Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder molecularly characterized by the formation of amyloid β (Aβ) plaques and type 2 microtubule-associated protein (Tau) abnormalities. Multiple studies have shown that many of the brain’s immunological cells, specifically microglia and astrocytes, are involved in AD pathogenesis. Cells of the innate immune system play an essential role in eliminating pathogens but also regulate brain homeostasis and AD. When activated, innate immune cells can cause programmed cell death through multiple pathways, including pyroptosis, apoptosis, necroptosis, and PANoptosis. The cell death often results in the release of proinflammatory cytokines that propagate the innate immune response and can eliminate Aβ plaques and aggregated Tau proteins. However, chronic neuroinflammation, which can result from cell death, has been linked to neurodegenerative diseases and can worsen AD. Therefore, the innate immune response must be tightly balanced to appropriately clear these AD-related structural abnormalities without inducing chronic neuroinflammation. In this review, we discuss neuroinflammation, innate immune responses, inflammatory cell death pathways, and cytokine secretion as they relate to AD. Therapeutic strategies targeting these innate immune cell death mechanisms will be critical to consider for future preventive or palliative treatments for AD.
Keywords:
neuroinflammation; innate immunity; cell death; pyroptosis; apoptosis; necroptosis; inflammasome; caspase-1; caspase-3; caspase-6; caspase-7; caspase-8; caspase-9; RIPK1; RIPK3; ZBP1; NLRP3; AIM2; RIPK1; MLKL; Toll-like receptor; PANoptosis; PANoptosome; Alzheimer’s disease; Amyloid β; Tau; microglia 1. Introduction
Alzheimer’s disease (AD) is a debilitating disease affecting approximately 44 million people worldwide [1]. The progression of this neurodegenerative disease varies between patients, and symptoms can take decades to develop. Dementia, characterized by progressive memory loss and cognitive impairment, is most often caused by AD. As dementia worsens, patients become unable to care for themselves and need constant monitoring and assistance before eventually succumbing to the effects of the disease. For these reasons, AD places an enormous burden on both individuals and society [2].
AD is characterized by the presence of two structural brain abnormalities caused by the proteins amyloid β (Aβ) and type 2 microtubule-associated protein (Tau). The characteristic AD-inducing plaques, caused by Aβ deposition between nerve cells in the brain, can manifest several years before clinical symptoms unfold, and plaques play a major role in cognitive decline and neuronal cell death [3,4]. Aβ is formed from amyloid precursor protein (APP) cleavage; APP is expressed in neurons and glial cells and is responsible for mediating cell-to-cell adhesion, neuronal signaling, and regulating neurotransmitter discharge [5]. APP can be cleaved into peptides (37–49 amino acids in length) by several proteolytic secretases. Depending on which secretases cleave APP, the process is either termed amyloidogenic, which is associated with plaque formation and disease, or nonamyloidogenic [6]. The nonamyloidogenic pathway is initiated by APP cleavage by α-secretase, followed by γ-secretase. The product is a non-toxic, soluble APPα fragment with essential neuroprotective functions [6,7]. Alternatively, induction of the amyloidogenic pathway involves APP cleavage by β-secretase, which first generates a soluble APPβ peptide. This processing is followed with cleavage by γ-secretase, which cleaves the APP C-terminal fragment, generating neurotoxic Aβ peptides. Various lengths of Aβs form, but Aβ1-42 is the major component of plaque formation; Aβ1-42 has a highly hydrophobic C-terminus that initiates Aβ aggregation and oligomerizes into higher order insoluble structures that diffuse throughout the brain [6,7,8]. These plaques interrupt cellular communication and can cause microglial activation and inflammation that ultimately can cause neuronal death and tissue damage in the brain [9].
Along with Aβ aggregation, the dysregulation of the protein Tau also results in AD [2]. Tau is expressed in neurons, astrocytes, and oligodendrocytes. Normally, Tau binds microtubules to stabilize them, impacting synaptic functions [10]. However, abnormal post-translational modifications can hyper-phosphorylate Tau, causing dissociation from microtubules and intracellular aggregation via neurofibrillary tangles (NFTs) [11]. Tau hyperphosphorylation and NFTs are associated with AD pathogenesis [12,13]. Additionally, Aβ deposition can drive Tau-mediated AD pathogenesis resulting in NFTs, cognitive impairment, and dementia [2].
While Aβ plaques and NFTs in neurons are key features of AD, the disease pathogenesis is not limited to the neuronal compartment, and many of the brain’s immunological cells (astrocytes, microglia, and peripheral infiltrating immune cells) are also involved. The activation of astrocytes and microglial cells releases proinflammatory cytokines, including IL-1β, TNF-α, and IL-6; these cytokines can trigger Tau hyper-phosphorylation [2]. Additionally, increased levels of proinflammatory cytokines have been found in the serum and brains of patients with AD compared with healthy patients, and this prolonged neuroinflammation can lead to the misfolding of Tau [14]. These results collectively lay the groundwork for a strong connection between innate immunity and pathology in the central nervous system (CNS) [15,16].
Innate Immune Signaling Pathways in the Central Nervous System
Innate immune signaling pathways in microglial cells are activated in response to a myriad of stimuli such as infection, injury, and chronic disease. As a result of an activated immune response, programmed cell death (PCD) can occur and has a significant role in pathogenesis in the CNS, with specific roles in brain development, homeostasis, and clearance of infected/transformed cells. There are several forms of PCD with defined molecular signatures [17], ranging from the traditionally noninflammatory apoptosis pathway to highly proinflammatory forms of cell death such as pyroptosis and necroptosis. Additionally, growing evidence of crosstalk among these three pathways has led to the conceptualization of PANoptosis, an inflammatory cell death pathway that integrates components from other cell death pathways. The totality of biological effects in PANoptosis cannot be individually accounted for by pyroptosis, apoptosis, or necroptosis alone [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Historically, apoptosis was considered to be the primary form of PCD employed in the CNS [37]. Apoptosis is initiated by microenvironmental perturbations that result in initiator caspases (caspase-8 or -9) activating downstream executioner caspases (caspase-3, -6, and -7) [17]. In neurological diseases, markers of apoptotic cell death have been identified, including the presence of pro-apoptotic B-cell lymphoma-2 (Bcl-2) family members, the activation of caspases, and the cleavage of caspase substrates [4]. However, nonapoptotic inflammatory PCD pathways also play a significant role in neurodegeneration. Pyroptosis is a proinflammatory, lytic PCD pathway that is associated with pathology in AD, multiple sclerosis, and traumatic brain injury [38]. Canonical pyroptosis is a caspase-1–mediated cell death pathway that involves inflammasome formation and the activation and release of proinflammatory cytokines IL-1β and IL-18 [38,39,40]. Necroptosis, another lytic form of PCD that occurs in response to caspase-8 inhibition and is RIPK3- and MLKL-dependent [17,41,42,43], has also been shown to be involved in the pathophysiology of neurological diseases such as ALS, schizophrenia, and ischemia–reperfusion injury [44,45,46]. The activation of RIPK1, a key inflammatory PCD molecule, may also lead to neuroinflammation and cell death [45].
Many questions remain about the precise mechanisms through which innate immunity and PCD pathways modulate neuronal pathogenesis. Here, we discuss the link between neuroinflammation, innate immune responses, and AD. Innate immune sensors and molecular mechanisms of PCD, as related to AD, are also described, along with proposed AD therapeutic strategies targeting fundamental regulators of PCD. Considering the innate immune components of AD pathogenesis and identifying strategies to therapeutically target these pathways will be critical for informing clinical interventions.
2. Neuroinflammation, Innate Immunity, and AD: A Complex Relationship
Neuroinflammation is involved in the propagation of several neurodegenerative disorders and has been shown to be a major contributor to AD pathogenesis and progression. Inflammation in the CNS can occur as a result of cells sensing Aβ or other damage- or pathogen-associated molecular patterns (DAMPs or PAMPs). Cells contain several pattern recognition receptors (PRRs), both on the cell surface and in the cytoplasm, that are responsible for recognizing DAMPs and PAMPs; sensing can induce inflammatory signaling pathways and immune responses. The five primary PRR families include Toll-like receptors (TLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), C-type lectin receptors (CLRs), and the absent in melanoma 2 (AIM2)-like receptors (ALRs) [47]. This PAMP/DAMP-mediated signaling response incites the production of inflammatory cytokines and chemokines, the induction of cell death to clear the infected cells, and in the case of AD, Tau protein misfolding or hyperphosphorylation and increased Aβ pathology [2,14]. Inflammatory responses in the CNS are largely carried out by glial cells, including microglial cells and astrocytes, though endothelial cells also play a role. Below, we highlight the key molecular pathways involved in DAMP and PAMP sensing and cytokine release in these cell types.
2.1. Microglia
Microglia are self-renewing immune cells of the CNS [48] that arise from yolk-sac fetal macrophages [49,50,51]. They play an essential role in regulating brain homeostasis and facilitating immune responses [52,53]. Microglial cells patrol the environment, assess and maintain synaptic health, clear debris, and assist in neuronal survival [54]. In response to stimuli, microglia can undergo classical activation to develop towards an M1 phenotype, which promotes inflammation, or they can undergo alternative/neuroprotective activation and develop towards an M2 phenotype, which is associated with anti-inflammatory functions. Healthy CNS maintenance requires a balance of M1 and M2 microglial activation to repair tissues, an M2 function, and also to clear cellular debris and aggregated misfolded proteins, an M1 function [55,56]. While these functions are essential for homeostasis, the overactivation of these pathways can lead to pathology.
Approximately 25% of the ~84 risk genes in AD that are associated with immune function have been identified to be enriched or exclusively expressed in microglia [57]. Some of these genes are TREM2, CD33, INPP5D, CLU, CR1, SPI1, ABCA7, EPHA7, MS4As, HLA-DRB5-DRB1, CASP7, and CASP8 (Table 1). Further research is needed to understand how these specific risk variants impact protein function, which could aid in identifying therapeutic targets for AD treatment [57]. In addition to these gene associations, several molecular mechanisms for innate immune activation in microglial cells have been implicated in AD pathogenesis. Microglia develop toward the proinflammatory M1 phenotype after sensing DAMPs and PAMPs through PRRs that include TLRs, RLRs, and NLRs [9,58,59,60,61]. In neurodegenerative diseases such as AD, PRRs are highly expressed in microglia; signaling through PRRs can provoke an inflammatory response and proinflammatory cytokine secretion [62,63] (Table 2). In healthy controls, activated microglia localize around Aβ plaques and neurons with NFTs [64,65]. Microglia use cell surface receptors (CD14, TLR2, TLR4, α6β1 integrin, CD47) and scavenger receptors (CD36) to phagocytose and subsequently clear Aβ [66,67,68,69]; specifically, the physical interaction of TLR2, TLR4, and the TLR4-coreceptor CD14 activates an immune response to fibrillar Aβ phagocytosis [70]. While microglial activation is beneficial in preventing AD-associated pathology, the chronic activation of microglia is detrimental, as prolonged TLR2 and TLR4 activation in microglia induces Aβ production [71]. If Aβ plaque formation is extensive, microglial cells cannot eliminate it [72,73,74,75]. In a common AD mouse model where mice express the double transgenic APP/PS1 (chimeric mouse or human APP/human presenilin 1 [76]), the inhibition of TLR2 reduces glial cell reactivity, reduces Aβ, and improves cognitive function [77]. Furthermore, TLR2/4-deficient C57BL/6 mice exhibit improved neuro-cognitive and behavioral patterns compared to wild-type mice in response to the Aβ1–42 peptide [78], and TLR2-deficient microglia induce proinflammatory cytokine release (TNF-α, IL-1β, IL-6) and enrich Aβ clearance [79,80]. These results highlight the importance of TLRs in driving AD pathology.

