PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Gene Transfection
2.2. In Vitro Coculture System
2.3. Animals and MSC Transplantation
2.4. Histopathological and Immunofluorescence Analysis
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Western Blotting
2.7. XF Mito Stress Assay
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
3. Results
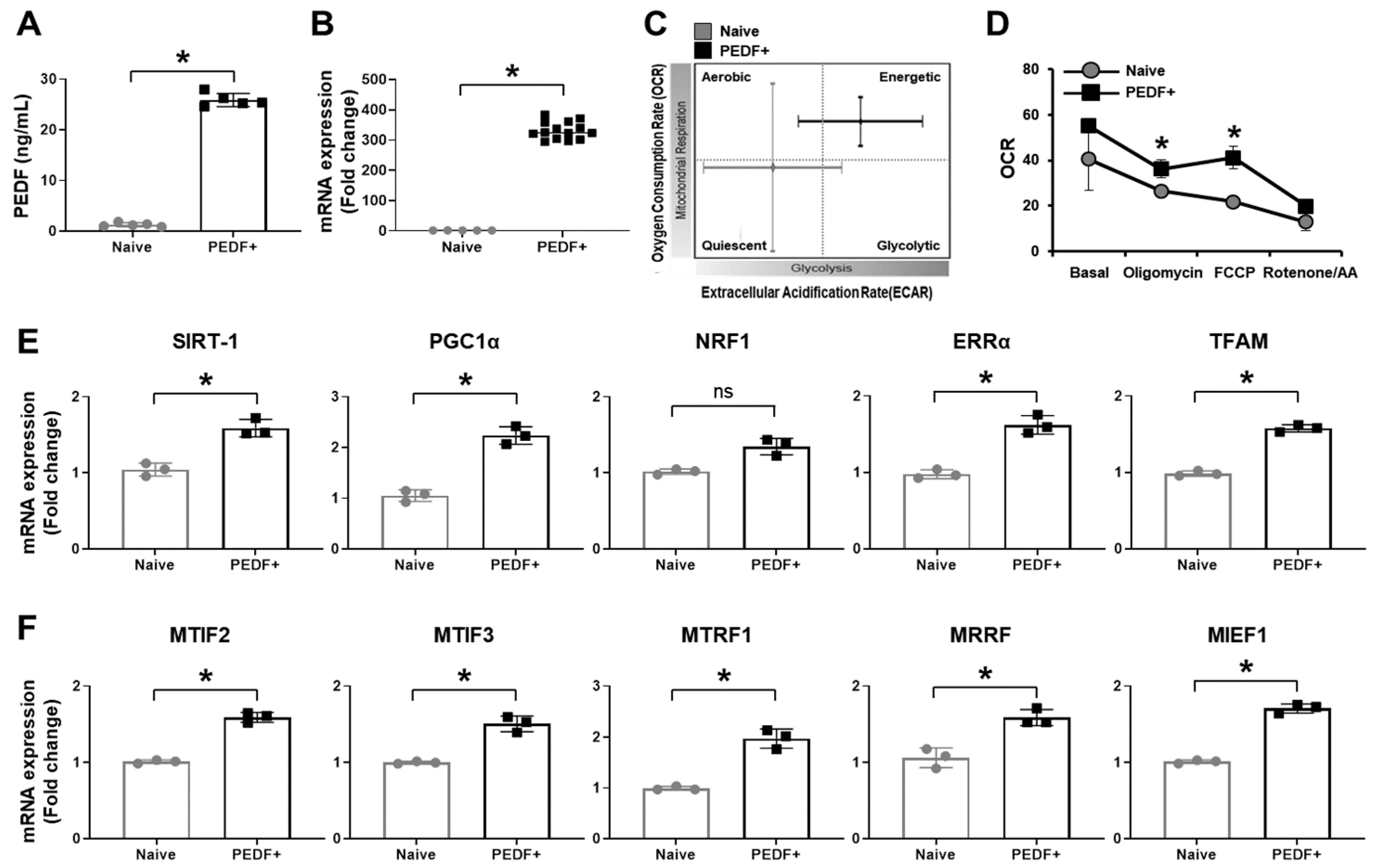
3.1. PD-MSCsPEDF Enhance Mitochondrial Activity
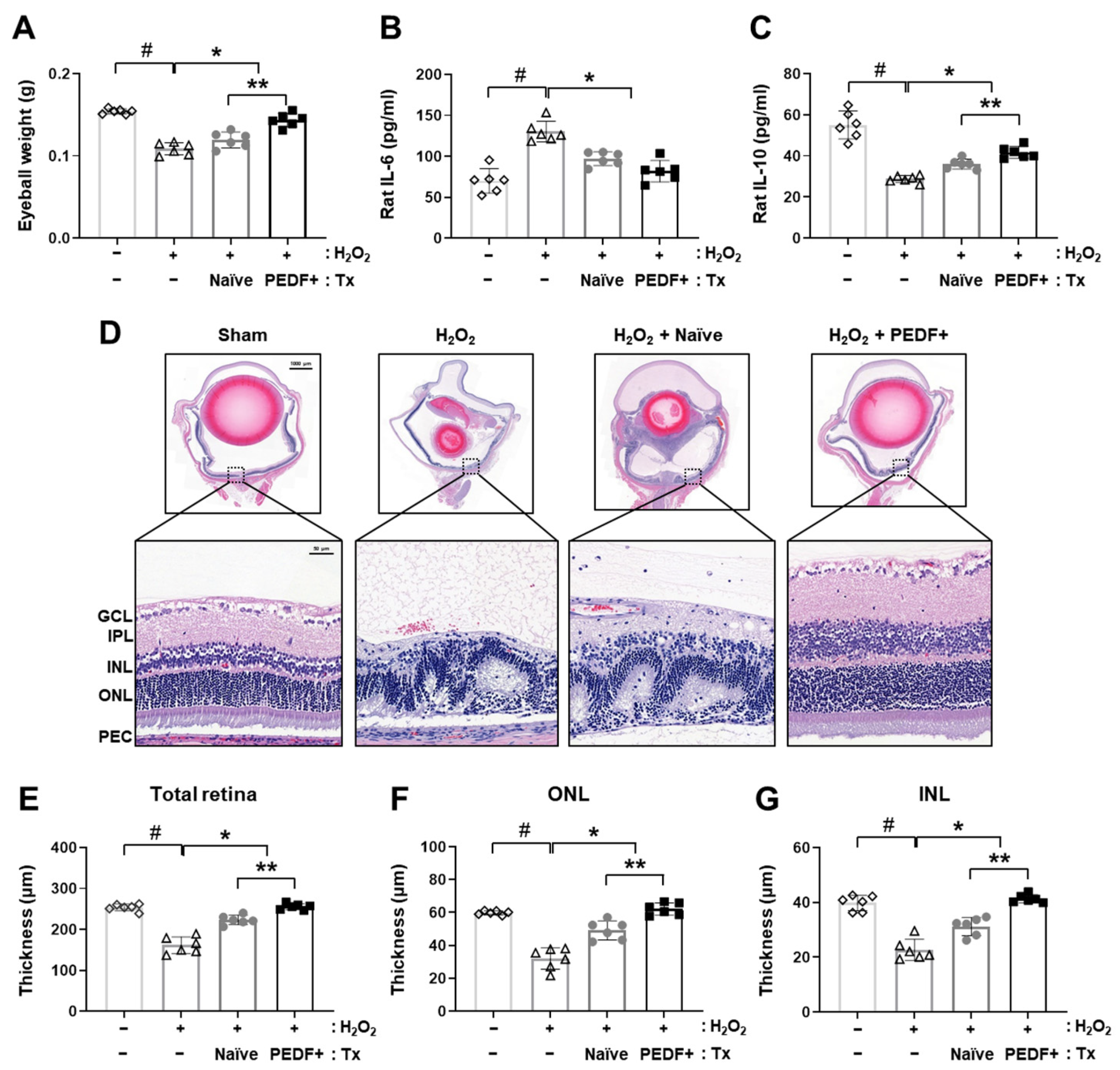
3.2. PD-MSCPEDF Transplantation Restores Retinal Function in a H2O2-Injured Rat Model
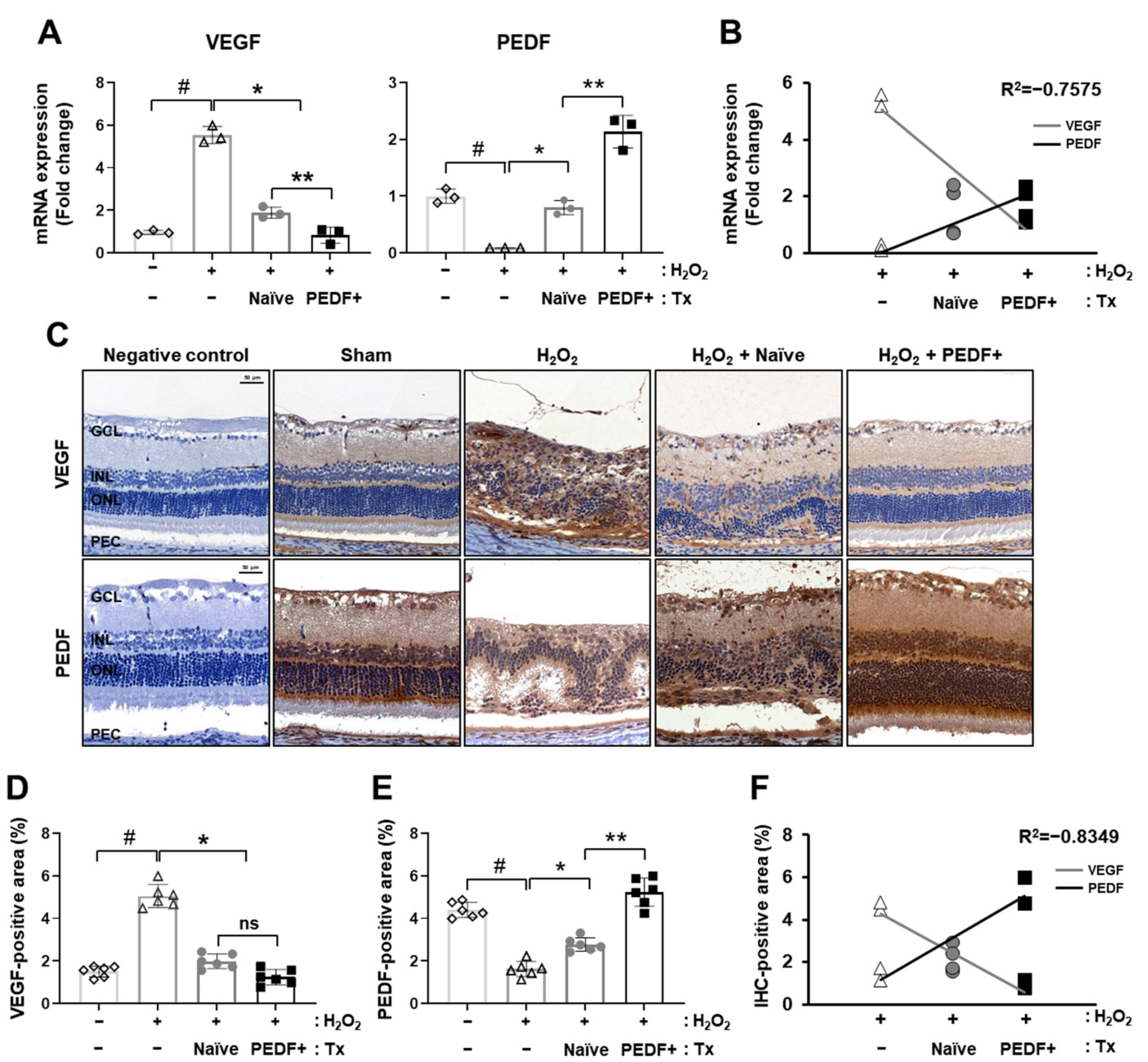
3.3. PD-MSCsPEDF Balanced VEGF and PEDF Levels in a H2O2-Induced Rat Model
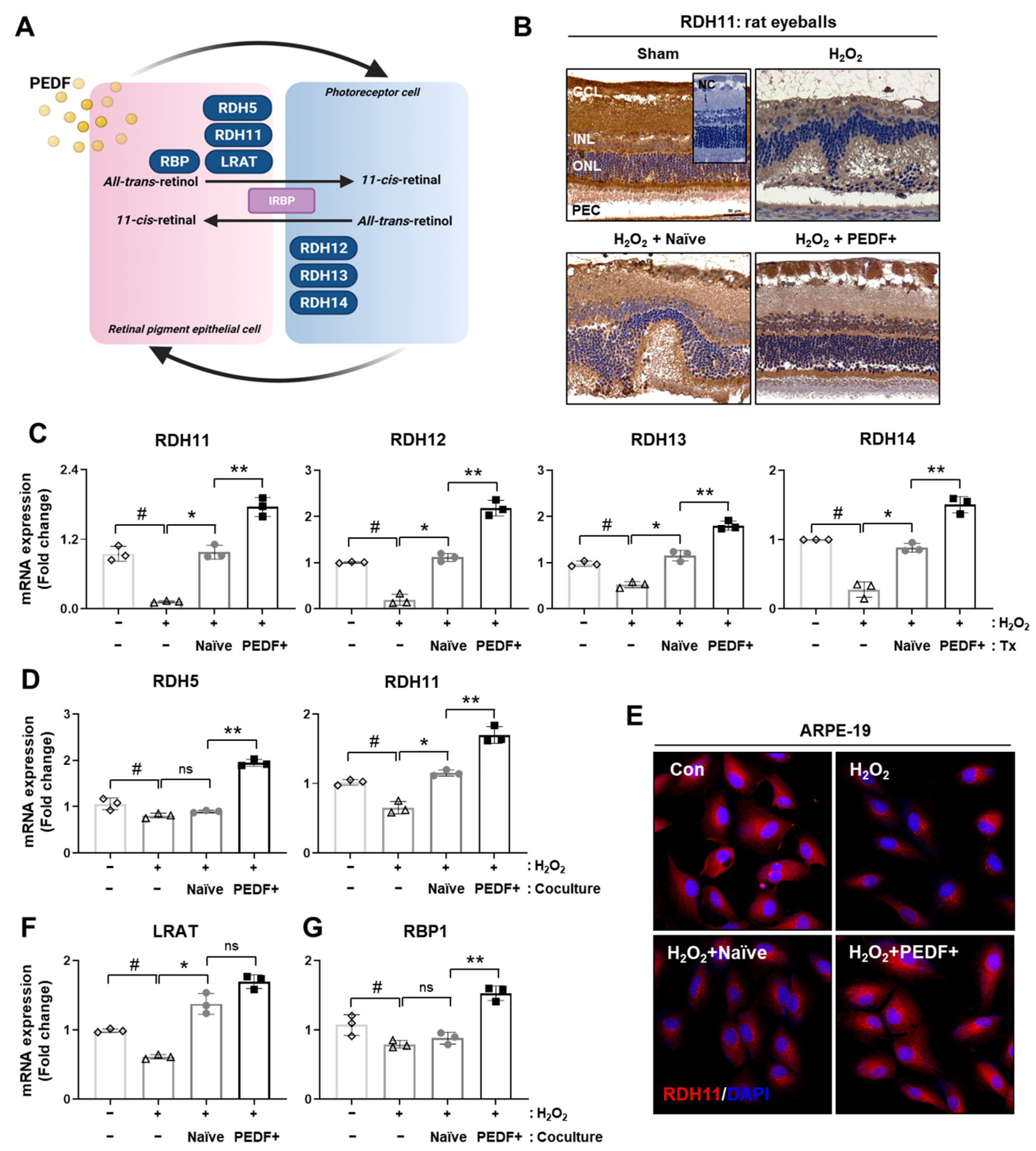
3.4. PD-MSCsPEDF Improved Visual Cycles in Rat Retinal Layers and RPE Cells
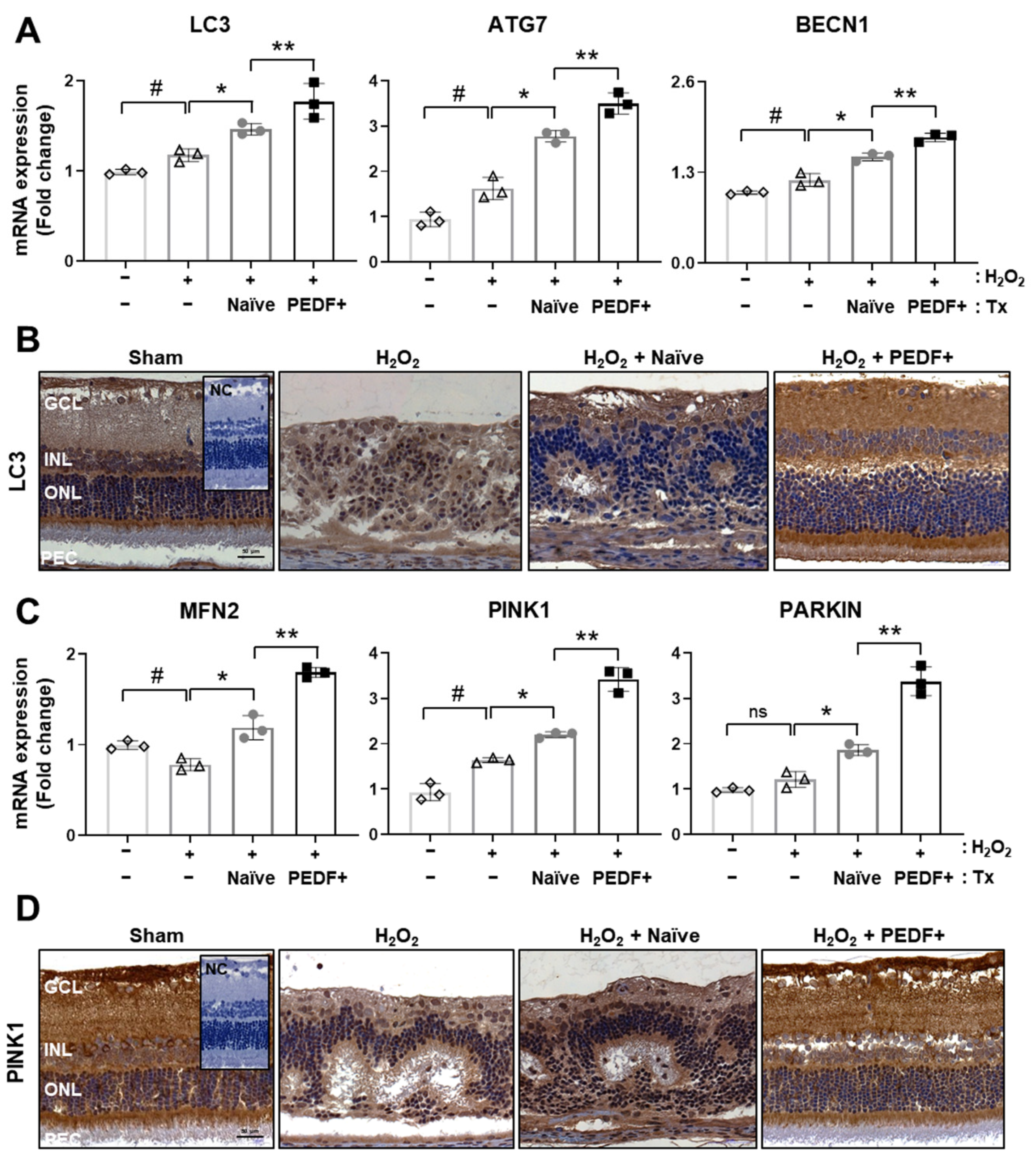
3.5. The Administration of PD-MSCsPEDF Induces Mitophagy in H2O2-Injured Rat Retinas
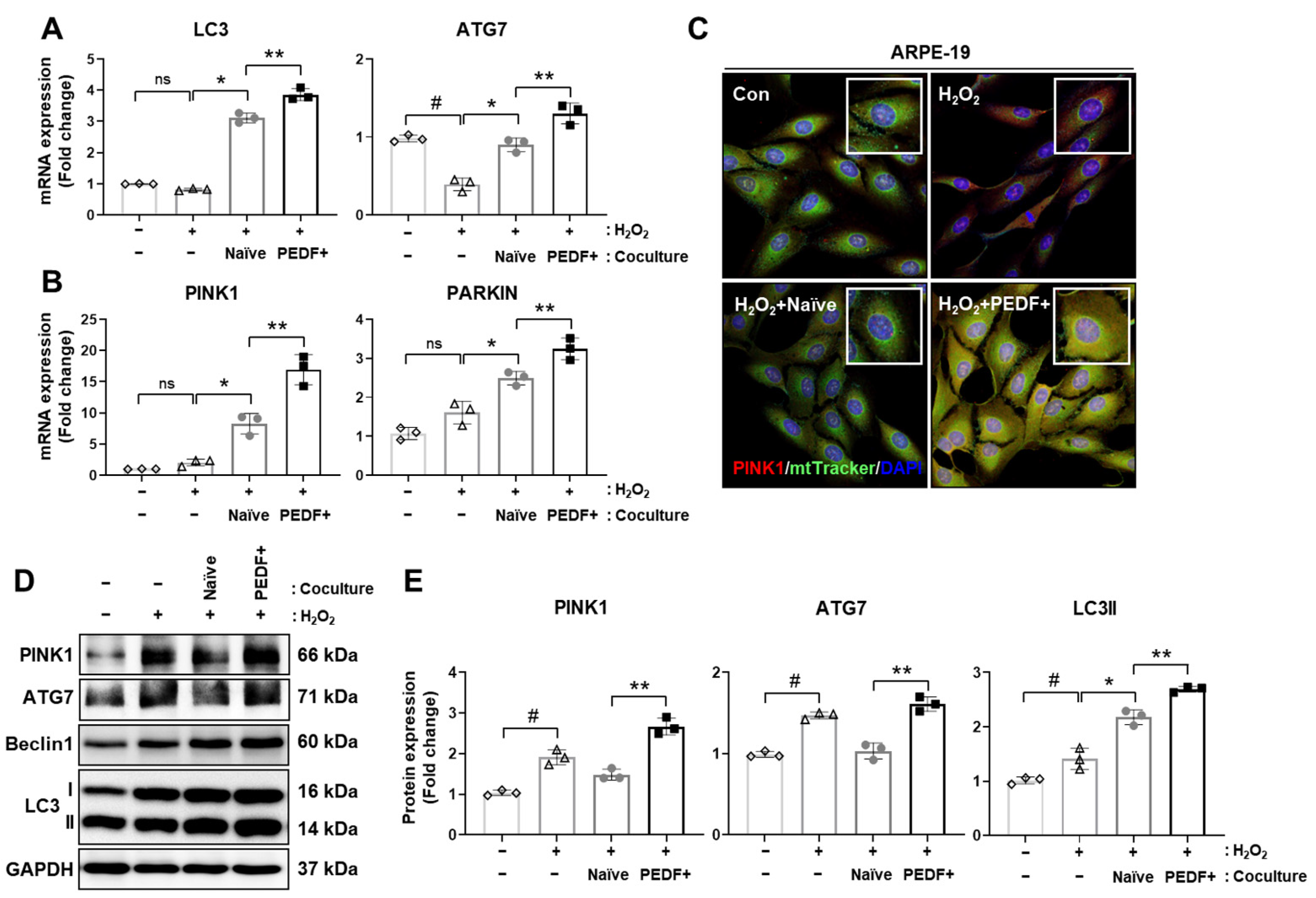
3.6. Coculturing RPE Cells with PD-MSCsPEDF Enhances Mitophagy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, S.L.S.; Kumar, S.; Mok, P.L. Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases. Int. J. Mol. Sci. 2017, 18, 1406. [Google Scholar] [CrossRef]
- Barot, M.; Gokulgandhi, M.R.; Mitra, A.K. Mitochondrial Dysfunction in Retinal Diseases. Curr. Eye Res. 2011, 36, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Felszeghy, S.; Viiri, J.; Paterno, J.J.; Hyttinen, J.M.T.; Koskela, A.; Chen, M.; Leinonen, H.; Tanila, H.; Kivinen, N.; Koistinen, A.; et al. Loss of Nrf-2 and Pgc-1alpha Genes Leads to Retinal Pigment Epithelium Damage Resembling Dry Age-Related Macular Degeneration. Redox Biol. