Untangling the Extracellular Matrix of Idiopathic Epiretinal Membrane: A Path Winding among Structure, Interactomics and Translational Medicine
Abstract
1. Introduction
2. Materials and Methods
3. ECM Components of iERMs
3.1. Collagens
3.1.1. Collagen I
3.1.2. Collagen II
3.1.3. Collagen III
3.1.4. Collagen IV
3.1.5. Collagen V
3.1.6. Collagen VI
3.1.7. Collagen VII
3.1.8. Other Collagens
3.2. Basement Membrane Proteins and Their Assembly in LNCP Matrix
3.2.1. Laminins
3.2.2. Nidogens/Entactins
3.2.3. Heparan Sulfate Proteoglycans
3.2.4. LNCP Matrix
3.3. Proteoglycans
3.4. Other Proteins
3.4.1. Fibrillin
3.4.2. Fibronectin
3.4.3. Vitronectin
3.4.4. Thrombospondin 1
3.4.5. Osteonectin/SPARC
3.4.6. Periostin
3.5. Glycosaminoglycans (GAG)
4. ECM Arrangement in iERMs
5. An Interactomic Overview of iERM ECM
5.1. Integrins
5.1.1. Integrins, VEGF and the Avascular Nature of iERMs
5.2. Epidermal Growth Factor Receptor (EGFR)
5.3. Galectin-3 Lattices
5.4. CD44 Antigen
5.5. ECM Synthesis and Degradation Loop
5.5.1. Cathepsins
5.5.2. Protein-Glutamine Gamma-Glutamyltransferase 2 (TGM2)
5.5.3. High-Temperature Requirement A Serine Peptidase 1 (HTRA1)
5.5.4. Prolow-Density Lipoprotein Receptor-Related Protein 1 (LRP1)
6. Fibrogenic Biomarkers in the Vitreous Humor: Growth Factors, Cytokines, mRNA and miRNA
7. Translational Medicine in iERM: The Pharmacologic Vitreolysis
7.1. Collagenase
7.2. Hyaluronidase
7.3. Chondroitinase
7.4. RGD Peptides
7.5. Dispase (Vitreolysin™)
7.6. Plasmin and Plasminogen Activators
7.7. Ocriplasmin
7.8. Nattokinase
7.9. Vitreosolve®
7.10. Reenginered Vibrio mimicus Collagenase-Derived Fusion Proteins
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertelli, E. Aqueous Humor, Lens, Ciliary Zonule, Vitreous. In Anatomy of the Eye and Human Visual System; Bertelli, E., Ed.; Piccin Nuova Libraria: Padova, Italy, 2019; pp. 249–267. [Google Scholar]
- Tosi, G.M.; Marignani, D.; Romeo, N.; Toti, P. Disease pathways in proliferative vitreoretinopathy: An ongoing challenge. J. Cell Physiol. 2014, 229, 1577–1583. [Google Scholar] [CrossRef]
- Patronas, M.; Kroll, A.J.; Lou, P.L.; Ryan, E.A. A review of the vitreoretinal interface pathology. Int. Ophthalmol. Clin. 2009, 49, 133–143. [Google Scholar] [CrossRef]
- Folk, J.C.; Adelman, R.A.; Flaxel, C.J.; Hyman, L.; Pulido, J.S.; Olsen, T.W. Idiopathic epiretinal membrane and vitreomacular traction preferred practice pattern (®) guidelines. Ophthalmology 2016, 123, P152–P181. [Google Scholar] [CrossRef]
- Hiscott, P.S.; Grierson, I.; McLeod, D. Natural history of fibrocellular epiretinal membranes: A quantitative, autoradiographic, and immunohistochemical study. Br. J. Ophthalmol. 1985, 69, 810–823. [Google Scholar] [CrossRef]
- Sramek, S.J.; Wallow, I.H.; Stevens, T.S.; Nork, T.M. Immunostaining of preretinal membranes for actin, fibronectin and glial fibrillary acidic protein. Ophthalmology 1989, 96, 835–841. [Google Scholar] [CrossRef]
- Bochaton-Piallat, M.-L.; Kapetanios, A.D.; Donati, G.; Redard, M.; Gabbiani, G.; Pournaras, C.J. TGFβ1, TGFβ receptor II and ED-A fibronectin expression in myofibroblast of vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2336–2342. [Google Scholar]
- Cabay, L.; Willermain, F.; Bruyns, C.; Verderbout, J.M.; Witta, Y.; Baffi, J.; Velu, T.; Libert, J.; Caspers-Velu, L.; Maho, A.; et al. CX3CR4 expression in vitreoretinal membranes. Br. J. Ophthalmol. 2003, 87, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Gandorfer, A.; Haritoglou, C.; Scheler, R.; Schaumberger, M.M.; Kampik, A.; Schumann, R.G. Epiretinal cell proliferation in macular pucker and vitreomacular traction syndrome. Analysis of flat-mounted internal limiting membrane specimens. Retina 2013, 33, 77–88. [Google Scholar] [CrossRef]
- Schumann, R.G.; Gandorfer, A.; Ziada, J.; Scheler, R.; Schaumberger, M.M.; Wolf, A.; Kampik, A.; Haritoglou, C. Hyalocytes in idiopathic epiretinal membranes: A correlative light and electron microscopic study. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1887–1894. [Google Scholar] [CrossRef]
- Bu, S.-C.; Kuijer, R.; van der Worp, R.J.; Postma, G.; de Lavalette, R.; Li, X.-R.; Hooymans, J.M.M.; Los, L.I. Immunohistochemical evaluation of idiopathic epiretinal membranes and in vitro studies on the effect of TGFβ on Müller cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6506–6514. [Google Scholar] [CrossRef]
- Tosi, G.M.; Regoli, M.; Altera, A.; Galvagni, F.; Arcuri, C.; Bacci, T.; Elia, I.; Realini, G.; Orlandini, M.; Bertelli, E. Heath shock protein 90 involvement in the development of idiopathic epiretinal membranes. Investig. Ophthalmol. Vis. Sci. 2020, 61, 34. [Google Scholar] [CrossRef]
- Christakopoulos, C.; Cehofski, L.J.; Christensen, S.R.; Vorum, H.; Honore, B. Proteomics reveals a set of highly enriched proteins in epiretina membrane compared with inner limiting membrane. Exp. Eye Res. 2019, 186, 107722. [Google Scholar] [CrossRef] [PubMed]
- Bini, L.; Schvartz, D.; Carnemolla, C.; Besio, R.; Garibaldi, N.; Sanchez, J.C.; Forlino, A.; Bianchi, L. Intracellular and extracellular markers of lethality in osteogenesis imperfecta: A quantitative proteomic approach. Int. J. Mol. Sci. 2021, 22, 429. [Google Scholar] [CrossRef]
- Bianchi, L.; Gagliardi, A.; Landi, C.; Focarelli, R.; De Leo, V.; Luddi, A.; Bini, L.; Piomboni, P. Protein pathways working in human follicular fluid: The future for tailored IVF? Expert Rev. Mol. Med. 2016, 18, e9. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Gagliardi, A.; Maruelli, S.; Besio, R.; Landi, C.; Gioia, R.; Kozloff, K.M.; Khoury, B.M.; Coucke, P.J.; Symoens, S.; et al. Altered cytoskeletal organization characterized lethal but not surviving Brtl+/− mice: Insight on phenotypic variability in osteogenesis imperfecta. Hum. Mol. Genet. 2015, 24, 6118–6133. [Google Scholar] [CrossRef]
- Bianchi, L.; Bruzzese, F.; Leone, A.; Gagliardi, A.; Puglia, M.; Di Gennaro, E.; Rocco, M.; Gimigliano, A.; Pucci, B.; Armini, A.; et al. Proteomic analysis identifies differentially expressed proteins after HDAC vorinostat and EGFR inhibitor gefitinib treatments in Hep-2 cancer cells. Proteomics 2011, 11, 3725–3742. [Google Scholar] [CrossRef]
- Okada, M.; Ogino, N.; Matsumura, M.; Honda, Y.; Nagai, Y. Histological and immunohistochemical study of idiopathic epiretinal membrane. Ophthalmic Res. 1995, 27, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Kritzenberger, M.; Junglas, B.; Framme, C.; Helbig, H.; Gabel, V.P.; Fuchshofer, R.; Tamm, E.R.; Hillenkamp, J. Different collagen types define two types of idiopathic epiretinal membranes. Histopathology 2011, 58, 953–965. [Google Scholar] [CrossRef]
- Regoli, M.; Tosi, G.M.; Neri, G.; Altera, A.; Orazioli, D.; Bertelli, E. The peculiar pattern of type IV collagen deposition in epiretinal membranes. J. Histochem. Cytochem. 2020, 68, 149–162. [Google Scholar] [CrossRef]
- Altera, A.; Tosi, G.M.; Regoli, M.; De Benedetto, E.; Bertelli, E. The extracellular matrix complexity of idiopathic epiretinal membranes and the bilaminar arrangement of the associated internal limiting membrane in the posterior retina. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2559–2571. [Google Scholar] [CrossRef]
- Jerdan, J.A.; Pepose, J.S.; Michels, R.G.; Hayashi, H.; De Bustros, S.; Sebag, M.; Glaser, B.M. Proliferative vitreoretinopathy membranes. An immunohistochemical study. Ophthalmology 1989, 96, 801–810. [Google Scholar] [CrossRef]
- Morino, I.; Hiscott, P.; McKechnie, N.; Grierson, I. Variation in epiretinal membrane components with clinical duration of the proliferative tissue. Br. J. Ophthlamol. 1990, 74, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Guenther, S.R.; Schumann, R.G.; Hagenau, F.; Wolf, A.; Priglinger, S.G.; Vogt, D. Comparison of surgically excised premacular membranes in eyes with macular pucker and proliferative vitreoretinopathy. Curr. Eye Res. 2019, 44, 341–349. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Chen, S.; Chaudhary, V.; Gonder, J.; Chakrabarti, S. Extracellular matrix proteins in epiretinal membranes and in diabetic retinopathy. Curr. Eye Res. 2009, 34, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Coltrini, D.; Belleri, M.; Gambicorti, E.; Romano, D.; Morescalchi, F.; Chandran, A.M.K.; Calza, S.; Semeraro, F.; Presta, M. Gene expression analysis identifies two distinct molecular clusters of idiopathic epiretinal membranes. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165934. [Google Scholar] [CrossRef]
