Prognostic Roles of BRAF, KIT, NRAS, IGF2R and SF3B1 Mutations in Mucosal Melanomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Findings and Histologic Features
2.2. Molecular Analyses
2.3. Statistical Analysis
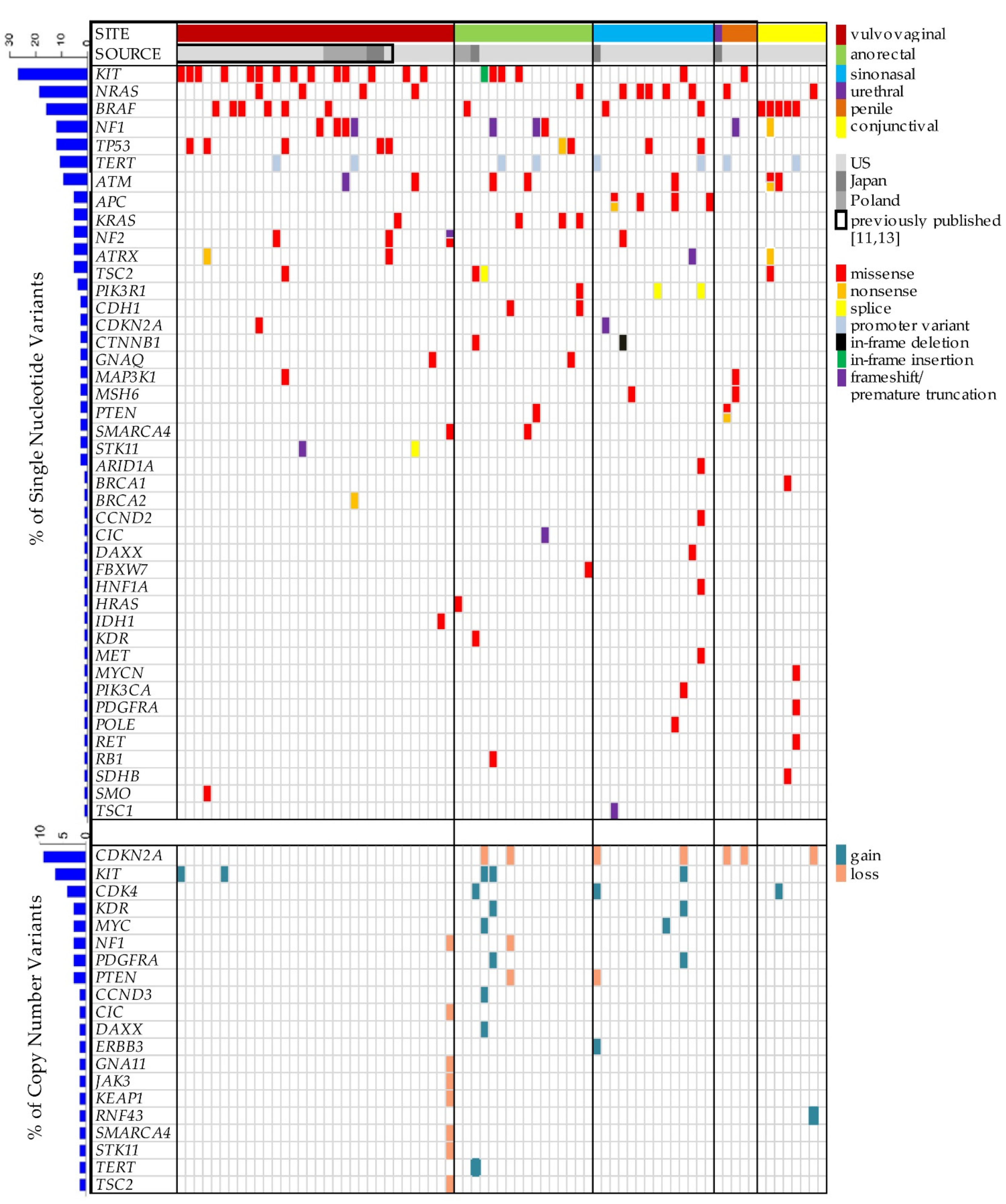
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.M.; Hsiao, S.J.; Schaeffer, D.F.; Lai, C.; Remotti, H.E.; Horst, D.; Mansukhani, M.M.; Horst, B.A. Identification of recurrent mutational events in anorectal melanoma. Mod. Pathol. 2017, 30, 286–296. [Google Scholar] [CrossRef]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Sarac, E.; Amaral, T.; Keim, U.; Leiter, U.; Forschner, A.; Eigentler, T.K.; Garbe, C. Prognostic factors in 161 patients with mucosal melanoma: A study of German Central Malignant Melanoma Registry. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2021–2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newell, F.; Kong, Y.; Wilmott, J.S.; Johansson, P.A.; Ferguson, P.M.; Cui, C.; Li, Z.; Kazakoff, S.H.; Burke, H.; Dodds, T.J.; et al. Whole-genome landscape of mucosal melanoma reveals diverse drivers and therapeutic targets. Nat. Commun. 2019, 10, 3163. [Google Scholar] [CrossRef]
- Cui, C.; Lian, B.; Zhou, L.; Song, X.; Zhang, X.; Wu, D.; Chi, Z.; Si, L.; Sheng, X.; Kong, Y.; et al. Multifactorial analysis of prognostic factors and survival rates among 706 mucosal melanoma patients. Ann. Surg. Oncol. 2018, 25, 2184–2192. [Google Scholar] [CrossRef]
- Amit, M.; Tam, S.; Abdelmeguid, A.S.; Roberts, D.B.; Takahashi, Y.; Raza, S.M.; Su, S.Y.; Kupferman, M.E.; DeMonte, F.; Hanna, E.Y. Mutation status among patients with sinonasal mucosal melanoma and its impact on survival. Br. J. Cancer 2017, 116, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Roesch, A.; Weide, B.; Gutzmer, R.; Meier, F.; Loquai, C.; Kähler, K.C.; Gesierich, A.; Meissner, M.; von Bubnoff, D.; et al. Prognostic factors and treatment outcomes in 444 patients with mucosal melanoma. Eur. J. Cancer 2017, 81, 36–44. [Google Scholar] [CrossRef]
- Zhou, R.; Shi, C.; Tao, W.; Li, J.; Wu, J.; Han, Y.; Yang, G.; Gu, Z.; Xu, S.; Wang, Y.; et al. Analysis of mucosal melanoma whole-genome landscapes reveals clinically relevant genomic aberrations. Clin. Cancer Res. 2019, 25, 3548–3560. [Google Scholar] [CrossRef] [Green Version]
