The Effects of Shear Force-Based Processing of Lipoaspirates on White Adipose Tissue and the Differentiation Potential of Adipose Derived Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Demographics
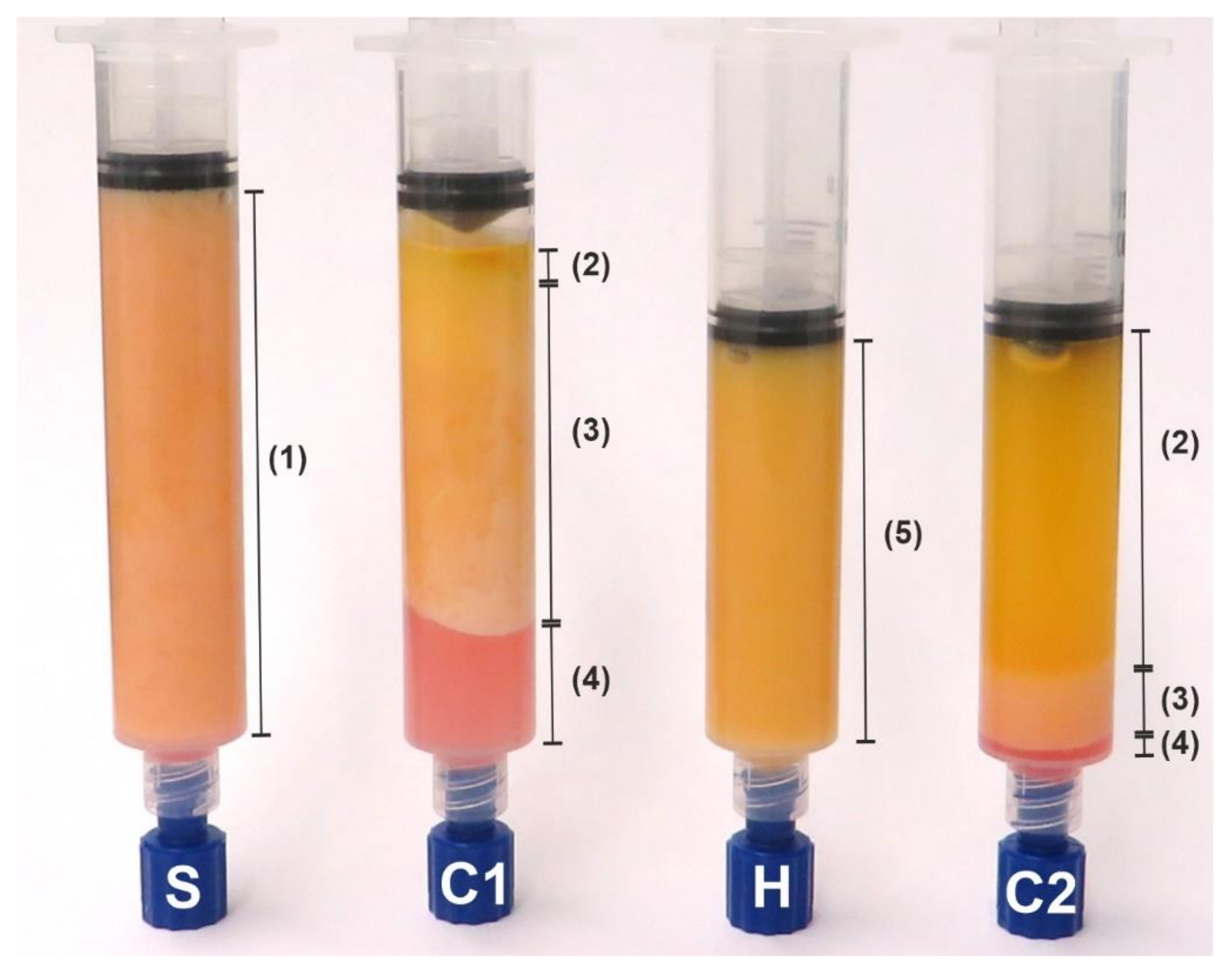
2.2. Liposuction Technique
2.3. Sample Preparation
2.4. Fixation and Staining
2.5. Histological Sample Evaluation
2.6. ADSC Isolation and Differentiation
2.7. Statistical Analysis
3. Results
3.1. Quantitative Description of the Histological Samples
3.2. Stromal Vascular Cells
3.3. Adipocyte Depots
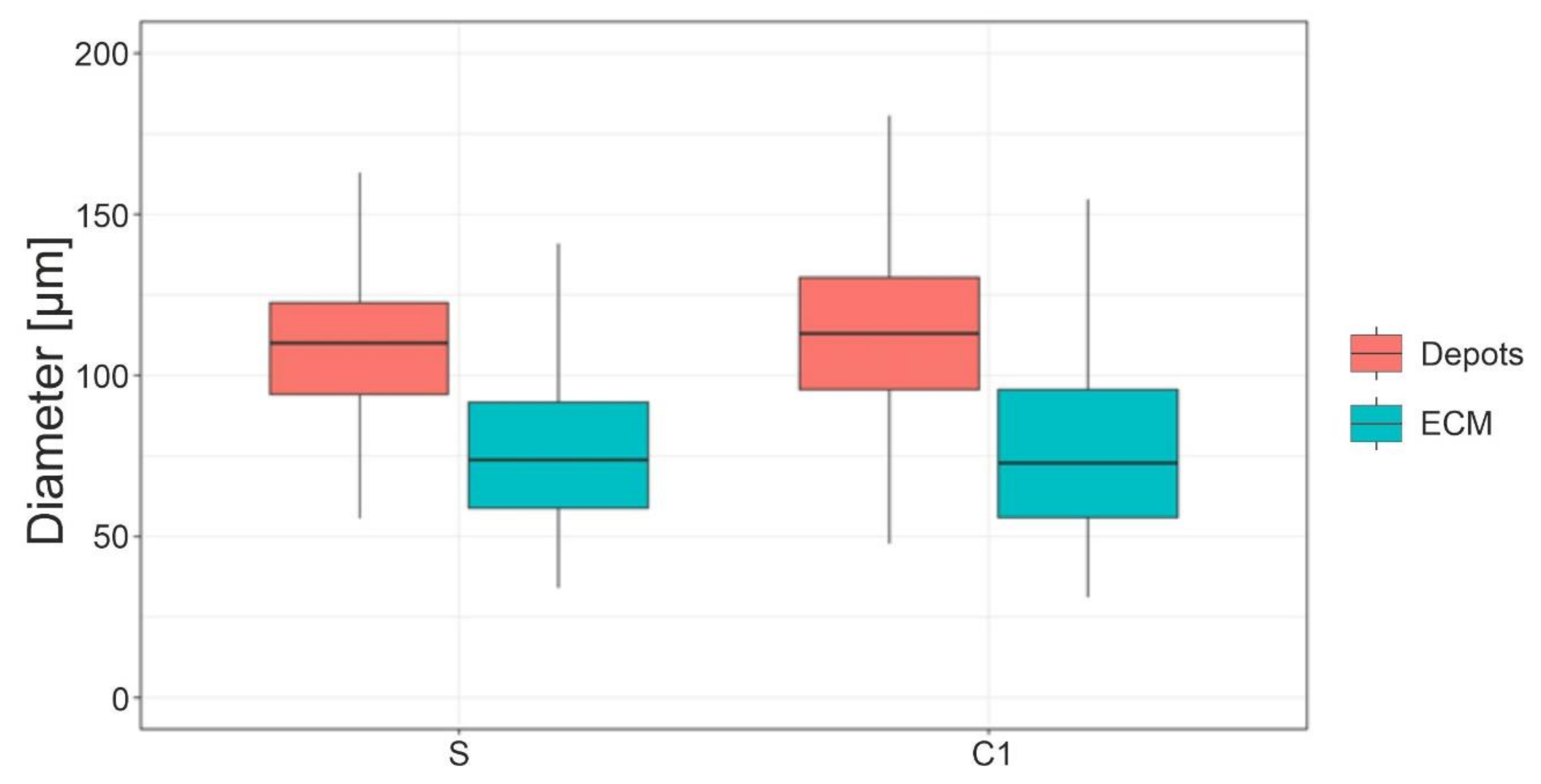
3.4. Adipocyte Size
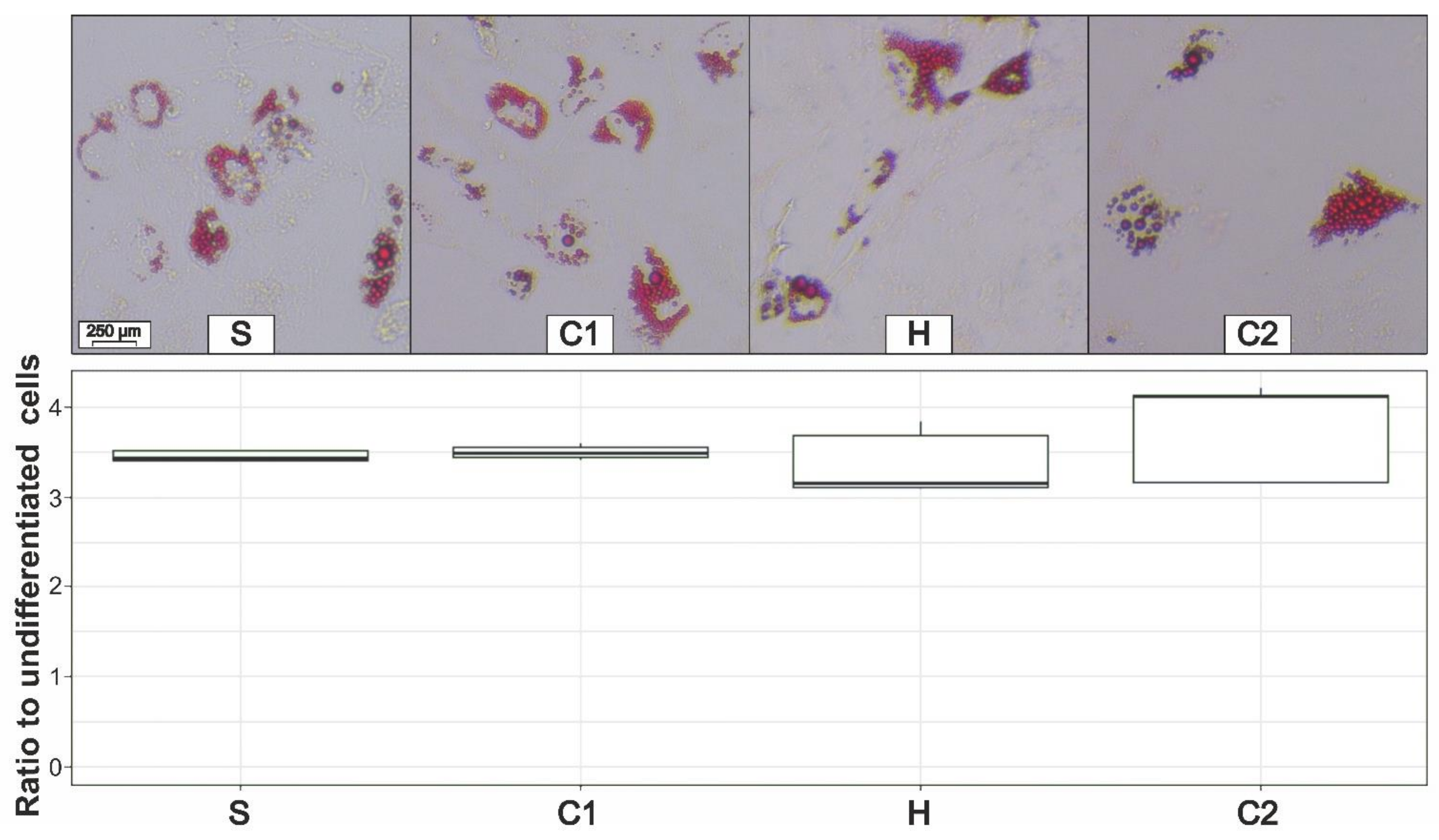
3.5. Adipogenic Differentiation
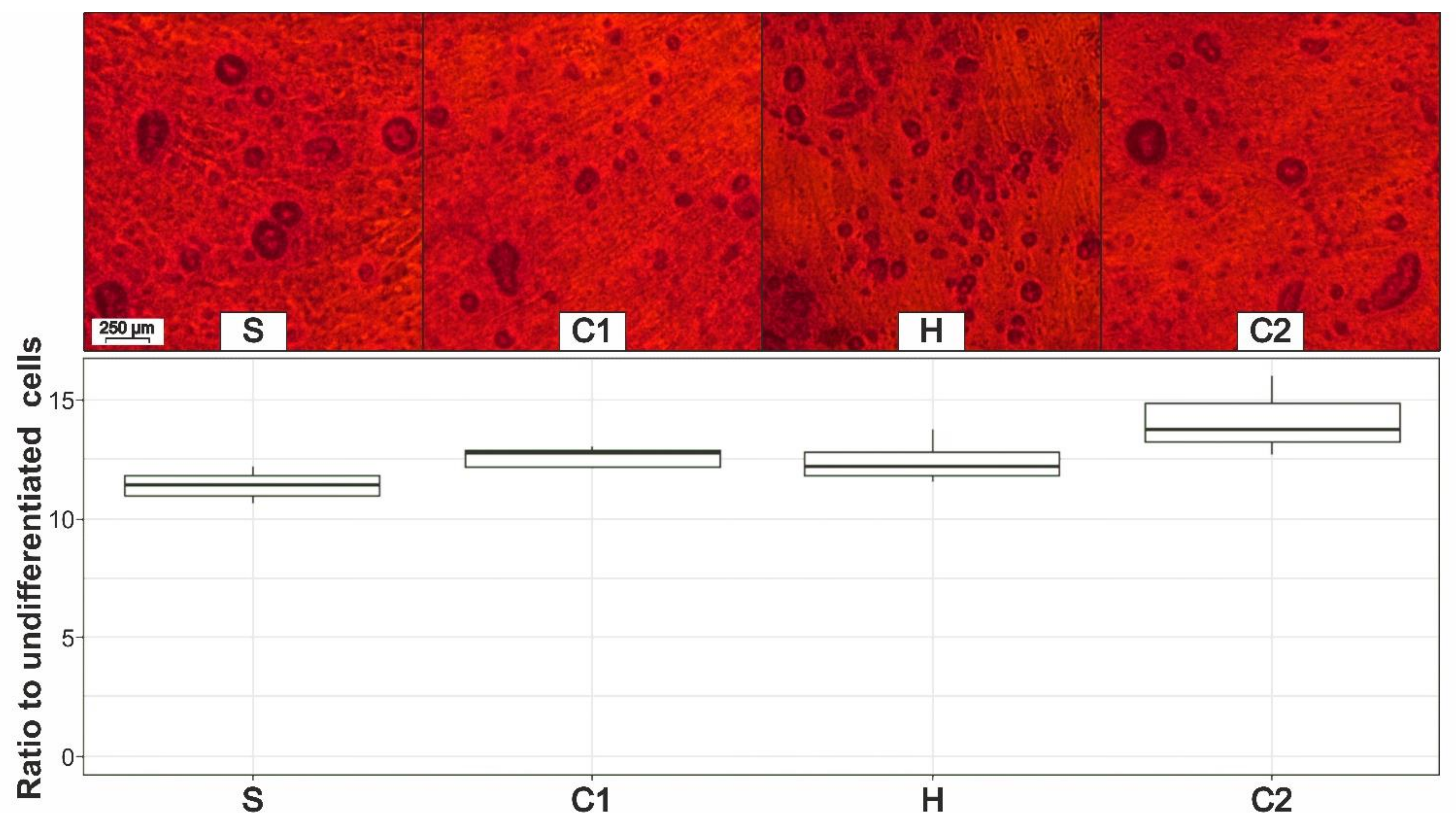
3.6. Osteogenic Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rohrich, R.J. The American Society of Plastic Surgeons’ procedural statistics: What they really mean. Plast. Reconstr. Surg. 2003, 112, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Simonacci, F.; Bertozzi, N.; Grieco, M.P.; Grignaffini, E.; Raposio, E. Procedure, applications, and outcomes of autologous fat grafting. Ann. Med. Surg. 2017, 20, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Nseir, I.; Delaunay, F.; Latrobe, C.; Bonmarchand, A.; Coquerel-Beghin, D.; Auquit-Auckbur, I. Use of adipose tissue and stromal vascular fraction in hand surgery. Orthop. Traumatol. Surg. Res. 2017, 103, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Luo, E.; Chen, X.; Liu, L.; Qiao, J.; Yan, Z.; Li, Z.; Tang, W.; Zheng, X.; Tian, W. Molecular and cellular characterization during chondrogenic differentiation of adipose tissue-derived