- Snead, D.R.J.; Cullen, N.; James, S.; Poulson, A.V.; Morris, A.H.C.; Lukaris, A.; Scott, J.D.; Richards, A.J.; Snead, M.P. Hyperconvolution of the inner limiting membrane in vitreomaculopathies. Graefes Arch. Clin. Exp. Ophthamol. 2004, 242, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Asato, R.; Yoshida, S.; Ogura, A.; Nakam, T.; Ishikawa, K.; Nakao, S.; Sassa, Y.; Enaida, H.; Osima, Y.; Ikeo, K.; et al. Comparison of gene expression profile of epiretinal membranes obtained from eyes with proliferative vitreoretinopathy to that of secondary epiretinal membranes. PLoS ONE 2013, 8, e54191. [Google Scholar] [CrossRef]
- Fleischmajer, R.; Jacobs, I.I.L.; Perfish, J.S.; Katchen, B.; Schwartz, E.; Timpl, R. Immunochemical analysis of human kidney reticulin. Am. J. Pathol. 1992, 140, 1225–1235. [Google Scholar]
- Yurchenko, P.D. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef]
- Pratt, B.M.; Madri, J.A. Immunolocalization of type IV collagen and laminin in nonbasement membrane structures of murine corneal stroma A light and electron microscopy. Lab. Investig. 1985, 52, 650–656. [Google Scholar]
- Oefner, C.M.; Sharkey, A.; Gardner, L.; Critchley, H.; Oyen, M.; Moffett, A. Collagen type IV at the fetal-maternal interface. Placenta 2015, 36, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Mauer, S.M.; Kim, Y.; Michael, A.F. Interstitial fibrosis in obstructive nephropathy. Kidney Int. 1993, 44, 774–788. [Google Scholar] [CrossRef] [PubMed]
- Urushiyama, H.; Terasaki, Y.; Nagasaka, S.; Terasaki, M.; Kunugi, S.; Nagase, T.; Fukuda, Y.; Shimizu, A. Role of α1 and α2 chains of type IV collagen in early fibrotic lesions of idiopathic interstitial pneumonias and migration of lung fibroblasts. Lab. Investig. 2015, 95, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Ioachim, E.; Stefaniotou, M.; Gorezis, S.; Tsanou, E.; Psilas, K.; Agnantis, N.J. Immunohistochemical study of extracellular matrix components in epiretinal membranes of vitreoproliferative retinopathy and proliferative diabetic retinopathy. Eur. J. Ophthalmol. 2005, 15, 384–391. [Google Scholar] [CrossRef]
- Birk, D.E. Type V collagen: Heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron 2001, 32, 223–227. [Google Scholar] [CrossRef]
- Bu, S.C.; Kuijer, R.; van der Worp, R.J.; Huiskamp, E.A.; Renardel de Lavalette, V.W.; Li, X.R.; Hooymans, J.M.; Los, L.I. Glial cells and collagens in epiretinal membranes associated with idiopathic macular holes. Retina 2014, 34, 897–906. [Google Scholar] [CrossRef]
- Wullink, B.; Pas, H.H.; Ven der Worp, R.J.; Kuijer, R.; Los, L.I. Type VII collagen expression in the human vitreoretinal interface, corpora amylacea and inner retinal layers. PLoS ONE 2015, 10, e0145502. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Koch, M.; Schulze, J.; Hansen, U.; Ashwodt, T.; Keene, D.R.; Brunken, W.J.; Burgeson, R.E.; Bruckner, P.; Breucknes-Tuderman, L. A novel marker of tissue junctions, collagen XXII. J. Biol. Chem. 2004, 279, 22514–22521. [Google Scholar] [CrossRef]
- Halfter, W.; Dong, S.; Schurer, B.; Cole, G.J. Collagen XVIII is a basement membrane heparin sulfate proteoglycan. J. Biol. Chem. 1998, 273, 25404–25412. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Pareja, J.C. Atypical basement membranes and basement membrane diversity–what is normal anyway? J. Cell Sci. 2020, 133, jcs241794. [Google Scholar] [CrossRef] [PubMed]
- Kvist, A.J.; Nyström, A.; Hultenby, K.; Sasaki, T.; Talts, J.F.; Aspberg, A. The major basement membrane components localize to the chondrocyte pericellular matrix–a cartilage basement membrane equivalent? Matrix Biol. 2008, 27, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, M.; Esser, P.; Wiedemann, P.; Heimann, K. Tenascin and decorin in epiretinal membranes of proliferative vitreoretinopathy and proliferative diabetic retinopathy. Germ. J. Ophthalmol. 1993, 2, 28–31. [Google Scholar]
- Immonen, I.; Tervo, K.; Virtanen, I.; Laatikainen, L.; Tervo, T. Immunohistochemical demonstration of cellular fibronectin and tenascin in human epiretinal membranes. Acta Ophthalmol. 1991, 69, 466–471. [Google Scholar] [CrossRef]
- Pattwell, D.M.; Sheridan, C.M.; Le Goff, M.; Bishop, P.N.; Hiscott, P. Localisation of opticin in human proliferative retinal disease. Exp. Eye Res. 2010, 90, 461–464. [Google Scholar] [CrossRef]
- Alexander, R.A.; Hiscott, P.; McGalliard, J.; Grierson, I. Oxytalan fibres in proliferative vitreoretinopathy. Germ. J. Ophthalmol. 1992, 1, 382–387. [Google Scholar]
- Weller, M.; Wiedemann, P.; Bresgen, M.; Heimann, K. Vitronectin and proliferative intraocular disorders. I. A colocalisation study of the serum spreading factor, vitronectin and fibronectin in traction membranes from patients with proliferative vitreoretinopathy. Int. Ophthalmol. 1991, 15, 93–101. [Google Scholar] [CrossRef]
- Hiscott, P.; Larkin, G.; Robey, H.L.; Orr, G.; Grierson, I. Thrombospondin as a component of the extracellular matrix of epiretinal membranes: Comparisons with cellular fibronectin. Eye 1992, 6, 566–569. [Google Scholar] [CrossRef][Green Version]
- Casaroli Marano, R.P.; Vilaró, S. The role of fibronectin, laminin, vitronectin and their receptors on cellular adhesion in proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2791–2803. [Google Scholar]
- Grisanti, S.; Guidry, C. Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Investig. Ophthalmol. Vis. Sci. 1995, 36, 391–405. [Google Scholar]
- Bornstein, P. Matricellular proteins: An overview. J. Cell Commun. Signal. 2009, 3, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Esser, P.; Bresgen, M.; Heimann, K.; Wiedemann, P. Thrombospondin: A new attachment protein in preretinal traction membranes. Eur. J. Ophthalmol. 1992, 2, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Hiscott, P.; Hagan, S.; Heathcote, L.; Sheridan, C.M.; Gronewald, C.P.; Grierson, I.; Wong, D.; Paraoan, L. Pathobiology of epiretinal and subretinal membranes: Possible roles for the matricellular proteins thrombospondin 1 and osteonectin (SPARC). Eye 2002, 16, 393–403. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshida, S.; Nakama, T.; Oshokawa, K.; Kakao, S.; Sonoda, K.; Ishibashi, T. Periostin in vitreoretinal diseases. Cell Mol. Life Sci. 2017, 74, 4329–4337. [Google Scholar] [CrossRef]
- Russel, S.R.; Hageman, G.S. Chondroitin sulfate-induced generation of epiretinal membranes. Arch. Ophthalmol. 1992, 110, 1000–1006. [Google Scholar] [CrossRef]
- Azzolini, C.; Congiu, T.; Donati, S.; Passi, A.; Basso, P.; Piantanida, E.; Mariotti, C.; Testa, F.; Caprani, S.M.; Cattaneo, J.; et al. Multilayer microstructure of idiopathic epiretinal macular membranes. Eur. J. Ophthalmol. 2017, 27, 762–768. [Google Scholar] [CrossRef]
- Pollreisz, A.; Funk, M.; Breitwieser, F.P.; Parapatics, K.; Sacu, S.; Georgopoulos, M.; Dunavoelgyi, R.; Zlabinger, G.J.; Colinge, J.; Bennett, K.L.; et al. Quantitative proteomics of aqueous and vitreous fluid from patients with idiopathic epiretinal membranes. Exp. Eye Res. 2013, 108, 48–58. [Google Scholar] [CrossRef]
- Mandal, N.; Kofod, M.; Vorum, H.; Villumsen, J.; Erikeìsen, J.; Heegaard, S.; Prause, J.U.; Ahuja, S.; Nonoré, B.; la Cour, M. Protemomic analysis of human vitreous associated with idiopathic epiretinal membranes. Acta Ophthalmol. 2013, 91, e333–e334. [Google Scholar] [CrossRef]
- Yu, J.; Feng, L.; Wu, Y.; Wang, H.; Ba, J.; Zhu, W.; Xie, C. Vitreous proteomic analysis of idiopathic epiretinal membranes. Mol. Biosyst. 2014, 10, 2558–2566. [Google Scholar] [CrossRef]
- Legate, K.R.; Wickström, S.A.; Fässler, R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009, 23, 397–418. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Kukkurainen, S.; Hytönen, V.P.; Wehrle-Haller, B. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef] [PubMed]
