Abstract
The calcium-sensing receptor (CaSR), a G-protein-coupled receptor (GPCR), is a cell-surface-located receptor that can induce highly diffusible messengers (IP3, Ca2+, cAMP) in the cytoplasm to activate various cellular responses. Recently, it has also been suggested that the CaSR mediates the intracellular communications between the endoplasmic reticulum (ER), mitochondria, nucleus, protease/proteasome, and autophagy–lysosome, which are involved in related cardiovascular diseases. The complex intracellular signaling of this receptor challenges it as a valuable therapeutic target. It is, therefore, necessary to understand the mechanisms behind the signaling characteristics of this receptor in intracellular communication. This review provides an overview of the recent research progress on the various regulatory mechanisms of the CaSR in related cardiovascular diseases and the heart–kidney interaction; the associated common causes are also discussed.
1. Introduction
The calcium-sensing receptor (CaSR) is a recognized member of G-protein-coupled receptors (GPCRs) Class III or Family C and plays multiple roles in animal cells, such as cell proliferation, apoptosis, autophagy, migration, and invasion, which contributes to CaSR signaling as a potential therapeutic target for related physiological disorders, diseases, or even cancer intervention [1,2,3]. However, constant activation of CaSR signaling will lead to Ca2+ homeostasis alteration [4].
The CaSR has three typical structural domains of GPCRs: a large extracellular amino (N)-terminal domain, with 612 amino acid residues; a 7 transmembrane domain (TMD) of 250 amino acid residues; and a 216-amino-acid-containing intracellular carboxy (C)-terminal domain [5]. It was revealed that receptor activation engenders intrinsic 7TM asymmetry in the homodimer that promoted G-protein coupling, which was stabilized by calcimimetic positive allosteric modulators but locked by calcilytic negative allosteric modulators [5]. The binding of the negative allosteric modulator (NPS-2143) in 7TMD rearranges the dimeric of two 7TMDs and pushes TM5, TM6, and TM7 away [6]. In addition, Chen et al. found a new Ca2+ binding site that stabilized the closure of active Venus flytraps, leading to conformational changes in the 7TMDs to initiate signaling [7]. These structural studies of the CaSR provide insights into the mechanism of CaSR signaling and the potential therapeutic strategies for related diseases. However, these in vitro studies still have limitations, as they cannot identify multiple CaSR-mediated intracellular signals.
Both the extracellular amino (N)-terminal domain and TMD participate in Ca2+ sensing. The cytoplasmic C-terminal domain forms dimers and couples with the G-protein subtypes, including Gαq/11, Gαi/o, Gα12/13, and Gαs, to induce various signaling pathways. For example, Gαq/11 activates the phospholipase C (PLC)/inositol 1, 4, 5-trisphosphate (IP3)/IP3 receptor/endoplasmic reticulum (ER)-released Ca2+ pathway, the PLC/diacylglycerol (DAG)/protein kinase C (PKC)/MAPK pathways, as well as the phospholipase A2 (PLA2)–arachidonic acid (AA) pathway [8,9]. CaSR-activated Gαi/o inhibits the adenylate cyclase (AC)/cyclic AMP (cAMP) pathway [8]. Gα12/13 induces Rho/PLD-mediated cytoskeletal remodeling [10,11], and Gαs elevates cAMP levels [12]. However, as these studies investigated CaSR signaling in limited cell lines, they only focused on parts of the whole picture.
These CaSR signaling pathways are dependent on the expression tissues of the CaSR. The CaSR is expressed in both calcitropic tissues such as the parathyroid glands, the kidneys, and the bone, and non-calcitropic tissues such as the lung, neurons, and heart [13]. The CaSR plays an important role in cardiovascular physiology, and the dysregulation of CaSR signaling participates in cardiovascular diseases [14]. In the vascular endothelial cell, extracellular Ca2+ activates the CaSR to increase the cytosolic Ca2+ level through intracellular Ca2+ release from the endoplasmic/sarcoplasmic reticulum (ER/SR) and extracellular Ca2+ entry [15,16]. However, whether the CaSR-induced increase in the cytosolic Ca2+ level caused the mitochondrial Ca2+ overload in vascular endothelial cells was not identified. The Ca2+ overload in the mitochondrial of vascular endothelial cells is associated with the loss of mitochondrial membrane potential, impaired mitochondrial respiration, and the excessive generation of mitochondrial reactive oxygen species (ROS) [17,18]. As one of the functions of the CaSR is Ca2+ redistribution and homeostasis, its role in vascular endothelial cells should be further investigated. A comparison between the spontaneous calcium transients and calcium handling proteins from human-induced pluripotent-stem-cell (hiPSC)-derived cardiomyocytes and those from human embryonic stem cells (hESCs) indicated that calcium homeostasis is a key mechanism underlying the calcium handling properties of hiPSC-derived cardiomyocytes [19]. However, this has not been further investigated in terms of the regulators of calcium homeostasis. In mouse ESCs, the CaSR is functionally expressed, and the activation of the CaSR is involved in the differentiation of mESCs into cardiomyocytes, while the differentiation rate was abolished by the inhibition of the CaSR, phospholipase C, the IP3 receptor, and Ca2+-ATPase, or by the depletion of the SR Ca2+ store [20]. This should be reinforced by further confirmation of in vivo results. In acute myocardial infarction (AMI) rats, CaSR expression was increased over time, and the inhibition of the CaSR by Calhex231 enhanced the efficacy of mESC transplantation for the treatment of AMI by inhibiting apoptosis and oxidative stress [21]. The effects of the CaSR inhibitor Calhex231 on the differentiation of mESCs have not been investigated, and the complications of the xenotransplantation of mouse cells into rats also need to be considered.
However, due to the highly specific cell-type specificity of CaSR regulation and signaling, the pharmacotherapy of cardiovascular diseases that directly target the CaSR may have adverse effects on other tissues, such as kidney injury. The CaSR has a pivotal role in maintaining Ca2+ homeostasis in the kidneys, and the activation of the CaSR might protect against kidney injury or chronic kidney disease (CKD) [22,23]. In mouse renal tubular cells, the activation of the CaSR by NPS R-467 could restore the intracellular Ca2+ level by switching the PLC pathway [22]. However, the mechanisms of the activation of the CaSR in regulating intracellular Ca2+ redistribution and homeostasis have not been investigated. There is considerable interest to use stem and progenitor cells, such as mesenchymal stem cells (MSCs), ESCs, iPSCs, and kidney-derived stem/progenitor cells (KSPCs) to recapitulate kidney development and restore renal function [24]. However, more preclinical studies are required because the molecular, morphological, and functional characterizations of the “kidney cells” generated from these cells have not been exhaustive. The long-term safety of renal stem cells and potential negative effects such as tumor formation also need consideration. In metanephric mesenchymal cells, the spontaneous calcium activity is dependent on the release of Ca2+ from the ER store and acts as a stochastic factor for the self-organizing process that controls branching morphogenesis and determines the ultimate number of nephrons in the kidney [25]. Since the CaSR has a central role in inducing Ca2+ release from the ER store, the regulation of the CaSR may play an important role in the branching morphogenesis of metanephric mesenchymal cells. In addition, the CaSR can protect podocytes from stress-related death and also mediates dysfunctional integrin signaling and the potassium channel function in podocyte-like cells derived from the induced pluripotent stem cells in Alport syndrome patients [26]. The limited sample size and the in vitro studies may not truly show the functional properties of podocytes in situ. Due to the multiple roles of the CaSR in kidney diseases, it is necessary to understand the potential of targeting the CaSR as a therapeutic approach in cardiovascular disease patients with cardiorenal syndrome.
The CaSR-regulated cytosolic and organellar Ca2+ levels activate various pathways, leading to two different endpoints: the sublethal (possibly reversible) or lethal (cell death) changes responsible for subsequent reactions to injury, such as remodeling (i.e., hypertrophy) and repair (i.e., diabetic cardiomyopathy and fibrosis). The disruption of Ca2+ homeostasis contributes to various types of cell death: (a) abnormal ROS production; (b) abnormal cytoskeletal contraction leading to catastrophic cell architecture disorganization; (c) the improper activation of cellular proteases (proteasomes, caspases, calpains, matrix metallopeptidases, etc.), leading to the rapid irreversible degradation of crucial substrates; (d) pore formation in the plasma membrane leading to pyroptosis and necroptosis [27,28,29]. The regulation of the CaSR on these types of cell death has been investigated in cardiovascular and kidney cells, as well as some other cell types [30,31,32]. Thereby, the clarification of the cellular signaling of the CaSR may help to treat cardiovascular diseases in the future. This review focuses on the CaSR-mediated intracellular communication in the non-calcitropic cells of the cardiovascular system and calcitropic kidney cells and the diverse regulation of CaSR signaling in protecting or promoting cardiovascular diseases. We present a general overview of CaSR signaling between the mitochondria, ER, nucleus, lysosome, and proteasomes in related cardiovascular diseases. In addition, the CaSR signaling pathways involved in various common causes of cardiovascular diseases are discussed based on the available data.
