The Role of AMPK Signaling in Brown Adipose Tissue Activation
Abstract
1. Introduction
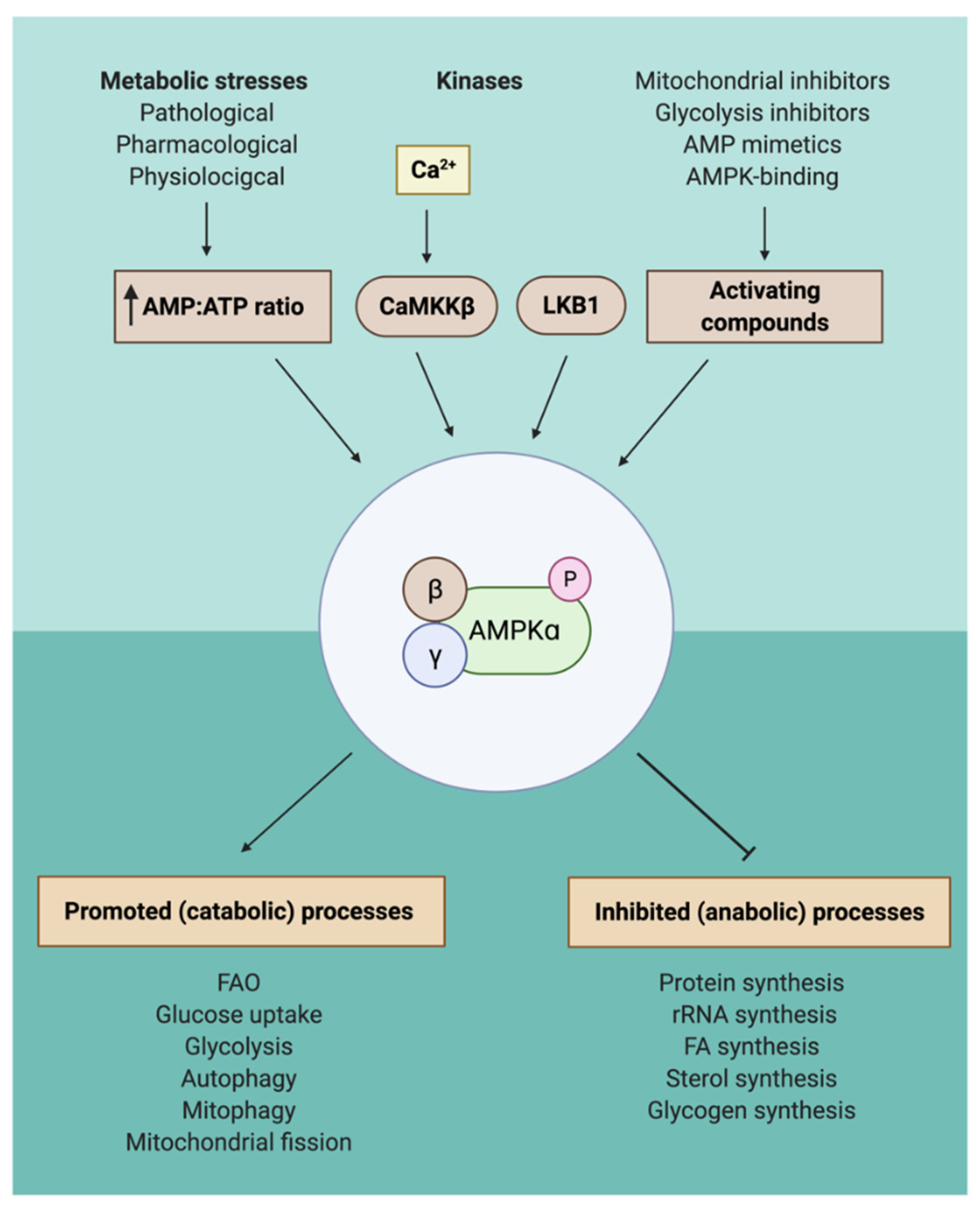
2. AMPK Activation through a Wide Variety of Different Compounds
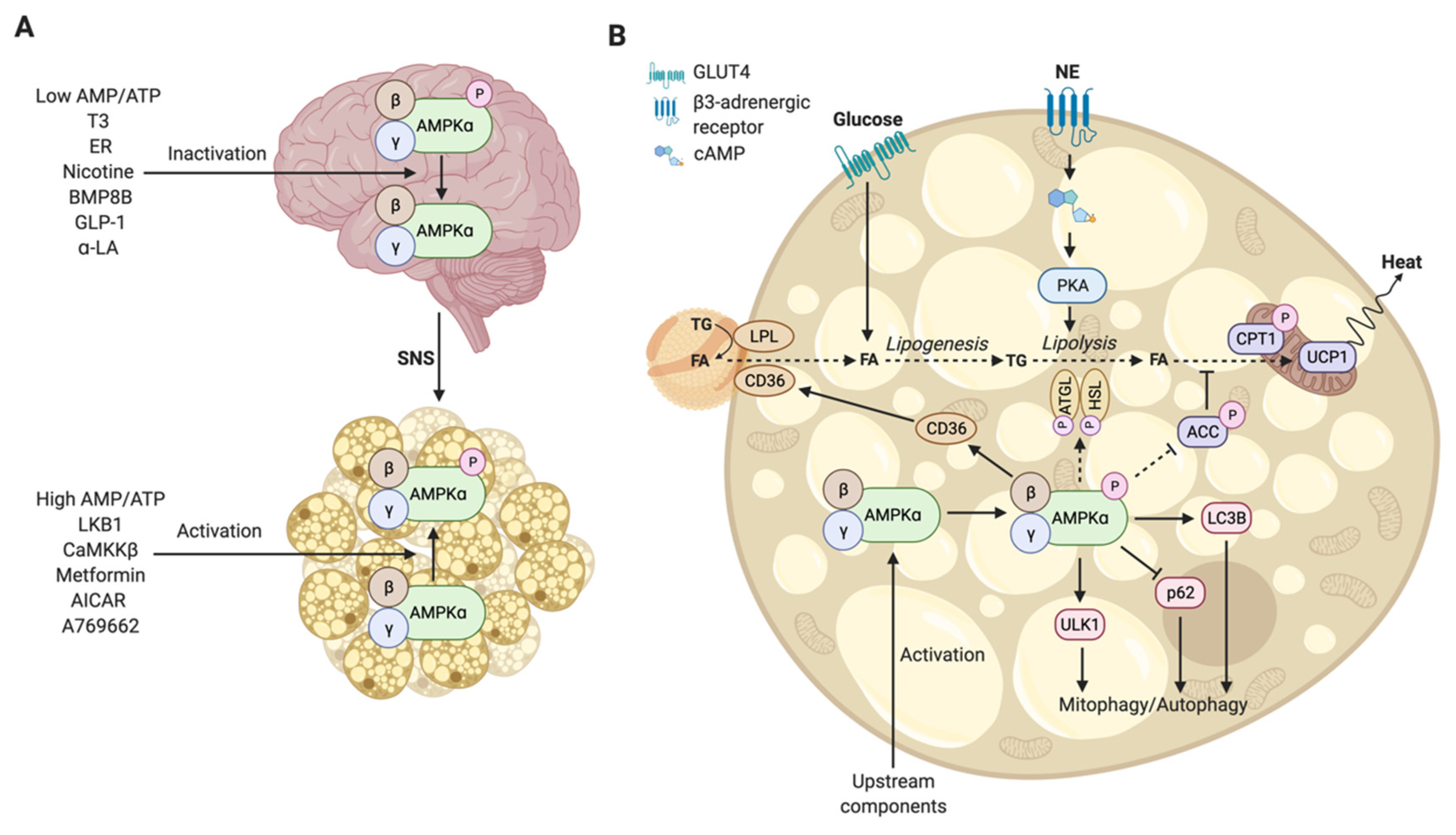
3. The Functional Mechanism of Regulation of Brown Adipose Tissue by Hypothalamic AMPK
Hormones, Compounds, and Metabolites Regulate AMPK in the Hypothalamus
4. The Functional Mechanism of AMPK-Mediated Activation in Brown Adipocytes
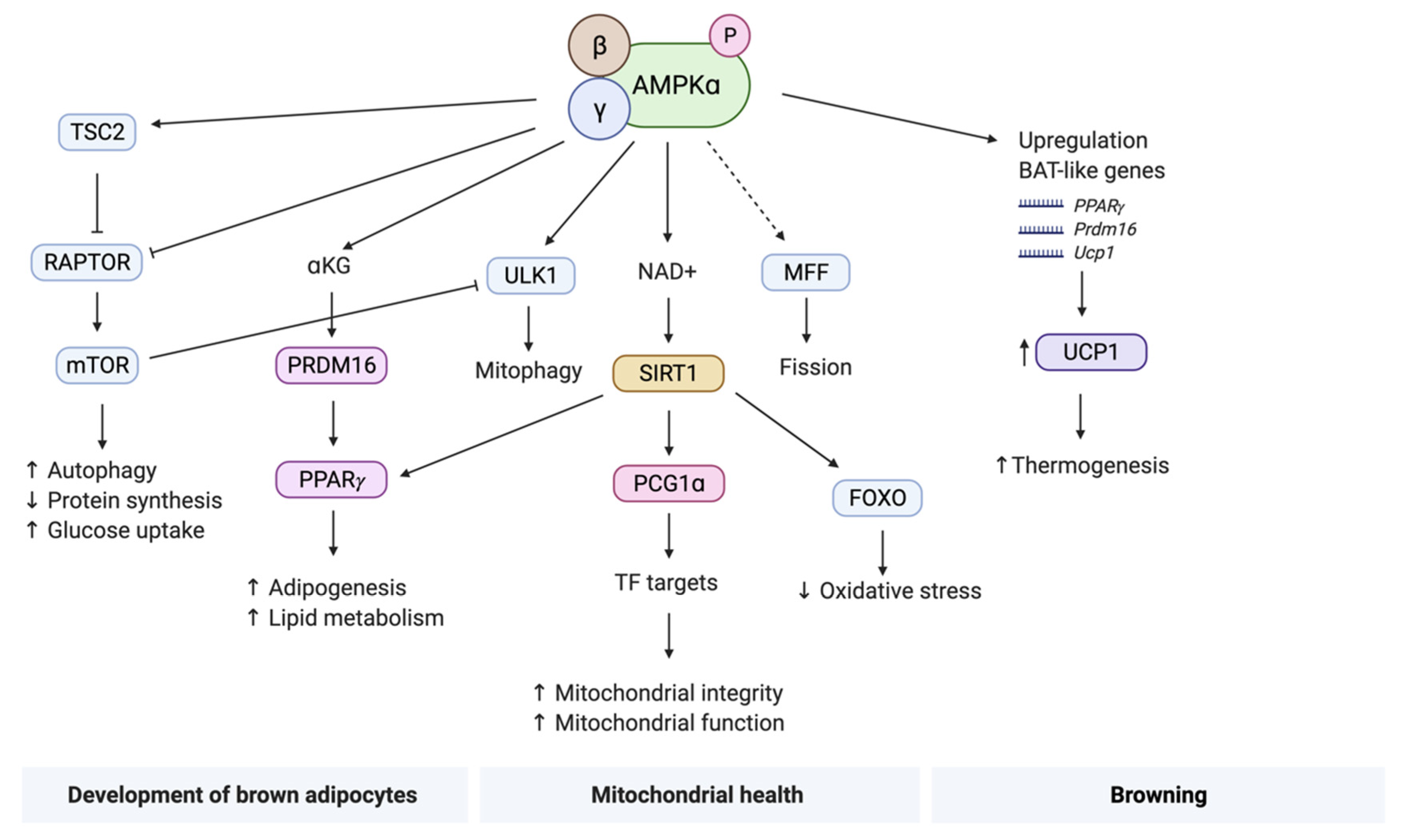
4.1. Development of Brown Adipocytes
4.2. Mitochondrial Health
4.3. Browning
5. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Oussaada, S.M.; van Galen, K.A.; Cooiman, M.I.; Kleinendorst, L.; Hazebroek, E.J.; van Haelst, M.M.; Ter Horst, K.W.; Serlie, M.J. The Pathogenesis of Obesity. Metabolism 2019, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Heshka, S.; Allison, D.B. Is obesity a disease? Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1401–1404. [Google Scholar] [CrossRef] [PubMed]
- Downey, M. Results of expert meetings: Obesity and cardiovascular disease. obesity as a disease entity. Am. Heart. J. 2001, 142, 1091–1094. [Google Scholar] [CrossRef]
- Via, M.A.; Mechanick, J.I. Obesity as a disease. Curr. Obes. Rep. 2014, 3, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: A chronic relapsing progressive disease process. A position statement of the world obesity federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef]
- Pillon, N.J.; Loos, R.J.F.; Marshall, S.M.; Zierath, J.R. Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care. Cell 2021, 184, 1530–1544. [Google Scholar] [CrossRef] [PubMed]
- Dodangeh, M.; Dodangeh, M. Metabolic regulation and the anti-obesity perspectives of Brown Adipose Tissue (BAT); a Systematic Review. Obes. Med. 2020, 17, 100163. [Google Scholar] [CrossRef]
- Bray, G.A. Medical consequences of obesity. J. Clin. Endocrinol. Metab. 2004, 89, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.-J.; Enerbäck, S.; et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Giralt, M. Brown Adipose Tissue as a Secretory Organ. Nat. Rev. Endocrinol. 2017, 13, 26–35. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-Activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Okamatsu-Ogura, Y.; Fukano, K.; Tsubota, A.; Nio-Kobayashi, J.; Nakamura, K.; Morimatsu, M.; Sakaue, H.; Saito, M.; Kimura, K. Cell-Cycle arrest in mature adipocytes impairs bat development but not wat browning, and reduces adaptive thermogenesis in mice. Sci. Rep. 2017, 7, 6648. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Barquissau, V.; Beuzelin, D.; Pisani, D.F.; Beranger, G.E.; Mairal, A.; Montagner, A.; Roussel, B.; Tavernier, G.; Marques, M.-A.; Moro, C.; et al. White-to-Brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Mol. Metab. 2016, 5, 352–365. [Google Scholar] [CrossRef]
- Blondin, D.P.; Nielsen, S.; Kuipers, E.N.; Severinsen, M.C.; Jensen, V.H.; Miard, S.; Jespersen, N.Z.; Kooijman, S.; Boon, M.R.; Fortin, M.; et al. Human brown adipocyte thermogenesis is driven by Β2-AR stimulation. Cell. Metab. 2020, 32, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Killick, R.; Banks, S.; Liu, P.Y. Implications of sleep restriction and recovery on metabolic outcomes. J. Clin. Endocrinol. Metab. 2012, 97, 3876–3890. [Google Scholar] [CrossRef] [PubMed]
- de Goede, P.; Sen, S.; Su, Y.; Foppen, E.; Poirel, V.-J.; Challet, E.; Kalsbeek, A. An ultradian feeding schedule in rats affects metabolic gene expression in liver, brown adipose tissue and skeletal muscle with only mild effects on circadian clocks. Int. J. Mol. Sci. 2018, 19, 3171. [Google Scholar] [CrossRef] [PubMed]
- Um, J.-H.; Pendergast, J.S.; Springer, D.A.; Foretz, M.; Viollet, B.; Brown, A.; Kim, M.K.; Yamazaki, S.; Chung, J.H. AMPK regulates circadian rhythms in a tissue- and isoform-specific manner. PLoS ONE 2011, 6, e18450. [Google Scholar] [CrossRef]
- Mulligan, J.D.; Gonzalez, A.A.; Stewart, A.M.; Carey, H.V.; Saupe, K.W. Upregulation of AMPK during cold exposure occurs via distinct mechanisms in brown and white adipose tissue of the mouse. J. Physiol. 2007, 580, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, A.D.; Kooijman, S.; Schilperoort, M.; Rensen, P.C.N.; Boon, M.R. Regulation of brown fat by AMP-activated protein kinase. Trends Mol. Med. 2015, 21, 571–579. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef]