- Iida, Y.; Salomon, M.P.; Hata, K.; Tran, K.; Ohe, S.; Griffiths, C.F.; Hsu, S.C.; Nelson, N.; Hoon, D.S.B. Predominance of triple wild-type and IGF2R mutations in mucosal melanomas. BMC Cancer 2018, 18, 1054. [Google Scholar] [CrossRef] [PubMed]
- Dias-Santagata, D.; Selim, M.A.; Su, Y.; Peng, Y.; Vollmer, R.; Chłopik, A.; Tell-Marti, G.; Paral, K.M.; Shalin, S.C.; Shea, C.R.; et al. KIT mutations and CD117 overexpression are markers of better progression-free survival in vulvar melanomas. Br. J. Dermatol. 2017, 177, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Wroblewska, J.P.; Mull, J.; Wu, C.L.; Fujimoto, M.; Ogawa, T.; Marszalek, A.; Hoang, M.P. SF3B1, NRAS, KIT, and BRAF mutation; CD117 and cMYC expression; and tumoral pigmentation in sinonasal melanomas: An analysis with newly found molecular alterations and some population-based molecular differences. Am. J. Surg. Pathol. 2019, 43, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.M.T.; Fujimoto, M.; Iwahashi, Y.; Cornejo, K.M.; Hoang, M.P. Molecular alterations in vaginal melanomas: Report of 4 cases and literature review. Am. J. Dermatopathol. 2021, 43, 45–48. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [Green Version]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ data to high confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013, 43, 11.10.1–11.10.33. [Google Scholar] [CrossRef]
- Cingolani, P.; Patel, V.M.; Coon, M.; Nguyen, T.; Land, S.J.; Ruden, D.M.; Lu, X. Using Drosophila melanogaster as a model for genotoxic chemical mutational studies with a new program, SnpSift. Front. Genet. 2012, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Liebers, M.; Zhelyazkova, B.; Cao, Y.; Panditi, D.; Lynch, K.D.; Chen, J.; Robinson, H.E.; Shim, H.S.; Chmielecki, J.; et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat. Med. 2014, 20, 1479–1484. [Google Scholar] [CrossRef]
- Kent, W.J. BLAT—The BLAST-like alignment tool. Genome Res. 2002, 12, 656–664. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 5 November 2020).
