Calcium and Redox Liaison: A Key Role of Selenoprotein N in Skeletal Muscle
Abstract
1. Introduction
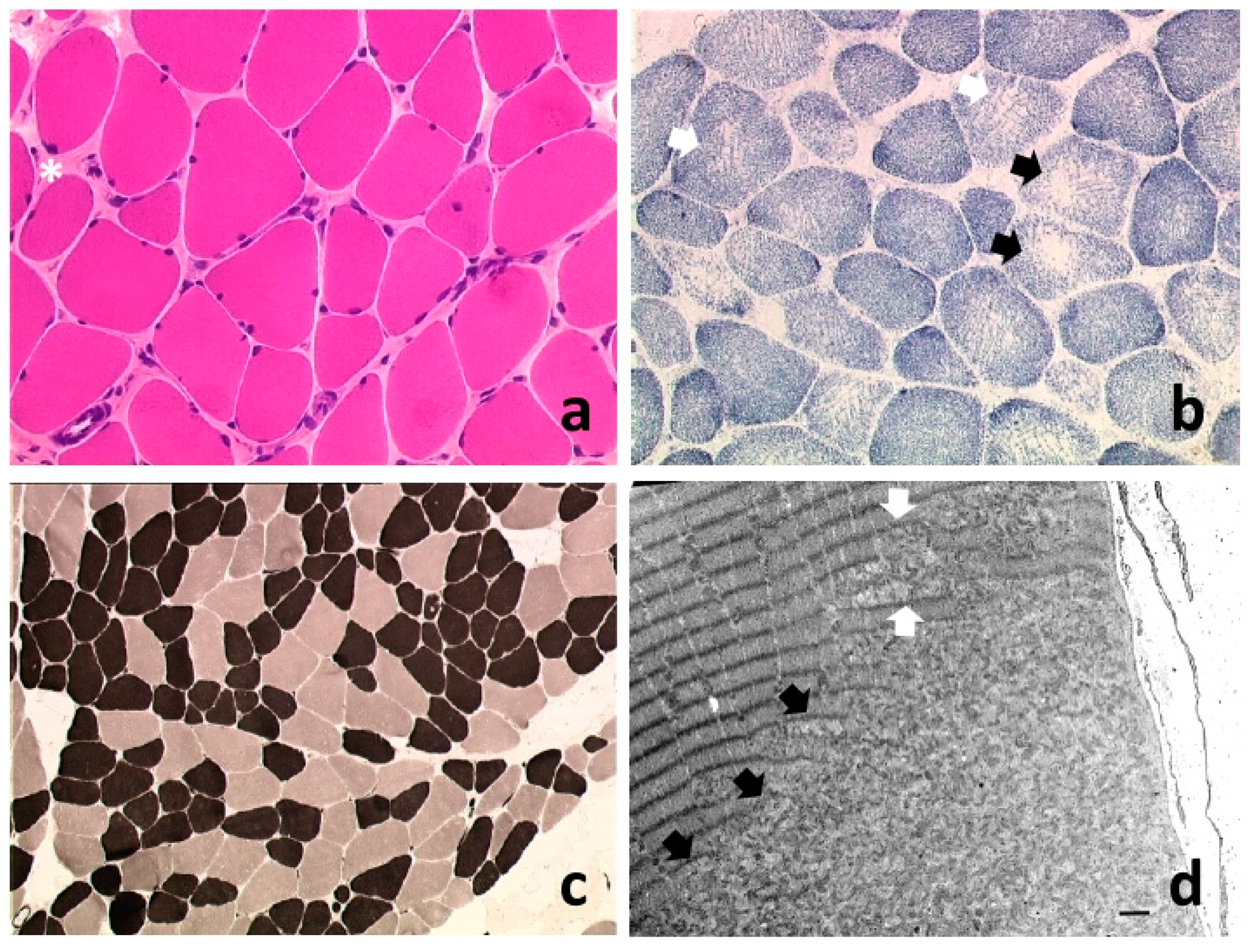
2. SEPN1-Related Myopathy
3. Redox Modulation of Calcium Handling in the ER and Mitochondria
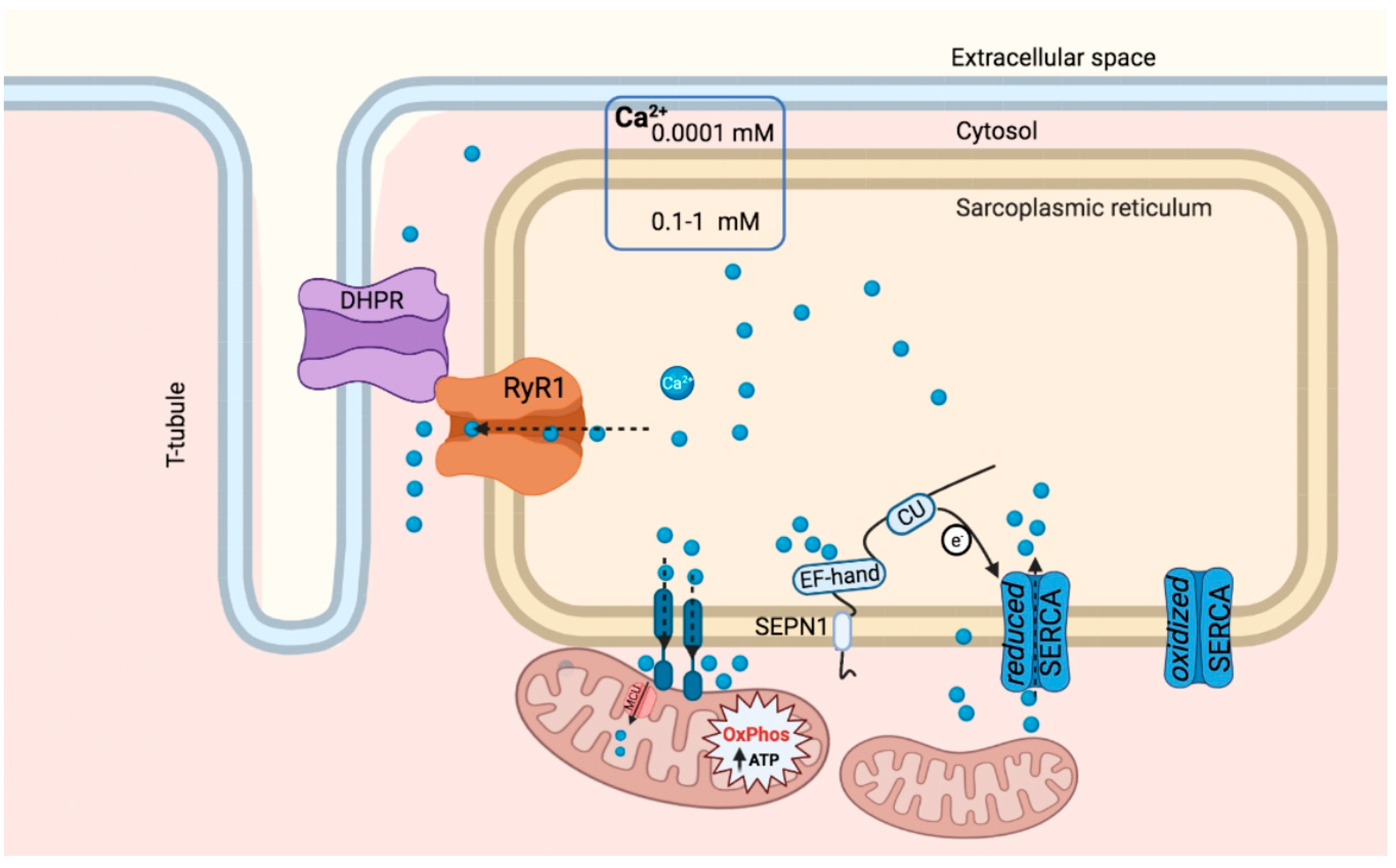
4. SEPN1-Mediated Calcium Handling in the ER and Mitochondria
5. SEPN1 at ER–Mitochondria Contact Sites
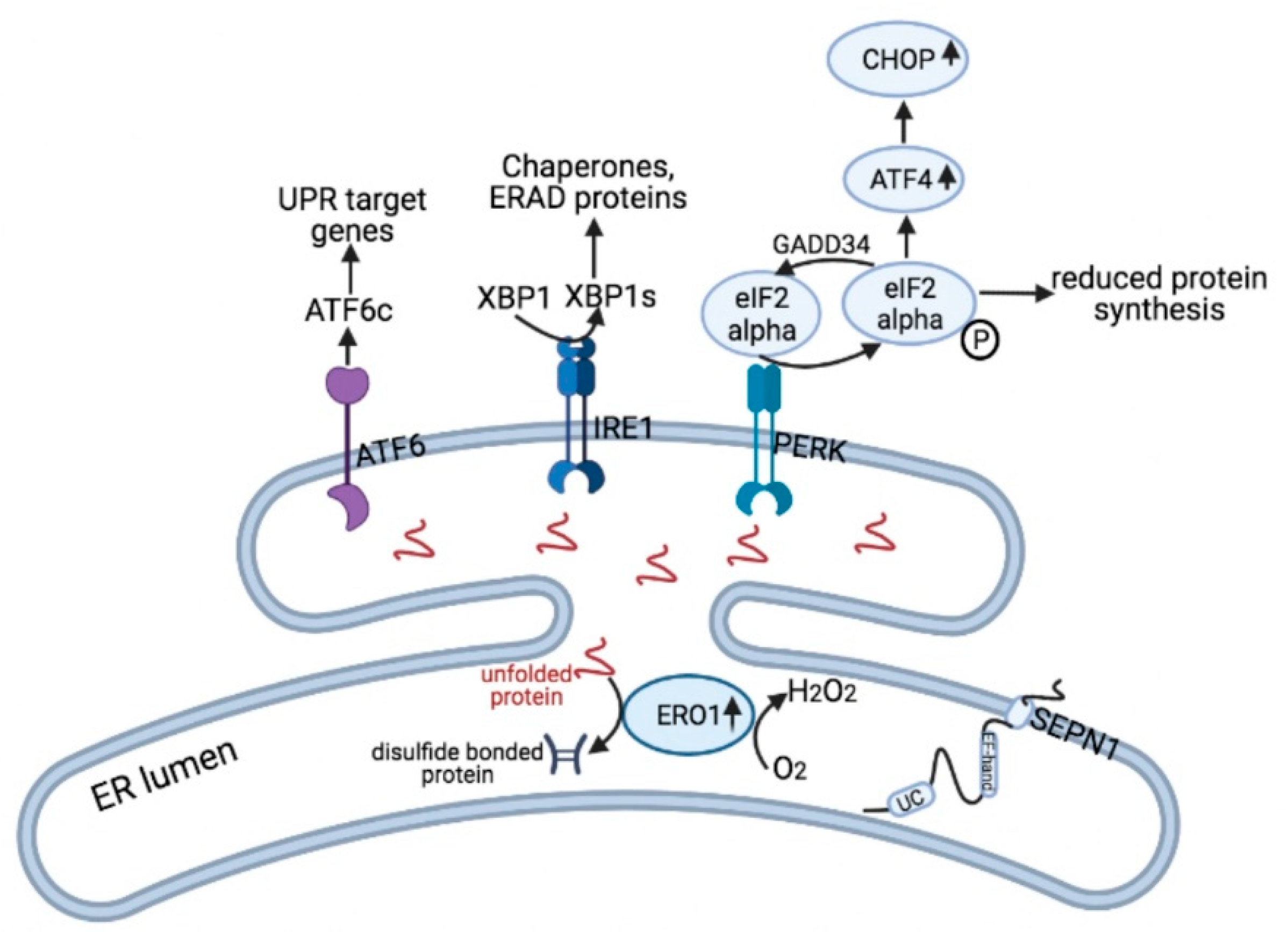
6. SEPN1 Loss and ER Stress
7. Conclusion and Therapeutic Perspectives of Core Diseases
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferreiro, A.; Quijano-Roy, S.; Pichereau, C.; Moghadaszadeh, B.; Goemans, N.; Bonnemann, C.; Jungbluth, H.; Straub, V.; Villanova, M.; Leroy, J.P.; et al. Mutations of the selenoprotein n gene, which is implicated in rigid spine muscular dystrophy, cause the classical phenotype of multiminicore disease: Reassessing the nosology of early-onset myopathies. Am. J. Hum. Genet. 2002, 71, 739–749. [Google Scholar] [CrossRef]
- Moghadaszadeh, B.; Petit, N.; Jaillard, C.; Brockington, M.; Quijano Roy, S.; Merlini, L.; Romero, N.; Estournet, B.; Desguerre, I.; Chaigne, D.; et al. Mutations in sepn1 cause congenital muscular dystrophy with spinal rigidity and restrictive respiratory syndrome. Nat. Genet. 2001, 29, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Moghadaszadeh, B.; Beggs, A.H. Selenoproteins and their impact on human health through diverse physiological pathways. Physiology 2006, 21, 307–315. [Google Scholar] [CrossRef]
- Chernorudskiy, A.; Varone, E.; Colombo, S.F.; Fumagalli, S.; Cagnotto, A.; Cattaneo, A.; Briens, M.; Baltzinger, M.; Kuhn, L.; Bachi, A.; et al. Selenoprotein n is an endoplasmic reticulum calcium sensor that links luminal calcium levels to a redox activity. Proc. Natl. Acad. Sci. USA 2020, 117, 21288–21298. [Google Scholar] [CrossRef]
- Pozzer, D.; Varone, E.; Chernorudskiy, A.; Schiarea, S.; Missiroli, S.; Giorgi, C.; Pinton, P.; Canato, M.; Germinario, E.; Nogara, L.; et al. A maladaptive er stress response triggers dysfunction in highly active muscles of mice with selenon loss. Redox. Biol. 2018, 20, 354–366. [Google Scholar] [CrossRef]