Table 1.
Summary of immune genes that have mutations associated with AD.

Table 2.
Innate immune molecules involved in Alzheimer’s disease.
Other PRRs, such as inflammasome sensors, have also been associated with AD. Inflammasomes are multiprotein complexes that form in response to PAMP or DAMP sensing, and they generally contain a sensor, the adaptor protein ASC, and caspase-1. Several inflammasome sensors have been described, with the most well-characterized being the NLR family sensors, NLRP1 [40], NLRP3 [95,96,97], and NAIP/NLRC4 [98,99,100], as well as other sensors containing pyrin domains, such as Pyrin [101] and AIM2 [102,103]. The NLRP3 inflammasome, which has been implicated in autoinflammatory diseases, obesity, colitis, cancers, and infections [104], has emerged as a trigger of AD pathology. Tau can activate the NLRP3 inflammasome in microglia, inducing Tau pathology and potentiating AD pathogenesis [105]. Similarly, NLRP3-deficient APP/PS1 mice are protected against neurobehavioral deficits and have reduced spatial memory deficits and Aβ buildup [88].
In addition to the direct role of PRRs in AD pathogenesis, PRR activation and signaling in microglia can also induce the release of proinflammatory cytokines that drive pathology. Inflammation or injury to the neuron can activate microglia to generate proinflammatory factors (IL-1β, TNF-α, and IL-6; an M1 phenotype). Nucleic acid-containing Aβ elicits a TLR- and RLR-derived type I IFN response for C3-dependent synapse destruction [106]. The expression of RIG-I, a key member of the RLR family, is elevated in the temporal cortex and plasma of patients with cognitive impairment [94]. However, the mechanistic details of RIG-I involvement in AD pathogenesis have yet to be discovered.
The activation of PRRs, especially inflammasome sensors, can lead to the release of IL-1β and IL-18, and these proinflammatory cytokines are also correlated with AD severity [107,108,109,110]. Though the release of proinflammatory cytokines is a natural result of the aging process, their continuous release can lead to Aβ production and neuronal distress [111,112,113,114,115,116].
2.2. Astrocytes
The CNS relies heavily on astrocytes for maintaining homeostasis and assisting with synapse formation and elimination [117,118]. Astrocytes are more numerous than neurons [119] and have a significant role in activation, neuroprotection, and neurotoxicity [120,121]. They respond to cytokines/chemokines and can identify Aβ aggregates [118,122,123], undergo hypertrophy upon activation, and upregulate glial fibrillary acidic protein (GFAP) expression [124]. Reactive astrocytes (atrophied or disrupted intralaminar astrocytes) are characteristically present in AD brains [125]. While innate immune sensing in astrocytes is not well understood, inflammasome activation in astrocytes has been associated with some neurodegenerative diseases. The AIM2 inflammasome was found to be activated during experimental autoimmune encephalomyelitis in astrocytes, although these cells failed to undergo cell death and had poor IL-1β expression [126]. Additionally, astroglial NLRP3 inflammasome complexes have also been reported to be involved in neuroinflammation in ALS, with higher levels of NLRP3, ASC, IL-18, and caspase-1 in comparison to non-diseased controls [127]. The role of innate immunity in astrocytes during AD requires further characterization.
2.3. Endothelial Cells
Single-nucleus transcriptome analyses from prefrontal cortical samples from patients with AD identified a variety of endothelial transcriptomic changes, specifically in angiogenesis and the immune response pathway when compared with healthy patients [128]. The endothelial cells of AD patients showed an elevated expression of EGFL7, FLT1, and VWF (angiogenic growth factors and their receptors) and B2M and HLA-E (antigen presentation machinery) [128], suggesting that these cells play a role in angiogenesis and immune responses in a diseased state. However, this cell type remains largely under-characterized for its roles in AD.
3. Cell Death and AD
The major pathological hallmarks of AD include extracellular Aβ deposition, the intraneuronal aggregation of NFTs, and neuronal loss (neurodegeneration) [129]. In mammalian hosts, Aβ and Tau deposits act as DAMPs and are recognized by multiple PRRs, as discussed above [68,88,94,130,131] (Table 2). The subsequent innate immune signaling can result in cell death, and these processes can provide significant protective responses against AD [62,88,131]. Conversely, inflammatory cell death also releases proinflammatory cytokines and cellular contents that can stimulate severe inflammation [132,133]. There is a delicate balance between an appropriate immune response, which clears Aβ and Tau deposits, and an exaggerated response, which promotes neuroinflammatory brain damage [132,133]. Below, we discuss the mechanics of canonical PCD pathways and what is known about their involvement in AD.
3.1. Pyroptosis in AD
Pyroptosis is an inflammatory PCD pathway initiated by the assembly of a multimeric protein complex called the inflammasome [40], as described above. The canonical inflammasome assembly cleaves pro–caspase-1, allowing its active form to cleave downstream substrates. These substrates include gasdermin D (GSDMD), leading to the release of its N-terminal fragment to form pores in the plasma membrane, and pro–IL-1β and pro–IL-18, which are processed into their active forms for release through the membrane pores [134,135,136,137,138]. Since activated caspase-1 mediates potent cell death and an inflammatory response, its catalytic activity is under tight regulation [139,140].
In pyroptosis, caspase-1-dependent GSDMD cleavage, and the subsequent release of IL-1β and IL-18, have been well characterized in immune cells such as macrophages [141]. However, pyroptosis and inflammasome activation also occur in brain cells; activated caspase-1 is elevated in AD brains and in the APP/PS1 mouse model [142], and Aβ1-42 can induce pyroptosis in cortical neurons [142,143] (Table 3). Additionally, Aβ fibrils can stimulate NLRP3 inflammasomes via lysosomal damage in mouse microglia [144]. NLRP3 or caspase-1 deficiency in the APP/PS1 mouse model reduces spatial memory impairment, hippocampal synaptic plasticity loss, behavioral disturbances, and AD consequences [88]. NLRP3 inflammasome deficiency also skews microglial cells from the M1 phenotype to the M2 phenotype [88]. Furthermore, microglial cells have been shown to secrete IL-1β after NLRP3 activation with recombinant Tau protein [105,130]. Together, these studies implicate pyroptosis in AD pathogenesis.

Table 3.
Cell death effector molecules involved in Alzheimer’s disease.
3.2. Apoptosis in AD
Apoptosis occurs through either the extrinsic or intrinsic pathway. Extrinsic apoptosis is activated by extracellular stimuli through the formation of a death-inducing signaling complex (DISC) that recruits adaptor proteins (including TRADD and FADD) and pro-caspase-8 [155,156]. Caspase-8 can then activate effector caspases, caspase-3, -6 and -7, to drive further substrate cleavage and the execution of apoptotic cell death [157,158]. Intrinsic apoptosis responds to intracellular changes in equilibrium and involves the activation of Bcl-2 family members followed by the release of mitochondrial proteins such as cytochrome c. Cytochrome c associates with APAF-1 and activates caspase-9, which enables downstream effectors, caspase-3, -6, and -7 [157,158,159,160].
Many studies have shown that compared to healthy brains, the expression profiles of several components of apoptosis, most notably the Bcl-2 family members, are altered in AD [161]. Bcl-2 family members can be classified as either pro- or anti-apoptotic, with Bax, Bak, BAD, Bid, Bim, and Bcl-x being canonically pro-apoptotic and Bcl-2, Bcl-xL, and Mcl-1 being anti-apoptotic [161]. Pro-apoptotic Bim is upregulated, anti-apoptotic Bcl-2 is downregulated, and pro-apoptotic Bax is activated in response to Aβ [162,163]. Furthermore, anti-apoptotic Bcl-xL expressed in microglia has been shown to co-localize with Aβ deposits and activate astrocytes [164]. Additionally, Bcl-2 proteins control intracellular calcium, and irregularities in calcium signaling have been implicated in AD progression [161], providing an additional link between apoptotic Bcl-2 proteins and AD.
In addition to the Bcl-2 family members, apoptotic caspases are also associated with AD. Stimulating microglia with inflammogens can activate caspase-8, -3, and -7 in BV2 cells and in mice, and these caspases are activated in the microglia of patients with AD [165]. Molecularly, caspase-3 can activate NF-κB, via protein kinase Cδ, and increase the production of neurotoxic proinflammatory mediators (IL-1β, TNF-α, and NO). Subsequently, in vitro inhibition of caspase-8 impedes microglia activation and neurotoxicity [166]. Caspase-3 can cleave APP, and this cleavage event can provoke Aβ plaque formation and synaptic loss in the brain as well as cause noticeable changes in behavior [147]. Along with caspase-3 activation, activating caspase-8 and -9 also induces Aβ plaque formation [151,153,167,168]. From a therapeutic standpoint, the pan-caspase inhibitor (Q-VD-OPh) and microglial activation inhibitor (minocycline) have been shown to provide neuroprotective effects in TgCRND8 and APP/PS1 mouse models, respectively [169,170] (Table 3). Collectively, these studies emphasize the involvement of apoptotic molecules in AD pathogenesis.
3.3. Necroptosis in AD
Necroptosis can be initiated in response to multiple signaling pathways, including TLR signaling, death receptor engagement, and IFN signaling [171]. The necroptotic cell death pathway is activated when caspase-8 is inhibited; RIPK1 is autophosphorylated and, through its interaction with phosphorylated RIPK3, recruits and phosphorylates MLKL [172,173,174,175,176,177,178]. Oligomerized MLKL translocates to the plasma membrane where it interacts with phospholipids to produce membrane pores [172,179,180].