- Schaefer, T.; Satzger, I.; Gutzmer, R. Clinics, prognosis and new therapeutic options in patients with mucosal melanoma: A retrospective analysis of 75 patients. Medicine 2017, 96, e5753. [Google Scholar] [CrossRef]
- Hahn, H.M.; Lee, K.G.; Choi, W.; Cheong, S.H.; Myung, K.B.; Hahn, H.J. An updated review of mucosal melanoma: Survival meta-analysis. Mol. Clin. Oncol. 2019, 11, 116–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altieri, L.; Eguchi, M.; Peng, D.H.; Cockburn, M. Predictors of mucosal melanoma survival in a population-based setting. J. Am. Acad. Dermatol. 2019, 81, 136–142.e2. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Chevallier, J.; Labeille, B.; Cambazard, F.; Thomas, L.; Balme, B.; Leccia, M.T.; D’Incan, M.; Vercherin, P.; Douchet, C.; et al. Mucosal melanoma: Clinical, histological and c-kit gene mutational profile of 86 French cases. J. Eur. Acad. Dermatol. Venereol 2017, 31, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, V.E.; Chan, J.K.; Shin, J.Y.; Berek, J.S.; Osann, K.; Kapp, D.S. Vulvar melanoma: A multivariable analysis of 644 patients. Obstet. Gynecol. 2007, 110, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Sinasac, S.E.; Petrella, T.M.; Rouzbahman, M.; Sade, S.; Ghazarian, D.; Vicus, D. Melanoma of the vulva and vagina: Surgical management and outcomes based on a clinicopathologic review of 68 cases. J. Obstet. Gynaecol. Can. 2019, 41, 762–771. [Google Scholar] [CrossRef]
- Gru, A.A.; Becker, N.; Dehner, L.P.; Pfeifer, J.D. Mucosal melanoma: Correlation of clinicopathologic, prognostic, and molecular features. Melanoma Res. 2014, 24, 360–370. [Google Scholar] [CrossRef]
- Seifried, S.; Haydu, L.E.; Quinn, M.J.; Scolyer, R.A.; Stretch, J.R.; Thompson, J.F. Melanoma of the vulva and vagina: Principles of staging and their relevance to management based on a clinicopathologic analysis of 85 cases. Ann. Surg. Oncol. 2015, 22, 1959–1966. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Liu, J.; Li, L.; Xu, X.; Yao, X.; Dai, Z.; Wang, X.; Yang, S.; Wu, H.; et al. Characterizations of gene alterations in melanoma patients from Chinese population. BioMed Res. Int. 2020, 2020, 6096814. [Google Scholar] [CrossRef]
- Lian, B.; Cui, C.L.; Zhou, L.; Song, X.; Zhang, X.S.; Wu, D.; Si, L.; Chi, Z.H.; Sheng, X.N.; Mao, L.L.; et al. The natural history and patterns of metastases from mucosal melanoma: An analysis of 706 prospectively-followed patients. Ann. Oncol. 2017, 28, 868–873. [Google Scholar] [CrossRef]
- Nagarajan, P.; Curry, J.L.; Ning, J.; Piao, J.; Torres-Cabala, C.A.; Aung, P.P.; Ivan, D.; Ross, M.I.; Levenback, C.F.; Frumovitz, M.; et al. Tumor thickness and mitotic rate robustly predict melanoma-specific survival in patients with primary vulvar melanoma: A retrospective review of 100 cases. Clin. Cancer Res. 2017, 23, 2093–2104. [Google Scholar] [CrossRef] [Green Version]