- Kooijman, S.; van den Berg, R.; Ramkisoensing, A.; Boon, M.R.; Kuipers, E.N.; Loef, M.; Zonneveld, T.C.M.; Lucassen, E.A.; Sips, H.C.M.; Chatzispyrou, I.A.; et al. Prolonged daily light exposure increases body fat mass through attenuation of brown adipose tissue activity. Proc. Natl. Acad. Sci. USA 2015, 112, 6748–6753. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular mechanisms of adipogenesis: The anti-adipogenic role of AMP-activated protein kinase. Front. Mol. Biosci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- López, M. Hypothalamic AMPK and energy balance. Eur. J. Clin. Invest. 2018, 48. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK as a direct sensor of long-chain fatty Acyl–CoA esters. Nat. Metab. 2020, 2, 799–800. [Google Scholar] [CrossRef]
- Hardie, D.G. Keeping the home fires burning: AMP-Activated Protein Kinase. J. R. Soc. Interface 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK–Sensing energy while talking to other signaling pathways. Cell Metab. 2014, 20, 939–952. [Google Scholar] [CrossRef]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef]
- Pinkosky, S.L.; Scott, J.W.; Desjardins, E.M.; Smith, B.K.; Day, E.A.; Ford, R.J.; Langendorf, C.G.; Ling, N.X.Y.; Nero, T.L.; Loh, K.; et al. Long-Chain fatty Acyl-CoA esters regulate metabolism via allosteric control of AMPK Β1 Isoforms. Nat. Metab. 2020, 2, 873–881. [Google Scholar] [CrossRef]
- Hardie, D.G.; Scott, J.W.; Pan, D.A.; Hudson, E.R. Management of cellular energy by the AMP-Activated protein kinase system. Febs. Lett. 2003, 546, 113–120. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell. 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Boudeau, J.; Reid, J.L.; Mustard, K.J.; Udd, L.; Mäkelä, T.P.; Alessi, D.R.; Hardie, D.G. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-Activated protein kinase cascade. J. Biol. 2003, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Johnstone, S.R.; Dickerson, K.; Leiper, F.C.; Fryer, L.G.D.; Neumann, D.; Schlattner, U.; Wallimann, T.; Carlson, M.; Carling, D. LKB1 is the upstream kinase in the AMP-Activated protein kinase cascade. Curr. Biol. 2003, 13, 2004–2008. [Google Scholar] [CrossRef]
- Hawley, S.A.; Davison, M.; Woods, A.; Davies, S.P.; Beri, R.K.; Carling, D.; Hardie, D.G. Characterization of the AMP-Activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-Activated protein kinase. J. Biol. Chem. 1996, 271, 27879–27887. [Google Scholar] [CrossRef]
- Shaw, R.J.; Kosmatka, M.; Bardeesy, N.; Hurley, R.L.; Witters, L.A.; DePinho, R.A.; Cantley, L.C. The tumor suppressor LKB1 kinase directly activates AMP-Activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 2004, 101, 3329–3335. [Google Scholar] [CrossRef]
- Hawley, S.A.; Pan, D.A.; Mustard, K.J.; Ross, L.; Bain, J.; Edelman, A.M.; Frenguelli, B.G.; Hardie, D.G. Calmodulin-Dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-Activated protein kinase. Cell. Metab. 2005, 2, 9–19. [Google Scholar] [CrossRef]
- Woods, A.; Dickerson, K.; Heath, R.; Hong, S.-P.; Momcilovic, M.; Johnstone, S.R.; Carlson, M.; Carling, D. Ca2+/Calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-Activated protein kinase in mammalian cells. Cell Metab. 2005, 2, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Geerling, J.J.; Boon, M.R.; van der Zon, G.C.; van den Berg, S.A.A.; van den Hoek, A.M.; Lombès, M.; Princen, H.M.G.; Havekes, L.M.; Rensen, P.C.N.; Guigas, B. Metformin lowers plasma triglycerides by promoting VLDL-Triglyceride clearance by brown adipose tissue in mice. Diabetes 2014, 63, 880–891. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Duca, F.A.; Côté, C.D.; Rasmussen, B.A.; Zadeh-Tahmasebi, M.; Rutter, G.A.; Filippi, B.M.; Lam, T.K.T. Metformin activates duodenal AMPK and a neuronal network to lower glucose production. Nat. Med. 2015, 21, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK: A target for drugs and natural products with effects on both diabetes and cancer. Diabetes 2013, 62, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xia, M.; Duan, Y.; Zhang, L.; Jiang, H.; Hu, X.; Yan, H.; Zhang, Y.; Gu, Y.; Shi, H.; et al. Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 2019, 10, 468. [Google Scholar] [CrossRef]