- Filipe, A.; Chernorudskiy, A.; Arbogast, S.; Varone, E.; Villar-Quiles, R.N.; Pozzer, D.; Moulin, M.; Fumagalli, S.; Cabet, E.; Dudhal, S.; et al. Defective endoplasmic reticulum-mitochondria contacts and bioenergetics in sepn1-related myopathy. Cell Death Differ. 2021, 28, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Varone, E.; Pozzer, D.; Di Modica, S.; Chernorudskiy, A.; Nogara, L.; Baraldo, M.; Cinquanta, M.; Fumagalli, S.; Villar-Quiles, R.N.; De Simoni, M.G.; et al. Selenon (sepn1) protects skeletal muscle from saturated fatty acid-induced er stress and insulin resistance. Redox. Biol. 2019, 24, 101176. [Google Scholar] [CrossRef] [PubMed]
- Arbogast, S.; Ferreiro, A. Selenoproteins and protection against oxidative stress: Selenoprotein n as a novel player at the crossroads of redox signaling and calcium homeostasis. Antioxid. Redox Signal. 2010, 12, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Boncompagni, S.; Pozzer, D.; Viscomi, C.; Ferreiro, A.; Zito, E. Physical and functional cross talk between endo-sarcoplasmic reticulum and mitochondria in skeletal muscle. Antioxid. Redox Signal. 2020, 32, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, A.; Monnier, N.; Romero, N.B.; Leroy, J.P.; Bonnemann, C.; Haenggeli, C.A.; Straub, V.; Voss, W.D.; Nivoche, Y.; Jungbluth, H.; et al. A recessive form of central core disease, transiently presenting as multi-minicore disease, is associated with a homozygous mutation in the ryanodine receptor type 1 gene. Ann. Neurol. 2002, 51, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Lawal, T.A.; Todd, J.J.; Witherspoon, J.W.; Bonnemann, C.G.; Dowling, J.J.; Hamilton, S.L.; Meilleur, K.G.; Dirksen, R.T. Ryanodine receptor 1-related disorders: An historical perspective and proposal for a unified nomenclature. Skelet. Muscle 2020, 10, 32. [Google Scholar] [CrossRef]
- Scoto, M.; Cirak, S.; Mein, R.; Feng, L.; Manzur, A.Y.; Robb, S.; Childs, A.M.; Quinlivan, R.M.; Roper, H.; Jones, D.H.; et al. Sepn1-related myopathies: Clinical course in a large cohort of patients. Neurology 2011, 76, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- Villar-Quiles, R.N.; von der Hagen, M.; Métay, C.; Gonzalez, V.; Donkervoort, S.; Bertini, E.; Castiglioni, C.; Chaigne, D.; Colomer, J.; Cuadrado, M.L.; et al. The clinical, histologic, and genotypic spectrum of sepn1-related myopathy: A case series. Neurology 2020, 95, e1512–e1527. [Google Scholar] [CrossRef]
