Differential Effects of Angiotensin-II Compared to Phenylephrine on Arterial Stiffness and Hemodynamics: A Placebo-Controlled Study in Healthy Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort and Design
2.2. Study Protocol
2.3. Biochemistry
2.4. Data Analysis
3. Results
3.1. Baseline Characteristics and Participant Drop-Outs
3.2. Hemodynamics
3.3. Parameters of Arterial Stiffness
3.4. Biochemistry
3.4.1. Electrolytes
3.4.2. Hormones
3.4.3. Inflammation and Endothelial Dysfunction
3.5. Analysis of Gender Effects
4. Discussion
4.1. Arterial Stiffness and Hemodynamics during Vasoactive Drug Infusion
4.2. Arterial Stiffness and Hemodynamics after Stopping Vasoactive Drug Infusion
4.3. Ang II—Sympathetic Baroreflex Interactions
4.4. Biochemistry and Markers of Endothelial Function
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baulmann, J.; Nurnberger, J.; Slany, J.; Schmieder, R.; Schmidt-Trucksass, A.; Baumgart, D.; Cremerius, P.; Hess, O.; Mortensen, K.; Weber, T. [Arterial stiffness and pulse wave analysis]. Dtsch. Med. Wochenschr. 2010, 135 (Suppl. 1), S4–S14. [Google Scholar] [CrossRef] [PubMed]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Avolio, A.P.; Chirinos, J.A.; Cockcroft, J.R.; Heffernan, K.S.; Lakatta, E.G.; McEniery, C.M.; Mitchell, G.F.; et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement from the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar] [CrossRef]
- Wilkinson, I.B.; MacCallum, H.; Flint, L.; Cockcroft, J.R.; Newby, D.E.; Webb, D.J. The influence of heart rate on augmentation index and central arterial pressure in humans. J. Physiol. 2000, 525 Pt 1, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.; Feely, J. Arterial stiffness and the renin-angiotensin-aldosterone system. J. Renin Angiotensin Aldosterone Syst. 2004, 5, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Franzen, K.; Reppel, M.; Koster, J.; Mortensen, K. Acute and chronic effects on central hemodynamics and arterial stiffness in professional rowers. Physiol. Meas. 2016, 37, 544–553. [Google Scholar] [CrossRef]