stromal cells in vitro and cartilage formation in vivo. J. Cell. Mol. Med. 2005, 9, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.; Guilak, F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy 2003, 5, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Doornaert, M.; Colle, J.; De Maere, E.; Declercq, H.; Blondeel, P. Autologous fat grafting: Latest insights. Ann. Med. Surg. 2019, 37, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Prantl, L.; Eigenberger, A.; Brix, E.; Kempa, S.; Baringer, M.; Felthaus, O. Adipose Tissue-Derived Stem Cell Yield Depends on Isolation Protocol and Cell Counting Method. Cells 2021, 10, 1113. [Google Scholar] [CrossRef]
- Hanke, A.; Prantl, L.; Wenzel, C.; Nerlich, M.; Brockhoff, G.; Loibl, M.; Gehmert, S. Semi-automated extraction and characterization of Stromal Vascular Fraction using a new medical device. Clin. Hemorheol. Microcirc. 2016, 64, 403–412. [Google Scholar] [CrossRef]
- Strong, A.L.; Cederna, P.S.; Rubin, J.P.; Coleman, S.R.; Levi, B. The Current State of Fat Grafting: A Review of Harvesting, Processing, and Injection Techniques. Plast. Reconstr. Surg. 2015, 136, 897–912. [Google Scholar] [CrossRef]
- Chan, T.-M.; Chen, J.Y.-R.; Ho, L.-I.; Lin, H.-P.; Hsueh, K.-W.; Liu, D.D.; Chen, Y.-H.; Hsieh, A.-C.; Tsai, N.-M.; Hueng, D.-Y.; et al. ADSC therapy in neurodegenerative disorders. Cell Transplant. 2014, 23, 549–557. [Google Scholar] [CrossRef]
- Kølle, S.-F.T.; Fischer-Nielsen, A.; Mathiasen, A.B.; Elberg, J.J.; Oliveri, R.S.; Glovinski, P.V.; Kastrup, J.; Kirchhoff, M.; Rasmussen, B.S.; Talman, M.-L.M.; et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: A randomised placebo-controlled trial. Lancet 2013, 382, 1113–1120. [Google Scholar] [CrossRef]