2. CaSR Signaling in Cardiovascular Diseases
The CaSR is expressed in different types of cardiovascular cells, such as vascular smooth muscle cells (VSMCs), endothelial cells, and ventricular cardiomyocytes, in which it plays important roles in the regulation of proliferation, blood pressure, and blood vessel tone [33,34]. To identify the role of the CaSR in related cardiovascular diseases, some in vivo and in vitro studies have been performed. In the following sections, we discuss the CaSR-mediated intracellular communication in several classical cardiovascular diseases, including ischemia/reperfusion damage, myocardial hypertrophy, diabetic cardiomyopathy, and cardiorenal syndrome, which is associated with the heart–kidney interaction.
2.1. Ischemia/Reperfusion Damage
Ischemia/reperfusion increases the intracellular and mitochondrial Ca2+ levels by reducing active Ca2+ reuptake into the ER and ATPase-dependent Ca2+ efflux across the plasma membrane [35]. The CaSR induces Ca2+ release from the ER and its transfer into the mitochondria, leading to mitochondrial depolarization, which is mediated by the mitochondria-associated ER membranes (MAMs), and it is a potential treatment target in cardiac ischemia–reperfusion injury and heart failure [36,37]. The increasing free radicals released from the mitochondria further aggravate ER stress. This indicates that protein kinase C (PKC) plays a central role in the CaSR-mediated communication between the mitochondria and the ER. The CaSR can be phosphorylated by PKC, which regulates cell growth, homeostasis, and programmed death. PKC phosphorylates the CaSR, which will cause the desensitization of the CaSR, and as a feedback mechanism, the activation of the CaSR also increases the phosphorylation of PKC. PKC-δ translocation from the membrane to the cytosol was found in the ischemic heart. Additionally, in the isolated mitochondria from hearts that had undergone ischemia/reperfusion (I/R) combined with the CaSR agonist GdCl3, a significant increase was observed in phosphorylated PKC-δ translocation to the mitochondria, as well as an increased release of cytochrome c from the mitochondria with an obvious decrease in the mitochondrial potential [38,39]. As the CaSR is sensitive to the environmental Ca2+ levels, its function as a buffer or its operation during the isolation process of the heart may also modulate CaSR-mediated intracellular Ca2+ signaling and homeostasis.
PKC-ε interacts with the CaSR in the ER–mitochondria crosstalk, which might be a potential target to prevent I/R-induced cardiovascular injury, and the underlying molecular mechanism can be further identified at a cellular level. PKC-α has been shown to be associated with the crosstalk of mitochondrial and ER stress in human hormone-refractory prostate cancer cells [40], and PKC-ε interacts with the CaSR to protect apoptosis via inhibiting the mitochondrial Ca2+ overload and the disruption of the mitochondrial function induced by the ER in ischemic post-conditioning cardiomyocytes [41]. It has been reported that I/R increased the expression of the CaSR and cardiomyocyte apoptosis, and the activation of the CaSR by GdCl3 induced PKC-δ translocation into the ER to induce ER stress-associated apoptotic pathways [42,43]. However, there is still no direct evidence to prove that PKC-δ interacts with the CaSR to regulate the ER–mitochondria crosstalk in the ischemia/reperfusion heart and cardiomyocytes.
In addition, PKC-ε translocates from the cytosol to the nucleus in the ischemic heart during cardiogenesis [39,44], while the function of the nuclear translocation of PKC-ε in cardiovascular diseases has not been identified. It was also observed that PKC-α and PKC-ε were proteolyzed by calpains in the ischemic heart and myocardium [45,46]. Calpain is an apoptotic protein in the mitochondria, and the nuclear translocation of calpain-2 promotes cardiomyocyte apoptosis and remodeling [47,48,49]. Moreover, PKC-α signaling by the calpain-generated free catalytic domains induces HDAC5 nuclear export and regulates cardiac transcription [50]. In these studies on tail-suspended rats, the results suggested that high-dose isoproterenol activated the CaSR by increasing the phosphorylation of the phospholamban of the nuclear envelope and increasing the intranuclear Ca2+ transients. However, the mechanism of the CaSR in the regulation of calpain translocation (from the mitochondria to the nucleus) involved in the mitochondria–nucleus crosstalk in cardiomyocytes has not been investigated.
The results of in vitro and in vivo studies in spinal cord neurons showed that the expressions of the CaSR and calpain increased during the spinal cord ischemia–reperfusion injury (SCIRI), and the expressions of calpains were enhanced or decreased by the CaSR agonist GdCl3 and antagonist NPS-2390, respectively [51]. The CaSR inhibitor NPS-2390 protects cardiomyocyte apoptosis by decreasing the CaSR-regulated calpain expression [52]. These studies indicate that the CaSR interacts with PKC and calpain proteins, which can translocate from the membrane to the mitochondria and/or to the nucleus in I/R hearts and cardiomyocytes; however, the mechanism of the CaSR–PKC/calpain pathway in the ER–mitochondria and mitochondria–nucleus communication, and its roles in cardiovascular diseases still need further studies (Figure 1).
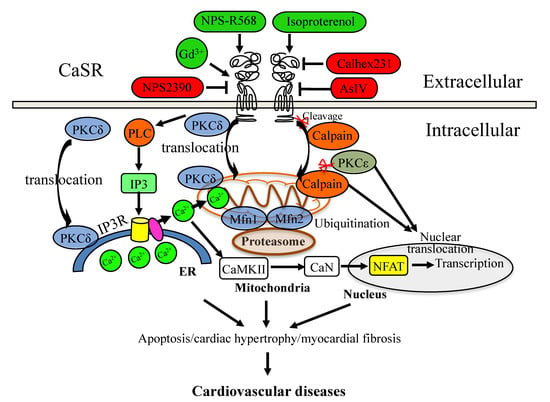
Figure 1.
CaSR signaling mediates cardiovascular diseases. The activation regulator is in red color, and the negative modulator is in green color.
2.2. Cardiac Hypertrophy
Hypertension is one of the cardiovascular risk factors for hypertrophy and the development of heart failure. Chronic hypertension causes left ventricular hypertrophy, which ultimately results in heart failure. Previous studies revealed that the CaSR could be a potential therapeutic target for hypertension. In spontaneously hypertensive rats (SHRs), calcimimetic R568 administration reduced blood pressure and myocardial remodeling and reversed the low expression of the CaSR [46]. As a negative allosteric modulator of the CaSR, Calhex231 decreased the heart-to-body weight ratio and the protein levels of the CaSR and attenuated myocardial apoptosis during hypertension [53]. In these two studies, they applied only one modulator of the CaSR as treatment, but it is interesting that both the activation and inhibition of the CaSR could protect the myocardia against hypertension.
Furthermore, previous studies suggested that the regulation of the CaSR could target the mitochondria and ER during cardiac hypertrophy. The inhibition of the CaSR by Calhex231 maintained the mitochondrial dynamic and function through regulating fission and fusion proteins in SHRs [53]. In the cardiac hypertrophy and heart failure model of Wistar rats, the subcutaneous injection of isoproterenol activated the CaSR, increased Ca2+ concentration in the mitochondria, decreased the mitochondrial membrane potential, and induced the release of cytochrome c from the mitochondria during ER stress and apoptosis [54]. The CaSR might mediate the Ca2+ transfer from the ER to mitochondria and regulate the mitochondrial dynamics, which contribute to cardiac hypertrophy and hypertension.
The CaSR-induced Ca2+ release from the ER could activate some Ca2+ binding proteins, such as calcineurin (CaN) and the Ca2+/calmodulin-dependent protein kinase type 2 (CaMK-II), which further promote the canonical nuclear factor of activated T cells (NFATs) to translocate into the nucleus to regulate the expressions of target genes. In the isolated neonatal rat ventricular myocytes, the CaSR specifically modulated nuclear Ca2+ signaling through the IP3R pathway, and the CaSR agonist GdCl3 induced cardiomyocyte hypertrophy through the CaN–NFAT pathway [55]. In the same cells, astragaloside IV (AsIV) attenuated cardiac hypertrophy and apoptosis and protected against cardiac injury by reducing the expression of the CaSR, reducing Ca2+ release from the ER through the PLC–IP3R pathway, and promoting Ca2+ reuptake into the ER through the sarcoplasmic reticulum Ca2+-ATPase2a (SERCA2a) pathway, suppressing the activation of the CaMK-II and CaN pathways and inhibiting NFAT-3 nuclear translocation [56]. The results in SHRs suggested that sodium ferulate protected against hypertension-induced cardiac hypertrophy by inhibiting the CaSR-mediated CaN-NFAT-3 signaling pathway [57]. Although these studies had not identified the downstream effectors of the CaSR-mediated CaN-NFAT-3 signaling pathway, they confirmed that the CaSR could regulate the ER–nucleus communication in hypertension-induced cardiac hypertrophy (Figure 1). It is worth noting that the CaSR may also mediate the crosstalk between the mitochondria and nucleus, as the nuclear translocation of mitochondria-related protein PKC-α has been found to induce hypertrophy in neonatal rat ventricular myocytes [58], and the calpain-2 mediated apoptosis of hypertrophied cardiomyocytes has also been observed in transverse aortic constriction rats [59].
