Thyroid Hormone Receptor Is Essential for Larval Epithelial Apoptosis and Adult Epithelial Stem Cell Development but Not Adult Intestinal Morphogenesis during Xenopus tropicalis Metamorphosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Generation of TR Double Knockout Xenopus tropicalis Animals and Genotyping
2.3. T3 Treatment
2.4. RNA Extraction and qRT-PCR
2.5. 5-Ethynyl-2-Deoxyuridine (EdU) Labeling
2.6. TUNEL Assays
2.7. Methyl Green-Pyronin Y (MGPY) Staining
2.8. Immunohistochemistry
2.9. Whole Transcriptome Sequencing (RNA-Seq)
2.10. Statistical Analysis
3. Results
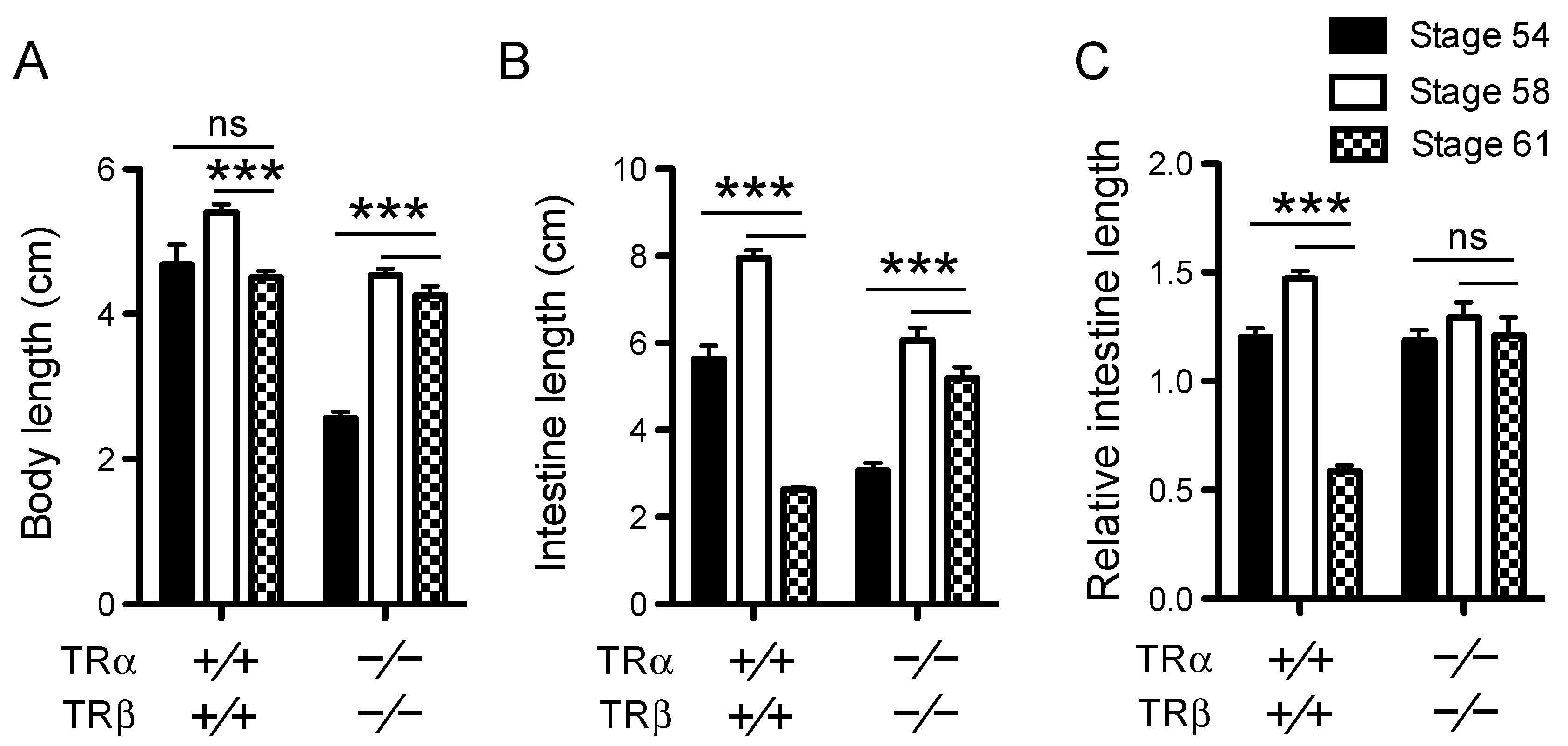
3.1. TR Double Knockout Blocks Intestinal Length Reduction During Metamorphosis
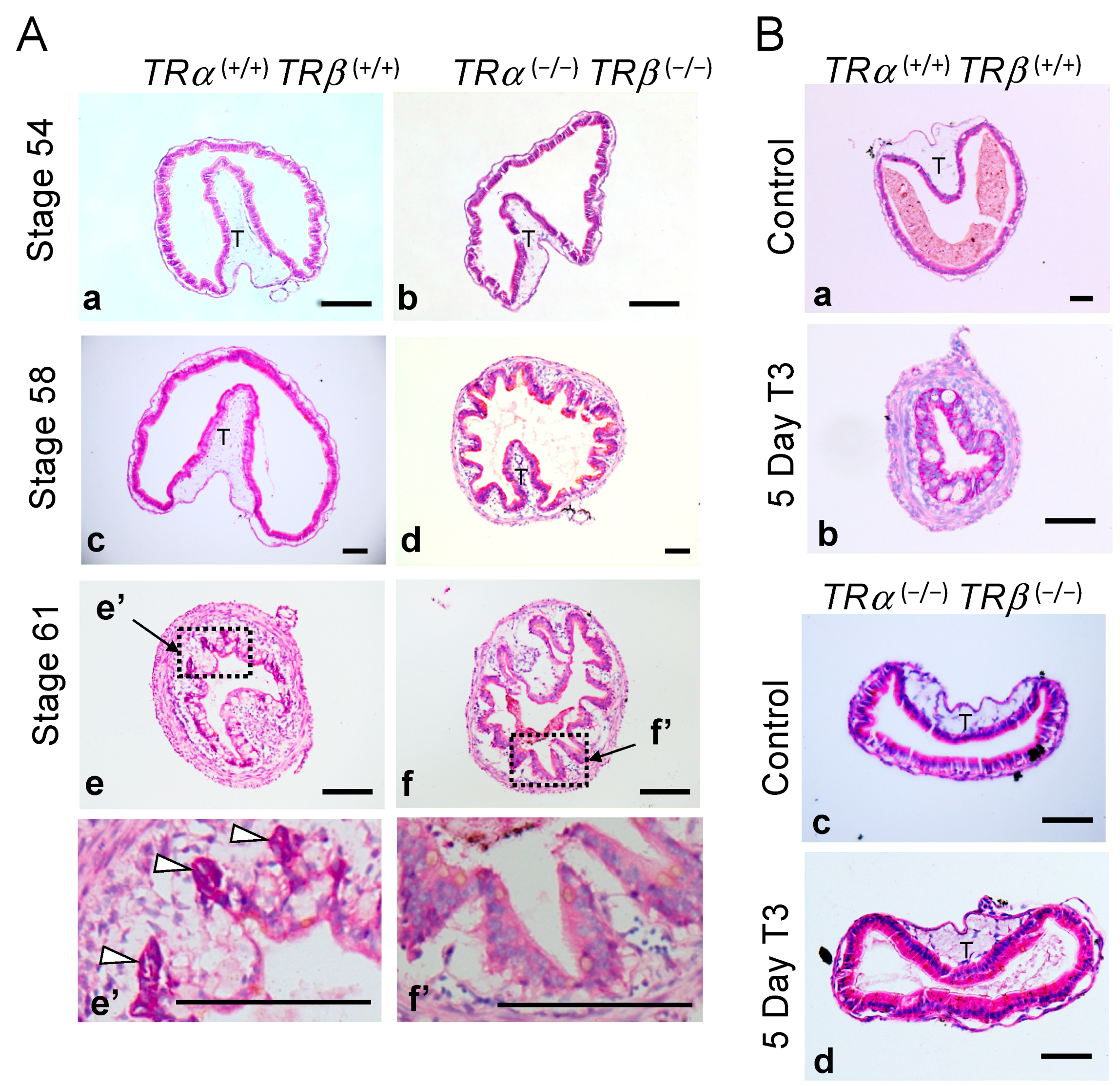
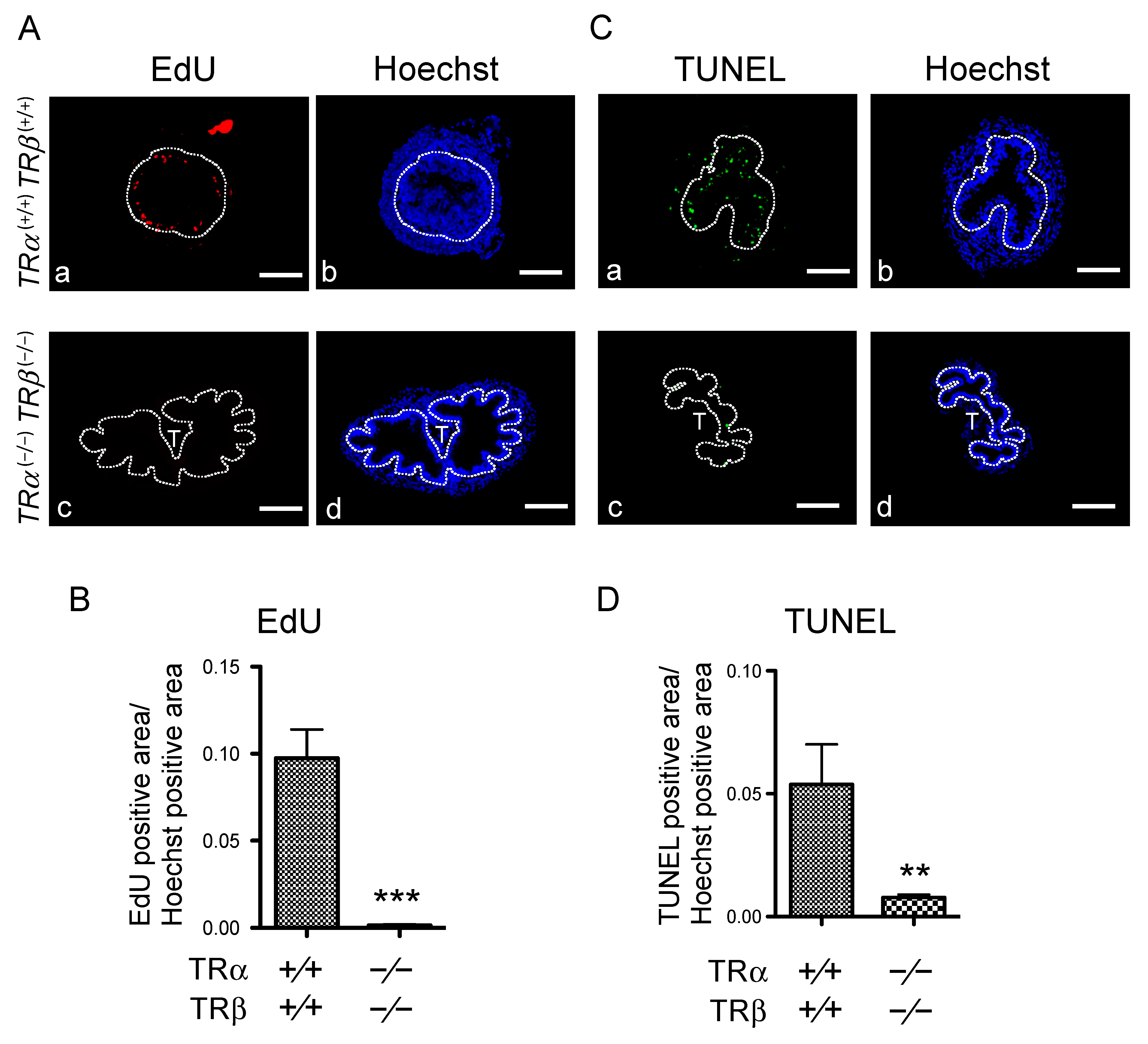
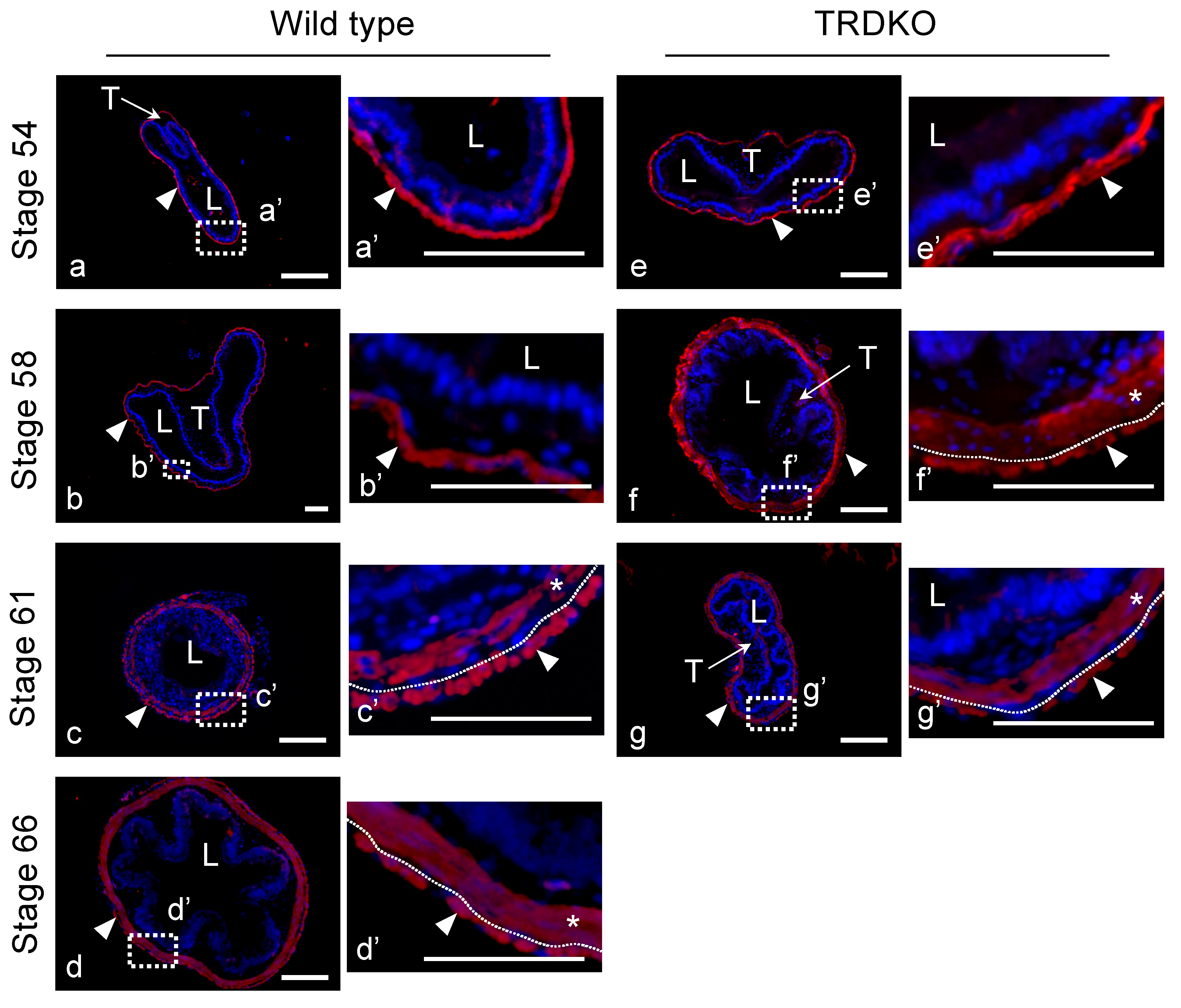
3.2. Abnormal Intestinal Development in TRDKO Xenopus tropicalis Tadpoles
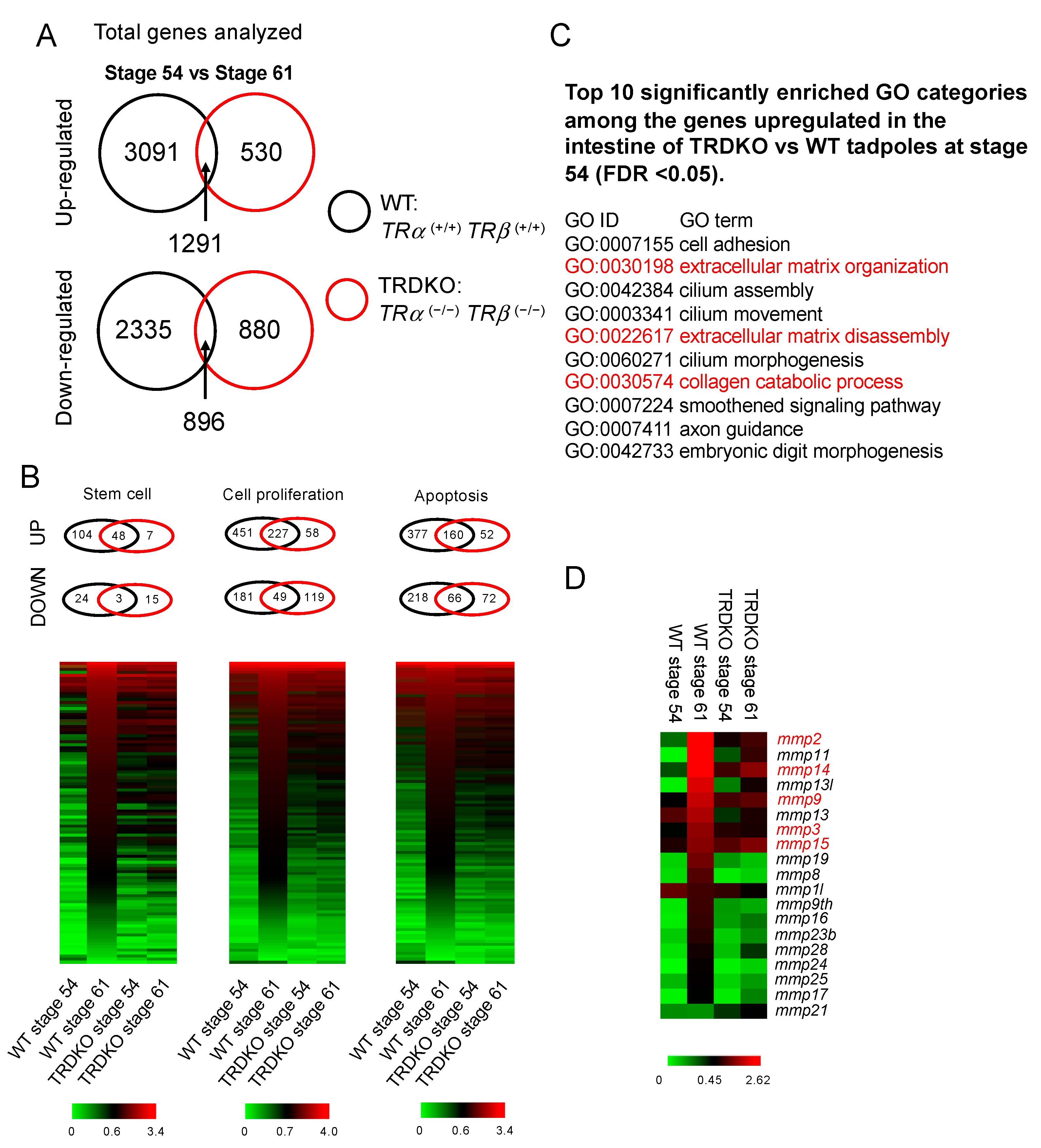
3.3. Gene Regulation Programs Underlying the Observed Abnormal Intestinal Development in TRDKO Tadpoles
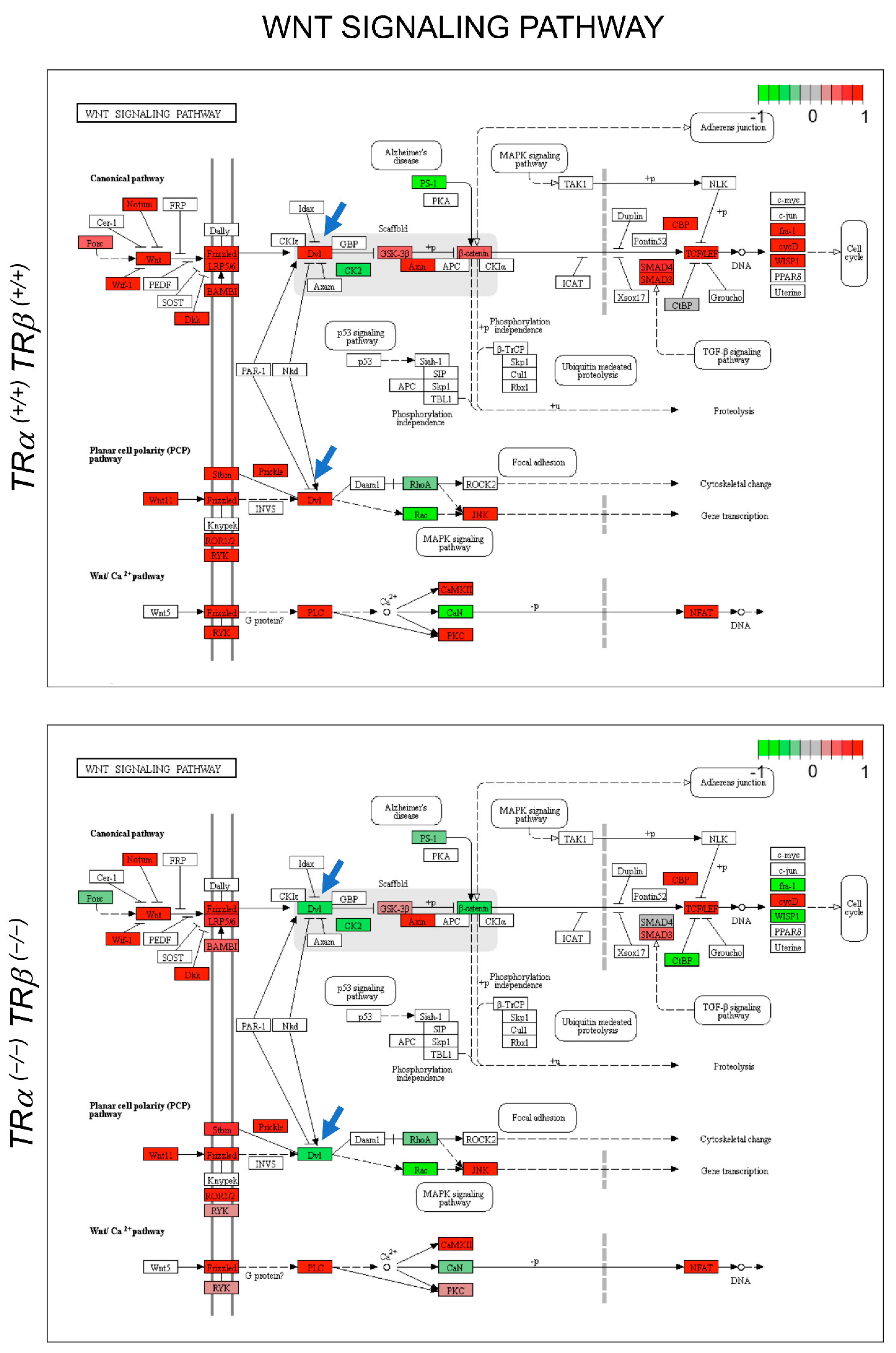
4. Discussion
4.1. TRα and TR® Are the Only Receptors Mediating Intestinal Epithelial Remodeling by T3 in Xenopus tropicalis
4.2. Liganded TR Induces Larval Epithelial Cell Death and Adult Stem Cell Development
4.3. Unliganded TR Prevents Precocious Adult Epithelial Morphogenesis and Smooth Muscle Development During Intestinal Remodeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hetzel, B.S. The story of Iodine Deficiency: An. International Challenge in Nutrition; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Vermiglio, F.; Presti, V.P.L.; Moleti, M.; Sidoti, M.; Tortorella, G.; Scaffidi, G.; Castagna, M.G.; Mattina, F.; Violi, M.A.; Crisà, A.; et al. Attention Deficit and Hyperactivity Disorders in the Offspring of Mothers Exposed to Mild-Moderate Iodine Deficiency: A Possible Novel Iodine Deficiency Disorder in Developed Countries. J. Clin. Endocrinol. Metab. 2004, 89, 6054–6060. [Google Scholar] [CrossRef]
- de Escobar, G.M.; Obregon, M.J.; del Rey, F.E. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr. 2007, 10, 1554–1570. [Google Scholar] [CrossRef]
- Tata, J.R. Gene expression during metamorphosis: An ideal model for post-embryonic development. Bioessays 1993, 15, 239–248. [Google Scholar] [CrossRef]
- Shi, Y.-B. Amphibian Metamorphosis: From Morphology to Molecular Biology; John Wiley and Sons: New York, NY, USA, 1999. [Google Scholar]