- Bellei, B.; Migliano, E.; Tedesco, M.; Caputo, S.; Picardo, M. Maximizing non-enzymatic methods for harvesting adipose-derived stem from lipoaspirate: Technical considerations and clinical implications for regenerative surgery. Sci. Rep. 2017, 7, 10015. [Google Scholar] [CrossRef] [PubMed]
- Jahr, H.; Hering, B.; Federlin, K.; Bretzel, R.G. Activation of human complement by collagenase and ficoll. Exp. Clin. Endocrinol. Diabetes 1995, 103 (Suppl. 2), 27–29. [Google Scholar] [CrossRef] [PubMed]
- Prantl, L.; Eigenberger, A.; Klein, S.; Limm, K.; Oefner, P.J.; Schratzenstaller, T.; Felthaus, O. Shear Force Processing of Lipoaspirates for Stem Cell Enrichment Does Not Affect Secretome of Human Cells Detected by Mass Spectrometry In Vitro. Plast. Reconstr. Surg. 2020, 146, 749e–758e. [Google Scholar] [CrossRef] [PubMed]
- Prantl, L.; Rennekampff, H.O.; Giunta, R.E.; Harder, Y.; von Heimburg, D.; Heine, N.; Herold, C.; Kneser, U.; Lampert, F.; Machens, H.G.; et al. Aktuelle Erkenntnisse zur Eigenfett Transplantation anhand der neuen Leitlinie „Autologe Fetttransplantation“. Handchir. Mikrochir. Plast. Chir. 2016, 48, 330–336. [Google Scholar] [CrossRef]
- Yingbo, Z.; Daping, Y. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann. Plast. Surg. 2012, 68, 111. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Sun, H.M.; Hwang, K.-C.; Kim, S.-W. Adipose-Derived Stromal Vascular Fraction Cells: Update on Clinical Utility and Efficacy. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Stenkula, K.G.; Erlanson-Albertsson, C. Adipose cell size: Importance in health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R284–R295. [Google Scholar] [CrossRef]