- Tcheung, W.J.; Selim, M.A.; Herndon, J.E., II; Abernethy, A.P.; Nelson, K.C. Clinicopathologic study of 85 cases of melanoma of the female genitalia. J. Am. Acad. Dermatol. 2012, 67, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.S.; Thomay, A.A.; Gaughan, J.; Olszanski, A.; Wu, H.; Berger, A.C.; Farma, J.M. Outcomes in patients with mucosal melanomas. J. Surg. Oncol. 2013, 108, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.J.; Shia, J.; Hwu, W.J.; Busam, K.J.; Paty, P.B.; Guillem, J.G.; Coit, D.G.; Wong, W.D.; Weiser, M.R. The role of abdominoperineal resection as surgical therapy for anorectal melanoma. Ann. Surg. 2006, 244, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Dumaz, N.; Jouenne, F.; Delyon, J.; Mourah, S.; Bensussan, A.; Lebbé, C. Atypical BRAF and NRAS mutations in mucosal melanoma. Cancers 2019, 11, 1133. [Google Scholar] [CrossRef] [Green Version]
- Adler, N.R.; Wolfe, R.; Kelly, J.W.; Haydon, A.; McArthur, G.A.; McLean, C.A.; Mar, V.J. Tumour mutation status and sites of metastasis in patients with cutaneous melanoma. Br. J. Cancer 2017, 117, 1026–1035. [Google Scholar] [CrossRef]
- Bai, X.; Kong, Y.; Chi, Z.; Sheng, X.; Cui, C.; Wang, X.; Mao, L.; Tang, B.; Li, S.; Lian, B.; et al. MAPK Pathway and TERT promoter gene mutation pattern and its prognostic value in melanoma patients: A retrospective study of 2,793 cases. Clin. Cancer Res. 2017, 23, 6120–6127. [Google Scholar] [CrossRef] [Green Version]
- Dummer, R.; Schadendorf, D.; Ascierto, P.A.; Arance, A.; Dutriaux, C.; Di Giacomo, A.M.; Rutkowski, P.; Del Vecchio, M.; Gutzmer, R.; Mandala, M.; et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 435–445. [Google Scholar] [CrossRef]
- Lebbé, C.; Dutriaux, C.; Lesimple, T.; Kruit, W.; Kerger, J.; Thomas, L.; Guillot, B.; Braud, F.; Garbe, C.; Grob, J.J.; et al. Pimasertib versus Dacarbazine in patients with unresectable NRAS-mutated cutaneous melanoma: Phase II, randomized, controlled trial with crossover. Cancers 2020, 12, 1727. [Google Scholar] [CrossRef]
- Greger, J.G.; Eastman, S.D.; Zhang, V.; Bleam, M.R.; Hughes, A.M.; Smitheman, K.N.; Dickerson, S.H.; Laquerre, S.G.; Liu, L.; Gilmer, T.M. Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol. Cancer Ther. 2012, 11, 909–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov. A Phase Ib/II Study of LEE011 in Combination with MEK162 in Patients with NRAS Mutant Melanoma (NCT01781572). Available online: https://clinicaltrials.gov/ct2/show/NCT01781572 (accessed on 22 August 2021).
- ClinicalTrials.gov. A Phase Ib Study of LXH254-Centric Combinations in NSCLC or Melanoma (NCT02974725). Available online: https://clinicaltrials.gov/ct2/show/NCT02974725 (accessed on 22 August 2021).
