The Many Faces of Post-Ischemic Tau Protein in Brain Neurodegeneration of the Alzheimer’s Disease Type
Abstract
1. Introduction
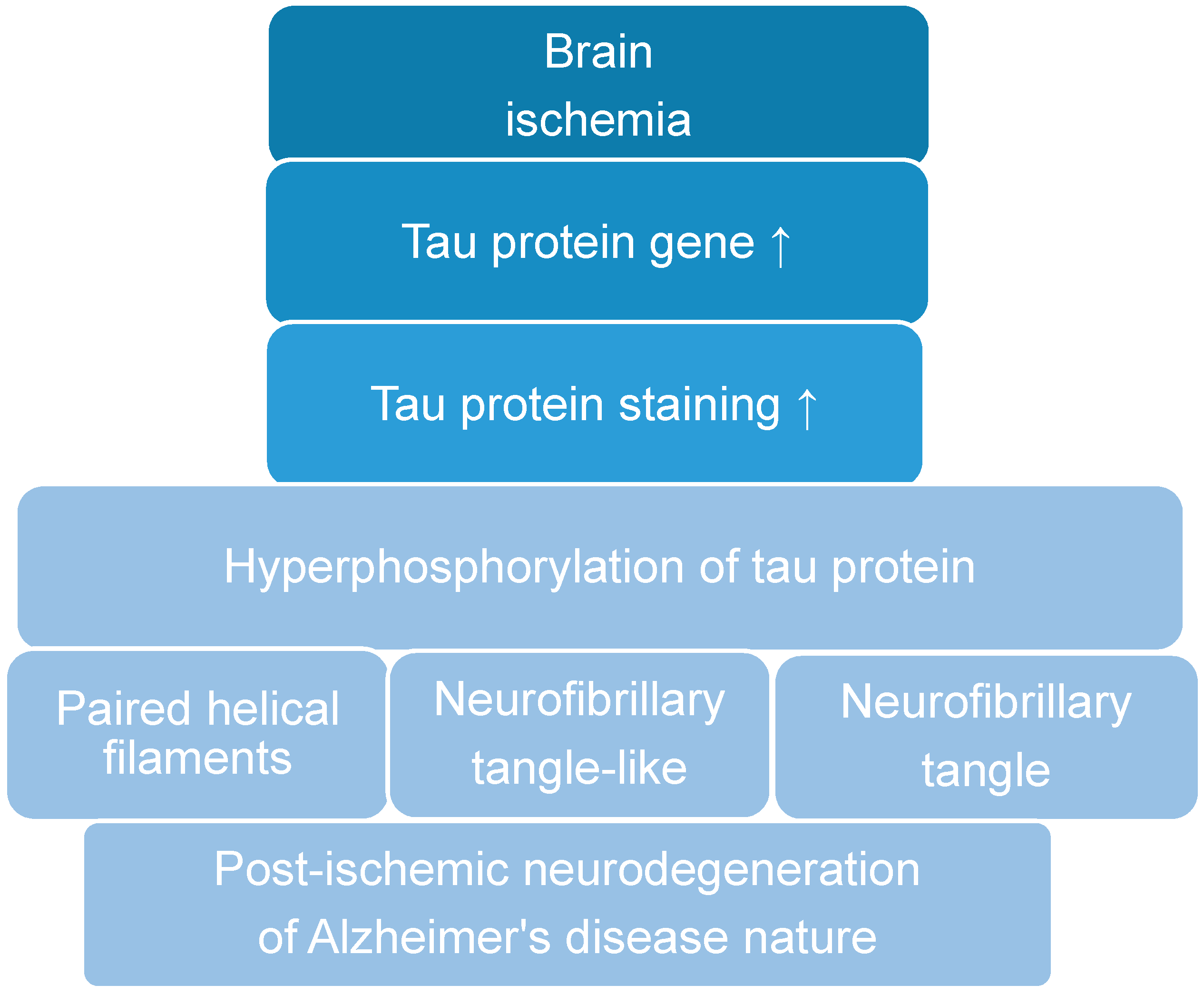
2. Post-Ischemic Tau Protein Gene Expression in the Brain
3. Post-Ischemic Tau Protein Accumulation in the Brain
4. Post-Ischemic Tau Protein in the Blood after Brain Injury
5. Post-Ischemic Tau Protein Hyperphosphorylation in the Brain
6. Post-Ischemic Tau Protein and Neurofibrillary Tangle Development in the Brain
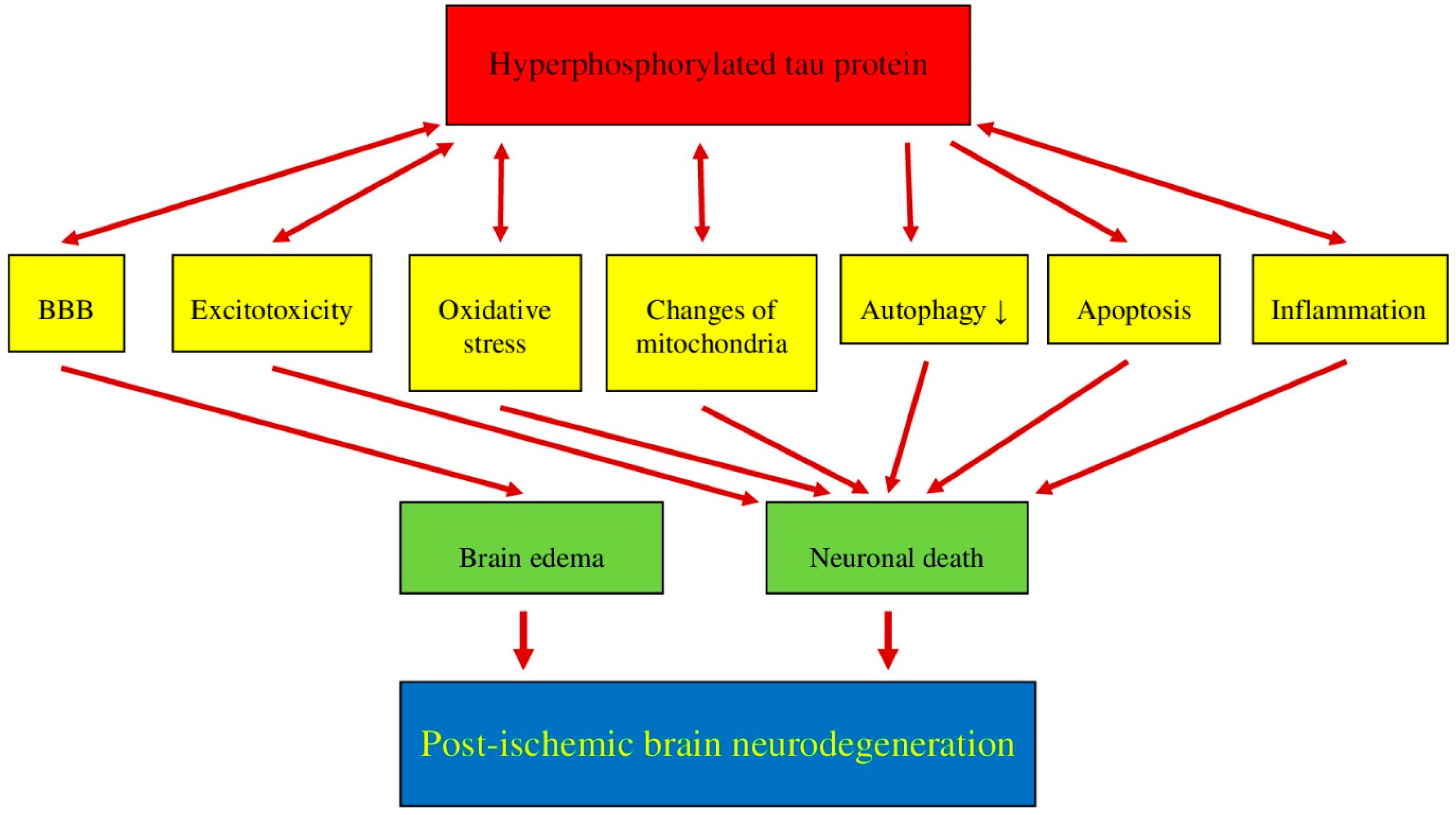
7. Post-Ischemia Tau Protein Intersection with Multiple Overlapping Phenomena/Pathologies in Brain Neurodegeneration
7.1. Post-Ischemic Tau Protein versus Blood-Brain Barrier
7.2. Post-Ischemic Tau Protein versus Excitotoxicity
7.3. Post-Ischemic Tau Protein versus Oxidative Stress
7.4. Post-Ischemic Tau Protein versus Mitochondria
7.5. Post-Ischemic Tau Protein versus Autophagy
7.6. Post-Ischemic Tau Protein versus Apoptosis
7.7. Post-Ischemic Tau Protein versus Neuroinflammation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gemmell, E.; Bosomworth, H.; Allan, L.; Hall, R.; Khundakar, A.; Oakley, A.E.; Deramecourt, V.; Polvikoski, T.M.; O’Brien, J.; Kalaria, R.N. Hippocampal Neuronal Atrophy and Cognitive Function in Delayed Poststroke and Aging-Related Dementias. Stroke 2012, 43, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Gemmell, E.; Tam, E.; Allan, L.; Hall, R.; Khundakar, A.; Oakley, A.E.; Thomas, A.; Deramecourt, V.; Kalaria, R.N. Neuron Volumes in Hippocampal Subfields in Delayed Poststroke and Aging-Related Dementias. J. Neuropathol. Exp. Neurol. 2014, 73, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Portegies, M.L.; Wolters, F.J.; Hofman, A.; Ikram, M.K.; Koudstaal, P.J.; Ikram, M.A. Prestroke vascular pathology and the risk of re-current stroke and poststroke dementia. Stroke 2016, 47, 2119–2122. [Google Scholar] [CrossRef] [PubMed]
- Surawan, J.; Areemit, S.; Tiamkao, S.; Sirithanawuthichai, T.; Saensak, S. Risk factors associated with post-stroke dementia: A sys-tematic review and meta-analysis. Neurol. Int. 2017, 9, 7216. [Google Scholar] [CrossRef][Green Version]
- Kim, J.-H.; Lee, Y. Dementia and death after stroke in older adults during a 10-year follow-up: Results from a competing risk model. J. Nutr. Health Aging 2017, 22, 297–301. [Google Scholar] [CrossRef]
- Goulay, R.; Romo, L.M.; Hol, E.M.; Dijkhuizen, R.M. From Stroke to Dementia: A Comprehensive Review Exposing Tight Interactions between Stroke and Amyloid-β Formation. Transl. Stroke Res. 2019, 11, 601–614. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Rothwell, P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A sys-tematic review and meta-analysis. Lancet Neurol. 2009, 8, 1006–1018. [Google Scholar] [CrossRef]
- Thal, D.R.; Ghebremedhin, E.; Orantes, M.; Wiestler, O.D. Vascular Pathology in Alzheimer Disease: Correlation of Cerebral Amyloid Angiopathy and Arteriosclerosis/Lipohyalinosis with Cognitive Decline. J. Neuropathol. Exp. Neurol. 2003, 62, 1287–1301. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- De la Tremblaye, P.B.; Plamondon, H. Impaired conditioned emotional response and object recognition are concomitant to neuronal damage in the amygdale and perirhinal cortex in middle-aged ischemic rats. Behav. Brain Res. 2011, 219, 227–233. [Google Scholar] [CrossRef]
- Kiryk, A.; Pluta, R.; Figiel, I.; Mikosz, M.; Ulamek, M.; Niewiadomska, G.; Jabłoński, M.; Kaczmarek, L. Transient brain ischemia due to cardiac arrest causes irreversible long-lasting cognitive injury. Behav. Brain Res. 2010, 219, 1–7. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.-J.; Zhang, M.; Fang, C.-Q.; Zhou, H.-D. Cerebral ischemia aggravates cognitive impairment in a rat model of Alzheimer’s disease. Life Sci. 2011, 89, 86–92. [Google Scholar] [CrossRef]
- Pluta, R.; Jolkkonen, J.; Cuzzocrea, S.; Pedata, F.; Cechetto, D.; Popa-Wagner, A. Cognitive Impairment with Vascular Impairment and Degeneration. Curr. Neurovascular. Res. 2011, 8, 342–350. [Google Scholar] [CrossRef]
- Cohan, C.H.; Neumann, J.T.; Dave, K.R.; Alekseyenko, A.; Binkert, M.; Stransky, K.; Lin, H.W.; Barnes, C.A.; Wright, C.B.; Perez-Pinzon, M.A. Effect of Cardiac Arrest on Cognitive Impairment and Hippocampal Plasticity in Middle-Aged Rats. PLoS ONE 2015, 10, e0124918. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Jabłoński, M.; Ułamek, M. Factors in Creepy Delayed Neuronal Death in Hippocampus Following Brain Ischemia–Reperfusion Injury with Long-Term Survival. Acta Neurochir. Suppl. 2009, 106, 37–41. [Google Scholar] [CrossRef]
- Sekeljic, V.; Bataveljic, D.; Stamenkovic, S.; Ułamek, M.; Jabłoński, M.; Radenovic, L.; Pluta, R.; Andjus, P.R. Cellular markers of neu-roinflammation and neurogenesis after ischemic brain injury in the long-term survival rat model. Brain Struct. Funct. 2012, 217, 411–420. [Google Scholar] [CrossRef]