- Laforest, S.; Michaud, A.; Paris, G.; Pelletier, M.; Vidal, H.; Géloën, A.; Tchernof, A. Comparative analysis of three human adipocyte size measurement methods and their relevance for cardiometabolic risk. Obesity 2017, 25, 122–131. [Google Scholar] [CrossRef]
- Sbarbati, A.; Accorsi, D.; Benati, D.; Marchetti, L.; Orsini, G.; Rigotti, G.; Panettiere, P. Subcutaneous adipose tissue classification. Eur. J. Histochem. 2010, 54, e48. [Google Scholar] [CrossRef]
- Comley, K.; Fleck, N.A. The toughness of adipose tissue: Measurements and physical basis. J. Biomech. 2010, 43, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I.; Trujillo, O.; Kristen, Q.; Isac, K.; Zorko, J.; Fam, M.; Okonkwo, K.; Mian, A.; Thanh, H.; Koban, K.; et al. The Facial Adipose Tissue: A Revision. Facial Plast. Surg. 2016, 32, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Bellini, E.; Grieco, M.P.; Raposio, E. The science behind autologous fat grafting. Ann. Med. Surg. 2017, 24, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R. Structural fat grafting: More than a permanent filler. Plast. Reconstr. Surg. 2006, 118, 108S–120S. [Google Scholar] [CrossRef] [PubMed]
- Pallua, N.; Pulsfort, A.K.; Suschek, C.; Wolter, T.P. Content of the growth factors bFGF, IGF-1, VEGF, and PDGF-BB in freshly harvested lipoaspirate after centrifugation and incubation. Plast. Reconstr. Surg. 2009, 123, 826–833. [Google Scholar] [CrossRef]