- Reppel, M.; Franzen, K.; Bode, F.; Weil, J.; Kurowski, V.; Schneider, S.A.; Baulmann, J.; von Lukowicz, T.; Mirau, W.; Mortensen, E.; et al. Central hemodynamics and arterial stiffness during the finals of the world cup soccer championship 2010. Int. J. Cardiol. 2011, 166, 627–632. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Sayk, F.; Wobbe, I.; Twesten, C.; Meusel, M.; Wellhöner, P.; Derad, I.; Dodt, C. Prolonged blood pressure elevation following continuous infusion of angiotensin II-a baroreflex study in healthy humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1406–R1414. [Google Scholar] [CrossRef]
- Agabiti-Rosei, E.; Mancia, G.; O’Rourke, M.F.; Roman, M.J.; Safar, M.E.; Smulyan, H.; Wang, J.G.; Wilkinson, I.B.; Williams, B.; Vlachopoulos, C. Central blood pressure measurements and antihypertensive therapy: A consensus document. Hypertension 2007, 50, 154–160. [Google Scholar] [CrossRef]
- Guyenet, P.G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006, 7, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L. Vascular and cardiac benefits of angiotensin receptor blockers. Am. J. Med. 2002, 113, 409–418. [Google Scholar] [CrossRef]
- Heeneman, S.; Sluimer, J.C.; Daemen, M.J. Angiotensin-converting enzyme and vascular remodeling. Circ. Res. 2007, 101, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Van Bortel, L.M.; Duprez, D.; Starmans-Kool, M.J.; Safar, M.E.; Giannattasio, C.; Cockcroft, J.; Kaiser, D.R.; Thuillez, C. Clinical applications of arterial stiffness, Task Force III: Recommendations for user procedures. Am. J. Hypertens. 2002, 15, 445–452. [Google Scholar] [CrossRef]
- Wassertheurer, S.; Kropf, J.; Weber, T.; van der Giet, M.; Baulmann, J.; Ammer, M.; Hametner, B.; Mayer, C.C.; Eber, B.; Magometschnigg, D. A new oscillometric method for pulse wave analysis: Comparison with a common tonometric method. J. Hum. Hypertens. 2010, 24, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Ammer, M.; Rammer, M.; Adji, A.; O’Rourke, M.F.; Wassertheurer, S.; Rosenkranz, S.; Eber, B. Noninvasive determination of carotid-femoral pulse wave velocity depends critically on assessment of travel distance: A comparison with invasive measurement. J. Hypertens. 2009, 27, 1624–1630. [Google Scholar] [CrossRef]