- Radenovic, L.; Nenadic, M.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J.; Andjus, P.R.; Pluta, R. Heterogeneity in brain distribution of activated microglia and astrocytes in a rat ischemic model of Alzheimer’s disease after 2 years of survival. Aging 2020, 12, 12251–12267. [Google Scholar] [CrossRef]
- Hossmann, K.A.; Schmidt-Kastner, R.; Ophoff, B.G. Recovery of integrative central nervous function after one hour global cere-bro-circulatory arrest in normothermic cat. J. Neurol. Sci. 1987, 77, 305–320. [Google Scholar] [CrossRef]
- Pluta, R. The role of apolipoprotein E in the deposition of β-amyloid peptide during ischemia–reperfusion brain injury. A model of early Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2000, 903, 324–334. [Google Scholar] [CrossRef]
- Pluta, R. Glial expression of the β-amyloid peptide in cardiac arrest. J. Neurol. Sci. 2002, 203-204, 277–280. [Google Scholar] [CrossRef]
- Pluta, R.; Ułamek, M.; Jabłoński, M. Alzheimer’s mechanisms in ischemic brain degeneration. Anat. Rec. 2009, 292, 1863–1881. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, M.; Maciejewski, R.; Januszewski, S.; Ułamek, M.; Pluta, R. One year follow up in ischemic brain injury and the role of Alzheimer factors. Physiol. Res. 2011, 60, S113–S119. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Salínska, E.; Puka, M.; Stafiej, A.; Lazarewicz, J. Early changes in extracellular amino acids and calcium concentrations in rabbit hippocampus following complete 15-min cerebral ischemia. Resuscitation 1988, 16, 193–210. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, B.; Yin, L.; Cai, B.; Shan, H.; Zhang, L.; Lu, Y.; Bi, Z. Tanshinone IIA Prevented Brain Iron Dyshomeostasis in Cerebral Ischemic Rats. Cell. Physiol. Biochem. 2011, 27, 23–30. [Google Scholar] [CrossRef]
- Pochwat, B.; Nowak, G.; Szewczyk, B. Relationship between Zinc (Zn2+) and Glutamate Receptors in the Processes Underlying Neurodegeneration. Neural Plast. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Yuan, Y.; Shan, X.; Men, W.; Zhai, H.; Qiao, X.; Geng, L.; Li, C. The effect of crocin on memory, hippocampal acetylcholine level, and apoptosis in a rat model of cerebral ischemia. Biomed. Pharmacother. 2020, 130, 110543. [Google Scholar] [CrossRef]
- Pluta, R.; Furmaga-Jabłońska, W.; Maciejewski, R.; Ułamek-Kozioł, M.; Jabłoński, M. Brain Ischemia Activates β- and γ-Secretase Cleavage of Amyloid Precursor Protein: Significance in Sporadic Alzheimer’s Disease. Mol. Neurobiol. 2012, 47, 425–434. [Google Scholar] [CrossRef]
- Pluta, R.; Jabłonski, M.; Ułamek-Kozioł, M.; Kocki, J.; Brzozowska, J.; Januszewski, S.; Furmaga-Jabłonska, W.; Bogucka-Kocka, A.; Maciejewski, R.; Czuczwar, S.J. Sporadic, Alzheimer’s disease begins as episodes of brain ischemia and ischemically dysregulated Alzheimer’s disease genes. Mol. Neurobiol. 2013, 48, 500–515. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Kocki, J.; Bogucka-Kocka, A.; Petniak, A.; Gil-Kulik, P.; Januszewski, S.; Bogucki, J.; Jabłoński, M.; Furmaga-Jabłońska, W.; Brzozowska, J.; et al. Dysregulation of autophagy, mitophagy and apoptotic genes in the medial temporal lobe cortex in an ischemic model of Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 54, 113–121. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Kocki, J.; Bogucka-Kocka, A.; Januszewski, S.; Bogucki, J.; Czuczwar, S.J.; Pluta, R. Autophagy, mitophagy and apoptotic gene changes in the hippocampal CA1 area in a rat ischemic model of Alzheimer’s disease. Pharmacol. Rep. 2017, 69, 1289–1294. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Kocki, J.; Januszewski, S.; Bogucki, J.; Bogucka-Kocka, A.; Pluta, R. Dysregulation of autophagy, mitophagy, and apoptosis genes in the CA3 region of the hippocampus in the ischemic model of Alzheimer’s disease in the rat. J. Alzheimer’s Dis. 2019, 72, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Maida, C.; Norrito, R.; Daidone, M.; Tuttolomondo, A.; Pinto, A. Neuroinflammatory Mechanisms in Ischemic Stroke: Focus on Cardioembolic Stroke, Background, and Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 6454. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Bogucka-Kocka, A.; Ułamek-Kozioł, M.; Bogucki, J.; Czuczwar, S.J. Ischemic tau protein gene induction as an additional key factor driving development of Alzheimer’s phenotype changes in CA1 area of hippocampus in an ischemic model of Alz-heimer’s disease. Pharmacol. Rep. 2018, 70, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J. Tau Protein Dysfunction after Brain Ischemia. J. Alzheimer’s Dis. 2018, 66, 429–437. [Google Scholar] [CrossRef]
- Pluta, R. Brain Ischemia: Alzheimer’s Disease Mechanisms; Nova, Science Publishers, Inc.: New York, NY, USA, 2019; p. 311. [Google Scholar]
- Pluta, R.; Ułamek-Kozioł, M.; Kocki, J.; Bogucki, J.; Januszewski, S.; Bogucka-Kocka, A.; Czuczwar, S.J. Expression of the Tau Protein and Amyloid Protein Precursor Processing Genes in the CA3 Area of the Hippocampus in the Ischemic Model of Alzheimer’s Disease in the Rat. Mol. Neurobiol. 2019, 57, 1281–1290. [Google Scholar] [CrossRef]
