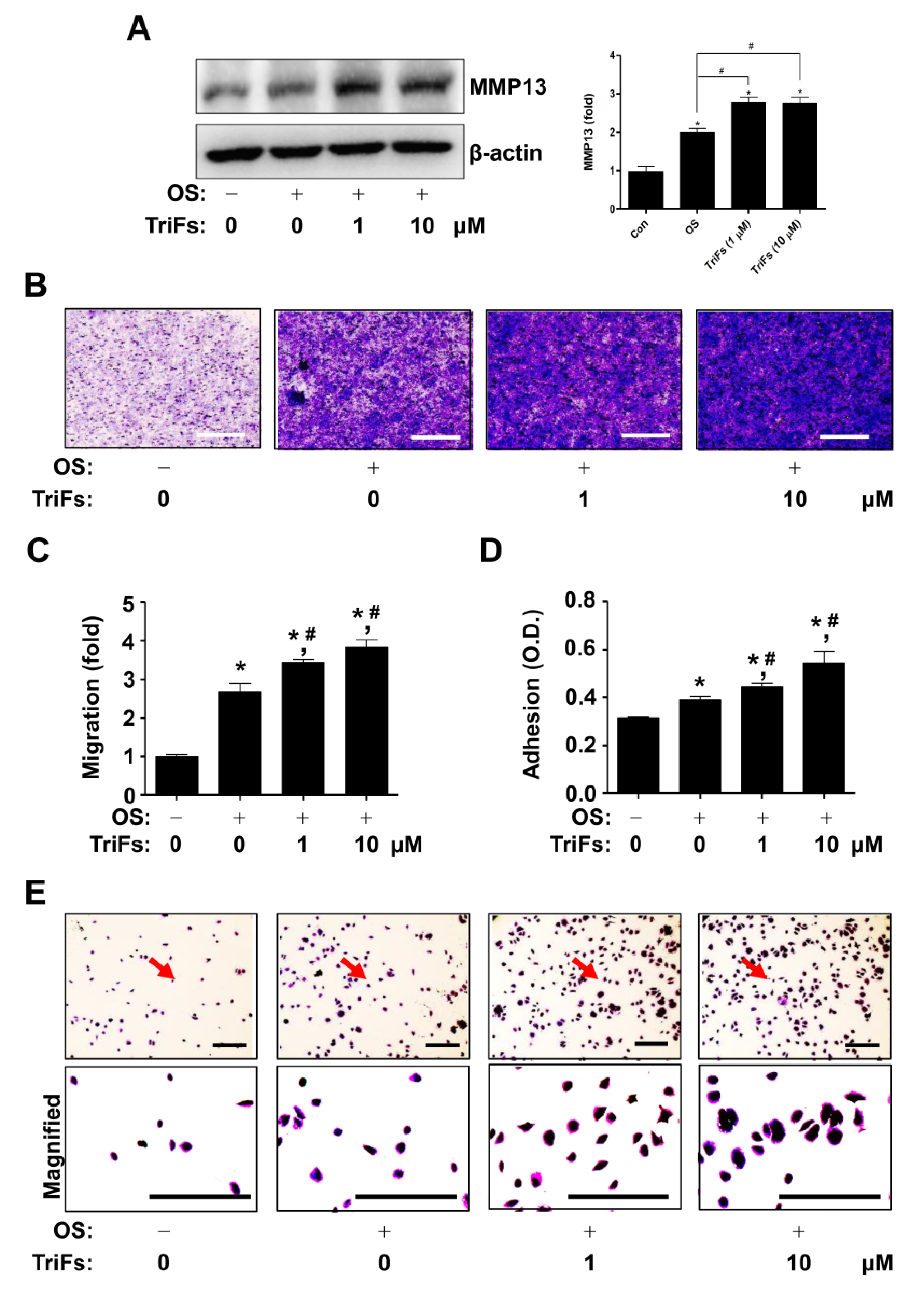
2.4. TriFs Accelerates Migration and Adhesion during Osteoblast Differentiation
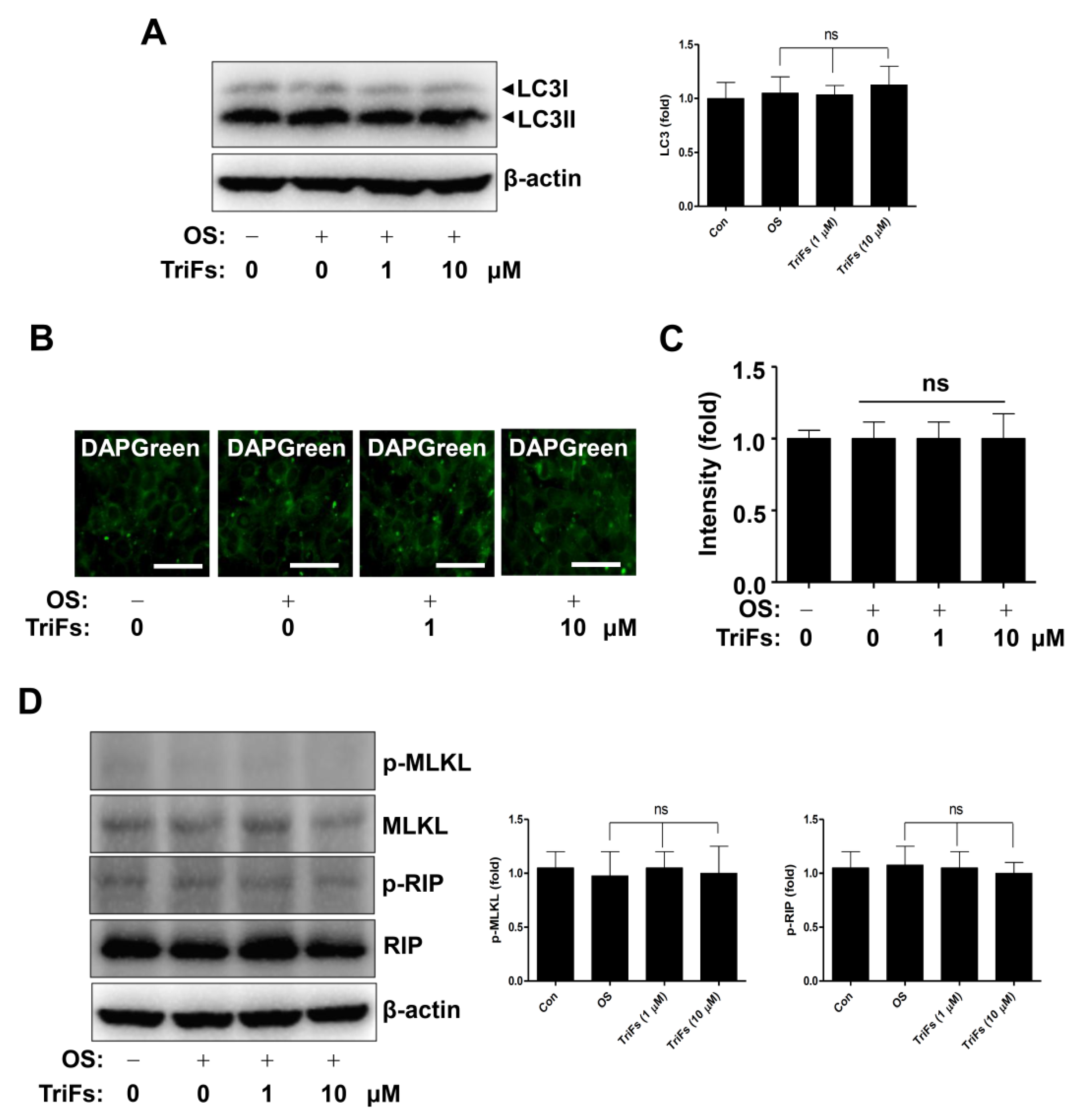
2.5. Effects of TriFs on Autophagy and Necroptosis in Osteoblast Differentiation
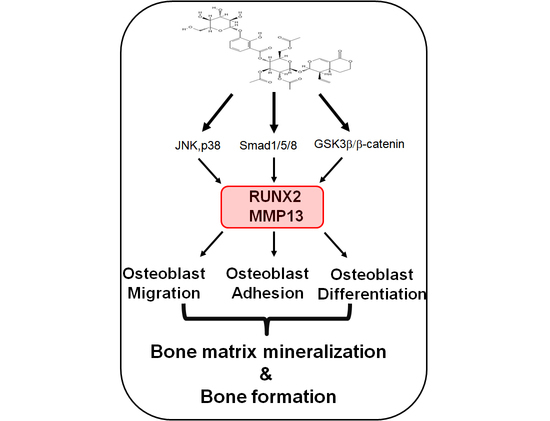
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Experimental Procedures of Plant Material
5.2. Pre-Osteoblast MC3T3E-1 Cells and Osteoblast Differentiation
5.3. Cell Viability
5.4. ALP and ARS Staining
5.5. Western Blotting
5.6. Immunofluorescence
5.7. Migration Assay
5.8. Cell Adhesion Assay
5.9. DAPGreen Autophagy Detection Assay
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase. |
| ARS | Alizarin Red S. |
| BMP2 | Bone morphogenetic protein 2. |
| β-GP | β-glycerophosphate. |
| L-AA | L-ascorbic acid. |
| LC3 | Microtubule associated protein light chain 3. |
| MAPKs | Mitogen-activated protein kinases. |
| MLKL | Mixed lineage kinase domain-like pseudokinase. |
| MMP | Matrix metalloproteinase. |
| MSCs | Mesenchymal stem cells. |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide. |
| OS | Osteogenic supplement medium. |
| RIP | Receptor-interacting serine/threonine-protein kinas. |
| RUNX2 | Runt-related transcription factor 2. |
| TriFs | Trifloroside. |
References
- Kobayashi, T.; Kronenberg, H.M. Overview of Skeletal Development. Methods Mol. Biol. 2021, 2230, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Khotib, J.; Gani, M.A.; Budiatin, A.S.; Lestari, M.; Rahadiansyah, E.; Ardianto, C. Signaling Pathway and Transcriptional Regulation in Osteoblasts during Bone Healing: Direct Involvement of Hydroxyapatite as a Biomaterial. Pharmaceuticals 2021, 14, 615. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Lee, J.Y.; Cho, M.; Hong, J.T.; Yun, H.M. Biological Mechanisms of Paeonoside in the Differentiation of Pre-Osteoblasts and the Formation of Mineralized Nodules. Int. J. Mol. Sci. 2021, 22, 6899. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhang, T.; Jiang, M.; Yin, X.; Luo, X.; Sun, H. Effect of the immune responses induced by implants in a integrated three-dimensional micro-nano topography on osseointegration. J. Biomed. Mater. Res. A 2021, 109, 1429–1440. [Google Scholar] [CrossRef]