- Torres-Gomez, A.; Cabañas, C.; Lafuente, E.M. Phagocytic integrins: Activation and signaling. Front. Immunol. 2020, 11, 738. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Wan, W.; Huang, G.; Li, Y.; Genin, G.M.; Mofrad, M.R.K.; Lu, T.J.; Xu, F.; Lin, M. Nanoscale integrin cluster dynamics controls cellular mechanosensing via FAKY397 phosphorylation. Sci. Adv. 2020, 6, eaax1909. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ogawa, R. Fibroproliferative disorders and their mechanobiology. Connect. Tissue Res. 2012, 53, 187–196. [Google Scholar] [CrossRef]
- Leask, A. Integrin β1: A mechanosignaling sensor essential for connective tissue deposition by fibroblasts. Adv. Wound Care 2013, 2, 160–166. [Google Scholar] [CrossRef]
- Reed, N.I.; Jo, H.; Chen, C.; Tsujino, K.; Arnold, T.D.; DeGrado, W.F.; Sheppard, D. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Sci. Transl. Med. 2015, 7, 288ra79. [Google Scholar] [CrossRef]
- Han, Z.; Ma, Y.; Cao, G.; Ma, Z.; Chen, R.; Cvijic, M.E.; Cheng, D. Integrin αVβ1 regulates procollagen I production through a non-canonical transforming growth factor β signaling pathway in human hepatic stellate cells. Biochem. J. 2021, 478, 1689–1703. [Google Scholar] [CrossRef]
- Boosani, C.S.; Mannam, A.P.; Cosgrove, D.; Silva, R.; Hodivala-Dilke, K.M.; Keshamouni, V.G.; Sudhakar, A. Regulation of COX-2 mediated signaling by alpha3 type IV noncollagenous domain in tumor angiogenesis. Blood 2007, 110, 1168–1177. [Google Scholar] [CrossRef]
- Dedhar, S.; Jewell, K.; Rojiani, M.; Gray, V. The receptor for the basement membrane glycoprotein entactin is the integrin alpha 3/beta 1. J. Biol. Chem. 1992, 267, 18908–18914. [Google Scholar] [CrossRef]
- Kim, K.K.; Wei, Y.; Szekeres, C.; Kugler, M.C.; Wolters, P.J.; Hill, M.L.; Frank, J.A.; Brumwell, A.N.; Wheeler, S.E.; Kreidberg, J.A.; et al. Epithelial cell alpha3beta1 integrin links beta-catenin and Smad signaling to promote myofibroblast formation and pulmonary fibrosis. J. Clin. Investig. 2009, 119, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, H.; Hagood, J.S. Cooperative signaling between integrins and growth factor receptors in fibrosis. J. Mol. Med. 2021, 99, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Sun, P.; Paller, A.S. Ganglioside GM3 blocks the activation of epidermal growth factor receptor induced by integrin at specific tyrosine sites. J. Biol. Chem. 2003, 278, 48770–48778. [Google Scholar] [CrossRef] [PubMed]
- Moro, L.; Dolce, L.; Cabodi, S.; Bergatto, E.; Boeri Erba, E.; Smeriglio, M.; Turco, E.; Retta, S.F.; Giuffrida, M.G.; Venturino, M.; et al. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J. Biol. Chem. 2002, 277, 9405–9414. [Google Scholar] [CrossRef]
- Jeong, B.Y.; Cho, K.H.; Jeong, K.J.; Park, Y.Y.; Kim, J.M.; Rha, S.Y.; Park, C.G.; Mills, G.B.; Cheong, J.H.; Lee, H.Y. Rab25 augments cancer cell invasiveness through a β1 integrin/EGFR/VEGF-A/Snail signaling axis and expression of fascin. Exp. Mol. Med. 2018, 50, e435. [Google Scholar] [CrossRef]
- da Silva, R.G.; Tavora, B.; Robinson, S.D.; Reynolds, L.E.; Szekeres, C.; Lamar, J.; Batista, S.; Kostourou, V.; Germain, M.A.; Reynolds, A.R.; et al. Endothelial alpha3beta1-integrin represses pathological angiogenesis and sustains endothelial-VEGF. Am. J. Pathol. 2010, 177, 1534–1548. [Google Scholar] [CrossRef]
- Kanda, A.; Noda, K.; Hirose, I.; Ishida, S. TGF-β-SNAIL axis induces Müller glial-mesenchymal transition in the pathogenesis of idiopathic epiretinal membrane. Sci. Rep. 2019, 9, 673. [Google Scholar] [CrossRef]
- Hata, Y.; Sassa, Y.; Kita, T.; Miura, M.; Kano, K.; Kawahara, S.; Arita, R.; Nakao, S.; Shih, J.L.; Ishibashi, T. Vascular endothelial growth factor expression by hyalocytes and its regulation by glucocorticoid. Br. J. Ophthalmol. 2008, 92, 1540–1544. [Google Scholar] [CrossRef]
- Yafai, Y.; Iandiev, I.; Wiedemann, P.; Reichenbach, A.; Eichler, W. Retinal endothelial angiogenic activity: Effects of hypoxia and glial (Müller) cells. Microcirculation 2004, 11, 577–586. [Google Scholar] [CrossRef]
- Smiddy, W.E.; Maguire, A.M.; Green, W.R.; Michels, R.G.; de la Cruz, Z.; Enger, C.; Jaeger, M.; Rice, T.A. Idiopathic epiretinal membranes. Ultrastructural characteristics and clinicopathologic correlation. Ophthalmology 1989, 96, 811–820. [Google Scholar] [CrossRef]
- Eichler, W.; Yafai, Y.; Keller, T.; Wiedemann, P.; Reichenbach, A. PEDF derived from glial Müller cells: A possible regulator of retinal angiogenesis. Exp. Cell Res. 2004, 299, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.S.; Santos, D.F.; Silva, G.A. The role of the retinal pigment epithelium and Müller cells secretome in neovascular retinal pathologies. Biochimie 2018, 155, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Bautch, V.L. VEGF-directed blood vessel patterning: From cells to organism. Cold Spring Harb. Perspect. Med. 2012, 2, a006452. [Google Scholar] [CrossRef] [PubMed]
- Mac Gabhann, F.; Popel, A.S. Dimerization of VEGF receptors and implications for signal transduction: A computational study. Biophys Chem. 2007, 128, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Cudmore, M.J.; Hewett, P.W.; Ahmad, S.; Wang, K.Q.; Cai, M.; Al-Ani, B.; Fujisawa, T.; Ma, B.; Sissaoui, S.; Ramma, W.; et al. The role of heterodimerization between VEGFR-1 and VEGFR-2 in the regulation of endothelial cell homeostasis. Nat. Commun. 2012, 3, 972. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.H.; Yao, J.Y.; Kuo, M.T.; See, L.C.; Lin, K.Y.; Chen, S.C.; Chen, J.K.; Chao, A.S.; Wang, S.F.; Lin, K.K. Generation of endostatin by matrix metalloproteinase and cathepsin from human limbocorneal epithelial cells cultivated on amniotic membrane. Investig. Ophthalmol. Vis. Sci. 2007, 48, 644–651. [Google Scholar] [CrossRef]
- Walia, A.; Yang, J.F.; Huang, Y.H.; Rosenblatt, M.I.; Chang, J.H.; Azar, D.T. Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim. Biophys. Acta 2015, 1850, 2422–2438. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, N.; Lai, W.; Yan, B.; Chen, L.; Liu, S.; Liu, S.; Wang, X.; Xiao, D.; Liu, X.; et al. Nuclear EGFR-PKM2 axis induces cancer stem cell-like characteristics in irradiation-resistant cells. Cancer Lett. 2018, 422, 81–93. [Google Scholar] [CrossRef]
- Epstein Shochet, G.; Brook, E.; Eyal, O.; Edelstein, E.; Shitrit, D. Epidermal growth factor receptor paracrine upregulation in idiopathic pulmonary fibrosis fibroblasts is blocked by nintedanib. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L1025–L1034. [Google Scholar] [CrossRef]
- Panizo, S.; Martínez-Arias, L.; Alonso-Montes, C.; Cannata, P.; Martín-Carro, B.; Fernández-Martín, J.L.; Naves-Díaz, M.; Carrillo-López, N.; Cannata-Andía, J.B. Fibrosis in chronic kidney disease: Pathogenesis and consequences. Int. J. Mol. Sci. 2021, 22, 408. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.X.; Ma, J.X.; Zhao, F.; An, J.B.; Geng, Y.X.; Liu, L.Y. Effects of curcumin on epidermal growth factor in proliferative vitreoretinopathy. Cell Physiol. Biochem. 2018, 47, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, H.B.; Meng, J.M.; Yuan, B.; Lin, W.J.; Feng, Y.; Chen, X.D. YM155 inhibits retinal pigment epithelium cell survival through EGFR/MAPK signaling pathway. Int. J. Ophthalmol. 2021, 14, 489–496. [Google Scholar] [CrossRef]
- Ni, Y.; Qin, Y.; Huang, Z.; Liu, F.; Zhang, S.; Zhang, Z. Distinct serum and vitreous inflammation-related factor profiles in patients with proliferative vitreoretinopathy. Adv. Ther. 2020, 37, 2550–2559. [Google Scholar] [CrossRef]
- Nabi, I.R.; Shankar, J.; Dennis, J.W. The galectin lattice at a glance. J. Cell Sci. 2015, 128, 2213–2219. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Andrade, L.N.; Bustos, S.O.; Chammas, R. Galectin-3 determines tumor cell adaptive strategies in stressed tumor microenvironments. Front. Oncol. 2016, 6, 127. [Google Scholar] [CrossRef]