Necroptosis can impair cognitive function; in the APP/PS1 mouse model, mice expressing constitutively active MLKL performed worse in the Morris water maze and had fewer neurons [154]. In addition, a robust increase in the levels of RIPK1 and MLKL have been seen in AD brains compared to healthy controls [154] (Table 3). Furthermore, inhibiting RIPK1 reduces Aβ deposits, inflammatory cytokines, and cognitive deficits in the APP/PS1 mouse model [181]. There is also a correlation between necroptosis and reduced brain weight, which is a pathological consequence of AD [154]. Additionally, aberrant protein phosphorylation is a well-recognized component of AD pathogenesis [182], and because the phosphorylation of RIPK1, RIPK3, and MLKL regulates necroptosis, it is possible that AD progression may modulate this cell death pathway.
3.4. PANoptosis in AD
PCD pathways have long been thought of as segregated pathways with little overlap. However, numerous studies have found significant crosstalk among the components of pyroptosis, apoptosis, and necroptosis. Components of noninflammatory apoptosis can undergo crosstalk with molecules involved in executing lytic, inflammatory PCD pathways [183,184]. For example, caspase-3 can activate GSDME, inducing a gasdermin-dependent cell death program [185], and caspase-8 can directly activate GSDMD in certain conditions [186,187]. The culmination of the observed experimental crosstalk has led to the conceptualization of PANoptosis. PANoptosis is an inflammatory cell death pathway that integrates components from other cell death pathways. The totality of biological effects in PANoptosis cannot be individually accounted for by pyroptosis, apoptosis, or necroptosis alone. PANoptosis is regulated by multifaceted macromolecular complexes termed PANoptosomes [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. PANoptosis is initiated by PAMP/DAMP sensing through an upstream sensor, followed by assembly of the multi-protein PANoptosome complex. PANoptosis involves the activation of multiple cell death molecules, which can include caspase-1, gasdermins, caspase-8, caspase-3, caspase-7, caspase-6, MLKL, and potentially others [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Molecules shown to be involved in PANoptosis, such as AIM2 [35], caspase-8, caspase-1, RIPK3, and MLKL [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36], have been implicated in neuroinflammation and neurodegenerative disorders, including AD (Table 1 and Table 3). Additionally, the increased expression of CASP1, CASP3, CASP6, CASP7, CASP8, and CASP9 have all been found in the entorhinal cortex of patients with AD with severe dementia [188], suggesting that pyroptosis or apoptosis alone may not account for the full picture of cell death in AD. Furthermore, inflammasome components are now well-established in their ability to drive PANoptotic cell death responses in mice in response to specific stimuli [18,22,30,34,189,190,191]. To date, Z-DNA binding protein 1 (ZBP1)- and AIM2-PANoptosomes have been characterized [18,22,30,35,36], with more PANoptosomes likely to exist. In the context of AD, AIM2 deficiency has been shown to reduce Aβ deposition and microglial activation in the 5xFAD mouse model [90], suggesting that the AIM2-PANoptosome may play a role in AD. PANoptosis is an emerging concept which, when applied to AD, may allow for a more complete understanding of how PCD impacts AD and other neurodegenerative diseases.
4. Cytokines and Chemokines as Modulators of Neuroinflammation
Innate immune signaling pathways and cell death mechanisms often culminate in the release of cytokines and chemokines. Cytokines, which are largely released by microglia and astrocytes, are major contributors of neuroinflammation. They are involved in chemoattraction, pro- and anti-inflammatory processes, neuronal injury, and the microglial response to Aβ deposits. Since cytokines modulate microglial activation, their presence or absence can be influential in the development and progression of AD [9] (Table 4). Several disease conditions can release cytokines into the brain; this has been associated with dementia and the loss of neurocognition. For example, associations between dementia and infectious diseases have been reported [192]. Additionally, high levels of intracranial proinflammatory cytokines have been reported during viral infections, including the neuroinvasive SARS-CoV-2 [193,194], suggesting that these infections may also exacerbate neuroinflammation and promote disease. Recent evidence has also identified a mechanistic link between cytokine storms, particularly synergism between TNF-α and IFN-γ, and PANoptotic cell death [31], defining cytokine storms as a life-threatening condition caused by excessive production of cytokines mediated by inflammatory cell death, PANoptosis [195]. Given the established role of TNF-α in AD [78] and the links between cytokine storm and neuroinflammation, this process is likely to further contribute to AD pathology (Figure 1).

Table 4.
Cytokines and interferons involved in Alzheimer’s disease.
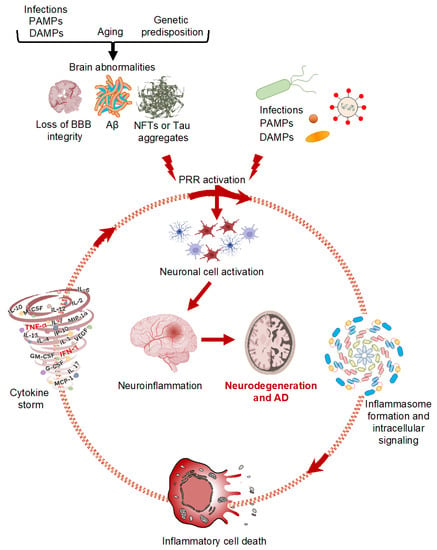
Figure 1.
Innate immune signaling and neurodegeneration. Schematic representation of different Alzheimer’s disease triggers activating innate immune signaling, neuroinflammation, and inflammatory cell death in neurodegenerative disorders. Created with Biorender (accessed on 12 May 2022).
Inflammation must be tightly balanced in the brain. A proinflammatory environment, such as that seen in AD brains, accounts for damaging pathology. The increased caspase-1 activation in AD leads to higher levels of IL-1β release [88,210]; this can be detrimental if prolonged, but there are conditions where a slightly inflammatory environment can be beneficial. For instance, IL-1β expression activates a form of neuroinflammation in the APP/PS1 mouse model that reduces amyloid plaque pathology [211,212], suggesting that a low level of inflammatory cell death could help prevent AD pathology. However, those with mild cognitive impairment are at risk for conversion to AD if they have high levels of the proinflammatory cytokine TNF-α and decreased anti-inflammatory TGF-β in their cerebrospinal fluid (CSF) [213]. Neuronal function is impaired with elevated levels of IL-1β, TNF-α, and other cytokines, as shown by the suppression of long-term potentiation of synaptic transmission [9]. Microglia in AD are skewed toward the M1 phenotype, indicated by a higher expression of proinflammatory cytokines/chemokines and innate immune receptors [9]. Additionally in an AD mouse model (Mo/Hu APPswe PS1dE9 mice), TLR4 mediates higher levels of TNF-α and MIP-1α [214]. Among the proinflammatory cytokines, interferon (IFN) is also a crucial molecule contributing to AD neuropathogenesis; RNA-seq data reported the upregulation of AD-related microglial-specific IFN-stimulated genes (ISGs) that had significant correlation with disease severity and complement activation [106].
In addition to cytokines, chemokines also enhance local inflammation in AD by regulating microglial migration to areas of neuroinflammation [215]. In AD, CCL2, CCR3, and CCR5 are elevated in reactive microglia [216,217], and CCL4 is detected in reactive astrocytes near the vicinity of Aβ plaques [216]. Aβ treatment to human astrocytes and macrophages generates CXCL8 (IL-8), CCL2, CCL3, and CCL4 [218], and microglia produce CXCL8, CCL2, and CCL3 [219]. Additionally, the CX3CR1/CX3CL1 AD mouse model modulates neuronal survival [220], plaque load [221], and cognition [222]. Overall, cytokines and chemokines are important to modulate signaling to help clear DAMPs in the brain, but prolonged production and exposure contributes to pathology and disease in AD.
5. Therapeutic Implications of Innate Immune Involvement for AD Management
Several AD therapeutics have attempted to target Aβ plaques and Tau. However, the clearance of Aβ plaques through anti-Aβ immunotherapy has failed to provide any cognitive benefit in patients with AD thus far [223]. The U.S. FDA granted accelerated approval for aducanumab, an anti-Aβ immunotherapy, to be used in patients with AD; however, the drug remains highly controversial with two phase III trials providing conflicting clinical efficacy results and lingering concerns over safety [224,225]. Previous anti-Tau therapies, such as those inhibiting kinases, Tau aggregation, or microtubule stabilization, have been withdrawn from clinical trials because of toxicity and efficacy issues [226]. However, several current Tau-targeted immunotherapies, including AADvac-1, ABBV-8E12, BIIB092, and RO71015705 [227], have shown promising preclinical results and are presently being evaluated in clinical trials [226]; their efficacy in patients with AD remains to be determined. The overall lack of clinical success to date in targeting Aβ plaques and Tau has led to a critical need to identify alternative treatment strategies that target upstream signaling, such as innate immune processes and PCD.
Mouse models, such as the APP/PS1 model as well as others, allow researchers to gain an understanding of relevant disease-related PCD signaling pathways and their regulation. The immediate hope is that PCD, in some capacity, can be targeted to modulate neurological disease severity and progression. However, this approach has also faced challenges. Historically, there has been an association between apoptosis and the expression of key apoptotic proteins with neurodegenerative disorders; yet in vivo targeting of apoptosis has failed to show effective therapeutic results. A recent clinical trial for minocycline, a 2nd generation tetracycline blocking cytochrome c release and upregulating Bcl-2 expression, was ineffective in patients with mild AD [228,229]. This may be due in part to crosstalk between PCD pathways. In contrast, the pharmacological inhibition of RIPK1 to prevent necroptosis results in neuroprotection in preclinical AD models [4], suggesting that targeting cell death remains a potentially viable therapeutic strategy for AD. Therefore, several other clinical trials and preclinical studies involving compounds that target cell death molecules are currently ongoing (Table 5).

Table 5.
Therapeutic options targeting neuro-inflammation and cell death pathways in Alzheimer’s disease.
A correlation has also been identified between inflammatory cytokines and their signaling pathways and AD, with microglia playing a key role in coupling inflammation with neurodegeneration [88,131,243,244]. Therefore, neuroinflammatory biomarkers are also being evaluated as potential targets (Table 5). However, AD pathogenesis may also be prevented through the adaptive immune system and the regulation of microglial function [245].
In addition to immune, inflammatory, and cell death pathways, the meningeal lymphatic vasculature (mLV) system may also be a promising therapeutic target for AD. The mLV system moves immune cells from the brain to the peripheral immune system; it is crucial in maintaining fluid homeostasis and enabling appropriate innate immune responses [246,247]. Mice with altered mLV have worsening AD symptoms, and the photoablation of mLV in 5xFAD mice leads to increased Aβ deposition, neurovascular dysfunction, and cognitive defects [248]. Dysfunctional mLV in murine models can also induce the activation of CNS immune cells and the increased secretion of proinflammatory cytokines IL-6, TNF-α, and IL-1β [246,249]. These data suggest that mLV, through modulating inflammation, could be involved in the progression of neurodegenerative diseases such as AD and warrants further study to determine whether mLV could be considered as a therapeutic target.