- Corton, J.M.; Gillespie, J.G.; Hawley, S.A.; Hardie, D.G. 5-Aminoimidazole-4-Carboxamide Ribonucleoside. A specific method for activating AMP-Activated protein kinase in intact cells? Eur. J. Biochem. 1995, 229, 558–565. [Google Scholar] [CrossRef]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell. Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.J.; Ali, Z.S.; Hegarty, B.D.; Heath, R.; Snowden, M.A.; Carling, D. Defining the mechanism of activation of AMP-Activated protein kinase by the small molecule A-769662, a member of the Thienopyridone family. J. Biol. Chem. 2007, 282, 32539–32548. [Google Scholar] [CrossRef]
- Salt, I.P.; Hardie, D.G. AMP-Activated protein kinase a ubiquitous signalling pathway with key roles in the cardiovascular system. Circ. Res. 2017, 120, 1825–1841. [Google Scholar] [CrossRef]
- Hawley, S.A.; Ross, F.A.; Chevtzoff, C.; Green, K.A.; Evans, A.; Fogarty, S.; Towler, M.C.; Brown, L.J.; Ogunbayo, O.A.; Evans, A.M.; et al. Use of cells expressing γ subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010, 11, 554–565. [Google Scholar] [CrossRef]
- de Ligt, M.; Timmers, S.; Schrauwen, P. Resveratrol and obesity: Can resveratrol relieve metabolic disturbances? Biochim. Biophys. Acta 2015, 1852, 1137–1144. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Sokolowski, A.A.; Graier, W.F. Dosis Facit Sanitatem—Concentration-Dependent effects of Resveratrol on Mitochondria. Nutrients 2017, 9, 1117. [Google Scholar] [CrossRef]
- Zheng, J.; Ramirez, V.D. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by Polyphenolic Phytochemicals. Br. J. Pharm. 2000, 130, 1115–1123. [Google Scholar] [CrossRef]
- Turner, N.; Li, J.-Y.; Gosby, A.; To, S.W.C.; Cheng, Z.; Miyoshi, H.; Taketo, M.M.; Cooney, G.J.; Kraegen, E.W.; James, D.E.; et al. Berberine and its more biologically available derivative, Dihydroberberine, inhibit mitochondrial respiratory complex i: A mechanism for the action of berberine to activate AMP-Activated protein kinase and improve insulin action. Diabetes 2008, 57, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Chang, J.S.; Jo, Y.-H. Intracellular glycolysis in brown adipose tissue is essential for optogenetically induced nonshivering thermogenesis in mice. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Held, N.M.; Kuipers, E.N.; van Weeghel, M.; van Klinken, J.B.; Denis, S.W.; Lombès, M.; Wanders, R.J.; Vaz, F.M.; Rensen, P.C.N.; Verhoeven, A.J.; et al. Pyruvate Dehydrogenase complex plays a central role in brown adipocyte energy expenditure and fuel utilization during short-term beta-adrenergic activation. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Anthony, N.M.; Gaidhu, M.P.; Ceddia, R.B. Regulation of visceral and subcutaneous Adipocyte Lipolysis by acute AICAR-induced AMPK activation. Obesity 2009, 17, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Cokorinos, E.C.; Delmore, J.; Reyes, A.R.; Albuquerque, B.; Kjøbsted, R.; Jørgensen, N.O.; Tran, J.-L.; Jatkar, A.; Cialdea, K.; Esquejo, R.M.; et al. Activation of skeletal muscle AMPK promotes glucose disposal and glucose lowering in non-human primates and mice. Cell Metab. 2017, 25, 1147–1159. [Google Scholar] [CrossRef]
- Salatto, C.T.; Miller, R.A.; Cameron, K.O.; Cokorinos, E.; Reyes, A.; Ward, J.; Calabrese, M.F.; Kurumbail, R.G.; Rajamohan, F.; Kalgutkar, A.S.; et al. Selective activation of AMPK Β1-Containing isoforms improves kidney function in a rat model of diabetic nephropathy. J. Pharm. Exp. 2017, 361, 303–311. [Google Scholar] [CrossRef]
- Myers, R.W.; Guan, H.-P.; Ehrhart, J.; Petrov, A.; Prahalada, S.; Tozzo, E.; Yang, X.; Kurtz, M.M.; Trujillo, M.; Gonzalez Trotter, D.; et al. Systemic Pan-AMPK Activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science 2017, 357, 507–511. [Google Scholar] [CrossRef]
- Feng, D.; Biftu, T.; Romero, F.A.; Kekec, A.; Dropinski, J.; Kassick, A.; Xu, S.; Kurtz, M.M.; Gollapudi, A.; Shao, Q.; et al. Discovery of MK-8722: A systemic, direct pan-activator of AMP-Activated protein kinase. ACS Med. Chem. Lett. 2018, 9, 39–44. [Google Scholar] [CrossRef]
