PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Gene Transfection
2.2. In Vitro Coculture System
2.3. Animals and MSC Transplantation
2.4. Histopathological and Immunofluorescence Analysis
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Western Blotting
2.7. XF Mito Stress Assay
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
3. Results
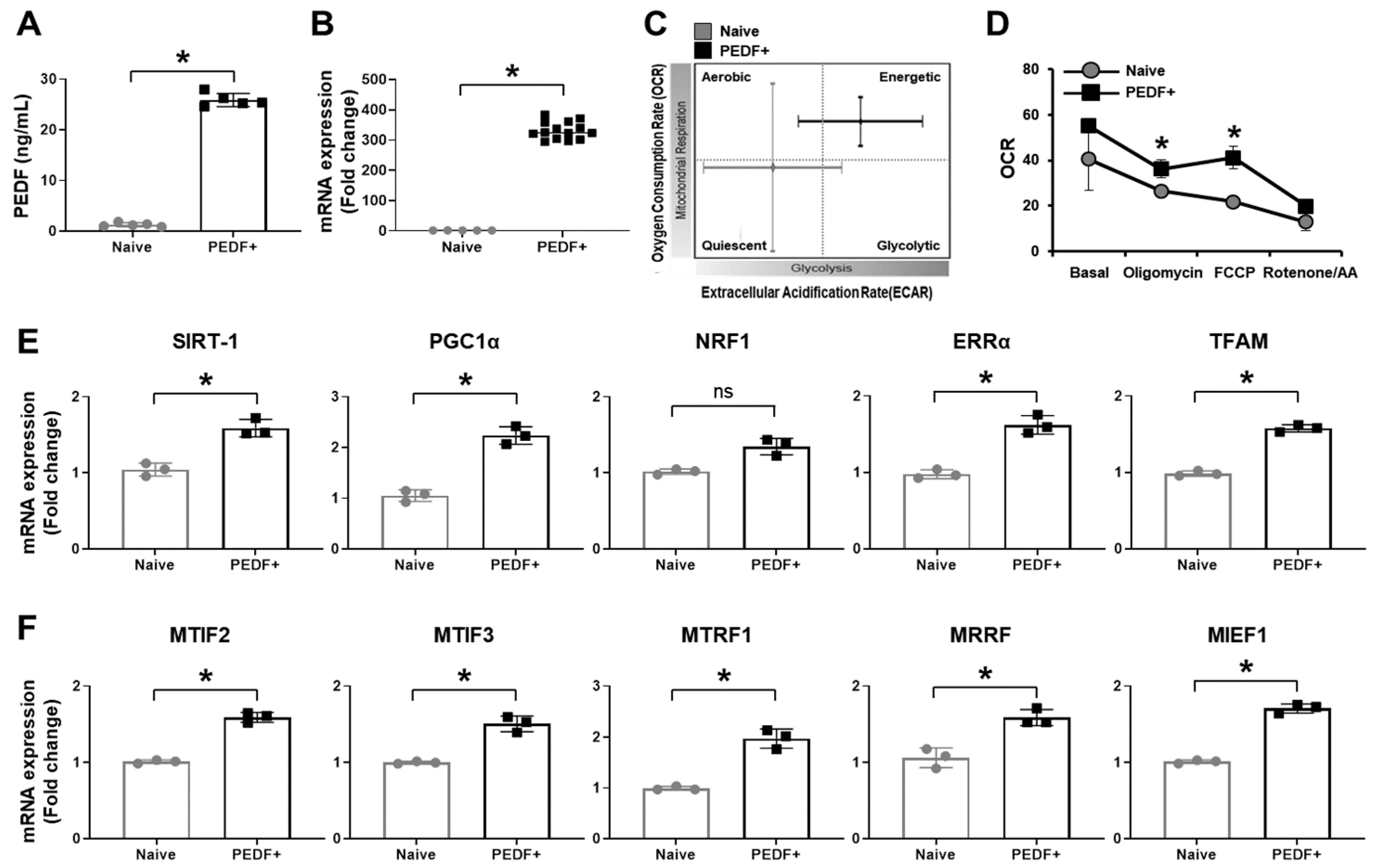
3.1. PD-MSCsPEDF Enhance Mitochondrial Activity
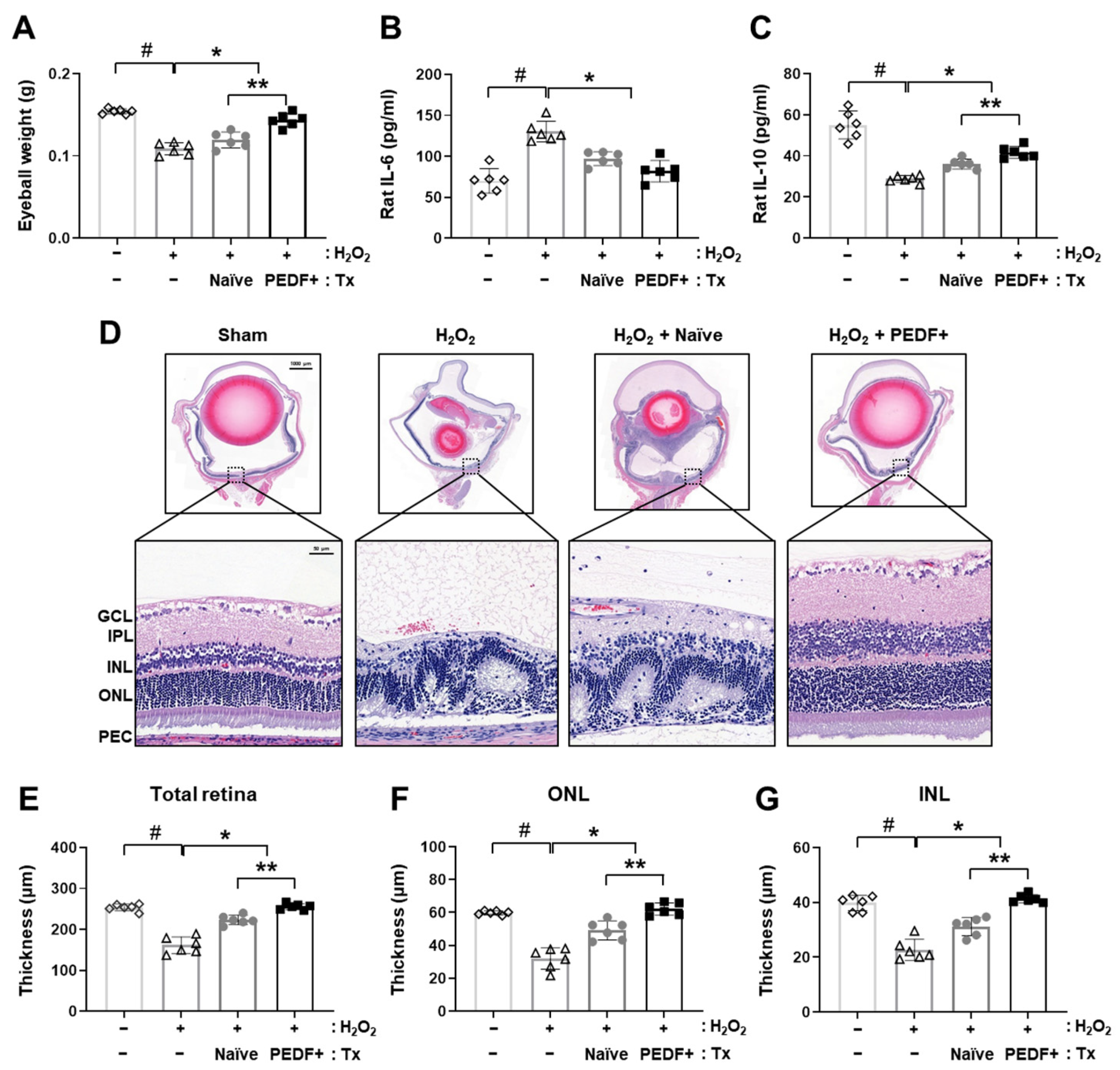
3.2. PD-MSCPEDF Transplantation Restores Retinal Function in a H2O2-Injured Rat Model
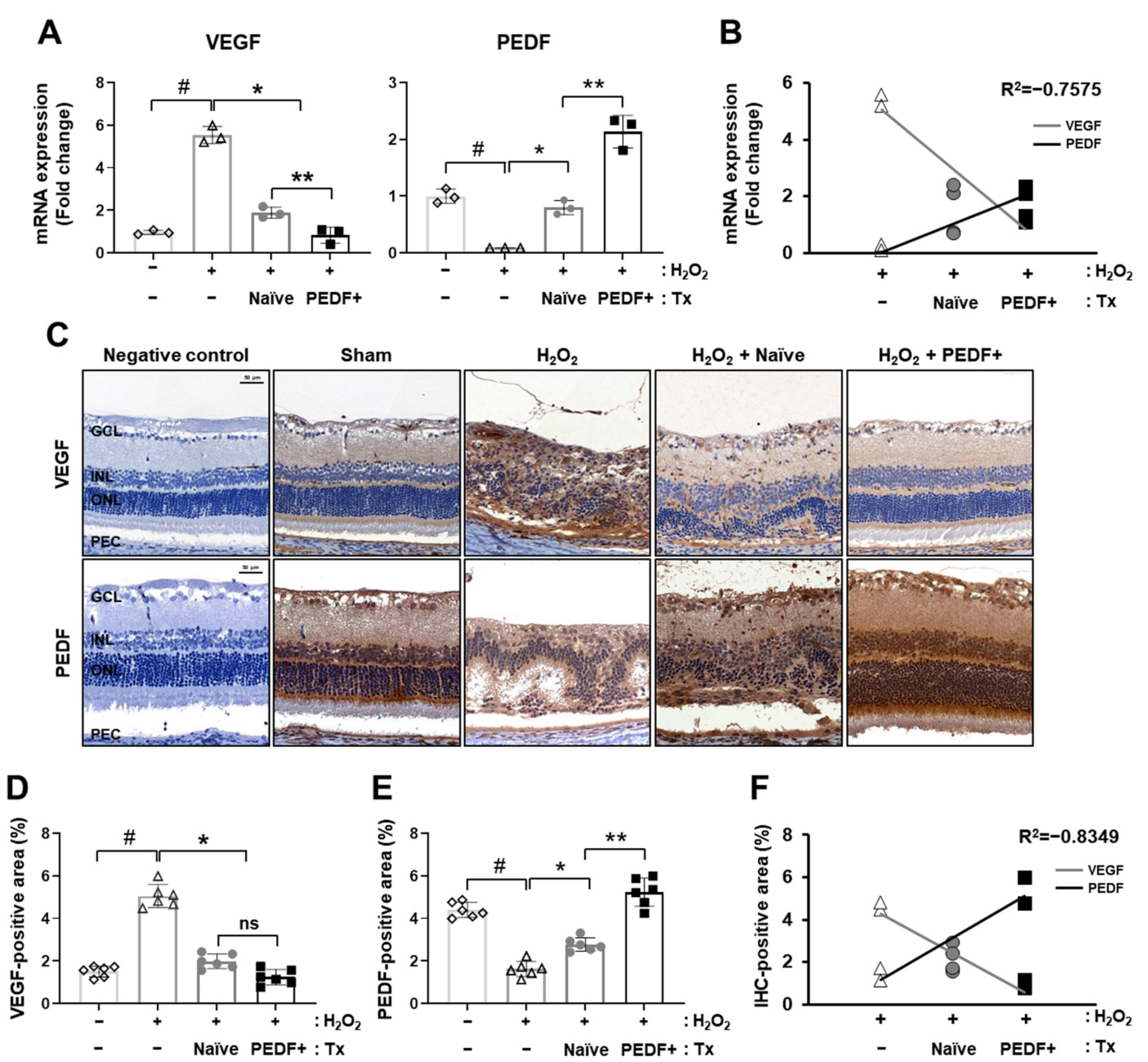
3.3. PD-MSCsPEDF Balanced VEGF and PEDF Levels in a H2O2-Induced Rat Model
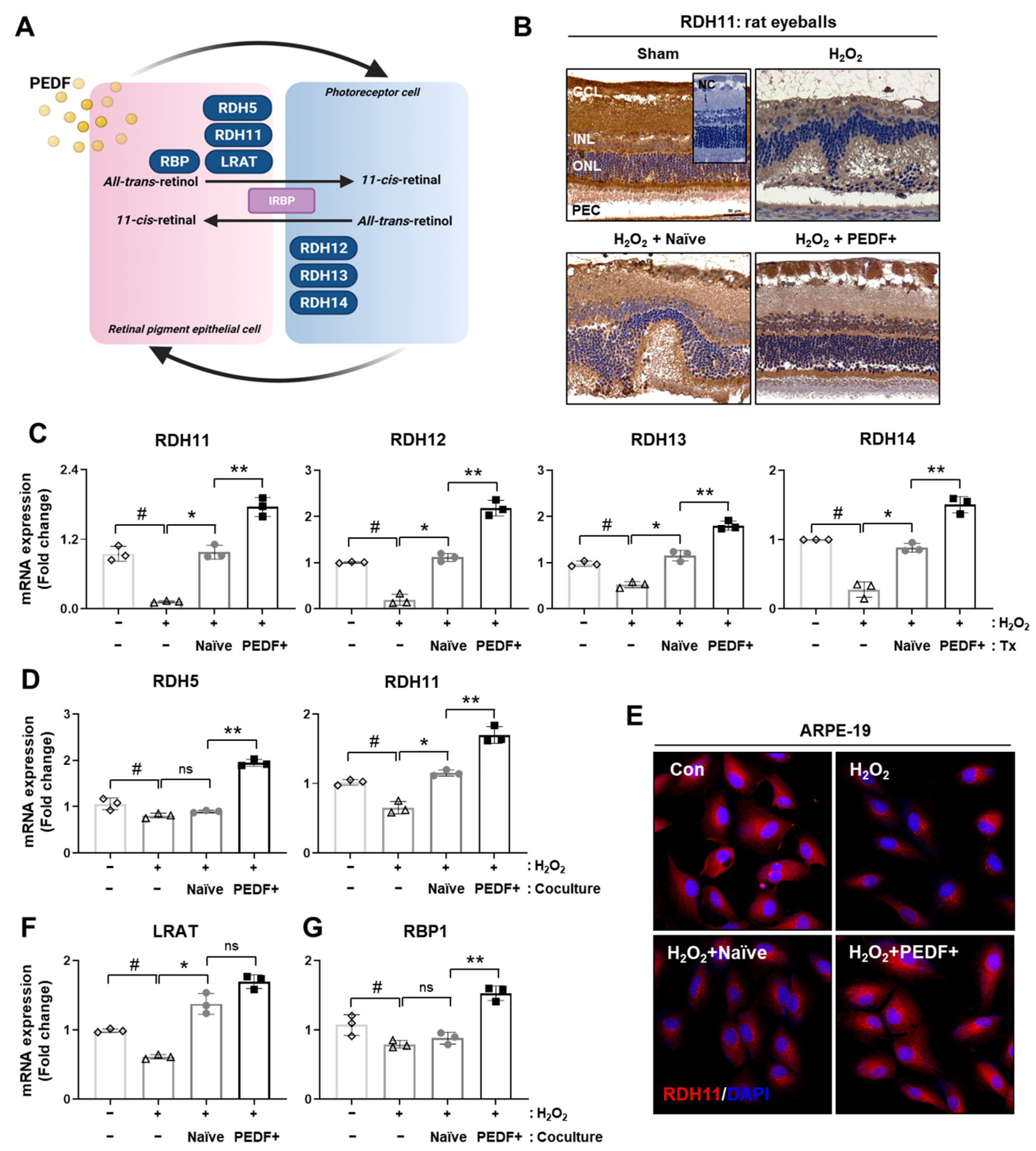
3.4. PD-MSCsPEDF Improved Visual Cycles in Rat Retinal Layers and RPE Cells
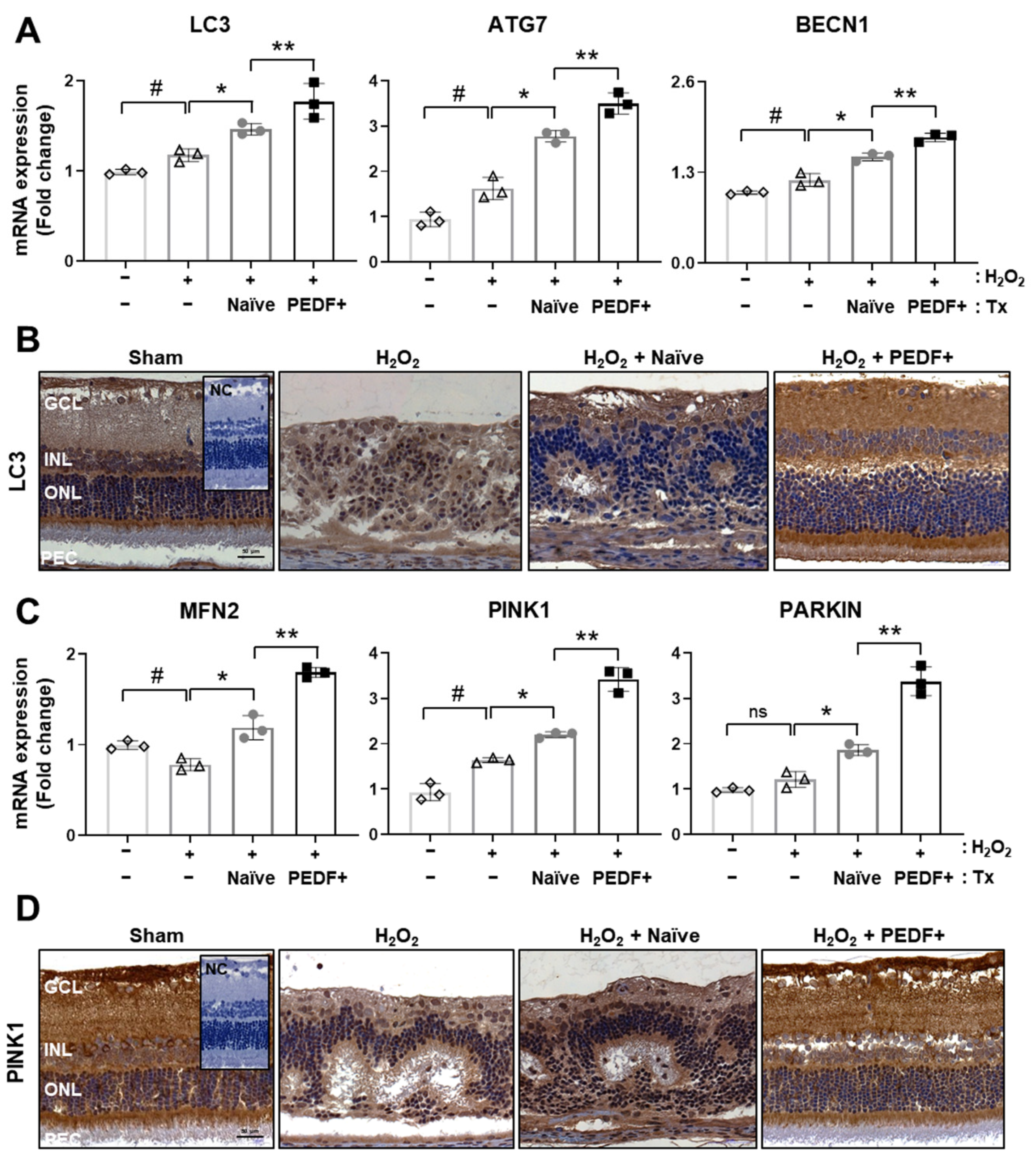
3.5. The Administration of PD-MSCsPEDF Induces Mitophagy in H2O2-Injured Rat Retinas
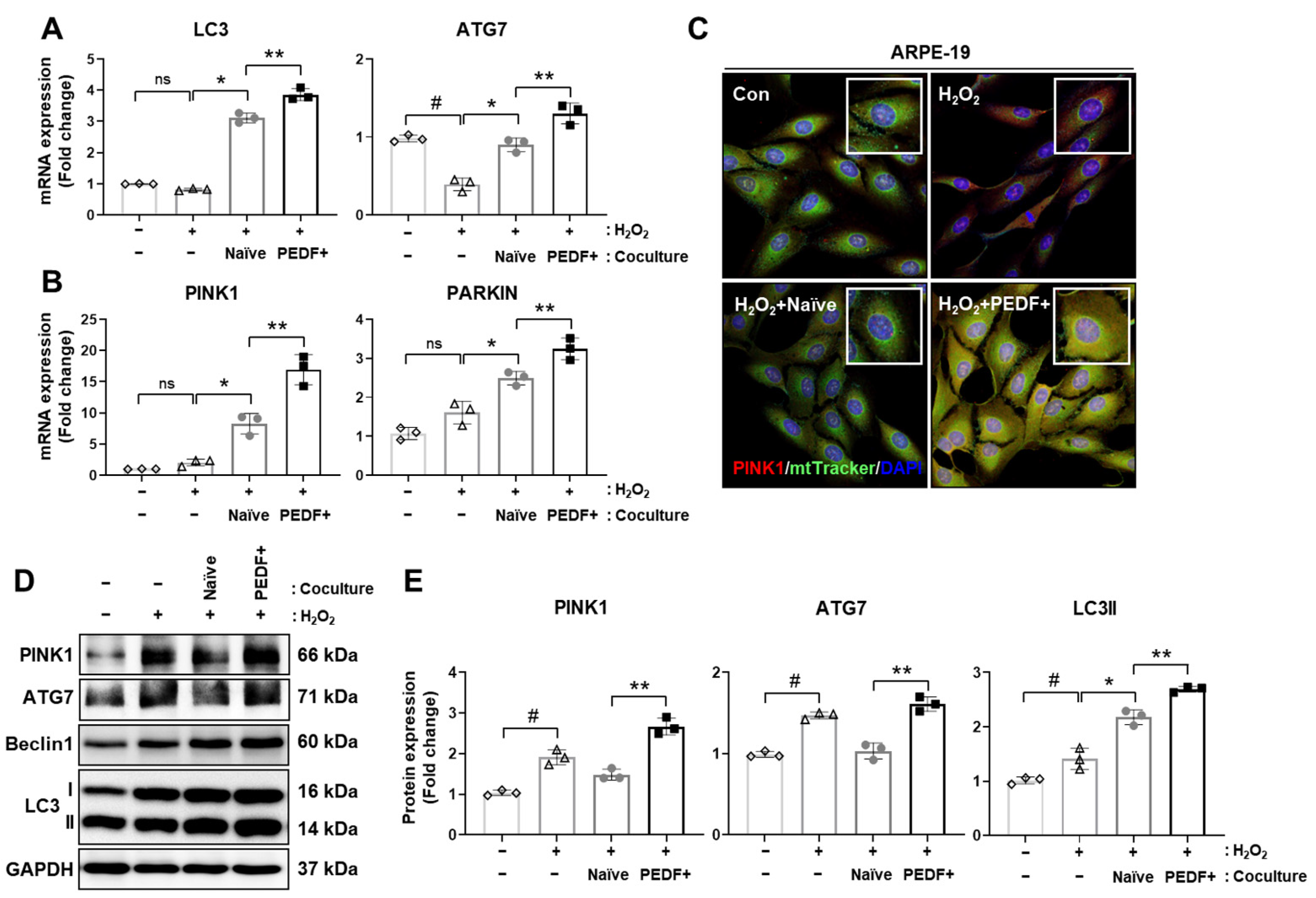
3.6. Coculturing RPE Cells with PD-MSCsPEDF Enhances Mitophagy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, S.L.S.; Kumar, S.; Mok, P.L. Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases. Int. J. Mol. Sci. 2017, 18, 1406. [Google Scholar] [CrossRef]
- Barot, M.; Gokulgandhi, M.R.; Mitra, A.K. Mitochondrial Dysfunction in Retinal Diseases. Curr. Eye Res. 2011, 36, 1069–1077. [Google Scholar] [CrossRef]
- Felszeghy, S.; Viiri, J.; Paterno, J.J.; Hyttinen, J.M.T.; Koskela, A.; Chen, M.; Leinonen, H.; Tanila, H.; Kivinen, N.; Koistinen, A.; et al. Loss of Nrf-2 and Pgc-1alpha Genes Leads to Retinal Pigment Epithelium Damage Resembling Dry Age-Related Macular Degeneration. Redox Biol. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-Related Macular Degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Farnoodian, M.; Sorenson, C.M.; Sheibani, N. Negative Regulators of Angiogenesis, Ocular Vascular Homeostasis, and Pathogenesis and Treatment of Exudative Amd. J. Ophthalmic Vis. Res. 2018, 13, 470–486. [Google Scholar] [PubMed]
- Li, R.; Du, J.H.; Yao, G.M.; Yao, Y.; Zhang, J. Autophagy: A New Mechanism for Regulating Vegf and Pedf Expression in Retinal Pigment Epithelium Cells. Int. J. Ophthalmol. 2019, 12, 57–62. [Google Scholar]
- Tombran-Tink, J.; Shivaram, S.M.; Chader, G.J.; Johnson, L.V.; Bok, D. Expression, Secretion, and Age-Related Downregulation of Pigment Epithelium-Derived Factor, a Serpin with Neurotrophic Activity. J. Neurosci. 1995, 15 Pt 1, 4992–5003. [Google Scholar] [CrossRef]
- He, X.; Cheng, R.; Benyajati, S.; Ma, J.X. Pedf and Its Roles in Physiological and Pathological Conditions: Implication in Diabetic and Hypoxia-Induced Angiogenic Diseases. Clin. Sci. (Lond.) 2015, 128, 805–823. [Google Scholar] [CrossRef]
- Spranger, J.; Osterhoff, M.; Reimann, M.; Mohlig, M.; Ristow, M.; Francis, M.K.; Cristofalo, V.; Hammes, H.P.; Smith, G.; Boulton, M.; et al. Loss of the Antiangiogenic Pigment Epithelium-Derived Factor in Patients with Angiogenic Eye Disease. Diabetes 2001, 50, 2641–2645. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yamagishi, S.; Matsui, T.; Nakamura, K.; Imaizumi, T.; Yoshimura, K.; Yamakawa, R. Positive Correlation of Pigment Epithelium-Derived Factor and Total Antioxidant Capacity in Aqueous Humour of Patients with Uveitis and Proliferative Diabetic Retinopathy. Br. J. Ophthalmol. 2007, 91, 1133–1134. [Google Scholar] [CrossRef]