- Hecht, M.; Krämer, L.M.; Von Arnim, C.A.F.; Otto, M.; Thal, D.R. Capillary cerebral amyloid angiopathy in Alzheimer’s disease: Association with allocortical/hippocampal microinfarcts and cognitive decline. Acta Neuropathol. 2018, 135, 681–694. [Google Scholar] [CrossRef]
- Wisniewski, H.M.; Pluta, R.; Lossinsky, A.S.; Mossakowski, M.J. Ultrastructural studies of cerebral vascular spasm after cardiac arrest-related global cerebral ischemia in rats. Acta Neuropathol. 1995, 90, 432–440. [Google Scholar] [CrossRef]
- Dewar, D.; Graham, D.I.; Teasdale, G.M.; McCulloch, J. Alz-50 and ubiquitin immunoreactivity is induced by permanent focal cerebral ischaemia in the cat. Acta Neuropathol. 1993, 86, 623–629. [Google Scholar] [CrossRef]
- Dewar, D.; Graham, D.; Teasdale, G.; McCulloch, J. Cerebral Ischemia Induces Alterations in Tau and Ubiquitin Proteins. Dement. Geriatr. Cogn. Disord. 1994, 5, 168–173. [Google Scholar] [CrossRef]
- Dewar, D.; Dawson, D. Tau protein is altered by focal cerebral ischaemia in the rat: An immunohistochemical and immunoblotting study. Brain Res. 1995, 684, 70–78. [Google Scholar] [CrossRef]
- Geddes, J.W.; Schwab, C.; Craddock, S.; Wilson, J.L.; Pettigrew, L.C. Alterations in tau immunostaining in the rat hippocampus following transient cerebral ischemia. J. Cereb. Blood Flow Metab. 1994, 14, 554–564. [Google Scholar] [CrossRef]
- Irving, E.A.; Yatsushiro, K.; McCulloch, J.; Dewar, D. Rapid alteration of tau in oligodendrocytes after focal ischemic injury in the rat: Involvement of free radicals. J. Cereb. Blood Flow Metab. 1997, 17, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Sinigaglia-Coimbra, R.; Cavalheiro, E.A.; Coimbra, C.G. Postischemic hypertermia induces Alzheimer like pathology in the rat brain. Acta Neuropathol. 2002, 103, 444–452. [Google Scholar] [CrossRef]
- Uchihara, T.; Tsuchiya, K.; Kondo, H.; Hayama, T.; Ikeda, K. Widespread appearance of Alz-50 immunoreactive neurons in the human brain with cerebral infarction. Stroke 1995, 26, 2145–2148. [Google Scholar] [CrossRef]
- Irving, E.; Nicoll, J.; Graham, D.; Dewar, D. Increased tau immunoreactivity in oligodendrocytes following human stroke and head injury. Neurosci. Lett. 1996, 213, 189–192. [Google Scholar] [CrossRef]
- Uchihara, T.; Nakamura, A.; Arai, T.; Ikeda, K.; Tsuchiya, K. Microglial tau undergoes phosphorylation-independent modification after ischemia. Glia 2003, 45, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Majd, S.; Power, J.H.; Koblar, S.A.; Grantham, H.J.M. Introducing a developed model of reversible cardiac arrest to produce global brain ischemia and its impact on microtubule-associated protein tau phosphorylation at Ser396. Int. J. Neurol. Neurother. 2016, 3, 040. [Google Scholar] [CrossRef]
- Fujii, H.; Takahashi, T.; Mukai, T.; Tanaka, S.; Hosomi, N.; Maruyama, H.; Sakai, N.; Matsumoto, M. Modifications of tau protein after cerebral ischemia and reperfusion in rats are similar to those occurring in Alzheimer’s disease—Hyperphosphorylation and cleavage of 4- and 3-repeat tau. Br. J. Pharmacol. 2016, 37, 2441–2457. [Google Scholar] [CrossRef] [PubMed]
- Stamer, K.; Vogel, R.; Thies, E.; Mandelkow, E.-M. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J. Cell Biol. 2002, 156, 1051–1063. [Google Scholar] [CrossRef]
- Schiefecker, A.J.; Putzer, G.; Braun, P.; Martini, J.; Strapazzon, G.; Antunes, A.P.; Mulino, M.; Pinggera, D.; Glodny, B.; Brugger, H.; et al. Total TauProtein as Investigated by Cerebral Microdialysis Increases in Hypothermic Cardiac Arrest: A Pig Study. Ther. Hypothermia Temp. Manag. 2021, 11, 28–34. [Google Scholar] [CrossRef]
- Mörtberg, E.; Zetterberg, H.; Nordmark, J.; Blennow, K.; Catry, C.; Decraemer, H.; Vanmechelen, E.; Rubertsson, S. Plasma tau protein in comatose patients after cardiac arrest treated with therapeutic hypothermia. Acta Anaesthesiol. Scand. 2011, 55, 1132–1138. [Google Scholar] [CrossRef]
- Randall, J.; Mörtberg, E.; Provuncher, G.K.; Fournier, D.R.; Duffy, D.C.; Rubertsson, S.; Blennow, K.; Zetterberg, H.; Wilson, D.H. Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: Results of a pilot study. Resuscitation 2012, 84, 351–356. [Google Scholar] [CrossRef]
- Bitsch, A.; Horn, C.; Kemmling, Y.; Seipelt, M.; Hellenbrand, U.; Stiefel, M.; Ciesielczyk, B.; Cepek, L.; Bahn, E.; Ratzka, P.; et al. Serum tau protein level as a marker of axonal damage in acute ischemic stroke. Eur. Neurol. 2002, 47, 45–51. [Google Scholar] [CrossRef]
- Kurzepa, J.; Bielewicz, J.; Grabarska, A.; Stelmasiak, Z.; Stryjecka-Zimmer, M.; Bartosik-Psujek, H. Matrix metalloproteinase-9 contributes to the increase of tau protein in serum during acute ischemic stroke. J. Clin. Neurosci. 2010, 17, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Bielewicz, J.; Kurzepa, J.; Czekajska-Chehab, E.; Stelmasiak, Z.; Bartosik-Psujek, H. Does serum tau protein predict the outcome of patients with ischemic stroke? J. Mol. Neurosci. 2011, 43, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Lasek-Bal, A.; Jedrzejowska-Szypulka, H.; Różycka, J.; Bal, W.; Kowalczyk, A.; Holecki, M.; Dulawa, J.; Lewin-Kowalik, J. The presence of Tau protein in blood as a potential prognostic factor in stroke patients. J. Physiol. Pharmacol. 2016, 67, 691–696. [Google Scholar]
