Fibrotic Events in the Progression of Cholestatic Liver Disease
Abstract
1. Introduction
2. Primary Biliary Cholangitis (PBC)
3. Primary Sclerosing Cholangitis (PSC)
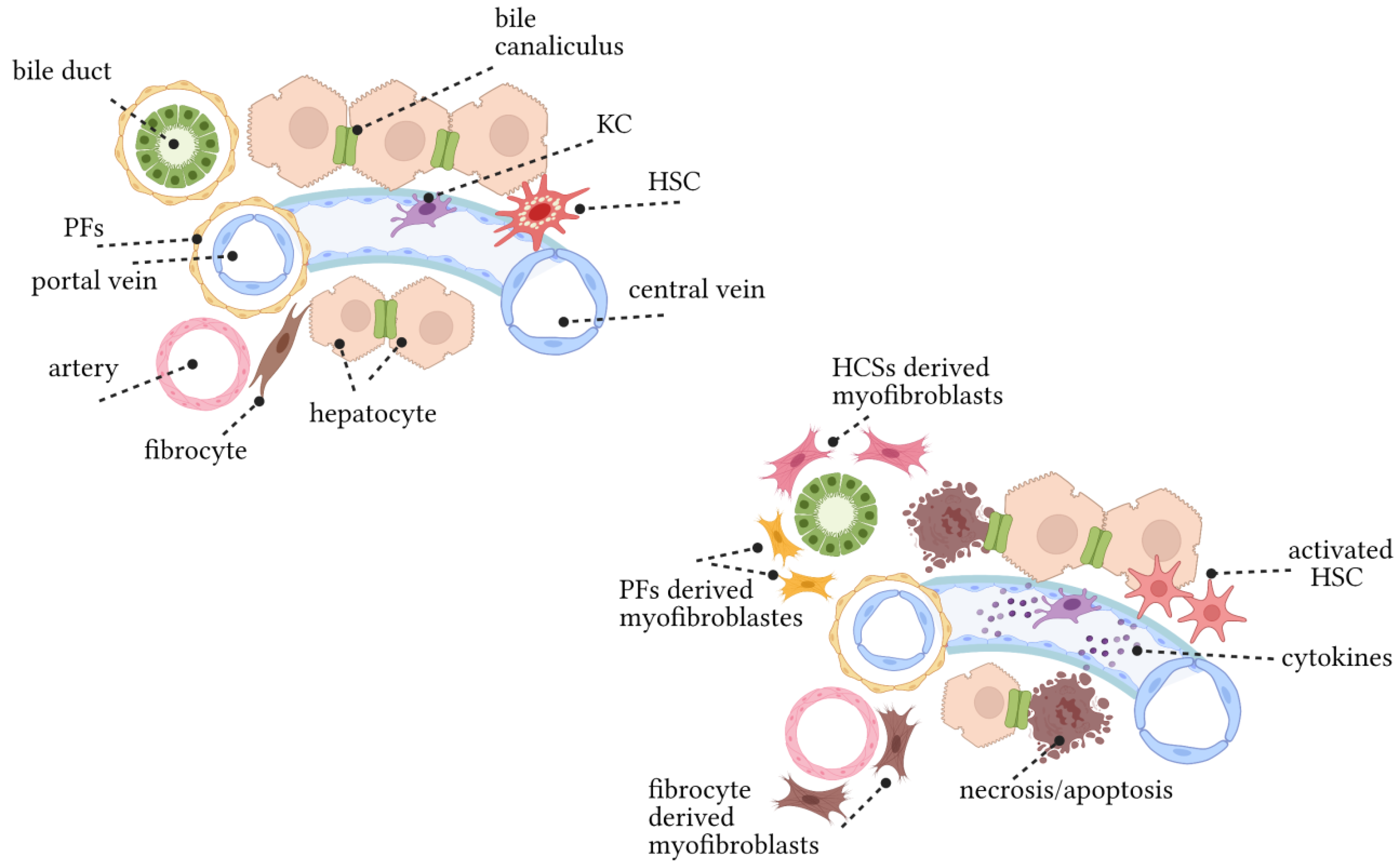
4. Key Hepatic Cells Involved in Disease Progression
4.1. Cholangiocytes and/or Biliary Epithelial Cells (BECs)
4.2. Periductular Fibroblasts and Hepatic Stellate Cells (HSCs)
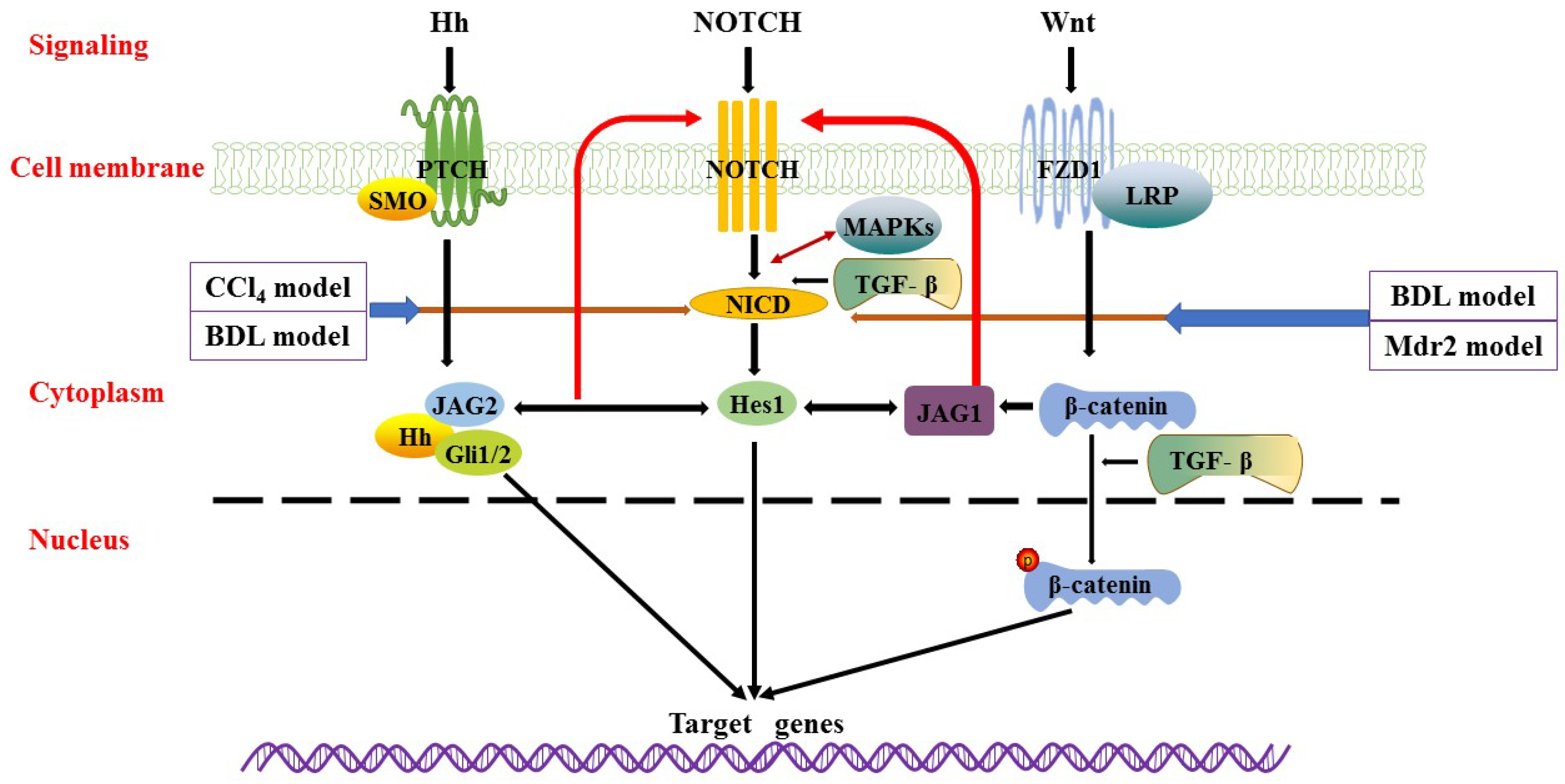
5. Signalling Pathways Involved in Pathogenesis
5.1. Notch Signalling Pathway
5.2. Hedgehog (Hh) Signalling Pathway
5.3. Wnt Signalling Pathway
5.4. Other Related Signalling Pathways
6. Pathophysiology
6.1. Hepatobiliary Acid-Base Balance Mitochondria miRNAs
6.2. Cholesterol Metabolism and Canalicular Transporters
6.3. Immune Targets
7. Mouse Models of Cholestasis Liver Disease
8. Therapeutic Opportunities
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2-OADCs | 2-oxo dehydrogenase complex |
| AE2 | anion exchanger 2 |
| AMAs | antimitochondrial antibodies |
| ATRA | all-trans retinoic acid |
| Bas | bile acids |
| BDL | bile duct ligation |
| BECs | biliary epithelial cells |
| CCA | Cholangiocarcinoma |
| CCl4 | C-C Motif Chemokine Ligand 4 |
| dnTGFβRII | dominant-negative form of TGF-β receptor type II |
| ECM | secrete extracellular matrix |
| EMT | epithelial-to-mesenchymal transition |
| FUT2 | glycocalyx stabilising enzyme fucosyltransferase 2 |
| UDCA | ursodeoxycholic acid |
| FZD1 | Frizzled 1 |
| GWAS | genome-wide association study |
| HCC | hepatocellular carcinoma |
| Hes1 | hairy and enhancer of split-1 |
| Hh | Hedgehog |
| HLA | human leukocyte antigen |
| HNFs | hepatocyte nuclear factors |
| HSCs | hepatic stellate cells |
| IBD | inflammatory bowel disease |
| IFNg | interferon gamma |
| IL-12 | interleukin 12 |
| IL-6 | interleukin 6 |
| JAG2 | jagged canonical notch ligand 2 |
| JNKs | c-Jun N-terminal kinases |
| LRP5/6 | low-density lipoprotein receptor-related protein5/6 |
| MAIT | mucosal-associated invariant T cells |
| MRC | magnetic resonance cholangiography |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NTPD2 | ecto-ATPase nucleoside triphosphate diphosphohydrolase-2 |
| OCA | obeticholic acid |
| PBC | primary biliary cholangitis |
| PC | primary cilium |
| PDC-E2 | pyruvate dehydrogenase complex |
| PFs | portal fibroblasts |
| PLD | polycystic liver disease |
| PSC | primary sclerosing cholangitis |
| p75NTR | p75 neurotrophin receptor |
| SOX4 | SRY-box transcription factor 4 |
| SOX9 | SRY-box transcription factor 9 |
| TGF-β | transforming growth factor beta |
| TGR5 | Takeda G protein-coupled receptor 5 |
| TNF-α | tumour necrosis factor-α |
| UDCA | ursodeoxycholic acid |
| YAP1 | Yes-associated protein 1 |
| αSMA | α-smooth muscle actin |
References
- Erlinger, S. What is cholestasis in 1985? J. Hepatol. 1985, 1, 687–693. [Google Scholar] [CrossRef]
- Fuchs, C.D.; Halilbasic, E.; Trauner, M. Pathophysiologic basis for alternative therapies for cholestasis. Liver Biol. Pathobiol. 2020, 364–377. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Luketic, V.; Chapman, R.; Hirschfield, G.M.; Poupon, R.; Schramm, C.; Vincent, C.; Rust, C.; Pares, A.; Mason, A.; et al. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology 2018, 67, 1890–1902. [Google Scholar] [CrossRef] [PubMed]
- Nevens, F.; Andreone, P.; Mazzella, G.; Strasser, S.I.; Bowlus, C.; Invernizzi, P.; Drenth, J.P.; Pockros, P.J.; Regula, J.; Beuers, U.; et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N. Engl. J. Med. 2016, 375, 631–643. [Google Scholar] [CrossRef]
- Reig, A.; Sese, P.; Pares, A. Effects of bezafibrate on outcome and pruritus in primary biliary cholangitis with suboptimal ursodeoxycholic acid response. Am. J. Gastroenterol. 2018, 113, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Dejman, A.; Clark, V.; Martin, P.; Levy, C. Fenofibrate improves alkaline phosphatase in primary sclerosing cholangitis. Gastroenterology 2013, 144, S1028–S1029. [Google Scholar] [CrossRef]
- Hommes, D.W.; Erkelens, W.; Ponsioen, C.; Stokkers, P.; Rauws, E.; van der Spek, M.; ten Kate, F.; van Deventer, S.J. A double-blind, placebo-controlled, randomized study of infliximab in primary sclerosing cholangitis. J. Clin. Gastroenterol. 2008, 42, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Gershwin, M.E.; Strauss, R.; Mayo, M.J.; Levy, C.; Zou, B.; Johanns, J.; Nnane, I.P.; Dasgupta, B.; Li, K.; et al. Ustekinumab for patients with primary biliary cholangitis who have an inadequate response to ursodeoxycholic acid: A proof-of-concept study. Hepatology 2016, 64, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Santiago, P.; Scheinberg, A.R.; Levy, C. Cholestatic liver diseases: New targets, new therapies. Therap. Adv. Gastroenterol. 2018, 11, 1756284818787400. [Google Scholar] [CrossRef]
- Cai, S.Y.; Li, M.; Boyer, J.L. The role of bile acid-mediated inflammation in cholestatic liver injury. Liver Biol. Pathobiol. 2020, 728–736. [Google Scholar] [CrossRef]
- Bull, L.N.; Thompson, R.J. Progressive familial intrahepatic cholestasis. Clin. Liver Dis. 2018, 22, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Zollner, G.; Trauner, M. Mechanisms of cholestasis. Clin. Liver Dis. 2008, 12, 1–26. [Google Scholar] [CrossRef]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.P.; Schwabe, R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef]
- Raynaud, P.; Carpentier, R.; Antoniou, A.; Lemaigre, F.P. Biliary differentiation and bile duct morphogenesis in development and disease. Int. J. Biochem Cell Biol. 2011, 43, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Cai, S.Y.; Mennone, A.; Vig, P.; Boyer, J.L. Cenicriviroc, a cytokine receptor antagonist, potentiates all-trans retinoic acid in reducing liver injury in cholestatic rodents. Liver Int. 2018, 38, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.T.; Chen, D.K. Immunological abnormalities in patients with primary biliary cholangitis. Clin. Sci. 2019, 133, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Mells, G.F.; Pells, G.; Dawwas, M.F.; Newton, J.L.; Heneghan, M.A.; Neuberger, J.M.; Day, D.B.; Ducker, S.J.; Consortium, U.P.; et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology 2013, 144, 560–569.e7. [Google Scholar] [CrossRef]
- Lammers, W.J.; van Buuren, H.R.; Hirschfield, G.M.; Janssen, H.L.; Invernizzi, P.; Mason, A.L.; Ponsioen, C.Y.; Floreani, A.; Corpechot, C.; Mayo, M.J.; et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: An international follow-up study. Gastroenterology 2014, 147, 1338–1349.e5. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Lammers, W.J.; van Buuren, H.R.; Pares, A.; Floreani, A.; Janssen, H.L.; Invernizzi, P.; Battezzati, P.M.; Ponsioen, C.Y.; Corpechot, C.; et al. Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: A multicentre international study. Gut 2016, 65, 321–329. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address, e.e.e.; European Association for the Study of the, L. Easl clinical practice guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef]
- Gulamhusein, A.F.; Hirschfield, G.M. Primary biliary cholangitis: Pathogenesis and therapeutic opportunities. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 93–110. [Google Scholar] [CrossRef]