2.3. Diabetic Cardiomyopathy
Diabetic cardiomyopathy (DCM) is a severe complication of type 1 and type 2 diabetic (T1D/T2D) patients characterized by the human pathophysiological conditions of dyslipidemia, hypertension, and coronary artery disease and results in heart failure. Myocardial fibrosis is a main pathological feature of DCM. High glucose is a common treatment in the studies of diabetic models. In T1D rats and primary neonatal rat cardiac fibroblasts, it was observed that high glucose induced myocardial fibrosis [60]. In cardiac fibroblasts, high glucose also caused excessive cardiac fibroblast proliferation and extensive collagen deposition [61]. Interestingly, the effects of high glucose on the expression of the CaSR depended on cell types. In T1D rats and the primary cultured neonatal rat cardiomyocytes, the CaSR was suppressed by high glucose [62,63], which is contrary to the effects of high glucose in cardiac fibroblasts [61]. However, the mechanism of the opposite effects of high glucose on the expression of the CaSR in cardiomyocytes and cardiac fibroblasts is still unclear, which may promote cell differentiation and contribute to myocardial fibrosis in DCM.
A high-fat diet induces the proteolytic cleavage of the CaSR by calpain, which contributes to the macrovascular late complications associated with diabetes [64]. The ubiquitin–proteasome pathway is another main proteolytic process, which can also be regulated by the CaSR. Previous studies suggested that the inhibition of the CaSR by Calhex231 could alleviate high-glucose-induced myocardial fibrosis via inhibiting the ubiquitin–proteasome pathway [60,61]. Furthermore, the CaSR also regulated mitochondrial function through the ubiquitin–proteasome pathway. The activation of the CaSR protected against a high-glucose-induced decrease in the mitochondrial fusion-related (Mfn1, Mfn2) protein levels via blocking the gp78-ubiquitin-proteasome pathway [65]. By targeting the gp78-ubiquitin-proteasome system, the supplement of spermine downregulated the expression of the CaSR following the decreased ubiquitination levels of mitochondrial fusion-related proteins (Mfn1, Mfn2), which in turn protected cardiomyocytes from high-glucose-induced energy disturbance [66]. These studies suggested that the regulation of CaSR-mediated protease/proteasome signaling with the mitochondria might be a potential treatment for DCM.
3. CaSR-Mediated Signaling in Heart-Kidney Interaction
Treatment for cardiac disease patients with kidney injury is particularly challenging, and the damage and dysfunction of one system are often associated with the dysfunction of the other through organ crosstalk. This observation in the clinical diagnosis is defined as a cardiorenal syndrome (CRS), which represents the crosstalk between heart failure and kidney injury and vice versa, as well as alterations in the inflammatory molecules of its clinical phenotypes [67]. For example, interleukin-6 (IL-6) was found to be associated with left-ventricular hypertrophy and systolic dysfunction in patients with CKD, and a high level of IL-6 in CKD contributed to chronic inflammation and subsequently left-ventricular dysfunction [68,69]. The tumor necrosis factor (TNF) and IL-10 contributed to cardiac remodeling and cardiovascular phenotype in the arteries of children with CKD [70].
Acute and chronic heart failure triggers acute kidney injury (AKI) and chronic kidney disease (CKD), which are defined, respectively, as type 1 and type 2 CRS. Types 3 and 4 CRS are the primary insults of renal injury with secondary cardiac dysfunction, and type 5 CRS reflects a systemic condition (e.g., sepsis) simultaneously causing both cardiac and renal dysfunction [71,72]. Recently, the CaSR was considered to be a novel target for the treatment of sepsis-induced CRS (type 5 CRS), as the CaSR mediates sepsis-induced oxidative stress, inflammation, apoptosis, and cardiorenal dysfunction [73]. In addition, the sepsis-increased CaSR expression in T lymphocytes (circulating or resident in heart or kidney tissues) promoted the release of TNF-α and IL-4 cytokines and induced tissue apoptosis and injury [74]. The CaSR mediates a series of pathways involved in CRS, which are described in the following sections.
3.1. Inflammatory-Reparative Response
Previous studies have demonstrated that the CaSR mediated the inflammation that influences the cardiovascular system, such as atherosclerosis, vascular calcification, myocardial infarction, hypertension, and obesity [75]. After acute myocardial infarction (AMI), the cardiac system undergoes a repair process, which is mediated by cytokines and inflammatory cells that infiltrate the infarcted sites and require an optimally orchestrated inflammatory-reparative response (IRR) [76]. IRR is indicated as a pathway constantly associated with the CaSR because of the following factors:
(a) Ca2+ or other CaSR agonists directly induce the activation of the inflammasome. The activation of the CaSR induced Ca2+ release from the ER stores through PLC-IP3 signaling and also reduced the intracellular cAMP, and both of them promoted the assembly of inflammasome components, which was reduced by the knockdown of the CaSR [77]. In addition, the AsIV-inhibited CaSR could suppress the high-glucose-induced NLRP3 inflammasome in human umbilical vein endothelial cells (HUVECs) [78]. The Calhex231-inhibited CaSR decreased the NLRP3 inflammasome activation and IL-1β release in neutrophils from patients with AMI [79]. The CaSR also mediated the inflammatory response in the kidney. The elevated extracellular calcium levels from necrotic cells recruited the monocytes/macrophages that express the CaSR to the kidney injury sites, and Ca2+ activated the CaSR to stimulate the assembly of inflammasomes resulting in the maturation of proinflammatory cytokine IL-1β by caspase-1 [80]. The activation of the CaSR by R-568 increased the renal injury in streptozotocin-induced diabetic rats associated with the promotion of the inflammatory response by enhancing the proinflammatory cytokine interleukin-6 (IL-6) levels and reducing the anti-inflammatory cytokine IL-10 levels [81], while an excessive inflammatory response may activate the proapoptosis and remodeling of cardiomyocytes, induce matrix degradation, and impair collagen deposition, which will influence the healing process.
(b) Another reason is the release of damage-associated molecular patterns (DAMPs) by cells under Ca2+-mediated stress or death. Clinical injury caused a systemic inflammatory response syndrome by sepsis, and cellular injury could release endogenous DAMPs into the circulation, which activated Ca2+ signaling and the MAPK pathway of polymorphonuclear neutrophils, thus leading to migration and degranulation and eliciting neutrophil-mediated organ injury [82]. As a DAMP, the high-mobility group box 1 (HMGB1) is involved in a large variety of different processes, such as inflammation, migration, invasion, proliferation, differentiation, and tissue regeneration, and it also serves as a regulator of inflammatory-reparative responses following MI, by inducing endothelial cells and modulating cardiac fibroblast activity [76,83].
(c) The activation of CaMK-IIδ pathways in response to pressure overload triggered inflammatory NLRP3 and IL-1β gene expressions and increased the caspase-1 activity and the IL-18 cleavage of the inflammasome in cardiomyocytes [84,85]. Additionally, targeting CaMK-IIδ might prevent cardiac ischemia/reperfusion injury by inhibiting myocardial inflammation [86,87]. Although the regulation of the CaSR on CaMK-II in cardiac hypertrophy and apoptosis has been investigated, the role of this special isotype, CaMK-IIδ, in CaSR-regulated cardiac inflammation still needs to be identified.
(d) The CaSR promoted cardiac apoptosis and fibrosis. In hereditary epileptic rats, it was shown that the CaSR protein, cardiac apoptosis, and fibrosis were increased, thus inducing the activation of the MAPK, transforming growth factor beta (TGF-β), and connective tissue growth factor (CTGF) pathways, which were aggravated by GdCl3 and attenuated by NPS-2390 [88]. In primary neonatal rat cardiac fibroblasts, high glucose induced myocardial fibrosis via the upregulation of the TGF-β1/Smads pathway, which was promoted by the CaSR agonist R568 but reduced by the CaSR inhibitor Calhex231 [89]. In T1D rats, Calhex231 could inhibit the TGF-β1/Smads pathway and then depress the proliferation of cardiac fibroblasts, along with the reduction deposition of collagen, alleviating glucose-induced myocardial fibrosis [60]. Collectively, these results suggested that TGF-β1 pathways were involved in the CaSR-promoted fibrosis of cardiac fibroblasts, but this needs to be further confirmed by investigating the special inhibitors of the pathways, and their downstream effectors also need to be identified. Engrailed 1 (EN1) re-expressed in multiple fibroblast subpopulations as a molecular amplifier of TGFβ signaling in myofibroblast differentiation, and a coordinator of the reorganization of the cytoskeleton during myofibroblast differentiation [90,91,92]. Thus, the targeted inactivation of EN1 may be a potential strategy to inhibit TGFβ signaling and fibroblast activation in fibrotic diseases.
(e) The hypoxia-associated activation of NFkB leads to IRR. Hypoxia occurred in a number of pathological conditions, such as MI, diabetes, obesity, myocardial ischemia, and cardiac hypertrophy. A hypoxia condition can dramatically increase the cytosolic Ca2+ levels and induce necrosis of cells and then release alarmins, which would trigger the activation of NFkB signaling to induce the IRR of the adaptation cells [93]. Chronic intermittent hypoxia-induced cardiac inflammation, apoptosis, and fibrosis in a rat obstructive sleep apnea model were associated with the increased expression of NFkB signaling molecules [94]. In neonatal rat ventricular myocytes and transverse aortic constriction mice, the hypoxia-induced development of cardiac hypertrophy could be prevented by inhibiting the CaSR with Calhex231 [95]. It has been indicated that the CaSR in human peripheral blood T lymphocytes is involved in the AMI onset and progression and promotes IL-6 and TNF-β releases and their mRNA expressions through the NFκB signaling pathway [96,97]. In addition, the CaSR gene transcription was upregulated by IL-1β and TNF-α via NFkB in kidney proximal tubule cells [98]. However, the study of the CaSR regulation of NFkB signaling under hypoxia stress is still limited.