- Silwal, A.; Sarkozy, A.; Scoto, M.; Ridout, D.; Schmidt, A.; Laverty, A.; Henriques, M.; D’Argenzio, L.; Main, M.; Mein, R.; et al. Selenoprotein n-related myopathy: A retrospective natural history study to guide clinical trials. Ann. Clin. Transl. Neurol. 2020, 7, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Hankiewicz, K.; Carlier, R.Y.; Lazaro, L.; Linzoain, J.; Barnerias, C.; Gomez-Andres, D.; Avila-Smirnow, D.; Ferreiro, A.; Estournet, B.; Guicheney, P.; et al. Whole-body muscle magnetic resonance imaging in sepn1-related myopathy shows a homogeneous and recognizable pattern. Muscle Nerve 2015, 52, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.F.; Kidson, W.; Quijano-Roy, S.; Estournet, B.; Ferreiro, A.; Guicheney, P.; Manson, J.I.; Kornberg, A.J.; Shield, L.K.; North, K.N.; et al. Sepn1: Associated with congenital fiber-type disproportion and insulin resistance. Ann. Neurol. 2006, 59, 546–552. [Google Scholar] [CrossRef]
- Burdakov, D.; Petersen, O.H.; Verkhratsky, A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium 2005, 38, 303–310. [Google Scholar] [CrossRef]
- Csordas, G.; Weaver, D.; Hajnoczky, G. Endoplasmic reticulum-mitochondrial contactology: Structure and signaling functions. Trends Cell Biol. 2018, 28, 523–540. [Google Scholar] [CrossRef]
- Csordas, G.; Renken, C.; Varnai, P.; Walter, L.; Weaver, D.; Buttle, K.F.; Balla, T.; Mannella, C.A.; Hajnoczky, G. Structural and functional features and significance of the physical linkage between er and mitochondria. J. Cell Biol. 2006, 174, 915–921. [Google Scholar] [CrossRef]
- Rowland, A.A.; Voeltz, G.K. Endoplasmic reticulum-mitochondria contacts: Function of the junction. Nat. Rev. Mol. Cell Biol. 2012, 13, 607–625. [Google Scholar] [CrossRef]