- Park, K.R.; Kim, S.; Cho, M.; Yun, H.M. Limonoid Triterpene, Obacunone Increases Runt-Related Transcription Factor 2 to Promote Osteoblast Differentiation and Function. Int. J. Mol. Sci. 2021, 22, 2483. [Google Scholar] [CrossRef]
- Deng, T.; Zhang, W.; Zhang, Y.; Zhang, M.; Huan, Z.; Yu, C.; Zhang, X.; Wang, Y.; Xu, J. Thyroid-stimulating hormone decreases the risk of osteoporosis by regulating osteoblast proliferation and differentiation. BMC Endocr. Disord. 2021, 21, 49. [Google Scholar] [CrossRef]
- Shalehin, N.; Hosoya, A.; Takebe, H.; Hasan, M.R.; Irie, K. Boric acid inhibits alveolar bone loss in rat experimental periodontitis through diminished bone resorption and enhanced osteoblast formation. J. Dent. Sci. 2020, 15, 437–444. [Google Scholar] [CrossRef]
- Martiniakova, M.; Babikova, M.; Omelka, R. Pharmacological agents and natural compounds: Available treatments for osteoporosis. J. Physiol. Pharmacol. 2020, 71. [Google Scholar] [CrossRef]
- He, Y.M.; Zhu, S.; Ge, Y.W.; Kazuma, K.; Zou, K.; Cai, S.Q.; Komatsu, K. The anti-inflammatory secoiridoid glycosides from gentianae scabrae radix: The root and rhizome of Gentiana scabra. J. Nat. Med. 2015, 69, 303–312. [Google Scholar] [CrossRef]
- Jiang, R.W.; Wong, K.L.; Chan, Y.M.; Xu, H.X.; But, P.P.; Shaw, P.C. Isolation of iridoid and secoiridoid glycosides and comparative study on Radix gentianae and related adulterants by HPLC analysis. Phytochemistry 2005, 66, 2674–2680. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.W.; Lee, K.B.; Kim, K.S.; Yang, H.J.; Choi, E.K.; Shin, M.H.; Park, Y.S.; Na, Y.C.; Ahn, K.S.; Jang, Y.P.; et al. A bitter herbal medicine Gentiana scabra root extract stimulates glucagon-like peptide-1 secretion and regulates blood glucose in db/db mouse. J. Ethnopharmacol. 2015, 172, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Lee, J.G.; Song, K.S. The Glycosidase Treatment of Gentianae Scabrae Radix Converts Trifloroside into Deglucosyltrifloroside with an Enhancement of Antioxidative Effects. J. Med. Food 2017, 20, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, C.; Marston, A.; Gauthier, R.; Hostettmann, K. Iridoids and secoiridoids from Gentiana linearis. Phytochemistry 1997, 44, 633–637. [Google Scholar] [CrossRef]
