Central vs. Peripheral Action of Thyroid Hormone in Adaptive Thermogenesis: A Burning Topic
Abstract
1. Introduction
2. Energy Expenditure and Adipose Tissues: The Main Route for Adaptive Thermogenesis
2.1. Adaptive Thermogenesis as a Way to Modulate Energy Expenditure
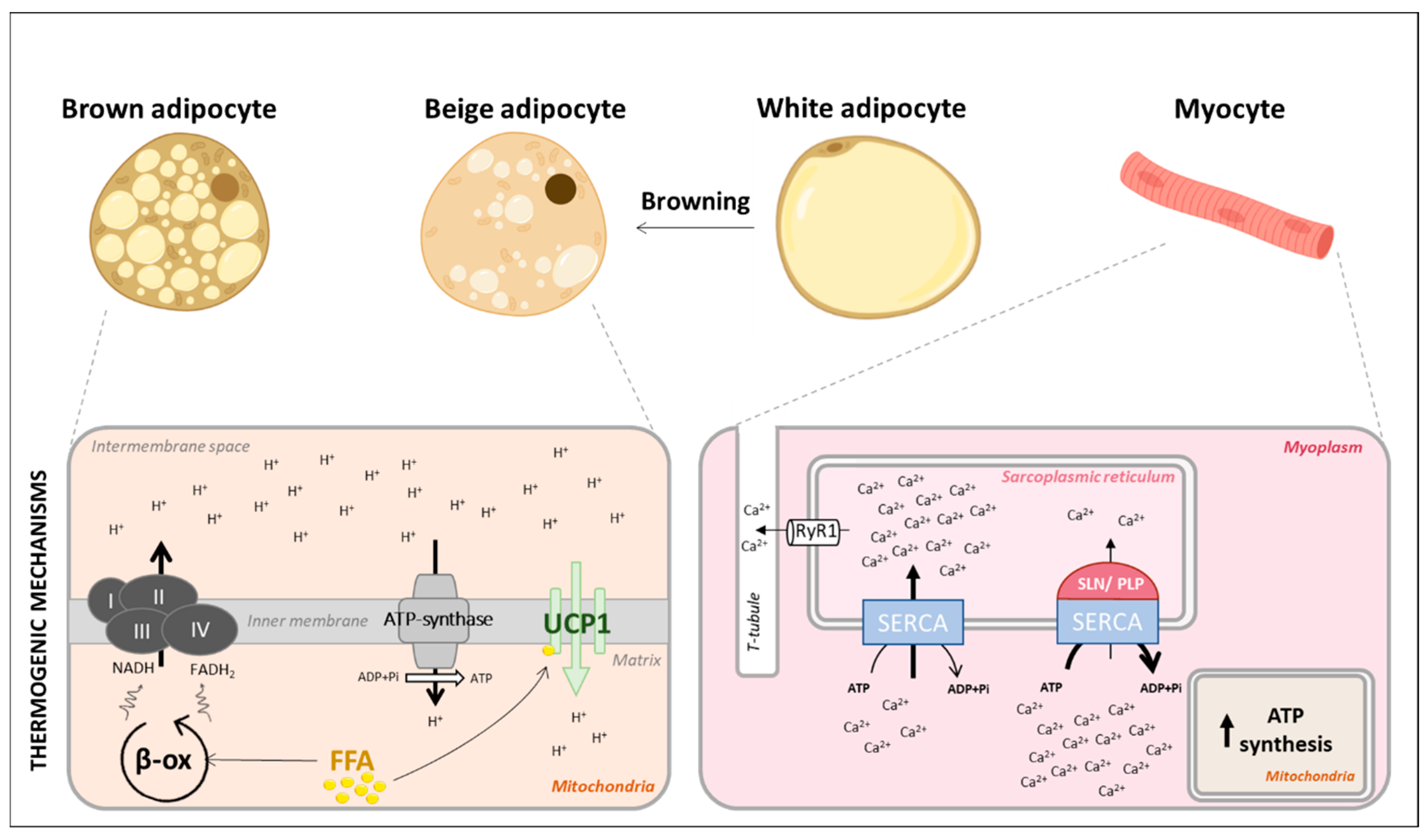
2.2. Adipose Tissues and Muscle, the Main Actors of Adaptive Thermogenesis
2.3. Adaptive Thermogenesis Is Induced by Cold Exposure and High Fat Diet
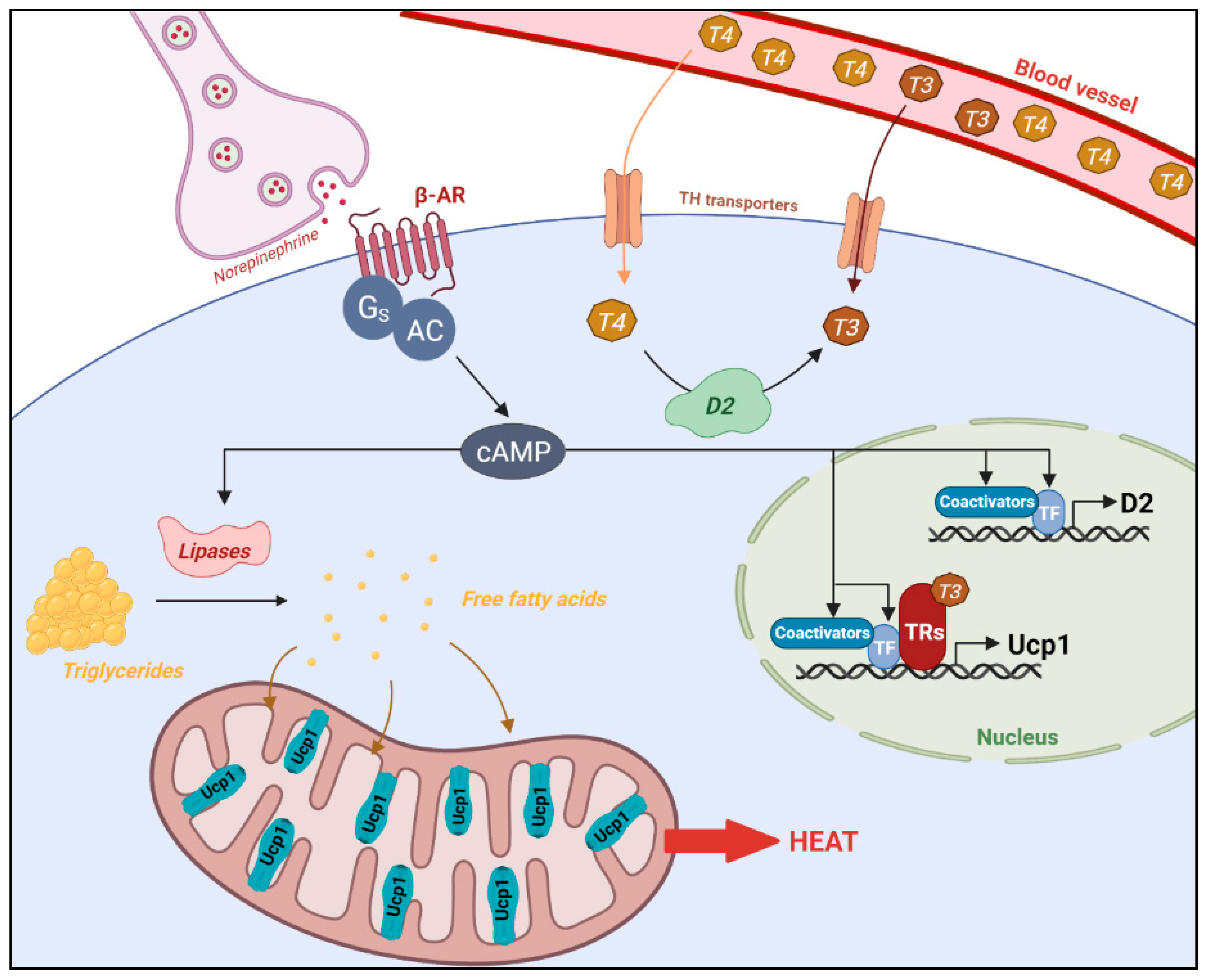
3. T3, an Important Component of Energy Expenditure
3.1. T3 Signaling Is Necessary for Cold-Induced Thermogenesis
3.2. The Role of T3 Signaling in Response to Diet-Induced Obesity
3.3. Tissue-Selective Metabolic Action for T3 Signaling
3.4. TR Isoform Selective Regulation of Adaptive Thermogenesis
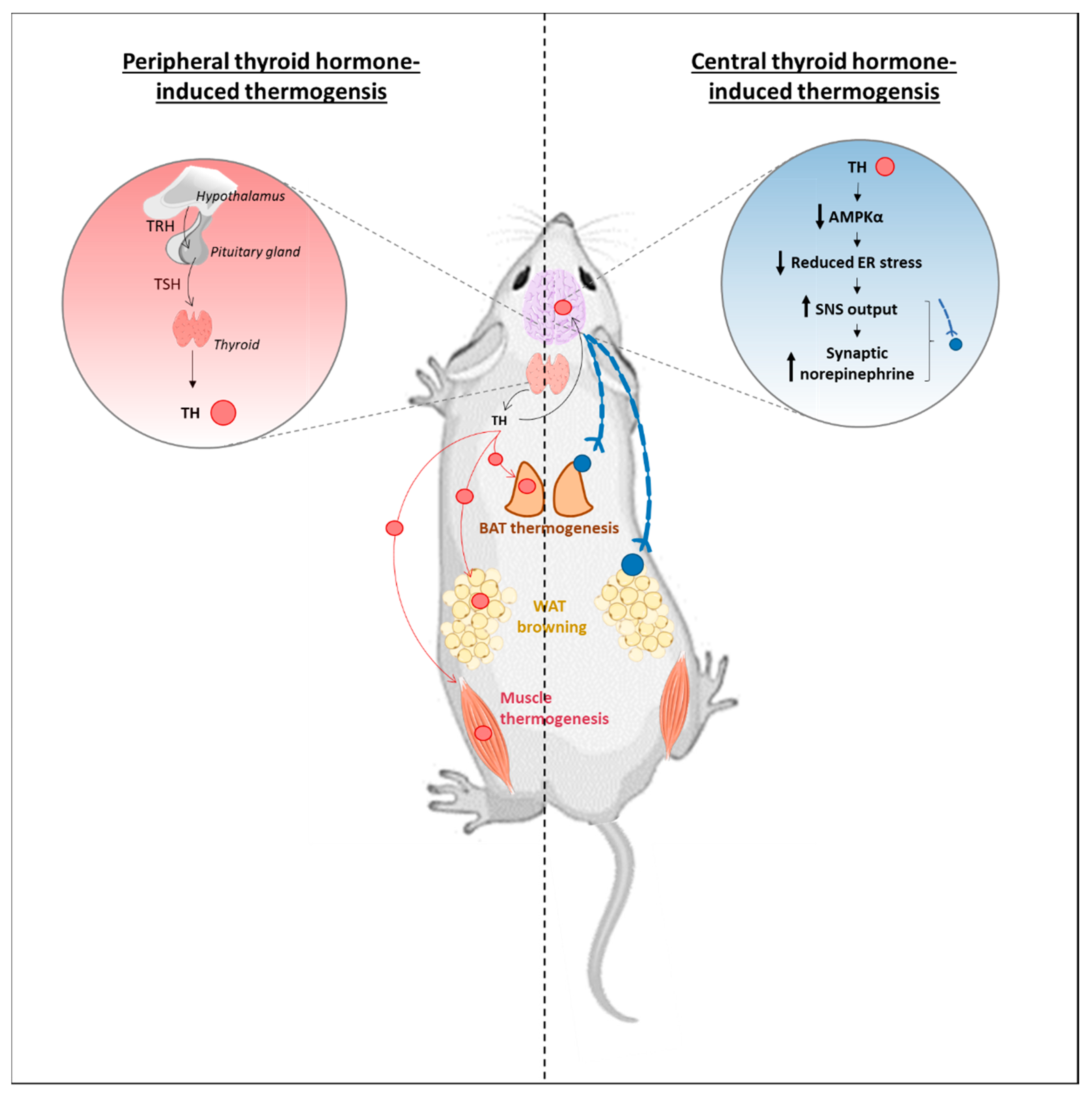
4. Central T3 Can Trigger Adaptive Thermogenesis: The Still Controversial Role of the Brain
4.1. Role of Central T3 in the Activation of BAT Thermogenesis
4.2. The Promising Metabolic Effects of WAT Browning: Also Concerned by a Central T3 Control?
4.3. Muscle: Another Thermogenic Actor, Same Conflict?
5. Roles for T3 Central Action in Regulating Other SNS-Sensitive Mechanisms?
5.1. T3 Regulation of Glucose Homeostasis Is a Composite Process
5.2. T3 Regulation of Heart Rate and Hypertrophy
6. Selective Mice Models and Pharmacology: New Perspectives to Better Understand and Take Advantage of T3 Metabolic Effects
6.1. Requirements for Elaborated Transgenic Models
6.2. Potential Therapeutical Applications: The Hope Raised by a New Class of Compounds
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Danforth, E.; Burger, A. The Role of Thyroid Hormones in the Control of Energy Expenditure. Clin. Endocrinol. Metab. 1984, 13, 581–595. [Google Scholar] [CrossRef]
- Yavuz, S.; Salgado Nunez del Prado, S.; Celi, F.S. Thyroid Hormone Action and Energy Expenditure. J. Endocr. Soc. 2019, 3, 1345–1356. [Google Scholar] [CrossRef]
- Abdi, H.; Kazemian, E.; Gharibzadeh, S.; Amouzegar, A.; Mehran, L.; Tohidi, M.; Rashvandi, Z.; Azizi, F. Association between Thyroid Function and Body Mass Index: A 10-Year Follow-Up. Ann. Nutr. Metab. 2017, 70, 338–345. [Google Scholar] [CrossRef]
- Weiss, R.E.; Murata, Y.; Cua, K.; Hayashi, Y.; Seo, H.; Refetoff, S. Thyroid Hormone Action on Liver, Heart, and Energy Expenditure in Thyroid Hormone Receptor Beta-Deficient Mice. Endocrinology 1998, 139, 4945–4952. [Google Scholar] [CrossRef]
- Nordyke, R.A.; Gilbert, F.I., Jr.; Harada, A.S.M. Graves’ Disease: Influence of Age on Clinical Findings. Arch. Intern. Med. 1988, 148, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liang, L.; Bray, G.A.; Qi, L.; Hu, F.B.; Rood, J.; Sacks, F.M.; Sun, Q. Thyroid Hormones and Changes in Body Weight and Metabolic Parameters in Response to Weight-Loss Diets: The POUNDS LOST Trial. Int. J. Obes. 2017, 41, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Delitala, A.P.; Scuteri, A.; Doria, C. Thyroid Hormone Diseases and Osteoporosis. J. Clin. Med. 2020, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Varela, L.; Vázquez, M.J.; Rodríguez-Cuenca, S.; González, C.R.; Velagapudi, V.R.; Morgan, D.A.; Schoenmakers, E.; Agassandian, K.; Lage, R.; et al. Hypothalamic AMPK and Fatty Acid Metabolism Mediate Thyroid Regulation of Energy Balance. Nat. Med. 2010, 16, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Spiegelman, B.M. Towards a Molecular Understanding of Adaptive Thermogenesis. Nature 2000, 404, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Commission for Thermal Physiology of the International Union of Physiological Sciences (IUPS Thermal Commission). Glossary of Terms for Thermal Physiology: Second Edition. Pflugers Arch. 1987, 410, 567–587. [Google Scholar] [CrossRef]
- Garretson, J.T.; Szymanski, L.A.; Schwartz, G.J.; Xue, B.; Ryu, V.; Bartness, T.J. Lipolysis Sensation by White Fat Afferent Nerves Triggers Brown Fat Thermogenesis. Mol. Metab. 2016, 5, 626–634. [Google Scholar] [CrossRef]
- Ameka, M.; Markan, K.R.; Morgan, D.A.; BonDurant, L.D.; Idiga, S.O.; Naber, M.C.; Zhu, Z.; Zingman, L.V.; Grobe, J.L.; Rahmouni, K.; et al. Liver Derived FGF21 Maintains Core Body Temperature During Acute Cold Exposure. Sci. Rep. 2019, 9, 630. [Google Scholar] [CrossRef]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10. [Google Scholar] [CrossRef]
- Scheele, C.; Wolfrum, C. Brown Adipose Crosstalk in Tissue Plasticity and Human Metabolism. Endocr. Rev. 2020, 41. [Google Scholar] [CrossRef]
- Villarroya, F.; Gavaldà-Navarro, A.; Peyrou, M.; Villarroya, J.; Giralt, M. Brown Adipokines. Handb. Exp. Pharmacol. 2019, 251, 239–256. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Oelkrug, R.; Polymeropoulos, E.T.; Jastroch, M. Brown Adipose Tissue: Physiological Function and Evolutionary Significance. J. Comp. Physiol. B 2015, 185, 587–606. [Google Scholar] [CrossRef]
- Marlatt, K.L.; Ravussin, E. Brown Adipose Tissue: An Update on Recent Findings. Curr. Obes. Rep. 2017, 6, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Ricquier, D. UCP1, the Mitochondrial Uncoupling Protein of Brown Adipocyte: A Personal Contribution and a Historical Perspective. Biochimie 2017, 134, 3–8. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Scheja, L. Brown Adipose Tissue and Lipid Metabolism. Curr. Opin Lipidol. 2018, 29, 180–185. [Google Scholar] [CrossRef]