- Shi, Y.-B.; Ishizuya-Oka, A. Biphasic intestinal development in amphibians: Embryogensis and remodeling during metamorphosis. Curr. Top. Dev. Biol. 1996, 32, 205–235. [Google Scholar]
- Bao, L.; Shi, B.; Shi, Y.-B. Intestinal homeostasis: A communication between life and death. Cell Biosci. 2020, 10, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Shi, Y.-B. Thyroid Hormone Regulation of Adult Intestinal Stem Cell Development: Mechanisms and Evolutionary Conservations. Int. J. Biol. Sci. 2012, 8, 1217–1224. [Google Scholar] [CrossRef]
- Wong, J.; Shi, Y.B.; Wolffe, A.P. A role for nucleosome assembly in both silencing and activation of the Xenopus TR beta A gene by the thyroid hormone receptor. Genes Dev. 1995, 9, 2696–2711. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A. Thyroid hormone receptors: Multiple forms, multiple possibilities. Endocr. Rev. 1993, 14, 184–193. [Google Scholar]
- Yen, P.M. Physiological and Molecular Basis of Thyroid Hormone Action. Physiol. Rev. 2001, 81, 1097–1142. [Google Scholar] [CrossRef]
- Evans, R.M. The steroid and thyroid hormone receptor superfamily. Science 1988, 240, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; O’Malley, B.W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann. Rev. Biochem. 1994, 63, 451–486. [Google Scholar] [CrossRef]
- Laudet, V.; Gronemeyer, H. The Nuclear Receptor Facts Book; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Shi, Y.-B.; Wong, J.; Puzianowska-Kuznicka, M.; Stolow, M.A. Tadpole competence and tissue-specific temporal regulation of amphibian metamorphosis: Roles of thyroid hormone and its receptors. BioEssays 1996, 18, 391–399. [Google Scholar] [CrossRef]
- Sachs, L.M.; Damjanovski, S.; Jones, P.L.; Li, Q.; Amano, T.; Ueda, S.; Shi, Y.-B.; Ishizuya-Oka, A. Dual functions of thyroid hormone receptors during Xenopus development. Comp. Biochem. Physiol. Part. B Biochem. Mol. Biol. 2000, 126, 199–211. [Google Scholar] [CrossRef]
- Schreiber, A.M.; Das, B.; Huang, H.; Marsh-Armstrong, N.; Brown, D.D. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 10739–10744. [Google Scholar] [CrossRef]