- Cameron, K.O.; Kung, D.W.; Kalgutkar, A.S.; Kurumbail, R.G.; Miller, R.; Salatto, C.T.; Ward, J.; Withka, J.M.; Bhattacharya, S.K.; Boehm, M.; et al. Discovery and preclinical characterization of 6-Chloro-5-[4-(1-Hydroxycyclobutyl)Phenyl]-1H-Indole-3-Carboxylic Acid (PF-06409577), a direct activator of Adenosine Monophosphate-Activated Protein Kinase (AMPK), for the potential treatment of diabetic nephropathy. J. Med. Chem. 2016, 59, 8068–8081. [Google Scholar] [CrossRef] [PubMed]
- Ryder, T.F.; Calabrese, M.F.; Walker, G.S.; Cameron, K.O.; Reyes, A.R.; Borzilleri, K.A.; Delmore, J.; Miller, R.; Kurumbail, R.G.; Ward, J.; et al. Acyl Glucuronide Metabolites of 6-Chloro-5-[4-(1-Hydroxycyclobutyl)Phenyl]-1 H-Indole-3-Carboxylic Acid (PF-06409577) and related Indole-3-Carboxylic acid derivatives are direct activators of Adenosine Monophosphate-Activated Protein Kinase (AMPK). J. Med. Chem. 2018, 61, 7273–7288. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Tena-Sempere, M. Estradiol effects on Hypothalamic AMPK and BAT Thermogenesis: A gateway for obesity treatment? Pharmacol. Ther. 2017, 178, 109–122. [Google Scholar] [CrossRef] [PubMed]
- DiMicco, J.A.; Zaretsky, D.V. The dorsomedial hypothalamus: A new player in thermoregulation. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2007, 292, R47–R63. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.; Gonzalez, F.; Fernø, J.; Diéguez, C.; Rahmouni, K.; Nogueiras, R.; López, M. The brain and brown fat. Ann. Med. 2015, 47, 150–168. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Nogueiras, R.; Tena-Sempere, M.; Diéguez, C. Hypothalamic AMPK: A canonical regulator of whole-body energy balance. Nat. Rev. Endocrinol. 2016, 12, 421–432. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Varela, L.; Vázquez, M.J.; Rodríguez-Cuenca, S.; González, C.R.; Velagapudi, V.R.; Morgan, D.A.; Schoenmakers, E.; Agassandian, K.; Lage, R.; et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat. Med. 2010, 16, 1001–1008. [Google Scholar] [CrossRef]
- Wang, B.; Cheng, K.K.-Y. Hypothalamic AMPK as a mediator of hormonal regulation of energy balance. Int. J. Mol. Sci. 2018, 19, 3552. [Google Scholar] [CrossRef]
- Majdic, G.; Young, M.; Gomez-Sanchez, E.; Anderson, P.; Szczepaniak, L.S.; Dobbins, R.L.; McGarry, J.D.; Parker, K.L. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology 2002, 143, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, N.; Seoane-Collazo, P.; Contreras, C.; Varela, L.; Villarroya, J.; Rial-Pensado, E.; Buqué, X.; Aurrekoetxea, I.; Delgado, T.C.; Vázquez-Martínez, R.; et al. Hypothalamic AMPK-ER Stress-JNK1 axis mediates the central actions of thyroid hormones on energy balance. Cell Metab. 2017, 26, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Collazo, P.; Roa, J.; Rial-Pensado, E.; Liñares-Pose, L.; Beiroa, D.; Ruíz-Pino, F.; López-González, T.; Morgan, D.A.; Pardavila, J.Á.; Sánchez-Tapia, M.J.; et al. SF1-Specific AMPKα1 deletion protects against diet-induced obesity. Diabetes 2018, 67, 2213–2226. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Ye, C.; McCrimmon, R.J.; Dhillon, H.; Choi, B.; Kramer, M.D.; Yu, J.; Yang, Z.; Christiansen, L.M.; Lee, C.E.; et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007, 5, 383–393. [Google Scholar] [CrossRef]
- Xu, Y.; Nedungadi, T.P.; Zhu, L.; Sobhani, N.; Irani, B.G.; Davis, K.E.; Zhang, X.; Zou, F.; Gent, L.M.; Hahner, L.D.; et al. Distinct Hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011, 14, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Crespo, M.; Csikasz, R.I.; Martínez-Sánchez, N.; Diéguez, C.; Cannon, B.; Nedergaard, J.; López, M. Essential role of UCP1 modulating the central effects of thyroid hormones on energy balance. Mol. Metab. 2016, 5, 271–282. [Google Scholar] [CrossRef]
- Martínez-Sánchez, N.; Moreno-Navarrete, J.M.; Contreras, C.; Rial-Pensado, E.; Fernø, J.; Nogueiras, R.; Diéguez, C.; Fernández-Real, J.-M.; López, M. Thyroid hormones induce browning of white fat. J. Endocrinol. 2016, 232, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Morentin, P.B.; González-García, I.; Martins, L.; Lage, R.; Fernández-Mallo, D.; Martínez-Sánchez, N.; Ruíz-Pino, F.; Liu, J.; Morgan, D.A.; Pinilla, L.; et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014, 20, 41–53. [Google Scholar] [CrossRef] [PubMed]