- Liang, J.; Bao, A.L.; Ma, H.Y.; Dong, W.; Li, W.H.; Wu, X.; Li, H.Y.; Hou, H.Y.; Chen, Y.Q.; Fu, J.L.; et al. Prevention of polycystic ovary syndrome and postmenopausal osteoporosis by inhibiting apoptosis with Shenling Baizhu powder compound. PeerJ 2022, 10, e13939. [Google Scholar] [CrossRef]
- Xue, C.; Pan, W.; Lu, X.; Guo, J.; Xu, G.; Sheng, Y.; Yuan, G.; Zhao, N.; Sun, J.; Guo, X.; et al. Effects of compound deer bone extract on osteoporosis model mice and intestinal microflora. J. Food Biochem. 2021, 45, e13740. [Google Scholar] [CrossRef]
- Di, Y.; Wasan, E.K.; Cawthray, J.; Syeda, J.; Ali, M.; Cooper, D.M.L.; Al-Dissi, A.; Ashjaee, N.; Cheng, W.; Johnston, J.; et al. Evaluation of La(XT), a novel lanthanide compound, in an OVX rat model of osteoporosis. Bone Rep. 2021, 14, 100753. [Google Scholar] [CrossRef]
- Li, M.; Shi, X.L.; Xu, C.; Wu, L.G.; He, B.; Li, Y.H.; Liang, B.C. Mechanism action of Chinese herbal compound and target network pharmacology of Yougui (YG) pill for the treatment of osteoporosis. Zhongguo Gu Shang 2020, 33, 933–937. [Google Scholar] [CrossRef]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef]
- Soelaiman, I.N.; Das, S.; Shuid, A.N.; Mo, H.; Mohamed, N. Use of medicinal plants and natural products for treatment of osteoporosis and its complications. Evid. Based Complement. Alternat. Med. 2013, 2013, 764701. [Google Scholar] [CrossRef]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef] [PubMed]
- Infante, A.; Rodriguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granero-Molto, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orimo, H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wennberg, C.; Hessle, L.; Lundberg, P.; Mauro, S.; Narisawa, S.; Lerner, U.H.; Millan, J.L. Functional characterization of osteoblasts and osteoclasts from alkaline phosphatase knockout mice. J. Bone Miner. Res. 2000, 15, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef]