- Szabadkai, G.; Bianchi, K.; Varnai, P.; De Stefani, D.; Wieckowski, M.R.; Cavagna, D.; Nagy, A.I.; Balla, T.; Rizzuto, R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial ca2+ channels. J. Cell Biol. 2006, 175, 901–911. [Google Scholar] [CrossRef]
- Eisner, V.; Csordas, G.; Hajnoczky, G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle-pivotal roles in ca(2)(+) and reactive oxygen species signaling. J. Cell Biol. 2013, 126, 2965–2978. [Google Scholar]
- Franzini-Armstrong, C. Er-mitochondria communication. How privileged? Physiology 2007, 22, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Missiroli, S.; Patergnani, S.; Duszynski, J.; Wieckowski, M.R.; Pinton, P. Mitochondria-associated membranes: Composition, molecular mechanisms, and physiopathological implications. Antioxid. Redox Signal. 2015, 22, 995–1019. [Google Scholar] [CrossRef]
- Li, Y.; Camacho, P. Ca2+-dependent redox modulation of serca 2b by erp57. J. Cell Biol. 2004, 164, 35–46. [Google Scholar] [CrossRef]
- Anelli, T.; Bergamelli, L.; Margittai, E.; Rimessi, A.; Fagioli, C.; Malgaroli, A.; Pinton, P.; Ripamonti, M.; Rizzuto, R.; Sitia, R. Ero1alpha regulates ca(2+) fluxes at the endoplasmic reticulum-mitochondria interface (mam). Antioxid. Redox Signal. 2012, 16, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Higo, T.; Hattori, M.; Nakamura, T.; Natsume, T.; Michikawa, T.; Mikoshiba, K. Subtype-specific and er lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by erp44. Cell 2005, 120, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Chernorudskiy, A.L.; Zito, E. Regulation of calcium homeostasis by er redox: A close-up of the er/mitochondria connection. J. Mol. Biol. 2017, 429, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Nakashima, R.; Yuan, Q.; Marks, A.R. Intracellular calcium release channels: An update. J. Physiol. 2017, 595, 3041–3051. [Google Scholar] [CrossRef]
- Carreras-Sureda, A.; Pihan, P.; Hetz, C. Calcium signaling at the endoplasmic reticulum: Fine-tuning stress responses. Cell Calcium 2018, 70, 24–31. [Google Scholar] [CrossRef]
- Petit, N.; Lescure, A.; Rederstorff, M.; Krol, A.; Moghadaszadeh, B.; Wewer, U.M.; Guicheney, P. Selenoprotein n: An endoplasmic reticulum glycoprotein with an early developmental expression pattern. Hum. Mol. Genet. 2003, 12, 1045–1053. [Google Scholar] [CrossRef]