- Sedlář, A.; Trávníčková, M.; Bojarová, P.; Vlachová, M.; Slámová, K.; Křen, V.; Bačáková, L. Interaction between galectin-3 and integrins mediates cell-matrix adhesion in endothelial cells and mesenchymal stem cells. Int. J. Mol. Sci. 2021, 22, 5144. [Google Scholar] [CrossRef]
- Slack, R.J.; Mills, R.; Mackinnon, A.C. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int. J. Biochem. Cell Biol. 2021, 130, 105881. [Google Scholar] [CrossRef] [PubMed]
- Luis, J.; Eastlake, K.; Khaw, P.T.; Limb, G.A. Galectins and their involvement in ocular disease and development. Exp. Eye Res. 2020, 197, 108120. [Google Scholar] [CrossRef]
- Mackinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Kipari, T.; Haslett, C.; Iredale, J.P.; Liu, F.T.; Hughes, J.; Sethi, T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am. J. Pathol. 2008, 172, 288–298. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.L.; Panera, N.; De Stefanis, C.; Mosca, A.; D’Oria, V.; Crudele, A.; De Vito, R.; Nobili, V.; Alisi, A. The number of liver galectin-3 positive cells is dually correlated with NAFLD severity in children. Int. J. Mol. Sci. 2019, 20, 3460. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Qian, X.; Chen, G.; Song, X. The role of galectin-3 in heart failure and cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2019, 46, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Burguillos, M.A.; Svensson, T.; Schulte, T.; Boza-Serrano, A.; Garcia-Quintanilla, A.; Kavanagh, E.; Santiago, M.; Viceconte, N.; Oliva-Martin, M.J.; Osman, A.M.; et al. Microglia-sereted galectin-3 acts as a toll-like receptor 4 ligand and contributes to microglial activation. Cell Rep. 2015, 10, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Alvarez, L.; Ortega, E. The many roles of galectin-3, a multifaceted molecule, in innate immune responses against pathogens. Mediators Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef]
- Woods, E.L.; Grigorieva, I.V.; Midgley, A.C.; Brown, C.V.M.; Lu, Y.A.; Phillips, A.O.; Bowen, T.; Meran, S.; Steadman, R. CD147 mediates the CD44s-dependent differentiation of myofibroblasts driven by transforming growth factor-β1. J. Biol. Chem. 2021, 297, 100987. [Google Scholar] [CrossRef]
- Guindolet, D.; Gabison, E.E. Role of CD147 (EMMPRIN/Basigin) in tissue remodeling. Anat. Rec. 2020, 303, 1584–1589. [Google Scholar] [CrossRef]
- Arima, M.; Cui, D.; Kimura, T.; Sonoda, K.H.; Ishibashi, T.; Matsuda, S.; Ikeda, E. Basigin can be a therapeutic target to restore the retinal vascular barrier function in the mouse model of diabetic retinopathy. Sci. Rep. 2016, 6, 38445. [Google Scholar] [CrossRef]
- Markowska, A.I.; Cao, Z.; Panjwani, N. Glycobiology of ocular angiogenesis. Glycobiology 2014, 24, 1275–1282. [Google Scholar] [CrossRef]
- Patrizz, A.; Doran, S.J.; Chauhan, A.; Ahnstedt, H.; Roy-O’Reilly, M.; Lai, Y.J.; Weston, G.; Tarabishy, S.; Patel, A.R.; Verma, R.; et al. EMMPRIN/CD147 plays a detrimental role in clinical and experimental ischemic stroke. Aging 2020, 12, 5121–5139. [Google Scholar] [CrossRef]
- Roesch, K.; Jadhav, A.P.; Trimarchi, J.M.; Stadler, M.B.; Roska, B.; Sun, B.B.; Cepko, C.L. The transcriptome of retinal Müller glial cells. J. Comp. Neurol. 2008, 509, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Priglinger, C.S.; Szober, C.M.; Priglinger, S.G.; Merl, J.; Euler, K.N.; Kernt, M.; Gondi, G.; Behler, J.; Geerlof, A.; Kampik, A.; et al. Galectin-3 induces clustering of CD147 and integrin-β1 transmembrane glycoprotein receptors on the RPE cell surface. PLoS ONE 2013, 8, e70011. [Google Scholar] [CrossRef]
- Xu, H.; Manivannan, A.; Liversidge, J.; Sharp, P.F.; Forrester, J.V.; Crane, I.J. Involvement of CD44 in leukocyte trafficking at the blood-retinal barrier. J. Leukoc. Biol. 2002, 72, 1133–1141. [Google Scholar]
- Sobue, Y.; Takahashi, N.; Ohashi, Y.; Suzuki, M.; Nishiume, T.; Kobayakawa, T.; Terabe, K.; Knudson, W.; Knudson, C.; Ishiguro, N.; et al. Inhibition of CD44 intracellular domain production suppresses bovine articular chondrocyte de-differentiation induced by excessive mechanical stress loading. Sci. Rep. 2019, 9, 14901. [Google Scholar] [CrossRef]
- Chaitin, M.H.; Wortham, H.S.; Brun-Zinkernagel, A.M. Immunocytochemical localization of CD44 in the mouse retina. Exp. Eye Res. 1994, 58, 359–365. [Google Scholar] [CrossRef]
- Nishina, S.; Hirakata, A.; Hida, T.; Sawa, H.; Azuma, N. CD44 expression in the developing human retina. Graefes Arch. Clin. Exp. Ophthalmol. 1997, 235, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Andresen, P.; Lumi, X.; Chen, Q.; Petrovski, G. Expression of progenitor cell markers in the glial-like cells of epiretinal membranes of different origins. J. Ophthalmol. 2018, 2018, 7096326. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Missotten, L.; Geboes, K. Expression of myofibroblast activation molecules in proliferve vitreoretinopathy epiretinal membranes. Acta Ophthalmol. 2011, 89, e115–e121. [Google Scholar] [CrossRef] [PubMed]
- Kampik, A.; Kenyon, K.R.; Michels, R.G.; Green, W.R.; de la Cruz, Z.C. Epiretinal and vitreous membranes. Comparative study of 56 cases. Arch. Ophthalmol. 1981, 99, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- McKeown-Longo, P.J.; Higgins, P.J. Hyaluronan, transforming growth factor β, and extra domain A-fibronectin: A fibrotic triad. Adv. Wound Care 2020, 10, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.K.; Saraswathy, S.; Parikh, J.G.; Rao, N.A. The role of TLR4 activation in photoreceptor mitochondrial oxidative stress. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5824–5835. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Sun, D.; Kaplan, H.J.; Shao, H. Retinal astrocytes pretreated with NOD2 and TLR2 ligands activate uveitogenic T cells. PLoS ONE 2012, 7, e40510. [Google Scholar] [CrossRef][Green Version]
- Midgley, A.C.; Rogers, M.; Hallett, M.B.; Clayton, A.; Bowen, T.; Phillips, A.O.; Steadman, R. Transforming growth factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J. Biol. Chem. 2013, 288, 14824–14838. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Leng, L.; Wang, T.; Wang, W.; Du, X.; Li, J.; McDonald, C.; Chen, Z.; Murphy, J.W.; Lolis, E.; et al. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity 2006, 25, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Osipyan, A.; Chen, D.; Dekker, F.J. Epigenetic regulation in macrophage migration inhibitory factor (MIF)-mediated signaling in cancer and inflammation. Drug Discov. Today 2021, 26, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, R.; Hausser, A.; Geiger, G.; Mischke, R.; Burger-Kentischer, A.; Flieger, O.; Johannes, F.J.; Roger, T.; Calandra, T.; Kapurniotu, A.; et al. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature 2000, 408, 211–216. [Google Scholar] [CrossRef]
- Man, A.L.; Lodi, F.; Bertelli, E.; Regoli, M.; Pin, C.; Mulholland, F.; Satoskar, A.R.; Taussig, M.J.; Nicoletti, C. Macrophage migration inhibitory factor plays a role in the regulation of microfold (M) cell-mediated transport in the gut. J. Immunol. 2008, 181, 5673–5680. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Ahmad, A.; Siddiquei, M.M.; De Zutter, A.; Allegaert, E.; Gikandi, P.W.; De Hertogh, G.; Van Damme, J.; Opdenakker, G.; Struyf, S. The proinflammatory and proangiogenic macrophage migration inhibitory factor is a potential regulator in proliferative diabetic retinopathy. Front. Immunol. 2019, 10, 2752. [Google Scholar] [CrossRef]
- Turk, B.; Turk, D.; Turk, V. Protease signalling: The cutting edge. EMBO J 2012, 31, 1630–1643. [Google Scholar] [CrossRef]
- Garcia-Cattaneo, A.; Gobert, F.X.; Müller, M.; Toscano, F.; Flores, M.; Lescure, A.; Del Nery, E.; Benaroch, P. Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 9053–9058. [Google Scholar] [CrossRef]
- Chevriaux, A.; Pilot, T.; Derangère, V.; Simonin, H.; Martine, P.; Chalmin, F.; Ghiringhelli, F.; Rébé, C. Cathepsin B is required for NLRP3 inflammasome activation in macrophages, through NLRP3 interaction. Front. Cell Dev. Biol. 2020, 8, 167. [Google Scholar] [CrossRef]