- Canalis, E.; Economides, A.N.; Gazzerro, E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr. Rev. 2003, 24, 218–235. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, V.; Bryant, H.U.; MacDougald, O.A. Regulation of bone mass by Wnt signaling. J. Clin. Invest. 2006, 116, 1202–1209. [Google Scholar] [CrossRef]
- Reya, T.; Clevers, H. Wnt signalling in stem cells and cancer. Nature 2005, 434, 843–850. [Google Scholar] [CrossRef]
- Gaur, T.; Lengner, C.J.; Hovhannisyan, H.; Bhat, R.A.; Bodine, P.V.; Komm, B.S.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 2005, 280, 33132–33140. [Google Scholar] [CrossRef]
- Huang, R.L.; Yuan, Y.; Tu, J.; Zou, G.M.; Li, Q. Opposing TNF-alpha/IL-1beta- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation. Cell Death Dis. 2014, 5, e1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, H.M.; Park, K.R.; Quang, T.H.; Oh, H.; Hong, J.T.; Kim, Y.C.; Kim, E.C. 2,4,5-Trimethoxyldalbergiquinol promotes osteoblastic differentiation and mineralization via the BMP and Wnt/beta-catenin pathway. Cell Death Dis. 2015, 6, e1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phimphilai, M.; Zhoa, Z.R.; Boules, H.; Roca, H.; Franceschi, R.T. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J. Bone Min. Res. 2006, 21, 637–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toriseva, M.; Laato, M.; Carpen, O.; Ruohonen, S.T.; Savontaus, E.; Inada, M.; Krane, S.M.; Kahari, V.M. MMP-13 regulates growth of wound granulation tissue and modulates gene expression signatures involved in inflammation, proteolysis, and cell viability. PLoS ONE 2012, 7, e42596. [Google Scholar] [CrossRef]
- Zaragoza, C.; Lopez-Rivera, E.; Garcia-Rama, C.; Saura, M.; Martinez-Ruiz, A.; Lizarbe, T.R.; Martin-de-Lara, F.; Lamas, S. Cbfa-1 mediates nitric oxide regulation of MMP-13 in osteoblasts. J. Cell Sci. 2006, 119, 1896–1902. [Google Scholar] [CrossRef] [Green Version]
- Uusitalo, H.; Hiltunen, A.; Soderstrom, M.; Aro, H.T.; Vuorio, E. Expression of cathepsins B, H, K, L, and S and matrix metalloproteinases 9 and 13 during chondrocyte hypertrophy and endochondral ossification in mouse fracture callus. Calcif. Tissue Int. 2000, 67, 382–390. [Google Scholar] [CrossRef]