- Beuers, U.; Gershwin, M.E.; Gish, R.G.; Invernizzi, P.; Jones, D.E.; Lindor, K.; Ma, X.; Mackay, I.R.; Pares, A.; Tanaka, A.; et al. Changing nomenclature for pbc: From ‘cirrhosis’ to ‘cholangitis’. Gastroenterology 2015, 149, 1627–1629. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.M.; Gershwin, M.E. Primary biliary cirrhosis. N. Engl. J. Med. 2005, 353, 1261–1273. [Google Scholar] [CrossRef]
- Dyson, J.K.; Wilkinson, N.; Jopson, L.; Mells, G.; Bathgate, A.; Heneghan, M.A.; Neuberger, J.; Hirschfield, G.M.; Ducker, S.J.; Consortium, U.-P.; et al. The inter-relationship of symptom severity and quality of life in 2055 patients with primary biliary cholangitis. Aliment. Pharmacol. Ther. 2016, 44, 1039–1050. [Google Scholar] [CrossRef]
- Mells, G.F.; Pells, G.; Newton, J.L.; Bathgate, A.J.; Burroughs, A.K.; Heneghan, M.A.; Neuberger, J.M.; Day, D.B.; Ducker, S.J.; Sandford, R.N.; et al. Impact of primary biliary cirrhosis on perceived quality of life: The uk-pbc national study. Hepatology 2013, 58, 273–283. [Google Scholar] [CrossRef]
- Li, T.; Chiang, J.Y. Bile acid metabolism in health and disease: An update. Liver Biol. Pathobiol. 2020, 269–285. [Google Scholar] [CrossRef]
- Gonzalez, R.S.; Washington, K. Primary biliary cholangitis and autoimmune hepatitis. Surg. Pathol. Clin. 2018, 11, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, K.; Kunst, A.E.; Stadhouders, P.H.; Tuynman, H.A.; Poen, A.C.; van Nieuwkerk, K.M.; Witteman, E.M.; Hamann, D.; Witteman, B.J.; Beuers, U.; et al. Rising incidence and prevalence of primary biliary cirrhosis: A large population-based study. Liver Int. 2014, 34, e31–e38. [Google Scholar] [CrossRef]
- Dahlqvist, G.; Gaouar, F.; Carrat, F.; Meurisse, S.; Chazouilleres, O.; Poupon, R.; Johanet, C.; Corpechot, C.; French network of Immunology, L. Large-scale characterization study of patients with antimitochondrial antibodies but nonestablished primary biliary cholangitis. Hepatology 2017, 65, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, L.; Dyson, J.K.; Jones, D.E. The new epidemiology of primary biliary cirrhosis. Semin. Liver Dis. 2014, 34, 318–328. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, Y.; Haller, I.V.; Romanelli, R.J.; VanWormer, J.J.; Rodriguez, C.V.; Anderson, H.; Boscarino, J.A.; Schmidt, M.A.; Daida, Y.G.; et al. Increasing prevalence of primary biliary cholangitis and reduced mortality with treatment. Clin. Gastroenterol. Hepatol. 2018, 16, 1342–1350.e1. [Google Scholar] [CrossRef]
- Gulamhusein, A.F.; Juran, B.D.; Lazaridis, K.N. Genome-wide association studies in primary biliary cirrhosis. Semin. Liver Dis. 2015, 35, 392–401. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Hirschfield, G.M. The immunogenetics of autoimmune cholestasis. Clin. Liver Dis. 2016, 20, 15–31. [Google Scholar] [CrossRef]
- Corpechot, C.; Chretien, Y.; Chazouilleres, O.; Poupon, R. Demographic, lifestyle, medical and familial factors associated with primary biliary cirrhosis. J. Hepatol. 2010, 53, 162–169. [Google Scholar] [CrossRef]
- Gershwin, M.E.; Selmi, C.; Worman, H.J.; Gold, E.B.; Watnik, M.; Utts, J.; Lindor, K.D.; Kaplan, M.M.; Vierling, J.M.; Group, U.P.E. Risk factors and comorbidities in primary biliary cirrhosis: A controlled interview-based study of 1032 patients. Hepatology 2005, 42, 1194–1202. [Google Scholar] [CrossRef]
- Hamlyn, A.N.; Macklon, A.F.; James, O. Primary biliary cirrhosis: Geographical clustering and symptomatic onset seasonality. Gut 1983, 24, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, P. Human leukocyte antigen in primary biliary cirrhosis: An old story now reviving. Hepatology 2011, 54, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Juran, B.D.; Lazaridis, K.N. Environmental factors in primary biliary cirrhosis. Semin. Liver Dis. 2014, 34, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Probert, P.M.; Leitch, A.C.; Dunn, M.P.; Meyer, S.K.; Palmer, J.M.; Abdelghany, T.M.; Lakey, A.F.; Cooke, M.P.; Talbot, H.; Wills, C.; et al. Identification of a xenobiotic as a potential environmental trigger in primary biliary cholangitis. J. Hepatol. 2018, 69, 1123–1135. [Google Scholar] [CrossRef]
- Wang, J.J.; Yang, G.X.; Zhang, W.C.; Lu, L.; Tsuneyama, K.; Kronenberg, M.; Vela, J.L.; Lopez-Hoyos, M.; He, X.S.; Ridgway, W.M.; et al. Escherichia coli infection induces autoimmune cholangitis and anti-mitochondrial antibodies in non-obese diabetic (nod).B6 (idd10/idd18) mice. Clin. Exp. Immunol. 2014, 175, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.; Eickmeier, I.; Kuhl, A.A.; Hamann, A.; Loddenkemper, C.; Schott, E. Cd8 t cells primed in the gut-associated lymphoid tissue induce immune-mediated cholangitis in mice. Hepatology 2014, 59, 601–611. [Google Scholar] [CrossRef]
- Li, Y.; Tang, R.; Leung, P.S.C.; Gershwin, M.E.; Ma, X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmun. Rev. 2017, 16, 885–896. [Google Scholar] [CrossRef]
- Chen, W.; Wei, Y.; Xiong, A.; Li, Y.; Guan, H.; Wang, Q.; Miao, Q.; Bian, Z.; Xiao, X.; Lian, M.; et al. Comprehensive analysis of serum and fecal bile acid profiles and interaction with gut microbiota in primary biliary cholangitis. Clin. Rev. Allergy Immunol. 2020, 58, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.J.; Lalor, P.F.; Salmi, M.; Jalkanen, S.; Adams, D.H. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet 2002, 359, 150–157. [Google Scholar] [CrossRef]
- Jimenez-Dalmaroni, M.J.; Gerswhin, M.E.; Adamopoulos, I.E. The critical role of toll-like receptors--from microbial recognition to autoimmunity: A comprehensive review. Autoimmun. Rev. 2016, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Chetwynd, A.; Newman, W.; Metcalf, J.V.; James, O.F. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: Follow-up for up to 28 years. Gastroenterology 2002, 123, 1044–1051. [Google Scholar] [CrossRef]
- Christensen, E.; Neuberger, J.; Crowe, J.; Altman, D.G.; Popper, H.; Portmann, B.; Doniach, D.; Ranek, L.; Tygstrup, N.; Williams, R. Beneficial effect of azathioprine and prediction of prognosis in primary biliary cirrhosis. Final results of an international trial. Gastroenterology 1985, 89, 1084–1091. [Google Scholar] [CrossRef]
- Jones, D.E.; Metcalf, J.V.; Collier, J.D.; Bassendine, M.F.; James, O.F. Hepatocellular carcinoma in primary biliary cirrhosis and its impact on outcomes. Hepatology 1997, 26, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Dyson, J.K.; Beuers, U.; Jones, D.E.J.; Lohse, A.W.; Hudson, M. Primary sclerosing cholangitis. Lancet 2018, 391, 2547–2559. [Google Scholar] [CrossRef]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Schrumpf, E.; Abdelnoor, M.; Fausa, O.; Elgjo, K.; Jenssen, E.; Kolmannskog, F. Risk factors in primary sclerosing cholangitis. J. Hepatol. 1994, 21, 1061–1066. [Google Scholar] [CrossRef]
- Tischendorf, J.J.; Hecker, H.; Kruger, M.; Manns, M.P.; Meier, P.N. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am. J. Gastroenterol. 2007, 102, 107–114. [Google Scholar] [CrossRef]
- Jepsen, P.; Gronbaek, L.; Vilstrup, H. Worldwide incidence of autoimmune liver disease. Dig. Dis. 2015, 33 (Suppl. 2), 2–12. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Kareemi, H.; Parab, R.; Barkema, H.W.; Quan, H.; Myers, R.P.; Kaplan, G.G. Incidence of primary sclerosing cholangitis: A systematic review and meta-analysis. Hepatology 2011, 53, 1590–1599. [Google Scholar] [CrossRef]