- Xiang, A.S.; Meikle, P.J.; Carey, A.L.; Kingwell, B.A. Brown Adipose Tissue and Lipid Metabolism: New Strategies for Identification of Activators and Biomarkers with Clinical Potential. Pharmacol. Ther. 2018, 192, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Chang, J.S.; Jo, Y.-H. Intracellular Glycolysis in Brown Adipose Tissue Is Essential for Optogenetically Induced Nonshivering Thermogenesis in Mice. Sci. Rep. 2018, 8, 6672. [Google Scholar] [CrossRef] [PubMed]
- Chitraju, C.; Fischer, A.W.; Farese, R.V.; Walther, T.C. Lipid Droplets in Brown Adipose Tissue Are Dispensable for Cold-Induced Thermogenesis. Cell Rep. 2020, 33, 108348. [Google Scholar] [CrossRef]
- Panic, V.; Pearson, S.; Banks, J.; Tippetts, T.S.; Velasco-Silva, J.N.; Lee, S.; Simcox, J.; Geoghegan, G.; Bensard, C.L.; van Ry, T.; et al. Mitochondrial Pyruvate Carrier Is Required for Optimal Brown Fat Thermogenesis. eLife 2020, 9. [Google Scholar] [CrossRef]
- Wang, Z.; Ning, T.; Song, A.; Rutter, J.; Wang, Q.A.; Jiang, L. Chronic Cold Exposure Enhances Glucose Oxidation in Brown Adipose Tissue. EMBO Rep. 2020, 21, e50085. [Google Scholar] [CrossRef]
- Rahbani, J.F.; Roesler, A.; Hussain, M.F.; Samborska, B.; Dykstra, C.B.; Tsai, L.; Jedrychowski, M.P.; Vergnes, L.; Reue, K.; Spiegelman, B.M.; et al. Creatine Kinase B Controls Futile Creatine Cycling in Thermogenic Fat. Nature 2021, 590, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L.; Chouchani, E.T.; Jedrychowski, M.P.; Erickson, B.K.; Shinoda, K.; Cohen, P.; Vetrivelan, R.; Lu, G.Z.; Laznik-Bogoslavski, D.; Hasenfuss, S.C.; et al. A Creatine-Driven Substrate Cycle Enhances Energy Expenditure and Thermogenesis in Beige Fat. Cell 2015, 163, 643–655. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamada, T. UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes. Front. Endocrinol. 2020, 11, 498. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7. [Google Scholar] [CrossRef]
- Pilkington, A.-C.; Paz, H.A.; Wankhade, U.D. Beige Adipose Tissue Identification and Marker Specificity-Overview. Front. Endocrinol. 2021, 12, 599134. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Wang, Q.A.; Song, A.; Vishvanath, L.; Busbuso, N.C.; Scherer, P.E.; Gupta, R.K. Cellular Origins of Beige Fat Cells Revisited. Diabetes 2019, 68, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
- Paulo, E.; Wang, B. Towards a Better Understanding of Beige Adipocyte Plasticity. Cells 2019, 8, 1552. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Leitner, B.P.; Huang, S.; Brychta, R.J.; Duckworth, C.J.; Baskin, A.S.; McGehee, S.; Tal, I.; Dieckmann, W.; Gupta, G.; Kolodny, G.M.; et al. Mapping of Human Brown Adipose Tissue in Lean and Obese Young Men. Proc. Natl. Acad. Sci. USA 2017, 114, 8649–8654. [Google Scholar] [CrossRef]
- Sharp, L.Z.; Shinoda, K.; Ohno, H.; Scheel, D.W.; Tomoda, E.; Ruiz, L.; Hu, H.; Wang, L.; Pavlova, Z.; Gilsanz, V.; et al. Human BAT Possesses Molecular Signatures That Resemble Beige/Brite Cells. PLoS ONE 2012, 7, e49452. [Google Scholar] [CrossRef]
- Lidell, M.E.; Betz, M.J.; Dahlqvist Leinhard, O.; Heglind, M.; Elander, L.; Slawik, M.; Mussack, T.; Nilsson, D.; Romu, T.; Nuutila, P.; et al. Evidence for Two Types of Brown Adipose Tissue in Humans. Nat. Med. 2013, 19, 631–634. [Google Scholar] [CrossRef]
- Chondronikola, M.; Volpi, E.; Børsheim, E.; Porter, C.; Annamalai, P.; Enerbäck, S.; Lidell, M.E.; Saraf, M.K.; Labbe, S.M.; Hurren, N.M.; et al. Brown Adipose Tissue Improves Whole-Body Glucose Homeostasis and Insulin Sensitivity in Humans. Diabetes 2014, 63, 4089–4099. [Google Scholar] [CrossRef]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Franquet Elía, E.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of Human Brown Adipose Tissue by a Β3-Adrenergic Receptor Agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef]
- van der Lans, A.A.J.J.; Hoeks, J.; Brans, B.; Vijgen, G.H.E.J.; Visser, M.G.W.; Vosselman, M.J.; Hansen, J.; Jörgensen, J.A.; Wu, J.; Mottaghy, F.M.; et al. Cold Acclimation Recruits Human Brown Fat and Increases Nonshivering Thermogenesis. J. Clin. Investig. 2013, 123, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kayahara, T.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Recruited Brown Adipose Tissue as an Antiobesity Agent in Humans. J. Clin. Investig. 2013, 123, 3404–3408. [Google Scholar] [CrossRef]
- Muzik, O.; Mangner, T.J.; Leonard, W.R.; Kumar, A.; Janisse, J.; Granneman, J.G. 15O PET Measurement of Blood Flow and Oxygen Consumption in Cold-Activated Human Brown Fat. J. Nucl. Med. 2013, 54, 523–531. [Google Scholar] [CrossRef]
- Din, M.U.; Raiko, J.; Saari, T.; Kudomi, N.; Tolvanen, T.; Oikonen, V.; Teuho, J.; Sipilä, H.T.; Savisto, N.; Parkkola, R.; et al. Human Brown Adipose Tissue [(15)O]O2 PET Imaging in the Presence and Absence of Cold Stimulus. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1878–1886. [Google Scholar] [CrossRef]
- Fernández-Verdejo, R.; Marlatt, K.L.; Ravussin, E.; Galgani, J.E. Contribution of Brown Adipose Tissue to Human Energy Metabolism. Mol. Aspects Med. 2019, 68, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Pant, M.; Bal, N.C.; Periasamy, M. Sarcolipin: A Key Thermogenic and Metabolic Regulator in Skeletal Muscle. Trends Endocrinol. Metab. 2016, 27, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, M.; Maurya, S.K.; Sahoo, S.K.; Singh, S.; Sahoo, S.K.; Reis, F.C.G.; Bal, N.C. Role of SERCA Pump in Muscle Thermogenesis and Metabolism. Compr. Physiol. 2017, 7, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, M.; Herrera, J.L.; Reis, F.C.G. Skeletal Muscle Thermogenesis and Its Role in Whole Body Energy Metabolism. Diabetes Metab. J. 2017, 41, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Gamu, D.; Juracic, E.S.; Hall, K.J.; Tupling, A.R. The Sarcoplasmic Reticulum and SERCA: A Nexus for Muscular Adaptive Thermogenesis. Appl. Physiol. Nutr. Metab. 2020, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Riley, C.L.; Dao, C.; Kenaston, M.A.; Muto, L.; Kohno, S.; Nowinski, S.M.; Solmonson, A.D.; Pfeiffer, M.; Sack, M.N.; Lu, Z.; et al. The Complementary and Divergent Roles of Uncoupling Proteins 1 and 3 in Thermoregulation. J. Physiol. 2016, 594, 7455–7464. [Google Scholar] [CrossRef] [PubMed]