- Castets, P.; Maugenre, S.; Gartioux, C.; Rederstorff, M.; Krol, A.; Lescure, A.; Tajbakhsh, S.; Allamand, V.; Guicheney, P. Selenoprotein n is dynamically expressed during mouse development and detected early in muscle precursors. BMC Dev. Biol. 2009, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Stoilova, T.; Giorgi, C.; Bachi, A.; Cattaneo, A.; Auricchio, A.; Pinton, P.; Zito, E. Sepn1, an endoplasmic reticulum-localized selenoprotein linked to skeletal muscle pathology, counteracts hyper-oxidation by means of redox-regulating serca2 pump activity. Hum. Mol. Genet. 2015, 24, 1843–1855. [Google Scholar] [CrossRef]
- Arbogast, S.; Beuvin, M.; Fraysse, B.; Zhou, H.; Muntoni, F.; Ferreiro, A. Oxidative stress in sepn1-related myopathy: From pathophysiology to treatment. Ann. Neurol. 2009, 65, 677–686. [Google Scholar] [CrossRef]
- Ushioda, R.; Miyamoto, A.; Inoue, M.; Watanabe, S.; Okumura, M.; Maegawa, K.I.; Uegaki, K.; Fujii, S.; Fukuda, Y.; Umitsu, M.; et al. Redox-assisted regulation of ca2+ homeostasis in the endoplasmic reticulum by disulfide reductase erdj5. Proc. Natl. Acad. Sci. USA 2016, 113, E6055–E6063. [Google Scholar] [CrossRef] [PubMed]
- Zito, E. Ero1: A protein disulfide oxidase and h2o2 producer. Free Radic. Biol. Med. 2015, 83, 299–304. [Google Scholar] [CrossRef]
- Moghadaszadeh, B.; Rider, B.E.; Lawlor, M.W.; Childers, M.K.; Grange, R.W.; Gupta, K.; Boukedes, S.S.; Owen, C.A.; Beggs, A.H. Selenoprotein n deficiency in mice is associated with abnormal lung development. FASEB J. 2013, 27, 1585–1599. [Google Scholar] [CrossRef]
- Pozzer, D.; Favellato, M.; Bolis, M.; Invernizzi, R.W.; Solagna, F.; Blaauw, B.; Zito, E. Endoplasmic reticulum oxidative stress triggers tgf-beta-dependent muscle dysfunction by accelerating ascorbic acid turnover. Sci. Rep. 2017, 7, 40993. [Google Scholar] [CrossRef] [PubMed]
- Rederstorff, M.; Castets, P.; Arbogast, S.; Laine, J.; Vassilopoulos, S.; Beuvin, M.; Dubourg, O.; Vignaud, A.; Ferry, A.; Krol, A.; et al. Increased muscle stress-sensitivity induced by selenoprotein n inactivation in mouse: A mammalian model for sepn1-related myopathy. PLoS ONE 2011, 6, e23094. [Google Scholar] [CrossRef]