- He, Y.; Leung, K.W.; Ren, Y.; Pei, J.; Ge, J.; Tombran-Tink, J. Pedf Improves Mitochondrial Function in Rpe Cells During Oxidative Stress. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6742–6755. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Maeda, A. Rpe Visual Cycle and Biochemical Phenotypes of Mutant Mouse Models. Methods Mol. Biol. 2018, 1753, 89–102. [Google Scholar] [PubMed]
- Kiser, P.D.; Golczak, M.; Maeda, A.; Palczewski, K. Key Enzymes of the Retinoid (Visual) Cycle in Vertebrate Retina. Biochim. Biophys. Acta 2012, 1821, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.F.; Chakarova, C.F.; El-Aziz, M.M.A.; Bhattacharya, S.S. Photoreceptor Degeneration: Genetic and Mechanistic Dissection of a Complex Trait. Nat. Rev. Genet. 2010, 11, 273–284. [Google Scholar] [CrossRef]
- Hernandez-Pinto, A.; Polato, F.; Subramanian, P.; Rocha-Munoz, A.; Vitale, S.; de la Rosa, E.J.; Becerra, S.P. Pedf Peptides Promote Photoreceptor Survival in Rd10 Retina Models. Exp. Eye Res. 2019, 184, 24–29. [Google Scholar] [CrossRef]
- Valiente-Soriano, F.J.; Di Pierdomenico, J.; Garcia-Ayuso, D.; Ortin-Martinez, A.; de Imperial-Ollero, J.A.M.; Gallego-Ortega, A.; Jimenez-Lopez, M.; Villegas-Perez, M.P.; Becerra, S.P.; Vidal-Sanz, M. Pigment Epithelium-Derived Factor (Pedf) Fragments Prevent Mouse Cone Photoreceptor Cell Loss Induced by Focal Phototoxicity In Vivo. Int. J. Mol. Sci. 2020, 21, 7242. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and Function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef]
- Ding, W.X.; Yin, X.M. Mitophagy: Mechanisms, Pathophysiological Roles, and Analysis. Biol. Chem. 2012, 393, 547–564. [Google Scholar] [CrossRef]