- Bambha, K.; Kim, W.R.; Talwalkar, J.; Torgerson, H.; Benson, J.T.; Therneau, T.M.; Loftus, E.V., Jr.; Yawn, B.P.; Dickson, E.R.; Melton, L.J., 3rd. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a united states community. Gastroenterology 2003, 125, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Escorsell, A.; Pares, A.; Rodes, J.; Solis-Herruzo, J.A.; Miras, M.; de la Morena, E. Epidemiology of primary sclerosing cholangitis in spain. Spanish association for the study of the liver. J. Hepatol. 1994, 21, 787–791. [Google Scholar] [CrossRef]
- Lindkvist, B.; Benito de Valle, M.; Gullberg, B.; Bjornsson, E. Incidence and prevalence of primary sclerosing cholangitis in a defined adult population in sweden. Hepatology 2010, 52, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Hov, J.R.; Folseraas, T.; Ellinghaus, E.; Rushbrook, S.M.; Doncheva, N.T.; Andreassen, O.A.; Weersma, R.K.; Weismuller, T.J.; Eksteen, B.; et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat. Genet. 2013, 45, 670–675. [Google Scholar] [CrossRef]
- Ji, S.G.; Juran, B.D.; Mucha, S.; Folseraas, T.; Jostins, L.; Melum, E.; Kumasaka, N.; Atkinson, E.J.; Schlicht, E.M.; Liu, J.Z.; et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat. Genet. 2017, 49, 269–273. [Google Scholar] [CrossRef]
- de Vries, A.B.; Janse, M.; Blokzijl, H.; Weersma, R.K. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J. Gastroenterol. 2015, 21, 1956–1971. [Google Scholar] [CrossRef]
- Katt, J.; Schwinge, D.; Schoknecht, T.; Quaas, A.; Sobottka, I.; Burandt, E.; Becker, C.; Neurath, M.F.; Lohse, A.W.; Herkel, J.; et al. Increased t helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology 2013, 58, 1084–1093. [Google Scholar] [CrossRef]
- Sabino, J.; Vieira-Silva, S.; Machiels, K.; Joossens, M.; Falony, G.; Ballet, V.; Ferrante, M.; Van Assche, G.; Van der Merwe, S.; Vermeire, S.; et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from ibd. Gut 2016, 65, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, R.H.; Grambsch, P.M.; Dickson, E.R.; Ludwig, J.; MacCarty, R.L.; Hunter, E.B.; Fleming, T.R.; Fisher, L.D.; Beaver, S.J.; LaRusso, N.F. Primary sclerosing cholangitis: Natural history, prognostic factors and survival analysis. Hepatology 1989, 10, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Bader, T.R.; Beavers, K.L.; Semelka, R.C. Mr imaging features of primary sclerosing cholangitis: Patterns of cirrhosis in relationship to clinical severity of disease. Radiology 2003, 226, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, E.; Lindqvist-Ottosson, J.; Asztely, M.; Olsson, R. Dominant strictures in patients with primary sclerosing cholangitis. Am. J. Gastroenterol. 2004, 99, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Broome, U.; Lofberg, R.; Veress, B.; Eriksson, L.S. Primary sclerosing cholangitis and ulcerative colitis: Evidence for increased neoplastic potential. Hepatology 1995, 22, 1404–1408. [Google Scholar]
- Chapman, R.; Fevery, J.; Kalloo, A.; Nagorney, D.M.; Boberg, K.M.; Shneider, B.; Gores, G.J.; American Association for the Study of Liver, D. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010, 51, 660–678. [Google Scholar] [CrossRef] [PubMed]
- Claessen, M.M.; Vleggaar, F.P.; Tytgat, K.M.; Siersema, P.D.; van Buuren, H.R. High lifetime risk of cancer in primary sclerosing cholangitis. J. Hepatol. 2009, 50, 158–164. [Google Scholar] [CrossRef]
- European Association for the Study of the, L. Easl clinical practice guidelines: Management of cholestatic liver diseases. J. Hepatol. 2009, 51, 237–267. [Google Scholar] [CrossRef]
- Linder, S.; Soderlund, C. Endoscopic therapy in primary sclerosing cholangitis: Outcome of treatment and risk of cancer. Hepatogastroenterology 2001, 48, 387–392. [Google Scholar] [PubMed]
- Bergquist, A.; Ekbom, A.; Olsson, R.; Kornfeldt, D.; Loof, L.; Danielsson, A.; Hultcrantz, R.; Lindgren, S.; Prytz, H.; Sandberg-Gertzen, H.; et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J. Hepatol. 2002, 36, 321–327. [Google Scholar] [CrossRef]
- Boberg, K.M.; Bergquist, A.; Mitchell, S.; Pares, A.; Rosina, F.; Broome, U.; Chapman, R.; Fausa, O.; Egeland, T.; Rocca, G.; et al. Cholangiocarcinoma in primary sclerosing cholangitis: Risk factors and clinical presentation. Scand. J. Gastroenterol. 2002, 37, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Fevery, J.; Henckaerts, L.; Van Oirbeek, R.; Vermeire, S.; Rutgeerts, P.; Nevens, F.; Van Steenbergen, W. Malignancies and mortality in 200 patients with primary sclerosering cholangitis: A long-term single-centre study. Liver Int. 2012, 32, 214–222. [Google Scholar] [CrossRef]
- Karlsen, T.H.; Folseraas, T.; Thorburn, D.; Vesterhus, M. Primary sclerosing cholangitis—A comprehensive review. J. Hepatol. 2017, 67, 1298–1323. [Google Scholar] [CrossRef]
- Carpentier, R.; Suner, R.E.; van Hul, N.; Kopp, J.L.; Beaudry, J.B.; Cordi, S.; Antoniou, A.; Raynaud, P.; Lepreux, S.; Jacquemin, P.; et al. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology 2011, 141, 1432–1438.e4. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.L. Bile formation and secretion. Compr. Physiol. 2013, 3, 1035–1078. [Google Scholar] [PubMed]
- Guicciardi, M.E.; Trussoni, C.E.; LaRusso, N.F.; Gores, G.J. The spectrum of reactive cholangiocytes in primary sclerosing cholangitis. Hepatology 2020, 71, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.M.; Brix, F.; Kohr, P. General and specific aspects of experimental dose measurement in total body irradiation (tbi). Strahlenther. Onkol. 1986, 162, 250–253. [Google Scholar]
- Poncy, A.; Antoniou, A.; Cordi, S.; Pierreux, C.E.; Jacquemin, P.; Lemaigre, F.P. Transcription factors sox4 and sox9 cooperatively control development of bile ducts. Dev. Biol. 2015, 404, 136–148. [Google Scholar] [CrossRef]
- Mansini, A.P.; Peixoto, E.; Thelen, K.M.; Gaspari, C.; Jin, S.; Gradilone, S.A. The cholangiocyte primary cilium in health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1245–1253. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, J.A.; Wells, R.G. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology 2010, 51, 1438–1444. [Google Scholar] [CrossRef]
- Kisseleva, T.; Cong, M.; Paik, Y.; Scholten, D.; Jiang, C.; Benner, C.; Iwaisako, K.; Moore-Morris, T.; Scott, B.; Tsukamoto, H.; et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 9448–9453. [Google Scholar] [CrossRef] [PubMed]
- Iwaisako, K.; Jiang, C.; Zhang, M.; Cong, M.; Moore-Morris, T.J.; Park, T.J.; Liu, X.; Xu, J.; Wang, P.; Paik, Y.H.; et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc. Natl. Acad. Sci. USA 2014, 111, E3297–E3305. [Google Scholar] [CrossRef]
- Chen, Y.; Gilbert, M.A.; Grochowski, C.M.; McEldrew, D.; Llewellyn, J.; Waisbourd-Zinman, O.; Hakonarson, H.; Bailey-Wilson, J.E.; Russo, P.; Wells, R.G.; et al. A genome-wide association study identifies a susceptibility locus for biliary atresia on 2p16.1 within the gene efemp1. PLoS Genet. 2018, 14, e1007532. [Google Scholar] [CrossRef] [PubMed]
- Fausther, M.; Dranoff, J.A. Beyond scar formation: Portal myofibroblast-mediated angiogenesis in the fibrotic liver. Hepatology 2015, 61, 766–768. [Google Scholar] [CrossRef]