3.2. CaSR/Wnt/β-Catenin Signaling
The activation of Wnt/β-catenin signaling is associated with chronic inflammation and organ fibrosis. Wnt/β-catenin signaling was s upregulated in CKD patients and contributed to remodeling and myocardial fibrosis [99,100]. The inhibition of the Wnt/β-catenin pathway ameliorated CKD-associated vascular calcification, which could be reversed by the PPARγ antagonist GW9662 [101]. CaSR activation modulated the crosstalk between Wnt and PPARγ pathways in adipocytes [102]. The CaSR was revealed to be co-distributed with β-catenin in Madin–Darby canine kidney (MDCK) cells and the β-catenin nuclear localization depended on the activation and expression of the CaSR in colonic epithelial cells [103,104]. It was reported that Wnt/β-catenin signaling mediated both heart and kidney inflammation and fibrosis in type 2 CRS [105]. However, the regulation of inflammation and fibrosis by CaSR/Wnt/β-catenin signaling in the hear-kidney interaction still needs to be clarified.
3.3. Autophagy–Lysosomal Pathway
The CaSR can modulate cell processes through the autophagy-lysosomal pathway, which has great potential as a treatment for damaged kidneys and cardiomyocyte injury. The CaSR-mediated autophagy pathway was not involved in the I/R-induced cardiomyocyte apoptosis [106]. Interestingly, both in vivo and in vitro studies suggested that the CaSR inhibitor Calhex231 inhibited autophagy by suppressing the CaMKKβ-AMPK-mammalian target of rapamycin (mTOR) pathway to ameliorate isoproterenol or Ang II-induced cardiomyocyte hypertrophy and cardiac fibrosis [107,108,109]. In kidney cells, it was shown that the CaSR stimulated the chaperone Hsp70-assisted protein degradation of inflammasome assemblage, including ASC, NLRP3, IRAK1, and TRAF6, through the combination of the ubiquitin–proteasome pathway, endosomal microautophagy, and macroautophagy [110]. Cardiovascular disorders are the most common causes of mortality in autosomal dominant polycystic kidney disease (ADPKD), which is mainly caused by the deficiency of polycystin-1 (PC1) or polycystin-2 (PC2) [111]. The lack of PKD1 impaired lysosomal acidification in a calpain protease-dependent manner, and this altered autophagy flux contributed to ADPKD progression [112]. By using the conditionally immortalized proximal tubular epithelial cells deficient for polycystin-1 (ciPTEC-PC1KD), it was shown that the calcimimetic NPS R-568-activated CaSR could reduce the mTOR activity and restore the lower mitochondrial calcium levels caused by PC1 deficiency, recover the mitochondrial ATP production and repair oxidative phosphorylation [113]. Our previous studies suggested that the activation of the CaSR by calcimimetic compound NPS R-467 could protect against the heavy metal cadmium-induced mouse kidney injury and cell apoptosis by restoring the autophagy–lysosome process [5,23].
3.4. Potential Target of Exosomes in Cardiorenal Syndrome
Due to their role in ischemic signaling, myocardial repair, and communication between the heart and the bone marrow, exosomes are considered novel targets in the CRS puzzle and the crosstalk between the heart and the kidneys [71,72]. The bone marrow mesenchymal stem cell-derived exosomes protected against myocardial infarction by promoting autophagy and inhibiting cell proliferation and migration, as well as cell apoptosis during hypoxia–reoxygenation [114]. However, the mechanism of the autophagy-related proteins involved was not identified. Although the production and function of exosomes in kidney tissues and cells remain unclear, the production of exosomes during hypoxia is protective in renal tubular cells [115]. In vitro, TGF-β1 stimulated kidney proximal tubular cells to produce exosome, which could induce renal interstitial fibroblast activation but was abolished by the exosome secretion inhibitor dimethyl amiloride. In vivo, the injections of tubular-cell-derived exosomes aggravated kidney injury and fibrosis, while these conditions were ameliorated by blocking exosome secretion [116]. In addition, the exosomes from endothelial progenitor cells improved survival, by suppressing renal vascular leakage and reducing kidney dysfunction in septic mice [117]. However, the mechanisms and methodologies of exosomal research in the context of cardiorenal pathology are still limited.
As a Ca2+ release channel on lysosomes, the transient receptor potential mucolipin 1 (TRPML1) channel plays an important role in maintaining cardiovascular and renal glomerular homeostasis and thereby contributes to some cardiovascular and kidney diseases. In arterial myocytes, TRPML1 induced intracellular Ca2+ homeostasis through an SR Ca2+ release. In podocytes, the TRPML1 channel controls lysosome trafficking and exosome release, and its deficiency contributes to podocyte dysfunction [118]. However, the role of CaSR-mediated intracellular Ca2+ in the exosome function is still unclear. An in vivo study suggested that the exosomes from CaSR-stimulated polymorphonuclear cells (PMNs) protected against AMI and reperfusion injury in the myocardial tissue by decreasing apoptosis, ROS production, and hypoxia-reoxygenation [30]. It was also shown that this might be related to the AKT signaling pathway, but the potential role of the CaSR–AKT signaling pathway in regulating autophagy needs to be further clarified.
4. Conclusions
The existing studies demonstrate that the CaSR mediates intracellular communications between the ER, mitochondria, nucleus, protease/proteasome, and autophagy–lysosome, and therefore, it plays complex roles in cardiovascular diseases, including the ischemia/reperfusion-induced myocardial injury, hypertension/hypoxia-induced cardiac hypertrophy, DCM, and cardiorenal syndrome. Moreover, it was also demonstrated that multiple regulations of the CaSR, such as upregulation/downregulation, activation/inhibition, mutation, degradation, or limited proteolytic cleavages, promote or prevent related cardiovascular diseases. In addition, the CaSR-mediated intracellular signaling or pathways contribute to inflammation and fibrosis and are involved in the heart–kidney interaction (Figure 2), which certainly does not rule out the interactions of the heart with other tissues.
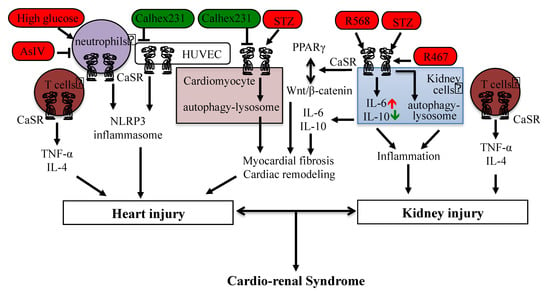
Figure 2.
CaSR-mediated signaling in cardiorenal syndrome and heart–kidney interaction. The activation regulator is in red color, and the negative modulator is in green color.
As the CaSR plays diverse and crucial roles in human physiology and pathophysiology, both in calcitropic and non-calcitropic tissues and cells, the drugs targeting the CaSR to treat human diseases, including cardiovascular diseases, have been considered. Various positive and negative allosteric modulators of the CaSR have been developed, which can increase or inhibit the sensitivity of this receptor by changing the conformation of the receptor. As cited in this review, the pharmaceutical activation or inhibition of the CaSR might be potential therapeutic strategies to prevent or treat vascular or cardiac diseases. However, both the heart and the kidneys are composed of various cells that have multiple crosstalk pathways and may perform diverse responses to these treatments. Even when considering the approved drugs, such as cinacalcet and etelcalcetide, which are applied for treating secondary hyperparathyroidism in cases of CKD and hypercalcemia with parathyroid carcinoma, it should be noted that etelcalcetide has some adverse reactions that seriously worsen heart failure and cardiovascular system. With the benefits derived from the studies of the CaSR structure, researchers can design more special drugs targeting the CaSR, and more advanced experimental tools and models will be helpful for screening the efficacy of therapeutic strategies [119,120]. We believe that in-depth research on the special CaSR signaling pathways in cellular compartments and targeting these pathways may lead to novel therapeutic strategies against related disorders or diseases.