- Brown, D.D.; Cai, L. Amphibian metamorphosis. Dev. Biol. 2007, 306, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, D.R.; Hsia, S.-C.V.; Fu, L.; Shi, Y.-B. A Dominant-Negative Thyroid Hormone Receptor Blocks Amphibian Metamorphosis by Retaining Corepressors at Target Genes. Mol. Cell. Biol. 2003, 23, 6750–6758. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, D.R.; Tomita, A.; Fu, L.; Paul, B.D.; Shi, Y.-B. Transgenic Analysis Reveals that Thyroid Hormone Receptor Is Sufficient To Mediate the Thyroid Hormone Signal in Frog Metamorphosis. Mol. Cell. Biol. 2004, 24, 9026–9037. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, D.R.; Paul, B.D.; Fu, L.; Shi, Y.-B. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen. Comp. Endocrinol. 2006, 145, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-B. Dual Functions of Thyroid Hormone Receptors in Vertebrate Development: The Roles of Histone-Modifying Cofactor Complexes. Thyroid 2009, 19, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Yaoita, Y. Dual mechanisms governing muscle cell death in tadpole tail during amphibian metamorphosis. Dev. Dyn. 2003, 227, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Denver, R.J.; Hu, F.; Scanlan, T.S.; Furlow, J.D. Thyroid hormone receptor subtype specificity for hormone-dependent neurogenesis in Xenopus laevis. Dev. Biol. 2009, 326, 155–168. [Google Scholar] [CrossRef]
- Bagamasbad, P.; Howdeshell, K.L.; Sachs, L.M.; Demeneix, B.A.; Denver, R.J. A role for basic transcription element-binding protein 1 (BTEB1) in the autoinduction of thyroid hormone receptor beta. J. Biol. Chem. 2008, 283, 2275–2285. [Google Scholar] [CrossRef]
- Schreiber, A.M.; Mukhi, S.; Brown, D.D. Cell–cell interactions during remodeling of the intestine at metamorphosis in Xenopus laevis. Dev. Biol. 2009, 331, 89–98. [Google Scholar] [CrossRef]
- Shi, Y.-B. Molecular biology of amphibian metamorphosis: A new approach to an old problem. Trends Endocrinol. Metab. 1994, 5, 14–20. [Google Scholar] [CrossRef]
- Shi, Y.-B.; Matsuura, K.; Fujimoto, K.; Wen, L.; Fu, L. Thyroid hormone receptor actions on transcription in amphibia: The roles of histone modification and chromatin disruption. Cell Biosci. 2012, 2, 42. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.; Buisine, N.; Miller, T.; Shi, Y.-B.; Sachs, L.M. Mechanisms of thyroid hormone receptor action during development: Lessons from amphibian studies. Biochim. Biophys. Acta—Gen. Subj. 2013, 1830, 3882–3892. [Google Scholar] [CrossRef] [PubMed]
- Puzianowska-Kuznicka, M.; Damjanovski, S.; Shi, Y.B. Both thyroid hormone and 9-cis retinoic acid receptors are required to efficiently mediate the effects of thyroid hormone on embryonic development and specific gene regulation in Xenopus laevis. Mol. Cell. Biol. 1997, 17, 4738–4749. [Google Scholar] [CrossRef][Green Version]