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in stage III BRAF-mutated melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, N.J.; Verdijk, R.M.; Heegaard, S.; Marinkovic, M.; Esmaeli, B.; Jager, M.J. Conjunctival melanoma: New insights in tumour genetics and immunology, leading to new therapeutic options. Prog. Retin. Eye Res. 2021, 17, 100971. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Okimoto, R.A.; Lin, L.; Olivas, V.; Chan, E.; Markegard, E.; Rymar, A.; Neel, D.; Chen, X.; Hemmati, G.; Bollag, G.; et al. Preclinical efficacy of a RAF inhibitor that evades paradoxical MAPK pathway activation in protein kinase BRAF-mutant lung cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 13456–13461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, D.; Carvajal, R.D. KIT as an oncogenic driver in melanoma: An update on clinical development. Am. J. Clin. Dermatol. 2019, 20, 315–323. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Antonescu, C.R.; Wolchok, J.D.; Chapman, P.B.; Roman, R.A.; Teitcher, J.; Panageas, K.S.; Busam, K.J.; Chmielowski, B.; Lutzky, J.; et al. KIT as a therapeutic target in metastatic melanoma. JAMA 2011, 305, 2327–2334. [Google Scholar] [CrossRef] [Green Version]
- Curtin, J.A.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic activation of KIT in distinct subtypes of melanoma. J. Clin. Oncol. 2006, 24, 4340–4346. [Google Scholar] [CrossRef]
- Kong, Y.; Si, L.; Zhu, Y.; Xu, X.; Corless, C.L.; Flaherty, K.T.; Li, L.; Li, H.; Sheng, X.; Cui, C.; et al. Large-scale analysis of KIT aberrations in Chinese patients with melanoma. Clin. Cancer Res. 2011, 17, 1684–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.A.; Chun, S.M.; Choi, Y.D.; Kweon, S.S.; Jung, S.T.; Shim, H.J.; Yun, S.J. BRAF mutations and KIT aberrations and their clinicopathological correlation in 202 Korean melanomas. J. Investig. Dermatol. 2013, 133, 579–582. [Google Scholar] [CrossRef] [Green Version]