- Weber, T.; Wassertheurer, S.; Rammer, M.; Maurer, E.; Hametner, B.; Mayer, C.C.; Kropf, J.; Eber, B. Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension 2011, 58, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Arnold, A.C. The renin-angiotensin system in cardiovascular autonomic control: Recent developments and clinical implications. Clin. Auton. Res. 2019, 29, 231–243. [Google Scholar] [CrossRef]
- Charkoudian, N.; Joyner, M.J.; Johnson, C.P.; Eisenach, J.H.; Dietz, N.M.; Wallin, B.G. Balance between cardiac output and sympathetic nerve activity in resting humans: Role in arterial pressure regulation. J. Physiol. 2005, 568, 315–321. [Google Scholar] [CrossRef]
- Vingerhoedt, N.M.; Gilles, R.; Howes, J.B.; Griffin, M.; Howes, L.G. Haemodynamic and pulse wave responses to intravenous infusions of angiotensin II during chronic telmisartan therapy in normal volunteers. J. Renin Angiotensin Aldosterone Syst. 2003, 4, 244–248. [Google Scholar] [CrossRef]
- Dudenbostel, T.; Glasser, S.P. Effects of antihypertensive drugs on arterial stiffness. Cardiol. Rev. 2012, 20, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Jekell, A.; Kalani, M.; Kahan, T. The effects of alpha 1-adrenoceptor blockade and angiotensin converting enzyme inhibition on central and brachial blood pressure and vascular reactivity: The doxazosin-ramipril study. Heart Vessel. 2017, 32, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Biancardi, V.C.; Son, S.J.; Ahmadi, S.; Filosa, J.A.; Stern, J.E. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension 2014, 63, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Koba, S.; Yoshida, T.; Hayashi, N. Differential sympathetic outflow and vasoconstriction responses at kidney and skeletal muscles during fictive locomotion. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H861–H868. [Google Scholar] [CrossRef]
- Matsukawa, T.; Miyamoto, T. Does infusion of ANG II increase muscle sympathetic nerve activity in patients with primary aldosteronism? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1873–R1879. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilbert-Lampen, U.; NPalmer, B.R.; Jones, L.M.; Young, J.M.; Faulkner, J. Inflammatory biomarkers for predicting cardiovascular disease. Clin. Biochem. 2013, 46, 1353–1371. [Google Scholar] [CrossRef]