- Jurynec, M.J.; Xia, R.; Mackrill, J.J.; Gunther, D.; Crawford, T.; Flanigan, K.M.; Abramson, J.J.; Howard, M.T.; Grunwald, D.J. Selenoprotein n is required for ryanodine receptor calcium release channel activity in human and zebrafish muscle. Proc. Natl. Acad. Sci. USA 2008, 105, 12485–12490. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewski, B. Regulation of oxidative phosphorylation in different muscles and various experimental conditions. Biochem. J. 2003, 375, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Willis, W.T.; Chess, D.J.; Balaban, R.S. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry 2013, 52, 2793–2809. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Alvear, D.; Zhou, Y.; Blais, A.; Tsikitis, M.; Lents, N.H.; Arias, C.; Lennon, C.J.; Kluger, Y.; Dynlacht, B.D. Xbp1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell 2007, 27, 53–66. [Google Scholar] [CrossRef]
- Wu, J.; Ruas, J.L.; Estall, J.L.; Rasbach, K.A.; Choi, J.H.; Ye, L.; Bostrom, P.; Tyra, H.M.; Crawford, R.W.; Campbell, K.P.; et al. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a pgc-1alpha/atf6alpha complex. Cell Metab. 2011, 13, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. Chop induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004, 18, 3066–3077. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.; et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Caggiano, S.; Khirani, S.; Dabaj, I.; Cavassa, E.; Amaddeo, A.; Arroyo, J.O.; Desguerre, I.; Richard, P.; Cutrera, R.; Ferreiro, A.; et al. Diaphragmatic dysfunction in sepn1-related myopathy. Neuromuscul. Disord. 2017, 27, 747–755. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. 2), S157–S163. [Google Scholar] [CrossRef]
- Volmer, R.; Ron, D. Lipid-dependent regulation of the unfolded protein response. Curr. Opin. Cell Biol. 2015, 33, 67–73. [Google Scholar] [CrossRef]