- Furney, S.J.; Pedersen, M.; Gentien, D.; Dumont, A.G.; Rapinat, A.; Desjardins, L.; Turajlic, S.; Piperno-Neumann, S.; de la Grange, P.; Roman-Roman, S.; et al. SF3B1 mutations are associated with alternative splicing in uveal melanoma. Cancer Discov. 2013, 3, 1122–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavuzyigitoglu, S.; Koopmans, A.E.; Verdijk, R.M.; Vaarwater, J.; Eussen, B.; van Bodegom, A.; Paridaens, D.; Kilic, E.; de Klein, A.; Rotterdam Ocular Melanoma Study Group. Uveal melanomas with SF3B1 mutations: A distinct subclass associated with late-onset metastases. Ophthalmology 2016, 123, 1118–1128. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Levy, C.; Khaled, M.; Iliopoulos, D.; Janas, M.M.; Schubert, S.; Pinner, S.; Chen, P.H.; Li, S.; Fletcher, A.L.; Yokoyama, S.; et al. Intronic miR-211 assumes the tumor suppressive function of its host gene in melanoma. Mol. Cell 2010, 40, 841–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dror, S.; Sander, L.; Schwartz, H.; Sheinboim, D.; Barzilai, A.; Dishon, Y.; Apcher, S.; Golan, T.; Greenberger, S.; Barshack, I.; et al. Melanoma miRNA trafficking controls tumour primary niche formation. Nat. Cell Biol. 2016, 18, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Hintzsche, J.D.; Gorden, N.T.; Amato, C.M.; Kim, J.; Wuensch, K.E.; Robinson, S.E.; Applegate, A.J.; Couts, K.L.; Medina, T.M.; Wells, K.R.; et al. Whole-exome sequencing identifies recurrent SF3B1 R625 mutation and comutation of NF1 and KIT in mucosal melanoma. Melanoma Res. 2017, 27, 189–199. [Google Scholar] [CrossRef] [PubMed]

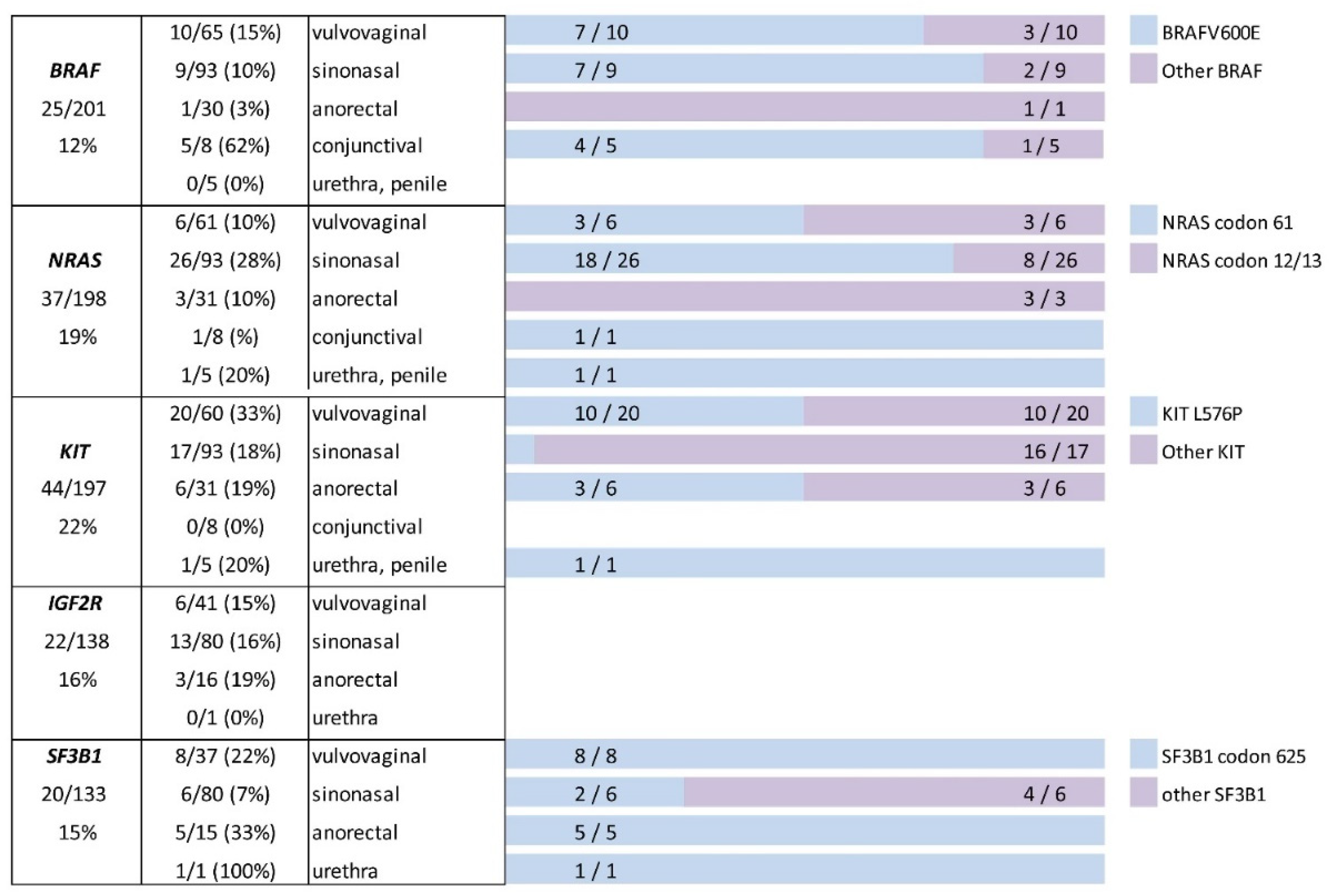
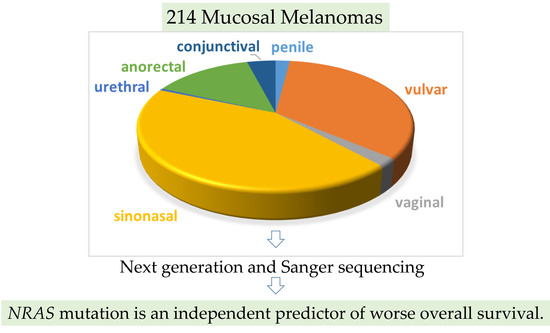
| Melanoma Subtype | BRAF NRAS KIT NGS | Sanger | ||||
|---|---|---|---|---|---|---|
| BRAF | NRAS | KIT | SF3B1 | IGF2R | ||