- Unanue, E.R.; Turk, V.; Neefjes, J. Variations in MHC Class II Antigen Processing and Presentation in Health and Disease. Annu. Rev. Immunol. 2016, 34, 265–297. [Google Scholar] [CrossRef]
- Vishwakarma, S.; Gupta, R.K.; Jakati, S.; Tyagi, M.; Pappuru, R.R.; Reddig, K.; Hendricks, G.; Volkert, M.R.; Khanna, H.; Chhablani, J.; et al. Molecular assessment of epiretinal membrane: Activated microglia, oxidative stress and inflammation. Antioxidants 2020, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Agrawal, S.; Christoforidis, J.B. Inflammatory mechanisms of idiopathic epiretinal membrane formation. Mediators Inflamm. 2013, 2013, 192582. [Google Scholar] [CrossRef] [PubMed]
- Yadati, T.; Houben, T.; Bitorina, A.; Shiri-Sverdlov, R. The ins and outs of cathepsins: Physiological function and role in disease management. Cells 2020, 9, 1679. [Google Scholar] [CrossRef]
- Buck, M.R.; Karustis, D.G.; Day, N.A.; Honn, K.V.; Sloane, B.F. Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochem. J. 1992, 282, 273–278. [Google Scholar] [CrossRef]
- Christensen, J.; Shastri, V.P. Matrix-metalloproteinase-9 is cleaved and activated by cathepsin K. BMC. Res. Notes 2015, 8, 322. [Google Scholar] [CrossRef]
- Ruan, H.; Hao, S.; Young, P.; Zhang, H. Targeting cathepsin B for cancer therapies. Horiz. Cancer Res. 2015, 56, 23–40. [Google Scholar]
- Ricard-Blum, S.; Vallet, S. Fragments generated upon extracellular matrix remodelling: Biological regulators and potential drugs. Matrix Biol. 2019, 75-76, 170–189. [Google Scholar] [CrossRef]
- Wells, J.M.; Gaggar, A.; Blalock, J.E. MMP generated matrikines. Matrix Biol. 2015, 44-46, 122–129. [Google Scholar] [CrossRef]
- Szmola, R.; Kukor, Z.; Sahin-Tóth, M. Human mesotrypsin is a unique digestive protease specialized for the degradation of trypsin inhibitors. J. Biol. Chem. 2003, 278, 48580–48589. [Google Scholar] [CrossRef] [PubMed]
- Paraoan, L.; Sharif, U.; Carlsson, E.; Supharattanasitthi, W.; Mahmud, N.M.; Kamalden, T.A.; Hiscott, P.; Jackson, M.; Grierson, I. Secretory proteostasis of the retinal pigmented epithelium: Impairment links to age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100859. [Google Scholar] [CrossRef] [PubMed]
- Tinklepaugh, J.; Smith, B.M.; Nie, Y.; Moody, K.; Grohn, K.; Bou-Abdallah, F.; Doyle, R.P. Saposin B binds the lipofuscin bisretinoid A2E and prevents its enzymatic and photooxidation. ChemPhotoChem 2017, 1, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Morrone, C.; Smirnova, N.F.; Jeridi, A.; Kneidinger, N.; Hollauer, C.; Schupp, J.C.; Kaminski, N.; Jenne, D.; Eickelberg, O.; Yildirim, A.Ö. Cathepsin B promotes collagen biosynthesis, which drives bronchiolitis obliterans syndrome. Eur. Respir. J. 2021, 57, 2001416. [Google Scholar] [CrossRef]
- Kasabova, M.; Joulin-Giet, A.; Lecaille, F.; Gilmore, B.F.; Marchand-Adam, S.; Saidi, A.; Lalmanach, G. Regulation of TGF-β1-driven differentiation of human lung fibroblasts: Emerging roles of cathepsin B and cystatin C. J. Biol. Chem. 2014, 289, 16239–16251. [Google Scholar] [CrossRef]
- Moles, A.; Tarrats, N.; Fernández-Checa, J.C.; Marí, M. Cathepsins B and D drive hepatic stellate cell proliferation and promote their fibrogenic potential. Hepatology 2009, 49, 1297–1307. [Google Scholar] [CrossRef]
- Priglinger, S.G.; May, C.A.; Neubauer, A.S.; Alge, C.S.; Schönfeld, C.L.; Kampik, A.; Welge-Lussen, U. Tissue transglutaminase as a modifying enzyme of the extracellular matrix in PVR membranes. Investig. Ophthalmol. Vis. Sci. 2003, 44, 355–364. [Google Scholar] [CrossRef]
- Quan, G.; Choi, J.Y.; Lee, D.S.; Lee, S.C. TGF-beta1 up-regulates transglutaminase two and fibronectin in dermal fibroblasts: A possible mechanism for the stabilization of tissue inflammation. Arch. Dermatol. Res. 2005, 297, 84–90. [Google Scholar] [CrossRef]
- Akimov, S.S.; Belkin, A.M. Cell-surface transglutaminase promotes fibronectin assembly via interaction with the gelatin-binding domain of fibronectin: A role in TGFbeta-dependent matrix deposition. J. Cell Sci. 2001, 114, 2989–3000. [Google Scholar] [CrossRef]
- Tovar-Vidales, T.; Clark, A.F.; Wordinger, R.J. Focus on molecules: Transglutaminase 2. Exp. Eye Res. 2011, 93, 2–3. [Google Scholar] [CrossRef]
- Aeschlimann, D.; Paulsson, M.; Mann, K. Identification of Gln726 in nidogen as the amine acceptor in transglutaminase-catalyzed crosslinking of laminin–nidogen complexes. J. Biol. Chem. 1992, 267, 11316–11321. [Google Scholar] [CrossRef]
- Kleman, J.P.; Aeschlimann, D.; Paulsson, M.; van der Rest, M. Transglutaminase-catalyzed cross-linking of fibrils of collagen V⁄XI in A204 rhabdomyosarcoma cells. Biochemistry 1995, 34, 13768–13775. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.S.; Lin, C.J.; Greenberg, C.S. Role of tissue transglutaminase-2 (TG2)-mediated aminylation in biological processes. Amino Acids 2017, 49, 501–515. [Google Scholar] [CrossRef]
- Melkonian, A.V.; Weng, N.; Palanski, B.A.; Khosla, C. In vivo measurement of redox-regulated TG2 activity. Methods Mol. Biol. 2019, 1967, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.S.; Lin, C.J.; Wu, Y.T.; Wu, C.J. Tissue transglutaminase (TG2) and mitochondrial function and dysfunction. Front. Biosci. 2017, 22, 1114–1137. [Google Scholar] [CrossRef]
- Campisi, A.; Caccamo, D.; Li Volti, G.; Currò, M.; Parisi, G.; Avola, R.; Vanella, A.; Ientile, R. Glutamate-evoked redox state alterations are involved in tissue transglutaminase upregulation in primary astrocyte cultures. FEBS Lett. 2004, 578, 80–84. [Google Scholar] [CrossRef]
- Kim, Y.; Eom, S.; Kim, K.; Lee, Y.S.; Choe, J.; Hahn, J.H.; Lee, H.; Kim, Y.M.; Ha, K.S.; Ro, J.Y.; et al. Transglutaminase II interacts with rac1, regulates production of reactive oxygen species, expression of snail, secretion of Th2 cytokines and mediates in vitro and in vivo allergic inflammation. Mol. Immunol. 2010, 47, 1010–1022. [Google Scholar] [CrossRef]
- Barrallo-Gimeno, A.; Nieto, M.A. The Snail genes as inducers of cell movement and survival: Implications in development and cancer. Development 2005, 132, 3151–3161. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Wang, F.; Gu, Q.; Xu, X. Snail involves in the transforming growth factor β1-mediated epithelial-mesenchymal transition of retinal pigment epithelial cells. PLoS ONE 2011, 6, e23322. [Google Scholar] [CrossRef]
- Oka, C.; Tsujimoto, R.; Kajikawa, M.; Koshiba-Takeuchi, K.; Ina, J.; Yano, M.; Tsuchiya, A.; Ueta, Y.; Soma, A.; Kanda, H.; et al. HtrA1 serine protease inhibits signaling mediated by Tgfbeta family proteins. Development 2004, 131, 1041–1053. [Google Scholar] [CrossRef]
- Yamawaki, S.; Naitoh, M.; Kubota, H.; Aya, R.; Katayama, Y.; Ishiko, T.; Tamura, T.; Yoshikawa, K.; Enoshiri, T.; Ikeda, M.; et al. HtrA1 is specifically up-regulated in active keloid lesions and stimulates keloid development. Int. J. Mol. Sci. 2018, 19, 1275. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.A.; Shirer, K.; Collazo, S.A.; Szczotka, K.; Baker, S.; Wood, B.; Carroll, L.; Haaland, B.; Iwata, T.; Katikaneni, L.D.; et al. The serine protease HTRA-1 is a biomarker for ROP and mediates retinal neovascularization. Front. Mol. Neurosci. 2020, 13, 605918. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Chen, C.Z.; Xing, Y.Q. Inhibition of cell proliferation, migration and apoptosis in blue-light illuminated human retinal pigment epithelium cells by down-regulation of HtrA1. Int. J. Ophthalmol. 2017, 10, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Acosta, H.; Iliev, D.; Grahn, T.H.; Gouignard, N.; Maccarana, M.; Griesbach, J.; Herzmann, S.; Sagha, M.; Climent, M.; Pera, E.M. The serpin PN1 is a feedback regulator of FGF signaling in germ layer and primary axis formation. Development 2015, 142, 1146–1158. [Google Scholar] [CrossRef]
- Romay, M.C.; Toro, C.; Iruela-Arispe, M.L. Emerging molecular mechanisms of vascular dementia. Curr. Opin. Hematol. 2019, 26, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Romaniuk, D.; Kimsa, M.W.; Strzalka-Mrozik, B.; Kimsa, M.C.; Kabiesz, A.; Romaniuk, W.; Mazurek, U. Gene expression of IGF1, IGF1R, and IGFBP3 in epiretinal membranes of patients with proliferative diabetic retinopathy: Preliminary study. Mediators Inflamm. 2013, 2013, 986217. [Google Scholar] [CrossRef]