- Sridevi Gurubaran, I.; Viiri, J.; Koskela, A.; Hyttinen, J.M.T.; Paterno, J.J.; Kis, G.; Antal, M.; Urtti, A.; Kauppinen, A.; Felszeghy, S.; et al. Mitophagy in the Retinal Pigment Epithelium of Dry Age-Related Macular Degeneration Investigated in the Nfe2l2/Pgc-1alpha(-/-) Mouse Model. Int. J. Mol. Sci. 2020, 21, 1976. [Google Scholar] [CrossRef]
- Zhang, Y.; Xi, X.; Mei, Y.; Zhao, X.; Zhou, L.; Ma, M.; Liu, S.; Zha, X.; Yang, Y. High-Glucose Induces Retinal Pigment Epithelium Mitochondrial Pathways of Apoptosis and Inhibits Mitophagy by Regulating Ros/Pink1/Parkin Signal Pathway. Biomed. Pharmacother. 2019, 111, 1315–1325. [Google Scholar] [CrossRef]
- Huang, B.; Liang, J.J.; Zhuang, X.; Chen, S.W.; Ng, T.K.; Chen, H. Intravitreal Injection of Hydrogen Peroxide Induces Acute Retinal Degeneration, Apoptosis, and Oxidative Stress in Mice. Oxid. Med. Cell Longev. 2018, 2018, 5489476. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.; Park, S.H.; Lee, D.; Kim, G.H.; Noh, J.E.; Lee, K.J.; Kim, G.J. Overexpression of Pigment Epithelium-Derived Factor in Placenta-Derived Mesenchymal Stem Cells Promotes Mitochondrial Biogenesis in Retinal Cells. Lab. Investig. 2021, 101, 51–69. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, J.H.; Kim, S.H.; Park, H.; Lee, D.; Kim, G.J. Efficacy of Gene Modification in Placenta-Derived Mesenchymal Stem Cells Based on Nonviral Electroporation. Int. J. Stem Cells 2021, 14, 12–18. [Google Scholar] [CrossRef]
- Brand, M.D.; Esteves, T.C. Physiological Functions of the Mitochondrial Uncoupling Proteins Ucp2 and Ucp3. Cell Metab. 2005, 2, 85–93. [Google Scholar] [CrossRef]
- Yokokawa, T.; Kido, K.; Suga, T.; Isaka, T.; Hayashi, T.; Fujita, S. Exercise-Induced Mitochondrial Biogenesis Coincides with the Expression of Mitochondrial Translation Factors in Murine Skeletal Muscle. Physiol. Rep. 2018, 6, e13893. [Google Scholar] [CrossRef]