- Yamagiwa, H.; Tokunaga, K.; Hayami, T.; Hatano, H.; Uchida, M.; Endo, N.; Takahashi, H.E. Expression of metalloproteinase-13 (Collagenase-3) is induced during fracture healing in mice. Bone 1999, 25, 197–203. [Google Scholar] [CrossRef]
- Hess, J.; Porte, D.; Munz, C.; Angel, P. AP-1 and Cbfa/runt physically interact and regulate parathyroid hormone-dependent MMP13 expression in osteoblasts through a new osteoblast-specific element 2/AP-1 composite element. J. Biol. Chem. 2001, 276, 20029–20038. [Google Scholar] [CrossRef] [Green Version]
- Javed, A.; Barnes, G.L.; Pratap, J.; Antkowiak, T.; Gerstenfeld, L.C.; van Wijnen, A.J.; Stein, J.L.; Lian, J.B.; Stein, G.S. Impaired intranuclear trafficking of Runx2 (AML3/CBFA1) transcription factors in breast cancer cells inhibits osteolysis in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 1454–1459. [Google Scholar] [CrossRef] [Green Version]
- Nollet, M.; Santucci-Darmanin, S.; Breuil, V.; Al-Sahlanee, R.; Cros, C.; Topi, M.; Momier, D.; Samson, M.; Pagnotta, S.; Cailleteau, L.; et al. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy 2014, 10, 1965–1977. [Google Scholar] [CrossRef]
- Kim, I.R.; Kim, S.E.; Baek, H.S.; Kim, B.J.; Kim, C.H.; Chung, I.K.; Park, B.S.; Shin, S.H. The role of kaempferol-induced autophagy on differentiation and mineralization of osteoblastic MC3T3-E1 cells. BMC Complement. Altern. Med. 2016, 16, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhang, S.; Liu, J.; Liu, Y.; Liang, Q. Vitamin K2 stimulates MC3T3E1 osteoblast differentiation and mineralization through autophagy induction. Mol. Med. Rep. 2019, 19, 3676–3684. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Yun, H.M. RANKL-induced osteoclastogenesis in bone marrow-derived macrophages is suppressed by cisapride. Toxicology 2019, 422, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Yun, H.M.; Yeo, I.J.; Cho, S.; Hong, J.T.; Jeong, Y.S. Peroxiredoxin 6 Inhibits Osteogenic Differentiation and Bone Formation Through Human Dental Pulp Stem Cells and Induces Delayed Bone Development. Antioxid. Redox Signal. 