- Sachs, L.M.; Shi, Y.-B. Targeted chromatin binding and histone acetylation in vivo by thyroid hormone receptor during amphibian development. Proc. Natl. Acad. Sci. USA 2000, 97, 13138–13143. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Ishizuya-Oka, A.; Buchholz, D.R. Growth, Development, and Intestinal Remodeling Occurs in the Absence of Thyroid Hormone Receptor α in Tadpoles of Xenopus tropicalis. Endocrinology 2017, 158, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Suzuki, K.I.; Sakuma, T.; Shewade, L.; Yamamoto, T.; Buchholz, D.R. Unliganded thyroid hormone receptor alpha regulates developmental timing via gene repression as revealed by gene disruption in Xenopus tropicalis. Endocrinology 2015, 156, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Sakane, Y.; Iida, M.; Hasebe, T.; Fujii, S.; Buchholz, D.R.; Ishizuya-Oka, A.; Yamamoto, T.; Suzuki, K.-I.T. Functional analysis of thyroid hormone receptor beta in Xenopus tropicalis founders using CRISPR-Cas. Biol. Open 2018, 7, bio030338. [Google Scholar] [CrossRef]
- Nakajima, K.; Tazawa, I.; Yaoita, Y. Thyroid Hormone Receptor alpha- and beta-Knockout Xenopus tropicalis Tadpoles Reveal Subtype-Specific Roles During Development. Endocrinology 2018, 159, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Shi, Y.-B. Unliganded Thyroid Hormone Receptor α Controls Developmental Timing in Xenopus tropicalis. Endocrinol. 2015, 156, 721–734. [Google Scholar] [CrossRef]
- Wen, L.; Shibata, Y.; Su, D.; Fu, L.; Luu, N.; Shi, Y.-B. Thyroid Hormone Receptor α Controls Developmental Timing and Regulates the Rate and Coordination of Tissue-Specific Metamorphosis in Xenopus tropicalis. Endocrinology 2017, 158, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Shi, Y.B. Regulation of growth rate and developmental timing by Xenopus thyroid hormone receptor alpha. Dev. Growth Differ. 2016, 58, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Sachs, L.M. Unliganded Thyroid Hormone Receptor Function: Amphibian Metamorphosis Got TALENs. Endocrinology 2015, 156, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.M. Unliganded TRs regulate growth and developmental timing during early embryogenesis: Evidence for a dual function mechanism of TR action. Cell Biosci. 