- Harada, T.; Harada, C.; Mitamura, Y.; Akazawa, C.; Ohtsuka, K.; Ohno, S.; Takeuchi, S.; Wada, K. Neurotrophic factor receptors in epiretinal membranes after human diabetic retinopathy. Diabetes Care 2002, 25, 1060–1065. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuznetsova, A.V.; Rzhanovaa, L.A.; Kurinova, A.M.; Aleksandrova, M.A. The effect of basic fibroblast growth factor on signaling pathways in adult human retinal pigment epithelial cells. Cell Tissue Biol. 2019, 13, 292–304. [Google Scholar] [CrossRef]
- Rehman, A.A.; Ahsan, H.; Khan, F.H. α-2-Macroglobulin: A physiological guardian. J. Cell Physiol. 2013, 228, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Bres, E.E.; Faissner, A. Low density receptor-related protein 1 interactions with the extracellular matrix: More than meets the eye. Front. Cell Dev. Biol. 2019, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Schnieder, J.; Mamazhakypov, A.; Birnhuber, A.; Wilhelm, J.; Kwapiszewska, G.; Ruppert, C.; Markart, P.; Wujak, L.; Rubio, K.; Barreto, G.; et al. Loss of LRP1 promotes acquisition of contractile-myofibroblast phenotype and release of active TGF-β1 from ECM stores. Matrix Biol. 2020, 88, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Rabiej, V.K.; Pflanzner, T.; Wagner, T.; Goetze, K.; Storck, S.E.; Eble, J.A.; Weggen, S.; Mueller-Klieser, W.; Pietrzik, C.U. Low density lipoprotein receptor-related protein 1 mediated endocytosis of β1-integrin influences cell adhesion and cell migration. Exp. Cell Res. 2016, 340, 102–115. [Google Scholar] [CrossRef]
- Strickland, D.K.; Muratoglu, S.C.; Antalis, T.M. Serpin-enzyme receptors LDL receptor-related protein 1. Methods Enzymol. 2011, 499, 17–31. [Google Scholar] [CrossRef]
- Salicioni, A.M.; Gaultier, A.; Brownlee, C.; Cheezum, M.K.; Gonias, S.L. Low density lipoprotein receptor-related protein-1 promotes beta1 integrin maturation and transport to the cell surface. J. Biol. Chem. 2004, 279, 10005–10012. [Google Scholar] [CrossRef]
- Wujak, L.; Böttcher, R.T.; Pak, O.; Frey, H.; El Agha, E.; Chen, Y.; Schmitt, S.; Bellusci, S.; Schaefer, L.; Weissmann, N.; et al. Low density lipoprotein receptor-related protein 1 couples β1 integrin activation to degradation. Cell Mol. Life Sci. 2018, 75, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Zemskov, E.A.; Mikhailenko, I.; Strickland, D.K.; Belkin, A.M. Cell-surface transglutaminase undergoes internalization and lysosomal degradation: An essential role for LRP1. J. Cell Sci. 2007, 120, 3188–3199. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, A.; Hui, D.Y. LDL receptor-related protein 1 and its interacting partners in tissue homeostasis. Curr. Opin. Lipidol. 2021, 32, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Wujak, L.; Schnieder, J.; Schaefer, L.; Wygrecka, M. LRP1: A chameleon receptor of lung inflammation and repair. Matrix Biol. 2018, 68-69, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Barcelona, P.F.; Jaldín-Fincati, J.R.; Sánchez, M.C.; Chiabrando, G.A. Activated α2-macroglobulin induces Müller glial cell migration by regulating MT1-MMP activity through LRP1. FASEB J. 2013, 27, 3181–3197. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.M.; Chess, J.; Henkind, P. Fibrinogen-induced vitreous membranes. Ophthalmic. Res. 1987, 19, 164–169. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, M.; Zhao, Y.; Qian, J.; Dufresne, C.; Turner, R.; Semba, R.D.; Solomon, S.D. A proteomic approach to understanding the pathogenesis of idiopathic macular hole formation. Clin. Proteom. 2017, 14, 37. [Google Scholar] [CrossRef]
- Trink, J.; Li, R.; Palarasah, Y.; Troyanov, S.; Andersen, T.E.; Sidelmann, J.J.; Inman, M.D.; Pizzo, S.V.; Gao, B.; Krepinsky, J.C. Activated alpha 2-macroglobulin is a novel mediator of mesangial cell profibrotic signaling in diabetic kidney disease. Biomedicines 2021, 9, 1112. [Google Scholar] [CrossRef] [PubMed]
- Khalkhali-Ellis, Z.; Hendrix, M.J. Elucidating the function of secreted maspin: Inhibiting cathepsin D-mediated matrix degradation. Cancer Res. 2007, 67, 3535–3539. [Google Scholar] [CrossRef]
- Kurz, S.; Thieme, R.; Amberg, R.; Groth, M.; Jahnke, H.G.; Pieroh, P.; Horn, L.C.; Kolb, M.; Huse, K.; Platzer, M.; et al. The anti-tumorigenic activity of A2M-A lesson from the naked mole-rat. PLoS ONE 2017, 12, e0189514. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.M.; Neri, G.; Caldi, E.; Fusco, F.; Bacci, T.; Tarantello, A.; Nuti, E.; Marigliani, D.; Baiocchi, S.; Traversi, C.; et al. TGF-β concentrations and activity are down-regulated in the aqueous humor of patients with neovascular age-related macular degeneration. Sci. Rep. 2018, 8, 8053. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.M.; Orlandini, M.; Galvagni, F. The controversial role of TGF-β in neovascular age-related macular degeneration pathogenesis. Int. J. Mol. Sci. 2018, 19, 3363. [Google Scholar] [CrossRef]
- Hashimoto, R.; Jiang, M.; Shiba, T.; Hiruta, N.; Takahashi, M.; Higashi, M.; Hori, Y.; Bujo, H.; Maeno, T. Soluble form of LR11 is highly increased in the vitreous fluids of patients with idiopathic epiretinal membrane. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Iannetti, L.; Accorinti, M.; Malagola, R.; Bozzoni-Pantaleoni, F.; Da Dalt, S.; Nicoletti, F.; Gradini, R.; Traficante, A.; Campanella, M.; Pivetti-Pezzi, P. Role of the intravitreal growth factors in the pathogenesis of idiopathic epiretinal membrane. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5786–5789. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Bujo, H.; Ohwaki, K.; Unoki, H.; Yamazaki, H.; Kanaki, T.; Shibasaki, M.; Azuma, K.; Harigaya, K.; Schneider, W.J.; et al. Ang II-stimulated migration of vascular smooth muscle cells is dependent on LR11 in mice. J. Clin. Investig. 2008, 118, 2733–2746. [Google Scholar] [CrossRef]
- Shiba, T.; Bujo, H.; Takahashi, M.; Sato, Y.; Jiang, M.; Hori, Y.; Maeno, T.; Shirai, K. Vitreous fluid and circulating levels of soluble lr11, a novel marker for progression of diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Ge, T.; Zhang, Y.; Li, M.; Yang, S.; Li, H.; Wang, F. BMP7 antagonizes proliferative vitreoretinopathy through retinal pigment epithelial fibrosis in vivo and in vitro. FASEB J. 2019, 33, 3212–3224. [Google Scholar] [CrossRef]
- Zandi, S.; Tappeiner, C.; Pfister, I.B.; Despont, A.; Rieben, R.; Garweg, J.G. Vitreal cytokine profile differences between eyes with epiretinal membranes or macular holes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6320–6326. [Google Scholar] [CrossRef] [PubMed]
- Minchiotti, S.; Stampachiacchiere, B.; Micera, A.; Lambiase, A.; Ripandelli, G.; Billi, B.; Bonini, S. Human idiopathic epiretinal membranes express NGF and NGF receptors. Retina. 2008, 28, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Hackett, S.F.; Schoenfeld, C.L.; Vinores, M.A.; Vinores, S.A.; Campochiaro, P.A. Localisation of vascular endothelial growth factor and its receptors to cells of vascular and avascular epiretinal membranes. Br. J. Ophthalmol. 1997, 81, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Simm, A.; Nestler, M.; Hoppe, V. PDGF-AA, a potent mitogen for cardiac fibroblasts from adult rats. J. Mol. Cell. Cardiol. 1997, 29, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Juhl, P.; Bondesen, S.; Hawkins, C.L.; Lambiase, A.; Ripandelli, G.; Billi, B.; Siebuhr, A.S. Dermal fibroblasts have different extracellular matrix profiles induced by TGF-β, PDGF and IL-6 in a model for skin fibrosis. Sci. Rep. 2020, 10, 17300. [Google Scholar] [CrossRef]
- Robbins, S.G.; Mixon, R.N.; Wilson, D.J.; Hart, C.E.; Robertson, J.E.; Westra, I.; Planck, S.R.; Rosenbaum, J.T. Platelet-derived growth factor ligands and receptors immunolocalized in proliferative retinal disease. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3649–3663. [Google Scholar]