2019, 30, 1969–1982. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Park, J.E.; Kim, B.; Kwon, I.K.; Hong, J.T.; Yun, H.M. Calycosin-7-O-beta-Glucoside Isolated from Astragalus membranaceus Promotes Osteogenesis and Mineralization in Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 11362. [Google Scholar] [CrossRef]
- Park, K.R.; Jeong, Y.; Lee, J.; Kwon, I.K.; Yun, H.M. Anti-tumor effects of jaceosidin on apoptosis, autophagy, and necroptosis in human glioblastoma multiforme. Am. J. Cancer Res. 2021, 11, 4919–4930. [Google Scholar]
- Park, K.R.; Leem, H.H.; Cho, M.; Kang, S.W.; Yun, H.M. Effects of the amide alkaloid piperyline on apoptosis, autophagy, and differentiation of pre-osteoblasts. Phytomedicine 2020, 79, 153347. [Google Scholar] [CrossRef]
- Park, K.R.; Leem, H.H.; Kwon, Y.J.; Kwon, I.K.; Hong, J.T.; Yun, H.M. Falcarindiol Stimulates Apoptotic and Autophagic Cell Death to Attenuate Cell Proliferation, Cell Division, and Metastasis through the PI3K/AKT/mTOR/p70S6K Pathway in Human Oral Squamous Cell Carcinomas. Am. J. Chin. Med. 2022, 50, 295–311. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.-M.; Kim, B.; Park, J.E.; Park, K.-R. Trifloroside Induces Bioactive Effects on Differentiation, Adhesion, Migration, and Mineralization in Pre-Osteoblast MC3T3E-1 Cells. Cells 2022, 11, 3887. https://doi.org/10.3390/cells11233887
Yun H-M, Kim B, Park JE, Park K-R. Trifloroside Induces Bioactive Effects on Differentiation, Adhesion, Migration, and Mineralization in Pre-Osteoblast MC3T3E-1 Cells. Cells. 2022; 11(23):3887. https://doi.org/10.3390/cells11233887
Chicago/Turabian StyleYun, Hyung-Mun, Bomi Kim, Ji Eun Park, and Kyung-Ran Park. 2022. "Trifloroside Induces Bioactive Effects on Differentiation, Adhesion, Migration, and Mineralization in Pre-Osteoblast MC3T3E-1 Cells" Cells 11, no. 23: 3887. https://doi.org/10.3390/cells11233887
APA StyleYun, H.-M., Kim, B., Park, J. E., & Park, K.-R. (2022). Trifloroside Induces Bioactive Effects on Differentiation, Adhesion, Migration, and Mineralization in Pre-Osteoblast MC3T3E-1 Cells. Cells, 11(23), 3887. https://doi.org/10.3390/cells11233887