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-Related Macular Degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Farnoodian, M.; Sorenson, C.M.; Sheibani, N. Negative Regulators of Angiogenesis, Ocular Vascular Homeostasis, and Pathogenesis and Treatment of Exudative Amd. J. Ophthalmic Vis. Res. 2018, 13, 470–486. [Google Scholar] [PubMed]
- Li, R.; Du, J.H.; Yao, G.M.; Yao, Y.; Zhang, J. Autophagy: A New Mechanism for Regulating Vegf and Pedf Expression in Retinal Pigment Epithelium Cells. Int. J. Ophthalmol. 2019, 12, 57–62. [Google Scholar]
- Tombran-Tink, J.; Shivaram, S.M.; Chader, G.J.; Johnson, L.V.; Bok, D. Expression, Secretion, and Age-Related Downregulation of Pigment Epithelium-Derived Factor, a Serpin with Neurotrophic Activity. J. Neurosci. 1995, 15 Pt 1, 4992–5003. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Cheng, R.; Benyajati, S.; Ma, J.X. Pedf and Its Roles in Physiological and Pathological Conditions: Implication in Diabetic and Hypoxia-Induced Angiogenic Diseases. Clin. Sci. (Lond.) 2015, 128, 805–823. [Google Scholar] [CrossRef] [Green Version]
- Spranger, J.; Osterhoff, M.; Reimann, M.; Mohlig, M.; Ristow, M.; Francis, M.K.; Cristofalo, V.; Hammes, H.P.; Smith, G.; Boulton, M.; et al. Loss of the Antiangiogenic Pigment Epithelium-Derived Factor in Patients with Angiogenic Eye Disease. Diabetes 2001, 50, 2641–2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, Y.; Yamagishi, S.; Matsui, T.; Nakamura, K.; Imaizumi, T.; Yoshimura, K.; Yamakawa, R. Positive Correlation of Pigment Epithelium-Derived Factor and Total Antioxidant Capacity in Aqueous Humour of Patients with Uveitis and Proliferative Diabetic Retinopathy. Br. J. Ophthalmol. 2007, 91, 1133–1134. [Google Scholar] [CrossRef]