- González-García, I.; Contreras, C.; Estévez-Salguero, Á.; Ruíz-Pino, F.; Colsh, B.; Pensado, I.; Liñares-Pose, L.; Rial-Pensado, E.; de Morentin, P.B.M.; Fernø, J.; et al. Estradiol regulates energy balance by ameliorating hypothalamic ceramide-induced ER stress. Cell Rep. 2018, 25, 413–423. [Google Scholar] [CrossRef]
- Martínez de Morentin, P.B.; Whittle, A.J.; Fernø, J.; Nogueiras, R.; Diéguez, C.; Vidal-Puig, A.; López, M. nicotine induces negative energy balance through hypothalamic AMP-Activated protein kinase. Diabetes 2012, 61, 807–817. [Google Scholar] [CrossRef]
- Seoane-Collazo, P.; de Morentin, P.B.M.; Fernø, J.; Diéguez, C.; Nogueiras, R.; López, M. nicotine improves obesity and hepatic steatosis and ER stress in diet-induced obese male rats. Endocrinology 2014, 155, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Kim, K.; Kaneko, K.; Iketani, M.; Otagiri, A.; Yamauchi, N.; Shibasaki, T. Nicotine infusion alters leptin and uncoupling protein 1 MRNA expression in adipose tissues of rats. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E867–E876. [Google Scholar] [CrossRef] [PubMed]
- Mano-Otagiri, A.; Iwasaki-Sekino, A.; Ohata, H.; Arai, K.; Shibasaki, T. Nicotine suppresses energy storage through activation of sympathetic outflow to brown adipose tissue via corticotropin-releasing factor type 1 receptor. Neurosci. Lett. 2009, 455, 26–29. [Google Scholar] [CrossRef]
- Nishii, N.; Arai, M.; Yanai, N.; Togari, A.; Nakabayashi, T. Effect of Bone Morphogenetic Protein-2 (BMP-2) or Troglitazone, as an inducer of osteogenic cells or adipocytes, on differentiation of a bone marrow mesenchymal progenitor cell line established from Temperature-Sensitive (Ts) Simian Virus (SV) 40 T-Antigen Gene Transgenic Mice. Biol. Pharm. Bull. 2009, 32, 10–17. [Google Scholar] [CrossRef]
- Sottile, V.; Seuwen, K. Bone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (Rosiglitazone). FEBS Lett. 2000, 475, 201–204. [Google Scholar] [CrossRef]
- Tang, Q.-Q.; Otto, T.C.; Lane, M.D. Commitment of C3H10T1/2 Pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad Sci. USA 2004, 101, 9607–9611. [Google Scholar] [CrossRef]
- Schulz, T.J.; Huang, T.L.; Tran, T.T.; Zhang, H.; Townsend, K.L.; Shadrach, J.L.; Cerletti, M.; McDougall, L.E.; Giorgadze, N.; Tchkonia, T.; et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl. Acad. Sci. USA 2011, 108, 143–148. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Whittle, A.J.; Carobbio, S.; Martins, L.; Slawik, M.; Hondares, E.; Vázquez, M.J.; Morgan, D.; Csikasz, R.I.; Gallego, R.; Rodriguez-Cuenca, S.; et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 2012, 149, 871–885. [Google Scholar] [CrossRef]
- Martins, L.; Seoane-Collazo, P.; Contreras, C.; González-García, I.; Martínez-Sánchez, N.; González, F.; Zalvide, J.; Gallego, R.; Diéguez, C.; Nogueiras, R.; et al. A functional link between AMPK and Orexin mediates the effect of BMP8B on energy balance. Cell Rep. 2016, 16, 2231–2242. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.G.M.; Nahon, K.J.; Bracké, K.F.M.; van den Broek, D.; Smit, R.; Sardjoe Mishre, A.S.D.; Koorneef, L.L.; Martinez-Tellez, B.; Burakiewicz, J.; Kan, H.E.; et al. Twelve weeks of exenatide treatment increases [18F]Fluorodeoxyglucose uptake by brown adipose tissue without affecting oxidative resting energy expenditure in nondiabetic males. Metabolism 2020, 106, 154167. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Sanchez-Watts, G.; Krieger, J.-P.; Pignalosa, A.; Norell, P.N.; Cortella, A.; Pettersen, K.G.; Vrdoljak, D.; Hayes, M.R.; Kanoski, S.E.; et al. Loss of dorsomedial hypothalamic GLP-1 signaling reduces BAT Thermogenesis and increases adiposity. Mol. Metab. 2018, 11, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Kooijman, S.; Wang, Y.; Parlevliet, E.T.; Boon, M.R.; Edelschaap, D.; Snaterse, G.; Pijl, H.; Romijn, J.A.; Rensen, P.C.N. Central GLP-1 receptor signalling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia 2015, 58, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Lockie, S.H.; Heppner, K.M.; Chaudhary, N.; Chabenne, J.R.; Morgan, D.A.; Veyrat-Durebex, C.; Ananthakrishnan, G.; Rohner-Jeanrenaud, F.; Drucker, D.J.; DiMarchi, R.; et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes 2012, 61, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Fernø, J.; Salvador, J.; Escalada, J.; et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Park, J.