- Prantl, L.; Brix, E.; Kempa, S.; Felthaus, O.; Eigenberger, A.; Brébant, V.; Anker, A.; Strauss, C. Facial Rejuvenation with Concentrated Lipograft-A 12 Month Follow-up Study. Cells 2021, 10, 594. [Google Scholar] [CrossRef]
- Varghese, J.; Griffin, M.; Mosahebi, A.; Butler, P. Systematic review of patient factors affecting adipose stem cell viability and function: Implications for regenerative therapy. Stem Cell Res. Ther. 2017, 8, 45. [Google Scholar] [CrossRef]
- Kølle, S.-F.T.; Oliveri, R.S.; Glovinski, P.V.; Elberg, J.J.; Fischer-Nielsen, A.; Drzewiecki, K.T. Importance of mesenchymal stem cells in autologous fat grafting: A systematic review of existing studies. J. Plast. Surg. Hand Surg. 2012, 46, 59–68. [Google Scholar] [CrossRef]
- Arderiu, G.; Lambert, C.; Ballesta, C.; Moscatiello, F.; Vilahur, G.; Badimon, L. Cardiovascular Risk Factors and Differential Transcriptomic Profile of the Subcutaneous and Visceral Adipose Tissue and Their Resident Stem Cells. Cells 2020, 9, 2235. [Google Scholar] [CrossRef]
- Eto, H.; Kato, H.; Suga, H.; Aoi, N.; Doi, K.; Kuno, S.; Yoshimura, K. The fate of adipocytes after nonvascularized fat grafting: Evidence of early death and replacement of adipocytes. Plast. Reconstr. Surg. 2012, 129, 1081–1092. [Google Scholar] [CrossRef]
- Pallua, N.; Grasys, J.; Kim, B.-S. Enhancement of Progenitor Cells by Two-Step Centrifugation of Emulsified Lipoaspirates. Plast. Reconstr. Surg. 2018, 142, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Gehmert, S.; Gehmert, S.; Hidayat, M.; Sultan, M.; Berner, A.; Klein, S.; Zellner, J.; Müller, M.; Prantl, L. Angiogenesis: The role of PDGF-BB on adipose-tissue derived stem cells (ASCs). Clin. Hemorheol. Microcirc. 