6. Discussion and Future Directions
Recent studies have significantly expanded our understanding of neuroinflammation, innate immune responses, and tightly orchestrated cell death signaling in AD pathogenesis. The brain immune cells (microglia) are integrally associated with innate immune cell death in AD pathology. Excessive neuroinflammation during Aβ and Tau clearance could be diminished by specifically regulating the innate immune cell death response. The continued elucidation of cell death pathways and the central innate immune sensor signaling pathways involved in regulating neuroinflammation and Aβ/Tau clearance will have a significant impact on the AD research field. The emerging concept of PANoptosis is likely to play a role in disease pathogenesis and understanding its relevance in AD therapeutics. Emerging AD studies are focusing on this network of inflammasomes, inflammatory cell death, and neuroinflammation. Further investigations into these processes will potentially aid in identifying novel therapeutic targets for AD management.
Author Contributions
Writing-original draft, Y.R.; writing-review and editing, Y.R., T.-D.K. All authors have read and agreed to the published version of the manuscript.
Funding
Work from the Kanneganti laboratory is supported by the National Institutes of Health (AI101935, AI124346, AI160179, AR056296, and CA253095 to T.-D.K.) and the American Lebanese Syrian Associated Charities (to T.-D.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Collaborators, G.B.D.D. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef] [Green Version]
- Maccioni, R.B.; Rojo, L.E.; Fernández, J.A.; Kuljis, R.O. The role of neuroimmunomodulation in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2009, 1153, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zou, X.; Zhu, R.; Shi, Y.; Wu, Z.; Zhao, F.; Chen, L. The correlation between accumulation of amyloid beta with enhanced neuroinflammation and cognitive impairment after intraventricular hemorrhage. J. Neurosurg. 2018, 131, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Moujalled, D.; Strasser, A.; Liddell, J.R. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021, 28, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta. Pharm. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Chasseigneaux, S.; Allinquant, B. Functions of Abeta, sAPPalpha and sAPPbeta: Similarities and differences. J. Neurochem. 2012, 120 (Suppl. S1), 99–108. [Google Scholar] [CrossRef]
- Katzmarski, N.; Ziegler-Waldkirch, S.; Scheffler, N.; Witt, C.; Abou-Ajram, C.; Nuscher, B.; Prinz, M.; Haass, C.; Meyer-Luehmann, M. Abeta oligomers trigger and accelerate Abeta seeding. Brain Pathol. 2020, 30, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Wesseling, H.; Mair, W.; Kumar, M.; Schlaffner, C.N.; Tang, S.; Beerepoot, P.; Fatou, B.; Guise, A.J.; Cheng, L.; Takeda, S.; et al. Tau PTM Profiles Identify Patient Heterogeneity and Stages of Alzheimer’s Disease. Cell 2020, 183, 1699–1713.e13. [Google Scholar] [CrossRef]
- Alonso, A.D.; Cohen, L.S.; Corbo, C.; Morozova, V.; ElIdrissi, A.; Phillips, G.; Kleiman, F.E. Hyperphosphorylation of Tau Associates With Changes in Its Function Beyond Microtubule Stability. Front. Cell Neurosci. 2018, 12, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2015, 11, 600–607.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.; Qiu, Q.; Zhang, H.; Chu, L.; Du, Y.; Zhang, J.; Zhou, C.; Liang, F.; Shi, S.; Wang, S.; et al. Concordance between the assessment of Abeta42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement. 2019, 15, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Cortes, N.; Andrade, V.; Guzman-Martinez, L.; Estrella, M.; Maccioni, R.B. Neuroimmune Tau Mechanisms: Their Role in the Progression of Neuronal Degeneration. Int. J. Mol. Sci. 2018, 19, 956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A protein factor essential for microtubule assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef] [Green Version]
- Drechsel, D.N.; Hyman, A.A.; Cobb, M.H.; Kirschner, M.W. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol. Biol. Cell 1992, 3, 1141–1154. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Kuriakose, T.; Man, S.M.; Malireddi, R.K.; Karki, R.; Kesavardhana, S.; Place, D.E.; Neale, G.; Vogel, P.; Kanneganti, T.D. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 2016, 1, aag2045. [Google Scholar] [CrossRef] [Green Version]
- Malireddi, R.K.S.; Karki, R.; Sundaram, B.; Kancharana, B.; Lee, S.; Samir, P.; Kanneganti, T.D. Inflammatory Cell Death, PANoptosis, Mediated by Cytokines in Diverse Cancer Lineages Inhibits Tumor Growth. Immunohorizons 2021, 5, 568–580. [Google Scholar] [CrossRef]
- Kesavardhana, S.; Malireddi, R.K.S.; Burton, A.R.; Porter, S.N.; Vogel, P.; Pruett-Miller, S.M.; Kanneganti, T.-D. The Zα2 domain of ZBP1 is a molecular switch regulating influenza-induced PANoptosis and perinatal lethality during development. J. Biol. Chem. 2020, 295, 8325–8330. [Google Scholar] [CrossRef]
- Banoth, B.; Tuladhar, S.; Karki, R.; Sharma, B.R.; Briard, B.; Kesavardhana, S.; Burton, A.; Kanneganti, T.-D. ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis). J. Biol. Chem. 2020, 295, 18276–18283. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Zheng, M.; Kesavardhana, S.; Karki, R.; Malireddi, R.K.S.; Banoth, B.; Place, D.E.; Briard, B.; Sharma, B.R.; Tuladhar, S.; et al. Identification of the PANoptosome: A molecular platform triggering pyroptosis, apoptosis, and necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Lee, E.; Banoth, B.; Malireddi, R.K.S.; Samir, P.; Tuladhar, S.; Mummareddy, H.; Burton, A.R.; Vogel, P.; et al. Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Williams, E.P.; Malireddi, R.K.S.; Karki, R.; Banoth, B.; Burton, A.; Webby, R.; Channappanavar, R.; Jonsson, C.B.; Kanneganti, T.-D. Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J. Biol. Chem. 2020, 295, 14040–14052. [Google Scholar] [CrossRef]
- Gurung, P.; Burton, A.; Kanneganti, T.D. NLRP3 inflammasome plays a redundant role with caspase 8 to promote IL-1beta-mediated osteomyelitis. Proc. Natl. Acad. Sci. USA 2016, 113, 4452–4457. [Google Scholar] [CrossRef] [Green Version]
- Lukens, J.R.; Gurung, P.; Vogel, P.; Johnson, G.R.; Carter, R.A.; McGoldrick, D.J.; Bandi, S.R.; Calabrese, C.R.; Vande Walle, L.; Lamkanfi, M.; et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature 2014, 516, 246–249. [Google Scholar] [CrossRef] [Green Version]
- Malireddi, R.K.; Ippagunta, S.; Lamkanfi, M.; Kanneganti, T.D. Cutting edge: Proteolytic inactivation of poly(ADP-ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. J. Immunol. 2010, 185, 3127–3130. [Google Scholar] [CrossRef] [Green Version]
- Malireddi, R.K.S.; Gurung, P.; Kesavardhana, S.; Samir, P.; Burton, A.; Mummareddy, H.; Vogel, P.; Pelletier, S.; Burgula, S.; Kanneganti, T.D. Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Malireddi, R.K.S.; Kesavardhana, S.; Karki, R.; Kancharana, B.; Burton, A.R.; Kanneganti, T.D. RIPK1 Distinctly Regulates Yersinia-Induced Inflammatory Cell Death, PANoptosis. Immunohorizons 2020, 4, 789–796. [Google Scholar] [CrossRef]
- Zheng, M.; Karki, R.; Vogel, P.; Kanneganti, T.D. Caspase-6 is a key regulator of innate immunity, inflammasome activation and host defense. Cell 2020, 181, 674–687.e13. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef] [PubMed]
- Malireddi, R.K.S.; Gurung, P.; Mavuluri, J.; Dasari, T.K.; Klco, J.M.; Chi, H.; Kanneganti, T.D. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J. Exp. Med. 2018, 215, 1023–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamkanfi, M.; Kanneganti, T.D.; Van Damme, P.; Vanden Berghe, T.; Vanoverberghe, I.; Vandekerckhove, J.; Vandenabeele, P.; Gevaert, K.; Nunez, G. Targeted peptidecentric proteomics reveals caspase-7 as a substrate of the caspase-1 inflammasomes. Mol. Cell Proteom. 2008, 7, 2350–2363. [Google Scholar] [CrossRef] [Green Version]
- Gurung, P.; Anand, P.K.; Malireddi, R.K.; Vande Walle, L.; Van Opdenbosch, N.; Dillon, C.P.; Weinlich, R.; Green, D.R.; Lamkanfi, M.; Kanneganti, T.D. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J. Immunol. 2014, 192, 1835–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Karki, R.; Wang, Y.; Nguyen, L.N.; Kalathur, R.C.; Kanneganti, T.D. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature 2021, 597, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sundaram, B.; Sharma, B.R.; Lee, S.; Malireddi, R.K.S.; Nguyen, L.N.; Christgen, S.; Zheng, M.; Wang, Y.; Samir, P.; et al. ADAR1 restricts ZBP1-mediated immune response and PANoptosis to promote tumorigenesis. Cell Rep. 2021, 37, 109858. [Google Scholar] [CrossRef]
- Mattson, M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000, 1, 120–129. [Google Scholar] [CrossRef]
- McKenzie, B.A.; Dixit, V.M.; Power, C. Fiery Cell Death: Pyroptosis in the Central Nervous System. Trends Neurosci. 2020, 43, 55–73. [Google Scholar] [CrossRef]
- Cookson, B.T.; Brennan, M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef] [Green Version]
- Nailwal, H.; Chan, F.K. Necroptosis in anti-viral inflammation. Cell Death Differ. 2019, 26, 4–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, T.B.; Ben-Moshe, T.; Varfolomeev, E.E.; Pewzner-Jung, Y.; Yogev, N.; Jurewicz, A.; Waisman, A.; Brenner, O.; Haffner, R.; Gustafsson, E.; et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J. Immunol. 2004, 173, 2976–2984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, B.; Pan, H.; Najafov, A.; Yuan, J. Necroptosis in development and diseases. Genes Dev. 2018, 32, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Ofengeim, D.; Yuan, J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat. Rev. Mol. Cell Biol. 2013, 14, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Weinlich, R.; Oberst, A.; Beere, H.M.; Green, D.R. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 127–136. [Google Scholar] [CrossRef]