- He, Y.; Leung, K.W.; Ren, Y.; Pei, J.; Ge, J.; Tombran-Tink, J. Pedf Improves Mitochondrial Function in Rpe Cells During Oxidative Stress. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6742–6755. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Maeda, A. Rpe Visual Cycle and Biochemical Phenotypes of Mutant Mouse Models. Methods Mol. Biol. 2018, 1753, 89–102. [Google Scholar] [PubMed]
- Kiser, P.D.; Golczak, M.; Maeda, A.; Palczewski, K. Key Enzymes of the Retinoid (Visual) Cycle in Vertebrate Retina. Biochim. Biophys. Acta 2012, 1821, 137–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, A.F.; Chakarova, C.F.; El-Aziz, M.M.A.; Bhattacharya, S.S. Photoreceptor Degeneration: Genetic and Mechanistic Dissection of a Complex Trait. Nat. Rev. Genet. 2010, 11, 273–284. [Google Scholar] [CrossRef]
- Hernandez-Pinto, A.; Polato, F.; Subramanian, P.; Rocha-Munoz, A.; Vitale, S.; de la Rosa, E.J.; Becerra, S.P. Pedf Peptides Promote Photoreceptor Survival in Rd10 Retina Models. Exp. Eye Res. 2019, 184, 24–29. [Google Scholar] [CrossRef]
- Valiente-Soriano, F.J.; Di Pierdomenico, J.; Garcia-Ayuso, D.; Ortin-Martinez, A.; de Imperial-Ollero, J.A.M.; Gallego-Ortega, A.; Jimenez-Lopez, M.; Villegas-Perez, M.P.; Becerra, S.P.; Vidal-Sanz, M. Pigment Epithelium-Derived Factor (Pedf) Fragments Prevent Mouse Cone Photoreceptor Cell Loss Induced by Focal Phototoxicity In Vivo. Int. J. Mol. Sci. 2020, 21, 7242. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and Function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.X.; Yin, X.M. Mitophagy: Mechanisms, Pathophysiological Roles, and Analysis. Biol. Chem. 2012, 393, 547–564. [Google Scholar] [CrossRef] [Green Version]