- Koyama, Y.; Wang, P.; Liang, S.; Iwaisako, K.; Liu, X.; Xu, J.; Zhang, M.; Sun, M.; Cong, M.; Karin, D.; et al. Mesothelin/mucin 16 signaling in activated portal fibroblasts regulates cholestatic liver fibrosis. J. Clin. Investig. 2017, 127, 1254–1270. [Google Scholar] [CrossRef]
- Nishio, T.; Hu, R.; Koyama, Y.; Liang, S.; Rosenthal, S.B.; Yamamoto, G.; Karin, D.; Baglieri, J.; Ma, H.Y.; Xu, J.; et al. Activated hepatic stellate cells and portal fibroblasts contribute to cholestatic liver fibrosis in mdr2 knockout mice. J. Hepatol. 2019, 71, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G. The portal fibroblast: Not just a poor man’s stellate cell. Gastroenterology 2014, 147, 41–47. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Koyama, Y.; Wang, P.; Lan, T.; Kim, I.G.; Kim, I.H.; Ma, H.Y.; Kisseleva, T. The types of hepatic myofibroblasts contributing to liver fibrosis of different etiologies. Front. Pharmacol. 2014, 5, 167. [Google Scholar] [CrossRef]
- Libbrecht, L.; Cassiman, D.; Desmet, V.; Roskams, T. The correlation between portal myofibroblasts and development of intrahepatic bile ducts and arterial branches in human liver. Liver 2002, 22, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Braet, F.; Taatjes, D.J.; Wisse, E. Probing the unseen structure and function of liver cells through atomic force microscopy. Semin. Cell Dev. Biol. 2018, 73, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Wisse, E. Ultrastructure and function of kupffer cells and other sinusoidal cells in the liver. Med. Chir. Digest. 1977, 6, 409–418. [Google Scholar]
- Klaas, M.; Kangur, T.; Viil, J.; Maemets-Allas, K.; Minajeva, A.; Vadi, K.; Antsov, M.; Lapidus, N.; Jarvekulg, M.; Jaks, V. The alterations in the extracellular matrix composition guide the repair of damaged liver tissue. Sci. Rep. 2016, 6, 27398. [Google Scholar] [CrossRef]
- Mallat, A.; Lotersztajn, S. Cellular mechanisms of tissue fibrosis. 5. Novel insights into liver fibrosis. Am. J. Physiol. Cell Physiol. 2013, 305, C789–C799. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Teratani, T.; Tomita, K.; Suzuki, T.; Oshikawa, T.; Yokoyama, H.; Shimamura, K.; Tominaga, S.; Hiroi, S.; Irie, R.; Okada, Y.; et al. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology 2012, 142, 152–164.e10. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Lee, Y.A.; Fujiwara, N.; Ybanez, M.; Allen, B.; Martins, S.; Fiel, M.I.; Goossens, N.; Chou, H.I.; Hoshida, Y.; et al. A simple diet- and chemical-induced murine nash model with rapid progression of steatohepatitis, fibrosis and liver cancer. J. Hepatol. 2018, 69, 385–395. [Google Scholar] [CrossRef]
- Furuhashi, H.; Tomita, K.; Teratani, T.; Shimizu, M.; Nishikawa, M.; Higashiyama, M.; Takajo, T.; Shirakabe, K.; Maruta, K.; Okada, Y.; et al. Vitamin a-coupled liposome system targeting free cholesterol accumulation in hepatic stellate cells offers a beneficial therapeutic strategy for liver fibrosis. Hepatol. Res. 2018, 48, 397–407. [Google Scholar] [CrossRef]
- Villeneuve, J.; Pelluard-Nehme, F.; Combe, C.; Carles, D.; Chaponnier, C.; Ripoche, J.; Balabaud, C.; Bioulac-Sage, P.; Lepreux, S. Immunohistochemical study of the phenotypic change of the mesenchymal cells during portal tract maturation in normal and fibrous (ductal plate malformation) fetal liver. Comp. Hepatol. 2009, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tanaka, M.; Watanabe, N.; Saito, S.; Nonaka, H.; Miyajima, A. P75 neurotrophin receptor is a marker for precursors of stellate cells and portal fibroblasts in mouse fetal liver. Gastroenterology 2008, 135, 270–281.e3. [Google Scholar] [CrossRef] [PubMed]
- Asahina, K.; Tsai, S.Y.; Li, P.; Ishii, M.; Maxson, R.E., Jr.; Sucov, H.M.; Tsukamoto, H. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology 2009, 49, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Sawitza, I.; Kordes, C.; Reister, S.; Haussinger, D. The niche of stellate cells within rat liver. Hepatology 2009, 50, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.D.; Xu, M.Y.; Cai, X.B.; Qu, Y.; Li, Z.H.; Lu, L.G. Myofibroblastic transformation of rat hepatic stellate cells: The role of notch signaling and epithelial-mesenchymal transition regulation. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4130–4138. [Google Scholar] [PubMed]
- Spee, B.; Carpino, G.; Schotanus, B.A.; Katoonizadeh, A.; Vander Borght, S.; Gaudio, E.; Roskams, T. Characterisation of the liver progenitor cell niche in liver diseases: Potential involvement of wnt and notch signalling. Gut 2010, 59, 247–257. [Google Scholar] [CrossRef]
- Tanriverdi, G.; Kaya-Dagistanli, F.; Ayla, S.; Demirci, S.; Eser, M.; Unal, Z.S.; Cengiz, M.; Oktar, H. Resveratrol can prevent ccl(4)-induced liver injury by inhibiting notch signaling pathway. Histol. Histopathol. 2016, 31, 769–784. [Google Scholar] [PubMed]
- Chen, Y.; Zheng, S.; Qi, D.; Zheng, S.; Guo, J.; Zhang, S.; Weng, Z. Inhibition of notch signaling by a gamma-secretase inhibitor attenuates hepatic fibrosis in rats. PLoS ONE 2012, 7, e46512. [Google Scholar]
- Adams, J.M.; Jafar-Nejad, H. The roles of notch signaling in liver development and disease. Biomolecules 2019, 9, 608. [Google Scholar] [CrossRef]
- Zhang, K.; Han, X.; Zhang, Z.; Zheng, L.; Hu, Z.; Yao, Q.; Cui, H.; Shu, G.; Si, M.; Li, C.; et al. The liver-enriched lnc-lfar1 promotes liver fibrosis by activating tgfbeta and notch pathways. Nat. Commun. 2017, 8, 144. [Google Scholar] [CrossRef]
- Aimaiti, Y.; Jin, X.; Wang, W.; Chen, Z.; Li, D. Tgf-beta1 signaling regulates mouse hepatic stellate cell differentiation via the jagged1/notch pathway. Life Sci. 2018, 192, 221–230. [Google Scholar] [CrossRef]
- Wallace, K.; Burt, A.D.; Wright, M.C. Liver fibrosis. Biochem. J. 2008, 411, 1–18. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Dropmann, A.; Dooley, S.; Dewidar, B.; Hammad, S.; Dediulia, T.; Werle, J.; Hartwig, V.; Ghafoory, S.; Woelfl, S.; Korhonen, H.; et al. Tgf-beta2 silencing to target biliary-derived liver diseases. Gut 2020, 69, 1677–1690. [Google Scholar] [CrossRef]
- Athwal, V.S.; Pritchett, J.; Llewellyn, J.; Martin, K.; Camacho, E.; Raza, S.M.; Phythian-Adams, A.; Birchall, L.J.; Mullan, A.F.; Su, K.; et al. Sox9 predicts progression toward cirrhosis in patients while its loss protects against liver fibrosis. EMBO Mol. Med. 2017, 9, 1696–1710. [Google Scholar] [CrossRef]
- Duan, J.L.; Ruan, B.; Yan, X.C.; Liang, L.; Song, P.; Yang, Z.Y.; Liu, Y.; Dou, K.F.; Han, H.; Wang, L. Endothelial notch activation reshapes the angiocrine of sinusoidal endothelia to aggravate liver fibrosis and blunt regeneration in mice. Hepatology 2018, 68, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Kim, K.; Wang, X.; Bartolome, A.; Salomao, M.; Dongiovanni, P.; Meroni, M.; Graham, M.J.; Yates, K.P.; Diehl, A.M.; et al. Hepatocyte notch activation induces liver fibrosis in nonalcoholic steatohepatitis. Sci. Transl. Med. 2018, 10, eaat0344. [Google Scholar] [CrossRef]
- Fabris, L.; Spirli, C.; Cadamuro, M.; Fiorotto, R.; Strazzabosco, M. Emerging concepts in biliary repair and fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G102–G116. [Google Scholar] [CrossRef]