- Kanneganti, T.D. Intracellular innate immune receptors: Life inside the cell. Immunol Rev. 2020, 297, 5–12. [Google Scholar] [CrossRef]
- Norris, G.T.; Kipnis, J. Immune cells and CNS physiology: Microglia and beyond. J. Exp. Med. 2019, 216, 60–70. [Google Scholar] [CrossRef]
- Ajami, B.; Bennett, J.L.; Krieger, C.; Tetzlaff, W.; Rossi, F.M. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007, 10, 1538–1543. [Google Scholar] [CrossRef]
- Tay, T.L.; Hagemeyer, N.; Prinz, M. The force awakens: Insights into the origin and formation of microglia. Curr. Opin. Neurobiol. 2016, 39, 30–37. [Google Scholar] [CrossRef]
- Elmore, M.R.; Najafi, A.R.; Koike, M.A.; Dagher, N.N.; Spangenberg, E.E.; Rice, R.A.; Kitazawa, M.; Matusow, B.; Nguyen, H.; West, B.L.; et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014, 82, 380–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clayton, K.A.; Van Enoo, A.A.; Ikezu, T. Alzheimer’s Disease: The Role of Microglia in Brain Homeostasis and Proteopathy. Front. Neurosci. 2017, 11, 680. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nelson, L.H. Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef] [Green Version]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Hodges, A.K.; Piers, T.M.; Collier, D.; Cousins, O.; Pocock, J.M. Pathways linking Alzheimer’s disease risk genes expressed highly in microglia. Neuroimmunol. Neuroinflammation 2021, 8, 245–268. [Google Scholar] [CrossRef]
- Baroja-Mazo, A.; Martin-Sanchez, F.; Gomez, A.I.; Martinez, C.M.; Amores-Iniesta, J.; Compan, V.; Barbera-Cremades, M.; Yague, J.; Ruiz-Ortiz, E.; Anton, J.; et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 2014, 15, 738–748. [Google Scholar] [CrossRef]
- Koenigsknecht-Talboo, J.; Landreth, G.E. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J. Neurosci. 2005, 25, 8240–8249. [Google Scholar] [CrossRef] [PubMed]
- Sondag, C.M.; Dhawan, G.; Combs, C.K. Beta amyloid oligomers and fibrils stimulate differential activation of primary microglia. J. Neuroinflammation 2009, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kigerl, K.A.; de Rivero Vaccari, J.P.; Dietrich, W.D.; Popovich, P.G.; Keane, R.W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 2014, 258, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef] [PubMed]
- McDonough, A.; Lee, R.V.; Weinstein, J.R. Microglial Interferon Signaling and White Matter. Neurochem. Res. 2017, 42, 2625–2638. [Google Scholar] [CrossRef] [PubMed]
- Nagele, R.G.; Wegiel, J.; Venkataraman, V.; Imaki, H.; Wang, K.C.; Wegiel, J. Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol. Aging 2004, 25, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Mielke, M.L.; Gomez-Isla, T.; Betensky, R.A.; Growdon, J.H.; Frosch, M.P.; Hyman, B.T. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am. J. Pathol. 2011, 179, 1373–1384. [Google Scholar] [CrossRef]
- Bamberger, M.E.; Harris, M.E.; McDonald, D.R.; Husemann, J.; Landreth, G.E. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J. Neurosci. 2003, 23, 2665–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ries, M.; Sastre, M. Mechanisms of Abeta Clearance and Degradation by Glial Cells. Front. Aging Neurosci. 2016, 8, 160. [Google Scholar] [CrossRef] [Green Version]
- Tahara, K.; Kim, H.D.; Jin, J.J.; Maxwell, J.A.; Li, L.; Fukuchi, K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain 2006, 129, 3006–3019. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, K.; El Khoury, J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. Int. J. Alzheimers Dis. 2012, 2012, 489456. [Google Scholar] [CrossRef] [Green Version]
- Reed-Geaghan, E.G.; Savage, J.C.; Hise, A.G.; Landreth, G.E. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J. Neurosci. 2009, 29, 11982–11992. [Google Scholar] [CrossRef]
- Chen, K.; Iribarren, P.; Hu, J.; Chen, J.; Gong, W.; Cho, E.H.; Lockett, S.; Dunlop, N.M.; Wang, J.M. Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid beta peptide. J. Biol. Chem. 2006, 281, 3651–3659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef] [PubMed]
- Theriault, P.; ElAli, A.; Rivest, S. The dynamics of monocytes and microglia in Alzheimer’s disease. Alzheimers Res. 2015, 7, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frackowiak, J.; Wisniewski, H.M.; Wegiel, J.; Merz, G.S.; Iqbal, K.; Wang, K.C. Ultrastructure of the microglia that phagocytose amyloid and the microglia that produce beta-amyloid fibrils. Acta Neuropathol. 1992, 84, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, G.; Halle, A.; Matyash, V.; Rinnenthal, J.L.; Eom, G.D.; Bernhardt, U.; Miller, K.R.; Prokop, S.; Kettenmann, H.; Heppner, F.L. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS ONE 2013, 8, e60921. [Google Scholar] [CrossRef] [PubMed]
- Lok, K.; Zhao, H.; Shen, H.; Wang, Z.; Gao, X.; Zhao, W.; Yin, M. Characterization of the APP/PS1 mouse model of Alzheimer’s disease in senescence accelerated background. Neurosci. Lett. 2013, 557, 84–89. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.L.; Hennessy, E.; Rubio-Araiz, A.; Keogh, B.; McCormack, W.; McGuirk, P.; Reilly, M.; Lynch, M.A. Inhibiting TLR2 activation attenuates amyloid accumulation and glial activation in a mouse model of Alzheimer’s disease. Brain Behav. Immun. 2016, 58, 191–200. [Google Scholar] [CrossRef]
- Vollmar, P.; Kullmann, J.S.; Thilo, B.; Claussen, M.C.; Rothhammer, V.; Jacobi, H.; Sellner, J.; Nessler, S.; Korn, T.; Hemmer, B. Active immunization with amyloid-beta 1-42 impairs memory performance through TLR2/4-dependent activation of the innate immune system. J. Immunol. 2010, 185, 6338–6347. [Google Scholar] [CrossRef] [Green Version]
- Jana, M.; Palencia, C.A.; Pahan, K. Fibrillar amyloid-beta peptides activate microglia via TLR2: Implications for Alzheimer’s disease. J. Immunol. 2008, 181, 7254–7262. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Hao, W.; Wolf, L.; Kiliaan, A.J.; Penke, B.; Rube, C.E.; Walter, J.; Heneka, M.T.; Hartmann, T.; et al. TLR2 is a primary receptor for Alzheimer’s amyloid beta peptide to trigger neuroinflammatory activation. J. Immunol. 2012, 188, 1098–1107. [Google Scholar] [CrossRef] [Green Version]
- Griciuc, A.; Tanzi, R.E. The role of innate immune genes in Alzheimer’s disease. Curr. Opin. Neurol. 2021, 34, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Wightman, D.P.; Jansen, I.E.; Savage, J.E.; Shadrin, A.A.; Bahrami, S.; Holland, D.; Rongve, A.; Borte, S.; Winsvold, B.S.; Drange, O.K.; et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 2021, 53, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Chatila, Z.K.; Bradshaw, E.M. Alzheimer’s Disease Genetics: A Dampened Microglial Response? Neuroscientist 2021, 10738584211024531. [Google Scholar] [CrossRef]
- Boutajangout, A.; Wisniewski, T. The innate immune system in Alzheimer’s disease. Int. J. Cell Biol. 2013, 2013, 576383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takatori, S.; Wang, W.; Iguchi, A.; Tomita, T. Genetic Risk Factors for Alzheimer Disease: Emerging Roles of Microglia in Disease Pathomechanisms. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019; Volume 1118. [Google Scholar]
- Song, M.; Jin, J.; Lim, J.E.; Kou, J.; Pattanayak, A.; Rehman, J.A.; Kim, H.D.; Tahara, K.; Lalonde, R.; Fukuchi, K. TLR4 mutation reduces microglial activation, increases Abeta deposits and exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. J. Neuroinflammation 2011, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, V.; Dye, R.; Pakavathkumar, P.; Foveau, B.; Flores, J.; Hyman, B.; Ghetti, B.; Koller, B.H.; LeBlanc, A.C. Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ. 2015, 22, 1676–1686. [Google Scholar] [CrossRef] [Green Version]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Freeman, L.; Guo, H.; David, C.N.; Brickey, W.J.; Jha, S.; Ting, J.P. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J. Exp. Med. 2017, 214, 1351–1370. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.J.; Hung, Y.F.; Liu, H.Y.; Hsueh, Y.P. Deletion of the Inflammasome Sensor Aim2 Mitigates Abeta Deposition and Microglial Activation but Increases Inflammatory Cytokine Expression in an Alzheimer Disease Mouse Model. Neuroimmunomodulation 2017, 24, 29–39. [Google Scholar] [CrossRef]
- Wang, Y.; Cella, M.; Mallinson, K.; Ulrich, J.D.; Young, K.L.; Robinette, M.L.; Gilfillan, S.; Krishnan, G.M.; Sudhakar, S.; Zinselmeyer, B.H.; et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 2015, 160, 1061–1071. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Liao, Y.; Xiao, L.; Wu, R.; Zhao, S.; Chen, H.; Hou, B.; Zhang, X.; Liang, C.; Xu, Y.; et al. Autophagy regulates MAVS signaling activation in a phosphorylation-dependent manner in microglia. Cell Death Differ. 2017, 24, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Paula Martorell, V.B.; Schwarz, S.; Heneka, M. cGAS-STING activation in Alzheimer’s disease. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjLvqyar-n3AhWRMM0KHTQCC6gQFnoECAoQAQ&url=https%3A%2F%2Fbonnbrain.de%2Fabstracts%2Fcgas-sting-activation-in-alzheimers-disease%2F&usg=AOvVaw0TTor6dH1LdB53mOX1R5xu (accessed on 24 March 2022).