| Vulvovaginal | 32 | 33 | 29 | 28 | 37 | 41 |
| Sinonasal | 13 | 80 | 80 | 80 | 80 | 80 |
| Anorectal | 17 | 13 | 14 | 14 | 15 | 16 |
| Conjunctival | 8 | 0 | 0 | 0 | 0 | 0 |
| Penile | 4 | 0 | 0 | 0 | 0 | 0 |
| Urethra | 1 | 0 | 0 | 0 | 1 | 1 |
| Total | 75 | 126 | 123 | 122 | 133 | 138 |
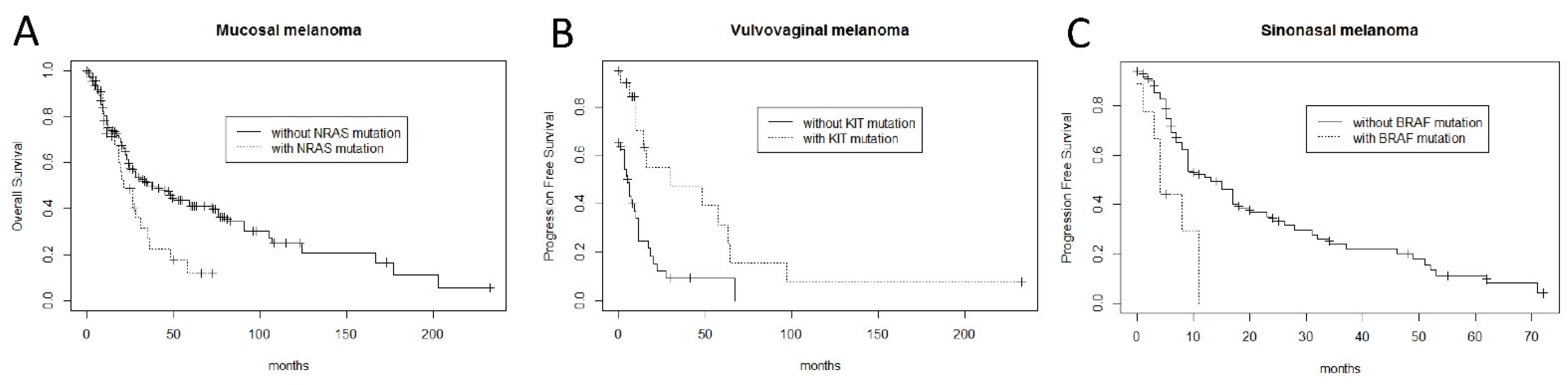
| Overall Survival | Melanoma-Specific Survival | |||
|---|---|---|---|---|
| Hazard Ratio | p-Value | Hazard Ratio | p-Value | |
| NRAS mutation | 1.71 | 0.036 * | 1.80 | 0.024 * |
| Stage (3–4 versus 1–2) | 1.71 | 0.026 * | 2.11 | 0.0012 * |
| Age (> 65 years) | 1.41 | 0.10 | - | - |
| Ulceration | 1.49 | 0.11 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewska, J.P.; Dias-Santagata, D.; Ustaszewski, A.; Wu, C.-L.; Fujimoto, M.; Selim, M.A.; Biernat, W.; Ryś, J.; Marszalek, A.; Hoang, M.P. Prognostic Roles of BRAF, KIT, NRAS, IGF2R and SF3B1 Mutations in Mucosal Melanomas. Cells 2021, 10, 2216. https://doi.org/10.3390/cells10092216
Wróblewska JP, Dias-Santagata D, Ustaszewski A, Wu C-L, Fujimoto M, Selim MA, Biernat W, Ryś J, Marszalek A, Hoang MP. Prognostic Roles of BRAF, KIT, NRAS, IGF2R and SF3B1 Mutations in Mucosal Melanomas. Cells. 2021; 10(9):2216. https://doi.org/10.3390/cells10092216
Chicago/Turabian StyleWróblewska, Joanna P., Dora Dias-Santagata, Adam Ustaszewski, Cheng-Lin Wu, Masakazu Fujimoto, M. Angelica Selim, Wojciech Biernat, Janusz Ryś, Andrzej Marszalek, and Mai P. Hoang. 2021. "Prognostic Roles of BRAF, KIT, NRAS, IGF2R and SF3B1 Mutations in Mucosal Melanomas" Cells 10, no. 9: 2216. https://doi.org/10.3390/cells10092216
APA StyleWróblewska, J. P., Dias-Santagata, D., Ustaszewski, A., Wu, C.-L., Fujimoto, M., Selim, M. A., Biernat, W., Ryś, J., Marszalek, A., & Hoang, M. P. (2021). Prognostic Roles of BRAF, KIT, NRAS, IGF2R and SF3B1 Mutations in Mucosal Melanomas. Cells, 10(9), 2216. https://doi.org/10.3390/cells10092216