- Xia, M.; Sui, Z. Recent developments in CCR2 antagonists. Expert Opin. Ther. Pat. 2009, 19, 295–303. [Google Scholar] [CrossRef]
- Cseh, E.K.; Veres, G.; Körtési, T.; Polyák, H.; Nánási, N.; Tajti, J.; Párdutz, Á.; Klivényi, P.; Vécsei, L.; Zádori, D. Neurotransmitter and tryptophan metabolite concentration changes in the complete Freund’s adjuvant model of orofacial pain. J. Headache Pain 2020, 21, 35. [Google Scholar] [CrossRef] [PubMed]
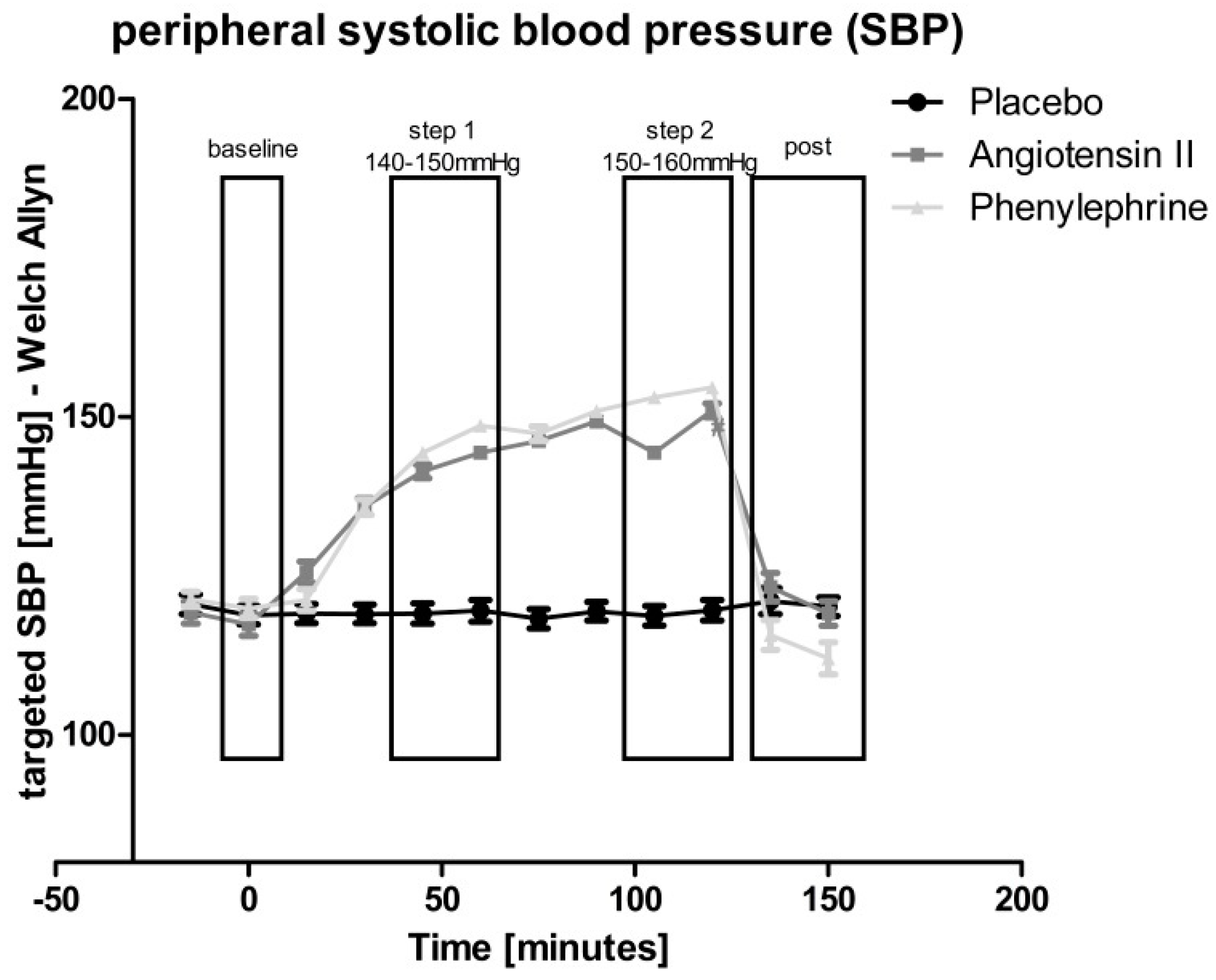
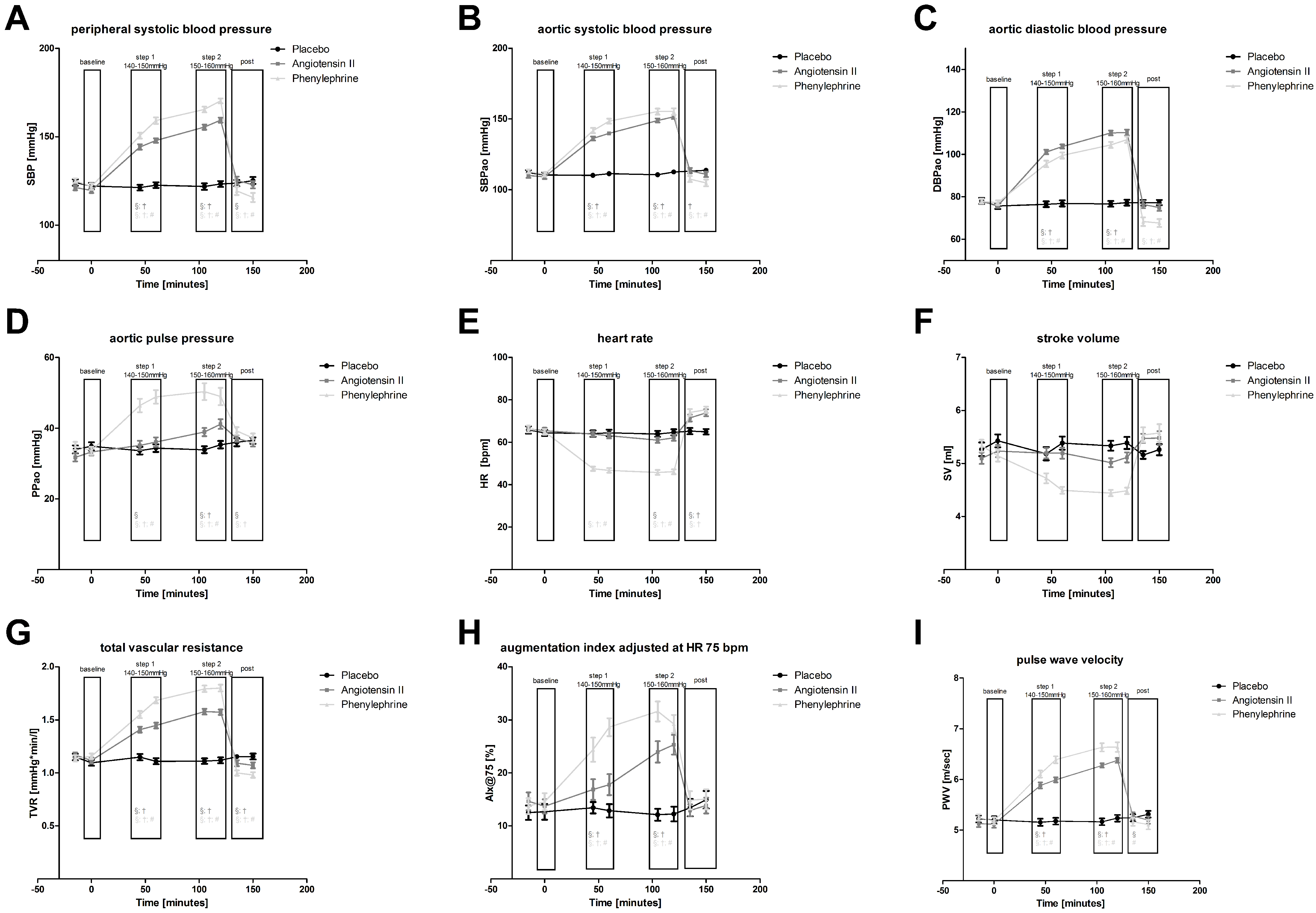
| Placebo | Angiotensin II | Phenylephrine | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sem | ANOVA | Mean | sem | ANOVA | Mean | sem | ANOVA | |||||
| Baseline | SBP [mmHg] | 123.1 | ± | 1.3 | 120.4 | ± | 1.0 | 123.4 | ± | 1.1 | |||
| DBP [mmHg] | 75.4 | ± | 1.0 | 75.7 | ± | 0.7 | 76.6 | ± | 0.8 | ||||
| PP [mmHg] | 47.6 | ± | 1.0 | 44.7 | ± | 0.7 | 46.8 | ± | 1.1 | ||||
| HR [bpm] | 65.1 | ± | 1.1 | 65.9 | ± | 1.0 | 65.6 | ± | 1.0 | ||||
| SV [mL] | 5.3 | ± | 0.1 | 5.2 | ± | 0.1 | 5.2 | ± | 0.1 | ||||
| TVR [mmHg × min/L] | 1.12 | ± | 0.02 | 1.14 | ± | 0.02 | 1.15 | ± | 0.02 | ||||
| Step 1 (140–150 mmHg) | SBP [mmHg] | 121.9 | ± | 1.1 | 146.0 | ± | 1.0 | §; † | 154.8 | ± | 1.3 | §; †; # | |
| DBP [mmHg] | 75.1 | ± | 1.0 | 100.8 | ± | 0.7 | §; † | 94.6 | ± | 1.1 | §; †; # | ||
| PP [mmHg] | 46.8 | ± | 0.9 | 45.1 | ± | 1.1 | 60.2 | ± | 1.4 | §; †; # | |||
| HR [bpm] | 64.3 | ± | 1.1 | 63.5 | ± | 0.9 | 47.1 | ± | 0.7 | §; †; # | |||
| SV [mL] | 5.3 | ± | 0.1 | 5.2 | ± | 0.1 | 4.6 | ± | 0.1 | §; †; # | |||
| TVR [mmHg × min/L] | 1.13 | ± | 0.02 | 1.43 | ± | 0.02 | §; † | 1.62 | ± | 0.02 | §; †; # | ||
| Step 2 (150–160 mmHg) | SBP [mmHg] | 122.5 | ± | 1.2 | 157.3 | ± | 1.0 | §; † | 167.8 | ± | 1.1 | §; †; # | |
| DBP [mmHg] | 75.4 | ± | 0.9 | 108.3 | ± | 0.9 | §; † | 103.6 | ± | 0.9 | §; †; # | ||
| PP [mmHg] | 47.1 | ± | 0.9 | 49.0 | ± | 1.0 | § | 64.2 | ± | 1.3 | §; †; # | ||
| HR [bpm] | 64.3 | ± | 1.0 | 61.5 | ± | 0.9 | § | 45.9 | ± | 0.8 | §; †; # | ||
| SV [mL] | 5.4 | ± | 0.1 | 5.1 | ± | 0.1 | † | 4.5 | ± | 0.0 | §; †; # | ||
| TVR [mmHg × min/L] | 1.11 | ± | 0.02 | 1.57 | ± | 0.02 | §; † | 1.80 | ± | 0.02 | §; †; # | ||
| Post | SBP [mmHg] | 124.5 | ± | 1.3 | 124.1 | ± | 1.4 | § | 117.5 | ± | 1.7 | §; †; # | |