- Planas-Paz, L.; Sun, T.; Pikiolek, M.; Cochran, N.R.; Bergling, S.; Orsini, V.; Yang, Z.; Sigoillot, F.; Jetzer, J.; Syed, M.; et al. Yap, but not rspo-lgr4/5, signaling in biliary epithelial cells promotes a ductular reaction in response to liver injury. Cell Stem Cell 2019, 25, 39–53.e10. [Google Scholar] [CrossRef] [PubMed]
- Furth, N.; Aylon, Y.; Oren, M. P53 shades of hippo. Cell Death Differ. 2018, 25, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Bird, T.G.; Lu, W.Y.; Boulter, L.; Gordon-Keylock, S.; Ridgway, R.A.; Williams, M.J.; Taube, J.; Thomas, J.A.; Wojtacha, D.; Gambardella, A.; et al. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated tweak signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 6542–6547. [Google Scholar] [CrossRef]
- Jakubowski, A.; Ambrose, C.; Parr, M.; Lincecum, J.M.; Wang, M.Z.; Zheng, T.S.; Browning, B.; Michaelson, J.S.; Baetscher, M.; Wang, B.; et al. Tweak induces liver progenitor cell proliferation. J. Clin. Investig. 2005, 115, 2330–2340. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Diehl, A.M. Hedgehog signalling in liver pathophysiology. J. Hepatol. 2018, 68, 550–562. [Google Scholar] [CrossRef]
- Strazzabosco, M.; Fabris, L. Notch signaling in hepatocellular carcinoma: Guilty in association! Gastroenterology 2012, 143, 1430–1434. [Google Scholar] [CrossRef]
- Omenetti, A.; Porrello, A.; Jung, Y.; Yang, L.; Popov, Y.; Choi, S.S.; Witek, R.P.; Alpini, G.; Venter, J.; Vandongen, H.M.; et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J. Clin. Investig. 2008, 118, 3331–3342. [Google Scholar] [CrossRef]
- Xie, G.; Karaca, G.; Swiderska-Syn, M.; Michelotti, G.A.; Kruger, L.; Chen, Y.; Premont, R.T.; Choi, S.S.; Diehl, A.M. Cross-talk between notch and hedgehog regulates hepatic stellate cell fate in mice. Hepatology 2013, 58, 1801–1813. [Google Scholar] [CrossRef]
- Stasiulewicz, M.; Gray, S.D.; Mastromina, I.; Silva, J.C.; Bjorklund, M.; Seymour, P.A.; Booth, D.; Thompson, C.; Green, R.J.; Hall, E.A.; et al. A conserved role for notch signaling in priming the cellular response to shh through ciliary localisation of the key shh transducer smo. Development 2015, 142, 2291–2303. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Guo, F.C.; Li, Z.; Yu, H.C.; Ma, P.F.; Zhao, J.L.; Feng, L.; Li, W.N.; Liu, X.W.; Qin, H.Y.; et al. Myeloid-specific disruption of recombination signal binding protein jkappa ameliorates hepatic fibrosis by attenuating inflammation through cylindromatosis in mice. Hepatology 2015, 61, 303–314. [Google Scholar] [CrossRef]
- Herrera, J.; Henke, C.A.; Bitterman, P.B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Investig. 2018, 128, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Monga, S.P. Beta-catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology 2015, 148, 1294–1310. [Google Scholar] [CrossRef]
- Ramirez, T.; Li, Y.M.; Yin, S.; Xu, M.J.; Feng, D.; Zhou, Z.; Zang, M.; Mukhopadhyay, P.; Varga, Z.V.; Pacher, P.; et al. Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J. Hepatol. 2017, 66, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Roeb, E. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol. 2018, 68-69, 463–473. [Google Scholar] [CrossRef]
- Beljaars, L.; Daliri, S.; Dijkhuizen, C.; Poelstra, K.; Gosens, R. Wnt-5a regulates tgf-beta-related activities in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G219–G227. [Google Scholar] [CrossRef]
- Carpino, G.; Nobili, V.; Renzi, A.; De Stefanis, C.; Stronati, L.; Franchitto, A.; Alisi, A.; Onori, P.; De Vito, R.; Alpini, G.; et al. Macrophage activation in pediatric nonalcoholic fatty liver disease (nafld) correlates with hepatic progenitor cell response via wnt3a pathway. PLoS ONE 2016, 11, e0157246. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.G.; Lv, X.D.; Zhan, L.L.; Chen, L.; Zou, Q.Y.; Xiang, J.Q.; Qin, J.L.; Zhang, W.W.; Zeng, Z.J.; Jin, H.; et al. Human urokinase-type plasminogen activator gene-modified bone marrow-derived mesenchymal stem cells attenuate liver fibrosis in rats by down-regulating the wnt signaling pathway. World J. Gastroenterol. 2016, 22, 2092–2103. [Google Scholar] [CrossRef]
- Xiong, W.J.; Hu, L.J.; Jian, Y.C.; Wang, L.J.; Jiang, M.; Li, W.; He, Y. Wnt5a participates in hepatic stellate cell activation observed by gene expression profile and functional assays. World J. Gastroenterol. 2012, 18, 1745–1752. [Google Scholar] [CrossRef]
- Cheng, J.H.; She, H.; Han, Y.P.; Wang, J.; Xiong, S.; Asahina, K.; Tsukamoto, H. Wnt antagonism inhibits hepatic stellate cell activation and liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G39–G49. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.K.Y.; Kweon, S.M.; Chi, F.; Hwang, E.; Kabe, Y.; Higashiyama, R.; Qin, L.; Yan, R.; Wu, R.P.; Lai, K.; et al. Stearoyl-coa desaturase promotes liver fibrosis and tumor development in mice via a wnt positive-signaling loop by stabilization of low-density lipoprotein-receptor-related proteins 5 and 6. Gastroenterology 2017, 152, 1477–1491. [Google Scholar] [CrossRef]
- Wang, J.N.; Li, L.; Li, L.Y.; Yan, Q.; Li, J.; Xu, T. Emerging role and therapeutic implication of wnt signaling pathways in liver fibrosis. Gene 2018, 674, 57–69. [Google Scholar] [CrossRef]
- Pradhan-Sundd, T.; Kosar, K.; Saggi, H.; Zhang, R.; Vats, R.; Cornuet, P.; Green, S.; Singh, S.; Zeng, G.; Sundd, P.; et al. Wnt/beta-catenin signaling plays a protective role in the mdr2 knockout murine model of cholestatic liver disease. Hepatology 2020, 71, 1732–1749. [Google Scholar] [CrossRef]
- Minden, A.; Karin, M. Regulation and function of the jnk subgroup of map kinases. Biochim Biophys Acta 1997, 1333, F85–F104. [Google Scholar] [CrossRef]
- Weston, C.R.; Davis, R.J. The jnk signal transduction pathway. Curr. Opin. Cell Biol. 2007, 19, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Hatting, M.; Nevzorova, Y.A.; Peng, J.; Hu, W.; Boekschoten, M.V.; Roskams, T.; Muller, M.; Gassler, N.; Liedtke, C.; et al. Jnk1 in murine hepatic stellate cells is a crucial mediator of liver fibrogenesis. Gut 2014, 63, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Kluwe, J.; Pradere, J.P.; Gwak, G.Y.; Mencin, A.; De Minicis, S.; Osterreicher, C.H.; Colmenero, J.; Bataller, R.; Schwabe, R.F. Modulation of hepatic fibrosis by c-jun-n-terminal kinase inhibition. Gastroenterology 2010, 138, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Bubici, C.; Zazzeroni, F.; Franzoso, G. Mechanisms of liver disease: Cross-talk between the nf-kappab and jnk pathways. Biol. Chem. 2009, 390, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Alper, S.L. Molecular physiology and genetics of na+-independent slc4 anion exchangers. J. Exp. Biol. 2009, 212, 1672–1683. [Google Scholar] [CrossRef]
- Trampert, D.C.; van de Graaf, S.F.J.; Jongejan, A.; Oude Elferink, R.P.J.; Beuers, U. Hepatobiliary acid-base homeostasis: Insights from analogous secretory epithelia. J. Hepatol. 2021, 74, 428–441. [Google Scholar] [CrossRef]
- Prieto, J.; Qian, C.; Garcia, N.; Diez, J.; Medina, J.F. Abnormal expression of anion exchanger genes in primary biliary cirrhosis. Gastroenterology 1993, 105, 572–578. [Google Scholar] [CrossRef]