- Pohl, E.E.; Rupprecht, A.; Macher, G.; Hilse, K.E. Important Trends in UCP3 Investigation. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Honek, J.; Xue, Y.; Seki, T.; Cao, Z.; Andersson, P.; Yang, X.; Hosaka, K.; Cao, Y. Cold-Induced Activation of Brown Adipose Tissue and Adipose Angiogenesis in Mice. Nat. Protoc. 2012, 7, 606–615. [Google Scholar] [CrossRef]
- Bachman, E.S.; Dhillon, H.; Zhang, C.-Y.; Cinti, S.; Bianco, A.C.; Kobilka, B.K.; Lowell, B.B. BetaAR Signaling Required for Diet-Induced Thermogenesis and Obesity Resistance. Science 2002, 297, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Himms-Hagen, J.; Hogan, S.; Zaror-Behrens, G. Increased Brown Adipose Tissue Thermogenesis in Obese (Ob/Ob) Mice Fed a Palatable Diet. Am. J. Physiol. 1986, 250, E274–E281. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, N.J.; Stock, M.J. A Role for Brown Adipose Tissue in Diet-Induced Thermogenesis. Nature 1979, 281, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Matsushita, M.; Yoneshiro, T.; Okamatsu-Ogura, Y. Brown Adipose Tissue, Diet-Induced Thermogenesis, and Thermogenic Food Ingredients: From Mice to Men. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef]
- Lumpkin, E.A.; Caterina, M.J. Mechanisms of Sensory Transduction in the Skin. Nature 2007, 445, 858–865. [Google Scholar] [CrossRef]
- Nakamura, K.; Morrison, S.F. A Thermosensory Pathway That Controls Body Temperature. Nat. Neurosci. 2008, 11, 62–71. [Google Scholar] [CrossRef]
- Moran, T.H.; Norgren, R.; Crosby, R.J.; McHugh, P.R. Central and Peripheral Vagal Transport of Cholecystokinin Binding Sites Occurs in Afferent Fibers. Brain Res. 1990, 526, 95–102. [Google Scholar] [CrossRef]
- Mönnikes, H.; Lauer, G.; Arnold, R. Peripheral Administration of Cholecystokinin Activates C-Fos Expression in the Locus Coeruleus/Subcoeruleus Nucleus, Dorsal Vagal Complex and Paraventricular Nucleus via Capsaicin-Sensitive Vagal Afferents and CCK-A Receptors in the Rat. Brain Res. 1997, 770, 277–288. [Google Scholar] [CrossRef]
- Paschos, G.K.; Tang, S.Y.; Theken, K.N.; Li, X.; Verginadis, I.; Lekkas, D.; Herman, L.; Yan, W.; Lawson, J.; FitzGerald, G.A. Cold-Induced Browning of Inguinal White Adipose Tissue Is Independent of Adipose Tissue Cyclooxygenase-2. Cell Rep. 2018, 24, 809–814. [Google Scholar] [CrossRef]
- Xu, Z.; You, W.; Zhou, Y.; Chen, W.; Wang, Y.; Shan, T. Cold-Induced Lipid Dynamics and Transcriptional Programs in White Adipose Tissue. BMC Biol. 2019, 17, 74. [Google Scholar] [CrossRef]
- García-Ruiz, E.; Reynés, B.; Díaz-Rúa, R.; Ceresi, E.; Oliver, P.; Palou, A. The Intake of High-Fat Diets Induces the Acquisition of Brown Adipocyte Gene Expression Features in White Adipose Tissue. Int. J. Obes. 2015, 39, 1619–1629. [Google Scholar] [CrossRef]
- Akagiri, S.; Naito, Y.; Ichikawa, H.; Mizushima, K.; Takagi, T.; Handa, O.; Kokura, S.; Yoshikawa, T. Bofutsushosan, an Oriental Herbal Medicine, Attenuates the Weight Gain of White Adipose Tissue and the Increased Size of Adipocytes Associated with the Increase in Their Expression of Uncoupling Protein 1 in High-Fat Diet-Fed Male KK/Ta Mice. J. Clin. Biochem. Nutr. 2008, 42, 158–166. [Google Scholar] [CrossRef]
- Jimenez, M.; Léger, B.; Canola, K.; Lehr, L.; Arboit, P.; Seydoux, J.; Russell, A.P.; Giacobino, J.P.; Muzzin, P.; Preitner, F. Beta(1)/Beta(2)/Beta(3)-Adrenoceptor Knockout Mice Are Obese and Cold-Sensitive but Have Normal Lipolytic Responses to Fasting. FEBS Lett. 2002, 530, 37–40. [Google Scholar] [CrossRef]
- Preite, N.Z.; do Nascimento, B.P.P.; Muller, C.R.; Américo, A.L.V.; Higa, T.S.; Evangelista, F.S.; Lancellotti, C.L.; dos Henriques, F.S.; Batista, M.L.; Bianco, A.C.; et al. Disruption of Beta3 Adrenergic Receptor Increases Susceptibility to DIO in Mouse. J. Endocrinol. 2016, 231, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Enerbäck, S.; Jacobsson, A.; Simpson, E.M.; Guerra, C.; Yamashita, H.; Harper, M.E.; Kozak, L.P. Mice Lacking Mitochondrial Uncoupling Protein Are Cold-Sensitive but Not Obese. Nature 1997, 387, 90–94. [Google Scholar] [CrossRef]
- Feldmann, H.M.; Golozoubova, V.; Cannon, B.; Nedergaard, J. UCP1 Ablation Induces Obesity and Abolishes Diet-Induced Thermogenesis in Mice Exempt from Thermal Stress by Living at Thermoneutrality. Cell Metab. 2009, 9, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L.; Chouchani, E.T.; Stavrovskaya, I.G.; Lu, G.Z.; Jedrychowski, M.P.; Egan, D.F.; Kumari, M.; Kong, X.; Erickson, B.K.; Szpyt, J.; et al. UCP1 Deficiency Causes Brown Fat Respiratory Chain Depletion and Sensitizes Mitochondria to Calcium Overload-Induced Dysfunction. Proc. Natl. Acad. Sci. USA 2017, 114, 7981–7986. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Silva, J.E. Intracellular Conversion of Thyroxine to Triiodothyronine Is Required for the Optimal Thermogenic Function of Brown Adipose Tissue. J. Clin. Investig. 1987, 79, 295–300. [Google Scholar] [CrossRef]
- Silva, J.E. The Thermogenic Effect of Thyroid Hormone and Its Clinical Implications. Ann. Intern. Med. 2003, 139, 205. [Google Scholar] [CrossRef]