2015, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Tazawa, I.; Shi, Y.B. A unique role of thyroid hormone receptor beta in regulating notochord resorption during Xenopus metamorphosis. Gen. Comp. Endocrinol. 2019, 277, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Wen, L.; Okada, M.; Shi, Y.-B. Organ-Specific Requirements for Thyroid Hormone Receptor Ensure Temporal Coordination of Tissue-Specific Transformations and Completion ofXenopusMetamorphosis. Thyroid 2020, 30, 300–313. [Google Scholar] [CrossRef]
- Shibata, Y.; Tanizaki, Y.; Shi, Y.-B. Thyroid hormone receptor beta is critical for intestinal remodeling during Xenopus tropicalis metamorphosis. Cell Biosci. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Tanizaki, Y.; Shibata, Y.; Zhang, H.; Shi, Y.-B. Analysis of Thyroid Hormone Receptor α-Knockout Tadpoles Reveals That the Activation of Cell Cycle Program Is Involved in Thyroid Hormone-Induced Larval Epithelial Cell Death and Adult Intestinal Stem Cell Development During Xenopus tropicalis Metamorphosis. Thyroid 2021, 31, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Tanizaki, Y.; Bao, L.; Shi, B.; Shi, Y.-B. A role of endogenous histone acetyltransferase steroid hormone receptor coactivator (SRC) 3 in thyroid hormone signaling during Xenopus intestinal metamorphosis. Thyroid 2020. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-B. Life without thyroid hormone receptor. Endocrinology 2021. [Google Scholar] [CrossRef] [PubMed]
- Nieuwkoop, P.D.; Faber, J. Normal Table of Xenopus laevis; North Holland Publishing: Amsterdam, The Netherlands, 1965. [Google Scholar]
- Okada, M.; Wen, L.; Miller, T.C.; Su, D.; Shi, Y.-B. Molecular and cytological analyses reveal distinct transformations of intestinal epithelial cells during Xenopus metamorphosis. Cell Biosci. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Roediger, J.; Park, S.; Fu, L.; Shi, B.; Cheng, S.-Y.; Shi, Y.-B. Thyroid Hormone Receptor Alpha Mutations Lead to Epithelial Defects in the Adult Intestine in a Mouse Model of Resistance to Thyroid Hormone. Thyroid 2019, 29, 439–448. [Google Scholar] [CrossRef]
- Nagasawa, K.; Tanizaki, Y.; Okui, T.; Watarai, A.; Ueda, S.; Kato, T. Significant modulation of the hepatic proteome induced by exposure to low temperature in Xenopus laevis. Biol. Open 2013, 2, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Huang Da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef]