- Medina, J.F.; Martinez, A.; Vazquez, J.J.; Prieto, J. Decreased anion exchanger 2 immunoreactivity in the liver of patients with primary biliary cirrhosis. Hepatology 1997, 25, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Melero, S.; Spirli, C.; Zsembery, A.; Medina, J.F.; Joplin, R.E.; Duner, E.; Zuin, M.; Neuberger, J.M.; Prieto, J.; Strazzabosco, M. Defective regulation of cholangiocyte cl-/hco3(-) and na+/h+ exchanger activities in primary biliary cirrhosis. Hepatology 2002, 35, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Salas, J.T.; Banales, J.M.; Sarvide, S.; Recalde, S.; Ferrer, A.; Uriarte, I.; Oude Elferink, R.P.; Prieto, J.; Medina, J.F. Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology 2008, 134, 1482–1493. [Google Scholar] [CrossRef]
- Castro, R.E.; Rodrigues, C.M.P. Cell death and micrornas in cholestatic liver diseases: Update on potential therapeutic applications. Curr. Drug Targets 2017, 18, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Erice, O.; Munoz-Garrido, P.; Vaquero, J.; Perugorria, M.J.; Fernandez-Barrena, M.G.; Saez, E.; Santos-Laso, A.; Arbelaiz, A.; Jimenez-Aguero, R.; Fernandez-Irigoyen, J.; et al. Microrna-506 promotes primary biliary cholangitis-like features in cholangiocytes and immune activation. Hepatology 2018, 67, 1420–1440. [Google Scholar] [CrossRef]
- Dyson, J.K.; Hirschfield, G.M.; Adams, D.H.; Beuers, U.; Mann, D.A.; Lindor, K.D.; Jones, D.E. Novel therapeutic targets in primary biliary cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Padgett, K.A.; Lan, R.Y.; Leung, P.C.; Lleo, A.; Dawson, K.; Pfeiff, J.; Mao, T.K.; Coppel, R.L.; Ansari, A.A.; Gershwin, M.E. Primary biliary cirrhosis is associated with altered hepatic microrna expression. J. Autoimmun. 2009, 32, 246–253. [Google Scholar] [CrossRef]
- Minagawa, N.; Nagata, J.; Shibao, K.; Masyuk, A.I.; Gomes, D.A.; Rodrigues, M.A.; Lesage, G.; Akiba, Y.; Kaunitz, J.D.; Ehrlich, B.E.; et al. Cyclic amp regulates bicarbonate secretion in cholangiocytes through release of atp into bile. Gastroenterology 2007, 133, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Saez, E.; Uriz, M.; Sarvide, S.; Urribarri, A.D.; Splinter, P.; Tietz Bogert, P.S.; Bujanda, L.; Prieto, J.; Medina, J.F.; et al. Up-regulation of microrna 506 leads to decreased cl-/hco3- anion exchanger 2 expression in biliary epithelium of patients with primary biliary cirrhosis. Hepatology 2012, 56, 687–697. [Google Scholar] [CrossRef]
- Monte, M.J.; Marin, J.J.; Antelo, A.; Vazquez-Tato, J. Bile acids: Chemistry, physiology, and pathophysiology. World J. Gastroenterol. 2009, 15, 804–816. [Google Scholar] [CrossRef]
- Song, K.H.; Li, T.; Owsley, E.; Chiang, J.Y. A putative role of micro rna in regulation of cholesterol 7alpha-hydroxylase expression in human hepatocytes. J. Lipid Res. 2010, 51, 2223–2233. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.M.; Marquart, T.J.; Albert, C.J.; Suchy, F.J.; Wang, D.Q.; Ananthanarayanan, M.; Ford, D.A.; Baldan, A. Mir-33 controls the expression of biliary transporters, and mediates statin- and diet-induced hepatotoxicity. EMBO Mol. Med. 2012, 4, 882–895. [Google Scholar] [CrossRef]
- Li, T.; Francl, J.M.; Boehme, S.; Chiang, J.Y. Regulation of cholesterol and bile acid homeostasis by the cholesterol 7alpha-hydroxylase/steroid response element-binding protein 2/microrna-33a axis in mice. Hepatology 2013, 58, 1111–1121. [Google Scholar] [CrossRef]
- Marin, J.J.; Bujanda, L.; Banales, J.M. Micrornas and cholestatic liver diseases. Curr. Opin. Gastroenterol. 2014, 30, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Gulamhusein, A.F.; Hirschfield, G.M. Pathophysiology of primary biliary cholangitis. Best Pract. Res. Clin. Gastroenterol. 2018, 34–35, 17–25. [Google Scholar] [CrossRef]
- Yeaman, S.J.; Fussey, S.P.; Danner, D.J.; James, O.F.; Mutimer, D.J.; Bassendine, M.F. Primary biliary cirrhosis: Identification of two major m2 mitochondrial autoantigens. Lancet 1988, 1, 1067–1070. [Google Scholar] [CrossRef]
- Kita, H.; Matsumura, S.; He, X.S.; Ansari, A.A.; Lian, Z.X.; Van de Water, J.; Coppel, R.L.; Kaplan, M.M.; Gershwin, M.E. Quantitative and functional analysis of pdc-e2-specific autoreactive cytotoxic t lymphocytes in primary biliary cirrhosis. J. Clin. Investig. 2002, 109, 1231–1240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kita, H.; Lian, Z.X.; Van de Water, J.; He, X.S.; Matsumura, S.; Kaplan, M.; Luketic, V.; Coppel, R.L.; Ansari, A.A.; Gershwin, M.E. Identification of hla-a2-restricted cd8(+) cytotoxic t cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J. Exp. Med. 2002, 195, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Lleo, A.; Bowlus, C.L.; Yang, G.X.; Invernizzi, P.; Podda, M.; Van de Water, J.; Ansari, A.A.; Coppel, R.L.; Worman, H.J.; Gores, G.J.; et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology 2010, 52, 987–998. [Google Scholar] [CrossRef]
- Tanaka, A.; Leung, P.S.C.; Gershwin, M.E. The genetics of primary biliary cholangitis. Curr. Opin. Gastroenterol. 2019, 35, 93–98. [Google Scholar] [CrossRef]
- Ellinghaus, D.; Jostins, L.; Spain, S.L.; Cortes, A.; Bethune, J.; Han, B.; Park, Y.R.; Raychaudhuri, S.; Pouget, J.G.; Hubenthal, M.; et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat. Genet. 2016, 48, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Goyette, P.; Boucher, G.; Mallon, D.; Ellinghaus, E.; Jostins, L.; Huang, H.; Ripke, S.; Gusareva, E.S.; Annese, V.; Hauser, S.L.; et al. High-density mapping of the mhc identifies a shared role for hla-drb1*01:03 in inflammatory bowel diseases and heterozygous advantage in ulcerative colitis. Nat. Genet. 2015, 47, 172–179. [Google Scholar] [CrossRef]
- Karlsen, T.H.; Franke, A.; Melum, E.; Kaser, A.; Hov, J.R.; Balschun, T.; Lie, B.A.; Bergquist, A.; Schramm, C.; Weismuller, T.J.; et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology 2010, 138, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Folseraas, T.; Melum, E.; Rausch, P.; Juran, B.D.; Ellinghaus, E.; Shiryaev, A.; Laerdahl, J.K.; Ellinghaus, D.; Schramm, C.; Weismuller, T.J.; et al. Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J. Hepatol. 2012, 57, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Beuers, U.; Hohenester, S.; de Buy Wenniger, L.J.; Kremer, A.E.; Jansen, P.L.; Elferink, R.P. The biliary hco(3)(-) umbrella: A unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology 2010, 52, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.Y.; Boyer, J.L. The role of inflammation in the mechanisms of bile acid-induced liver damage. Dig. Dis. 2017, 35, 232–234. [Google Scholar] [CrossRef]
- Penz-Osterreicher, M.; Osterreicher, C.H.; Trauner, M. Fibrosis in autoimmune and cholestatic liver disease. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 245–258. [Google Scholar] [CrossRef]
- Trauner, M.; Fickert, P.; Halilbasic, E.; Moustafa, T. Lessons from the toxic bile concept for the pathogenesis and treatment of cholestatic liver diseases. Wien. Med. Wochenschr. 2008, 158, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Fickert, P.; Fuchsbichler, A.; Wagner, M.; Zollner, G.; Kaser, A.; Tilg, H.; Krause, R.; Lammert, F.; Langner, C.; Zatloukal, K.; et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in mdr2 (abcb4) knockout mice. Gastroenterology 2004, 127, 261–274. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; Barua, L.; Bowen, W.C. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology 2005, 41, 535–544. [Google Scholar] [CrossRef]