- Sridevi Gurubaran, I.; Viiri, J.; Koskela, A.; Hyttinen, J.M.T.; Paterno, J.J.; Kis, G.; Antal, M.; Urtti, A.; Kauppinen, A.; Felszeghy, S.; et al. Mitophagy in the Retinal Pigment Epithelium of Dry Age-Related Macular Degeneration Investigated in the Nfe2l2/Pgc-1alpha(-/-) Mouse Model. Int. J. Mol. Sci. 2020, 21, 1976. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xi, X.; Mei, Y.; Zhao, X.; Zhou, L.; Ma, M.; Liu, S.; Zha, X.; Yang, Y. High-Glucose Induces Retinal Pigment Epithelium Mitochondrial Pathways of Apoptosis and Inhibits Mitophagy by Regulating Ros/Pink1/Parkin Signal Pathway. Biomed. Pharmacother. 2019, 111, 1315–1325. [Google Scholar] [CrossRef]
- Huang, B.; Liang, J.J.; Zhuang, X.; Chen, S.W.; Ng, T.K.; Chen, H. Intravitreal Injection of Hydrogen Peroxide Induces Acute Retinal Degeneration, Apoptosis, and Oxidative Stress in Mice. Oxid. Med. Cell Longev. 2018, 2018, 5489476. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Park, S.; Park, S.H.; Lee, D.; Kim, G.H.; Noh, J.E.; Lee, K.J.; Kim, G.J. Overexpression of Pigment Epithelium-Derived Factor in Placenta-Derived Mesenchymal Stem Cells Promotes Mitochondrial Biogenesis in Retinal Cells. Lab. Investig. 2021, 101, 51–69. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, J.H.; Kim, S.H.; Park, H.; Lee, D.; Kim, G.J. Efficacy of Gene Modification in Placenta-Derived Mesenchymal Stem Cells Based on Nonviral Electroporation. Int. J. Stem Cells 2021, 14, 12–18. [Google Scholar] [CrossRef]
- Brand, M.D.; Esteves, T.C. Physiological Functions of the Mitochondrial Uncoupling Proteins Ucp2 and Ucp3. Cell Metab. 2005, 2, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Yokokawa, T.; Kido, K.; Suga, T.; Isaka, T.; Hayashi, T.; Fujita, S. Exercise-Induced Mitochondrial Biogenesis Coincides with the Expression of Mitochondrial Translation Factors in Murine Skeletal Muscle. Physiol. Rep. 2018, 6, e13893. [Google Scholar] [CrossRef]