- Bhutto, I.A.; McLeod, D.S.; Hasegawa, T.; Kim, S.Y.; Merges, C.; Tong, P.; Lutty, G.A. Pigment Epithelium-Derived Factor (Pedf) and Vascular Endothelial Growth Factor (Vegf) in Aged Human Choroid and Eyes with Age-Related Macular Degeneration. Exp. Eye Res. 2006, 82, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.; Ahn, J.; Sohn, J.; Hwang, D.D. Evaluation of the Safety of Bilateral Same-Day Intravitreal Injections of Anti-Vascular Endothelial Growth Factor Agents: Experience of a Large Korean Retina Center. Clin. Ophthalmol. 2020, 14, 3211–3218. [Google Scholar] [CrossRef]
- Han, F.; Xu, G. Stem Cell Transplantation Therapy for Retinal Degenerative Diseases. Adv. Exp. Med. Biol. 2020, 1266, 127–139. [Google Scholar] [PubMed]
- Feng, X.; Chen, P.; Zhao, X.; Wang, J.; Wang, H. Transplanted Embryonic Retinal Stem Cells Have the Potential to Repair the Injured Retina in Mice. BMC Ophthalmol. 2021, 21, 26. [Google Scholar] [CrossRef]
- Jeon, S.; Oh, I.H. Regeneration of the Retina: Toward Stem Cell Therapy for Degenerative Retinal Diseases. BMB Rep. 2015, 48, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.K.; Tosi, J.; Kasanuki, J.M.; Chou, C.L.; Kong, J.; Parmalee, N.; Wert, K.J.; Allikmets, R.; Lai, C.C.; Chien, C.L.; et al. Transplantation of Reprogrammed Embryonic Stem Cells Improves Visual Function in a Mouse Model for Retinitis Pigmentosa. Transplantation 2010, 89, 911–919. [Google Scholar] [CrossRef]
- Adak, S.; Magdalene, D.; Deshmukh, S.; Das, D.; Jaganathan, B.G. A Review on Mesenchymal Stem Cells for Treatment of Retinal Diseases. Stem Cell Rev. Rep. 2021, 1–20. [Google Scholar] [CrossRef]