| DBP [mmHg] | 75.8 | ± | 0.9 | 74.1 | ± | 1.1 | § | 66.9 | ± | 1.4 | §; †; # | ||
| PP [mmHg] | 48.6 | ± | 1.0 | 50.0 | ± | 0.9 | § | 50.5 | ± | 1.2 | §; † | ||
| HR [bpm] | 65.1 | ± | 1.0 | 72.7 | ± | 1.1 | §; † | 74.7 | ± | 1.1 | §; † | ||
| SV [mL] | 5.2 | ± | 0.1 | 5.5 | ± | 0.1 | §; † | 5.6 | ± | 0.1 | §; † | ||
| TVR [mmHg × min/L] | 1.15 | ± | 0.02 | 1.08 | ± | 0.02 | §; † | 0.99 | ± | 0.02 | §; †; # | ||
| Placebo | Angiotensin II | Phenylephrine | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sem | ANOVA | Mean | sem | ANOVA | Mean | sem | ANOVA | |||||
| Baseline | SBPao [mmHg] | 111.3 | ± | 1.2 | 109.5 | ± | 1.1 | 111.9 | ± | 1.0 | |||
| DBPao [mmHg] | 76.8 | ± | 1.0 | 76.9 | ± | 0.8 | 77.5 | ± | 0.8 | ||||
| PPao [mmHg] | 34.4 | ± | 0.8 | 32.5 | ± | 0.8 | 34.3 | ± | 0.8 | ||||
| AIx [%] | 12.6 | ± | 1.0 | 14.2 | ± | 1.1 | 14.1 | ± | 1.1 | ||||
| PWV [m/s] | 5.2 | ± | 0.0 | 5.1 | ± | 0.0 | 5.2 | ± | 0.0 | ||||
| Step 1 (140–150 mmHg) | SBPao [mmHg] | 110.7 | ± | 1.1 | 138.0 | ± | 0.9 | §; † | 145.1 | ± | 1.4 | §; †; # | |
| DBPao [mmHg] | 76.7 | ± | 1.0 | 102.4 | ± | 0.7 | §; † | 97.4 | ± | 1.1 | §; †; # | ||
| PPao [mmHg] | 34.0 | ± | 0.7 | 35.6 | ± | 0.9 | § | 47.6 | ± | 1.3 | §; †; # | ||
| AIx [%] | 13.1 | ± | 0.8 | 17.3 | ± | 1.4 | §; † | 26.5 | ± | 1.4 | §; †; # | ||
| PWV [m/s] | 5.2 | ± | 0.0 | 5.9 | ± | 0.0 | §; † | 6.2 | ± | 0.1 | §; †; # | ||
| Step 2 (150–160 mmHg) | SBPao [mmHg] | 111.5 | ± | 1.0 | 150.2 | ± | 1.0 | §; † | 155.2 | ± | 1.6 | §; † | |
| DBPao [mmHg] | 76.9 | ± | 1.0 | 110.2 | ± | 0.8 | §; † | 105.7 | ± | 1.0 | §; †; # | ||
| PPao [mmHg] | 34.6 | ± | 0.7 | 40.0 | ± | 0.9 | §; † | 49.6 | ± | 1.7 | §; †; # | ||
| AIx [%] | 12.2 | ± | 0.9 | 24.6 | ± | 1.4 | §; † | 30.4 | ± | 1.3 | §; †; # | ||
| PWV [m/s] | 5.2 | ± | 0.0 | 6.3 | ± | 0.0 | §; † | 6.6 | ± | 0.1 | §; †; # | ||
| Post | SBPao [mmHg] | 113.4 | ± | 1.1 | 112.2 | ± | 1.3 | † | 106.1 | ± | 1.6 | §; †; # | |
| DBPao [mmHg] | 77.2 | ± | 0.9 | 75.6 | ± | 1.2 | 67.9 | ± | 1.3 | §; †; # | |||
| PPao [mmHg] | 36.2 | ± | 0.7 | 36.5 | ± | 0.7 | § | 38.2 | ± | 0.9 | §; † | ||
| AIx [%] | 14.2 | ± | 1.2 | 13.4 | ± | 0.9 | 14.9 | ± | 1.3 | ||||
| PWV [m/s] | 5.3 | ± | 0.0 | 5.2 | ± | 0.0 | § | 5.1 | ± | 0.1 | # | ||