- De Rivero Vaccari, J.P.; Brand, F.J., 3rd; Sedaghat, C.; Mash, D.C.; Dietrich, W.D.; Keane, R.W. RIG-1 receptor expression in the pathology of Alzheimer’s disease. J. Neuroinflammation 2014, 11, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanneganti, T.D.; Ozoren, N.; Body-Malapel, M.; Amer, A.; Park, J.H.; Franchi, L.; Whitfield, J.; Barchet, W.; Colonna, M.; Vandenabeele, P.; et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 2006, 440, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Mariathasan, S.; Weiss, D.S.; Newton, K.; McBride, J.; O’Rourke, K.; Roose-Girma, M.; Lee, W.P.; Weinrauch, Y.; Monack, D.M.; Dixit, V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006, 440, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchi, L.; Amer, A.; Body-Malapel, M.; Kanneganti, T.D.; Ozoren, N.; Jagirdar, R.; Inohara, N.; Vandenabeele, P.; Bertin, J.; Coyle, A.; et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat. Immunol. 2006, 7, 576–582. [Google Scholar] [CrossRef]
- Miao, E.A.; Alpuche-Aranda, C.M.; Dors, M.; Clark, A.E.; Bader, M.W.; Miller, S.I.; Aderem, A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat. Immunol. 2006, 7, 569–575. [Google Scholar] [CrossRef]
- Mariathasan, S.; Newton, K.; Monack, D.M.; Vucic, D.; French, D.M.; Lee, W.P.; Roose-Girma, M.; Erickson, S.; Dixit, V.M. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 2004, 430, 213–218. [Google Scholar] [CrossRef]
- Xu, H.; Yang, J.; Gao, W.; Li, L.; Li, P.; Zhang, L.; Gong, Y.N.; Peng, X.; Xi, J.J.; Chen, S.; et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 2014, 513, 237–241. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Yu, J.W.; Datta, P.; Wu, J.; Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 2009, 458, 509–513. [Google Scholar] [CrossRef] [Green Version]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009, 458, 514–518. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Stancu, I.C.; Cremers, N.; Vanrusselt, H.; Couturier, J.; Vanoosthuyse, A.; Kessels, S.; Lodder, C.; Brône, B.; Huaux, F.; Octave, J.N.; et al. Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol. 2019, 137, 599–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, E.R.; Wang, B.; Wan, Y.W.; Chiu, G.; Cole, A.; Yin, Z.; Propson, N.E.; Xu, Y.; Jankowsky, J.L.; Liu, Z.; et al. Type I interferon response drives neuroinflammation and synapse loss in Alzheimer disease. J. Clin. Investig. 2020, 130, 1912–1930. [Google Scholar] [CrossRef]
- Forlenza, O.V.; Diniz, B.S.; Talib, L.L.; Mendonca, V.A.; Ojopi, E.B.; Gattaz, W.F.; Teixeira, A.L. Increased serum IL-1beta level in Alzheimer’s disease and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2009, 28, 507–512. [Google Scholar] [CrossRef] [PubMed]
- King, E.; O’Brien, J.T.; Donaghy, P.; Morris, C.; Barnett, N.; Olsen, K.; Martin-Ruiz, C.; Taylor, J.P.; Thomas, A.J. Peripheral inflammation in prodromal Alzheimer’s and Lewy body dementias. J. Neurol. Neurosurg. Psychiatry 2018, 89, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Ojala, J.; Alafuzoff, I.; Herukka, S.K.; van Groen, T.; Tanila, H.; Pirttila, T. Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiol. Aging 2009, 30, 198–209. [Google Scholar] [CrossRef]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1beta, IL-6, TNF- alpha and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12050. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol 2018, 217, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Hemonnot, A.L.; Hua, J.; Ulmann, L.; Hirbec, H. Microglia in Alzheimer Disease: Well-Known Targets and New Opportunities. Front. Aging Neurosci. 2019, 11, 233. [Google Scholar] [CrossRef] [Green Version]
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017, 127, 3240–3249. [Google Scholar] [CrossRef] [PubMed]
- Tejera, D.; Heneka, M.T. Microglia in Alzheimer’s disease: The good, the bad and the ugly. Curr. Alzheimer Res. 2016, 13, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Rogers, J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012, 2, a006346. [Google Scholar] [CrossRef]
- Biber, K.; Neumann, H.; Inoue, K.; Boddeke, H.W. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007, 30, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Preman, P.; Alfonso-Triguero, M.; Alberdi, E.; Verkhratsky, A.; Arranz, A.M. Astrocytes in Alzheimer’s Disease: Pathological Significance and Molecular Pathways. Cells 2021, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.; Allen, N.J.; Eroglu, C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020370. [Google Scholar] [CrossRef] [Green Version]
- Sheeler, C.; Rosa, J.G.; Ferro, A.; McAdams, B.; Borgenheimer, E.; Cvetanovic, M. Glia in Neurodegeneration: The Housekeeper, the Defender and the Perpetrator. Int. J. Mol. Sci. 2020, 21, 9188. [Google Scholar] [CrossRef] [PubMed]
- Gamage, R.; Wagnon, I.; Rossetti, I.; Childs, R.; Niedermayer, G.; Chesworth, R.; Gyengesi, E. Cholinergic Modulation of Glial Function During Aging and Chronic Neuroinflammation. Front. Cell Neurosci 2020, 14, 577912. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Hol, E.M.; Pekny, M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Onyango, I.G.; Jauregui, G.V.; Carna, M.; Bennett, J.P., Jr.; Stokin, G.B. Neuroinflammation in Alzheimer’s Disease. Biomedicines 2021, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Barclay, W.E.; Aggarwal, N.; Deerhake, M.E.; Inoue, M.; Nonaka, T.; Nozaki, K.; Luzum, N.A.; Miao, E.A.; Shinohara, M.L. The AIM2 inflammasome is activated in astrocytes during the late phase of EAE. JCI Insight 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Johann, S.; Heitzer, M.; Kanagaratnam, M.; Goswami, A.; Rizo, T.; Weis, J.; Troost, D.; Beyer, C. NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia 2015, 63, 2260–2273. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.F.; Cao, H.; Fu, A.K.Y.; Ip, N.Y. Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2020, 117, 25800–25809. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; Del Tredici, K.; et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Venegas, C.; Kumar, S.; Franklin, B.S.; Dierkes, T.; Brinkschulte, R.; Tejera, D.; Vieira-Saecker, A.; Schwartz, S.; Santarelli, F.; Kummer, M.P.; et al. Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature 2017, 552, 355–361. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Pasparakis, M.; Vandenabeele, P. Necroptosis and its role in inflammation. Nature 2015, 517, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.C.; Shao, F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sborgi, L.; Ruhl, S.; Mulvihill, E.; Pipercevic, J.; Heilig, R.; Stahlberg, H.; Farady, C.J.; Muller, D.J.; Broz, P.; Hiller, S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016, 35, 1766–1778. [Google Scholar] [CrossRef]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Van de Veerdonk, F.L.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011, 32, 110–116. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Yang, Y.; Guan, Q.; Zhang, X.; Shen, H.; Sheng, Y.; Wang, J.; Zhou, X.; Li, W.; Guo, L.; et al. New mechanism of nerve injury in Alzheimer’s disease: β-amyloid-induced neuronal pyroptosis. J. Cell Mol. Med. 2020, 24, 8078–8090. [Google Scholar] [CrossRef]
- Tan, M.S.; Yu, J.T.; Jiang, T.; Zhu, X.C.; Guan, H.S.; Tan, L. IL12/23 p40 inhibition ameliorates Alzheimer’s disease-associated neuropathology and spatial memory in SAMP8 mice. J. Alzheimers Dis. 2014, 38, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monks, B.G.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008, 9, 857–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccioni, R.B. Molecular Cytology of Microtubules; Springer: Berlin/Heidelberg, Germany, 1986; Volume 8, pp. 1–124. [Google Scholar]
- Shen, H.; Han, C.; Yang, Y.; Guo, L.; Sheng, Y.; Wang, J.; Li, W.; Zhai, L.; Wang, G.; Guan, Q. Pyroptosis executive protein GSDMD as a biomarker for diagnosis and identification of Alzheimer’s disease. Brain Behav. 2021, 11, e02063. [Google Scholar] [CrossRef] [PubMed]
- Gervais, F.G.; Xu, D.; Robertson, G.S.; Vaillancourt, J.P.; Zhu, Y.; Huang, J.; LeBlanc, A.; Smith, D.; Rigby, M.; Shearman, M.S.; et al. Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell 1999, 97, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, S.; Bourdeau, M.; Bennett, D.; Mufson, E.J.; Bhattacharjee, M.; LeBlanc, A.C. Activation of caspase-6 in aging and mild cognitive impairment. Am. J. Pathol. 2007, 170, 1200–1209. [Google Scholar] [CrossRef] [Green Version]
- Ramcharitar, J.; Albrecht, S.; Afonso, V.M.; Kaushal, V.; Bennett, D.A.; Leblanc, A.C. Cerebrospinal fluid tau cleaved by caspase-6 reflects brain levels and cognition in aging and Alzheimer disease. J. Neuropathol. Exp. Neurol. 2013, 72, 824–832. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, C.; Beecham, G.; Vardarajan, B.N.; Ma, Y.; Lancour, D.; Farrell, J.J.; Chung, J.; Alzheimer’s Disease Sequencing, P.; Mayeux, R.; et al. A rare missense variant of CASP7 is associated with familial late-onset Alzheimer’s disease. Alzheimers Dement. 2019, 15, 441–452. [Google Scholar] [CrossRef]
- Rohn, T.T.; Head, E.; Nesse, W.H.; Cotman, C.W.; Cribbs, D.H. Activation of caspase-8 in the Alzheimer’s disease brain. Neurobiol. Dis. 2001, 8, 1006–1016. [Google Scholar] [CrossRef] [Green Version]
- Rehker, J.; Rodhe, J.; Nesbitt, R.R.; Boyle, E.A.; Martin, B.K.; Lord, J.; Karaca, I.; Naj, A.; Jessen, F.; Helisalmi, S.; et al. Caspase-8, association with Alzheimer’s Disease and functional analysis of rare variants. PLoS ONE 2017, 12, e0185777. [Google Scholar] [CrossRef] [Green Version]
- Rohn, T.T.; Rissman, R.A.; Davis, M.C.; Kim, Y.E.; Cotman, C.W.; Head, E. Caspase-9 activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol. Dis. 2002, 11, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Caccamo, A.; Branca, C.; Piras, I.S.; Ferreira, E.; Huentelman, M.J.; Liang, W.S.; Readhead, B.; Dudley, J.T.; Spangenberg, E.E.; Green, K.N.; et al. Necroptosis activation in Alzheimer’s disease. Nat. Neurosci. 2017, 20, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, F.C.; Hellbardt, S.; Behrmann, I.; Germer, M.; Pawlita, M.; Krammer, P.H.; Peter, M.E. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995, 14, 5579–5588. [Google Scholar] [CrossRef] [PubMed]