- Kahraman, N.S.; Oner, A. Umbilical Cord Derived Mesenchymal Stem Cell Implantation in Retinitis Pigmentosa: A 6-Month Follow-up Results of a Phase 3 Trial. Int. J. Ophthalmol. 2020, 13, 1423–1429. [Google Scholar] [CrossRef]
- Zhou, Z.; Doggett, T.A.; Sene, A.; Apte, R.S.; Ferguson, T.A. Autophagy Supports Survival and Phototransduction Protein Levels in Rod Photoreceptors. Cell Death Differ. 2015, 22, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, T.; Golczak, M.; Palczewski, K. Retinopathy in Mice Induced by Disrupted All-Trans-Retinal Clearance. J. Biol. Chem. 2008, 283, 26684–26693. [Google Scholar] [CrossRef]
- Kang, M.K.; Lee, E.J.; Kim, Y.H.; Kim, D.Y.; Oh, H.; Kim, S.I.; Kang, Y.H. Chrysin Ameliorates Malfunction of Retinoid Visual Cycle through Blocking Activation of Age-Rage-Er Stress in Glucose-Stimulated Retinal Pigment Epithelial Cells and Diabetic Eyes. Nutrients 2018, 10, 1046. [Google Scholar] [CrossRef] [PubMed]
- Ferrington, D.A.; Ebeling, M.C.; Kapphahn, R.J.; Terluk, M.R.; Fisher, C.R.; Polanco, J.R.; Roehrich, H.; Leary, M.M.; Geng, Z.; Dutton, J.R.; et al. Altered Bioenergetics and Enhanced Resistance to Oxidative Stress in Human Retinal Pigment Epithelial Cells from Donors with Age-Related Macular Degeneration. Redox Biol. 2017, 13, 255–265. [Google Scholar] [CrossRef]
- Becerra, S.P.; Fariss, R.N.; Wu, Y.Q.; Montuenga, L.M.; Wong, P.; Pfeffer, B.A. Pigment Epithelium-Derived Factor in the Monkey Retinal Pigment Epithelium and Interphotoreceptor Matrix: Apical Secretion and Distribution. Exp. Eye Res. 2004, 78, 223–234. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of Mitochondrial Dysfunction and Their Impact on Age-Related Macular Degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Zhang, Y.; Zhao, Q.; Wang, X.; Lu, P.; Zhang, H.; Wang, Z.; Dong, H.; Zhang, Z. Pedf Protects Cardiomyocytes by Promoting Fundc1mediated Mitophagy Via Pedf-R under Hypoxic Condition. Int. J. Mol. Med. 2018, 41, 3394–3404. [Google Scholar]