- De Vos, A.; Bjerke, M.; Brouns, R.; De Roeck, N.; Jacobs, D.; Van den Abbeele, L.; Guldolf, K.; Zetterberg, H.; Blennow, K.; Engelborghs, S.; et al. Neurogranin and tau in cerebrospinal fluid and plasma of patients with acute ischemic stroke. BMC Neurol. 2017, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Mailliot, C.; Podevin-Dimster, V.; Rosenthal, R.E.; Sergeant, N.; Delacourte, A.; Fiskum, G.; Buée, L. Rapid tau protein dephosphorylation and differential rephosphorylation during cardiac arrest-induced cerebral ischemia and reperfusion. J. Cereb. Blood Flow Metab. 2000, 20, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Yang, S.; Liu, R.; Simpkins, J.W. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain Res. 2004, 1022, 30–38. [Google Scholar] [CrossRef]
- Morioka, M.; Kawano, T.; Yano, S.; Kai, Y.; Tsuiki, H.; Yoshinaga, Y.; Matsumoto, J.; Maeda, T.; Hamada, J.; Yamamoto, H.; et al. Hyperphosphorylation at serine 199/202 of tau factor in the gerbil hippocampus after transient forebrain ischemia. Biochem. Biophys. Res. Commun. 2006, 347, 273–278. [Google Scholar] [CrossRef]
- Wen, Y.; Yang, S.; Liu, R.; Brun-Zinkernagel, A.M.; Koulen, P.; Simpkins, J.W. Transient Cerebral Ischemia Induces Aberrant Neuronal Cell Cycle Re-entry and Alzheimer’s Disease-like Tauopathy in Female Rats. J. Biol. Chem. 2004, 279, 22684–22692. [Google Scholar] [CrossRef]
- Wen, Y.; Yang, S.-H.; Liu, R.; Perez, E.J.; Brun-Zinkernagel, A.M.; Koulen, P.; Simpkins, J.W. Cdk5 is involved in NFT-like tauopathy induced by transient cerebral ischemia in female rats. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2007, 1772, 473–483. [Google Scholar] [CrossRef]
- Majd, S.; Power, J.H.T.; Koblar, S.; Grantham, H. Early glycogen synthase kinase-3β and protein phosphatase 2A independent tau dephosphorylation during global brain ischaemia and reperfusion following cardiac arrest and the role of the adenosine monophosphate kinase pathway. Eur. J. Neurosci. 2016, 44, 1987–1997. [Google Scholar] [CrossRef]
- Kovalska, M.; Tothova, B.; Kovalska, L.; Tatarkova, Z.; Kalenska, D.; Tomascova, A.; Adamkov, M.; Lehotsky, J. Association of Induced Hyperhomocysteinemia with Alzheimer’s Disease-Like Neurodegeneration in Rat Cortical Neurons After Global Ischemia-Reperfusion Injury. Neurochem. Res. 2018, 43, 1766–1778. [Google Scholar] [CrossRef]
- Gordon-Krajcer, W.; Kozniewska, E.; Lazarewicz, J.W.; Ksiezak-Reding, H. Differential changes in phosphorylation of tau at PHF-1 and 12E8 epitopes during brain ischemia and reperfusion in gerbils. Neurochem. Res. 2007, 32, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Basurto-Islas, G.; Gu, J.-H.; Tung, Y.C.; Liu, F.; Iqbal, K. Mechanism of Tau Hyperphosphorylation Involving Lysosomal Enzyme Asparagine Endopeptidase in a Mouse Model of Brain Ischemia. J. Alzheimers Dis. 2018, 63, 821–833. [Google Scholar] [CrossRef]
- Khan, S.; Yuldasheva, N.Y.; Batten, T.F.C.; Pickles, A.R.; Kellett, K.A.B.; Saha, S. Tau pathology and neurochemical changes associ-ated with memory dysfunction in an optimized murine model of global cerebral ischaemia—A potential model for vascular dementia? Neurochem. Int. 2018, 118, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, S.N.; Hachinski, V.C.; Cechetto, D.F. Interaction between a rat model of cerebral ischemia and beta-amyloid toxicity: Inflammatory responses. Stroke 2005, 36, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hirano, A.; Katagiri, T.; Sasaki, H.; Yamada, S. Neurofibrillary tangle formation in the nucleus basalis of meynert ipsilateral to a massive cerebral infarct. Ann. Neurol. 1988, 23, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Hatsuta, H.; Takao, M.; Nogami, A.; Uchino, A.; Sumikura, H.; Takata, T.; Morimoto, S.; Kanemaru, K.; Adachi, T.; Arai, T.; et al. Tau and TDP-43 accumulation of the basal nucleus of Meynert in individuals with cerebral lobar infarcts or hemorrhage. Acta Neuropathol. Commun. 2019, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Kovac, A.; Zilkova, M.; Deli, M.A.; Zilka, N.; Novak, M. Human truncated tau is using a different mechanism from amyloid-beta to damage the blood-brain barrier. J. Alzheimer’s Dis. 2009, 18, 897–906. [Google Scholar] [CrossRef]
- Kovac, A.; Zilka, N.; Kazmerova, Z.; Cente, M.; Zilkova, M.; Novak, M. Misfolded Truncated Protein τ Induces Innate Immune Response via MAPK Pathway. J. Immunol. 2011, 187, 2732–2739. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, H. Tau as a potential therapeutic target for ischemic stroke. Aging 2019, 11, 12827–12843. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.; Maeda, S.; Vossel, K.; Mucke, L. The Many Faces of Tau. Neuron 2011, 70, 410–426. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, K.K.; Beard, D.C.; Lehman, R.A.; Billingsley, M.L. Alterations in Tau Phosphorylation in Rat and Human Neocortical Brain Slices Following Hypoxia and Glucose Deprivation. Exp. Neurol. 1998, 154, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, D.A.; Yeh, R.Y. Dephosphorylation of tau during transient forebrain ischemia in the rat. Mol. Chem. Neuropathol. 1998, 34, 103–120. [Google Scholar] [CrossRef]