| Placebo | Angiotensin II | Phenylephrine | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | sem | ANOVA | Mean | sem | ANOVA | Mean | sem | ANOVA | |||||
| Baseline | Cortisol | 9.2 | ± | 0.6 | 8.7 | ± | 0.8 | 9.1 | ± | 0.7 | |||
| Aldosterone | 62.6 | ± | 4.2 | 51.1 | ± | 2.8 | 47.4 | ± | 3.0 | ||||
| Renin | 4.6 | ± | 0.5 | 4.7 | ± | 0.6 | 4.9 | ± | 0.6 | ||||
| Angiotensin | 7.8 | ± | 0.9 | 15.0 | ± | 2.9 | 8.2 | ± | 1.4 | ||||
| Step 1 (140–150 mmHg) | Angiotensin | 10.7 | ± | 1.4 | 53.8 | ± | 7.7 | §; † | 9.4 | ± | 2.1 | †; # | |
| Step 2 (150–160 mmHg) | Angiotensin | 9.3 | ± | 2.3 | 77.8 | ± | 10.4 | §; † | 8.1 | ± | 1.5 | †; # | |
| Post | Cortisol | 4.6 | ± | 0.4 | § | 7.7 | ± | 1.0 | † | 8.4 | ± | 1.0 | † |
| Aldosterone | 47.0 | ± | 3.1 | § | 68.1 | ± | 3.7 | §; † | 39.3 | ± | 2.8 | §; †; # | |
| Renin | 4.1 | ± | 0.4 | 3.4 | ± | 0.3 | § | 4.8 | ± | 0.6 | # | ||
| Angiotensin | 7.7 | ± | 0.9 | 20.0 | ± | 6.8 | 7.8 | ± | 1.5 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzen, K.F.; Meusel, M.; Engel, J.; Röcker, T.; Drömann, D.; Sayk, F. Differential Effects of Angiotensin-II Compared to Phenylephrine on Arterial Stiffness and Hemodynamics: A Placebo-Controlled Study in Healthy Humans. Cells 2021, 10, 1108. https://doi.org/10.3390/cells10051108
Franzen KF, Meusel M, Engel J, Röcker T, Drömann D, Sayk F. Differential Effects of Angiotensin-II Compared to Phenylephrine on Arterial Stiffness and Hemodynamics: A Placebo-Controlled Study in Healthy Humans. Cells. 2021; 10(5):1108. https://doi.org/10.3390/cells10051108
Chicago/Turabian StyleFranzen, Klaas F., Moritz Meusel, Julia Engel, Tamara Röcker, Daniel Drömann, and Friedhelm Sayk. 2021. "Differential Effects of Angiotensin-II Compared to Phenylephrine on Arterial Stiffness and Hemodynamics: A Placebo-Controlled Study in Healthy Humans" Cells 10, no. 5: 1108. https://doi.org/10.3390/cells10051108
APA StyleFranzen, K. F., Meusel, M., Engel, J., Röcker, T., Drömann, D., & Sayk, F. (2021). Differential Effects of Angiotensin-II Compared to Phenylephrine on Arterial Stiffness and Hemodynamics: A Placebo-Controlled Study in Healthy Humans. Cells, 10(5), 1108. https://doi.org/10.3390/cells10051108