- Bhutto, I.A.; McLeod, D.S.; Hasegawa, T.; Kim, S.Y.; Merges, C.; Tong, P.; Lutty, G.A. Pigment Epithelium-Derived Factor (Pedf) and Vascular Endothelial Growth Factor (Vegf) in Aged Human Choroid and Eyes with Age-Related Macular Degeneration. Exp. Eye Res. 2006, 82, 99–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, K.; Ahn, J.; Sohn, J.; Hwang, D.D. Evaluation of the Safety of Bilateral Same-Day Intravitreal Injections of Anti-Vascular Endothelial Growth Factor Agents: Experience of a Large Korean Retina Center. Clin. Ophthalmol. 2020, 14, 3211–3218. [Google Scholar] [CrossRef]
- Han, F.; Xu, G. Stem Cell Transplantation Therapy for Retinal Degenerative Diseases. Adv. Exp. Med. Biol. 2020, 1266, 127–139. [Google Scholar] [PubMed]
- Feng, X.; Chen, P.; Zhao, X.; Wang, J.; Wang, H. Transplanted Embryonic Retinal Stem Cells Have the Potential to Repair the Injured Retina in Mice. BMC Ophthalmol. 2021, 21, 26. [Google Scholar] [CrossRef]
- Jeon, S.; Oh, I.H. Regeneration of the Retina: Toward Stem Cell Therapy for Degenerative Retinal Diseases. BMB Rep. 2015, 48, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.K.; Tosi, J.; Kasanuki, J.M.; Chou, C.L.; Kong, J.; Parmalee, N.; Wert, K.J.; Allikmets, R.; Lai, C.C.; Chien, C.L.; et al. Transplantation of Reprogrammed Embryonic Stem Cells Improves Visual Function in a Mouse Model for Retinitis Pigmentosa. Transplantation 2010, 89, 911–919. [Google Scholar] [CrossRef]
- Adak, S.; Magdalene, D.; Deshmukh, S.; Das, D.; Jaganathan, B.G. A Review on Mesenchymal Stem Cells for Treatment of Retinal Diseases. Stem Cell Rev. Rep. 2021, 1–20. [Google Scholar] [CrossRef]