- Maushart, C.I.; Loeliger, R.; Gashi, G.; Christ-Crain, M.; Betz, M.J. Resolution of Hypothyroidism Restores Cold-Induced Thermogenesis in Humans. Thyroid 2019, 29, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Ueta, C.B.; Olivares, E.L.; Bianco, A.C. Responsiveness to Thyroid Hormone and to Ambient Temperature Underlies Differences between Brown Adipose Tissue and Skeletal Muscle Thermogenesis in a Mouse Model of Diet-Induced Obesity. Endocrinology 2011, 152, 3571–3581. [Google Scholar] [CrossRef][Green Version]
- Bianco, A.; da Conceição, R. The Deiodinase Trio and Thyroid Hormone Signaling. Methods Mol. Biol. 2018, 1801, 67–83. [Google Scholar] [CrossRef]
- Margolis, R.N. The Nuclear Receptor Signaling Atlas: Catalyzing Understanding of Thyroid Hormone Signaling and Metabolic Control. Thyroid 2008, 18, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Minakhina, S.; Bansal, S.; Zhang, A.; Brotherton, M.; Janodia, R.; De Oliveira, V.; Tadepalli, S.; Wondisford, F.E. A Direct Comparison of Thyroid Hormone Receptor Protein Levels in Mice Provides Unexpected Insights into Thyroid Hormone Action. Thyroid 2020, 30, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Flamant, F. Futures Challenges in Thyroid Hormone Signaling Research. Front. Endocrinol. 2016, 7, 58. [Google Scholar] [CrossRef]
- Flamant, F.; Cheng, S.-Y.; Hollenberg, A.N.; Moeller, L.C.; Samarut, J.; Wondisford, F.E.; Yen, P.M.; Refetoff, S. Thyroid Hormone Signaling Pathways: Time for a More Precise Nomenclature. Endocrinology 2017, 158, 2052–2057. [Google Scholar] [CrossRef]
- Sinha, R.; Yen, P.M. Cellular Action of Thyroid Hormone. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Grossman, A., Hershman, J.M., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Bianco, A.C.; Silva, J.E. Cold Exposure Rapidly Induces Virtual Saturation of Brown Adipose Tissue Nuclear T3 Receptors. Am. J. Physiol. 1988, 255, E496–E503. [Google Scholar] [CrossRef]
- Silva, J.E.; Larsen, P.R. Potential of Brown Adipose Tissue Type II Thyroxine 5’-Deiodinase as a Local and Systemic Source of Triiodothyronine in Rats. J. Clin. Investig. 1985, 76, 2296–2305. [Google Scholar] [CrossRef]
- Mohácsik, P.; Erdélyi, F.; Baranyi, M.; Botz, B.; Szabó, G.; Tóth, M.; Haltrich, I.; Helyes, Z.; Sperlágh, B.; Tóth, Z.; et al. A Transgenic Mouse Model for Detection of Tissue-Specific Thyroid Hormone Action. Endocrinology 2017, 159, 1159–1171. [Google Scholar] [CrossRef]
- Schneider, M.J.; Fiering, S.N.; Pallud, S.E.; Parlow, A.F.; St. Germain, D.L.; Galton, V.A. Targeted Disruption of the Type 2 Selenodeiodinase Gene (DIO2) Results in a Phenotype of Pituitary Resistance to T4. Mol. Endocrinol. 2001, 15, 2137–2148. [Google Scholar] [CrossRef]
- de Jesus, L.A.; Carvalho, S.D.; Ribeiro, M.O.; Schneider, M.; Kim, S.W.; Harney, J.W.; Larsen, P.R.; Bianco, A.C. The Type 2 Iodothyronine Deiodinase Is Essential for Adaptive Thermogenesis in Brown Adipose Tissue. J. Clin. Investig. 2001, 108, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Christoffolete, M.A.; Linardi, C.C.G.; de Jesus, L.; Ebina, K.N.; Carvalho, S.D.; Ribeiro, M.O.; Rabelo, R.; Curcio, C.; Martins, L.; Kimura, E.T.; et al. Mice with Targeted Disruption of the Dio2 Gene Have Cold-Induced Overexpression of the Uncoupling Protein 1 Gene but Fail to Increase Brown Adipose Tissue Lipogenesis and Adaptive Thermogenesis. Diabetes 2004, 53, 577–584. [Google Scholar] [CrossRef]
- Castillo, M.; Hall, J.A.; Correa-Medina, M.; Ueta, C.; Kang, H.W.; Cohen, D.E.; Bianco, A.C. Disruption of Thyroid Hormone Activation in Type 2 Deiodinase Knockout Mice Causes Obesity with Glucose Intolerance and Liver Steatosis Only at Thermoneutrality. Diabetes 2011, 60, 1082–1089. [Google Scholar] [CrossRef]
- Fischer, A.W.; Cannon, B.; Nedergaard, J. The Answer to the Question “What Is the Best Housing Temperature to Translate Mouse Experiments to Humans?” Is: Thermoneutrality. Mol. Metab. 2019, 26, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Hoo, R.L.C.; Wu, X.; Pan, Y.; Lee, I.P.C.; Cheong, L.Y.; Bornstein, S.R.; Rong, X.; Guo, J.; Xu, A. A-FABP Mediates Adaptive Thermogenesis by Promoting Intracellular Activation of Thyroid Hormones in Brown Adipocytes. Nat. Commun. 2017, 8, 14147. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.E.; Larsen, P.R. Adrenergic Activation of Triiodothyronine Production in Brown Adipose Tissue. Nature 1983, 305, 712–713. [Google Scholar] [CrossRef]
- Grozovsky, R.; Ribich, S.; Rosene, M.L.; Mulcahey, M.A.; Huang, S.A.; Patti, M.E.; Bianco, A.C.; Kim, B.W. Type 2 Deiodinase Expression Is Induced by Peroxisomal Proliferator-Activated Receptor-Gamma Agonists in Skeletal Myocytes. Endocrinology 2009, 150, 1976–1983. [Google Scholar] [CrossRef][Green Version]
- Hosoi, Y.; Murakami, M.; Mizuma, H.; Ogiwara, T.; Imamura, M.; Mori, M. Expression and Regulation of Type II Iodothyronine Deiodinase in Cultured Human Skeletal Muscle Cells. J. Clin. Endocrinol. Metab. 1999, 84, 3293–3300. [Google Scholar] [CrossRef]
- Salvatore, D.; Bartha, T.; Harney, J.W.; Larsen, P.R. Molecular Biological and Biochemical Characterization of the Human Type 2 Selenodeiodinase. Endocrinology 1996, 137, 3308–3315. [Google Scholar] [CrossRef]