- Ezure, T.; Sakamoto, T.; Tsuji, H.; Lunz, J.G., 3rd; Murase, N.; Fung, J.J.; Demetris, A.J. The development and compensation of biliary cirrhosis in interleukin-6-deficient mice. Am. J. Pathol. 2000, 156, 1627–1639. [Google Scholar] [CrossRef]
- Lunz, J.G., 3rd; Contrucci, S.; Ruppert, K.; Murase, N.; Fung, J.J.; Starzl, T.E.; Demetris, A.J. Replicative senescence of biliary epithelial cells precedes bile duct loss in chronic liver allograft rejection: Increased expression of p21(waf1/cip1) as a disease marker and the influence of immunosuppressive drugs. Am. J. Pathol. 2001, 158, 1379–1390. [Google Scholar] [CrossRef]
- Matsumoto, K.; Fujii, H.; Michalopoulos, G.; Fung, J.J.; Demetris, A.J. Human biliary epithelial cells secrete and respond to cytokines and hepatocyte growth factors in vitro: Interleukin-6, hepatocyte growth factor and epidermal growth factor promote DNA synthesis in vitro. Hepatology 1994, 20, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, V.; Cadamuro, M.; Spirli, C.; Fiorotto, R.; Strazzabosco, M.; Fabris, L. Animal models of cholestasis: An update on inflammatory cholangiopathies. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, L.; Flavell, R.A. Abrogation of tgfbeta signaling in t cells leads to spontaneous t cell differentiation and autoimmune disease. Immunity 2000, 12, 171–181. [Google Scholar] [CrossRef]
- Ishigame, H.; Mosaheb, M.M.; Sanjabi, S.; Flavell, R.A. Truncated form of tgf-betarii, but not its absence, induces memory cd8+ t cell expansion and lymphoproliferative disorder in mice. J. Immunol. 2013, 190, 6340–6350. [Google Scholar] [CrossRef]
- Vergani, D.; Alvarez, F.; Bianchi, F.B.; Cancado, E.L.; Mackay, I.R.; Manns, M.P.; Nishioka, M.; Penner, E.; International Autoimmune Hepatitis, G. Liver autoimmune serology: A consensus statement from the committee for autoimmune serology of the international autoimmune hepatitis group. J. Hepatol. 2004, 41, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Sudo, Y.; Kono, N.; Ozaki, S.; Tsuneyama, K.; Gershwin, M.E.; Nakanuma, Y. In situ nucleic acid detection of pdc-e2, bcoadc-e2, ogdc-e2, pdc-e1alpha, bcoadc-e1alpha, ogdc-e1, and the e3 binding protein (protein x) in primary biliary cirrhosis. Hepatology 1999, 30, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Habior, A. Detection of autoantibodies against nucleoporin p62 in sera of patients with primary biliary cholangitis. Ann. Lab. Med. 2019, 39, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Habior, A.; Wieszczy, P.; Gawel, D. Analysis of autoantibodies against promyelocytic leukemia nuclear body components and biochemical parameters in sera of patients with primary biliary cholangitis. Diagnostics 2021, 11, 587. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Xu, L.; Liu, B. Detection of anti-kelch-like 12 and anti-hexokinase 1 antibodies in primary biliary cholangitis patients in china. Rev. Esp. Enferm. Dig. 2020. [Google Scholar] [CrossRef]
- Hausdorf, G.; Roggenbuck, D.; Feist, E.; Buttner, T.; Jungblut, P.R.; Conrad, K.; Berg, C.; Klein, R. Autoantibodies to asialoglycoprotein receptor (asgpr) measured by a novel elisa--revival of a disease-activity marker in autoimmune hepatitis. Clin. Chim. Acta 2009, 408, 19–24. [Google Scholar] [CrossRef]
- Reig, A.; Norman, G.L.; Garcia, M.; Shums, Z.; Ruiz-Gaspa, S.; Bentow, C.; Mahler, M.; Romera, M.A.; Vinas, O.; Pares, A. Novel anti-hexokinase 1 antibodies are associated with poor prognosis in patients with primary biliary cholangitis. Am. J. Gastroenterol. 2020, 115, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Terjung, B.; Spengler, U.; Sauerbruch, T.; Worman, H.J. “Atypical p-anca” in ibd and hepatobiliary disorders react with a 50-kilodalton nuclear envelope protein of neutrophils and myeloid cell lines. Gastroenterology 2000, 119, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Jendrek, S.T.; Gotthardt, D.; Nitzsche, T.; Widmann, L.; Korf, T.; Michaels, M.A.; Weiss, K.H.; Liaskou, E.; Vesterhus, M.; Karlsen, T.H.; et al. Anti-gp2 iga autoantibodies are associated with poor survival and cholangiocarcinoma in primary sclerosing cholangitis. Gut 2017, 66, 137–144. [Google Scholar] [CrossRef]
- Beuers, U.; Trauner, M.; Jansen, P.; Poupon, R. New paradigms in the treatment of hepatic cholestasis: From udca to fxr, pxr and beyond. J. Hepatol. 2015, 62, S25–S37. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Kowdley, K.V.; Luketic, V.A.; Harrison, M.E.; McCashland, T.; Befeler, A.S.; Harnois, D.; Jorgensen, R.; Petz, J.; Keach, J.; et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology 2009, 50, 808–814. [Google Scholar] [CrossRef]
- Poupon, R.E.; Poupon, R.; Balkau, B. Ursodiol for the long-term treatment of primary biliary cirrhosis. The udca-pbc study group. N. Engl. J. Med. 1994, 330, 1342–1347. [Google Scholar] [CrossRef]
- Fiorucci, S.; Rizzo, G.; Antonelli, E.; Renga, B.; Mencarelli, A.; Riccardi, L.; Orlandi, S.; Pruzanski, M.; Morelli, A.; Pellicciari, R. A farnesoid x receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J. Pharmacol. Exp. Ther. 2005, 314, 584–595. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Mason, A.; Luketic, V.; Lindor, K.; Gordon, S.C.; Mayo, M.; Kowdley, K.V.; Vincent, C.; Bodhenheimer, H.C., Jr.; Pares, A.; et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology 2015, 148, 751–761.e8. [Google Scholar] [CrossRef]
- Levy, C.; Peter, J.A.; Nelson, D.R.; Keach, J.; Petz, J.; Cabrera, R.; Clark, V.; Firpi, R.J.; Morelli, G.; Soldevila-Pico, C.; et al. Pilot study: Fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Aliment. Pharmacol. Ther. 2011, 33, 235–242. [Google Scholar] [CrossRef]
- Ghonem, N.S.; Auclair, A.M.; Hemme, C.L.; Gallucci, G.M.; de la Rosa Rodriguez, R.; Boyer, J.L.; Assis, D.N. Fenofibrate improves liver function and reduces the toxicity of the bile acid pool in patients with primary biliary cholangitis and primary sclerosing cholangitis who are partial responders to ursodiol. Clin. Pharmacol. Ther. 2020, 108, 1213–1223. [Google Scholar] [CrossRef]
- Corpechot, C.; Chazouilleres, O.; Lemoinne, S.; Rousseau, A. Letter: Reduction in projected mortality or need for liver transplantation associated with bezafibrate add-on in primary biliary cholangitis with incomplete udca response. Aliment. Pharmacol. Ther. 2019, 49, 236–238. [Google Scholar] [CrossRef]
- de Vries, E.; Bolier, R.; Goet, J.; Pares, A.; Verbeek, J.; de Vree, M.; Drenth, J.; van Erpecum, K.; van Nieuwkerk, K.; van der Heide, F.; et al. Fibrates for itch (fitch) in fibrosing cholangiopathies: A double-blind, randomized, placebo-controlled trial. Gastroenterology 2020, 160, 734–743.e6. [Google Scholar] [CrossRef]
- Lemoinne, S.; Corpechot, C.; Fankem, A.K.; Gaouar, F.; Poupon, R.; Chazouillères, O. Fibrates improve liver tests in primary sclerosing cholangitis with incomplete biochemical response to ursodeoxycholic acid: Update of a pilote study: 335. Hepatology 2014, 60, 521–528. [Google Scholar]