- Tubbs, E.; Chanon, S.; Robert, M.; Bendridi, N.; Bidaux, G.; Chauvin, M.A.; Ji-Cao, J.; Durand, C.; Gauvrit-Ramette, D.; Vidal, H.; et al. Disruption of mitochondria-associated endoplasmic reticulum membrane (mam) integrity contributes to muscle insulin resistance in mice and humans. Diabetes 2018, 67, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Ariyama, Y.; Shimizu, H.; Satoh, T.; Tsuchiya, T.; Okada, S.; Oyadomari, S.; Mori, M.; Mori, M. Chop-deficient mice showed increased adiposity but no glucose intolerance. Obesity 2007, 15, 1647–1656. [Google Scholar] [CrossRef]
- Lee, C.S.; Hanna, A.D.; Wang, H.; Dagnino-Acosta, A.; Joshi, A.D.; Knoblauch, M.; Xia, Y.; Georgiou, D.K.; Xu, J.; Long, C.; et al. A chemical chaperone improves muscle function in mice with a RYR1 mutation. Nat. Commun. 2017, 8, 14659. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.J.; Arbogast, S.; Hur, J.; Nelson, D.D.; McEvoy, A.; Waugh, T.; Marty, I.; Lunardi, J.; Brooks, S.V.; Kuwada, J.Y.; et al. Oxidative stress and successful antioxidant treatment in models of RYR1-related myopathy. Brain 2012, 135, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.J.; Lawal, T.A.; Witherspoon, J.W.; Chrismer, I.C.; Razaqyar, M.S.; Punjabi, M.; Elliott, J.S.; Tounkara, F.; Kuo, A.; Shelton, M.O.; et al. Randomized controlled trial of n-acetylcysteine therapy for RYR1-related myopathies. Neurology 2020, 94, e1434–e1444. [Google Scholar] [CrossRef] [PubMed]
- Zito, E. Targeting er stress/er stress response in myopathies. Redox. Biol. 2019, 26, 101232. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zito, E.; Ferreiro, A. Calcium and Redox Liaison: A Key Role of Selenoprotein N in Skeletal Muscle. Cells 2021, 10, 1116. https://doi.org/10.3390/cells10051116
Zito E, Ferreiro A. Calcium and Redox Liaison: A Key Role of Selenoprotein N in Skeletal Muscle. Cells. 2021; 10(5):1116. https://doi.org/10.3390/cells10051116
Chicago/Turabian StyleZito, Ester, and Ana Ferreiro. 2021. "Calcium and Redox Liaison: A Key Role of Selenoprotein N in Skeletal Muscle" Cells 10, no. 5: 1116. https://doi.org/10.3390/cells10051116
APA StyleZito, E., & Ferreiro, A. (2021). Calcium and Redox Liaison: A Key Role of Selenoprotein N in Skeletal Muscle. Cells, 10(5), 1116. https://doi.org/10.3390/cells10051116