- Gao, Y.-L.; Wang, N.; Sun, F.-R.; Cao, X.-P.; Zhang, W.; Yu, J.-T. Tau in neurodegenerative disease. Ann. Transl. Med. 2018, 6, 175. [Google Scholar] [CrossRef]
- Tuo, Q.Z.; Lei, P.; Jackman, K.A.; Li, X.L.; Xiong, H.; Li, X.L.; Liuyang, Z.Y.; Roisman, L.; Zhang, S.T.; Ayton, S.; et al. Tau mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatry 2017, 22, 1520–1530. [Google Scholar] [CrossRef]
- Bi, M.; Gladbach, A.; van Eersel, J.; Ittner, A.; Przybyla, M.; van Hummel, A.; Chua, S.W.; van der Hoven, J.; Lee, W.S.; Muller, J.; et al. Tau exacerbates excitotoxic brain damage in an animal model of stroke. Nat. Commun. 2017, 8, 473. [Google Scholar] [CrossRef]
- Pluta, R.; Lossinsky, A.; Wisniewski, H.; Mossakowski, M. Early blood-brain barrier changes in the rat following transient complete cerebral ischemia induced by cardiac arrest. Brain Res. 1994, 633, 41–52. [Google Scholar] [CrossRef]
- Pluta, R. Blood-brain barrier dysfunction and amyloid precursor protein accumulation in microvascular compartment following ischemia-reperfusion brain injury with 1-year survival. Acta Neurochir. Suppl. 2003, 86, 117–122. [Google Scholar] [CrossRef]
- Pluta, R. Pathological Opening of the Blood-Brain Barrier to Horseradish Peroxidase and Amyloid Precursor Protein following Ischemia-Reperfusion Brain Injury. Chemotherapy 2005, 51, 223–226. [Google Scholar] [CrossRef]
- Pluta, R.; Ułamek, M.; Januszewski, S. Micro-blood-brain barrier openings and cytotoxic fragments of amyloid precursor protein accumulation in white matter after ischemic brain injury in long-lived rats. Pain 2006, 96, 267–271. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Ulamek, M. Ischemic blood-brain barrier and amyloid in white matter as etiological factors in leukoaraiosis. Acta Neurochir. Suppl. 2008, 102, 353–356. [Google Scholar] [CrossRef]
- Ramos-Cejudo, J.; Wisniewski, T.; Marmar, C.; Zetterberg, H.; Blennow, K.; de Leon, M.J.; Fossati, S. Traumatic brain injury and Alzheimer’s disease: The cerebrovascular link. EBio Med. 2018, 28, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Bang, O.Y.; Hwang, E.M.; Lee, J.S.; Joo, U.S.; Mook-Jung, I.; Huh, K. Circulating beta amyloid protein is elevated in patients with acute ischemic stroke. J. Neural. Transm. 2005, 112, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, H.; Mortberg, E.; Song, L.; Chang, L.; Provuncher, G.K.; Patel, P.P.; Ferrell, E.; Fournier, D.R.; Kan, C.W.; Campbell, T.G.; et al. Hypoxia due to cardiac arrest induces a time-dependent increase in serum amyloidβ levels in humans. PLoS ONE 2011, 6, e28263. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Stetler, R.A.; Leak, R.; Shi, Y.; Li, Y.; Yu, W.; Bennett, M.V.; Chen, J. Oxidative stress and DNA damage after cerebral ischemia: Potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology 2017, 134, 208–217. [Google Scholar] [CrossRef]
- Kumfu, S.; Charununtakorn, S.T.; Jaiwongkam, T.; Chattipakorn, N.; Chattipakorn, S.C. Humanin Exerts Neuroprotection During Cardiac Ischemia-Reperfusion Injury. J. Alzheimer’s Dis. 2018, 61, 1343–1353. [Google Scholar] [CrossRef]
- Banks, W.A.; Kovac, A.; Majerova, P.; Bullock, K.M.; Shi, M.; Zhang, J. Tau Proteins Cross the Blood-Brain Barrier. J. Alzheimer’s Dis. 2016, 55, 411–419. [Google Scholar] [CrossRef]
- Ueno, M.; Chiba, Y.; Murakami, R.; Matsumoto, K.; Kawauchi, M.; Fujihara, R. Blood-brain barrier and blood–cerebrospinal fluid barrier in normal and pathological conditions. Brain Tumor Pathol. 2016, 33, 89–96. [Google Scholar] [CrossRef]
- Ojo, O.B.; Amoo, Z.A.; Saliu, I.O.; Olaleye, M.T.; Farombi, E.O.; Akinmoladun, A.C. Neurotherapeutic potential of kolaviron on neurotransmitter dysregulation, excitotoxicity, mitochondrial electron transport chain dysfunction and redox imbalance in 2-VO brain ischemia/reperfusion injury. Biomed. Pharmacother. 2019, 111, 859–872. [Google Scholar] [CrossRef]
- Tejeda, G.S.; Esteban-Ortega, G.M.; San Antonio, E.; Vidaurre, O.G.; Díaz-Guerra, M. Prevention of excitotoxicity-induced processing of BDNF receptor TrkB-FL leads to stroke neuroprotection. EMBO Mol. Med. 2019, 11, e9950. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.I.; Ortiz, D.; Rogers, E.; Shea, T.B. Multiple aspects of homocysteine neurotoxicity: Glutamate excitotoxicity, kinase hyperactivation and DNA damage. J. Neurosci. Res. 2002, 70, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, F.J.; Malik, K.U.; Shea, T.B. Activation of the L voltage-sensitive calcium channel by mitogen-activated protein (MAP) kinase following exposure of neuronal cells to beta-amyloid. MAP kinase mediates beta-amyloid-induced neurodegeneration. J. Biol. Chem. 1999, 274, 30322–30327. [Google Scholar] [CrossRef] [PubMed]