Author Contributions
H.C., Z.Q., J.G. and B.S. discussed the topic of this article. H.C., J.M. and Y.X. wrote the draft of the manuscript. Y.X., Z.Q., J.G. and B.S. participated in the interpretation of the literature. Y.X., Z.Q., H.S., J.G. and B.S. revised the review. All authors revised the draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Development Medical and Health Guidance Plan Project of Yixing City (202038), the Science and Technology Development Medical and Health Guidance Plan Project of Wuxi City (202061), the Science and Technology Innovation (Social Development) Project of Yixing Science and Technology Bureau (2020SF08), and the Scientific and Technological Achievements and Suitable Technology Promotion Project of Wuxi Health Commission (T202126).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alfadda, T.I.; Saleh, A.M.; Houillier, P.; Geibel, J.P. Calcium-sensing receptor 20 years later. Am. J. Physiol. Cell Physiol. 2014, 307, C221–C231. [Google Scholar] [CrossRef] [PubMed]
- Kosiba, A.A.; Wang, Y.; Chen, D.; Wong, C.K.C.; Gu, J.; Shi, H. The roles of calcium-sensing receptor (CaSR) in heavy metals-induced nephrotoxicity. Life. Sci. 2020, 242, 117183. [Google Scholar] [CrossRef] [PubMed]
- Tuffour, A.; Kosiba, A.A.; Zhang, Y.; Peprah, F.A.; Gu, J.; Shi, H. Role of the calcium-sensing receptor (CaSR) in cancer metastasis to bone: Identifying a potential therapeutic target. Biochim. Biophys. Acta. Rev. Cancer. 2021, 1875, 188528. [Google Scholar] [CrossRef]
- Leach, K.; Hannan, F.M.; Josephs, T.M.; Keller, A.N.; Møller, T.C.; Ward, D.T.; Kallay, E.; Mason, R.S.; Thakker, R.V.; Riccardi, D.I.; et al. International union of basic and clinical pharmacology. CVIII. calcium-sensing receptor nomenclature, pharmacology, and function. Pharmacol. Rev. 2020, 72, 558–604. [Google Scholar] [CrossRef]
- Gao, Y.; Robertson, M.J.; Rahman, S.N.; Seven, A.B.; Zhang, C.; Meyerowitz, J.G.; Panova, O.; Hannan, F.M.; Thakker, R.V.; Bräuner-Osborne, H.; et al. Asymmetric activation of the calcium-sensing receptor homodimer. Nature 2021, 595, 455–459. [Google Scholar] [CrossRef]
- Wen, T.; Wang, Z.; Chen, X.; Ren, Y.; Lu, X.; Xing, Y.; Lu, J.; Chang, S.; Zhang, X.; Shen, Y.; et al. Structural basis for activation and allosteric modulation of full-length calcium-sensing receptor. Sci. Adv. 2021, 7, eabg1483. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, L.; Cui, Q.; Ding, Z.; Han, L.; Kou, Y.; Zhang, W.; Wang, H.; Jia, X.; Dai, M.; et al. Structural insights into the activation of human calcium-sensing receptor. Elife 2021, 10, e68578. [Google Scholar] [CrossRef] [PubMed]
- Conigrave, A.D.; Ward, D.T. Calcium-sensing receptor (CaSR): Pharmacological properties and signaling pathways. Best. Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 315–331. [Google Scholar] [CrossRef]
- Smajilovic, S.; Yano, S.; Jabbari, R.; Tfelt-Hansen, J. The calcium-sensing receptor and calcimimetics in blood pressure modulation. Br. J. Pharmacol. 2011, 164, 884–893. [Google Scholar] [CrossRef]
- Davies, S.L.; Gibbons, C.E.; Vizard, T.; Ward, D.T. Ca2+-sensing receptor induces Rho kinase-mediated actin stress fiber assembly and altered cell morphology, but not in response to aromatic amino acids. Am. J. Physiol. Cell. Physiol. 2006, 290, 1543–1551. [Google Scholar] [CrossRef]
- Huang, C.; Hujer, K.M.; Wu, Z.; Miller, R.T. The Ca2+-sensing receptor couples to Galpha12/13 to activate phospholipase D in Madin-Darby canine kidney cells. Am. J. Physiol. Cell. Physiol. 2004, 286, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Mamillapalli, R.; VanHouten, J.; Zawalich, W.; Wysolmerski, J. Switching of G-protein usage by the calcium-sensing receptor reverses its effect on parathyroid hormone-related protein secretion in normal versus malignant breast cells. J. Biol. Chem. 2008, 283, 24435–24447. [Google Scholar] [CrossRef] [PubMed]
- Hannan, F.M.; Kallay, E.; Chang, W.; Brandi, M.L.; Thakker, R.V. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat. Rev. Endocrinol. 2018, 15, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Schreckenberg, R.; Schlüter, K.D. Calcium sensing receptor expression and signalling in cardiovascular physiology and disease. Vascul. Pharmacol. 2018, 107, 35–42. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, X.; He, J.; Liu, J.; Yang, S.; Dong, H. Important roles of the Ca2+-sensing receptor in vascular health and disease. Life. Sci. 2018, 209, 217–227. [Google Scholar] [CrossRef]
- Leong, I.L.; Tsai, T.Y.; Shiao, L.R.; Zhang, Y.M.; Wong, K.L.; Chan, P.; Leung, Y.M. Characterization of Ca2+-sensing receptor-mediated Ca2+ influx in microvascular bEND.3 endothelial cells. Chin. J. Physiol. 2021, 64, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Lian, Q.; Jiang, D.; Ho, J.T.K.; Cheung, Y.F.; Chan, G.C. Deferiprone inhibits iron overload-induced tissue factor bearing endothelial microparticle generation by inhibition oxidative stress induced mitochondrial injury, and apoptosis. Toxicol. Appl. Pharmacol. 2018, 338, 148–158. [Google Scholar] [CrossRef]
- Otto, M.; Bucher, C.; Liu, W.; Müller, M.; Schmidt, T.; Kardell, M.; Driessen, M.N.; Rossaint, J.; Gross, E.R.; Wagner, N.M. 12(S)-HETE mediates diabetes-induced endothelial dysfunction by activating intracellular endothelial cell TRPV1. J. Clin. Invest. 2020, 130, 4999–5010. [Google Scholar] [CrossRef]
- Lee, G.S.; Subramanian, N.; Kim, A.I.; Aksentijevich, I.; Goldbach-Mansky, R.; Sacks, D.B.; Germain, R.N.; Kastner, D.L.; Chae, J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012, 492, 123–127. [Google Scholar] [CrossRef]
- Sun, J.; He, W.; Bai, S.Z.; Peng, X.; Zhang, N.; Li, H.X.; Zhang, W.H.; Wang, L.N.; Shao, X.Q.; He, Y.Q.; et al. The expression of calcium-sensing receptor in mouse embryonic stem cells (mESCs) and its influence on differentiation of mESC into cardiomyocytes. Differentiation 2013, 85, 32–40. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, G.; Li, S.; Li, L.; Wei, T.; Song, G.; Luan, H.; Meng, J.; Wang, Q.; Yu, Y.; et al. Calcium sensing receptor involving in therapy of embryonic stem cell transplantation alleviates acute myocardial infarction by inhibiting apoptosis and oxidative stress in rats. Iran. J. Basic. Med. Sci. 2020, 23, 1353–1359. [Google Scholar]
- Gu, J.; Dai, S.; Liu, Y.; Liu, H.; Zhang, Y.; Ji, X.; Yu, F.; Zhou, Y.; Chen, L.; Tse, W.K.F.; et al. Activation of Ca(2+)-sensing receptor as a protective pathway to reduce cadmium-induced cytotoxicity in renal proximal tubular cells. Sci. Rep. 2018, 8, 1092. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ren, Z.; Zhao, J.; Peprah, F.A.; Xie, Y.; Cheng, D.; Wang, Y.; Liu, H.; Chu Wong, C.K.; Zhou, Y.; et al. Calcimimetic compound NPS R-467 protects against chronic cadmium-induced mouse kidney injury by restoring autophagy process. Ecotoxicol Environ. Saf. 2020, 189, 110052. [Google Scholar] [CrossRef]
- Murray, P.A.; Woolf, A.S. Using stem and progenitor cells to recapitulate kidney development and restore renal function. Curr. Opin. Organ. Transplant. 2014, 19, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.M.; Khodus, G.R.; Unnersjö-Jess, D.; Blom, H.; Aperia, A.; Brismar, H. Spontaneous calcium activity in metanephric mesenchymal cells regulates branching morphogenesis in the embryonic kidney. FASEB J. 2019, 33, 4089–4096. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.M.; Selby, J.N.; Vandekolk, T.H.; Abad, I.P.L.; Ho, J.K.; Lieuw, W.L.; Leach, K.; Savige, J.; Saini, S.; Fisher, C.L.; et al. Induced pluripotent stem cell-derived podocyte-like cells as models for assessing mechanisms underlying heritable disease phenotype: Initial studies using two alport syndrome patient lines indicate impaired potassium channel activity. J. Pharm. Exp. Ther. 2018, 367, 335–347. [Google Scholar] [CrossRef]
- Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G.; Rimessi, A.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell. Calcium. 2018, 69, 62–72. [Google Scholar] [CrossRef]