- Cui, J.Z.; Chiu, A.; Maberley, D.; Ma, P.; Samad, A.; Matsubara, J.A. Stage specificity of novel growth factor expression during development of proliferative vitreoretinopathy. Eye 2007, 21, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Micera, A.; Vigneti, E.; Pickholtz, D.; Ma, P.; Samad, A.; Matsubara, J.A.; Maquart, F.X.; Aloe, L.; Levi-Schaffer, F. Nerve growth factor displays stimulatory effects on human skin and lung fibroblasts, demonstrating a direct role for this factor in tissue repair. Proc Natl Acad Sci, USA 2001, 98, 6162–6167. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, Y.; Liu, G.; Yin, S.; Ma, J.; Liu, J.; Zhang, M.; Wang, Y. p75 neurotrophin receptor regulates NGF-induced myofibroblast differentiation and collagen synthesis through MRTF-A. Exp. Cell Res. 2019, 383, 1111504. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, A.; Sonar, S.S.; Yildirim, A.O.; Fehrenbach, H.; Nockher, W.A.; Renz, H. Nerve growth factor induces type III collagen production in chronic allergic airway inflammation. J. Allergy Clin. Immunol. 2011, 128, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.W.; Zhang, Q.L.; Qiao, L.-Y. Endogenous nerve growth factor regulates collagen expression and bladder hypertrophy through Akt and MAPK pathways during cystitis. J. Biol. Chem. 2010, 285, 4206–4216. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Augustin, A.J.; Spengler, R.; Al-Jada, A.; Nickola, T.; Grus, F.; Koch, F. Detection of vascular endothelial growth factor and tumor necrosis factor alpha in epiretinal membranes of proliferative diabetic retinopathy, proliferative vitreoretinopathy and macular pucker. Ophthalmologica 1998, 212, 410–414. [Google Scholar] [CrossRef]
- Watanabe, D.; Suzuma, K.; Suzuma, I.; Ohashi, H.; Ojima, T.; Kurimoto, M.; Murakami, T.; Kimura, T.; Takagi, H. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am. J. Ophthalmol. 2005, 139, 476–481. [Google Scholar] [CrossRef]
- Applewhite, B.P.; Babapoor-Farrokhran, S.; Poon, D.; Hassan, S.J.; Wellmann, E.; Ying, H.S.; Semenza, G.L.; Montaner, S.; Sodhi, A. Lack of evidence for vasoactive and inflammatory mediators in the promotion of macular edema associated with epiretinal membranes. Sci. Rep. 2017, 7, 10608. [Google Scholar] [CrossRef]
- Harada, C.; Mitamura, Y.; Harada, T. The role of cytokines and trophic factors in epiretinal membranes: Involvement of signal transduction in glial cells. Prog. Retin. Eye Res. 2006, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.M.; Caldi, E.; Neri, G.; Nuti, E.; Marigliani, D.; Baiocchi, S.; Traversi, C.; Cevenini, G.; Tarantello, A.; Fusco, F.; et al. HTRA1 and TGF-β1 concentrations in the aqueous humor of patients with neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 162–167. [Google Scholar] [CrossRef]
- Saint-Geniez, M.; Maharaj, A.S.; Walshe, T.E.; Tucker, B.A.; Sekiyama, E.; Kurihara, T.; Darland, D.C.; Young, M.J.; D’Amore, P.A. Endogenous VEGF is required for visual function: Evidence for a survival role on müller cells and photoreceptors. PLoS ONE 2008, 3, e3554. [Google Scholar] [CrossRef]
- Myojin, S.; Yoshimura, T.; Yoshida, S.; Takeda, A.; Murakami, Y.; Kawano, Y.; Oshima, Y.; Ishibashi, T.; Sonoda, K.H. Gene expression analysis of the irrigation solution samples collected during vitrectomy for idiopathic epiretinal membrane. PLoS ONE 2016, 11, e0164355. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Ragusa, M.; Barbagallo, C.; Longo, A.; Avitabile, T.; Uva, M.G.; Bonfiglio, V.; Toro, M.D.; Caltabiano, R.; Mariotti, C.; et al. miRNAs in the vitreous humor of patients affected by idiopathic epiretinal membrane and macular hole. PLoS ONE 2017, 12, e0174297. [Google Scholar] [CrossRef]
- Mestdagh, P.; Boström, A.K.; Impens, F.; Fredlund, E.; Van Peer, G.; De Antonellis, P.; von Stedingk, K.; Ghesquière, B.; Schulte, S.; Dews, M.; et al. The miR-17-92 microRNA cluster regulates multiple components of the TGF-β pathway in neuroblastoma. Mol. Cell. 2010, 40, 762–773. [Google Scholar] [CrossRef]
- Dews, M.; Fox, J.L.; Hultine, S.; Sundaram, P.; Wang, W.; Liu, Y.Y.; Furth, E.; Enders, G.H.; El-Deiry, W.; Schelter, J.M.; et al. The myc-miR-17~92 axis blunts TGF{beta} signaling and production of multiple TGF{beta}-dependent antiangiogenic factors. Cancer Res 2010, 70, 8233–8246. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.; Yang, G.; Chen, X.; Zhang, Y.; Cao, G.; Wang, J.; Sun, Y.; Zhang, P.; Fan, M.; et al. Transforming growth factor-beta-regulated miR-24 promotes skeletal muscle differentiation. Nucleic Acids Res. 2008, 36, 2690–2699. [Google Scholar] [CrossRef]
- Chan, M.C.; Hilyard, A.C.; Wu, C.; Davis, B.N.; Hill, N.S.; Lal, A.; Lieberman, J.; Lagna, G.; Hata, A. Molecular basis for antagonism between PDGF and the TGFbeta family of signalling pathways by control of miR-24 expression. EMBO J. 2010, 29, 559–573. [Google Scholar] [CrossRef]
- Wickham, L.; Gregor, Z. Epiretinal Membranes. In Retina; Ryan, S.J., Ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; pp. 1954–1961. [Google Scholar]
- Yusuf, A.M.; Bizrah, M.; Bunce, C.; Bainbridge, J.W. Surgery for idiopathic epiretinal membrane. Cochrane Database Syst. Rev. 2021, 3, CD013297. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.D.; Zacks, D.N.; Grossman, D.; Grabe, H.; Johnson, M.W.; Sloan, F.A. Trends in rates of adverse events after pars plana vitrectomy among medicare beneficiaries. Arch. Ophthalmol. 2009, 127, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Sebag, J. Pharmacologic vitreolysis. Retina 1998, 18, 1–3. [Google Scholar] [CrossRef] [PubMed]
- de Smet, M.D.; Gandorfer, A.; Stalmans, P.; Veckeneer, M.; Feron, E.; Pakola, S.; Kampik, A. Microplasmin intravitreal administration in patients with vitreomacular traction scheduled for vitrectomy. Ophthalmology 2009, 116, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Stalmans, P.; Delaey, C.; de Smet, M.D.; van Dijkman, E.; Pakola, S. Intravitreal injection of microplasmin for treatment of vitreomacular adhesion: Results of a prospective, randomized, sham-controlled phase II trial (the MIVI-IIT trial). Retina 2010, 30, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Stalmans, P.; Benz, M.S.; Gandorfer, A.; van Dijkman, E.; Pakola, S. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N. Engl. J. Med. 2012, 367, 606–615. [Google Scholar] [CrossRef]
- Kampik, A. Pathology of epiretinal membrane, idiopathic macular hole, and vitreomacular traction syndrome. Retina 2012, 32, S194–S198. [Google Scholar] [CrossRef] [PubMed]
- Trese, M.T. Enzymatic-assisted vitrectomy. Eye 2002, 16, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Schumann, R.G.; Wolf, A.; Mayer, W.J.; Compera, D.; Hagenau, F.; Ziada, J.; Kampik, A.; Haritoglou, C. Pathology of internal limiting membrane specimens following intravitreal injection of ocriplasmin. Am. J. Ophthalmol. 2015, 160, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Sorgenre, N.; Ishibashi, T.; Goodnight, R.; Ryan, S.J. Immunofluorescent studies of fibronectin and laminin in the humon eye. Investig. Ophthalmol. Vis. Sci. 1987, 28, 506–513. [Google Scholar]
- Ponsioen, T.L.; van Luyn, M.J.A.; van der Worp, R.J.; van Meurs, J.C.; Hooymans, J.M.; Los, L.I. Collagen distribution in the human vitreoretinal interface. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4089. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Keenan, T.D.L.; Fielder, H.L.; Collinson, L.J.; Holley, R.J.; Merry, C.L.R.; Kampik, A.; Haritoglou, C. Mapping the differential distribution of glycosaminoglycans in the adult human retina, choroid, and sclera. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6511. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.D.L.; Clark, S.J.; Unwin, R.D.; Ridge, L.A.; Day, A.J.; Bishop, P.N. Mapping the differential distribution of proteoglycan core proteins in the adult human retina, choroid, and sclera. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7528. [Google Scholar] [CrossRef] [PubMed]
- Halfter, W.; Monnier, C.; Müller, D.; Oertle, P.; Uechi, G.; Balasubramani, M.; Safi, F.; Lim, R.; Loparic, M.; Henrich, P.B. The bi-functional organization of human basement membranes. PLoS ONE 2013, 8, e67660. [Google Scholar] [CrossRef]