2011, 48, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Wang, Q.; Qiu, L.; Yang, J.; Yi, C. Regenerative Therapeutic Applications of Mechanized Lipoaspirate Derivatives. Chin. J. Plast. Reconstr. Surg. 2020, 2, 120–127. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, W.; Xu, X.; Gould, O.E.C.; Kratz, K.; Ma, N.; Lendlein, A. Polymeric sheet actuators with programmable bioinstructivity. Proc. Natl. Acad. Sci. USA 2020, 117, 1895–1901. [Google Scholar] [CrossRef]
- Yogi, A.; Rukhlova, M.; Charlebois, C.; Tian, G.; Stanimirovic, D.B.; Moreno, M.J. Differentiation of Adipose-Derived Stem Cells into Vascular Smooth Muscle Cells for Tissue Engineering Applications. Biomedicines 2021, 9, 797. [Google Scholar] [CrossRef]
- Gao, S.; Guo, X.; Zhao, S.; Jin, Y.; Zhou, F.; Yuan, P.; Cao, L.; Wang, J.; Qiu, Y.; Sun, C.; et al. Differentiation of human adipose-derived stem cells into neuron/motoneuron-like cells for cell replacement therapy of spinal cord injury. Cell Death Dis. 2019, 10, 597. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef]
- Luck, J.; Weil, B.D.; Lowdell, M.; Mosahebi, A. Adipose-Derived Stem Cells for Regenerative Wound Healing Applications: Understanding the Clinical and Regulatory Environment. Aesthet. Surg. J. 2020, 40, 784–799. [Google Scholar] [CrossRef]
- Prantl, L.; Schreml, J.; Gehmert, S.; Klein, S.; Bai, X.; Zeitler, K.; Schreml, S.; Alt, E.; Gehmert, S.; Felthaus, O. Transcription Profile in Sporadic Multiple Symmetric Lipomatosis Reveals Differential Expression at the Level of Adipose Tissue-Derived Stem Cells. Plast. Reconstr. Surg. 2016, 137, 1181–1190. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, L.; Chen, P.; Zhang, C.; Tang, S.; Chen, X. Comparison of the Efficacy and Safety of Cell-Assisted Lipotransfer and Platelet-Rich Plasma Assisted Lipotransfer: What Should We Expect from a Systematic Review with Meta-Analysis? Cell Transplant. 2021, 30, 963689721989607. [Google Scholar] [CrossRef]
- Huayllani, M.T.; Sarabia-Estrada, R.; Restrepo, D.J.; Boczar, D.; Sisti, A.; Nguyen, J.H.; Rinker, B.D.; Moran, S.L.; Quiñones-Hinojosa, A.; Forte, A.J. Adipose-derived stem cells in wound healing of full-thickness skin defects: A review of the literature. J. Plast. Surg. Hand Surg. 2020, 54, 263–279. [Google Scholar] [CrossRef]
- Shaker, R.F.; Abdel Aal, A.R.M.; El Gazzar, K.M.A.; Abu Zahra, F.A.K.; Elshahat, A. In Vitro Comparative Study of Emulsified Fat Grafts. Eplasty 2020, 20, e1. [Google Scholar] [PubMed]
- Pu, L.L.Q.; Coleman, S.R.; Cui, X.; Ferguson, R.E.H.; Vasconez, H.C. Autologous fat grafts harvested and refined by the Coleman technique: A comparative study. Plast. Reconstr. Surg. 2008, 122, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Caggiati, A.; Germani, A.; Di Carlo, A.; Borsellino, G.; Capogrossi, M.C.; Picozza, M. Naturally Adipose Stromal Cell-Enriched Fat Graft: Comparative Polychromatic Flow Cytometry Study of Fat Harvested by Barbed or Blunt Multihole Cannula. Aesthet. Surg. J. 2017, 37, 591–602. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferguson, R.E.H.; Cui, X.; Fink, B.F.; Vasconez, H.C.; Pu, L.L.Q. The viability of autologous fat grafts harvested with the LipiVage system: A comparative study. Ann. Plast. Surg. 2008, 60, 594–597. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef]