- Ning, B.; Guo, C.; Kong, A.; Li, K.; Xie, Y.; Shi, H.; Gu, J. Calcium signaling mediates cell death and crosstalk with autophagy in kidney disease. Cells 2021, 10, 3204. [Google Scholar] [CrossRef]
- Sridhar, K.C.; Hersch, N.; Dreissen, G.; Merkel, R.; Hoffmann, B. Calcium mediated functional interplay between myocardial cells upon laser-induced single-cell injury: An in vitro study of cardiac cell death signaling mechanisms. Cell. Commun. Signal. 2020, 18, 191. [Google Scholar] [CrossRef]
- Zhai, T.Y.; Cui, B.H.; Zhou, Y.; Xu, X.Y.; Zou, L.; Lin, X.; Zhu, X.S.; Zhang, S.W.; Xie, W.L.; Cheng, Y.Y.; et al. Exosomes released from CaSR-stimulated PMNs reduce ischaemia/reperfusion injury. Oxid. Med. Cell. Longev. 2021, 2021, 3010548. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, T.; Shi, N.; Yao, L.; Yang, X.; Han, C.; Wen, L.; Du, D.; Szatmary, P.; Mukherjee, R.; et al. Mechanisms of pancreatic injury induced by basic amino acids differ between L-Arginine, L-Ornithine, and L-Histidine. Front. Physiol. 2019, 9, 1922. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, G.; Chen, P.; Liu, K.; Xu, Y.; Liu, Y.; Liu, J. Effects of Cr(VI)-induced calcium-sensing receptor activation on DF-1 cell pyroptosis. Ecotoxicol. Environ. Saf. 2019, 179, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Molostvov, G.; Fletcher, S.; Bland, R.; Zehnder, D. Extracellular calcium-sensing receptor mediated signalling is involved in human vascular smooth muscle cell proliferation and apoptosis. Cell. Physiol. Biochem. 2008, 22, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Schepelmann, M. The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure. Am. J. Physiol. Cell. Physiol. 2016, 310, 193–204. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/reperfusion. Compr. Physiol. 2016, 7, 113–170. [Google Scholar]
- Gao, P.; Yan, Z.; Zhu, Z. Mitochondria-associated endoplasmic reticulum membranes in cardiovascular diseases. Front. Cell. Dev. Biol. 2020, 8, 604240. [Google Scholar] [CrossRef]
- Gong, Y.; Lin, J.; Ma, Z.; Yu, M.; Wang, M.; Lai, D.; Fu, G. Mitochondria-associated membrane-modulated Ca2+ transfer: A potential treatment target in cardiac ischemia reperfusion injury and heart failure. Life Sci. 2021, 278, 119511. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, J.; Liu, C.; Lu, F.; Zhao, Y.; Jin, Z.; Ren, H.; Leng, X.; Jia, J.; Hu, G.; et al. Calcium-sensing receptor activating phosphorylation of PKCdelta translocation on mitochondria to induce cardiomyocyte apoptosis during ischemia/reperfusion. Mol. Cell. Biochem. 2011, 358, 335–343. [Google Scholar] [CrossRef]
- Yoshida, K.; Hirata, T.; Akita, Y.; Mizukami, Y.; Yamaguchi, K.; Sorimachi, Y.; Ishihara, T.; Kawashiama, S. Translocation of protein kinase C-alpha, delta and epsilon isoforms in ischemic rat heart. Biochim. Biophys. Acta 1996, 1317, 36–44. [Google Scholar] [CrossRef]
- Kuo, T.C.; Huang, W.J.; Guh, J.H. WJ9708012 exerts anticancer activity through PKC-α related crosstalk of mitochondrial and endoplasmic reticulum stresses in human hormone-refractory prostate cancer cells. Acta Pharmacol. Sin. 2011, 32, 89–98. [Google Scholar] [CrossRef]
- Dong, S.; Teng, Z.; Lu, F.H.; Zhao, Y.J.; Li, H.; Ren, H.; Chen, H.; Pan, Z.W.; Lv, Y.J.; Yang, B.F.; et al. Post-conditioning protects cardiomyocytes from apoptosis via PKC(epsilon)-interacting with calcium-sensing receptors to inhibit endo(sarco)plasmic reticulum-mitochondria crosstalk. Mol. Cell. Biochem. 2010, 41, 195–206. [Google Scholar]
- Jiang, C.M.; Han, L.P.; Li, H.Z.; Qu, Y.B.; Zhang, Z.R.; Wang, R.; Xu, C.Q.; Li, W.M. Calcium-sensing receptors induce apoptosis in cultured neonatal rat ventricular cardiomyocytes during simulated ischemia/reperfusion. Cell. Biol. Int. 2008, 32, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, H.; Zheng, H.; Zhai, M.; Lu, F.; Dong, S.; Fang, T.; Zhang, W. CaSR activates PKCδ to induce cardiomyocyte apoptosis via ER stress-associated apoptotic pathways during ischemia/reperfusion. Int. J. Mol. Med. 2019, 44, 1117–1126. [Google Scholar] [CrossRef]
- Ventura, C.; Zinellu, E.; Maninchedda, E.; Fadda, M.; Maioli, M. Protein kinase C signaling transduces endorphin-primed cardiogenesis in GTR1 embryonic stem cells. Circ. Res. 2003, 92, 617–622. [Google Scholar] [CrossRef]
- Urthaler, F.; Wolkowicz, P.E.; Digerness, S.B.; Harris, K.D.; Walker, A.A. MDL-28170, a membrane-permeant calpain inhibitor, attenuates stunning and PKC epsilon proteolysis in reperfused ferret hearts. Cardiovasc. Res. 1997, 35, 60–67. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, N.; Xi, D.; Zhao, Y.; Liu, Y.; Wang, L.; Tang, Y.; Zhang, X.; Zhong, H.; He, F. Calcimimetic R568 improved cardiac remodeling by classic and novel renin-angiotensin system in spontaneously hypertensive rats. Exp. Biol. Med. 2019, 244, 789–801. [Google Scholar] [CrossRef]
- Lahiri, S.K.; Quick, A.P.; Samson-Couterie, B.; Hulsurkar, M.; Elzenaar, I.; van Oort, R.J.; Chang, H.; Zhang, L.; Xu, P.T.; Li, Q.; et al. Nuclear translocation of calpain-2 regulates propensity toward apoptosis in cardiomyocytes of tail-suspended rats. J. Cell. Biochem. 2011, 112, 571–580. [Google Scholar]
- Wehrens, X.H.T. Nuclear localization of a novel calpain-2 mediated junctophilin-2 C-terminal cleavage peptide promotes cardiomyocyte remodeling. Basic. Res. Cardiol. 2020, 115, 49. [Google Scholar]
- Chang, H.; Sheng, J.J.; Zhang, L.; Yue, Z.J.; Jiao, B.; Li, J.S.; Yu, Z.B. ROS-induced nuclear translocation of calpain-2 facilitates cardiomyocyte apoptosis in tail-suspended rats. J. Cell. Biochem. 2015, 116, 2258–2269. [Google Scholar] [CrossRef]
- Zhang, Y.; Matkovich, S.J.; Duan, X.; Diwan, A.; Kang, M.Y.; Dorn, G.W. Receptor-independent protein kinase C alpha (PKCalpha) signaling by calpain-generated free catalytic domains induces HDAC5 nuclear export and regulates cardiac transcription. J. Biol. Chem. 2011, 286, 26943–26951. [Google Scholar] [CrossRef]
- Sun, J.F.; Yang, H.L.; Huang, Y.H.; Chen, Q.; Cao, X.B.; Li, D.P.; Shu, H.M.; Jiang, R.Y. CaSR and calpain contribute to the ischemia reperfusion injury of spinal cord. Neurosci. Lett. 2017, 646, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, W.H.; Yang, F.; An, Y.Q.; Zhou, W.; Yu, H.; Xie, H.; Zhang, Y.L.; Zhu, Y.; Shen, X.C.; et al. VEGF121 mediates post-hypoxia cardioprotective effects via casr and mitochondria-dependent protease pathway. Arq. Bras. Cardiol. 2021, 117, 476–483. [Google Scholar] [CrossRef]
- Hong, S.; Zhang, X.; Zhang, X.; Liu, W.; Fu, Y.; Liu, Y.; Shi, Z.; Chi, J.; Zhao, M.; Yin, X. Role of the calcium sensing receptor in cardiomyocyte apoptosis via mitochondrial dynamics in compensatory hypertrophied myocardium of spontaneously hypertensive rat. Biochem. Biophys. Res. Commun. 2017, 487, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.H.; Fu, S.B.; Leng, X.; Zhang, X.; Dong, S.; Zhao, Y.J.; Ren, H.; Li, H.; Zhong, X.; Xu, C.Q.; et al. Role of the calcium-sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure. Cell. Physiol. Biochem. 2013, 31, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Liu, J.; Lu, F.; Wang, Y.; Zhao, Y.; Dong, S.; Leng, X.; Jia, J.; Ren, H.; Xu, C.; et al. Calcium sensing receptor regulates cardiomyocyte function through nuclear calcium. Cell. Biol. Int. 2012, 36, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Leng, B.; He, X.; Zhang, Z.; Wang, H.; Tang, F. Calcium sensing receptor-related pathway contributes to cardiac injury and the mechanism of astragaloside IV on cardioprotection. Front. Pharmacol. 2018, 9, 1163. [Google Scholar] [CrossRef]
- Chen, P.; Wen, Z.; Shi, W.; Li, Z.; Chen, X.; Gao, Y.; Xu, S.; Gong, Q.; Deng, J. Effects of sodium ferulate on cardiac hypertrophy are via the CaSR-mediated signaling pathway. Front. Pharmacol. 2021, 12, 674570. [Google Scholar] [CrossRef]
- Vijayan, K.; Szotek, E.L.; Martin, J.L.; Samarel, A.M. Protein kinase C-alpha-induced hypertrophy of neonatal rat ventricular myocytes. Am. J. Physiol. Heart. Circ. Physiol. 2004, 287, 2777–2789. [Google Scholar] [CrossRef]