-Y.; Namkoong, C.; Jang, P.-G.; Ryu, J.-W.; Song, H.-S.; Yun, J.-Y.; Namgoong, I.-S.; Ha, J.; Park, I.-S.; et al. Anti-Obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-Activated protein kinase. Nat. Med. 2004, 10, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yan, W.; Hu, X.; Huang, Y.; Wang, F.; Zhang, W.; Wang, Q.; Wang, X.; Sun, K. Effects of Oral α-Lipoic acid administration on body weight in overweight or obese subjects: A crossover randomized, double-blind, placebo-controlled trial. Clin. Endocrinol. 2017, 86, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.H.; Lee, W.J.; Lee, S.A.; Kim, W.H.; Cho, E.H.; Jeong, E.; Kim, D.W.; Kim, M.S.; Park, J.Y.; Park, K.G.; et al. Efects of alpha-lipoic acid on body weight in obese subjects. Am. J. Med. 2011, 124, e1–e85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Li, B.; Meng, X.; Wang, J.; Zhang, Y.; Yao, S.; Ma, Q.; Jin, L.; Yang, J.; et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 2014, 5, 5493. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, E.P.; Desjardins, E.M.; Crane, J.D.; Smith, B.K.; Green, A.E.; Ducommun, S.; Henriksen, T.I.; Rebalka, I.A.; Razi, A.; Sakamoto, K.; et al. Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through brown and beige adipose tissue function. Cell Metab. 2016, 24, 118–129. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Q.; Zhang, L.; Liang, X.; Sun, X.; Wang, B.; Chen, Y.; Zhu, M.; Du, M. AMPKα1 deficiency suppresses brown adipogenesis in favor of fibrogenesis during brown adipose tissue development. Biochem. Biophys. Res. Commun. 2017, 491, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Vila-Bedmar, R.; Lorenzo, M.; Fernández-Veledo, S. Adenosine 5’-Monophosphate-Activated protein kinase-mammalian target of rapamycin cross talk regulates brown adipocyte differentiation. Endocrinology 2010, 151, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J. LKB1 and AMP-Activated protein kinase control of MTOR signalling and growth. Acta Physiol. 2009, 196, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Zhu, T.; Guan, K.-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Agarwal, S.; Bell, C.M.; Rothbart, S.B.; Moran, R.G. AMP-Activated Protein Kinase (AMPK) control of MTORC1 Is P53- and TSC2-Independent in pemetrexed-treated carcinoma cells. J. Biol. Chem. 2015, 290, 27473–27486. [Google Scholar] [CrossRef]
- Desjardins, E.M.; Steinberg, G.R. Emerging Role of AMPK in Brown and Beige Adipose Tissue (BAT): Implications for obesity, insulin resistance, and type 2 diabetes. Curr. Diab. Rep. 2018, 18, 80. [Google Scholar] [CrossRef]
- Olsen, J.M.; Sato, M.; Dallner, O.S.; Sandström, A.L.; Pisani, D.F.; Chambard, J.-C.; Amri, E.-Z.; Hutchinson, D.S.; Bengtsson, T. Glucose uptake in brown fat cells is dependent on MTOR complex 2-promoted GLUT1 translocation. J. Cell. Biol. 2014, 207, 365–374. [Google Scholar] [CrossRef]
- Olsen, J.M.; Csikasz, R.I.; Dehvari, N.; Lu, L.; Sandström, A.; Öberg, A.I.; Nedergaard, J.; Stone-Elander, S.; Bengtsson, T. Β3-adrenergically induced glucose uptake in brown adipose tissue is independent of UCP1 presence or activity: Mediation through the MTOR pathway. Mol. Metab. 2017, 6, 611–619. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, X.; Sun, X.; Zhang, L.; Fu, X.; Rogers, C.J.; Berim, A.; Zhang, S.; Wang, S.; Wang, B.; et al. AMPK/α-Ketoglutarate axis dynamically mediates DNA demethylation in the prdm16 promoter and brown adipogenesis. Cell Metab. 2016, 24, 542–554. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, X.; Zhao, J.; Du, M. AMPKα1 regulates Idh2 transcription through H2B O-GlcNAcylation during brown adipogenesis. Acta Biochim. Biophys. Sin. 2021, 53, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell. Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.; Liu, X.; Dagda, R.K.; Zhang, Y. How AMPK and PKA interplay to regulate mitochondrial function and survival in models of ischemia and diabetes. Oxid. Med. Cell. Longev. 2017, 2017, 4353510. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell. Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Munk-Hansen, N.; Birk, J.B.; Foretz, M.; Viollet, B.; Björnholm, M.; Zierath, J.R.; Treebak, J.T.; Wojtaszewski, J.F.P. Enhanced muscle insulin sensitivity after contraction/exercise is mediated by AMPK. Diabetes 2017, 66, 598–612. [Google Scholar] [CrossRef]