- Schneider, E.W.; Johnson, M.W. Emerging non surgical methods for the treatment of vitreomacular adhesion: A review. Clin. Ophthalmol. 2011, 5, 1151–1165. [Google Scholar] [CrossRef]
- Koleva-Georgieva, D.N. Pharmacologic vitreolysis: New strategy for treatment of anomalous vitreo-macular adhesion. World. J. Ophthalmol. 2015, 5, 99–105. [Google Scholar] [CrossRef]
- Dolz-Marco, R.; Gallego-Pinazo, R.; Diaz-Llopis, M.; Arévalo, J.F. Pharmacovitrectomy. Dev. Ophthalmol. 2014, 54, 126–134. [Google Scholar] [CrossRef]
- Wan, R.; Hong, T.; Tariq, Y.; Chang, A. Pharmacotherapy of vitreomacular traction. Curr. Pharm. Des. 2018, 24, 4874–4881. [Google Scholar] [CrossRef]
- Sebag, J. Pharmacologic Vitreolysis. In Vitreous in Health and Disease; Sebag, J., Ed.; Springer: New York, NY, USA, 2014; pp. 799–815. [Google Scholar] [CrossRef]
- Moorhead, L.C.; Redburn, D.A.; Kirkpatrick, D.S.; Kretzer, F. Bacterial collagenase. Proposed adjunct to vitrectomy with membranectomy. Arch. Ophthalmol. 1980, 98, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Kuppermann, B.D.; Thomas, E.L.; de Smet, M.D.; Grillone, L.R. Pooled efficacy results from two multinational randomized controlled clinical trials of a single intravitreous injection of highly purified ovine hyaluronidase (Vitrase®) for the management of vitreous hemorrhage. Am. J. Ophthalmol. 2005, 140, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.R. What we know (and what do not know) about vitreoretinal adhesion. Retina. 2012, 32, S181–S186. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.B.; Meyer, C.H.; Kumar, J.; Tatebayashi, M.; Toth, C.A.; Wong, F.; Epstein, D.L.; McCuen, B.W., 2nd. RGD peptide-assisted vitrectomy to facilitate induction of a posterior vitreous detachment: A new principle in pharmacological vitreolysis. Curr. Eye Res. 2002, 25, 333–340. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Sun, X.; Wang, F.; Xu, X.; Zhang, X. Safety and efficacy of dispase and plasmin in pharmacologic vitreolysis. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3286. [Google Scholar] [CrossRef]
- Liotta, L.A.; Goldfarb, R.H.; Brundage, R.; Siegal, G.P.; Terranova, V.; Garbisa, S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981, 41, 4629–4636. [Google Scholar]
- Uemura, A.; Nakamura, M.; Kachi, S.; Nishizawa, Y.; Asami, T.; Miyake, Y.; Terasaki, H. Effect of plasmin on laminin and fibronectin during plasmin-assisted vitrectomy. Arch. Ophthalmol. 2005, 123, 209–213. [Google Scholar] [CrossRef]
- Hikichi, T.; Yanagiya, N.; Kado, M.; Akiba, J.; Yoshida, A. Posterior vitreous detachment induced by injection of plasmin and sulfur hexafluoride in the rabbit vitreous. Retina 1999, 19, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Gandorfer, A.; Putz, E.; Welge-Lussen, U.; Gruterich, M.; Ulbig, M.; Kampik, A. Ultrastructure of the vitreoretinal interface following plasmin assisted vitrectomy. Br. J. Ophthalmol. 2001, 85, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Gandorfer, A.; Priglinger, S.; Schebitz, K.; Hoops, J.; Ulbig, M.; Ruckhofer, J.; Grabner, G.; Kampik, A. Vitreoretinal morphology of plasmin treated human eyes. Am. J. Ophthalmol. 2002, 133, 156–159. [Google Scholar] [CrossRef]
- Rizzo, S.; Pellegrini, G.; Benocci, F.; Belting, C.; Baicchi, U.; Vispi, M. Autologous plasmin for pharmacologic vitreolysis prepared 1 hour before surgery. Retina 2006, 26, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, Y.; Honda, S.; Imai, H.; Kondo, N.; Fujii, S.; Yokoyama, N.; Hirata, A.; Kawaji, T.; Fukushima, M.; Tanihara, H. Autologous plasmin-assisted vitrectomy for stage 5 retinopathy of prematurity: A preliminary trial. Am. J. Ophthalmol. 2007, 144, 139–141. [Google Scholar] [CrossRef]
- Hirata, A.; Takano, A.; Inomata, Y.; Yonemura, N.; Sagara, N.; Tanihara, H. Plasmin-assisted vitrectomy for management of proliferative membrane in proliferative diabetic retinopathy: A pilot study. Retina 2007, 27, 1074–1078. [Google Scholar] [CrossRef]
- Wu, W.C.; Drenser, K.A.; Capone, A.; Williams, G.A.; Trese, M.T. Plasmin enzyme-assisted vitreoretinal surgery in congenital X-linked retinoschisis: Surgical techniques based on a new classification system. Retina 2007, 27, 1079–1085. [Google Scholar] [CrossRef]
- Esser, P.; Heimann, K.; Bartz-Schmidt, K.U.; Walter, P.; Krott, R.; Weller, M. Plasminogen in proliferative vitreoretinal disorders. Br. J. Ophthalmol. 1997, 81, 590–594. [Google Scholar] [CrossRef]
- Unal, M.; Peyman, G.A. The efficacy of plasminogen-urokinase combination in inducing posterior vitreous detachment. Retina 2000, 20, 69–75. [Google Scholar] [CrossRef]
- Hesse, L.; Nebeling, B.; Schroeder, B.; Heller, G.; Kroll, P. Induction of posterior vitreous detachment in rabbits by intravitreal injection of tissue plasminogen activator following cryopexy. Exp. Eye Res. 2000, 70, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Gandorfer, A.; Rohleder, M.; Sethi, C.; Eckle, D.; Welge-Lüssen, U.; Kampik, A.; Luthert, P.; Charteris, D. Posterior Vitreous Detachment Induced by Microplasmin. Investig. Ophthalmol. Vis. Sci. 2004, 45, 641. [Google Scholar] [CrossRef]
- Neffendorf, J.E.; Kirthi, V.; Pringle, E.; Jackson, T.L. Ocriplasmin for symptomatic vitreomacular adhesion. Cochrane Database Syst. Rev. 2017, 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Morescalchi, F.; Gambicorti, E.; Duse, S.; Costagliola, C.; Semeraro, F. From the analysis of pharmacologic vitreolysis to the comprehension of ocriplasmin safety. Expert Opin. Drug Saf. 2016, 15, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Hirata, A.; Ogasawara, K.; Sagara, N.; Inomata, Y.; Kawaji, T.; Tanihara, H. Posterior vitreous detachment induced by Nattokinase (Subtilisin NAT): A novel enzyme for pharmacologic vitreolysis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2075. [Google Scholar] [CrossRef] [PubMed]
- Santra, M.; Sharma, M.; Katoch, D.; Jain, S.; Saikia, U.N.; Dogra, M.R.; Luthra-Guptasarma, M. Induction of posterior vitreous detachment (PVD) by non-enzymatic reagents targeting vitreous collagen liquefaction as well as vitreoretinal adhesion. Sci. Rep. 2020, 10, 8250. [Google Scholar] [CrossRef] [PubMed]
- Santra, M.; Sharma, M.; Katoch, D.; Jain, S.; Saikia, U.N.; Dogra, M.R.; Luthra-Guptasarma, M. Enzymatic vitreolysis using reengineered Vibrio mimicus-derived collagenase. Mol. Vis. 2021, 27, 125–141. [Google Scholar] [PubMed]
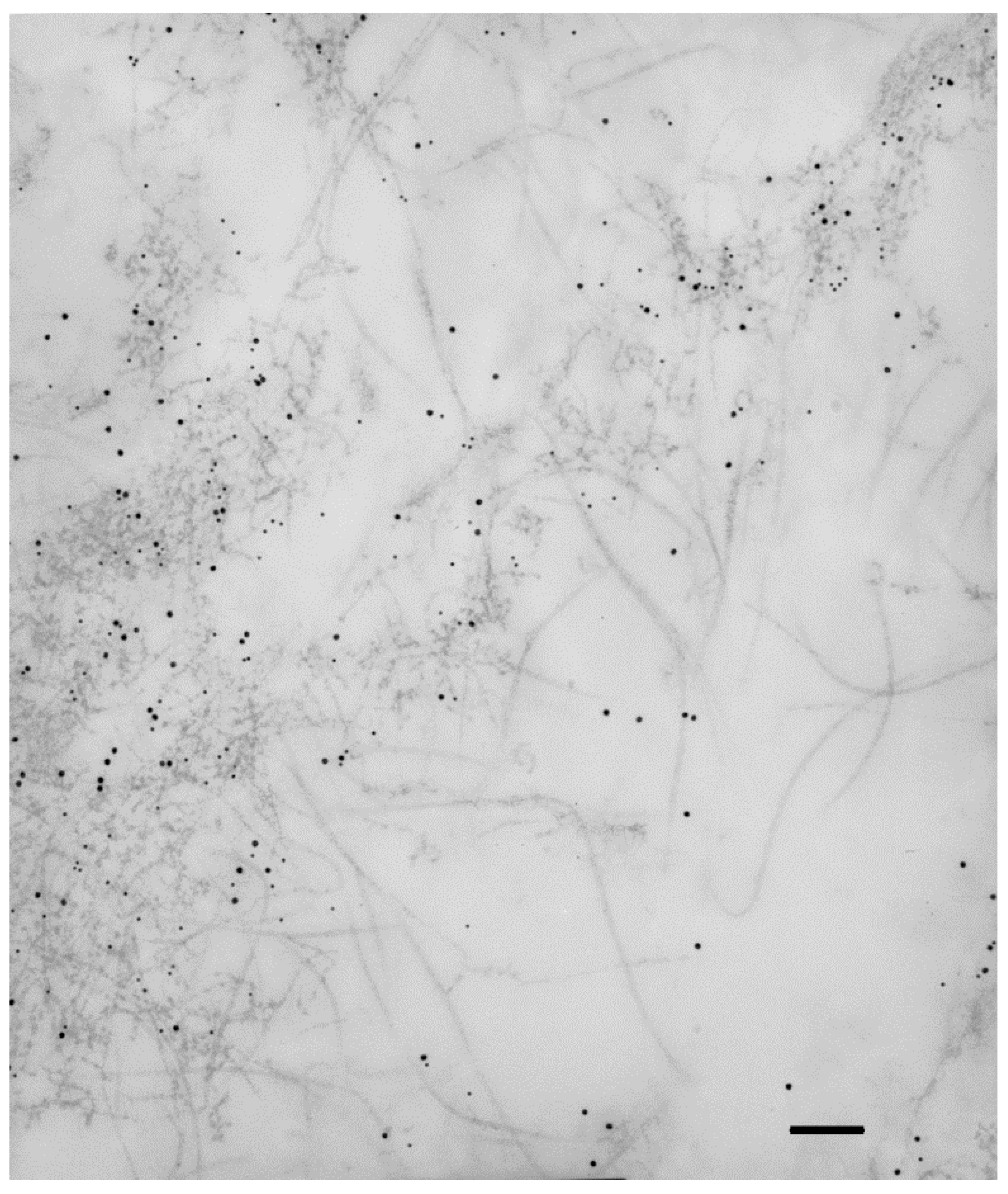
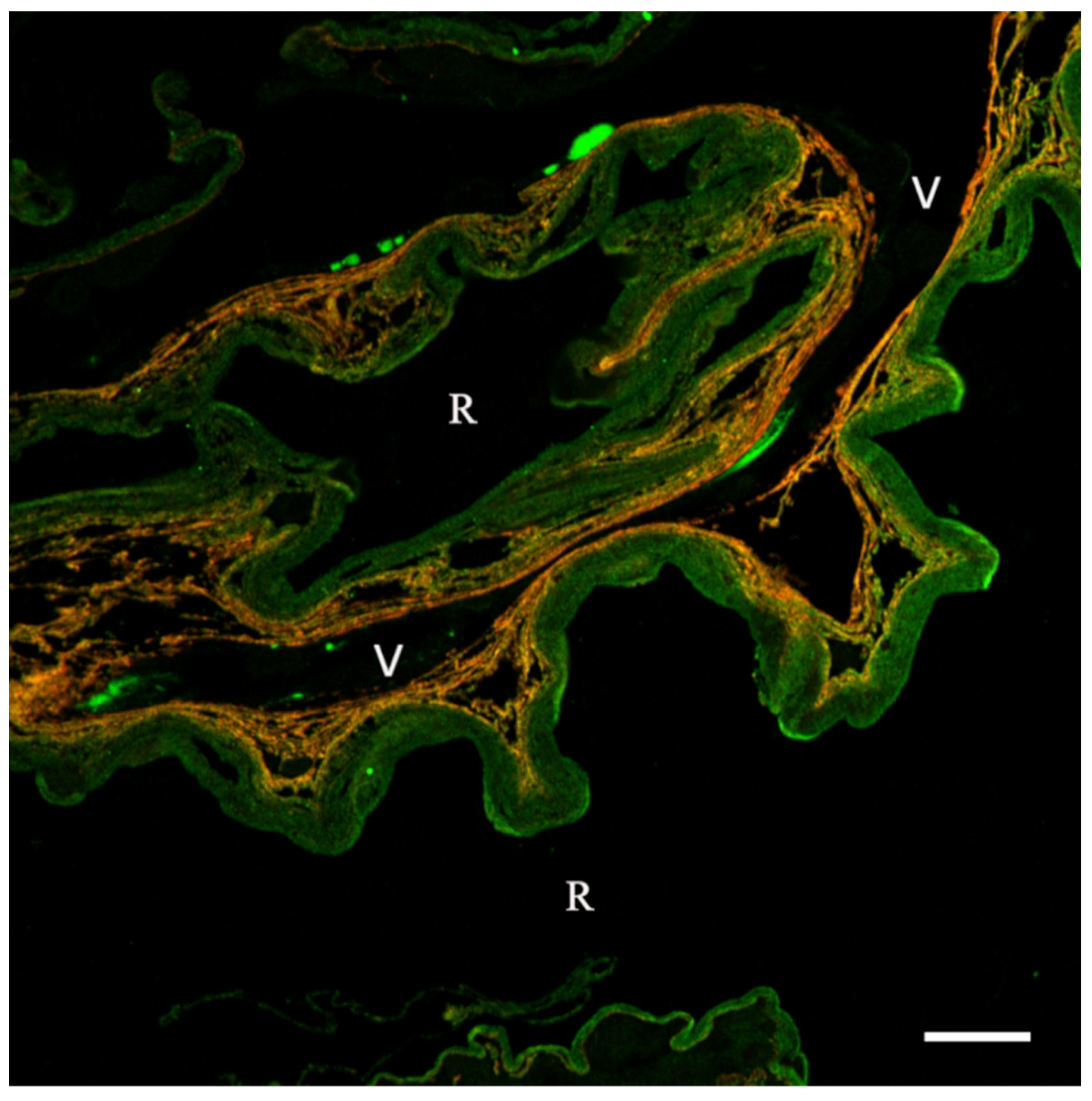
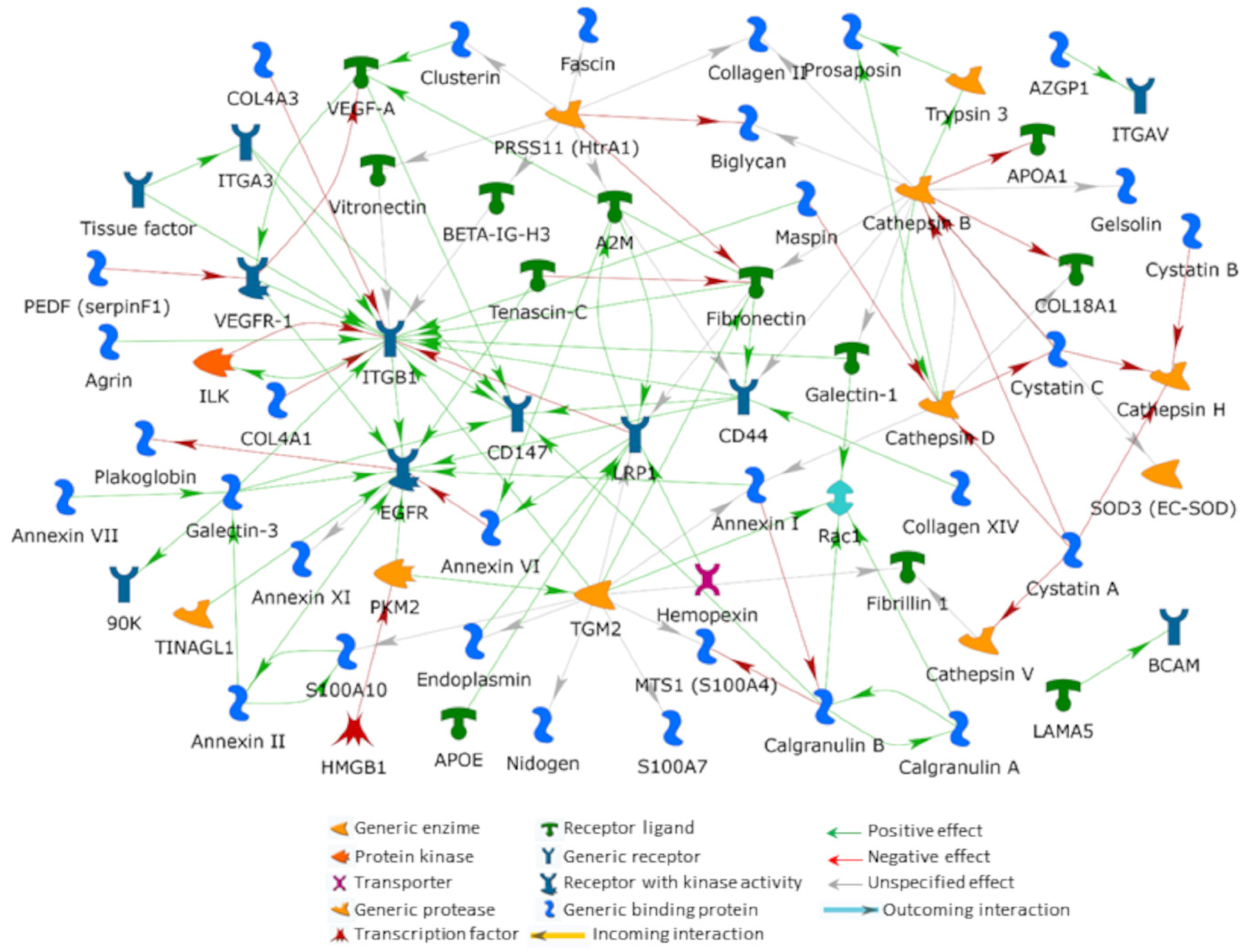
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, L.; Altera, A.; Barone, V.; Bonente, D.; Bacci, T.; De Benedetto, E.; Bini, L.; Tosi, G.M.; Galvagni, F.; Bertelli, E. Untangling the Extracellular Matrix of Idiopathic Epiretinal Membrane: A Path Winding among Structure, Interactomics and Translational Medicine. Cells 2022, 11, 2531. https://doi.org/10.3390/cells11162531
Bianchi L, Altera A, Barone V, Bonente D, Bacci T, De Benedetto E, Bini L, Tosi GM, Galvagni F, Bertelli E. Untangling the Extracellular Matrix of Idiopathic Epiretinal Membrane: A Path Winding among Structure, Interactomics and Translational Medicine. Cells. 2022; 11(16):2531. https://doi.org/10.3390/cells11162531
Chicago/Turabian StyleBianchi, Laura, Annalisa Altera, Virginia Barone, Denise Bonente, Tommaso Bacci, Elena De Benedetto, Luca Bini, Gian Marco Tosi, Federico Galvagni, and Eugenio Bertelli. 2022. "Untangling the Extracellular Matrix of Idiopathic Epiretinal Membrane: A Path Winding among Structure, Interactomics and Translational Medicine" Cells 11, no. 16: 2531. https://doi.org/10.3390/cells11162531
APA StyleBianchi, L., Altera, A., Barone, V., Bonente, D., Bacci, T., De Benedetto, E., Bini, L., Tosi, G. M., Galvagni, F., & Bertelli, E. (2022). Untangling the Extracellular Matrix of Idiopathic Epiretinal Membrane: A Path Winding among Structure, Interactomics and Translational Medicine. Cells, 11(16), 2531. https://doi.org/10.3390/cells11162531