- Petroni, D.; Tsai, J.; Mondal, D.; George, W. Attenuation of low dose methylmercury and glutamate induced-cytotoxicity and tau phosphorylation by anN-methyl-D-aspartate antagonist in human neuroblastoma (SHSY5Y) cells. Environ. Toxicol. 2011, 28, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Amadoro, G.; Ciotti, M.T.; Costanzi, M.; Cestari, V.; Calissano, P.; Canu, N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc. Natl. Acad. Sci. USA 2006, 103, 2892–2897. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef]
- Holth, J.K.; Bomben, V.C.; Reed, J.G.; Inoue, T.; Younkin, L.; Younkin, S.G.; Pautler, R.G.; Botas, J.; Noebels, J.L. Tau Loss Attenuates Neuronal Network Hyperexcitability in Mouse and Drosophila Genetic Models of Epilepsy. J. Neurosci. 2013, 33, 1651–1659. [Google Scholar] [CrossRef]
- Mehta, A.; Prabhakar, M.; Kumar, P.; Deshmukh, R.; Sharma, P. Excitotoxicity: Bridge to various triggers in neurodegenerative disorders. Eur. J. Pharmacol. 2012, 698, 6–18. [Google Scholar] [CrossRef]
- Hunsberger, H.C.; Rudy, C.C.; Batten, S.R.; Gerhardt, G.A.; Reed, M.N. P301L tau expression affects glutamate release and clearance in the hippocampal trisynaptic pathway. J. Neurochem. 2015, 132, 169–182. [Google Scholar] [CrossRef]
- Pallo, S.P.; DiMaio, J.; Cook, A.; Nilsson, B.; Johnson, G.V. Mechanisms of tau and Aβ-induced excitotoxicity. Brain Res. 2015, 1634, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Decker, J.M.; Krüger, L.; Sydow, A.; Dennissen, F.J.; Siskova, Z.; Mandelkow, E.; Mandelkow, E.M. The Tau/A152T mutation, a risk factor for frontotemporal-spectrum disorders, leads to NR2B receptor-mediated excitotoxicity. EMBO Rep. 2016, 17, 552–569. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Stein, L.; Thomas, R.; Djukic, B.; Taneja, P.; Knox, J.; Vossel, K.; Mucke, L. Phosphorylation of tau at Y18, but not tau-fyn binding, is required for tau to modulate NMDA receptor-dependent excitotoxicity in primary neuronal culture. Mol. Neurodegener. 2017, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yu, G.; Chi, L.; Zhu, J.; Zhang, W.; Zhang, Y.; Zhang, L. Neuroprotective effects of edaravone on cognitive deficit, oxidative stress and tau hyperphosphorylation induced by intracerebroventricular streptozotocin in rats. NeuroToxicology 2013, 38, 136–145. [Google Scholar] [CrossRef]
- Kang, S.-W.; Kim, S.J.; Kim, M.-S. Oxidative stress with tau hyperphosphorylation in memory impaired 1,2-diacetylbenzene-treated mice. Toxicol. Lett. 2017, 279, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.; Adlard, P.A.; Morten, K.; Johnson, F.; Golden, T.R.; Hinerfeld, D.; Schilling, B.; Mavros, C.; Masters, C.L.; Volitakis, I.; et al. Mitochondrial Oxidative Stress Causes Hyperphosphorylation of Tau. PLoS ONE 2007, 2, e536. [Google Scholar] [CrossRef]
- Chen, S.; Liu, A.-R.; An, F.-M.; Yao, W.-B.; Gao, X.-D. Amelioration of neurodegenerative changes in cellular and rat models of diabetes-related Alzheimer’s disease by exendin-4. AGE 2011, 34, 1211–1224. [Google Scholar] [CrossRef]
- Clausen, A.; Xu, X.; Bi, X.; Baudry, M. Effects of the Superoxide Dismutase/Catalase Mimetic EUK-207 in a Mouse Model of Alzheimer’s Disease: Protection Against and Interruption of Progression of Amyloid and Tau Pathology and Cognitive Decline. J. Alzheimer’s Dis. 2012, 30, 183–208. [Google Scholar] [CrossRef]
- Sanderson, T.H.; Reynolds, C.; Kumar, R.; Przyklenk, K.; Hüttemann, M. Molecular Mechanisms of Ischemia–Reperfusion Injury in Brain: Pivotal Role of the Mitochondrial Membrane Potential in Reactive Oxygen Species Generation. Mol. Neurobiol. 2012, 47, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Du Boff, B.; Götz, J.; Feany, M.B. Tau promotes neurodegeneration via DRP1 mislocalization in vivo. Neuron 2012, 75, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Fujioka, H.; Hoppel, C.; Whone, A.; Caldwell, M.; Cullen, P.; Liu, J.; Zhu, X. Parkinson’s disease–associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat. Med. 2015, 22, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Manczak, M.; Fry, D.; Suneetha, Y.; Sesaki, H.; Reddy, P.H. Reduced dynamin-related protein 1 protects against phosphorylated Tau-induced mitochondrial dysfunction and synaptic damage in Alzheimer’s disease. Hum. Mol. Genet. 2016, 25, 4881–4897. [Google Scholar] [CrossRef] [PubMed]
- Kopeikina, K.J.; Carlson, G.A.; Pitstick, R.; Ludvigson, A.; Peters, A.; Luebke, J.; Koffie, R.M.; Frosch, M.P.; Hyman, B.T.; Spires-Jones, T. Tau Accumulation Causes Mitochondrial Distribution Deficits in Neurons in a Mouse Model of Tauopathy and in Human Alzheimer’s Disease Brain. Am. J. Pathol. 2011, 179, 2071–2082. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Nguyen, H.M.; Maezawa, I.; Grössinger, E.M.; Garing, A.L.; Kohler, R.; Jin, L.-W.; Wulff, H. The potassium channel KCa3.1 constitutes a pharmacological target for neuroinflammation associated with ischemia/reperfusion stroke. Br. J. Pharmacol. 2016, 36, 2146–2161. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, L.; Yu, J.-T. Axonal Transport Defects in Alzheimer’s Disease. Mol. Neurobiol. 2014, 51, 1309–1321. [Google Scholar] [CrossRef]