- Hirschfield, G.; Boudes, P.; Bowlus, C.; Gitlin, N.; Michael, G.; Harrison, S.; Gordon, S.; Aspinall, R.; Doerffel, Y.; Kremer, A. Treatment efficacy and safety of seladelpar, a selective peroxisome proliferator-activated receptor delta agonist, in primary biliary cholangitis patients: 12-and 26-week analysis from an ongoing international, randomized, dose raging phase 2 study. J. Hepatol. 2018, 68, S105–S106. [Google Scholar] [CrossRef]
- Schattenberg, J.M.; Pares, A.; Kowdley, K.V.; Heneghan, M.A.; Caldwell, S.; Pratt, D.; Bonder, A.; Hirschfield, G.M.; Levy, C.; Vierling, J.; et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to udca. J. Hepatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Gershwin, M.E.; Poupon, R.; Kaplan, M.; Bergasa, N.V.; Heathcote, E.J.; American Association for Study of Liver, D. Primary biliary cirrhosis. Hepatology 2009, 50, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Trauner, M.; Gulamhusein, A.; Hameed, B.; Caldwell, S.; Shiffman, M.L.; Landis, C.; Eksteen, B.; Agarwal, K.; Muir, A.; Rushbrook, S.; et al. The nonsteroidal farnesoid x receptor agonist cilofexor (gs-9674) improves markers of cholestasis and liver injury in patients with primary sclerosing cholangitis. Hepatology 2019, 70, 788–801. [Google Scholar] [CrossRef]
- Goldstein, J.; Levy, C. Novel and emerging therapies for cholestatic liver diseases. Liver Int. 2018, 38, 1520–1535. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Fickert, P. Time for the dawn of multimodal therapies and the dusk for mono-therapeutic trials for cholestatic liver diseases? Liver Int. 2018, 38, 991–994. [Google Scholar] [CrossRef]
- Khanna, A.; Jopson, L.; Howel, D.; Bryant, A.; Blamire, A.; Newton, J.L.; Jones, D.E. Rituximab is ineffective for treatment of fatigue in primary biliary cholangitis: A phase 2 randomized controlled trial. Hepatology 2019, 70, 1646–1657. [Google Scholar] [CrossRef]
- Muir, A.J.; Levy, C.; Janssen, H.L.A.; Montano-Loza, A.J.; Shiffman, M.L.; Caldwell, S.; Luketic, V.; Ding, D.; Jia, C.; McColgan, B.J.; et al. Simtuzumab for primary sclerosing cholangitis: Phase 2 study results with insights on the natural history of the disease. Hepatology 2019, 69, 684–698. [Google Scholar] [CrossRef]
- Ali, A.H.; Damman, J.; Shah, S.B.; Davies, Y.; Hurwitz, M.; Stephen, M.; Lemos, L.M.; Carey, E.J.; Lindor, K.D.; Buness, C.W.; et al. Open-label prospective therapeutic clinical trials: Oral vancomycin in children and adults with primary sclerosing cholangitis. Scand. J. Gastroenterol. 2020, 55, 941–950. [Google Scholar] [CrossRef]
- Tabibian, J.H.; Weeding, E.; Jorgensen, R.A.; Petz, J.L.; Keach, J.C.; Talwalkar, J.A.; Lindor, K.D. Randomised clinical trial: Vancomycin or metronidazole in patients with primary sclerosing cholangitis—A pilot study. Aliment. Pharmacol. Ther. 2013, 37, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, J.H.; Gossard, A.; El-Youssef, M.; Eaton, J.E.; Petz, J.; Jorgensen, R.; Enders, F.B.; Tabibian, A.; Lindor, K.D. Prospective clinical trial of rifaximin therapy for patients with primary sclerosing cholangitis. Am. J. Ther. 2017, 24, e56–e63. [Google Scholar] [CrossRef]
- Assis, D.N.; Abdelghany, O.; Cai, S.Y.; Gossard, A.A.; Eaton, J.E.; Keach, J.C.; Deng, Y.; Setchell, K.D.; Ciarleglio, M.; Lindor, K.D.; et al. Combination therapy of all-trans retinoic acid with ursodeoxycholic acid in patients with primary sclerosing cholangitis: A human pilot study. J. Clin. Gastroenterol. 2017, 51, e11–e16. [Google Scholar] [CrossRef]
- Gochanour, E.M.; Kowdley, K.V. Investigational drugs in early phase development for primary biliary cholangitis. Expert Opin. Investig. Drugs 2021, 30, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Chazouilleres, O.; Drenth, J.P.; Thorburn, D.; Harrison, S.A.; Landis, C.S.; Mayo, M.J.; Muir, A.J.; Trotter, J.F.; Leeming, D.J.; et al. Effect of ngm282, an fgf19 analogue, in primary sclerosing cholangitis: A multicenter, randomized, double-blind, place-bo-controlled phase ii trial. J. Hepatol. 2019, 70, 483–493. [Google Scholar] [CrossRef] [PubMed]
| Autoantibody | Target | Found Positive in Liver Disease |
|---|---|---|
| AMAs | PDC-E2 | PBC [185,186] |
| OGDC-E2 | ||
| BCOADC-E2 | ||
| E3BP | ||
| ANAs | gp210 | PBC and PSC [21,187,188] |
| p62 | ||
| sp100 | ||
| PML | ||
| Anti-Kelch | KLHL12 | PBC [189] |
| Anti-ASGPR | ASGPR | PBC [190] |
| Anti-hexokinase | HK1 | PBC [191] |
| p-ANCA/p-ANNA | Unclear | PSC [192] |
| Anti-GP2 IgA | Gp2 | PSC [193] |
| Drug | Mechanism of Action | Reference and NCT Number | |
|---|---|---|---|
| PBS | PCS | ||
| Agonist (Target) | |||
| Fenofibrate | PPARα | Levy et al. [199] NCT00575042 | Ghonem et al. [200] NCT01142323 |
| Bezafibrate | Pan PPAR | Corpechot et al. [201] NCT01654731 | Elsemieke et al. [202] NCT02701166 |
| Fenofibrate and bezafibrate | PPARα | Lemoinne et al. [203] | |
| Seladelpar | Selective PPARδ | Hirschfield et al. [204] NCT02955602 | NCT04024813 |
| Elafibranor | PPARα | Schattenberg et al. [205] NCT03124108 | |
| Saroglitazar | PPARα and PPARγ | Lindor et al. [206] NCT03112681 | |
| Cilofexor | FXR | NCT02943447 | Trauner et al. [207] NCT02943460 |
| Tropifexor | FXR | NCT02516605 | |
| EDP-305 | FXR | Goldstein et al. [208] NCT03394924 | |
| Etrasimod | S1PR1 and S1PR4 | NCT03155932 | |
| Inhibitor (Target) | |||
| GKT137831 | NOX1 and NOX4 | Goldstein et al. [208] NCT03226067 | |
| Baricitinib | JAK1 and JAK2 | NCT03742973 | |
| Monoclonal antibody against (Target) | |||
| Ustekinumab | IL-12 and IL-23 | Hirschfield et al. [9] NCT01389973 | |
| Abatacept | CD80 and CD86 interferes with T-cell activation | Wagner et al. [209] NCT02078882 | |
| Rituximab | CD20 B-cell depletion | Khanna et al. [210] NCT02376335 | |
| Infliximab | TNF-α | Hommes et al. [8] | |
| Simtuzumab | LOXL2 | Muir et al. [211] NCT01672853 | |
| E6011 | CX3CL1 (fractalkine) | Goldstein et al. [208] NCT03092765 | |
| Antibiotics Gut microbiome: antimicrobial and immunomodulation | |||
| Vancomycin | Ali et al. [212] NCT01322386 | Ali et al. [212] NCT01802073 | |
| Vancomycin and metronidazole | Tabibian et al. [213] NCT01085760 | ||
| Rifaximin | Tabibian et al. [214] NCT01695174 | ||
| Probiotics | NCT03521297 | ||
| ATRA | Permissive activator of the nuclear receptor FXR/RXR | Assis et al. [215] NCT01456468 | |
| NGM282 | FGF 19 analogue | Gochanour et al. [216] NCT02026401 | Hirschfield et al. [217] NCT02704364 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Chen, C.; Ziani, S.; Nelson, L.J.; Ávila, M.A.; Nevzorova, Y.A.; Cubero, F.J. Fibrotic Events in the Progression of Cholestatic Liver Disease. Cells 2021, 10, 1107. https://doi.org/10.3390/cells10051107
Wu H, Chen C, Ziani S, Nelson LJ, Ávila MA, Nevzorova YA, Cubero FJ. Fibrotic Events in the Progression of Cholestatic Liver Disease. Cells. 2021; 10(5):1107. https://doi.org/10.3390/cells10051107
Chicago/Turabian StyleWu, Hanghang, Chaobo Chen, Siham Ziani, Leonard J. Nelson, Matías A. Ávila, Yulia A. Nevzorova, and Francisco Javier Cubero. 2021. "Fibrotic Events in the Progression of Cholestatic Liver Disease" Cells 10, no. 5: 1107. https://doi.org/10.3390/cells10051107
APA StyleWu, H., Chen, C., Ziani, S., Nelson, L. J., Ávila, M. A., Nevzorova, Y. A., & Cubero, F. J. (2021). Fibrotic Events in the Progression of Cholestatic Liver Disease. Cells, 10(5), 1107. https://doi.org/10.3390/cells10051107