| Measurement | Magnification | Area Size | Number of Areas per Sample |
|---|---|---|---|
| Adipocyte depots | 20× | 0.31 mm² | 10 |
| Adipocyte diameter | 40× | 0.08 mm² | >10 |
| Number of SVCs | 63× | 0.03 mm² | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eigenberger, A.; Felthaus, O.; Schratzenstaller, T.; Haerteis, S.; Utpatel, K.; Prantl, L. The Effects of Shear Force-Based Processing of Lipoaspirates on White Adipose Tissue and the Differentiation Potential of Adipose Derived Stem Cells. Cells 2022, 11, 2543. https://doi.org/10.3390/cells11162543
Eigenberger A, Felthaus O, Schratzenstaller T, Haerteis S, Utpatel K, Prantl L. The Effects of Shear Force-Based Processing of Lipoaspirates on White Adipose Tissue and the Differentiation Potential of Adipose Derived Stem Cells. Cells. 2022; 11(16):2543. https://doi.org/10.3390/cells11162543
Chicago/Turabian StyleEigenberger, Andreas, Oliver Felthaus, Thomas Schratzenstaller, Silke Haerteis, Kirsten Utpatel, and Lukas Prantl. 2022. "The Effects of Shear Force-Based Processing of Lipoaspirates on White Adipose Tissue and the Differentiation Potential of Adipose Derived Stem Cells" Cells 11, no. 16: 2543. https://doi.org/10.3390/cells11162543
APA StyleEigenberger, A., Felthaus, O., Schratzenstaller, T., Haerteis, S., Utpatel, K., & Prantl, L. (2022). The Effects of Shear Force-Based Processing of Lipoaspirates on White Adipose Tissue and the Differentiation Potential of Adipose Derived Stem Cells. Cells, 11(16), 2543. https://doi.org/10.3390/cells11162543