- Li, X.-C.; Xia-Chun, L.; Wang, Z.-H.; Luo, Y.; Zhang, Y.; Liu, X.-P.; Feng, Q.; Wang, Q.; Ye, K.; Liu, G.-P.; et al. Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins. Sci. Rep. 2016, 6, 24756. [Google Scholar] [CrossRef]
- Ittner, L.M.; Ke, Y.; Götz, J. Phosphorylated Tau Interacts with c-Jun N-terminal Kinase-interacting Protein 1 (JIP1) in Alzheimer Disease. J. Biol. Chem. 2009, 284, 20909–20916. [Google Scholar] [CrossRef]
- Kanaan, N.; Morfini, G.A.; Lapointe, N.E.; Pigino, G.F.; Patterson, K.R.; Song, Y.; Andreadis, A.; Fu, Y.; Brady, S.T.; Binder, L.I. Pathogenic Forms of Tau Inhibit Kinesin-Dependent Axonal Transport through a Mechanism Involving Activation of Axonal Phosphotransferases. J. Neurosci. 2011, 31, 9858–9868. [Google Scholar] [CrossRef]
- Ibáñez-Salazar, A.; Bañuelos-Hernandez, B.; Rodriguez-Leyva, I.; Chi-Ahumada, E.; Monreal-Escalante, E.; Jiménez-Capdeville, M.E.; Rosales-Mendoza, S. Oxidative Stress Modifies the Levels and Phosphorylation State of Tau Protein in Human Fibroblasts. Front. Neurosci. 2017, 11, 495. [Google Scholar] [CrossRef]
- Boland, B.; Kumar, A.; Lee, S.; Platt, F.M.; Wegiel, J.; Yu, W.H.; Nixon, R.A. Autophagy induction and autophagosome clearance in neurons: Relationship to autophagic pathology in Alzheimer’s disease. J. Neurosci. 2008, 28, 6926–6937. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.L.; Matus, S.; Bargsted, L.; Hetz, C. Targeting autophagy in neurodegenerative diseases. Trends Pharmacol. Sci. 2014, 35, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Maday, S.; Holzbaur, E.L.F. Compartment-Specific Regulation of Autophagy in Primary Neurons. J. Neurosci. 2016, 36, 5933–5945. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, X.; Shen, J. Reactive nitrogen species as therapeutic targets for autophagy: Implication for ischemic stroke. Expert Opin. Ther. Targets 2017, 21, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Koike, M.A.; Green, K.N.; Blurton-Jones, M.; Laferla, F.M. Oligemic hypoperfusion differentially affects tau and amyloid-beta. Am. J Pathol. 2010, 177, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Huuskonen, M.T.; Loppi, S.; Dhungana, H.; Keksa-Goldsteine, V.; Lemarchant, S.; Korhonen, P.; Wojciechowski, S.; Pollari, E.; Valonen, P.; Koponen, J.; et al. Bexarotene targets autophagy and is protective against thromboembolic stroke in aged mice with tauopathy. Sci. Rep. 2016, 6, 33176. [Google Scholar] [CrossRef] [PubMed]
- Falcon, B.; Noad, J.; McMahon, H.; Randow, F.; Goedert, M. Galectin-8–mediated selective autophagy protects against seeded tau aggregation. J. Biol. Chem. 2018, 293, 2438–2451. [Google Scholar] [CrossRef]
- Scott, I.S.; Lowe, J.S. The ubiquitin-binding protein p62 identifies argyrophilic grain pathology with greater sensitivity than conventional silver stains. Acta Neuropathol. 2006, 113, 417–420. [Google Scholar] [CrossRef]
- Ozcelik, S.; Fraser, G.; Castets, P.; Schaeffer, V.; Skachokova, Z.; Breu, K.; Clavaguera, F.; Sinnreich, M.; Kappos, L.; Goedert, M.; et al. Rapamycin Attenuates the Progression of Tau Pathology in P301S Tau Transgenic Mice. PLoS ONE 2013, 8, e62459. [Google Scholar] [CrossRef]
- Xu, Y.; Martini-Stoica, H.; Zheng, H. A seeding based cellular assay of tauopathy. Mol. Neurodegener. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Hasegawa, M. Molecular Mechanisms in the Pathogenesis of Alzheimer’s disease and Tauopathies-Prion-Like Seeded Aggregation and Phosphorylation. Biomolecules 2016, 6, 24. [Google Scholar] [CrossRef]
- Ghetti, B.; Oblak, A.L.; Boeve, B.F.; Johnson, K.A.; Dickerson, B.C.; Goedert, M. Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: A chameleon for neuropathology and neuroimaging. Neuropathol. Appl. Neurobiol. 2014, 41, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Gusev, G.P.; Govekar, R.; Gadewal, N.; Agalakova, N.I. Understanding quasi-apoptosis of the most numerous enucleated components of blood needs detailed molecular autopsy. Ageing Res. Rev. 2017, 35, 46–62. [Google Scholar] [CrossRef]
- Ma, X.; Liu, L.; Meng, J. MicroRNA-125b promotes neurons cell apoptosis and Tau phosphorylation in Alzheimer’s disease. Neurosci. Lett. 2017, 661, 57–62. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, W.; Wang, P.; Chu, J. Asiatic acid protects differentiated PC12 cells from Aβ25–35-induced apoptosis and tau hyperphosphorylation via regulating PI3K/Akt/GSK-3β signaling. Life Sci. 2018, 208, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Zhang, F.; Zhu, B.; Liu, C.; Lin, Z.; Wang, H.; Xie, W.-B. CDK5-mediated tau accumulation triggers methamphetamine-induced neuronal apoptosis via endoplasmic reticulum-associated degradation pathway. Toxicol. Lett. 2018, 292, 97–107. [Google Scholar] [CrossRef]