- Chinnaiyan, A.M.; O’Rourke, K.; Tewari, M.; Dixit, V.M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 1995, 81, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slee, E.A.; Harte, M.T.; Kluck, R.M.; Wolf, B.B.; Casiano, C.A.; Newmeyer, D.D.; Wang, H.G.; Reed, J.C.; Nicholson, D.W.; Alnemri, E.S.; et al. Ordering the cytochrome c-initiated caspase cascade: Hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J. Cell Biol. 1999, 144, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Zou, H.; Li, Y.; Liu, X.; Wang, X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 1999, 274, 11549–11556. [Google Scholar] [CrossRef] [Green Version]
- Callens, M.; Kraskovskaya, N.; Derevtsova, K.; Annaert, W.; Bultynck, G.; Bezprozvanny, I.; Vervliet, T. The role of Bcl-2 proteins in modulating neuronal Ca(2+) signaling in health and in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118997. [Google Scholar] [CrossRef]
- Kudo, W.; Lee, H.P.; Smith, M.A.; Zhu, X.; Matsuyama, S.; Lee, H.G. Inhibition of Bax protects neuronal cells from oligomeric Abeta neurotoxicity. Cell Death Dis. 2012, 3, e309. [Google Scholar] [CrossRef]
- Paradis, E.; Douillard, H.; Koutroumanis, M.; Goodyer, C.; LeBlanc, A. Amyloid beta peptide of Alzheimer’s disease downregulates Bcl-2 and upregulates bax expression in human neurons. J. Neurosci. 1996, 16, 7533–7539. [Google Scholar] [CrossRef] [Green Version]
- Drache, B.; Diehl, G.E.; Beyreuther, K.; Perlmutter, L.S.; Konig, G. Bcl-xl-specific antibody labels activated microglia associated with Alzheimer’s disease and other pathological states. J. Neurosci. Res. 1997, 47, 98–108. [Google Scholar] [CrossRef]
- Burguillos, M.A.; Deierborg, T.; Kavanagh, E.; Persson, A.; Hajji, N.; Garcia-Quintanilla, A.; Cano, J.; Brundin, P.; Englund, E.; Venero, J.L.; et al. Caspase signalling controls microglia activation and neurotoxicity. Nature 2011, 472, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.; Vilalta, A.; Tolkovsky, A.M.; Brown, G.C. Caspase inhibitors protect neurons by enabling selective necroptosis of inflamed microglia. J. Biol. Chem. 2013, 288, 9145–9152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadelmann, C.; Deckwerth, T.L.; Srinivasan, A.; Bancher, C.; Bruck, W.; Jellinger, K.; Lassmann, H. Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer’s disease. Evidence for apoptotic cell death. Am. J. Pathol. 1999, 155, 1459–1466. [Google Scholar] [CrossRef]
- Su, J.H.; Zhao, M.; Anderson, A.J.; Srinivasan, A.; Cotman, C.W. Activated caspase-3 expression in Alzheimer’s and aged control brain: Correlation with Alzheimer pathology. Brain Res. 2001, 898, 350–357. [Google Scholar] [CrossRef]
- Rohn, T.T.; Kokoulina, P.; Eaton, C.R.; Poon, W.W. Caspase activation in transgenic mice with Alzheimer-like pathology: Results from a pilot study utilizing the caspase inhibitor, Q-VD-OPh. Int. J. Clin. Exp. Med. 2009, 2, 300–308. [Google Scholar]
- Biscaro, B.; Lindvall, O.; Tesco, G.; Ekdahl, C.T.; Nitsch, R.M. Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive deficits in a transgenic mouse model for Alzheimer’s disease. Neurodegener Dis. 2012, 9, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Grootjans, S.; Vanden Berghe, T.; Vandenabeele, P. Initiation and execution mechanisms of necroptosis: An overview. Cell Death Differ. 2017, 24, 1184–1195. [Google Scholar] [CrossRef]
- Dondelinger, Y.; Declercq, W.; Montessuit, S.; Roelandt, R.; Goncalves, A.; Bruggeman, I.; Hulpiau, P.; Weber, K.; Sehon, C.A.; Marquis, R.W.; et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014, 7, 971–981. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Sun, L.; Su, L.; Rizo, J.; Liu, L.; Wang, L.F.; Wang, F.S.; Wang, X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 2014, 54, 133–146. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Yang, Y.; Mei, Y.; Ma, L.; Zhu, D.E.; Hoti, N.; Castanares, M.; Wu, M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell. Signal. 2007, 19, 2056–2067. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Hitomi, J.; Germscheid, M.; Ch’en, I.L.; Korkina, O.; Teng, X.; Abbott, D.; Cuny, G.D.; Yuan, C.; Wagner, G.; et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008, 4, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.S.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009, 137, 1112–1123. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009, 137, 1100–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.W.; Shao, J.; Lin, J.; Zhang, N.; Lu, B.J.; Lin, S.C.; Dong, M.Q.; Han, J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 2009, 325, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Jitkaew, S.; Cai, Z.; Choksi, S.; Li, Q.; Luo, J.; Liu, Z.G. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 5322–5327. [Google Scholar] [CrossRef] [Green Version]
- Ofengeim, D.; Mazzitelli, S.; Ito, Y.; DeWitt, J.P.; Mifflin, L.; Zou, C.; Das, S.; Adiconis, X.; Chen, H.; Zhu, H.; et al. RIPK1 mediates a disease-associated microglial response in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, E8788–E8797. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.H. Aberrant phosphorylation in the pathogenesis of Alzheimer’s disease. BMB Rep. 2009, 42, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Rogers, C.; Fernandes-Alnemri, T.; Mayes, L.; Alnemri, D.; Cingolani, G.; Alnemri, E.S. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 2017, 8, 14128. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.; Erkes, D.A.; Nardone, A.; Aplin, A.E.; Fernandes-Alnemri, T.; Alnemri, E.S. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 2019, 10, 1689. [Google Scholar] [CrossRef] [PubMed]
- Orning, P.; Weng, D.; Starheim, K.; Ratner, D.; Best, Z.; Lee, B.; Brooks, A.; Xia, S.; Wu, H.; Kelliher, M.A.; et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 2018, 362, 1064–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarhan, J.; Liu, B.C.; Muendlein, H.I.; Li, P.; Nilson, R.; Tang, A.Y.; Rongvaux, A.; Bunnell, S.C.; Shao, F.; Green, D.R.; et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. USA 2018, 115, E10888–E10897. [Google Scholar] [CrossRef] [Green Version]
- Pompl, P.N.; Yemul, S.; Xiang, Z.; Ho, L.; Haroutunian, V.; Purohit, D.; Mohs, R.; Pasinetti, G.M. Caspase gene expression in the brain as a function of the clinical progression of Alzheimer disease. Arch. Neurol. 2003, 60, 369–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesavardhana, S.; Kanneganti, T.D. ZBP1: A STARGᐰTE to decode the biology of Z-nucleic acids in disease. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Malireddi, R.K.S.; Tweedell, R.E.; Kanneganti, T.D. PANoptosis components, regulation, and implications. Aging 2020, 12, 11163–11164. [Google Scholar] [CrossRef] [PubMed]
- Place, D.E.; Lee, S.; Kanneganti, T.D. PANoptosis in microbial infection. Curr. Opin. Microbiol. 2021, 59, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Dunn, N.; Mullee, M.; Perry, V.H.; Holmes, C. Association between dementia and infectious disease: Evidence from a case-control study. Alzheimer Dis. Assoc. Disord. 2005, 19, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Castro, P.J.; Estivill-Torrus, G.; Cabezudo-Garcia, P.; Reyes-Bueno, J.A.; Ciano Petersen, N.; Aguilar-Castillo, M.J.; Suarez-Perez, J.; Jimenez-Hernandez, M.D.; Moya-Molina, M.A.; Oliver-Martos, B.; et al. Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: A delayed pandemic? Neurología 2020, 35, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Kanneganti, T.D. The ‘cytokine storm’: Molecular mechanisms and therapeutic prospects. Trends Immunol. 2021, 42, 681–705. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.S.; Paris, D.; Mathura, V.; Quadros, A.N.; Crawford, F.C.; Mullan, M.J. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer’s disease. J. Neuroinflammation 2005, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Minter, M.R.; Taylor, J.M.; Crack, P.J. The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J. Neurochem. 2016, 136, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.T.; Howell, J.C.; Ozturk, T.; Gangishetti, U.; Kollhoff, A.L.; Hatcher-Martin, J.M.; Anderson, A.M.; Tyor, W.R. CSF Cytokines in Aging, Multiple Sclerosis, and Dementia. Front. Immunol. 2019, 10, 480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genazzani, A.D.; Forti, G.; Guardabasso, V.; Maggi, M.; Milloni, M.; Cianfanelli, F.; Serio, M. Frequency of prolactin pulsatile release in normal men and in agonadal patients is neither coupled to LH release nor influenced by androgen modulation. Clin. Endocrinol. 1992, 37, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Vom Berg, J.; Prokop, S.; Miller, K.R.; Obst, J.; Kalin, R.E.; Lopategui-Cabezas, I.; Wegner, A.; Mair, F.; Schipke, C.G.; Peters, O.; et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat. Med. 2012, 18, 1812–1819. [Google Scholar] [CrossRef]
- Rentzos, M.; Paraskevas, G.P.; Kapaki, E.; Nikolaou, C.; Zoga, M.; Rombos, A.; Tsoutsou, A.; Vassilopoulos, D.D. Interleukin-12 is reduced in cerebrospinal fluid of patients with Alzheimer’s disease and frontotemporal dementia. J. Neurol. Sci. 2006, 249, 110–114. [Google Scholar] [CrossRef]
- Decourt, B.; Lahiri, D.K.; Sabbagh, M.N. Targeting Tumor Necrosis Factor Alpha for Alzheimer’s Disease. Curr Alzheimer Res. 2017, 14, 412–425. [Google Scholar] [CrossRef] [Green Version]
- Meda, L.; Cassatella, M.A.; Szendrei, G.I.; Otvos, L., Jr.; Baron, P.; Villalba, M.; Ferrari, D.; Rossi, F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature 1995, 374, 647–650. [Google Scholar] [CrossRef]
- Tan, J.; Town, T.; Paris, D.; Mori, T.; Suo, Z.; Crawford, F.; Mattson, M.P.; Flavell, R.A.; Mullan, M. Microglial activation resulting from CD40-CD40L interaction after beta-amyloid stimulation. Science 1999, 286, 2352–2355. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Town, T.; Crawford, F.; Mori, T.; DelleDonne, A.; Crescentini, R.; Obregon, D.; Flavell, R.A.; Mullan, M.J. Role of CD40 ligand in amyloidosis in transgenic Alzheimer’s mice. Nat. Neurosci. 2002, 5, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, P.; Ceballos-Diaz, C.; Beccard, A.; Janus, C.; Dickson, D.; Golde, T.E.; Das, P. IFN-gamma promotes complement expression and attenuates amyloid plaque deposition in amyloid beta precursor protein transgenic mice. J. Immunol. 2010, 184, 5333–5343. [Google Scholar] [CrossRef] [PubMed]