- Fonseca, T.L.; Werneck-De-Castro, J.P.; Castillo, M.; Bocco, B.M.L.C.; Fernandes, G.W.; McAninch, E.A.; Ignacio, D.L.; Moises, C.C.S.; Ferreira, A.R.; Gereben, B.; et al. Tissue-Specific Inactivation of Type 2 Deiodinase Reveals Multilevel Control of Fatty Acid Oxidation by Thyroid Hormone in the Mouse. Diabetes 2014, 63, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Carmody, C.; Ogawa-Wong, A.N.; Martin, C.; Luongo, C.; Zuidwijk, M.; Sager, B.; Petersen, T.; Roginski Guetter, A.; Janssen, R.; Wu, E.Y.; et al. A Global Loss of Dio2 Leads to Unexpected Changes in Function and Fiber Types of Slow Skeletal Muscle in Male Mice. Endocrinology 2019, 160, 1205–1222. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hurtado, E.; Lee, J.; Choi, J.; Wolfgang, M.J. Fatty Acid Oxidation Is Required for Active and Quiescent Brown Adipose Tissue Maintenance and Thermogenic Programing. Mol. Metab. 2017, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ellis, J.M.; Wolfgang, M.J. Adipose Fatty Acid Oxidation Is Required for Thermogenesis and Potentiates Oxidative Stress Induced Inflammation. Cell Rep. 2015, 10, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Flamant, F.; Samarut, J. Thyroid Hormone Receptors: Lessons from Knockout and Knock-in Mutant Mice. Trends Endocrinol. Metab. 2003, 14, 85–90. [Google Scholar] [CrossRef]
- Gauthier, K.; Plateroti, M.; Harvey, C.B.; Williams, G.R.; Weiss, R.E.; Refetoff, S.; Willott, J.F.; Sundin, V.; Roux, J.P.; Malaval, L.; et al. Genetic Analysis Reveals Different Functions for the Products of the Thyroid Hormone Receptor Alpha Locus. Mol. Cell Biol. 2001, 21, 4748–4760. [Google Scholar] [CrossRef]
- Golozoubova, V.; Gullberg, H.; Matthias, A.; Cannon, B.; Vennström, B.; Nedergaard, J. Depressed Thermogenesis but Competent Brown Adipose Tissue Recruitment in Mice Devoid of All Hormone-Binding Thyroid Hormone Receptors. Mol. Endocrinol. 2004, 18, 384–401. [Google Scholar] [CrossRef]
- Marrif, H.; Schifman, A.; Stepanyan, Z.; Gillis, M.-A.; Calderone, A.; Weiss, R.E.; Samarut, J.; Silva, J.E. Temperature Homeostasis in Transgenic Mice Lacking Thyroid Hormone Receptor-Alpha Gene Products. Endocrinology 2005, 146, 2872–2884. [Google Scholar] [CrossRef][Green Version]
- Kaneshige, M.; Kaneshige, K.; Zhu, X.; Dace, A.; Garrett, L.; Carter, T.A.; Kazlauskaite, R.; Pankratz, D.G.; Wynshaw-Boris, A.; Refetoff, S.; et al. Mice with a Targeted Mutation in the Thyroid Hormone β Receptor Gene Exhibit Impaired Growth and Resistance to Thyroid Hormone. Proc. Natl. Acad. Sci. USA 2000, 97, 13209–13214. [Google Scholar] [CrossRef]
- Ribeiro, M.O.; Bianco, S.D.C.; Kaneshige, M.; Schultz, J.J.; Cheng, S.; Bianco, A.C.; Brent, G.A. Expression of Uncoupling Protein 1 in Mouse Brown Adipose Tissue Is Thyroid Hormone Receptor-β Isoform Specific and Required for Adaptive Thermogenesis. Endocrinology 2010, 151, 432–440. [Google Scholar] [CrossRef]
- Chiellini, G.; Apriletti, J.W.; Yoshihara, H.A.; Baxter, J.D.; Ribeiro, R.C.; Scanlan, T.S. A High-Affinity Subtype-Selective Agonist Ligand for the Thyroid Hormone Receptor. Chem. Biol. 1998, 5, 299–306. [Google Scholar] [CrossRef]
- Nedergaard, J.; Dicker, A.; Cannon, B. The Interaction between Thyroid and Brown-Fat Thermogenesis. Central or Peripheral Effects? Ann. N. Y. Acad. Sci. 1997, 813, 712–717. [Google Scholar] [CrossRef]
- Bernardis, L.L.; Goldman, J.K. Origin of Endocrine-Metabolic Changes in the Weanling Rat Ventromedial Syndrome. J. Neurosci. Res. 1976, 2, 91–116. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.N.; Rothwell, N.J.; Stock, M.J.; Stone, T.W. Activation of Brown Adipose Tissue Thermogenesis by the Ventromedial Hypothalamus. Nature 1981, 289, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Lechan, R.M.; Fekete, C. Role of Thyroid Hormone Deiodination in the Hypothalamus. Thyroid 2005, 15, 883–897. [Google Scholar] [CrossRef]
- Mayerl, S.; Müller, J.; Bauer, R.; Richert, S.; Kassmann, C.M.; Darras, V.M.; Buder, K.; Boelen, A.; Visser, T.J.; Heuer, H. Transporters MCT8 and OATP1C1 Maintain Murine Brain Thyroid Hormone Homeostasis. J. Clin. Investig. 2014, 124, 1987–1999. [Google Scholar] [CrossRef]
- Wallis, K.; Dudazy, S.; van Hogerlinden, M.; Nordström, K.; Mittag, J.; Vennström, B. The Thyroid Hormone Receptor Alpha1 Protein Is Expressed in Embryonic Postmitotic Neurons and Persists in Most Adult Neurons. Mol. Endocrinol. 2010, 24, 1904–1916. [Google Scholar] [CrossRef]
- Hameed, S.; Patterson, M.; Dhillo, W.S.; Rahman, S.A.; Ma, Y.; Holton, C.; Gogakos, A.; Yeo, G.S.H.; Lam, B.Y.H.; Polex-Wolf, J.; et al. Thyroid Hormone Receptor Beta in the Ventromedial Hypothalamus Is Essential for the Physiological Regulation of Food Intake and Body Weight. Cell Rep. 2017, 19, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Whittle, A.J.; Carobbio, S.; Martins, L.; Slawik, M.; Hondares, E.; Vázquez, M.J.; Morgan, D.; Csikasz, R.I.; Gallego, R.; Rodriguez-Cuenca, S.; et al. BMP8B Increases Brown Adipose Tissue Thermogenesis through Both Central and Peripheral Actions. Cell 2012, 149, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Lockie, S.H.; Heppner, K.M.; Chaudhary, N.; Chabenne, J.R.; Morgan, D.A.; Veyrat-Durebex, C.; Ananthakrishnan, G.; Rohner-Jeanrenaud, F.; Drucker, D.J.; DiMarchi, R.; et al. Direct Control of Brown Adipose Tissue Thermogenesis by Central Nervous System Glucagon-like Peptide-1 Receptor Signaling. Diabetes 2012, 61, 2753–2762. [Google Scholar] [CrossRef]
- Martínez de Morentin, P.B.; González-García, I.; Martins, L.; Lage, R.; Fernández-Mallo, D.; Martínez-Sánchez, N.; Ruíz-Pino, F.; Liu, J.; Morgan, D.A.; Pinilla, L.; et al. Estradiol Regulates Brown Adipose Tissue Thermogenesis via Hypothalamic AMPK. Cell Metab. 2014, 20, 41–53. [Google Scholar] [CrossRef]
- Tanida, M.; Yamamoto, N.; Shibamoto, T.; Rahmouni, K. Involvement of Hypothalamic AMP-Activated Protein Kinase in Leptin-Induced Sympathetic Nerve Activation. PLoS ONE 2013, 8, e56660. [Google Scholar] [CrossRef]