- Wikstrom, J.D.; Mahdaviani, K.; Liesa, M.; Sereda, S.B.; Si, Y.; Las, G.; Twig, G.; Petrovic, N.; Zingaretti, C.; Graham, A.; et al. Hormone-Induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. Embo. J. 2014, 33, 418–436. [Google Scholar] [CrossRef] [PubMed]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.L.; Losón, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. Metabolism. AMP-Activated protein kinase mediates mitochondrial fission in response to energy stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Perdikari, A.; Kulenkampff, E.; Rudigier, C.; Neubauer, H.; Luippold, G.; Redemann, N.; Wolfrum, C. A high-throughput, image-based screen to identify kinases involved in brown adipocyte development. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef]
- Perdikari, A.; Leparc, G.G.; Balaz, M.; Pires, N.D.; Lidell, M.E.; Sun, W.; Fernandez-Albert, F.; Müller, S.; Akchiche, N.; Dong, H.; et al. BATLAS: Deconvoluting brown adipose tissue. Cell Rep. 2018, 25, 784–797.e4. [Google Scholar] [CrossRef] [PubMed]
- Finlin, B.S.; Memetimin, H.; Zhu, B.; Confides, A.L.; Vekaria, H.J.; El Khouli, R.H.; Johnson, Z.R.; Westgate, P.M.; Chen, J.; Morris, A.J.; et al. The Β3-Adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J. Clin. Investig. 2020, 130, 2319–2331. [Google Scholar] [CrossRef]
- O’Mara, A.E.; Johnson, J.W.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Fletcher, L.A.; Fink, Y.A.; Kapuria, D.; Cassimatis, T.M.; Kelsey, N.; et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Investig. 2020, 130, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.; Lu, M.; Bandyopadhyay, G.; Li, P.; Zhou, Y.; Huang, J.; Levin, N.; Tomas, E.M.; Calle, R.A.; Erion, D.M.; et al. FGF21 does not require interscapular brown adipose tissue and improves liver metabolic profile in animal models of obesity and insulin-resistance. Sci. Rep. 2015, 5, 11382. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.D.L.; Gao, J.; Yang, Q.; Wu, Z.; Gromada, J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 12553–12558. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, E.P.; Desjardins, E.M.; Fritzen, A.M.; Zou, V.Z.; Crane, J.D.; Yabut, J.M.; Kiens, B.; Erion, D.M.; Lanba, A.; Granneman, J.G.; et al. FGF21 does not require adipocyte AMP-Activated Protein Kinase (AMPK) or the Phosphorylation of Acetyl-CoA Carboxylase (ACC) to mediate improvements in whole-body glucose homeostasis. Mol. Metab. 2017, 6, 471–481. [Google Scholar] [CrossRef]
- Bertholet, A.M.; Kazak, L.; Chouchani, E.T.; Bogaczyńska, M.G.; Paranjpe, I.; Wainwright, G.L.; Bétourné, A.; Kajimura, S.; Spiegelman, B.M.; Kirichok, Y. Mitochondrial patch clamp of beige adipocytes reveals UCP1-Positive and UCP1-Negative cells both exhibiting futile creatine cycling. Cell Metab. 2017, 25, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Kang, Q.; Yoneshiro, T.; Camporez, J.P.; Maki, H.; Homma, M.; Shinoda, K.; Chen, Y.; Lu, X.; Maretich, P.; et al. UCP1-independent signaling involving SERCA2b-Mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 2017, 23, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L.; Chouchani, E.T.; Jedrychowski, M.P.; Erickson, B.K.; Shinoda, K.; Cohen, P.; Vetrivelan, R.; Lu, G.Z.; Laznik-Bogoslavski, D.; Hasenfuss, S.C.; et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige Fat. Cell 2015, 163, 643–655. [Google Scholar] [CrossRef]
- Hanssen, M.J.W.; Hoeks, J.; Brans, B.; van der Lans, A.A.J.J.; Schaart, G.; van den Driessche, J.J.; Jörgensen, J.A.; Boekschoten, M.V.; Hesselink, M.K.C.; Havekes, B.; et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 2015, 21, 863–865. [Google Scholar] [CrossRef]
- Remie, C.M.E.; Moonen, M.P.B.; Roumans, K.H.M.; Nascimento, E.B.M.; Gemmink, A.; Havekes, B.; Schaart, G.; Kornips, E.; Joris, P.J.; Schrauwen-Hinderling, V.B.; et al. Metabolic responses to mild cold acclimation in type 2 diabetes patients. Nat. Commun. 2021, 12, 1516. [Google Scholar] [CrossRef]