- Zilka, N.; Kazmerova, Z.; Jadhav, S.; Neradil, P.; Madari, A.; Obetkova, D.; Bugos, O.; Novak, M. Who fans the flames of Alz-heimer’s disease brains? Misfolded tau on the crossroad of neurodegenerative and inflammatory pathways. J. Neuroinflam. 2012, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Asai, H.; Ikezu, S.; Woodbury, M.E.; Yonemoto, G.M.; Cui, L.; Ikezu, T. Accelerated Neurodegeneration and Neuroinflammation in Transgenic Mice Expressing P301L Tau Mutant and Tau-Tubulin Kinase 1. Am. J. Pathol. 2014, 184, 808–818. [Google Scholar] [CrossRef]
- Majerova, P.; Zilkova, M.; Kazmerova, Z.; Kovac, A.; Paholikova, K.; Kovacech, B.; Zilka, N.; Novak, M. Microglia display modest phagocytic capacity for extracellular tau oligomers. J. Neuroinflam. 2014, 11, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Barger, S.; Griffin, W.S.T. Interleukin-1 Mediates Pathological Effects of Microglia on Tau Phosphorylation and on Synaptophysin Synthesis in Cortical Neurons through a p38-MAPK Pathway. J. Neurosci. 2003, 23, 1605–1611. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Kitazawa, M.; Oddo, S.; Yamasaki, T.R.; Green, K.N.; LaFerla, F.M. Lipopolysaccharide-induced inflammation exacer-bates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J. Neurosci. 2005, 25, 8843–8853. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Mastrangelo, M.A.; Park, K.M.; Sudol, K.L.; Narrow, W.C.; Oddo, S.; LaFerla, F.M.; Callahan, L.M.; Federoff, H.J.; Bowers, W.J. Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am. J. Pathol. 2008, 173, 1768–1782. [Google Scholar] [CrossRef]
- Sy, M.; Kitazawa, M.; Medeiros, R.; Whitman, L.; Cheng, D.; Lane, T.E.; LaFerla, F.M. Inflammation Induced by Infection Potentiates Tau Pathological Features in Transgenic Mice. Am. J. Pathol. 2011, 178, 2811–2822. [Google Scholar] [CrossRef] [PubMed]
- Maphis, N.; Xu, G.; Kokiko-Cochran, O.N.; Cardona, A.E.; Ransohoff, R.M.; Lamb, B.T.; Bhaskar, K. Loss of tau rescues inflammation-mediated neurodegeneration. Front. Neurosci. 2015, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, M.A.; Sudol, K.L.; Narrow, W.C.; Bowers, W.J. Interferon-gamma differentially affects Alzheimer’s disease pathologies and induces neurogenesis in triple transgenic-AD mice. Am. J. Pathol. 2009, 175, 2076–2088. [Google Scholar] [CrossRef] [PubMed]
- Bolós, M.; Llorens-Martín, M.; Jurado-Arjona, J.; Hernández, F.; Rábano, A.; Avila, J. Direct Evidence of Internalization of Tau by Microglia In Vitro and In Vivo. J. Alzheimer’s Dis. 2015, 50, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Ułamek-Kozioł, M. Genes associated with Alzheimer’s disease affecting ischemic neurodegeneration of the hippocampal CA3 region. Neural. Regen. Res. 2021, 16, 1392–1393. [Google Scholar] [CrossRef]
- Pluta, R.; Ouyang, L.; Januszewski, S.; Li, Y.; Czuczwar, S.J. Participation of amyloid and tau protein in post-ischemic neurodegeneration of the hippocampus of a aature identical to Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 2460. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. Brain ischemia as a prelude to Alzheimer’s disease. Front. Aging Neurosci. 2021, 13, 636653. [Google Scholar] [CrossRef]
| Days | 2 | 7 | 30 | |
|---|---|---|---|---|
| Area | ||||
| CA1 | ↑↑↑ | ↓ | ↓ | |
| CA3 | ↓ | ↑ | ↑ | |
| Tau Protein | Ischemia | Human/Animal | Sites of Phosphorylation | Reference |
|---|---|---|---|---|
| Hyperphosphorylation | Focal | Rat | Asp421, pT181, pT205 pT212, pT231, pS202 pS214, pS262, pS396, pS404, pS422 | [49,60] |
| Hyperphosphorylation | Global | Rat | Ser202, Ser262, Ser396 Thr205 | [48,65] |
| Hyperphosphorylation | Forebrain | Gerbil | Ser199, Ser202 | [61,66] |
| Hyperphosphorylation | Focal | Mouse | Ser262, Ser 356 | [67] |
| Hyperphosphorylation | Stroke | Human | Ser101 | [47] |
| Paired helical filaments | Forebrain | Mouse | pS396, pS404 | [68] |
| Fibrillar tau protein | Focal + amyloid | Rat | Tau 2 | [69] |
| Neurofibrillary tangle-like | Focal | Rat | P-396, P-404 | [63] |
| Neurofibrillary tangles | Stroke | Human | Tau 1 | [70,71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pluta, R.; Czuczwar, S.J.; Januszewski, S.; Jabłoński, M. The Many Faces of Post-Ischemic Tau Protein in Brain Neurodegeneration of the Alzheimer’s Disease Type. Cells 2021, 10, 2213. https://doi.org/10.3390/cells10092213
Pluta R, Czuczwar SJ, Januszewski S, Jabłoński M. The Many Faces of Post-Ischemic Tau Protein in Brain Neurodegeneration of the Alzheimer’s Disease Type. Cells. 2021; 10(9):2213. https://doi.org/10.3390/cells10092213
Chicago/Turabian StylePluta, Ryszard, Stanisław J. Czuczwar, Sławomir Januszewski, and Mirosław Jabłoński. 2021. "The Many Faces of Post-Ischemic Tau Protein in Brain Neurodegeneration of the Alzheimer’s Disease Type" Cells 10, no. 9: 2213. https://doi.org/10.3390/cells10092213
APA StylePluta, R., Czuczwar, S. J., Januszewski, S., & Jabłoński, M. (2021). The Many Faces of Post-Ischemic Tau Protein in Brain Neurodegeneration of the Alzheimer’s Disease Type. Cells, 10(9), 2213. https://doi.org/10.3390/cells10092213