- Martínez-Sánchez, N.; Seoane-Collazo, P.; Contreras, C.; Varela, L.; Villarroya, J.; Rial-Pensado, E.; Buqué, X.; Aurrekoetxea, I.; Delgado, T.C.; Vázquez-Martínez, R.; et al. Hypothalamic AMPK-ER Stress-JNK1 Axis Mediates the Central Actions of Thyroid Hormones on Energy Balance. Cell Metab. 2017, 26, 212–229.e12. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.; González-García, I.; Seoane-Collazo, P.; Martínez-Sánchez, N.; Liñares-Pose, L.; Rial-Pensado, E.; Fernø, J.; Tena-Sempere, M.; Casals, N.; Diéguez, C.; et al. Reduction of Hypothalamic Endoplasmic Reticulum Stress Activates Browning of White Fat and Ameliorates Obesity. Diabetes 2017, 66, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bisschop, P.H.; Foppen, E.; van Beeren, H.C.; Kalsbeek, A.; Boelen, A.; Fliers, E. A Model for Chronic, Intrahypothalamic Thyroid Hormone Administration in Rats. J. Endocrinol. 2016, 229, 37–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Z.; Foppen, E.; Su, Y.; Bisschop, P.H.; Kalsbeek, A.; Fliers, E.; Boelen, A. Metabolic Effects of Chronic T3 Administration in the Hypothalamic Paraventricular and Ventromedial Nucleus in Male Rats. Endocrinology 2016, 157, 4076–4085. [Google Scholar] [CrossRef][Green Version]
- Bachman, E.S.; Hampton, T.G.; Dhillon, H.; Amende, I.; Wang, J.; Morgan, J.P.; Hollenberg, A.N. The Metabolic and Cardiovascular Effects of Hyperthyroidism Are Largely Independent of Beta-Adrenergic Stimulation. Endocrinology 2004, 145, 2767–2774. [Google Scholar] [CrossRef]
- Morte, B.; Bernal, J. Thyroid Hormone Action: Astrocyte–Neuron Communication. Front. Endocrinol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Gavaldà-Navarro, A.; Giralt, M. Toward an Understanding of How Immune Cells Control Brown and Beige Adipobiology. Cell Metab. 2018, 27, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Villicev, C.M.; Freitas, F.R.S.; Aoki, M.S.; Taffarel, C.; Scanlan, T.S.; Moriscot, A.S.; Ribeiro, M.O.; Bianco, A.C.; Gouveia, C.H.A. Thyroid Hormone Receptor Beta-Specific Agonist GC-1 Increases Energy Expenditure and Prevents Fat-Mass Accumulation in Rats. J. Endocrinol. 2007, 193, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Z.; Martagón, A.J.; Cimini, S.L.; Gonzalez, D.D.; Tinkey, D.W.; Biter, A.; Baxter, J.D.; Webb, P.; Gustafsson, J.-Å.; Hartig, S.M.; et al. Pharmacological Activation of Thyroid Hormone Receptors Elicits a Functional Conversion of White to Brown Fat. Cell Rep. 2015, 13, 1528–1537. [Google Scholar] [CrossRef]
- Johann, K.; Cremer, A.L.; Fischer, A.W.; Heine, M.; Pensado, E.R.; Resch, J.; Nock, S.; Virtue, S.; Harder, L.; Oelkrug, R.; et al. Thyroid-Hormone-Induced Browning of White Adipose Tissue Does Not Contribute to Thermogenesis and Glucose Consumption. Cell Rep. 2019, 27, 3385–3400.e3. [Google Scholar] [CrossRef]
- Martínez-Sánchez, N.; Moreno-Navarrete, J.M.; Contreras, C.; Rial-Pensado, E.; Fernø, J.; Nogueiras, R.; Diéguez, C.; Fernández-Real, J.-M.; López, M. Thyroid Hormones Induce Browning of White Fat. J. Endocrinol. 2016, 232, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Crespo, M.; Csikasz, R.I.; Martínez-Sánchez, N.; Diéguez, C.; Cannon, B.; Nedergaard, J.; López, M. Essential Role of UCP1 Modulating the Central Effects of Thyroid Hormones on Energy Balance. Mol. Metab. 2016, 5, 271–282. [Google Scholar] [CrossRef]
- Bamshad, M.; Aoki, V.T.; Adkison, M.G.; Warren, W.S.; Bartness, T.J. Central Nervous System Origins of the Sympathetic Nervous System Outflow to White Adipose Tissue. Am. J. Physiol. 1998, 275, R291–R299. [Google Scholar] [CrossRef]
- Bamshad, M.; Song, C.K.; Bartness, T.J. CNS Origins of the Sympathetic Nervous System Outflow to Brown Adipose Tissue. Am. J. Physiol. 1999, 276, R1569–R1578. [Google Scholar] [CrossRef]
- Weiner, J.; Kranz, M.; Klöting, N.; Kunath, A.; Steinhoff, K.; Rijntjes, E.; Köhrle, J.; Zeisig, V.; Hankir, M.; Gebhardt, C.; et al. Thyroid Hormone Status Defines Brown Adipose Tissue Activity and Browning of White Adipose Tissues in Mice. Sci. Rep. 2016, 6, 38124. [Google Scholar] [CrossRef] [PubMed]
- Dehvari, N.; Sato, M.; Bokhari, M.H.; Kalinovich, A.; Ham, S.; Gao, J.; Nguyen, H.T.M.; Whiting, L.; Mukaida, S.; Merlin, J.; et al. The Metabolic Effects of Mirabegron Are Mediated Primarily by Β3 -Adrenoceptors. Pharmacol. Res. Perspect. 2020, 8, e00643. [Google Scholar] [CrossRef] [PubMed]
- Nomura, E.; Toyoda, N.; Harada, A.; Nishimura, K.; Ukita, C.; Morimoto, S.; Kosaki, A.; Iwasaka, T.; Nishikawa, M. Type 2 Iodothyronine Deiodinase Is Expressed in Human Preadipocytes. Thyroid 2011, 21, 305–310. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Takahashi, N.; Yasubuchi, M.; Kim, Y.-I.; Hashizaki, H.; Kim, M.-J.; Sakamoto, T.; Goto, T.; Kawada, T. Triiodothyronine Induces UCP-1 Expression and Mitochondrial Biogenesis in Human Adipocytes. Am. J. Physiol. Cell Physiol. 2012, 302, C463–C472. [Google Scholar] [CrossRef]
- de Oliveira, M.; Mathias, L.S.; Rodrigues, B.M.; Mariani, B.G.; Graceli, J.B.; De Sibio, M.T.; Castro Olimpio, R.M.; Fontes Moretto, F.C.; Deprá, I.C.; Nogueira, C.R. The Roles of Triiodothyronine and Irisin in Improving the Lipid Profile and Directing the Browning of Human Adipose Subcutaneous Cells. Mol. Cell Endocrinol. 2020, 506, 110744. [Google Scholar] [CrossRef]