- Lue, L.F.; Rydel, R.; Brigham, E.F.; Yang, L.B.; Hampel, H.; Murphy, G.M., Jr.; Brachova, L.; Yan, S.D.; Walker, D.G.; Shen, Y.; et al. Inflammatory repertoire of Alzheimer’s disease and nondemented elderly microglia in vitro. Glia 2001, 35, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Laske, C.; Stransky, E.; Hoffmann, N.; Maetzler, W.; Straten, G.; Eschweiler, G.W.; Leyhe, T. Macrophage colony-stimulating factor (M-CSF) in plasma and CSF of patients with mild cognitive impairment and Alzheimer’s disease. Curr. Alzheimer Res. 2010, 7, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Hirsch-Reinshagen, V.; Maia, L.F.; Burgess, B.L.; Blain, J.F.; Naus, K.E.; McIsaac, S.A.; Parkinson, P.F.; Chan, J.Y.; Tansley, G.H.; Hayden, M.R.; et al. The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J. Biol. Chem. 2005, 280, 43243–43256. [Google Scholar] [CrossRef] [Green Version]
- Akama, K.T.; Van Eldik, L.J. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J. Biol. Chem. 2000, 275, 7918–7924. [Google Scholar] [CrossRef] [Green Version]
- Shaftel, S.S.; Carlson, T.J.; Olschowka, J.A.; Kyrkanides, S.; Matousek, S.B.; O’Banion, M.K. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J. Neurosci. 2007, 27, 9301–9309. [Google Scholar] [CrossRef]
- Ghosh, S.; Wu, M.D.; Shaftel, S.S.; Kyrkanides, S.; LaFerla, F.M.; Olschowka, J.A.; O’Banion, M.K. Sustained interleukin-1beta overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer’s mouse model. J. Neurosci. 2013, 33, 5053–5064. [Google Scholar] [CrossRef]
- Tarkowski, E.; Andreasen, N.; Tarkowski, A.; Blennow, K. Intrathecal inflammation precedes development of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1200–1205. [Google Scholar] [CrossRef]
- Jin, J.J.; Kim, H.D.; Maxwell, J.A.; Li, L.; Fukuchi, K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer’s disease. J. Neuroinflammation 2008, 5, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savarin-Vuaillat, C.; Ransohoff, R.M. Chemokines and chemokine receptors in neurological disease: Raise, retain, or reduce? Neurotherapeutics 2007, 4, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.Q.; Qin, S.X.; Wu, L.J.; Mackay, C.R.; Hyman, B.T. Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer’s disease brains. Am. J. Pathol. 1998, 153, 31–37. [Google Scholar] [CrossRef]
- Ishizuka, K.; Kimura, T.; Igata-yi, R.; Katsuragi, S.; Takamatsu, J.; Miyakawa, T. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer’s disease. Psychiatry Clin. Neurosci. 1997, 51, 135–138. [Google Scholar] [CrossRef]
- Smits, H.A.; Rijsmus, A.; van Loon, J.H.; Wat, J.W.; Verhoef, J.; Boven, L.A.; Nottet, H.S. Amyloid-beta-induced chemokine production in primary human macrophages and astrocytes. J. Neuroimmunol. 2002, 127, 160–168. [Google Scholar] [CrossRef]
- Lue, L.F.; Walker, D.G.; Rogers, J. Modeling microglial activation in Alzheimer’s disease with human postmortem microglial cultures. Neurobiol. Aging 2001, 22, 945–956. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Bittner, T.; Jung, C.K.; Burgold, S.; Page, R.M.; Mitteregger, G.; Haass, C.; LaFerla, F.M.; Kretzschmar, H.; Herms, J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2010, 13, 411–413. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Varvel, N.H.; Konerth, M.E.; Xu, G.; Cardona, A.E.; Ransohoff, R.M.; Lamb, B.T. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am. J. Pathol. 2010, 177, 2549–2562. [Google Scholar] [CrossRef]
- Cho, S.H.; Sun, B.; Zhou, Y.; Kauppinen, T.M.; Halabisky, B.; Wes, P.; Ransohoff, R.M.; Gan, L. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J. Biol. Chem. 2011, 286, 32713–32722. [Google Scholar] [CrossRef] [Green Version]
- Holmes, C.; Boche, D.; Wilkinson, D.; Yadegarfar, G.; Hopkins, V.; Bayer, A.; Jones, R.W.; Bullock, R.; Love, S.; Neal, J.W.; et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet 2008, 372, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Rabinovici, G.D. Controversy and Progress in Alzheimer’s Disease—FDA Approval of Aducanumab. N. Engl. J. Med. 2021, 385, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Budd-Haeberlein, S.; Von Hein, C.; Tian, Y.; Chalkias, S.; Muralidharan, K.K.; Chen, T.; Wu, S.; Skordos, L.; Nisenbaum, L.; Rajagovindan, R.; et al. EMERGE and ENGAGE topline results: Two phase 3 studies to evaluate aducanumab in patients with early Alzheimer’s disease. In Proceedings of the Advances in Alzheimer’s and Parkinson’s Therapies: An AAT-AD/PD Focus Meeting, Vienna, Austria, 2–5 April 2020. [Google Scholar]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Bittar, A.; Bhatt, N.; Kayed, R. Advances and considerations in AD tau-targeted immunotherapy. Neurobiol. Dis. 2020, 134, 104707. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Suh, Y.H. Minocycline and neurodegenerative diseases. Behav. Brain Res. 2009, 196, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; Zubko, O.; Gray, R.; Bradley, R.; Harper, E.; Kelly, L.; Pank, L.; O’Brien, J.; Fox, C.; Tabet, N.; et al. Minocycline 200 mg or 400 mg versus placebo for mild Alzheimer’s disease: The MADE Phase II, three-arm RCT. Effic. Mech. Eval. 2020, 7, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018, 17, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Primiano, M.J.; Lefker, B.A.; Bowman, M.R.; Bree, A.G.; Hubeau, C.; Bonin, P.D.; Mangan, M.; Dower, K.; Monks, B.G.; Cushing, L.; et al. Efficacy and Pharmacology of the NLRP3 Inflammasome Inhibitor CP-456,773 (CRID3) in Murine Models of Dermal and Pulmonary Inflammation. J. Immunol. 2016, 197, 2421–2433. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili, M.H.; Enayati, M.; Khabbaz Abkenar, F.; Ebrahimian, F.; Salari, A.A. Glibenclamide mitigates cognitive impairment and hippocampal neuroinflammation in rats with type 2 diabetes and sporadic Alzheimer-like disease. Behav. Brain Res. 2020, 379, 112359. [Google Scholar] [CrossRef]
- Flores, J.; Noel, A.; Foveau, B.; Lynham, J.; Lecrux, C.; LeBlanc, A.C. Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer’s disease mouse model. Nat. Commun. 2018, 9, 3916. [Google Scholar] [CrossRef] [Green Version]
- Mandrioli, J.; D’Amico, R.; Zucchi, E.; Gessani, A.; Fini, N.; Fasano, A.; Caponnetto, C.; Chio, A.; Dalla Bella, E.; Lunetta, C.; et al. Rapamycin treatment for amyotrophic lateral sclerosis: Protocol for a phase II randomized, double-blind, placebo-controlled, multicenter, clinical trial (RAP-ALS trial). Medicine 2018, 97, e11119. [Google Scholar] [CrossRef]
- Wang, S.; Mustafa, M.; Yuede, C.M.; Salazar, S.V.; Kong, P.; Long, H.; Ward, M.; Siddiqui, O.; Paul, R.; Gilfillan, S.; et al. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Brazier, D.; Perry, R.; Keane, J.; Barrett, K.; Elmaleh, D.R. Pharmacokinetics of Cromolyn and Ibuprofen in Healthy Elderly Volunteers. Clin. Drug Investig. 2017, 37, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Griciuc, A.; Hudry, E.; Wan, Y.; Quinti, L.; Ward, J.; Forte, A.M.; Shen, X.; Ran, C.; Elmaleh, D.R.; et al. Cromolyn Reduces Levels of the Alzheimer’s Disease-Associated Amyloid beta-Protein by Promoting Microglial Phagocytosis. Sci. Rep. 2018, 8, 1144. [Google Scholar] [CrossRef] [PubMed]
- Blacher, E.; Dadali, T.; Bespalko, A.; Haupenthal, V.J.; Grimm, M.O.; Hartmann, T.; Lund, F.E.; Stein, R.; Levy, A. Alzheimer’s disease pathology is attenuated in a CD38-deficient mouse model. Ann. Neurol. 2015, 78, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, S.; Privat, A.L.; Bressac, L.; Toulorge, D. CD38 in Neurodegeneration and Neuroinflammation. Cells 2020, 9, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, J.; Mei, Z.L.; Wang, H.; Hu, M.; Long, Y.; Miao, M.X.; Li, N.; Hong, H. Montelukast rescues primary neurons against Abeta1-42-induced toxicity through inhibiting CysLT1R-mediated NF-kappaB signaling. Neurochem. Int. 2014, 75, 26–31. [Google Scholar] [CrossRef]
- Morin, N.; Jourdain, V.A.; Morissette, M.; Gregoire, L.; Di Paolo, T. Long-term treatment with l-DOPA and an mGlu5 receptor antagonist prevents changes in brain basal ganglia dopamine receptors, their associated signaling proteins and neuropeptides in parkinsonian monkeys. Neuropharmacology 2014, 79, 688–706. [Google Scholar] [CrossRef]
- Estus, S.; Shaw, B.C.; Devanney, N.; Katsumata, Y.; Press, E.E.; Fardo, D.W. Evaluation of CD33 as a genetic risk factor for Alzheimer’s disease. Acta Neuropathol. 2019, 138, 187–199. [Google Scholar] [CrossRef]
- Friker, L.L.; Scheiblich, H.; Hochheiser, I.V.; Brinkschulte, R.; Riedel, D.; Latz, E.; Geyer, M.; Heneka, M.T. beta-Amyloid Clustering around ASC Fibrils Boosts Its Toxicity in Microglia. Cell Rep. 2020, 30, 3743–3754.e6. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Marsh, S.E.; Abud, E.M.; Lakatos, A.; Karimzadeh, A.; Yeung, S.T.; Davtyan, H.; Fote, G.M.; Lau, L.; Weinger, J.G.; Lane, T.E.; et al. The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proc. Natl. Acad. Sci. USA 2016, 113, E1316–E1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.C.; Kingsmore, K.M.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018, 560, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Olate-Briones, A.; Escalona, E.; Salazar, C.; Herrada, M.J.; Liu, C.; Herrada, A.A.; Escobedo, N. The meningeal lymphatic vasculature in neuroinflammation. FASEB J. 2022, 36, e22276. [Google Scholar] [CrossRef] [PubMed]
- Da Mesquita, S.; Papadopoulos, Z.; Dykstra, T.; Brase, L.; Farias, F.G.; Wall, M.; Jiang, H.; Kodira, C.D.; de Lima, K.A.; Herz, J.; et al. Meningeal lymphatics affect microglia responses and anti-Abeta immunotherapy. Nature 2021, 593, 255–260. [Google Scholar] [CrossRef]
- Zou, W.; Pu, T.; Feng, W.; Lu, M.; Zheng, Y.; Du, R.; Xiao, M.; Hu, G. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated alpha-synuclein. Transl. Neurodegener 2019, 8, 7. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).