- Miao, H.; Qiu, F.; Huang, B.; Liu, X.; Zhang, H.; Liu, Z.; Yuan, Y.; Zhao, Q.; Zhang, H.; Dong, H.; et al. Pkcalpha Replaces Ampk to Regulate Mitophagy: Another Pedf Role on Ischaemic Cardioprotection. J. Cell Mol. Med. 2018, 22, 5732–5742. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sawada, O.; Kohno, H.; Le, Y.Z.; Subauste, C.; Maeda, T.; Maeda, A. Autophagy Protects the Retina from Light-Induced Degeneration. J. Biol. Chem. 2013, 288, 7506–7518. [Google Scholar] [CrossRef]
- Justilien, V.; Pang, J.J.; Renganathan, K.; Zhan, X.; Crabb, J.W.; Kim, S.R.; Sparrow, J.R.; Hauswirth, W.W.; Lewin, A.S. Sod2 Knockdown Mouse Model of Early Amd. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4407–4420. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.E.; DeWeerd, A.J.; Ildefonso, C.J.; Lewin, A.S.; Ash, J.D. Mitochondrial Oxidative Stress in the Retinal Pigment Epithelium (Rpe) Led to Metabolic Dysfunction in Both the Rpe and Retinal Photoreceptors. Redox Biol. 2019, 24, 101201. [Google Scholar] [CrossRef]
- Chalam, K.V.; Khetpal, V.; Rusovici, R.; Balaiya, S. A Review: Role of Ultraviolet Radiation in Age-Related Macular Degeneration. Eye Contact Lens 2011, 37, 225–232. [Google Scholar] [CrossRef]
- Zerti, D.; Hilgen, G.; Dorgau, B.; Collin, J.; Ader, M.; Armstrong, L.; Sernagor, E.; Lako, M. Transplanted Pluripotent Stem Cell-Derived Photoreceptor Precursors Elicit Conventional and Unusual Light Responses in Mice with Advanced Retinal Degeneration. Stem Cells 2021. [Google Scholar] [CrossRef]