- Dittner, C.; Lindsund, E.; Cannon, B.; Nedergaard, J. At Thermoneutrality, Acute Thyroxine-Induced Thermogenesis and Pyrexia Are Independent of UCP1. Mol. Metab. 2019, 25, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Kang, Q.; Yoneshiro, T.; Camporez, J.P.; Maki, H.; Homma, M.; Shinoda, K.; Chen, Y.; Lu, X.; Maretich, P.; et al. UCP1-Independent Signaling Involving SERCA2b-Mediated Calcium Cycling Regulates Beige Fat Thermogenesis and Systemic Glucose Homeostasis. Nat. Med. 2017, 23, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L.; Chouchani, E.T.; Lu, G.Z.; Jedrychowski, M.P.; Bare, C.J.; Mina, A.I.; Kumari, M.; Zhang, S.; Vuckovic, I.; Laznik-Bogoslavski, D.; et al. Genetic Depletion of Adipocyte Creatine Metabolism Inhibits Diet-Induced Thermogenesis and Drives Obesity. Cell Metab. 2017, 26, 693. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, K.G.; Krause, K.; Linder, N.; Rullmann, M.; Volke, L.; Gebhardt, C.; Busse, H.; Stumvoll, M.; Blüher, M.; Sabri, O.; et al. Effects of Hyperthyroidism on Adipose Tissue Activity and Distribution in Adults. Thyroid 2021, 31, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.S.; Savage, D.B.; Dufour, S.; Schoenmakers, N.; Murgatroyd, P.; Befroy, D.; Halsall, D.; Northcott, S.; Raymond-Barker, P.; Curran, S.; et al. Resistance to Thyroid Hormone Is Associated with Raised Energy Expenditure, Muscle Mitochondrial Uncoupling, and Hyperphagia. J. Clin. Investig. 2010, 120, 1345–1354. [Google Scholar] [CrossRef]
- Nicolaisen, T.S.; Klein, A.B.; Dmytriyeva, O.; Lund, J.; Ingerslev, L.R.; Fritzen, A.M.; Carl, C.S.; Lundsgaard, A.-M.; Frost, M.; Ma, T.; et al. Thyroid Hormone Receptor α in Skeletal Muscle Is Essential for T3-Mediated Increase in Energy Expenditure. FASEB J. 2020, 34, 15480–15491. [Google Scholar] [CrossRef]
- Miniou, P.; Tiziano, D.; Frugier, T.; Roblot, N.; Le Meur, M.; Melki, J. Gene Targeting Restricted to Mouse Striated Muscle Lineage. Nucleic Acids Res. 1999, 27, e27. [Google Scholar] [CrossRef]
- Quignodon, L.; Vincent, S.; Winter, H.; Samarut, J.; Flamant, F. A Point Mutation in the Activation Function 2 Domain of Thyroid Hormone Receptor A1 Expressed after CRE-Mediated Recombination Partially Recapitulates Hypothyroidism. Mol. Endocrinol. 2007, 21, 2350–2360. [Google Scholar] [CrossRef]
- Autry, J.M.; Thomas, D.D.; Espinoza-Fonseca, L.M. Sarcolipin Promotes Uncoupling of the SERCA Ca2+ Pump by Inducing a Structural Rearrangement in the Energy-Transduction Domin. Biochemistry 2016, 55, 6083–6086. [Google Scholar] [CrossRef]
- Bal, N.C.; Maurya, S.K.; Sopariwala, D.H.; Sahoo, S.K.; Gupta, S.C.; Shaikh, S.A.; Pant, M.; Rowland, L.A.; Bombardier, E.; Goonasekera, S.A.; et al. Sarcolipin Is a Newly Identified Regulator of Muscle-Based Thermogenesis in Mammals. Nat. Med. 2012, 18, 1575–1579. [Google Scholar] [CrossRef]
- Werneck-de-Castro, J.P.; Fonseca, T.L.; Ignacio, D.L.; Fernandes, G.W.; Andrade-Feraud, C.M.; Lartey, L.J.; Ribeiro, M.B.; Ribeiro, M.O.; Gereben, B.; Bianco, A.C. Thyroid Hormone Signaling in Male Mouse Skeletal Muscle Is Largely Independent of D2 in Myocytes. Endocrinology 2015, 156, 3842–3852. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jiang, Y.; Meltzer, P.; Yen, P.M. Thyroid Hormone Regulation of Hepatic Genes in Vivo Detected by Complementary DNA Microarray. Mol. Endocrinol. 2000, 14, 947–955. [Google Scholar] [CrossRef]
- Santiago, L.A.; Santiago, D.A.; Faustino, L.C.; Cordeiro, A.; Lisboa, P.C.; Wondisford, F.E.; Pazos-Moura, C.C.; Ortiga-Carvalho, T.M. The Δ337T Mutation on the TRβ Causes Alterations in Growth, Adiposity, and Hepatic Glucose Homeostasis in Mice. J. Endocrinol. 2011, 211, 39–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jornayvaz, F.R.; Lee, H.-Y.; Jurczak, M.J.; Alves, T.C.; Guebre-Egziabher, F.; Guigni, B.A.; Zhang, D.; Samuel, V.T.; Silva, J.E.; Shulman, G.I. Thyroid Hormone Receptor-α Gene Knockout Mice Are Protected from Diet-Induced Hepatic Insulin Resistance. Endocrinology 2012, 153, 583–591. [Google Scholar] [CrossRef]
- Ramadoss, P.; Abraham, B.J.; Tsai, L.; Zhou, Y.; Costa-e-Sousa, R.H.; Ye, F.; Bilban, M.; Zhao, K.; Hollenberg, A.N. Novel Mechanism of Positive versus Negative Regulation by Thyroid Hormone Receptor Β1 (TRβ1) Identified by Genome-Wide Profiling of Binding Sites in Mouse Liver. J. Biol. Chem. 2014, 289, 1313–1328. [Google Scholar] [CrossRef]
- Klieverik, L.P.; Sauerwein, H.P.; Ackermans, M.T.; Boelen, A.; Kalsbeek, A.; Fliers, E. Effects of Thyrotoxicosis and Selective Hepatic Autonomic Denervation on Hepatic Glucose Metabolism in Rats. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E513–E520. [Google Scholar] [CrossRef] [PubMed]
- Klieverik, L.P.; Janssen, S.F.; van Riel, A.; Foppen, E.; Bisschop, P.H.; Serlie, M.J.; Boelen, A.; Ackermans, M.T.; Sauerwein, H.P.; Fliers, E.; et al. Thyroid Hormone Modulates Glucose Production via a Sympathetic Pathway from the Hypothalamic Paraventricular Nucleus to the Liver. Proc. Natl. Acad. Sci. USA 2009, 106, 5966–5971. [Google Scholar] [CrossRef]
- Mai, W.; Janier, M.F.; Allioli, N.; Quignodon, L.; Chuzel, T.; Flamant, F.; Samarut, J. Thyroid Hormone Receptor Alpha Is a Molecular Switch of Cardiac Function between Fetal and Postnatal Life. Proc. Natl. Acad. Sci. USA 2004, 101, 10332–10337. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Payumo, A.Y.; Cutie, S.; Hoang, A.; Zhang, H.; Guyot, R.; Lunn, D.; Bigley, R.B.; Yu, H.; Wang, J.; et al. Evidence for Hormonal Control of Heart Regenerative Capacity during Endothermy Acquisition. Science 2019, 364, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Pachucki, J.; Hopkins, J.; Peeters, R.; Tu, H.; Carvalho, S.D.; Kaulbach, H.; Abel, E.D.; Wondisford, F.E.; Ingwall, J.S.; Larsen, P.R. Type 2 Iodothyronin Deiodinase Transgene Expression in the Mouse Heart Causes Cardiac-Specific Thyrotoxicosis. Endocrinology 2001, 142, 13–20. [Google Scholar] [CrossRef][Green Version]