- Sheng, J.J.; Chang, H.; Yu, Z.B. Nuclear translocation of Calpain-2 mediates apoptosis of hypertrophied cardiomyocytes in transverse aortic constriction rat. J. Cell. Physiol. 2015, 230, 2743–2754. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, J.; Xu, X.; Gao, T.; Wang, Y.; Fan, Y.; Hu, J.; Shao, Y.; Zhao, B.; Li, H.; et al. Calhex231 alleviates high glucose-induced myocardial fibrosis via inhibiting itch-ubiquitin proteasome pathway in vitro. Biol. Pharm. Bull. 2019, 42, 1337–1344. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, J.; Zhu, Y.; Li, L.; Wang, Q.; Yu, Y.; Zhou, B.; Liu, Y.; Xu, X.; Wang, Z. Activation of calcium sensing receptor mediated autophagy in high glucoseinduced cardiac fibrosis in vitro. Mol. Med. Rep. 2020, 22, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.Z.; Sun, J.; Wu, H.; Zhang, N.; Li, H.X.; Li, G.W.; Li, H.Z.; He, W.; Zhang, W.H.; Zhao, Y.J.; et al. Decrease in calcium-sensing receptor in the progress of diabetic cardiomyopathy. Diabetes. Res. Clin. Pract. 2012, 95, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Li, S.; Zhang, X.; Guo, Z.; Hu, J.; Shao, X.; Song, N.; Zhao, Y.; Li, H.; et al. Exogenous spermine attenuates rat diabetic cardiomyopathy via suppressing ROS-p53 mediated downregulation of calcium-sensitive receptor. Redox. Biol. 2020, 32, 101514. [Google Scholar] [CrossRef] [PubMed]
- Loot, A.E.; Pierson, I.; Syzonenko, T.; Elgheznawy, A.; Randriamboavonjy, V.; Zivkovic, A.; Stark, H.; Fleming, I. Ca2+-sensing receptor cleavage by calpain partially accounts for altered vascular reactivity in mice fed a high-fat diet. J. Cardiovasc. Pharmacol. 2013, 61, 528–535. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, P.; Wei, C.; Li, H.; Zhang, L.; Zhao, Y.; Wu, B.; Tian, Y.; Zhang, W.; Wu, L.; et al. Calcium sensing receptor protects high glucose-induced energy metabolism disorder via blocking gp78-ubiquitin proteasome pathway. Cell. Death Dis. 2017, 8, e2799. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, F.; Zhang, X.; Li, H.; Yang, G.; Xu, C.; Wei, C. Spermine protects cardiomyocytes from high glucose-induced energy disturbance by targeting the CaSR-gp78-Ubiquitin proteasome system. Cardiovasc. Drugs Ther. 2021, 35, 73–85. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal syndrome: Classification, pathophysiology, diagnosis, and treatment strategies: A scientific statement from the american heart association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef]
- Gupta, J.; Dominic, E.A.; Fink, J.C.; Ojo, A.O.; Barrows, I.R.; Reilly, M.P.; Townsend, R.R.; Joffe, M.M.; Rosas, S.E.; Wolman, M.; et al. Association between inflammation and cardiac geometry in chronic kidney disease: Findings from the CRIC study. PLoS ONE 2015, 10, e0124772. [Google Scholar] [CrossRef]
- Hassan, M.O.; Duarte, R.; Dix-Peek, T.; Vachiat, A.; Naidoo, S.; Dickens, C.; Grinter, S.; Manga, P.; Naicker, S. Correlation between volume overload, chronic inflammation, and left ventricular dysfunction in chronic kidney disease patients. Clin. Nephrol. 2016, 86, 131–135. [Google Scholar] [CrossRef]
- Freise, C.; Schaefer, B.; Bartosova, M.; Bayazit, A.; Bauer, U.; Pickardt, T.; Berger, F.; Rasmussen, L.M.; Jensen, P.S.; Laube, G.; et al. Arterial tissue transcriptional profiles associate with tissue remodeling and cardiovascular phenotype in children with end-stage kidney disease. Sci. Rep. 2019, 9, 10316. [Google Scholar] [CrossRef]
- Gonzalez-Calero, L.; Martin-Lorenzo, M.; Alvarez-Llamas, G. Exosomes: A potential key target in cardio-renal syndrome. Front. Immunol. 2014, 5, 465. [Google Scholar] [CrossRef]
- Taub, P.R.; Borden, K.C.; Fard, A.; Maisel, A. Role of biomarkers in the diagnosis and prognosis of acute kidney injury in patients with cardiorenal syndrome. Expert. Rev. Cardiovasc. Ther. 2012, 10, 657–667. [Google Scholar] [CrossRef]
- Yadav, S.; Gupta, K.; Deshmukh, K.; Bhardwaj, L.; Dahiya, A.; Krishan, P.; Singh, G. Calcium sensing receptor as a novel target for treatment of sepsis induced cardio-renal syndrome: Need for exploring mechanisms. Drug. Dev. Res. 2021, 82, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Wu, Q.Y.; Du, J.J.; Zeng, J.Y.; Li, T.T.; Xu, C.Q.; Sun, Y.H. Calcium-sensing receptor in the T lymphocyte enhanced the apoptosis and cytokine secretion in sepsis. Mol. Immunol. 2015, 63, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Sundararaman, S.S.; van der Vorst, E.P.C. Calcium-sensing receptor (CaSR), its impact on inflammation and the consequences on cardiovascular health. Int. Mol. Sci. 2021, 22, 2478. [Google Scholar] [CrossRef] [PubMed]
- Foglio, E.; Pellegrini, L.; Russo, M.A.; Limana, F. HMGB1-mediated activation of the inflammatory-reparative response following myocardial infarction. Cells 2022, 11, 216. [Google Scholar] [CrossRef]
- Lee, Y.K.; Ng, K.M.; Lai, W.H.; Chan, Y.C.; Lau, Y.M.; Lian, Q.; Tse, H.F.; Siu, C.W. Calcium homeostasis in human induced pluripotent stem cell-derived cardiomyocytes. Stem. Cell. Rev. Rep. 2011, 7, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Leng, B.; Zhang, Y.; Liu, X.; Zhang, Z.; Liu, Y.; Wang, H.; Lu, M. Astragaloside IV suppresses high glucose-induced NLRP3 inflammasome activation by inhibiting TLR4/NF-kappaB and CaSR. Mediat. Inflamm. 2019, 2019, 1082497. [Google Scholar] [CrossRef]
- Ren, Z.; Yang, K.; Zhao, M.; Liu, W.; Zhang, X.; Chi, J.; Shi, Z.; Zhang, X.; Fu, Y.; Liu, Y.; et al. Calcium-sensing receptor on neutrophil promotes myocardial apoptosis and fibrosis after acute myocardial infarction via NLRP3 inflammasome activation. Can. J. Cardiol. 2020, 36, 893–905. [Google Scholar] [CrossRef]
- Canaff, L.; Hendy, G.N. Calcium-sensing receptor, proinflammatory cytokines and calcium homeostasis. Semin. Cell. Dev. Biol. 2016, 49, 37–43. [Google Scholar]
- Hu, B.; Tong, F.; Xu, L.; Shen, Z.; Yan, L.; Xu, G.; Shen, R. Role of calcium sensing receptor in streptozotocin-induced diabetic rats exposed to renal ischemia reperfusion injury. Kidney Blood Press. Res. 2018, 43, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Harutyunyan, K.R.; Abrahamyan, H.T.; Adamyan, S.H.; Mkrtchian, S.; Ter-Markosyan, A.S. Calcium-regulating hormonal system and HMGB1 in cardiomyopathies. Endocr. Metab. Immune Disord. Drug Targets, 2022; ahead of print. [Google Scholar] [CrossRef]
- Suetomi, T.; Miyamoto, S.; Brown, J.H. Inflammation in nonischemic heart disease: Initiation by cardiomyocyte CaMKII and NLRP3 inflammasome signaling. Am. J. Physiol. Heart. Circ. Physiol. 2019, 317, H877–H890. [Google Scholar] [CrossRef] [PubMed]
- Suetomi, T.; Willeford, A.; Brand, C.S.; Cho, Y.; Ross, R.S.; Miyamoto, S.; Brown, J.H. Inflammation and NLRP3 inflammasome activation initiated in response to pressure overload by Ca2+/calmodulin-dependent protein kinase II δ signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation 2018, 138, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, F.; Zhang, M.; Jin, L.; Xie, P.; Liu, D.; Zhang, J.; Hu, X.; Lv, F.; Shang, H.; et al. Targeting CaMKII-δ9 ameliorates cardiac ischemia/reperfusion injury by inhibiting myocardial inflammation. Circ. Res. 2022, 130, 887–903. [Google Scholar] [CrossRef]
- Willeford, A.; Suetomi, T.; Nickle, A.; Hoffman, H.M.; Miyamoto, S.; Heller Brown, J. CaMKIIδ-mediated inflammatory gene expression and inflammasome activation in cardiomyocytes initiate inflammation and induce fibrosis. JCI Insight 2018, 3, e97054. [Google Scholar] [CrossRef]
- Li, L.; Chen, F.; Cao, Y.G.; Qi, H.P.; Huang, W.; Wang, Y.; Jing, S.; Sun, H.L. Role of calcium-sensing receptor in cardiac injury of hereditary epileptic rats. Pharmacology 2015, 95, 10–21. [Google Scholar] [CrossRef]
- Yuan, H.; Fan, Y.; Wang, Y.; Gao, T.; Shao, Y.; Zhao, B.; Li, H.; Xu, C.; Wei, C. Calcium-sensing receptor promotes high glucose-induced myocardial fibrosis via upregulation of the TGF-β1/Smads pathway in cardiac fibroblasts. Mol. Med. Rep. 2019, 20, 1093–1102. [Google Scholar] [CrossRef]