- Gonzalez Fleitas, M.F.; Devouassoux, J.; Aranda, M.L.; Dieguez, H.H.; Calanni, J.S.; Iaquinandi, A.; Sande, P.H.; Dorfman, D.; Rosenstein, R.E. Melatonin Prevents Non-Image-Forming Visual System Alterations Induced by Experimental Glaucoma in Rats. Mol. Neurobiol. 2021, 1–12. [Google Scholar] [CrossRef]
- Mao, H.; Seo, S.J.; Biswal, M.R.; Li, H.; Conners, M.; Nandyala, A.; Jones, K.; Le, Y.Z.; Lewin, A.S. Mitochondrial Oxidative Stress in the Retinal Pigment Epithelium Leads to Localized Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4613–4627. [Google Scholar] [CrossRef]
- Roberts, J.E. Ultraviolet Radiation as a Risk Factor for Cataract and Macular Degeneration. Eye Contact Lens 2011, 37, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Charng, J.; Nguyen, C.T.; Bui, B.V.; Vingrys, A.J. Age-Related Retinal Function Changes in Albino and Pigmented Rats. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8891–8899. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Park, S.; Park, H.J.; Kim, S.H.; Lew, H.; Kim, G.J. PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model. Cells 2021, 10, 1117. https://doi.org/10.3390/cells10051117
Kim JY, Park S, Park HJ, Kim SH, Lew H, Kim GJ. PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model. Cells. 2021; 10(5):1117. https://doi.org/10.3390/cells10051117
Chicago/Turabian StyleKim, Jae Yeon, Sohae Park, Hee Jung Park, Se Ho Kim, Helen Lew, and Gi Jin Kim. 2021. "PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model" Cells 10, no. 5: 1117. https://doi.org/10.3390/cells10051117
APA StyleKim, J. Y., Park, S., Park, H. J., Kim, S. H., Lew, H., & Kim, G. J. (2021). PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model. Cells, 10(5), 1117. https://doi.org/10.3390/cells10051117