- Kahraman, N.S.; Oner, A. Umbilical Cord Derived Mesenchymal Stem Cell Implantation in Retinitis Pigmentosa: A 6-Month Follow-up Results of a Phase 3 Trial. Int. J. Ophthalmol. 2020, 13, 1423–1429. [Google Scholar] [CrossRef]
- Zhou, Z.; Doggett, T.A.; Sene, A.; Apte, R.S.; Ferguson, T.A. Autophagy Supports Survival and Phototransduction Protein Levels in Rod Photoreceptors. Cell Death Differ. 2015, 22, 488–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, A.; Maeda, T.; Golczak, M.; Palczewski, K. Retinopathy in Mice Induced by Disrupted All-Trans-Retinal Clearance. J. Biol. Chem. 2008, 283, 26684–26693. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.K.; Lee, E.J.; Kim, Y.H.; Kim, D.Y.; Oh, H.; Kim, S.I.; Kang, Y.H. Chrysin Ameliorates Malfunction of Retinoid Visual Cycle through Blocking Activation of Age-Rage-Er Stress in Glucose-Stimulated Retinal Pigment Epithelial Cells and Diabetic Eyes. Nutrients 2018, 10, 1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrington, D.A.; Ebeling, M.C.; Kapphahn, R.J.; Terluk, M.R.; Fisher, C.R.; Polanco, J.R.; Roehrich, H.; Leary, M.M.; Geng, Z.; Dutton, J.R.; et al. Altered Bioenergetics and Enhanced Resistance to Oxidative Stress in Human Retinal Pigment Epithelial Cells from Donors with Age-Related Macular Degeneration. Redox Biol. 2017, 13, 255–265. [Google Scholar] [CrossRef]
- Becerra, S.P.; Fariss, R.N.; Wu, Y.Q.; Montuenga, L.M.; Wong, P.; Pfeffer, B.A. Pigment Epithelium-Derived Factor in the Monkey Retinal Pigment Epithelium and Interphotoreceptor Matrix: Apical Secretion and Distribution. Exp. Eye Res. 2004, 78, 223–234. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of Mitochondrial Dysfunction and Their Impact on Age-Related Macular Degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Zhang, Y.; Zhao, Q.; Wang, X.; Lu, P.; Zhang, H.; Wang, Z.; Dong, H.; Zhang, Z. Pedf Protects Cardiomyocytes by Promoting Fundc1mediated Mitophagy Via Pedf-R under Hypoxic Condition. Int. J. Mol. Med. 2018, 41, 3394–3404. [Google Scholar]