- Howe, E.A.; Sinha, R.; Schlauch, D.; Quackenbush, J. RNA-Seq analysis in MeV. Bioinformatics 2011, 27, 3209–3210. [Google Scholar] [CrossRef]
- Sterling, J.; Fu, L.; Matsuura, K.; Shi, Y.-B. Cytological and Morphological Analyses Reveal Distinct Features of Intestinal Development during Xenopus tropicalis Metamorphosis. PLoS ONE 2012, 7, e47407. [Google Scholar] [CrossRef]
- Shi, Y.-B.; Ishizuya-Oka, A. Thyroid hormone regulation of apoptotic tissue remodeling: Implications from molecular analysis of amphibian metamorphosis. Prog. Nucleic Acid Res. Mol. Biol. 2000, 65, 53–100. [Google Scholar] [CrossRef]
- Schreiber, A.M.; Cai, L.; Brown, D.D. Remodeling of the intestine during metamorphosis of Xenopus laevis. Proc. Natl. Acad. Sci. USA 2005, 102, 3720–3725. [Google Scholar] [CrossRef]
- Ishizuya-Oka, A.; Ueda, S. Apoptosis and cell proliferation in the Xenopus small intestine during metamorphosis. Cell Tissue Res. 1996, 286, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Ishizuya-Oka, A.; Ueda, S.; Damjanovski, S.; Li, Q.; Liang, V.C.; Shi, Y.-B. Anteroposterior gradient of epithelial transformation during amphibian intestinal remodeling: Immunohistochemical detection of intestinal fatty acid-binding protein. Dev. Biol. 1997, 192, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Hasebe, T.; Ishizuya-Oka, A.; Shi, Y.-B. Roles of Matrix Metalloproteinases and ECM Remodeling during Thyroid Hormone-Dependent Intestinal Metamorphosis in Xenopus laevis. Organogenesis 2007, 3, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chen, Y.-G. Dishevelled: The hub of Wnt signaling. Cell. Signal. 2010, 22, 717–727. [Google Scholar] [CrossRef]
- Morvan-Dubois, G.; Demeneix, B.A.; Sachs, L.M. Xenopus laevis as a model for studying thyroid hormone signalling: From development to metamorphosis. Mol. Cell. Endocrinol. 2008, 293, 71–79. [Google Scholar] [CrossRef][Green Version]
- Takashi, H.; Hasebe, T.; Fu, L.; Fujimoto, K.; Ishizuya-Oka, A. The development of the adult intestinal stem cells: Insights from studies on thyroid hormone-dependent amphibian metamorphosis. Cell Biosci. 2011, 1, 1–9. [Google Scholar] [CrossRef]
- Ishizuya-Oka, A.; Hasebe, T.; Buchholz, D.R.; Kajita, M.; Fu, L.; Shi, Y. Origin of the adult intestinal stem cells induced by thyroid hormone in Xenopus laevis. FASEB J. 2009, 23, 2568–2575. [Google Scholar] [CrossRef] [PubMed]