- Chleilat, E.; Pethe, A.; Pfeifer, D.; Krieglstein, K.; Roussa, E. TGF-β signaling regulates SLC8A3 expression and prevents oxidative stress in developing midbrain dopaminergic and dorsal raphe serotonergic neurons. Int. J. Mol. Sci. 2020, 21, 2735. [Google Scholar] [CrossRef]
- Mascharak, S.; desJardins-Park, H.E.; Davitt, M.F.; Griffin, M.; Borrelli, M.R.; Moore, A.L.; Chen, K.; Duoto, B.; Chinta, M.; Foster, D.; et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 2021, 372, eaba2374. [Google Scholar] [CrossRef]
- Györfi, A.H.; Matei, A.E.; Fuchs, M.; Liang, C.; Rigau, A.R.; Hong, X.; Zhu, H.; Luber, M.; Bergmann, C.; Dees, K.C.; et al. Engrailed 1 coordinates cytoskeletal reorganization to induce myofibroblast differentiation. J. Exp. Med. 2021, 218, e20201916. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.A.; Sansone, L.; Carnevale, I.; Limana, F.; Runci, A.; Polletta, L.; Perrone, G.A.; De Santis, E.; Tafani, M. One special question to start with: Can HIF/NFkB be a target in inflammation? Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Bian, Y.; Yu, F.; Zhang, Q.; Zhang, G.; Li, Y.; Song, S.; Ren, X.; Tong, J. Chronic intermittent hypoxia induces cardiac inflammation and dysfunction in a rat obstructive sleep apnea model. J. Biomed. Res. 2016, 30, 490–495. [Google Scholar] [PubMed]
- Kumar, S.; Wang, G.; Liu, W.; Ding, W.; Dong, M.; Zheng, N.; Ye, H.; Liu, J. Hypoxia-induced mitogenic factor promotes cardiac hypertrophy via calcium-dependent and hypoxia-inducible factor-1alpha mechanisms. Hypertension 2018, 72, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, M.; Yin, X.; Wu, C.; Wu, Q.; Feng, S.; Li, H.; Luan, Y.; Wen, J.; Yan, L. Expression of the calcium sensing receptor in human peripheral blood T lymphocyte and its contribution to cytokine secretion through MAPKs or NF-κB pathways. Mol. Immunol. 2013, 53, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.Y.; Du, J.J.; Pan, Y.; Wu, J.; Bi, H.L.; Cui, B.H.; Zhai, T.Y.; Sun, Y.; Sun, Y.H. Calcium-sensing receptor in human peripheral blood T lymphocytes is involved in the ami onset and progression through the NF-κB signaling pathway. Int. J. Mol. Sci. 2016, 17, 1397. [Google Scholar] [CrossRef]
- Canaff, L.; Hendy, G.N. Calcium-sensing receptor gene transcription is up-regulated by the proinflammatory cytokine, interleukin-1β. J. Biol. Chem. 2005, 14177–14185. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, L.J.; Waaga-Gasser, A.M.; Ding, Y.; Cao, M.; Jadhav, S.J.; Kirollos, S.; Shekar, P.S.; Padera, R.F.; Chang, Y.C.; et al. The axis of local cardiac endogenous Klotho-TGF-beta1-Wnt signaling mediates cardiac fibrosis in human. J. Mol. Cell. Cardiol. 2019, 136, 113–124. [Google Scholar] [CrossRef]
- Bogdanova, E.; Beresneva, O.; Galkina, O.; Zubina, I.; Ivanova, G.; Parastaeva, M.; Semenova, N.; Dobronravov, V. Myocardial hypertrophy and fibrosis are associated with cardiomyocyte beta-catenin and TRPC6/Calcineurin/NFAT signaling in spontaneously hypertensive rats with 5/6 nephrectomy. Int. J. Mol. Sci. 2021, 22, 4645. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, X.; Guo, M.; Guo, R.; Wang, L.; Zhang, Z.; Lin, Z.; Dong, M.; Dai, H.; Ji, X.; et al. Ginsenoside Rb1 ameliorates CKD-associated vascular calcification by inhibiting the Wnt/beta-catenin pathway. J. Cell. Mol. Med. 2019, 23, 7088–7098. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, H.; Majumder, K.; Zhang, W.; Mine, Y. Gamma-glutamylvaline prevents low-grade chronic inflammation via activation of a calcium-sensing receptor pathway in 3T3-L1mouse adipocytes. J. Agric. Food. Chem. 2019, 67, 8361–8369. [Google Scholar] [CrossRef] [PubMed]
- Jouret, F.; Wu, J.; Hull, M.; Rajendran, V.; Mayr, B.; Schofl, C.; Geibel, J.; Caplan, M.J. Activation of the Ca(2)+-sensing receptor induces deposition of tight junction components to the epithelial cell plasma membrane. J. Cell Sci. 2013, 126, 5132–5142. [Google Scholar]
- Rey, O.; Chang, W.; Bikle, D.; Rozengurt, N.; Young, S.H.; Rozengurt, E. Negative cross-talk between calcium-sensing receptor and beta-catenin signaling systems in colonic epithelium. J. Biol. Chem. 2012, 287, 1158–1167. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Hong, X.; Miao, J.; Liao, Y.; Hou, F.F.; Zhou, L.; Liu, Y. Wnt/beta-catenin signaling mediates both heart and kidney injury in type 2 cardiorenal syndrome. Kidney Int. 2019, 95, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, T.; Chen, L.; Luo, W.; Chao, J. MCP-1 mediates ischemia-reperfusion-induced cardiomyocyte apoptosis via MCPIP1 and CaSR. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H59–H71. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Wang, L.; Zhang, X.; Fu, Y.; Liu, Y.; Chen, W.; Liu, W.; Shi, Z.; Yin, X. Activation of calcium-sensing receptor-mediated autophagy in angiotensinII-induced cardiac fibrosis in vitro. Biochem. Biophys. Res. Commun. 2018, 497, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, C.; Sun, D.; Jiang, S.; Li, H.; Zhang, W.; Zhao, Y.; Xi, Y.; Shi, S.; Lu, F.; et al. Calhex231 ameliorates cardiac hypertrophy by inhibiting cellular autophagy in vivo and in vitro. Cell. Physiol. Biochem. 2015, 36, 1597–1612. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.; Lin, Y.; Xi, Y.; Li, H.; Shi, S.; Li, H.; Zhang, W.; Zhao, Y.; Tian, Y.; et al. Suppression of calcium sensing receptor ameliorates cardiac hypertrophy through inhibition of autophagy. Mol. Med. Rep. 2016, 14, 111–120. [Google Scholar] [CrossRef]
- Gutierrez-Lopez, T.Y.; Orduna-Castillo, L.B.; Hernandez-Vasquez, M.N.; Vazquez-Prado, J.; Reyes-Cruz, G. Calcium sensing receptor activates the NLRP3 inflammasome via a chaperone-assisted degradative pathway involving Hsp70 and LC3-II. Biochem. Biophys. Res. Commun. 2018, 505, 1121–1127. [Google Scholar] [CrossRef]
- Ecder, T.; Schrier, R.W. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat. Rev. Nephrol. 2009, 5, 221–228. [Google Scholar] [CrossRef]
- Peintner, L.; Venkatraman, A.; Waeldin, A.; Hofherr, A.; Busch, T.; Voronov, A.; Viau, A.; Kuehn, E.W.; Kottgen, M.; Borner, C. Loss of PKD1/polycystin-1 impairs lysosomal activity in a CAPN (calpain)-dependent manner. Autophagy 2021, 17, 2384–2400. [Google Scholar] [CrossRef] [PubMed]
- Di Mise, A.; Ranieri, M.; Centrone, M.; Venneri, M.; Tamma, G.; Valenti, D.; Valenti, G. Activation of the calcium-sensing receptor corrects the impaired mitochondrial energy status observed in renal polycystin-1 knockdown cells modeling autosomal dominant polycystic kidney disease. Front. Mol. Biosci. 2018, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Ma, X.; Lin, S.; Wu, B.; Chen, Y.; Peng, C. Bone marrow mesenchymal stem cell-derived exosomes protect against myocardial infarction by promoting autophagy. Exp. Ther. Med. 2019, 18, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, X.; Yao, Q.; Liu, Y.; Zhang, H.; Dong, Z. HIF-1-mediated production of exosomes during hypoxia is protective in renal tubular cells. Am. J. Physiol. Renal. Physiol. 2017, 313, F906–F913. [Google Scholar] [CrossRef]
- Liu, X.; Miao, J.; Wang, C.; Zhou, S.; Chen, S.; Ren, Q.; Hong, X.; Wang, Y.; Hou, F.F.; Zhou, L.; et al. Tubule-derived exosomes play a central role in fibroblast activation and kidney fibrosis. Kidney. Int. 2020, 97, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, P.; Goodwin, A.J.; Cook, J.A.; Halushka, P.V.; Chang, E.; Fan, H. Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol. Ther. 2018, 26, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Li, G.B.; Li, P.L. Lysosomal TRPML1 channel: Implications in cardiovascular and kidney diseases. Adv. Exp. Med. Biol. 2021, 1349, 275–301. [Google Scholar] [PubMed]
- Trivedi, R.; Mithal, A.; Chattopadhyay, N. Recent updates on the calcium-sensing receptor as a drug target. Curr. Med. Chem. 2008, 15, 178–186. [Google Scholar] [CrossRef]
- Abdelsayed, M.; Kort, E.J.; Jovinge, S.; Mercola, M. Repurposing drugs to treat cardiovascular disease in the era of precision medicine. Nat. Rev. Cardiol. 2022. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).