- Miao, H.; Qiu, F.; Huang, B.; Liu, X.; Zhang, H.; Liu, Z.; Yuan, Y.; Zhao, Q.; Zhang, H.; Dong, H.; et al. Pkcalpha Replaces Ampk to Regulate Mitophagy: Another Pedf Role on Ischaemic Cardioprotection. J. Cell Mol. Med. 2018, 22, 5732–5742. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sawada, O.; Kohno, H.; Le, Y.Z.; Subauste, C.; Maeda, T.; Maeda, A. Autophagy Protects the Retina from Light-Induced Degeneration. J. Biol. Chem. 2013, 288, 7506–7518. [Google Scholar] [CrossRef] [Green Version]
- Justilien, V.; Pang, J.J.; Renganathan, K.; Zhan, X.; Crabb, J.W.; Kim, S.R.; Sparrow, J.R.; Hauswirth, W.W.; Lewin, A.S. Sod2 Knockdown Mouse Model of Early Amd. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4407–4420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.E.; DeWeerd, A.J.; Ildefonso, C.J.; Lewin, A.S.; Ash, J.D. Mitochondrial Oxidative Stress in the Retinal Pigment Epithelium (Rpe) Led to Metabolic Dysfunction in Both the Rpe and Retinal Photoreceptors. Redox Biol. 2019, 24, 101201. [Google Scholar] [CrossRef]
- Chalam, K.V.; Khetpal, V.; Rusovici, R.; Balaiya, S. A Review: Role of Ultraviolet Radiation in Age-Related Macular Degeneration. Eye Contact Lens 2011, 37, 225–232. [Google Scholar] [CrossRef]
- Zerti, D.; Hilgen, G.; Dorgau, B.; Collin, J.; Ader, M.; Armstrong, L.; Sernagor, E.; Lako, M. Transplanted Pluripotent Stem Cell-Derived Photoreceptor Precursors Elicit Conventional and Unusual Light Responses in Mice with Advanced Retinal Degeneration. Stem Cells 2021. [Google Scholar] [CrossRef]