- Ishizuya-Oka, A.; Hasebe, T. Establishment of Intestinal Stem Cell Niche During Amphibian Metamorphosis. Curr. Top. Dev. Biol. 2013, 103, 305–327. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Shi, Y.-B. The balance of two opposing factors Mad and Myc regulates cell fate during tissue remodeling. Cell Biosci. 2018, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Fu, L.; Shi, Y.-B. Epigenetic regulation of thyroid hormone-induced adult intestinal stem cell development during anuran metamorphosis. Cell Biosci. 2014, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Ishizuya-Oka, A.; Kajita, M.; Hasebe, T. Thyroid Hormone-Regulated Wnt5a/Ror2 Signaling Is Essential for Dedifferentiation of Larval Epithelial Cells into Adult Stem Cells in the Xenopus laevis Intestine. PLoS ONE 2014, 9, e107611. [Google Scholar] [CrossRef]
- Hasebe, T.; Fujimoto, K.; Kajita, M.; Fu, L.; Shi, Y.; Ishizuya-Oka, A. Thyroid Hormone-Induced Activation of Notch Signaling is Required for Adult Intestinal Stem Cell Development DuringXenopus LaevisMetamorphosis. Stem Cells 2017, 35, 1028–1039. [Google Scholar] [CrossRef]
- Buchholz, D.R.; Shi, Y.-B. Dual function model revised by thyroid hormone receptor alpha knockout frogs. Gen. Comp. Endocrinol. 2018, 265, 214–218. [Google Scholar] [CrossRef]
- Wen, L.; Hasebe, T.; Miller, T.C.; Ishizuya-Oka, A.; Shi, Y.-B. A requirement for hedgehog signaling in thyroid hormone-induced postembryonic intestinal remodeling. Cell Biosci. 2015, 5, 13. [Google Scholar] [CrossRef]
- Muncan, V.; Heijmans, J.; Krasinski, S.D.; Büller, N.V.; Wildenberg, M.E.; Meisner, S.; Radonjic, M.; Stapleton, K.A.; Lamers, W.H.; Biemond, I.; et al. Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat. Commun. 2011, 2, 1–9. [Google Scholar] [CrossRef]
- Harper, J.; Mould, A.; Andrews, R.M.; Bikoff, E.K.; Robertson, E.J. The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 10585–10590. [Google Scholar] [CrossRef]
- Matsuda, H.; Shi, Y.-B. An Essential and Evolutionarily Conserved Role of Protein Arginine Methyltransferase 1 for Adult Intestinal Stem Cells During Postembryonic Development. Stem Cells 2010, 28, 2073–2083. [Google Scholar] [CrossRef] [PubMed]
- Plateroti, M.; Gauthier, K.; Domon-Dell, C.; Freund, J.N.; Samarut, J.; Chassande, O. Functional interference between thyroid hormone receptor alpha (TRalpha) and natural truncated TRDeltaalpha isoforms in the control of intestine development. Mol. Cell Biol. 2001, 21, 4761–4772. [Google Scholar] [CrossRef]