- Vujovic, M.; Nordström, K.; Gauthier, K.; Flamant, F.; Visser, T.J.; Vennström, B.; Mittag, J. Interference of a Mutant Thyroid Hormone Receptor Alpha1 with Hepatic Glucose Metabolism. Endocrinology 2009, 150, 2940–2947. [Google Scholar] [CrossRef] [PubMed]
- Mittag, J.; Davis, B.; Vujovic, M.; Arner, A.; Vennström, B. Adaptations of the Autonomous Nervous System Controlling Heart Rate Are Impaired by a Mutant Thyroid Hormone Receptor-Alpha1. Endocrinology 2010, 151, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.; Dratman, M.B.; Crutchfield, F.L.; Jennings, A.S.; Maruniak, J.A.; Gibbons, R. Intrathecal Triiodothyronine Administration Causes Greater Heart Rate Stimulation in Hypothyroid Rats than Intravenously Delivered Hormone. Evidence for a Central Nervous System Site of Thyroid Hormone Action. J. Clin. Investig. 1985, 76, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, H.L.; Strait, K.A.; Ling, N.C.; Oppenheimer, J.H. Quantitation of Rat Tissue Thyroid Hormone Binding Receptor Isoforms by Immunoprecipitation of Nuclear Triiodothyronine Binding Capacity. J. Biol. Chem. 1992, 267, 11794–11799. [Google Scholar] [CrossRef]
- Swanson, E.A.; Gloss, B.; Belke, D.D.; Kaneshige, M.; Cheng, S.-Y.; Dillmann, W.H. Cardiac Expression and Function of Thyroid Hormone Receptor Beta and Its PV Mutant. Endocrinology 2003, 144, 4820–4825. [Google Scholar] [CrossRef][Green Version]
- Bates, J.M.; St Germain, D.L.; Galton, V.A. Expression Profiles of the Three Iodothyronine Deiodinases, D1, D2, and D3, in the Developing Rat. Endocrinology 1999, 140, 844–851. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Im, S.-K.; Fang, S. Mouse Cre-LoxP System: General Principles to Determine Tissue-Specific Roles of Target Genes. Lab Anim. Res. 2018, 34, 147–159. [Google Scholar] [CrossRef]
- Tinnikov, A.; Nordström, K.; Thorén, P.; Kindblom, J.M.; Malin, S.; Rozell, B.; Adams, M.; Rajanayagam, O.; Pettersson, S.; Ohlsson, C.; et al. Retardation of Post-Natal Development Caused by a Negatively Acting Thyroid Hormone Receptor Alpha1. EMBO J. 2002, 21, 5079–5087. [Google Scholar] [CrossRef]
- Gouveia, C.H.A.; Miranda-Rodrigues, M.; Martins, G.M.; Neofiti-Papi, B. Thyroid Hormone and Skeletal Development. Vitam Horm. 2018, 106, 383–472. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, G.; Giannini, C.; Chiarelli, F. Effect of Thyroid Hormones on Neurons and Neurodevelopment. Horm. Res. Paediatr. 2018, 90, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Ribich, S.; Christoffolete, M.A.; Simovic, G.; Correa-Medina, M.; Patti, M.E.; Bianco, A.C. Absence of Thyroid Hormone Activation during Development Underlies a Permanent Defect in Adaptive Thermogenesis. Endocrinology 2010, 151, 4573–4582. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Carvalho, S.D.; Carvalho, C.R.; Rabelo, R.; Moriscot, A.S. Thyroxine 5’-Deiodination Mediates Norepinephrine-Induced Lipogenesis in Dispersed Brown Adipocytes. Endocrinology 1998, 139, 571–578. [Google Scholar] [CrossRef][Green Version]
- Negron, S.G.; Ercan-Sencicek, A.G.; Freed, J.; Walters, M.; Lin, Z. Both Proliferation and Lipogenesis of Brown Adipocytes Contribute to Postnatal Brown Adipose Tissue Growth in Mice. Sci. Rep. 2020, 10, 20335. [Google Scholar] [CrossRef]
- Saunders, T.L. Inducible Transgenic Mouse Models. Methods Mol. Biol. 2011, 693, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Brocard, J.; Warot, X.; Wendling, O.; Messaddeq, N.; Vonesch, J.L.; Chambon, P.; Metzger, D. Spatio-Temporally Controlled Site-Specific Somatic Mutagenesis in the Mouse. Proc. Natl. Acad. Sci. USA 1997, 94, 14559–14563. [Google Scholar] [CrossRef]
- Shoemaker, T.J.; Kono, T.; Mariash, C.N.; Evans-Molina, C. Thyroid Hormone Analogues for the Treatment of Metabolic Disorders: New Potential for Unmet Clinical Needs? Endocr. Pract. 2012, 18, 954–964. [Google Scholar] [CrossRef]
- Finan, B.; Yang, B.; Ottaway, N.; Stemmer, K.; Müller, T.D.; Yi, C.-X.; Habegger, K.; Schriever, S.C.; García-Cáceres, C.; Kabra, D.G.; et al. Targeted Estrogen Delivery Reverses the Metabolic Syndrome. Nat. Med. 2012, 18, 1847–1856. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, Physiology, and Mechanisms of Incretin Hormone Action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef]
- Finan, B.; Clemmensen, C.; Zhu, Z.; Stemmer, K.; Gauthier, K.; Müller, L.; De Angelis, M.; Moreth, K.; Neff, F.; Perez-Tilve, D.; et al. Chemical Hybridization of Glucagon and Thyroid Hormone Optimizes Therapeutic Impact for Metabolic Disease. Cell 2016, 167, 843–857.e14. [Google Scholar] [CrossRef]
- Svoboda, M.; Tastenoy, M.; Vertongen, P.; Robberecht, P. Relative Quantitative Analysis of Glucagon Receptor MRNA in Rat Tissues. Mol. Cell Endocrinol. 1994, 105, 131–137. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zekri, Y.; Flamant, F.; Gauthier, K. Central vs. Peripheral Action of Thyroid Hormone in Adaptive Thermogenesis: A Burning Topic. Cells 2021, 10, 1327. https://doi.org/10.3390/cells10061327
Zekri Y, Flamant F, Gauthier K. Central vs. Peripheral Action of Thyroid Hormone in Adaptive Thermogenesis: A Burning Topic. Cells. 2021; 10(6):1327. https://doi.org/10.3390/cells10061327
Chicago/Turabian StyleZekri, Yanis, Frédéric Flamant, and Karine Gauthier. 2021. "Central vs. Peripheral Action of Thyroid Hormone in Adaptive Thermogenesis: A Burning Topic" Cells 10, no. 6: 1327. https://doi.org/10.3390/cells10061327
APA StyleZekri, Y., Flamant, F., & Gauthier, K. (2021). Central vs. Peripheral Action of Thyroid Hormone in Adaptive Thermogenesis: A Burning Topic. Cells, 10(6), 1327. https://doi.org/10.3390/cells10061327