- Gonzalez Fleitas, M.F.; Devouassoux, J.; Aranda, M.L.; Dieguez, H.H.; Calanni, J.S.; Iaquinandi, A.; Sande, P.H.; Dorfman, D.; Rosenstein, R.E. Melatonin Prevents Non-Image-Forming Visual System Alterations Induced by Experimental Glaucoma in Rats. Mol. Neurobiol. 2021, 1–12. [Google Scholar] [CrossRef]
- Mao, H.; Seo, S.J.; Biswal, M.R.; Li, H.; Conners, M.; Nandyala, A.; Jones, K.; Le, Y.Z.; Lewin, A.S. Mitochondrial Oxidative Stress in the Retinal Pigment Epithelium Leads to Localized Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4613–4627. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.E. Ultraviolet Radiation as a Risk Factor for Cataract and Macular Degeneration. Eye Contact Lens 2011, 37, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Charng, J.; Nguyen, C.T.; Bui, B.V.; Vingrys, A.J. Age-Related Retinal Function Changes in Albino and Pigmented Rats. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8891–8899. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Park, S.; Park, H.J.; Kim, S.H.; Lew, H.; Kim, G.J. PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model. Cells 2021, 10, 1117. https://doi.org/10.3390/cells10051117
Kim JY, Park S, Park HJ, Kim SH, Lew H, Kim GJ. PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model. Cells. 2021; 10(5):1117. https://doi.org/10.3390/cells10051117
Chicago/Turabian StyleKim, Jae Yeon, Sohae Park, Hee Jung Park, Se Ho Kim, Helen Lew, and Gi Jin Kim. 2021. "PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model" Cells 10, no. 5: 1117. https://doi.org/10.3390/cells10051117
APA StyleKim, J. Y., Park, S., Park, H. J., Kim, S. H., Lew, H., & Kim, G. J. (2021). PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model. Cells, 10(5), 1117. https://doi.org/10.3390/cells10051117