- Fernández-Verdejo, R.; Marlatt, K.L.; Ravussin, E.; Galgani, J.E. Contribution of brown adipose tissue to human energy metabolism. Mol. Asp. Med. 2019, 68, 82–89. [Google Scholar] [CrossRef]
- Kong, X.; Yao, T.; Zhou, P.; Kazak, L.; Tenen, D.; Lyubetskaya, A.; Dawes, B.A.; Tsai, L.; Kahn, B.B.; Spiegelman, B.M.; et al. Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab. 2018, 28, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-X.; Zhao, X.-Y.; Meng, Z.-X.; Kern, M.; Dietrich, A.; Chen, Z.; Cozacov, Z.; Zhou, D.; Okunade, A.L.; Su, X.; et al. The brown fat-enriched secreted factor nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med. 2014, 20, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
| Compound Name | Mechanism of Action | Binding Site | Type | Function | Reference |
|---|---|---|---|---|---|
| Metformin | Indirect activator | AMP binds γ subunit | Mitochondrial inhibitor | Mimics glucose deprivation by lowering the glucose blood levels. Inhibits Complex I of the respiratory chain causing transient energy stress in the cell, elevating the AMP:ATP and ADP:ATP ratio. | [26,40,41,42,49] |
| Oligomycin | Indirect activator | AMP binds γ subunit | Mitochondrial inhibitor | Mitochondrial poison that activates AMPK through inhibition of Complex V of the respiratory chain, elevating the AMP:ATP and ADP:ATP ratio. | [33,43] |
| Resveratrol | Indirect activator | AMP binds γ subunit | Mitochondrial inhibitor | Plant-derived drug that activates AMPK through inhibition of Complex I, III, and V of the respiratory chain, elevating the AMP:ATP and ADP:ATP ratio. | [33,43,50,51,52,53] |
| Berberine | Indirect activator | AMP binds γ subunit | Mitochondrial inhibitor | Plant-derived drug that activates AMPK through inhibition of Complex I of the respiratory chain, elevating the AMP:ATP and ADP:ATP ratio. | [33,43,44,54] |
| 2-deoxy-glucose | Indirect activator | AMP binds γ subunit | Glycolysis inhibitor | A non-metabolizable glucose analog. Elevates the AMP:ATP and ADP:ATP ratio by suppressing glycolysis. | [33,49,55,56] |
| AICAR | Direct activator | ZMP binds γ subunit | AMP mimetic | Pro-drug that is converted to ZMP, mimics energy stress. | [22,33,57] |
| A769662 | Direct activator | Binds the ADaM site. Selective for AMPKβ1 isoform. | AMPK binding | Small molecule that activates allosterically by binding AMPK. Prevents dephosphorylation of Thr-172. | [23,46,47,48] |
| PF-249 | Direct activator | Binds the ADaM site. Selective for AMPKβ1 isoform. | AMPK binding | Small molecule that activates allosterically by binding AMPK. Prevents dephosphorylation of Thr-172. | [48,58,59] |
| PF-739 | Direct activator | Binds the ADaM site. Act on all 12 mammalian AMPK isoforms. | AMPK binding | Small molecule that activates allosterically by binding AMPK. Prevents dephosphorylation of Thr-172. | [58] |
| MK-8722 | Direct activator | Binds the ADaM site. Act on all 12 mammalian AMPK isoforms. | AMPK binding | Small molecule that activates allosterically by binding AMPK. Prevents dephosphorylation of Thr-172. | [60,61] |
| PF-06409577 | Direct activator | Binds the ADaM site. Selective for AMPKβ1 isoform. | AMPK binding | Small molecule that activates allosterically by binding AMPK. Prevents dephosphorylation of Thr-172. | [48,59,62,63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Vaart, J.I.; Boon, M.R.; Houtkooper, R.H. The Role of AMPK Signaling in Brown Adipose Tissue Activation. Cells 2021, 10, 1122. https://doi.org/10.3390/cells10051122
van der Vaart JI, Boon MR, Houtkooper RH. The Role of AMPK Signaling in Brown Adipose Tissue Activation. Cells. 2021; 10(5):1122. https://doi.org/10.3390/cells10051122
Chicago/Turabian Stylevan der Vaart, Jamie I., Mariëtte R. Boon, and Riekelt H. Houtkooper. 2021. "The Role of AMPK Signaling in Brown Adipose Tissue Activation" Cells 10, no. 5: 1122. https://doi.org/10.3390/cells10051122
APA Stylevan der Vaart, J. I., Boon, M. R., & Houtkooper, R. H. (2021). The Role of AMPK Signaling in Brown Adipose Tissue Activation. Cells, 10(5), 1122. https://doi.org/10.3390/cells10051122