- Flamant, F.; Poguet, A.L.; Plateroti, M.; Chassande, O.; Gauthier, K.; Streichenberger, N.; Mansouri, A.; Samarut, J. Congenital hypothyroid Pax8(-/-) mutant mice can be rescued by inactivating the TRalpha gene. Mol. Endocrinol. 2002, 16, 24–32. [Google Scholar] [PubMed]
- Kress, E.; Rezza, A.; Nadjar, J.; Samarut, J.; Plateroti, M. The frizzled-related sFRP2 gene is a target of thyroid hormone receptor alpha1 and activates beta-catenin signaling in mouse intestine. J. Biol. Chem. 2009, 284, 1234–1241. [Google Scholar] [CrossRef]
- Plateroti, M.; Chassande, O.; Fraichard, A.; Gauthier, K.; Freund, J.N.; Samarut, J.; Kedinger, M. Involvement of T3Ralpha- and beta-receptor subtypes in mediation of T3 functions during postnatal murine intestinal development. Gastroenterology 1999, 116, 1367–1378. [Google Scholar] [CrossRef]
- Plateroti, M.; Kress, E.; Mori, J.I.; Samarut, J. Thyroid hormone receptor alpha1 directly controls transcription of the beta-catenin gene in intestinal epithelial cells. Mol. Cell Biol. 2006, 26, 3204–3214. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibata, Y.; Tanizaki, Y.; Zhang, H.; Lee, H.; Dasso, M.; Shi, Y.-B. Thyroid Hormone Receptor Is Essential for Larval Epithelial Apoptosis and Adult Epithelial Stem Cell Development but Not Adult Intestinal Morphogenesis during Xenopus tropicalis Metamorphosis. Cells 2021, 10, 536. https://doi.org/10.3390/cells10030536
Shibata Y, Tanizaki Y, Zhang H, Lee H, Dasso M, Shi Y-B. Thyroid Hormone Receptor Is Essential for Larval Epithelial Apoptosis and Adult Epithelial Stem Cell Development but Not Adult Intestinal Morphogenesis during Xenopus tropicalis Metamorphosis. Cells. 2021; 10(3):536. https://doi.org/10.3390/cells10030536
Chicago/Turabian StyleShibata, Yuki, Yuta Tanizaki, Hongen Zhang, Hangnoh Lee, Mary Dasso, and Yun-Bo Shi. 2021. "Thyroid Hormone Receptor Is Essential for Larval Epithelial Apoptosis and Adult Epithelial Stem Cell Development but Not Adult Intestinal Morphogenesis during Xenopus tropicalis Metamorphosis" Cells 10, no. 3: 536. https://doi.org/10.3390/cells10030536
APA StyleShibata, Y., Tanizaki, Y., Zhang, H., Lee, H., Dasso, M., & Shi, Y.-B. (2021). Thyroid Hormone Receptor Is Essential for Larval Epithelial Apoptosis and Adult Epithelial Stem Cell Development but Not Adult Intestinal Morphogenesis during Xenopus tropicalis Metamorphosis. Cells, 10(3), 536. https://doi.org/10.3390/cells10030536






